Background: The molecular identity of the lysosomal transporter for cationic amino acids, system c, remains unknown.
Results: SLC7A14 is a lysosomal localized protein with a functional domain that mediates arginine transport.
Conclusion: SLC7A14 may mediate cationic amino acid transport across lysosomal membranes.
Significance: As system c represents a salvage pathway in the therapy of cystinosis, characterization of SLC7A14 might help to develop better drugs.
Keywords: Amino acid transport, Lysosomal Storage Disease, Lysosomes, Membrane Transport, Protein Chimeras, SLC7A14, System c, Arginine Transport, Cystinosis, Human Cationic Amino Acid Transporter (hCATs)
Abstract
In human skin fibroblasts, a lysosomal transport system specific for cationic amino acids has been described and named system c. We asked if SLC7A14 (solute carrier family 7 member A14), an orphan protein assigned to the SLC7 subfamily of cationic amino acid transporters (CATs) due to sequence homology, may represent system c. Fusion proteins between SLC7A14 and enhanced GFP localized to intracellular vesicles, co-staining with the lysosomal marker LysoTracker®. To perform transport studies, we first tried to redirect SLC7A14 to the plasma membrane (by mutating putative lysosomal targeting motifs) but without success. We then created a chimera carrying the backbone of human (h) CAT-2 and the protein domain of SLC7A14 corresponding to the so-called “functional domain” of the hCAT proteins, a protein stretch of 81 amino acids that determines the apparent substrate affinity, sensitivity to trans-stimulation, and (as revealed in this study) pH dependence. The chimera mediated arginine transport and exhibited characteristics similar but not identical to hCAT-2A (the low affinity hCAT-2 isoform). Western blot and microscopic analyses confirmed localization of the chimera in the plasma membrane of Xenopus laevis oocytes. Noticeably, arginine transport by the hCAT-2/SLC7A14 chimera was pH-dependent, trans-stimulated, and inhibited by α-trimethyl-l-lysine, properties assigned to lysosomal transport system c in human skin fibroblasts. Expression analysis showed strong expression of SLC7A14 mRNA in these cells. Taken together, these data strongly suggest that SLC7A14 is a lysosomal transporter for cationic amino acids.
Introduction
After degradation of proteins in lysosomes, amino acids have to be carried to the cytoplasm by specialized transport proteins. A lysosomal transport system for cationic amino acids has been described in human skin fibroblasts by Pisoni et al. (1, 2) and designated system c. However, its molecular identity has not been identified so far. System c has therapeutic interest because it provides a salvage pathway in the therapy of patients with cystinosis (3). In these individuals, the loss of function of the lysosomal cystine transporter cystinosin leads to accumulation of the disulfide cystine, resulting in crystal deposition and cell damage (4). Patients are treated with oral application of the aminothiol cysteamine (5, 6), which enters the cells and lysosomes and forms a mixed disulfide with one cysteine molecule. This mixed disulfide resembles the cationic amino acid l-lysine and is transported out of the lysosome by a system c transporter. Administration is problematic because cysteamine has an offensive taste and smell and has to be taken according to a strict timetable (every 6 h) (7). Therefore, better therapeutic substances are needed. The knowledge of the salvage transporter would facilitate screening for such compounds. Because of their substrate specificity for cationic amino acids and their partial localization to lysosomes (at least in overexpressing cells), members of the SLC7 (solute carrier family 7) subfamily of cationic amino acid transporters are potential candidates for system c (8, 9).
Recently, an orphan protein has been assigned to the SLC7 family as member A14 due to sequence homology. The SLC7 family is divided into two subgroups (9). The light chains of the heteromeric amino acid transporters (lcHATs)2 are predicted to comprise 12 transmembrane domains. They have to associate with a glycoprotein of the SLC3 family (heavy chain) to localize to the plasma membrane. The substrate selectivity and ion coupling of those glycoprotein-associated amino acid transporters are diversified. In contrast, the cationic amino acid transporters are predicted to comprise 14 transmembrane domains, are glycosylated, and localize to the plasma membrane without coexpression of a second protein. They mediate exclusively Na+-independent transport of cationic l-amino acids. SLC7A14 exhibits a higher sequence identity to the human cationic amino acid transporter (hCAT) compared with the lcHAT subfamily (supplemental Fig. 1). It is also predicted to have 14 putative transmembrane domains, relating it even more closely to the hCAT subfamily (10). In our initial experiments, Xenopus laevis oocytes and U373MG glioblastoma cells expressing an SLC7A14-enhanced GFP (EGFP) fusion protein exhibited an almost exclusive intracellular staining that coincided with the lysosomal marker LysoTracker® in the latter. This suggests that SLC7A14 may represent a lysosomal transport protein. In addition, SLC7A14 was expressed in human skin fibroblasts, where system c has initially been described. We thus hypothesized that SLC7A14 may represent system c. To measure the transport activity of the protein, we first attempted to direct SLC7A14 to the plasma membrane by mutating putative lysosomal targeting sequences, a strategy that has successfully been used in the case of cystinosin (11). However, here we were without success. Therefore, we created a chimera carrying the functional domain of human SLC7A14 in the backbone of hCAT-2. This domain is defined by a stretch of 81 amino acids that resemble the fourth intracellular and fifth extracellular loops and transmembrane domain (TM) 9 and TM10 of all cationic amino acid transporter proteins according to the 14-TM model. It has already been reported that this domain determines the apparent substrate affinity and sensitivity to trans-stimulation (12). The latter refers to a transport property typical for carrier proteins and distinguishing them from channels: carriers often work better or even exclusively in an exchange mode as opposed to unidirectional transport. This can be seen by acceleration of transport by substrate at the trans-side of the membrane (the side to which substrate is transported) and is referred to as trans-stimulation. The hCAT-2/A14_BK chimera mediated arginine transport and was characterized regarding its localization, substrate affinity, pH dependence, trans-stimulation properties, and substrate specificity. The results from these experiments support the notion that SLC7A14 may indeed represent a system c transporter.
EXPERIMENTAL PROCEDURES
Cell Culture
The U373MG glioblastoma cell line was obtained from ATCC (Manassas, VA). Stably transfected cells were grown in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with GlutaMAX, 10% FBS, and 0.2 mg/ml G418. Human skin fibroblasts were a kind gift from Martijn Wilmer (Department of Pharmacology/Toxicology, Nijmegen Centre for Molecular Life Sciences, Nijmegen, The Netherlands) and were cultured in M199 medium, 10% FCS, and 100 units/ml penicillin/streptomycin.
Quantitative RT-PCR
Total RNA from human cells was isolated using an RNeasy mini kit (Qiagen). The expression of amino acid transporters and GAPDH (used as a reference) was determined using a QuantiTect RT-PCR kit (Qiagen) and specific TaqMan hybridization probes as described (13). The following oligonucleotides and hybridization probe were used to detect SLC7A14: ss, 5′-CTGGTGAACATCTATCTCATGC-3′; as, 5′-CTGTTCCAGATGCCATATCC-3′; and TaqMan probe, 5′-6-FAM-AAGCTCTCCACCATCACATGGATCC-TAMRA-Q-3′.
Site-directed Mutagenesis and Generation of Chimeric cDNAs
Site-directed mutagenesis was performed using a QuikChange mutagenesis kit (Stratagene). Restriction sites for BamHI and KpnI were introduced or deleted in the SLC7A14-coding sequence as silent mutations. The sequence of each oligonucleotide pair is presented in supplemental Table I. The introduction of BamHI sites into the hCAT-2A and hCAT-1 sequences and other chimeras have been described previously (4). This and a conserved KpnI site were used to create chimeric cDNAs between SLC7A14 and hCAT-2.
Fluorescent Protein Fusion Constructs and Expression in U373MG Cells
A full-length cDNA clone from human KIAA1613/SLC7A14 (IRAKp961A1742Q2) was obtained from imaGenes (Source BioScience LifeScience, Berlin, Germany). The fluorescent protein fusion construct for mammalian expression was realized by amplification of the cDNA insert of human SLC7A14 by PCR (HotStar HiFidelity polymerase, Qiagen) using oligonucleotides TCAGATCTCTCAAGATGAGTGGCTTCTTCACCTC (which contains a BglII site (underlined) and a start codon (italic)) and CGACCGGTCGCAACTCTGGAGAGTAATCTAACTC (which removes the stop codon and introduces an AgeI site (underlined)) as sense and antisense primers, respectively, and subcloned into the BglII/AgeI sites of pEGFP-N1. The resulting plasmid was named pSLC7A14-EGFP. Constructs for EGFP fusion proteins with hCAT-2A (3) and hCAT-1 (15) have been described previously. Cells were seeded into 6-well plates (2 × 108 cells/well) 1 day before transfection. 8 μl of FuGENE HD (Promega) was added to 160 μl of plasmid DNA (2 μg) in water, mixed, and incubated at room temperature for 10 min. The reaction was then added dropwise to the cells in 1 ml of medium containing FBS. The cells were incubated with transfection reagent for 48 h at 37 °C. Stably transfected cell clones were selected in medium containing 0.2 mg/ml G418. For each construct, several independent clones were selected.
Fluorescence Microscopy
U373MG cells expressing SLC7A14-EGFP were seeded in glass-bottom dishes; stained with 50 nm LysoTracker® Red DND-99, 1 μm MitoTracker® Orange CM-H2TMRos, or 1 μm ER-TrackerTM Red (Invitrogen); and analyzed by confocal laser scanning microscopy.
Expression of cRNA in X. laevis Oocytes
All cDNAs were inserted between the SacII and ClaI sites of pSGEM (14). The plasmids were linearized, and cRNA was prepared by in vitro transcription from the T7 promoter (mMESSAGE mMACHINE in vitro transcription kit, Ambion). 10 ng of cRNA in 20 nl of water was injected in each X. laevis oocyte (Dumont stages V and VI). Non-injected oocytes were used as controls. All experiments were performed 3 days after cRNA injection.
Transport Studies in X. laevis Oocytes
To measure arginine uptake, oocytes were washed three times with ice-cold uptake solution (100 mm NaCl, 2 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm HEPES, and 5 mm Tris, pH 7.5). The oocytes were then transferred to the same solution supplemented with the indicated concentrations of unlabeled l-amino acids (0.01–10 mm) and l-[3H]arginine (10 μCi/ml; ICN). After a 15-min incubation at 20 °C, oocytes were washed five times with ice-cold uptake solution and solubilized individually in 2% SDS. The radioactivity of the lysates was determined in a liquid scintillation counter. For trans-stimulation and efflux experiments, cRNA-injected oocytes were each injected a second time with 3.6 nmol of l-[3H]arginine (3.6 nCi) in 36 nl of water. The oocytes were then immediately transferred into uptake solution (with pH and amino acid concentration as indicated). After a 30-min incubation at 20 °C, the l-[3H]arginine that had accumulated in the extracellular buffer was determined by liquid scintillation counting.
Cell Lysates and Biotinylation of Cell Surface Proteins
All steps were performed at 4 °C. Oocytes were rinsed three times with PBS (0.1 m NaCl, 2 mm KCl, 1.76 mm KH2PO4, and 10.1 mm Na2HPO4) containing 0.1 mm CaCl2 and 1 mm MgCl2 (PBS/CM) and then incubated in PBS/CM containing 1 mg/ml sulfosuccinimidobiotin (EZ-LinkTM, sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate, Thermo Fisher Scientific) for 30 min. The biotinylation reaction was stopped by incubating oocytes in PBS/CM containing 50 mm NH4Cl for 10 min and rinsing four times with PBS/CM. After lysis in radioimmune precipitation assay buffer (0.15 mm NaCl, 1 mm EDTA, pH 8, 0.1 mm Tris, pH 7.2, 1% Triton X-100, 1% deoxycholate, and 0.1% SDS) containing protease inhibitors (Complete mini EDTA-free protease inhibitor tablets, Roche), an aliquot of each whole oocyte lysate was mixed directly with an equal volume of 2× sample buffer (125 mm Tris base, 20% (v/v) glycerol, 5% SDS, 0.001% (w/v) bromophenol blue, 8 m urea, and 2% mercaptoethanol) and incubated for 10 min at 37 °C. Biotinylated surface proteins from the remaining lysate were incubated overnight with avidin-coated Sepharose beads (immobilized NeutrAvidinTM, Thermo Fisher Scientific). The beads were then washed three times with 50 mm Tris, pH 8, 0.5 mm, NaCl, 1 mm EDTA, pH 8, 0.5% Triton X-100, and 0.1% SDS and once with 50 mm Tris, pH 7.4, 1 mm EDTA, 0.5% Triton X-100, and 0.1% SDS. All washing solutions contained 0.2 mm PMSF. Biotinylated proteins were released from the beads by incubation in 1× sample buffer for 10 min at 37 °C and analyzed by Western blotting.
Western Blotting
Lysates were separated by 10% SDS-PAGE and then blotted onto nitrocellulose transfer membranes (Protran 83, Whatman). Staining for EGFPs was achieved by sequential incubations in Blotto (50 mm Tris, pH 8, 2 mm CaCl2, 0.01% antifoam A (Sigma-Aldrich), 0.05% Tween 20, and 5% nonfat dry milk) for 2 h at room temperature to block unspecific binding sites; a 1:3000 dilution of rabbit anti-EGFP antibody (Living Colors® full-length A.v. polyclonal antibody, Clontech) in Blotto overnight at 4 °C; three times in Blotto for 10 min at room temperature; a 1:15,000 dilution of peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Calbiochem) in Blotto for 1 h at room temperature; three times in 10 mm Tris, pH 8, 150 mm NaCl, and 0.05% Tween 20; once in 10 mm Tris-HCl, pH 8, and 150 mm NaCl; and finally, in chemiluminescence reagent (Western Lightning® Plus ECL, PerkinElmer Life Sciences) for 1 min. Chemiluminescence films (Amersham Biosciences HyperfilmTM ECL, GE Healthcare) were then immediately exposed to the membranes. For standardization, membranes were stained with anti-β-tubulin monoclonal antibody (1:3000; Sigma-Aldrich) and peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5000; Sigma-Aldrich).
RESULTS
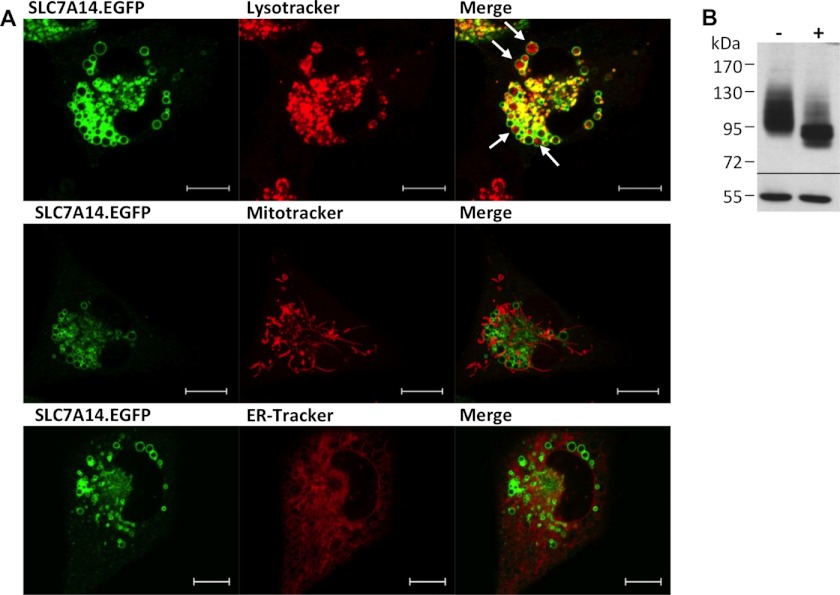
Our initial experiments in which we expressed the orphan protein SLC7A14 in X. laevis oocytes revealed no increase in arginine transport compared with control oocytes (data not shown). To prove protein expression and investigate the subcellular localization of the orphan, we fused EGFP to the C terminus of SLC7A14. Expression of this fusion protein revealed no plasma membrane staining in either X. laevis oocytes or human U373MG glioblastoma cells. In the latter, staining of intracellular vesicles that also lit up with the lysosomal marker LysoTracker® was observed (Fig. 1A). In contrast, overexpressed hCAT-1-EGFP (and all other hCATs) was clearly localized to the plasma membrane in these cells, in addition to intracellular membranes (data not shown). EGFP alone was equally distributed in the cytoplasm (data not shown). SLC7A14 and hCAT-1 coincided in intracellular membranes, but not at the plasma membrane (data not shown). For SLC7A14-EGFP, localization in the mitochondria and endoplasmic reticulum could be excluded by staining of the cells with MitoTracker® and ER-TrackerTM. Western blot analyses revealed that like hCAT proteins (and unlike lcHATs), SLC7A14 was glycosylated (Fig. 1B). As in X. laevis oocytes, overexpression of SLC7A14-EGFP in U373MG cells did not lead to enhanced arginine transport in comparison with control cells (data not shown). We thus hypothesized that SLC7A14 may represent a lysosomal transporter for cationic amino acids as described for human skin fibroblasts and designated system c. In fact, SLC7A14 mRNA was expressed in primary human skin fibroblasts from six different donors (Fig. 2). Related to GAPDH (and assuming equal efficiency of the RT-PCR), mRNA expression of SLC7A14 was about half of that of hCAT-1. hCAT-2B and hCAT-3 mRNA expression was marginal, and hCAT-2A mRNA could not be detected at all. There was no difference in SLC7A14 expression between cystinotic and wild-type cells (data not shown).
FIGURE 1.
Localization of SLC7A14 in human U373MG glioblastoma cells. A, U373MG cells overexpressing the SLC7A14-EGFP fusion protein (stable transfection; green) were stained with LysoTracker®, MitoTracker®, or ER-TrackerTM (red). Cells were analyzed by confocal laser scanning microscopy. Scale bars = 10 μm. B, U373MG cells overexpressing SLC7A14-EGFP were lysed and analyzed by Western blotting. Proteins with (+) or without (−) glycosidase treatment were separated by 7.5% SDS-PAGE, blotted, and probed with anti-GFP antibody (upper panel) and anti-tubulin antibody as a loading control (lower panel).
FIGURE 2.
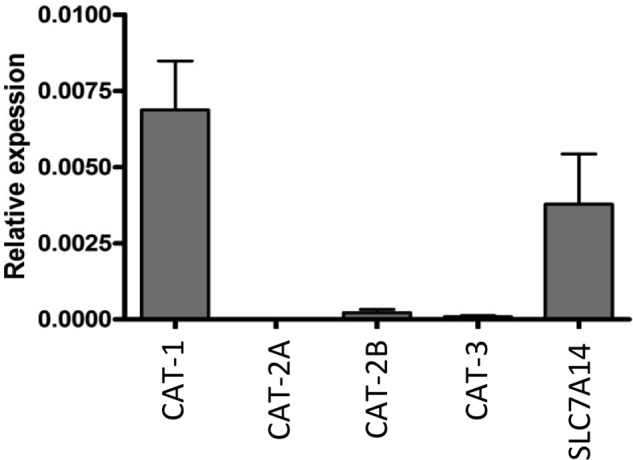
mRNA expression of SLC7A14 and hCATs in human skin fibroblasts. Total RNA was isolated from skin fibroblasts of six donors. mRNAs of SLC7 family members were measured by quantitative RT-PCR. GAPDH was chosen as the housekeeping gene for relative determinations and was set at 1. Bars represent means ± S.E. (n = 6).
To measure transport activity, we first tried to redirect SLC7A14 to the plasma membrane by mutating putative lysosomal targeting sequences but without success. Therefore, a chimera carrying the so-called functional domain of SLC7A14 in the backbone of hCAT-2 was created. This chimera was named hCAT-2/A14_BK, as the exchanged fragment in the respective cDNAs is framed by BamHI and KpnI restriction sites (Fig. 3A). According to the 14-TM model, the SLC7A14 fragment introduced into hCAT-2 comprised the fourth intracellular and fifth extracellular loops, part of TM8, TM9, and TM10 (supplemental Fig. 2). Like hCAT-2A, an EGFP fusion protein of the hCAT-2/A14_BK chimera localized to the plasma membrane of X. laevis oocytes as revealed by fluorescence microscopy (Fig. 3B). In contrast, EGFP alone and SLC7A14-EGFP remained intracellular. Western blot analyses of whole cell lysates and cell surface-biotinylated protein fractions confirmed plasma membrane localization of the chimera. In contrast to fluorescence microscopy, this method revealed a very small portion of SLC7A14-EGFP to be localized to the plasma membrane (Fig. 3C).
FIGURE 3.
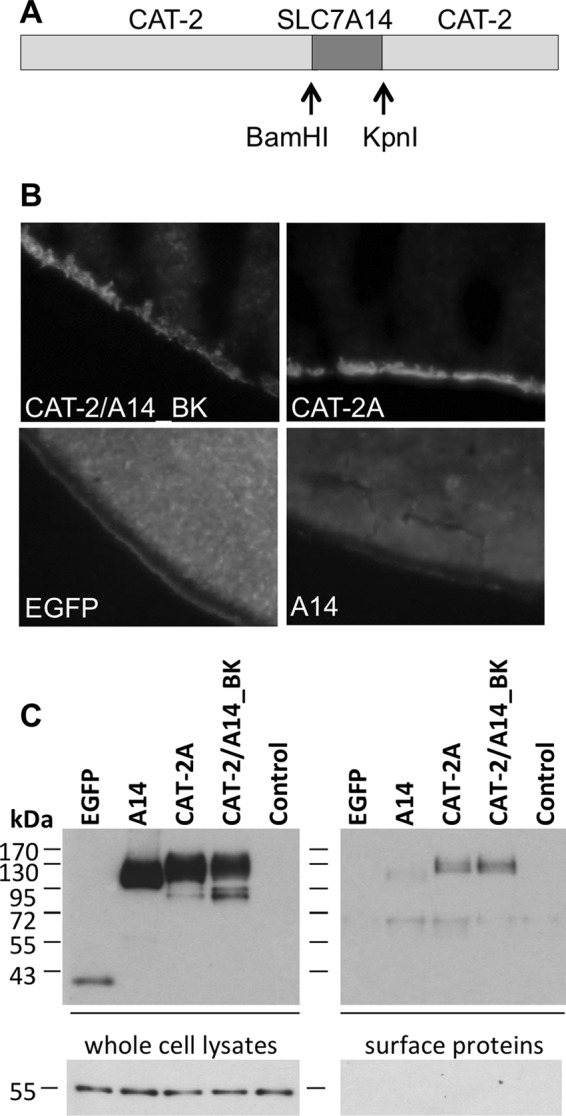
Localization of SLC7A14, hCAT-2/A14_BK, and hCAT-2A in Xenopus oocytes. A, scheme of the chimeric protein hCAT-2/A14_BK. B, Xenopus oocytes were frozen in tissue freezing medium 3 days after cRNA injection. Cryosections (12 μm) were analyzed by fluorescence microscopy. C, proteins of Xenopus oocytes expressing the EGFP fusion protein or EGFP alone as indicated were biotinylated at the cell surface. Non-injected oocytes served as controls. Subsequently, oocytes were lysed for Western blot analysis. Total lysates (left panels) and surface proteins (separated from non-biotinylated intracellular proteins by avidin-coated beads; right panels) were separated by 10% SDS-PAGE, blotted, and probed with anti-GFP antibody (upper panels) and subsequently with anti-tubulin antibody as a loading control (lower left panel) and successful withdrawal of intracellular proteins (lower right panel).
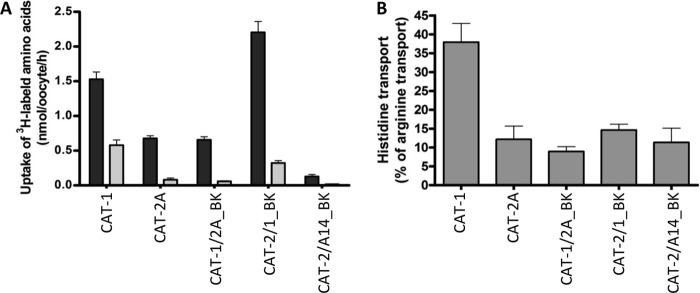
Transport measurements using 1 mm l-[3H]arginine and an incubation time of 15 min (to stay in the linear part of the uptake) (supplemental Fig. 3) demonstrated that the hCAT-2/A14_BK chimera mediated arginine transport (0.61 ± 0.06 compared with 1.38 ± 0.16 nmol of arginine/oocyte/h mediated by hCAT-2A at pH 7.5). The apparent substrate affinity of the chimera was determined at arginine concentrations between 0.01 and 10 mm (Table 1). The arginine concentrations at which half-maximal transport rates were reached (apparent Km) were calculated by fitting the data according to the Eadie-Hofstee equation after subtraction of the values obtained with non-injected oocytes. The apparent affinity of the hCAT-2/A14_BK chimera (Km = 1.93 ± 0.19 mm) was low, similar to the apparent affinity of hCAT-2A (Km = 1.45 ± 0.02 mm). As reported earlier, hCAT-1 exhibited a significantly higher apparent affinity for arginine (Km = 0.6 ± 0.06 mm) (15).
TABLE 1.
Apparent Km of the hCAT-2/A14_BK chimera compared with those of hCAT-1 and hCAT-2A
For expression of hCAT-2/A14_BK, hCAT-2A and hCAT-1 cRNAs were injected in X. laevis oocytes. Non-injected oocytes served as controls. The dependence of l-[3H]arginine uptake on the extracellular amino acid concentration (0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mm) was measured. The results represent means ± S.E. (n = 3–4).
| Km for l-arginine | Vmax | |
|---|---|---|
| μm | nmol l-arginine/oocyte/h | |
| CAT-1 | 0.567 ± 0.055 | 3.083 ± 0.466 |
| CAT-2A | 1.445 ± 0.018 | 4.208 ± 0.615 |
| CAT-2/A14_BK | 1.929 ± 0.194 | 2.511 ± 0.38 |
Next, we determined in efflux experiments in which labeled substrate was injected into the oocytes whether the chimera is trans-stimulated (Fig. 4), e.g. if it exhibits higher transport rates, when substrate is present at the opposite side of the membrane. This is characteristic for hCAT-1. In the absence of trans-substrate, efflux mediated by the chimera was reduced by 47 ± 4.5% (compared with efflux into buffer containing 1 mm arginine). Although trans-stimulation was not as pronounced as for hCAT-1 (87 ± 0.7% reduced transport without trans-substrate), it was clearly different from hCAT-2A, which exhibited no trans-stimulation.
FIGURE 4.
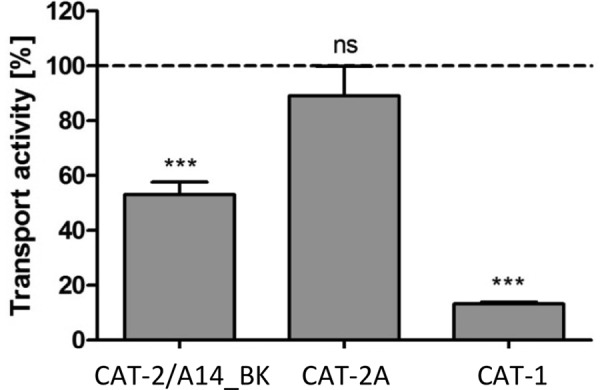
Trans-stimulation of hCAT-2/A14_BK in comparison with hCAT-1 and hCAT-2A. Xenopus oocytes expressing the indicated transporters were incubated in amino acid-free medium for 4–6 h and then injected with 36 nl of 100 mm l-[3H]arginine (100 μCi/ml). Three oocytes each were incubated for 30 min in pH 7.5 buffer containing either no amino acids or 1 mm arginine. Subsequently, the radioactivity in the supernatants was determined. Values obtained with non-injected oocytes were subtracted, and the radioactivity in buffer containing no amino acids is expressed as a percentage of the respective radioactivity detected in the extracellular buffer containing 1 mm l-arginine (100%, dashed line). Bars represent means ± S.E. (n = 9–15), with at least three different batches of oocytes. Statistical analysis was performed using analysis of variance with the Bonferroni post hoc test. ***, p ≤ 0.001; n.s., not significant.
We then asked if pH differences influence the transport activity of the chimera by varying the extracellular pH from 5 to 8.5 in influx experiments. Indeed, the transport activity of the chimera was continually increased from pH 5 to 8.5: transport rates at pH 5 were only 24.7 ± 3.2% of the rates measured at pH 7.5 (Fig. 5A). The pH dependence of the chimera was thus similar to that of hCAT-2 (A or B) and significantly different from that of hCAT-1 (Fig. 5A), which is largely pH-independent (15). Until now, it was not clear if the functional domain also defines the pH dependence of the hCAT proteins. To answer this question, a chimera carrying the functional domain of hCAT-1 in the backbone of hCAT-2 (hCAT-2/1_BK) was investigated. Like hCAT-1, hCAT-2/1_BK was pH-independent (Fig. 5A), suggesting that pH dependence is indeed determined by the functional domain. Arginine efflux mediated by hCAT-2/A14_BK and hCAT-2A was also reduced at extracellular pH 5 compared with pH 7.5, whereas hCAT-1-mediated efflux was again pH-independent (Fig. 5B).
FIGURE 5.
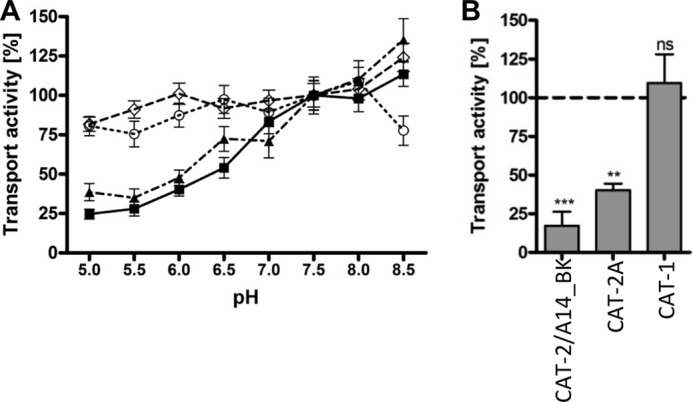
pH dependence of l-arginine transport mediated by hCAT-2/A14_BK in comparison with hCAT-1 and hCAT-2A. Xenopus oocytes were injected with cRNAs encoding individual transporters. A) the dependence of uptake of 1 mm l-[3H]arginine into oocytes expressing hCAT-2/A14_BK (■), hCAT-2A (▴), hCAT-1 (○), or hCAT-2/1_BK (♢) on pH in the extracellular buffer was measured. B, 36 nl of 100 mm l-[3H]arginine (100 μCi/ml) were injected into the oocytes. Efflux measured in the extracellular buffer containing 1 mm arginine at pH 5 is expressed as a percentage of efflux at pH 7.5 (dashed line). Data points and bars represent means ± S.E. (n = 10–20 for influx and 9–15 for efflux experiments), with at least two different batches of oocytes. Statistical analysis was performed using analysis of variance with the Bonferroni post hoc test. ***, p ≤ 0.001; **, p ≤ 0.01; n.s., not significant.
Pisoni et al. (2) identified cationic amino acid derivatives that interfere with transport by lysosomal system c, but not system y+. In accordance with these observations, transport of the chimera, but not of hCAT-1, hCAT-2B, and hCAT-3 (all system y+ transporters), was inhibited by ϵ-trimethyl-l-lysine (Fig. 6). However, inhibition of the chimera was less pronounced than reported for system c in human skin fibroblasts (34% versus 50%). Transport by hCAT-2A (which is not expressed in skin fibroblasts and is not a y+ transporter) was inhibited to a similar extent as the chimera. In contrast, hCAT-2/1_BK remained unaffected. This suggests the functional domain to be involved in substrate recognition.
FIGURE 6.
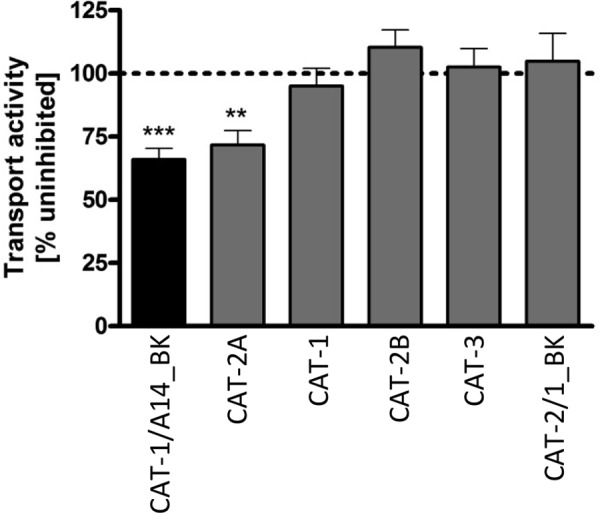
Transport activity in the presence of the system c inhibitor ϵ-trimethyl-l-lysine. Xenopus oocytes were injected with cRNAs encoding the indicated transporters. The uptake of 30 μm l-[3H]arginine in the absence or presence of 3 mm ϵ-trimethyl-l-lysine was determined. Values obtained in the absence of the putative inhibitor were set as 100% (data not shown). Bars represent means ± S.E. (n = 10–20), with at least two different batches of oocytes. Statistical analysis was performed using analysis of variance with the Bonferroni post hoc test. ***, p ≤ 0.001; **, p ≤ 0.01 compared with untreated controls.
To further elucidate this question, we investigated whether histidine recognition is determined by the functional domain. We have previously established that histidine is a substrate for hCAT-1 at low pH (when it carries a positive charge) (19). Here we found, that in contrast, hCAT-2A transported only very little histidine under this condition (Fig. 7A). The hCAT-1/2A_BK chimera was very similar to hCAT-2A, supporting the notion that the functional domain is involved in substrate recognition. The rates of histidine transport by the reciprocal chimera hCAT-2/1 were higher compared with hCAT-2A or hCAT-1/2A. However, when related to arginine transport rates (which were higher at pH 5.5 in the transporters carrying the pH-independent functional domain of hCAT-1), histidine recognition by hCAT-2/1 was not different compared with hCAT-2A (Fig. 7B). This suggests that the functional domain is necessary but not sufficient for substrate recognition. The hCAT-2/A14_BK chimera was similar to hCAT-2A in histidine recognition (relative to arginine).
FIGURE 7.
Substrate recognition of several chimeric proteins in comparison with the wild-type transporters. Xenopus oocytes were injected with cRNAs encoding the indicated transporters. A, the uptake of 1 mm l-[3H]arginine (black bars) or 1 mm [3H]histidine (gray bars) was measured at pH 5.5. Data points represent means ± S.E. (n = 13–28), with at least two different batches of oocytes. B, the same data as shown in A, but histidine transport is expressed as a percentage of the arginine transport measured in parallel by the same transporter.
DISCUSSION
The aim of our study was to elucidate the physiological function of SLC7A14, an orphan protein assigned to the amino acid transporter family SLC7 due to sequence homology. Within this family, SLC7A14 is most closely related to the subfamily of cationic amino acid transporters (42–45% identity in amino acid sequence compared with 16–20% identity to the subfamily of lcHATs) (supplemental Fig. 1). In addition, like all hCATs, SLC7A14 is predicted to exhibit 14 TMs by most analysis software. This is in contrast to lcHATS, with only 12 putative TMs.
In mammalian cells, SLC7A14 localized almost exclusively to intracellular vesicles that were not stained with MitoTracker® or ER-TrackerTM but with the lysosomal marker LysoTracker®. This is in contrast to all other known hCAT proteins that are clearly residents of the plasma membrane (Fig. 1) (8). However, hCAT proteins were also found in lysosomes, where they coincided with lysosomal markers.3 If they function as amino acid transporters in these compartments remains to be determined. The intracellular localization of SLC7A14 is different from the intracellular localization of lcHATs that are trapped in the endoplasmic reticulum when their respective glycoprotein partner is missing (16). In contrast, hCAT proteins do not seem to need a partner protein to traffic to the plasma membrane. Using fluorescence microscopy, the SLC7A14-EGFP fusion protein was detected solely in lysosomes also in all other cell types tested (U373 glioblastoma, Huh-7 hepatoma, A673 neuroepithelioma, NT2 teratocarcinoma, and TGW neuroblastoma cells) (data not shown), including two that exhibited endogenous expression of SLC7A14. This was also true when the fluorescent protein DsRed (dimeric or monomeric) was fused to the C or N terminus of SLC7A14, indicating that the subcellular localization is independent of the position or type of fluorescent protein in the fusion protein (data not shown). We found a very small portion of SLC7A14 to be biotinylated at the cell surface of X. laevis oocytes, suggesting that the transporter may first be incorporated into the plasma membrane before it traffics to lysosomes. This has also been observed for other lysosomal proteins (17). Taken together, these results indicate that SLC7A14 is truly a lysosomal resident. However, antibodies against SLC7A14 are necessary to examine the subcellular localization of the native protein in primary cells and tissues.
In addition to its expression in human skin fibroblasts, high levels of SLC7A14 mRNA were detected in the CNS (brain, cerebellum, and spinal cord) using a human multi-tissue RNA panel (supplemental Fig. 4A). This is consistent with findings in other bilateral species (18). Marginal mRNA levels could be detected in trachea, kidney, prostate, salivary gland, testis, and whole skin, whereas no SLC7A14 mRNA was detected in heart, fetal liver, placenta, thymus, and thyroid gland. Among various human cell lines tested, human umbilical vein endothelial cells and NB-OK-1 and TGW-1 neuroblastoma cells exhibited considerable SLC7A14 expression (supplemental Fig. 4B). SLC7A14 thus seems to be expressed primarily in skin fibroblasts, neuronal cells, and primary endothelial cells. If SLC7A14 indeed represents system c in these cells, a different transporter has to mediate lysosomal transport of cationic amino acids in tissues lacking SLC7A14 expression.
Our mRNA analyses showed that hCAT-1 and SLC7A14 are the only members of the hCAT subfamily that are expressed at an appreciable level in human skin fibroblasts. As Pisoni et al. (1, 2) found clear differences in the transport activities of systems c and y+ (hCAT-1), it seems unlikely that hCAT-1 is responsible for the system c activity described in fibroblast lysosomes. On the other hand, the chimera carrying the functional domain of SLC7A14 had transport properties very similar to system c (and different from system y+/hCAT-1): apparent low substrate affinity, pH dependence, moderate trans-stimulation, and inhibition by ϵ-trimethyl-l-lysine.
A crucial question is thus if the transport properties of the chimera reflect those of SLC7A14. Several findings indicate that this is indeed the fact. (i) The observation that the chimera mediated arginine transport suggests that its functional domain is derived from a transporter that recognizes this amino acid as substrate. In addition, the cationic amino acid lysine was recognized by the chimeric protein equally well as arginine (supplemental Fig. 5). (ii) Our previous work has shown that the functional domain defines the apparent substrate affinity and sensitivity to trans-stimulation (12). Here we have further shown that the pH dependence of the transport activity is also determined by this domain. Like the donor of the functional domain in the chimera hCAT-2/1_BK (hCAT-1), the chimera itself was pH-independent. As the chimera hCAT-2/A14_BK was pH-dependent, it seems very likely that the donor of its functional domain, SLC7A14, is a pH-dependent transporter as described for system c. (iii) In contrast to hCAT-1, the chimera hCAT-1/2A_BK recognized histidine very poorly, demonstrating that the functional domain is necessary for substrate recognition. However, other protein regions seem also to be involved in this process, as the introduction of the functional domain of hCAT-1 into the hCAT-2 backbone was not sufficient to fully confer histidine recognition. However, the importance of the functional domain in substrate recognition, together with the observed arginine transport activity for hCAT-2/A14_BK, strongly suggests that SLC7A14 is also an arginine transporter. In addition, this indicates that α-trimethyl-l-lysine is most likely recognized by SLC7A14. (iv) The transport properties of hCAT-2/A14_BK were similar but not identical to those of hCAT-2A. The chimera exported a 2-fold higher amount of arginine into buffer containing 1 mm arginine than into buffer without any cationic amino acid, whereas hCAT-2A was not trans-stimulated. Therefore, hCAT-2/A14_BK is clearly different from its backbone donor, hCAT-2A, and also from the system y+ transporter hCAT-1, which exhibited a more pronounced (7.6-fold) trans-stimulation than the chimera. The trans-stimulation of the chimera in fact resembles system c-mediated lysine efflux from lysosomes, which is 2-fold higher into lysine-containing buffer compared with buffer without amino acids (1). Taken together, our data suggest that the system c-like transport properties of the chimera hCAT-2/A14_BK reflect the transport properties of SLC7A14.
The “wrong” pH dependence of system c has already been discussed by Pisoni et al. (2). For a lysosomal exporter, one would rather expect that the transport activity increases at low luminal pH (which is equivalent to extracellular pH in our transport assay). Instead, the activities of system c and the functional domain of SLC7A14 decrease at low pH, suggesting that they mediate substrate uptake into rather than efflux out of lysosomes. In our experiments, we varied only the pH at the extracellular space that corresponds to the intralysosomal space when considering the orientation of the transporter. In contrast, measuring uptake in isolated lysosomes, Pisoni et al. varied the pH at the extralysosomal side (corresponding to the cytoplasmic side). Efflux out of oocytes (corresponding to uptake into lysosomes) was just as poor at low pH as influx into oocytes (corresponding to export out of lysosomes). In addition, the pH dependence was identical under trans-stimulated and non-trans-stimulated conditions (data not shown). This indicates that, in general, SLC7A14 has only weak transport activity at low pH.
hCAT-2/A14_BK showed an apparent low substrate affinity, similar to the low affinity of hCAT-2A and significantly different from the high affinity of hCAT-1. In comparison with system y+, low substrate affinity has also been described by Pisoni et al. (2) for system c.
Until now, it was not clear whether the functional domain also determines pH dependence. The pH independence of hCAT-2/1 (a chimeric protein with the functional domain of pH-independent hCAT-1 in the backbone of pH-dependent hCAT-2) supports this notion. It seems thus likely that the pH-dependent arginine transport of the hCAT-2/A14 chimera is a genuine characteristic of SLC7A14.
In summary, the data presented here suggest that SLC7A14 may represent a lysosomal transporter for cationic amino acids resembling system c because (i) it localizes almost exclusively to lysosomes in mammalian cells; (ii) it is expressed in human skin fibroblasts, in which system c was first described; and most important, (iii) its functional domain confers arginine transport activity similar to system c. However, final proof has to await establishment of an assay in which transport of the native protein can be measured directly. In addition, other lysosomal transporters for cationic amino acids must exist in cells not expressing SLC7A14.
Supplementary Material
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grant Cl 100/4-3 (to E. I. C.) and a Cystinosis Research Foundation common grant (to B. G. and E. I. C.).

This article contains supplemental Figs. 1–5 and Table I.
I. Jaenecke, J.-P. Boissel, M. Lemke, J. Rupp, B. Gasnier, and E. I. Closs, unpublished data.
- lcHAT
- light chain of heteromeric amino acid transporter
- hCAT
- human cationic amino acid transporter
- EGFP
- enhanced GFP
- TM
- transmembrane domain.
REFERENCES
- 1. Pisoni R. L., Thoene J. G., Christensen H. N. (1985) Detection and characterization of carrier-mediated cationic amino acid transport in lysosomes of normal and cystinotic human fibroblasts. Role in therapeutic cystine removal? J. Biol. Chem. 260, 4791–4798 [PubMed] [Google Scholar]
- 2. Pisoni R. L., Thoene J. G., Lemons R. M., Christensen H. N. (1987) Important differences in cationic amino acid transport by lysosomal system c and system y+ of the human fibroblast. J. Biol. Chem. 262, 15011–15018 [PubMed] [Google Scholar]
- 3. Gahl W. A., Thoene J. G., Schneider J. A. (2002) Cystinosis. N. Engl. J. Med. 347, 111–121 [DOI] [PubMed] [Google Scholar]
- 4. Schulman J. D., Bradley K. H., Seegmiller J. E. (1969) Cystine: compartmentalization within lysosomes in cystinotic leukocytes. Science 166, 1152–1154 [DOI] [PubMed] [Google Scholar]
- 5. Cairns D., Anderson R. J., Coulthard M., Terry J. (2002) Cystinosis and its treatment. Pharm. J. 269, 615–616 [Google Scholar]
- 6. Markello T. C., Bernardini I. M., Gahl W. A. (1993) Improved renal function in children with cystinosis treated with cysteamine. N. Engl. J. Med. 328, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 7. Levtchenko E. N., van Dael C. M., de Graaf-Hess A. C., Wilmer M. J., van den Heuvel L. P., Monnens L. A., Blom H. J. (2006) Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr. Nephrol. 21, 110–113 [DOI] [PubMed] [Google Scholar]
- 8. Wolf S., Janzen A., Vékony N., Martiné U., Strand D., Closs E. I. (2002) Expression of solute carrier 7A4 (SLC7A4) in the plasma membrane is not sufficient to mediate amino acid transport activity. Biochem. J. 364, 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch. Eur. J. Physiol. 447, 532–542 [DOI] [PubMed] [Google Scholar]
- 10. Closs E. I., Boissel J. P., Habermeier A., Rotmann A. (2006) Structure and function of cationic amino acid transporters (CATs). J. Membr. Biol. 213, 67–77 [DOI] [PubMed] [Google Scholar]
- 11. Kalatzis V., Cherqui S., Antignac C., Gasnier B. (2001) Cystinosin, the protein defective in cystinosis, is a H+-driven lysosomal cystine transporter. EMBO J. 20, 5940–5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Habermeier A., Wolf S., Martiné U., Gräf P., Closs E. I. (2003) Two amino acid residues determine the low substrate affinity of human cationic amino acid transporter 2A. J. Biol. Chem. 278, 19492–19499 [DOI] [PubMed] [Google Scholar]
- 13. Rotmann A., Simon A., Martiné U., Habermeier A., Closs E. I. (2007) Activation of classical protein kinase C decreases transport via systems y+ and y+L. Am. J. Physiol. Cell Physiol. 292, C2259–C2268 [DOI] [PubMed] [Google Scholar]
- 14. Liman E. R., Tytgat J., Hess P. (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871 [DOI] [PubMed] [Google Scholar]
- 15. Closs E. I., Gräf P., Habermeier A., Cunningham J. M., Förstermann U. (1997) Human cationic amino acid transporters hCAT-1, hCAT-2A, and hCAT-2B: three related carriers with distinct transport properties. Biochemistry 36, 6462–6468 [DOI] [PubMed] [Google Scholar]
- 16. Reig N., Chillarón J., Bartoccioni P., Fernández E., Bendahan A., Zorzano A., Kanner B., Palacín M., Bertran J. (2002) The light subunit of system bo,+ is fully functional in the absence of the heavy subunit. EMBO J. 21, 4906–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janvier K., Bonifacino J. S. (2005) Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol. Biol. Cell 16, 4231–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sreedharan S., Stephansson O., Schiöth H. B., Fredriksson R. (2011) Long evolutionary conservation and considerable tissue specificity of several atypical solute carrier transporters. Gene 478, 11–18 [DOI] [PubMed] [Google Scholar]
- 19. Vékony N., Wolf S., Boissel J.-P., Gnauert K., Closs E. I. (2001) Human cationic amino acid transporter hCAT-3 is preferentially expressed in peripheral tissues. Biochemistry 40, 12387–12394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.