Abstract
There is considerable evidence that glucosamine exerts an inhibitory effect on inflammatory cytokine expression in cells. Glucosamine has been recommended as a promising anti-inflammatory modulator, which has been applied in clinical trials for attenuation of the inflammatory process. However, it is unknown whether glucosamine reduces the expression of TNF-α-induced inflammatory cytokines in HaCaT cells. The anti-inflammatory effects of curcumin in HaCaT cells have been extensively investigated in several studies. Thus, in this study we investigated the expression of IL-6, IL-8, TNF-α and IL-1β in glucosamine-treated HaCaT cells, and the effects of glucosamine were compared to those of curcumin-treated HaCaT cells. Our data showed that the expression of IL-6, IL-8, TNF-α and IL-1β was decreased by glucosamine treatment in the HaCaT cells. In contrast, the expression of IL-6, IL-8, TNF-α and IL-1β was not attenuated by glucosamine treatment in the TNF-α-treated HaCaT cells. Notably, curcumin induced an increased expression of IL-8 and IL-1β in the HaCaT cells, but not that of IL-6 and TNF-α. On the other hand, curcumin attenuated the expression of IL-6 and IL-8 in the TNF-α-treated HaCaT cells. Our data indicated that glucosamine induced the down-regulation of IL-6, IL-8, TNF-α and IL-1β expression in the HaCaT cells. However, the stimulation of TNF-α abolished the inhibitory effects of glucosamine on the expression of inflammatory cytokines in the HaCaT cells. Thus, even though glucosamine induces the down-regulation of inflammatory cytokines in HaCaT cells, the anti-inflammatory role of glucosamine in TNF-α-mediated inflammatory skin diseases should be investigated.
Keywords: interleukin, tumor necrosis factor-α, glucosamine, HaCaT cells
Introduction
The inflammatory cytokines IL-6, IL-8, TNF-α and IL-1β play roles in mediating the cellular injury and pathogenesis of chronic inflammatory diseases (1–3). TNF-α and IL-1β initiate the cascade of destructive events, in part, through the activation of transcription factor NF-κB, which in turn induces several proinflammatory genes. In addition, mitogen-activated protein kinases (MAPKs) regulate key proinflammatory pathways following stimulation with UV and TNF-α (4,5). Three MAPK proteins, i.e., extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK are thought to play different roles in chronic inflammatory diseases and homeostasis in the skin (6–8).
Glucosamine, an amino sugar, plays a role in improving arthritis in patients due to the anti-inflammatory action of glucosamine compounds that are associated with the suppression of neutrophil functions and proinflammatory cytokines (9–11). Moreover, structural modifications to glucosamine by introducing new functional groups can be expected to improve its therapeutic effects (12). As in the case of glucosamine, curcumin, extracted from C. longa, is a promising anti-inflammatory agent under various experimental conditions (13,14). Curcumin attenuates the expression of TNF-α or ultraviolet-induced inflammatory cytokines in cells (15–17). However, it is still largely unknown whether glucosamine inhibits the TNF-α-induced expression of inflammatory cytokines in the HaCaT keratinocyte cell line. Thus, the present study investigated the anti-inflammatory effect of glucosamine in HaCaT keratinocyte cells with or without TNF-α treatment. In addition, the inhibitory effects of glucosamine were compared to those of curcumin in the HaCaT keratinocyte cell line.
Materials and methods
Materials
Curcumin, glucosamine and TNF-α were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against phospho-ERK (p-ERK), ERK, phospho-p38 (p-p38), p38, phospho-JNK (p-JNK) and JNK were purchased from Cell Signaling (Beverly, MA, USA).
Cell culture
The HaCaT keratinocyte cell line was maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. For the experiments, cells (5×104/ml) were seeded in a culture dish and maintained in the tissue culture incubator.
Chemical agent treatment
Cells were cultured and treated with glucosamine (1–10 mM), curcumin (1–20 μM) or TNF-α (20 ng/ml) for 24 h.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the cells using RNAzol™ B (Biotech Laboratories, Houston, TX, USA) according to the manufacturer’s instructions and then quantitated with a spectrophotometer. Total RNA (1 μg) was reverse transcribed using M-MLV Reverse Transcriptase (Promega Co., Madison, WI, USA). The PCR reaction was carried out under the conditions recommended by the manufacturer (Takara Co., Otsu, Japan). The primer sequences and product sizes were as follows: GAPDH forward, 5′-CGT CTT CAC CAC CAT GGA GA-3′; reverse, 5′-CGG CCA TCA CGC CAC AGT TT-3′; IL-6 forward, 5′-GTG TGA AAG CAG CAA AGA GGC-3′; reverse, 5′-CTG GAG GTA CTC TAG GTA TAC-3′; IL-8 forward, 5′-ATG ACT TCC AAG CTG GGC CGT G-3′; reverse, 5′-TAT GAA TTC TCA GCC CTC TTC AAAA-3′; TNF-α forward, 5′-CAA AGT AGA CCT GCC CAG AC-3′; reverse, 5′-GAC CTC TCT CTA ATC AGC CC-3′; IL-1β forward, 5′-AAA AGC TTG GTG ATG TCT GG-3′; reverse, 5′-TTT CAA CAC GCA GGA CAG G-3′.
Western blot analysis
Cells were lysed in lysis buffer [10 mM Tris (pH 7.4), 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, 10 μg/ml PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 5 mM phenanthroline and 28 mM benzamidine-HCl] for 30 min on ice. Lysates were clarified by centrifugation and quantitated using the Bradford assay (Life Science Co., CA, USA) with bovine serum albumin as a reference standard. Proteins (35 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gels and transferred to an Immobilon-P Transfer Membrane (Millipore Co., Billerica, MA, USA). After incubation with the primary antibodies, proteins were visualized by incubation with horseradish peroxidase-conjugated secondary antibodies, followed by ECL according to the manufacturer’s instructions (Amersham Life Science Co., Buckinghamshire, UK).
Results
Effect of glucosamine and curcumin on the expression of proinflammatory cytokines in HaCaT cells
To study the effect of glucosamine and curcumin treatment on the expression of IL-6, IL-8, TNF-α and IL-1β, HaCaT cells were exposed to glucosamine at doses ranging from 1 to 10 mM, and curcumin at doses ranging from 1 to 20 μM. Cells were then harvested for 24 h after treatment for RT-PCR analysis. As shown in Fig. 1A, IL-6, IL-8, TNF-α and IL-1β expression was clearly decreased in a dose-dependent manner in the glucosamine-treated HaCaT cells. In contrast to glucosamine treatment, the expression of IL-8, TNF-α and IL-1β was increased in the curcumin-treated HaCaT cells (Fig. 1B). Increased expression of IL-8 and IL-1β was clearly visualized at >10 μM curcumin treatment, following a sustained increased expression of IL-8 and IL-1β. The results indicate that glucosamine induces the down-regulation of IL-6, IL-8, TNF-α and IL-1β, while curcumin induces the up-regulation of IL-8 and IL-1β in HaCaT cells.
Figure 1.
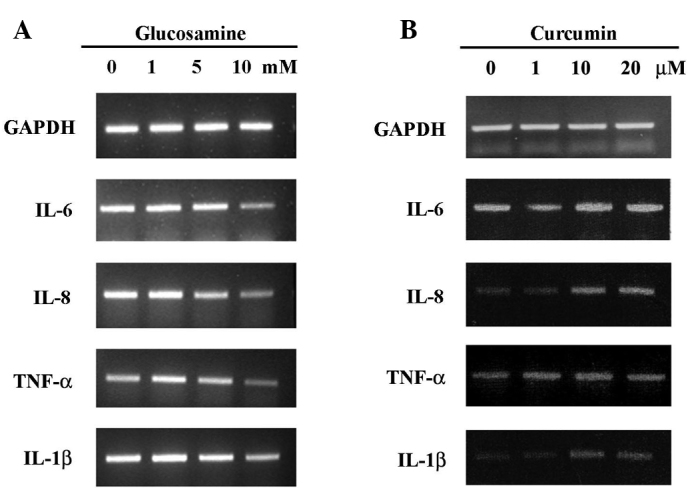
Differential expression of IL-6, IL-8, TNF-α and IL-1β mRNA in glucosamine- or curcumin-treated HaCaT cells. The cells were cultured to 90% confluence in DMEM supplemented with 10% fetal bovine serum at 37°C and in 5% CO2. After glucosamine (A) or curcumin (B) treatment for 24 h for the indicted concentrations, cells were harvested and the cell lysates were prepared for RT-PCR analysis. Similar results were obtained in two different experiments.
Effect of TNF-α on the expression of proinflammatory cytokines in HaCaT cells
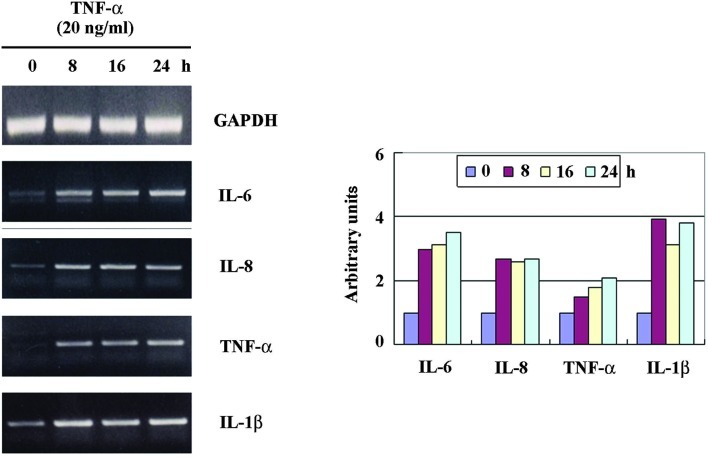
To study the effect of TNF-α treatment on the expression of IL-6, IL-8, TNF-α and IL-1β, HaCaT cells were exposed to TNF-α at doses of 20 ng/ml. The cells were then harvested for 24 h after treatment for RT-PCR analysis. As shown in Fig. 2, IL-6, IL-8, TNF-α and IL-1β expression was increased and visualized at 8 h, following the sustained increased expression. Thus, we investigated whether glucosamine inhibits the TNF-α-induced up-regulation of IL-6, IL-8, TNF-α and IL-1β in HaCaT cells.
Figure 2.
Up-regulation of IL-6, IL-8, TNF-α and IL-1β mRNA expression in TNF-α-treated HaCaT cells. The cells were cultured to 90% confluence in DMEM supplemented with 10% fetal bovine serum at 37°C and in 5% CO2. After TNF-α treatment (20 ng/ml), cells were harvested at the indicated times, and the cell lysates were prepared for RT-PCR analysis. Similar results were obtained in two different experiments.
Effect of glucosamine on TNF-α-induced expression of proinflammatory cytokines in HaCaT cells
To investigate whether glucosamine inhibits TNF-α-induced IL-6, IL-8, TNF-α and IL-1β expression, HaCaT cells were treated with TNF-α (20 ng/ml) with or without glucosamine (10 mM). These results were compared to those of curcumin treatment. As shown in Fig. 3A, the up-regulation of IL-6, IL-8, TNF-α and IL-1β by TNF-α treatment was not decreased by glucosamine treatment. These results indicate that, even though glucosamine reduces the expression of proinflammatory cytokines in cells, the glucosamine-induced inhibitory effect on the expression of proinflammatory cytokines, such as IL-6, IL-8, TNF-α and IL-1β, is eliminated by the presence of a potent inflammatory stimulus such as TNF-α. However, the up-regulation of IL-6, IL-8, TNF-α and IL-1β by TNF-α treatment was decreased by curcumin treatment (Fig. 3B).
Figure 3.
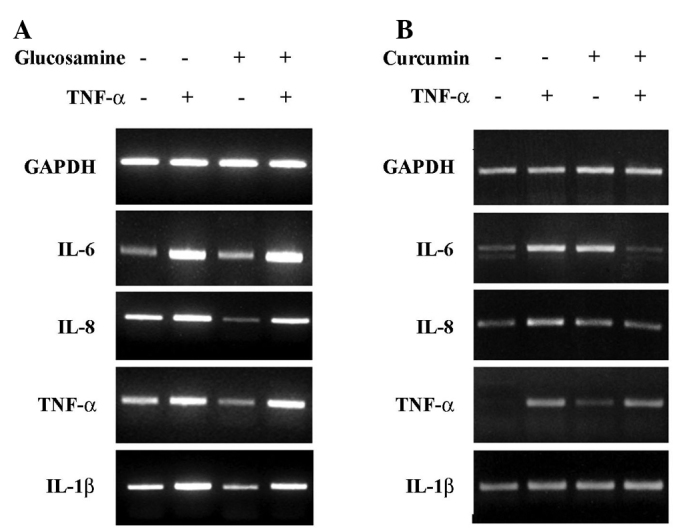
Differential expression of TNF-α-induced IL-6, IL-8, TNF-α and IL-1β by glucosamine or curcumin. Cells were treated with TNF-α (20 ng/ml), with or without glucosamine (10 mM) (A) or curcumin (20 μM) (B) for 24 h. The cells were then harvested to extract RNA. The expression of IL-6, IL-8, TNF-α and IL-1β was analyzed by RT-PCR analysis. Similar results were obtained in two experiments.
Effect of glucosamine on TNF-α induced the activation of MAPKs
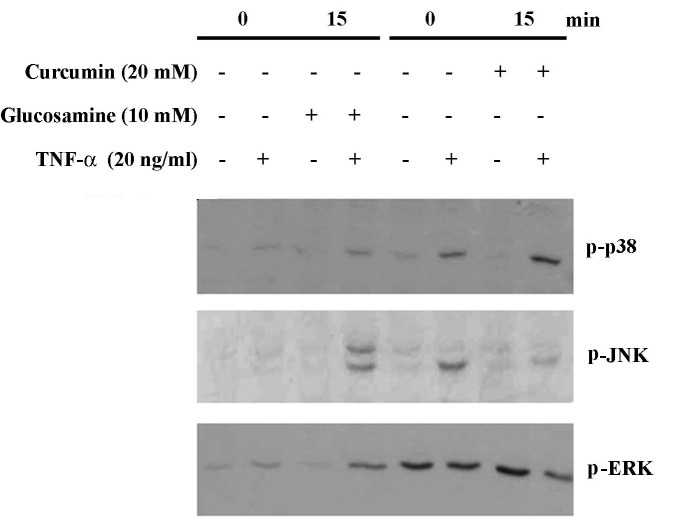
Accumulating data suggest that TNF-α-treated cells show an increased expression of IL-6, IL-8, TNF-α and IL-1β through MAPK-dependent pathways such as p38 MAPK and JNK. However, it is unknown whether glucosamine modulates the expression of IL-6, IL-8 and IL-1β by inhibiting the MAPK pathways in TNF-α-treated HaCaT cells. We compared the MPK inhibitory effects between glucosamine and curcumin. We examined the effect of glucosamine (10 mM) or curcumin (10 μM) on the activation of p38 MAPK, JNK and ERK in HaCaT cells. As shown in Fig. 4, treatment of glucosamine (10 mM) did not inhibit the activation of p38, JNK and ERK in HaCaT cells treated with TNF-α. However, in the presence of curcumin (20 μM), TNF-α-induced JNK and ERK activation was dramatically inhibited, evidenced by decreased phospho-p38 and ERK. These results suggest that TNF-α-induced MAPK activation is not inhibited by glucosamine treatment in HaCaT cells, but is inhibited by curcumin. Stripping and reprobing the same membrane with antibodies against p38, JNK and ERK revealed no change in the total protein levels of each kinase (data not shown).
Figure 4.
Non-altered expression of TNF-α-induced p38, JNK and ERK activations by glucosamine. The cells were harvested 15 min after TNF-α treatment (20 ng/ml) with or without glucosamine (10 mM) or curcumin (20 μM), and whole cell lysates and nuclear extracts were used for Western blot analysis. Whole cell lysates were also prepared 2 h after TNF-α treatment and used for p-p38, p-JNK, p-ERK Western with the respective antibodies. Similar results were shown in two experiments.
Discussion
Glucosamine is thought to exert anti-inflammatory effects, including the suppression of inflammatory cytokines under various conditions. In addition, glucosamine was found to inhibit inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide (LPS)-stimulated RAW264.7 cells (18) and suppress the proliferation of human prostate carcinoma DU145 cells through the inhibition of STAT3 signaling (19). Numerous studies have repoted on the anti-inflammatory effects of glucosamine and chondroitin sulfate. The two sulfates are agents that relieve pain, improve functional activity and slow disease progression in osteoarthritis especially that of the hip and knee (20). However, the anti-inflammatory effect of glucosamine in inflammatory skin disease still remains unknown. Our data showed that glucosamine inhibited the growth of the HaCaT keratinocyte cell line and induced the down-regulation of IL-6, IL-8, TNF-α and IL-1β mRNA, suggesting that glucosamine also plays a role in alleviating inflammatory skin diseases.
TNF-α is a primary inflammatory cytokine with a broad range of proinflammatory and immunostimulatory activities. TNF-α plays an important role in the development of inflammatory skin diseases such as psoriasis and atopic dermatitis (21–23). Down-stream inflammatory responses to TNF-α are mediated through the up-regulation of IL-1, IL-2, IL-6, IL-10 and IFN-γ (5,24). Thus, broad anti-inflammatory effects may be achieved through the inhibition of the TNF-α pathways. However, we did not observe an inhibitory effect of glucosamine on the TNF-α-induced up-regulation of IL-6, IL-8, TNF-α and IL-1β in HaCaT cells. In addition, the activation of MAPKs such as p38, JNK and ERK in TNF-α-treated HaCaT cells was not inhibited by glucosamine. These results were similar to a previous study in which glucosamine was noted to have no effect on the activation of p38 MAPK, JNK-1/2 and ERK-1/2 in IL-1β-stimulated A549 cells (9). In contrast, glucosamine was shown to block LPS-induced activation of p38 MAPK and JNK in RAW264.7 cells (18). Thus, the glucosamine-induced down-regulation of the MAPK pathway requires further study under various conditions. Curcumin is known to exert its anti-inflammatory activity in various cell lines through the inhibition of the MAPK pathways. In this study, curcumin attenuated the expression of TNF-α-induced IL-6 and IL-8 in HaCaT cells, as well as the inhibition of TNF-α-induced JNK and ERK activation.
In conclusion, the findings of the present study demonstrate, for the first time, to the best of our knowledge, that glucosamine inhibits the expression of IL-6, IL-8, TNF-α and IL-1β in HaCaT cells, but not in the presence of TNF-α. These results suggest that glucosamine can be used as a candidate immunomodulatory agent in inflammatory skin disease, while its use in the case of TNF-α-overexpressing skin disease should be further investigated.
References
- 1.Damjanov N, Vojinovic J. Biologic therapy of rheumatoid arthritis. Srp Arh Celok Lek. 2009;137:205–210. doi: 10.2298/sarh0904205d. [DOI] [PubMed] [Google Scholar]
- 2.Ding C, Cicuttini F, Li J, Jones G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin Investig Drugs. 2009;18:1457–1466. doi: 10.1517/13543780903203789. [DOI] [PubMed] [Google Scholar]
- 3.Kovarikova M, Hofmanova J, Soucek K, Kozubik A. The effects of TNF-alpha and inhibitors of arachidonic acid metabolism on human colon HT-29 cells depend on differentiation status. Differentiation. 2004;72:23–31. doi: 10.1111/j.1432-0436.2004.07201006.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen H, Alexander G, Austenaa LM, Ebihara K, Blomhoff R. Molecular imaging of the transcription factor NF-kappaB, a primary regulator of stress response. Mutat Res. 2004;551:199–211. doi: 10.1016/j.mrfmmm.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2009 Sep 16; doi: 10.1007/s00403-009-0994-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 6.Leng H, Luo X, Ma L, Kang K, Zheng Z. Reversal of ultraviolet B-induced immunosuppression by inhibition of the extracellular signal-regulated mitogen-activated protein kinase. Photodermatol Photoimmunol Photomed. 2009;25:264–269. doi: 10.1111/j.1600-0781.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 7.Neves BM, Cruz MT, Francisco V, Garcia-Rodriguez C, Silvestre R, Cordeiro-da-Silva A, Dinis AM, Batista MT, Duarte CB, Lopes MC. Differential roles of PI3-kinase, MAPKs and NF-kappaB on the manipulation of dendritic cell T(h)1/T(h)2 cytokine/chemokine polarizing profile. Mol Immunol. 2009;46:2481–2492. doi: 10.1016/j.molimm.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Ivanova IA, Nakrieko KA, Dagnino L. Phosphorylation by p38 MAP kinase is required for E2F1 degradation and keratinocyte differentiation. Oncogene. 2009;28:52–62. doi: 10.1038/onc.2008.354. [DOI] [PubMed] [Google Scholar]
- 9.Hong H, Park YK, Choi MS, Ryu NH, Song DK, Suh SI, Nam KY, Park GY, Jang BC. Differential down-regulation of COX-2 and MMP-13 in human skin fibroblasts by glucosamine-hydrochloride. J Dermatol Sci. 2009;56:43–50. doi: 10.1016/j.jdermsci.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SM, Chen CY, Chen SS, Chen JC. Chitinous materials inhibit nitric oxide production by activated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2000;271:229–233. doi: 10.1006/bbrc.2000.2602. [DOI] [PubMed] [Google Scholar]
- 11.Xing D, Feng W, Not LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol. 2008;295:335–342. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kneass ZT, Marchase RB. Protein O-GlcNAc modulates motility-associated signaling intermediates in neutrophils. J Biol Chem. 2005;280:14579–14585. doi: 10.1074/jbc.M414066200. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R, Anderson DE, McDonald SS, Greenwald P. Cancer risk and diet in India. J Postgrad Med. 2003;49:222–228. [PubMed] [Google Scholar]
- 14.Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem. 2008;114:127–149. doi: 10.1080/13813450802033958. [DOI] [PubMed] [Google Scholar]
- 15.Jiang ZY, Zou L, Shi SS, Lu YR, Dong J, Yang CH, Lu YC, Dai GK. Effects of curcumin on TNF-alpha and TGF-beta1 in serum and lung tissue of SiO(2)-induced fibrosis in mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:399–401. [PubMed] [Google Scholar]
- 16.Reuter S, Charlet J, Juncker T, Teiten MH, Dicato M, Diederich M. Effect of curcumin on nuclear factor kappaB signaling pathways in human chronic myelogenous K562 leukemia cells. Ann NY Acad Sci. 2009;1171:436–447. doi: 10.1111/j.1749-6632.2009.04731.x. [DOI] [PubMed] [Google Scholar]
- 17.Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li ST, Sun CH. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomed Environ Sci. 2009;22:32–39. doi: 10.1016/S0895-3988(09)60019-2. [DOI] [PubMed] [Google Scholar]
- 18.Rafi MM, Yadav PN, Rossi AO. Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and transcription factor NF-kappaB. Mol Nutr Food Res. 2007;51:587–593. doi: 10.1002/mnfr.200600226. [DOI] [PubMed] [Google Scholar]
- 19.Chesnokov V, Sun C, Itakura K. Glucosamine suppresses proliferation of human prostate carcinoma DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int. 2009;9:25–28. doi: 10.1186/1475-2867-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox BA, Stephens MM. Glucosamine/chondroitin/primorine combination therapy for osteoarthritis. Drugs Today. 2009;45:21–31. doi: 10.1358/dot.2009.45.1.1314053. [DOI] [PubMed] [Google Scholar]
- 21.Bonness S, Bieber T. Molecular basis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007;7:382–386. doi: 10.1097/ACI.0b013e3282a643c3. [DOI] [PubMed] [Google Scholar]
- 22.Capon F, Trembath RC, Barker JN. An update on the genetics of psoriasis. Dermatol Clin. 2004;22:339–347. doi: 10.1016/S0733-8635(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 23.Deane JA, Hickey MJ. Molecular mechanisms of leukocyte trafficking in T-cell-mediated skin inflammation: insights from intravital imaging. Expert Rev Mol Med. 2009;11:e25. doi: 10.1017/S146239940900115X. [DOI] [PubMed] [Google Scholar]
- 24.Escobar J, Pereda J, Arduini A, Sandoval J, Sabater L, Aparisi L, Lopez-Rodas G, Sastre J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15:3027–3042. doi: 10.2174/138161209789058075. [DOI] [PubMed] [Google Scholar]