Abstract
Abnormal activation of the Sonic hedgehog (Shh) signaling pathway has been demonstrated in a number of human tumors, including prostate cancer. The study aimed to assess the activity of Shh pathway components (Shh, Gli1, Gli2 and Gli3), as well as the proliferation markers FoxA1 and Notch1 during cancer progression in the transgenic adenocarcinoma of the mouse prostate (TRAMP). We evaluated changes in respective proteins by immunohistochemistry at three time points (12, 17 and 21 weeks of age) in the tissue of TRAMP and C57Bl/6 mice. Moreover, the expression of mRNA of these proteins was assessed. The present study shows a significant age-dependent increase in the number of Shh, Gli1, Gli3 and FoxA1-positive prostate cells and a decrease in Gli2-positive cells in TRAMP. The study also supports the hypothesis that the development of prostate cancer and its metastasis is associated with activation of the Shh signaling pathway.
Keywords: transgenic adenocarcinoma of the mouse prostate, mice, immunohistochemistry
Introduction
The Hedgehog (Hh) signaling pathway plays an important role in embryogenesis and histogenesis (1). There are three mammalian Hh genes: Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh). Ihh plays an important role in chondrogenesis (2), whereas Dhh is necessary for the development of peripheral nerves and spermatogenesis (3,4). In mammals, the role of Shh is well established in limb development, tissue polarity and patterning of the central nervous system (1). Shh is also involved in the morphogenesis of the axial skeleton, lungs, skin, hair and teeth (1,5). Hh proteins activate the signaling cascade by binding to the membrane-bound receptor Patched that leads to the activation of transmembrane protein Smoothened and the sequential activation of intracellular signal transduction (1). As a result, the expression of Hh target genes is initialized through the activation of Gli transcription factors, which in mammals contains Gli1, Gli2 and Gli3 homologues (5,6).
The role of Shh is not restricted to embryonic development. Previous studies proved that Shh is also required for adult stem cell (SC) proliferation and differentiation (7). Thus, anti-Shh antibodies block the proliferation of human haematopoietic SC; the addition of purified Shh accordingly induces cell proliferation and differentiation in vitro (8). However, an inappropriate activation of Hh signaling can initiate tumorigenesis in different tissues (9–11). It has been shown that human tumors such as basal cell carcinoma, medulloblastoma, rhabdomyosarcoma, fibroma and meningioma are associated with abnormalities in the Shh signaling pathway (12).
Recent data have shown that the Shh pathway is involved in prostate development (13). These data have also implicated the Shh pathway in the development of prostate cancer, the second most prevalent cause of male cancer-related death in the world. Thus, in vitro blocking of the Shh pathway inhibits the proliferation of human prostate cancer cell lines and cells in primary prostate cancer cultures (10,14,15). Moreover, the activation of the Shh pathway has been detected in human metastatic and high-grade prostate tumors (15). Human prostate cancer is associated with mutations and loss of heterozygosity in the Shh pathway negative regulator suppressor of fused (15). In contrast to accumulated human data, recent in vivo data from the LADY prostate cancer mice model have shown that the expression level of Shh and other components of the Hh signaling pathway (Ptc1 and Gli1) are not increased during prostate tumor development (16).
The present study aimed to evaluate the activity of the Shh pathway during prostate cancer progression. To assess a possible correlation between activation of the Shh pathway and prostate cancer development, we examined the expression of the different Shh pathway proteins (Shh, Gli1, Gli2 and Gli3) and the proliferation markers FoxA1 and Notch1 in prostate biopsies in C57Bl/6 (WT) and transgenic adenocarcinoma of the mouse prostate (TRAMP) mice at 12, 17 and 21 weeks of age.
Materials and methods
Animals and tissue preparation
Heterozygous male TRAMP and WT mice (Jackson Laboratory, USA) were maintained under conventional housing conditions. Animal procedures were approved by the Ethics Committee of the University of Tartu. Animals were euthanized using cervical dislocation. After excision, a part of each prostate was immediately frozen for RNA isolation. For immunohistochemistry, tissues were embedded in Tissue-Tek O.C.T (Sakura Finetek, The Netherlands) and frozen. Samples were stored at −70°C until further analysis.
Immunohistochemistry
Prostate blocks were cut into 10-μm sections (Microm HM500OM; Carl Zeiss, Germany). After fixation, peroxidase was blocked in 0.75% hydrogen peroxide in methanol. After incubation with 3% normal goat serum (Sigma-Aldrich, USA), sections were incubated overnight at 4°C with either rabbit anti-Gli1 IgG polyclonal antibody (pAb) (1:200; Santa Cruz Biotechnology, USA) (17,18), rabbit anti-Gli2 IgG, pAb (1:200; Santa Cruz Biotechnology) (19), mouse anti-Gli3 IgM, monoclonal antibody (mAb) (1:100, clone 5E1; reviewed in ref. 20), mouse anti-Shh IgG, mAb (1:50, clone 5E1; Developmental Studies Hybridoma Bank, USA) (21,22), mouse anti-FoxA1 IgG pAb (1:50; Cemines, USA) (23) or rat anti-Notch1 IgG pAb (1:10; Developmental Studies Hybridoma Bank) (24,25). Sections were then incubated with secondary antibodies for 1 h at room temperature. HRP-conjugated goat anti-rabbit IgG and goat anti-rat IgG antibodies were used for rabbit and rat pAb, respectively (1:200; Jackson ImmunoResearch Laboratories, USA). HRP-conjugated goat anti-mouse IgG -positive IgM antibody (1:200; Jackson ImmunoResearch Laboratories) was used for mouse pAb. Mouse on mouse staining protocol (Jackson ImmunoResearch Laboratories) was used if the pAB had mouse origin. As a negative control pAB was replaced with vehicle. Positive staining was visualized using diaminobenzidine (Vector Laboratories, USA). Counterstaining was performed with Harris hematoxylin, followed by tissue dehydration and mounting with Assistant Histokitt (Chemi-Teknik, Norway). Enumeration of the cells was determined using a Zeiss light microscope. Cells were counted in two randomly selected high-power fields (HPF; magnification, ×400) pro animal.
RNA isolation and RT-PCR
Total prostate RNA was isolated using TRI Reagent (Ambion, USA). mRNA expression analysis of Shh, Gli1, Gli2 and Gli3 was carried out by RT-PCR. RNA was reversibly transcribed using oligo-dT primers and Stratascript III Reverse Transcriptase (Invitrogen, USA). The specific primer pairs used were: Gli1 5′-GTCCACCAAC CAACTATG-3′ forward, 5′-TCCAGGTCAAGAGAGTCC-3′ reverse (product is 481 bp); Gli2 5′-GAACGAAGAGA CAGCTCCAC-3′ forward, 5′-CTGTGGAAACGTTGCACT-3′ reverse (337 bp); Gli3 5′-CTGGAAAGGAGCGGGAAAG-3′ forward, 5′-CTGAGGCTGCAGTGGGATTAC-3′ reverse (256 bp); Shh 5′-TACAAGCAGTTTATTCCCAACGT-3′ forward, 5′-GACCCTCATAGTGTAGAGACT-3′ reverse (243 bp); actin 5′-TACCACAGGCATTGTGATGGA-3′ forward, 5′-CAACGTCACACTTCATGATGG-3′ reverse (272 bp). Reaction was initially performed at 95°C for 2 min, followed by 35 cycles of denaturation (at 94°C for 30 sec), annealing (for Gli1 at 52°C, for Gli2 at 54°C, for Gli3 at 60°C, for Shh at 58°C and for actin at 57°C, for 30 sec) and extension (at 72°C for 1 min). The PCR products were identified by electrophoresis in 1.5% agarose gels. DNA bands were visualized using ethidium bromide.
Statistical analysis
Data are expressed as the mean number of positive cells pro HPF ± SEM. Statistical analysis was carried out using non-parametric analysis of variance (Kruskal-Wallis test) to evaluate variance among the groups studied. If a significant variance was found, an unpaired two-group test (Mann-Whitney U test) was used to determine significant differences between individual groups. The Spearman-rank correlation test was used to detect the relationship between variables. P<0.05 was considered to be a statistically significant difference.
Results
Shh pathway components in prostate
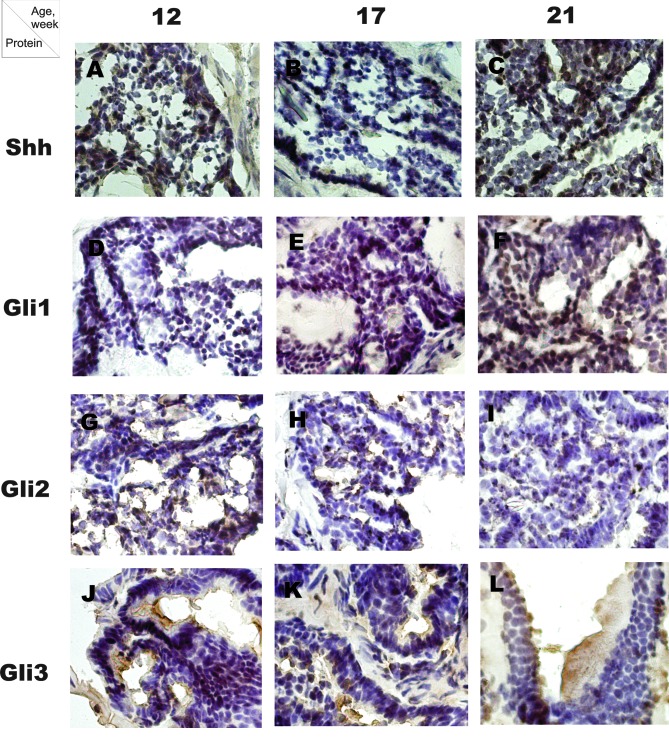
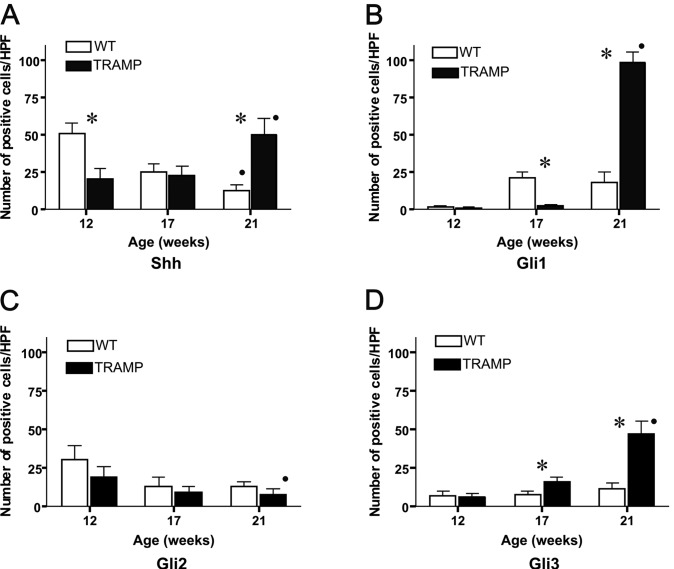
In the WT mice the number of Shh-positive cells decreased significantly in an age-dependent manner, and were 50±6, 25±5 and 13±4 cells/HPF at 12, 17 and 21 weeks of age, respectively (P=0.002, Rs=−0.95). In the TRAMP mice, the number of Shh-positive cells increased significantly during the period studied (20±7, 23±6 and 50±10 cells/HPF at 12, 17 and 21 weeks of age, respectively; P=0.01, Rs=0.73) (Fig. 1A-C, Fig. 2A). The number of Shh-positive cells was higher in WT compared to TRAMP mice at 12 weeks of age, whereas in older mice, at 21 weeks of age, the number of Shh-positive cells was higher in TRAMP mice (P=0.02 for both comparisons).
Figure 1.
Photographs of the immunohistochemical staining of Shh, Gli1, Gli2 and Gli3 proteins in TRAMP mice prostate (x400). Brown staining indicates the positive staining of the studied proteins.
Figure 2.
The number of (A) Shh+, (B) Gli1+, (C) Gli2+ and (D) Gli3+ cells in prostate tissue at 12, 17 and 21 weeks of age in the WT and TRAMP mice. *P<0.05 between WT and TRAMP mice. •P<0.05 for age-dependency within the same mouse strain.
The number of Gli1-positive cells in WT was 2±0.9 cells/HPF at 12 weeks, 21±4 at 17 weeks and 18±7 cells/HPF at 21 weeks of age. In the TRAMP mice, the number of Gli1-positive cells increased from 2±0.5 cells/HPF at 12 weeks to 4±0.8 cells at 17 weeks and 100±7 cells/HPF at 21 weeks of age (P=0.002, Rs=0.93) (Fig. 1D-F, Fig. 2B). At 12 weeks of age no difference was noted in the number of Gli1-positive cells between WT and TRAMP mice. At 17 weeks the number of Gli1-positive cells was significantly lower in TRAMP compared to WT mice, but in older mice, at 21 weeks of age, the number of Gli1-positive cells was higher in TRAMP compared to WT mice (P=0.02 for both comparisons).
In the WT mice, the number of Gli2-positive cells was 30±9 cells/HPF at 12 weeks, 13±6 at 17 weeks and 13±3 cells/HPF at 21 weeks of age. In the TRAMP mice, the number of Gli2-positive cells decreased in an age-dependent manner and were 19±7 cells/HPF at 12 weeks, 10±3 at 17 weeks and 8±3 cells/HPF at 21 weeks of age (P=0.03, Rs=−0.7) (Fig. 1G-I, Fig. 2C). No significant differences were noted in the number of Gli2-positive cells between WT and TRAMP mice at all time points studied.
In the WT mice, the number of Gli3-positive cells at 12 weeks of age was 7±3 cells/HPF, and at 17 and 21 weeks 8±2 and 11±3 cells/HPF, respectively. The number of Gli3-positive cells in the TRAMP mice increased during the study period, and were 6±2 cells/HPF at 12 weeks, 16±3 at 17 weeks and 48±8 cells/HPF at 21 weeks of age (P=0.002, Rs=0.095) (Fig. 1J-L, Fig. 2D). No significant difference was noted in the number of Gli3-positive cells between WT and TRAMP mice at 12 weeks of age. The number of Gli3-positive cells was higher in TRAMP compared to WT mice at 17 and 21 weeks of age (P=0.02 for both comparisons).
Messenger RNA expression of the Shh signaling pathway components
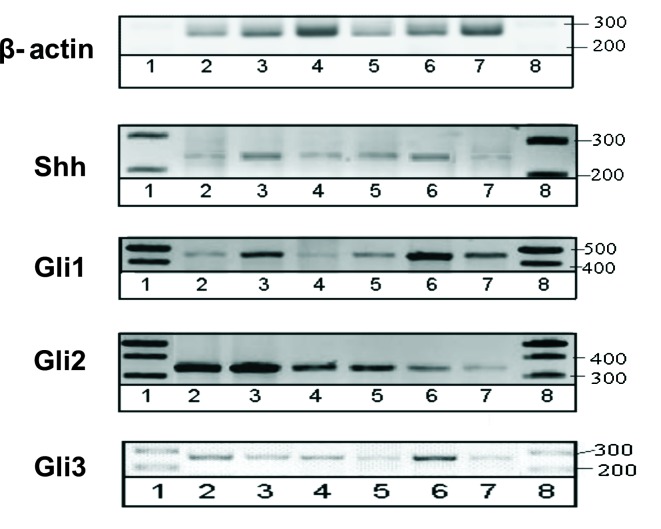
RT-PCR yields semi-quantitative results and, therefore, was performed to verify the presence of respective genes in mouse prostate. The scan of representative gels is shown in Fig. 4.
Figure 4.
The mRNA expression of Shh and transcription factors Gli1, Gli2 and Gli3 in the TRAMP and WT mice with prostate tumors at 12 (columns 2 and 3), 17 (columns 4 and 5) and 21 (columns 6 and 7) weeks of age, respectively. Marker GeneRuler 1 kb DNA Ladder (columns 1 and 8).
FoxA1 protein in prostate
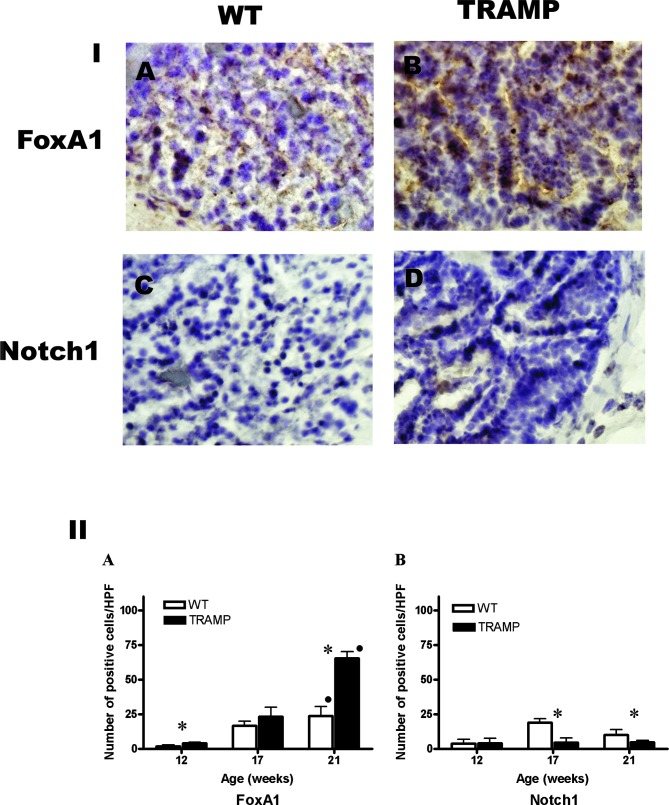
In the WT mice, the number of FoxA1-positive cells increased significantly at 2±0.9, 16±3 and 24±6 cells/HPF at 12, 17 and 21 weeks of age, respectively (P=0.004, Rs=0.9). In TRAMP mice, the number of FoxA1-positive cells increased significantly at 4±0.8, 24±6 and 65±5 cells/HPF at 12, 17 and 21 weeks of age, respectively (P=0.002, Rs=0.95) (Fig. 3, Panel IA-B and Panel IIA). The number of FoxA1-positive cells was higher in WT compared to TRAMP mice at 12 and 21 weeks of age (P=0.03 and 0.02, respectively).
Figure 3.
(Panel I) Photographs of the immunohistochemical staining of FoxA1 and Notch1 proteins in WT and TRAMP mice with prostate tumors at 21 weeks of age (x400). Brown staining indicates positive staining for studied proteins. (Panel II) The number of (A) FoxA1+ and (B) Notch1+ cells in prostate tissue at 12, 17 and 21 weeks of age in WT and TRAMP mice. *P<0.05 between WT and TRAMP mice. •P<0.05 for age-dependency within the same mouse strain.
Notch1 protein in prostate
In WT mice, the number of Notch1-positive cells was 4±2.5, 18±3 and 10±4 cells/HPF at 12, 17 and 21 weeks of age, respectively. In TRAMP mice, there were 4±3, 4.5 ±3 and 5±1 Notch1-positive cells/HPF at 12, 17 and 21 weeks of age, respectively (Fig. 3, Panel IC-D and Panel IIB). The number of Notch1-positive cells was higher in WT compared to TRAMP mice at 17 and 21 weeks of age (P=0.02 and 0.03, respectively).
Discussion
Our study proves that with aging the number of Shh-positive cells increased in TRAMP and decreased in wild-type mice. In the TRAMP mice, the increase in the number of Shh-positive cells was paralleled by the age-dependent increase in Gli1-and Gli3-positive cells that in turn reflects activation of the whole Shh pathway.
Prostate cancer is the most common type of cancer in males and is the second cause of male cancer-related death. In Estonia, frequency of prostate cancer is 107/100,000 individuals, making it the first cause of cancer-related death in males (www.cancer.ee). The risk for developing prostate cancer rises significantly with age, and 60% of newly diagnosed cases occur in men over the age of 70.
Mouse experimental models are the most frequently used in vivo research tools in the study of human diseases. However, due to species-specific differences there are no suitable wild-type mice strains to study human prostate cancer, mainly because prostate cancer does not develop in rodents spontaneously (26). Possible reasons for this include the short life span of the rodent and that in contrast to human prostate, mouse prostate atrophies with aging (26). Additionally, prostate anatomy considerably differs between human and rodents (26,27). Currently, well-validated prostate cancer transgenic mouse strains TRAMP (28,29) and LADY (30) are available. In these mice strains rat probasin gene promoter is used to direct the expression of T/t-antigens of the SV40 virus in the prostate epithelium. Besides the different size of the promoter, another interesting difference between the two models is that the TRAMP mice epithelial cells may express both large and small T/t-antigens of the SV40 virus, while LADY is designed to express only the large T-antigen. Notably, TRAMP mice develop high-grade prostatic intraepithelial neoplasia and adenocarcinoma, which later metastasize primarily to the lungs and lymph nodes. On the other hand, LADY mice develop low-grade prostatic intraepithelial neoplasia and invasive carcinoma, which generally fail to metastasize (31). Therefore, taking into account the fact that human prostate adenocarcinoma is an early metastasizing cancer, we utilized a more relevant TRAMP model.
Involvement of the Shh pathway in prostate cancer has previously been demonstrated. Currently, the activation of Ptch1 and Gli1, but not Shh, is considered to be a feature of the Shh pathway activation in a signal-receiving cell (5,6). Thus, despite the constant presence of Shh transcripts in human metastatic prostate tumors, the Shh pathway is activated in only 25% of tumors (10). Nevertheless, human prostate carcinomas as well as human metastatic and high-grade prostate tumors are associated with a high expression level of Shh mRNA (14,15).
The present study demonstrated that the number of prostate Shh-positive cells increased in an age-dependent manner in the TRAMP mouse model for prostate cancer. A significant difference in the number of Shh-positive cells as well as the Shh mRNA expression level between transgenic and wild-type mice was detected only in older mice, at the 21st week of age. Based on the TRAMP mice breeder description, prostate adenocarcinoma develops by the 24th week and metastasizes by the 30th week of age (www.jax.org). Therefore, the increase in the number of Shh-positive cells preceded the estimated appearance of adenocarcinoma per se or its metastasis in TRAMP mice. The presence of Shh mRNA in normal adult murine and human prostate tissue has been demonstrated (14,16). This observation was extended and it was shown that Shh is present in normal prostate on the protein level. Furthermore, we detected a significant age-dependent decrease in the number of cells in wild-type mice. Thus, our data showed that the Shh protein expression decreases with age in healthy conditions, whereas in prostate cancer development the Shh protein expression increases with age and precedes cancer manifestation.
Shh signal transduction leads to the activation of the Gli transcription factors, Gli1, Gli2 and Gli3. Gli1 serves as a direct readout of Hh signaling and as a transcriptional activator of the Shh pathway (5). Increased expression level of Gli1 in human prostate cancer cell lines and human prostate carcinomas was previously demonstrated (10,14). We showed an age-dependent increase in the number of Gli1-positive cells in transgenic mice. Similar to the number of the Shh-positive cells, the number of Gli1-positive cells and its mRNA expression was higher only in older TRAMP mice. It was shown that while Gli1 acts solely as an activator, Gli2 and Gli3 are bi-potential transcriptional regulators (32). Gli2 and Gli3 are activators or repressors, depending on the context. Although we detected an age-dependent decrease in the number of Gli2-positive cells and the level of its mRNA expression in transgenic mice, no significant differences were noted in the number of cells between transgenic and wild-type mice. On the other hand, the number of Gli3-positive cells increased in an age-dependent manner in transgenic mice, with the number of cells beng significantly higher compared to wild-type mice in the 17th and 21st weeks of age. This is consistent with our mRNA data and earlier observations (33). Our data showed that tumor development in prostate cancer-prone TRAMP mice is associated with an age-dependent increase in Shh, Gli1 and Gli3 and decrease in Gli2 mRNA and protein levels in prostate tissue. Our data support the hypothesis that the following order of events may occur during prostate carcinogenesis (34): i) a small amount of Shh activates Gli3 and Gli1 proteins; ii) Gli3 binds to the Gli1 promoter and induces the additional expression of Gli1 and iii) Shh pathway proteins activate relevant target genes that lead to tumorigenesis. Whether this hypothesis is correct remains to be tested in further studies.
Tumor development in TRAMP mice is associated with an increased rate of epithelial proliferation (28). FoxA1 is an important regulator of cell proliferation and differentiation, and is continuously expressed in human and mice prostate from its development till aging (35,36). FoxA1 protein-enhanced expression was repeatedly detected in human prostate carcinomas (35). We detected an age-dependent increase in the number of prostate FoxA1-positive cells in both transgenic and wild-type mice. Transgenic mice had an increased number of prostate FoxA1-positive cells compared to wild-type mice. Thus, in the current model prostate tumor progression is associated with an increase in the number of FoxA1-positive cells, which is in agreement with human data (35). Taking into account that FoxA1 is constantly expressed in the human prostate and the fact that its activation is time-dependently associated with prostate carcinoma development, the possible role of this marker in prostate tumor development as well as its possible use as a diagnostic marker warrants further investigation.
Notch1 is crucial for prostate development and the differentiation of prostate cells (37). An interesting aspect of Notch1 is its apparently opposite functions in tumor development, where it can act either as an oncogene or as a tumor suppressor. For example, a high expression of Notch1 was shown in 14 human prostate cancer cell lines in vitro and in TRAMP prostate tissue in vivo (38,39). On the other hand, Notch1 prostate tumor suppressor features were demonstrated in human prostate adenocarcinomas in vivo and Notch1 knock-out mice (37). Nevertheless, although the role of Notch1 in the prostate cancer remains controversial, this proliferation marker certainly warrants further investigation in prostate tumors (reviewed in ref. 40). In this study, the number of Notch1-positive cells was decreased at the 17th and 21st week of age in transgenic compared to wild-type mice, suggesting that Notch1 functions as a tumor-suppressor protein in the current prostate cancer model.
Our study showed age-dependent activation of the Shh pathway in the TRAMP transgenic mice. As this particular mouse strain is likely to develop metastasizing prostate cancer, our data actually implicate Shh pathway activation not only in the development of primary prostate carcinomas but also in its metastatic spread. Therefore, further use of this particular strain as a valid tool for prostate cancer research is significant.
Acknowledgements
The authors thank Dr Tõnis Timmusk for providing the FoxA1 and Notch1 antibodies. We are also grateful to Dr Illar Pata for the help with the mouse work and to Drs Torben Østerlund, Piret Michelson and Robert Tsanev for critical review of the manuscript. The study was supported by the Estonian Science Foundation (grants ETF7242 to S.S. and ETF8116 to P.K.), and the Estonian Ministry of Education (Directional financing to P.K.). P.K. was supported by a Wellcome Trust International Senior Research Fellowship for part of these studies.
References
- 1.Osterlund T, Kogerman P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends Cell Biol. 2006;16:176–180. doi: 10.1016/j.tcb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharghi-Namini S, Turmaine M, Meier C, et al. The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J Neurosci. 2006;26:6364–6376. doi: 10.1523/JNEUROSCI.0157-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 5.Kinzler KW, Ruppert JM, Bigner SH, Vogelstein B. The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature. 1988;332:371–374. doi: 10.1038/332371a0. [DOI] [PubMed] [Google Scholar]
- 6.Ruppert JM, Kinzler KW, Wong AJ, et al. The GLI-Kruppel family of human genes. Mol Cell Biol. 1988;8:3104–3113. doi: 10.1128/mcb.8.8.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowitch DH, BSJ, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 9.Stecca B, Mas C, Ruiz i Altaba A. Interference with HH-GLI signaling inhibits prostate cancer. Trends Mol Med. 2005;11:199–203. doi: 10.1016/j.molmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz i Altaba A. Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets? Cancer Cell. 2008;14:281–283. doi: 10.1016/j.ccr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Oldak M, Grzela T, Lazarczyk M, Malejczyk J, Skopinski P. Clinical aspects of disrupted Hedgehog signaling (Review) Int J Mol Med. 2001;8:445–452. [PubMed] [Google Scholar]
- 13.Datta S, Datta MW. Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006;63:435–448. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez P, Hernandez AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with Sonic Hedgehog-Gli1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng T, Li C, Zhang X, et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gipp J, Gu G, Crylen C, Kasper S, Bushman W. Hedgehog pathway activity in the LADY prostate tumor model. Mol Cancer. 2007;6:19. doi: 10.1186/1476-4598-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S, Frolova N, Sadlonova A, et al. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5:674–683. doi: 10.4161/cbt.5.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Chen G, Cao D, et al. Expression of Gli1 correlates with the transition of breast cancer cells to estrogen-independent growth. Breast Cancer Res Treat. 2010;119:39–51. doi: 10.1007/s10549-009-0323-3. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen SK, Mollgard K, Clement CA, et al. Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev Dyn. 2008;237:2039–2052. doi: 10.1002/dvdy.21610. [DOI] [PubMed] [Google Scholar]
- 20.Hunt R, Bragina O, Drews M, et al. Generation and characterization of mouse monoclonal antibody 5E1 against human transcription factor GLI3. Hybridoma (Larchmt) 2007;26:231–240. doi: 10.1089/hyb.2007.0507. [DOI] [PubMed] [Google Scholar]
- 21.Unger S, Copland I, Tibboel D, Post M. Down-regulation of sonic hedgehog expression in pulmonary hypoplasia is associated with congenital diaphragmatic hernia. Am J Pathol. 2003;162:547–555. doi: 10.1016/S0002-9440(10)63848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang BE, Shou J, Ross S, Koeppen H, De Sauvage FJ, Gao WQ. Inhibition of epithelial ductal branching in the prostate by sonic hedgehog is indirectly mediated by stromal cells. J Biol Chem. 2003;278:18506–18513. doi: 10.1074/jbc.M300968200. [DOI] [PubMed] [Google Scholar]
- 23.Minoo P, Hu L, Zhu N, et al. SMAD3 prevents binding of NKX2.1 and FOXA1 to the SpB promoter through its MH1 and MH2 domains. Nucleic Acids Res. 2008;36:179–188. doi: 10.1093/nar/gkm871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox V, Gokhale PJ, Walsh JR, Matin M, Jones M, Andrews PW. Cell-cell signaling through NOTCH regulates human embryonic stem cell proliferation. Stem Cells. 2008;26:715–723. doi: 10.1634/stemcells.2007-0368. [DOI] [PubMed] [Google Scholar]
- 25.Djalilian AR, Namavari A, Ito A, et al. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–1049. [PMC free article] [PubMed] [Google Scholar]
- 26.Maini A, Archer C, Wang CY, Haas GP. Comparative pathology of benign prostatic hyperplasia and prostate cancer. In Vivo. 1997;11:293–299. [PubMed] [Google Scholar]
- 27.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 28.Gingrich JR, Barrios RJ, Morton RA, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–4102. [PubMed] [Google Scholar]
- 29.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–4691. [PubMed] [Google Scholar]
- 30.Kasper S, Sheppard PC, Yan Y, et al. Development, progression and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:319–333. [PubMed] [Google Scholar]
- 31.Abate-Shen C, Shen MM. Mouse models of prostate carcinogenesis. Trends Genet. 2002;18:S1–S5. doi: 10.1016/s0168-9525(02)02683-5. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–3924. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 33.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Ruther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol. 2007;305:460–469. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66:1013–1028. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 36.Gao N, Ishii K, Mirosevich J, et al. Forkhead box A1 regulates prostate ductal morphogenesis and promotes epithelial cell maturation. Development. 2005;132:3431–3443. doi: 10.1242/dev.01917. [DOI] [PubMed] [Google Scholar]
- 37.Wang XD, Leow CC, Zha J, et al. Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation. Dev Biol. 2006;290:66–80. doi: 10.1016/j.ydbio.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- 39.Shou J, Ross S, Koeppen H, de Sauvage FJ, Gao WQ. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res. 2001;61:7291–7297. [PubMed] [Google Scholar]
- 40.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]