Abstract
The present study investigated the similarities between rodent and human urothelial carcinogenesis models using DNA content, p53 and Ki-67 immunoexpression as surrogate markers of bladder carcinogenesis. Following N-butyl-N-(4-hydroxybutyl)-nitrosamine exposure, 49 human cystectomy specimens of bladder cancer and 53 rat bladder specimens were studied. All of the tumours and adjacent mucosa present in each specimen were evaluated. High similarities were observed between the rodent urothelium carcinogenesis process and the corresponding process in humans, in regards to the histopathological features and biological alteration profile: DNA aneuploidy, p53 overexpression and high proliferative index measured by Ki-67 immunoexpression. Despite these similarities, a higher frequency of alterations was observed in earlier stages in the rat chemical-induced carcinogenesis, namely in 5c aneuploid cells, p53 overexpression and higher Ki-67 labelling index. These results confirm that this experimental animal model is a suitable and reproducible model of bladder carcinogenesis, particularly in regards to high-risk non-invasive and invasive urothelial carcinomas. These features mandate its use in the identification of new molecular targets and evaluation of tumour response to new cytotoxic drugs or drug combinations in bladder cancer therapeutic intervention.
Keywords: human, rat, bladder carcinogenesis model, DNA content, p53, Ki-67
Introduction
The importance of chemical carcinogens in the development of bladder cancer has been well established and generally accepted. This carcinogenesis process occurs as a multistep process. Preneoplastic lesions, differently progressed, but with clonally related genomes, exist prior to in situ and invasive carcinoma, and may locally contribute to the independent formation of tumours (1).
Animal models of cancer have been fundamental for the demonstration of this multistep nature (2). Several rodent models have been established to study these different stages of urinary bladder chemical carcinogenesis, namely chemical initiation and promotion, and the stages of progression (3,4). The administration of N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) to mice (5–7) or rats (7–11) is one of the most widely used chemical carcinogens.
The resultant induced tumours in these experimental models have been histologically well studied. However, little is known regarding the biological features and genetic similarities between rodent and human bladder cancer models.
This study aimed to identify biological similarities between human bladder carcinogenesis and rat chemical-induced bladder carcinogenesis by assessing DNA content alterations and p53 and Ki-67 immunoexpression, in order to use this preclinical animal model in future therapeutic experimental studies.
Materials and methods
In this study, we compared biological data collected from our previous studies, in which human (12) and rat (10) bladder samples with premalignant and malignant lesions and adjacent mucosa were studied with regards to histopathology, DNA content, p53 immunoexpression and the Ki-67 labelling index.
Human bladder samples
Consecutive radical cystectomy specimens from 49 patients with previous bladder cancer (non-invasive and invasive urothelial cell carcinomas), consecutively admitted and treated at the Instituto Português de Oncologia do Porto, Portugal, between 1989 and 1996, with available archival material for the image cytometric study and patient consent, were included in the present study. Only high-risk non-invasive urothelial cell carcinomas received prior intravesical Bacillus Calmette-Guérin treatment. Clinical data were collected from the patient records and the paraffin blocks from the Department of Pathology. All of the lesions present in each cystectomy specimen were studied, including the tumour area and the adjacent mucosa (12).
Rat bladder samples
Fifty-three female Fisher 344 rats were obtained at the age of 5 weeks from Harlan (Amsterdam, The Netherlands). Details concerning the animals, diet, carcinogens and experimental protocol were approved by the Portuguese Ethics Committee for Animal Experimentation (Direcção Geral de Veterinária, approval no. 520/000/000/2003) and are fully described in our previously published study (10). Briefly, animals were randomly separated in five groups. BBN was administered in drinking water at a concentration of 0.05% during a period of 20 weeks to all animal groups, with the exception of group 1, which served as the control and was not given any chemical supplement. After this BBN exposure, animals were maintained on normal tap water until the end of the experiment. Groups 2 and 3 were exposed only to BBN, and animals were sacrificed after an intraperitoneal administration of sodium pentobarbital 1 week (group 2) and 7 weeks (group 3) after BBN exposure. One week after BBN exposure, groups 4 and 5 began intravesical instillations with physiological saline solution (PSS) and mitomycin C (MMC), respectively, once a week for 6 weeks. After this treatment period, the animals were sacrificed. The tumours and adjacent mucosa were studied for each bladder specimen.
For intravesical immunotherapy, the dosing schedules were based on those commonly used in clinical work; 300 μl of MMC solution (1 mg/1 ml) were used (10). The preparation procedures of the bladder for macroscopic examination and tissue processing were previously described (10).
Histopathological analysis
The diagnostic pathology slides were re-evaluated by experienced uropathologists (T.A. for the human series, and C.L. and P.O. for the rat specimens). Pathological staging was performed according to the American Joint Committee on Cancer Staging (13). The histological classification and grading were performed using the criteria from the 2004 World Health Organization (WHO) guidelines (14,15).
DNA content analysis
The DNA content quantification was performed using image cytometric analysis and a CAS 200 Image Analysis System (Cell Analysis Systems, Inc., Elmhurst, IL, USA). All methodological and DNA histogram analysis procedures were previously described in detail (6). Briefly, after the calibration of the image system using a control slide with rat hepatocytes with a known quantity of DNA, 20–30 lymphocytes and a minimum of 100 intact non-overlapping urothelial nuclei were measured and analysed for each case. The G0/G1 peak was visually identified in each DNA histogram, and the mean, standard deviation (SD) and coefficient of variation (CV) values were calculated. The DNA index (DI) describes the relative DNA content of the study population and was defined as the ratio of mean DNA content of the urothelial G0/G1 peak divided by the mean DNA content of the resting diploid lymphocyte G0/G1 peak. The 5cER was also evaluated and defined as the percentage of cells with values >5n. Lesions were considered aneuploid only if a separate G0/G1 peak was distinguishable on the histogram and it differed from the reference lymphocyte population by >2 SD. A DNA diploid lesion showed a single distinct G0/G1 peak with a DI within 2 SD of the control lymphocytes and usually with <1% 5cER.
Immunohistochemical analysis
Human specimens
For the immunohistochemical analysis a standard avidin-biotin peroxidase method was used, as described in a previous study (16). The immunoexpression of p53 and Ki-67 was evaluated with the primary antibodies D07 (1:50 dilution; Dako®) and MIB1 (1:50 dilution; Novocastra®), respectively.
Paraffin-embedded tissues, known to express nuclear p53 (colon carcinoma) and have a high proliferation (lymphoma), were used for titration and positive controls. Negative controls were performed replacing the primary antibody with 2.5% bovine serum albumin in PBS.
Rat specimens
A three-step streptavidin-biotin immunoperoxidase method was used, as described in a previous study (10). The primary antibodies AB-1 (1:50 dilution; Neomarkers-Labvision) for p53 expression and Ki-67 (1:20 dilution; Dako) for proliferative activity, were used. Paraffin sections from colon and breast cancer with known immunoreactivity to p53 and Ki-67 antigens, respectively, were used as positive controls. Negative controls were carried out by replacing the primary antibodies with PBS.
Slides were re-evaluated by two independent observers (T.A. and L.S. for the human cases and C.L. and P.O. for the rat cases) in a blinded fashion, without knowledge of the clinical data. The evaluation method was also described previously (16). The entire lesion was screened to find the region with the maximum fraction of positive- and contiguous-stained nuclei for p53 and the region with the maximum fraction of positivity for Ki-67. The percentage of positive-stained nuclei was scored in this region using a ×40 objective. Whenever possible, at least 100 cells were scored in each histological lesion. In the human series, the cut-off values used to distinguish positivity for p53 and Ki-67 were >18 and >32%, respectively, based on studies reported previously (16). In the rat samples, the p53 and Ki-67 immunoexpression was calculated as the percentage of positive nuclei divided by the total number of cells examined (10).
Statistical analysis
The statistical analysis was carried out using the SPSS 15.0 statistical package for Windows (SPSS Inc.). A descriptive analysis was performed for the variables studied. The incidences of differences in molecular alterations between the histological groups were evaluated by the Pearson’s Chi-square and the Fisher’s exact (when n<5) tests. The mean percentage of positivity of p53 and Ki-67 of the histological types was compared by analysis of variance (ANOVA). P<0.05 was defined as statistically significant.
Results
Evaluation of human carcinogenesis
Of the 49 patients included in the present study, 19 cases (38.8%) were classified as high-grade papillary urothelial carcinoma (HGP) and 30 (61.2%) as invasive urothelial carcinoma (INV). For all of the tumours the adjacent mucosa was evaluated and showed differential histological patterns dependent on the adjacent tumour. Morphological normal urothelium (n=13, 68.5%), hyperplasia (n=3, 15.8%) and carcinoma in situ (CIS) (n=3, 15.7%) lesions were found in the HGP adjacent mucosa, while the INV adjacent mucosa showed morphological normal urothelium (n=14, 46.7%), hyperplasia (n=6, 19.9%), dysplasia (n=2, 6.7%) and CIS (n=8, 26.7%) lesions.
Each one of these histological patterns found in the adjacent mucosa showed the same molecular profile, in regards to the biological variables studied, independently of the adjacent tumour.
Considering each histological type, we compared the distribution of the molecular alterations for the biological variables studied. Statistical significant differences were observed according to the potential of aggressiveness of the lesion (Table I).
Table I.
Frequency of molecular alterations for each histological type observed in the human series.
| Variables | Normal | HYP | DYS | CIS | HGP | INV | p-value |
|---|---|---|---|---|---|---|---|
| n=27 | n=9 | n=2 | n=11 | n=19 | n=30 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| DNA ploidy | <0.001 | ||||||
| Diploid | 26 (96.3) | 7 (77.8) | 1 (50) | 1 (9.1) | 7 (36.8) | 2 (6.7) | |
| Aneuploid | 1 (3.7) | 2 (22.2) | 1 (50) | 10 (90.9) | 12 (63.2) | 28 (93.3) | |
| 5cER | <0.001 | ||||||
| Without | 26 (96.3) | 7 (77.8) | 1 (50) | 1 (9.1) | 9 (47.4) | 2 (6.7) | |
| With | 1 (3.7) | 2 (22.2) | 1 (50) | 10 (90.9) | 10 (52.6) | 28 (93.3) | |
| p53 | 0.001 | ||||||
| Negative | 26 (96.3) | 8 (88.9) | 1 (50) | 7 (63.6) | 14 (73.7) | 15 (50.0) | |
| Positive | 1 (3.7) | 1 (11.1) | 1 (50) | 4 (36.4) | 5 (26.3) | 15 (50.0) | |
| Ki-67 | <0.001 | ||||||
| Negative | 26 (96.3) | 9 (100) | 2 (100) | 4 (36.4) | 7 (36.8) | 8 (26.7) | |
| Positive | 1 (3.7) | 0 (0) | 0 (0) | 7 (63.6) | 12 (63.2) | 22 (73.3) |
Normal, morphological normal urothelium; HYP, hyperplasia; DYS, dysplasia; CIS, carcinoma in situ; HGP, high-grade papillary urothelial carcinoma; INV, invasive urothelial carcinoma.
Evaluation of rat carcinogenesis
The histopathological lesions observed in each experimental group of rats are described in Table II. No histopathological alterations in urothelial cells were observed in the control group (group 1). The frequency of lesions increased with a longer observation period after BBN exposure. A higher number of HGP and INV cases was observed in the animal group exposed to BBN and submitted to the intravesical instillation of physiological saline solution (group 4). CIS lesions were detected only in animals in group 5, exposed to BBN and treated with MMC.
Table II.
Incidence of urothelial histopathological lesions in rats exposed to N-butyl-N-(4-hydroxybutyl)-nitrosamine and treated with Mitomycin C and physiological saline solution.
| Group (n) | Normal | HYP | DYS | CIS | LGP | HGP | INV |
|---|---|---|---|---|---|---|---|
| n=10 | n=6 | n=41 | n=7 | n=40 | n=30 | n=10 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| 1 (10) | 10 (100) | 0 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 (12) | 0 (0) | 2 (16.6) | 10 (87.3) | 0 (0) | 10 (83.3) | 2 (16.6) | 2 (16.6) |
| 3 (10) | 0 (0) | 2 (20.0) | 10 (100.0) | 0 (0) | 10 (100.0) | 9 (90.0) | 1 (10.0) |
| 4 (11) | 0 (0) | 1 (9.0) | 11 (100.0) | 0 (0) | 11 (100.0) | 11 (100.0) | 5 (45.4) |
| 5 (10) | 0 (0) | 1 (10.0) | 10 (100.0) | 7 (70) | 9 (90.0) | 8 (80.0) | 2 (20.0) |
Normal, morphological normal urothelium; HYP, hyperplasia; DYS, dysplasia; CIS, carcinoma in situ; LGP, low-grade papillary urothelial carcinoma; HGP, high-grade papillary urothelial carcinoma; INV, invasive urothelial carcinoma.
The molecular alterations found in each histological type are presented in Table III. Despite the period of observation and type of intravesical instillation, the frequency of DNA aneuploidy, 5c aneuploid cells, p53 overexpression and abnormal Ki-67 immunoexpression increased with the degree of aggressiveness of the urothelial lesion.
Table III.
Frequency of molecular alterations for each histological type observed in the rat series.
| Variables | Normal | HYP | DYS | CIS | LGP | HGP | INV | p-value |
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| DNA ploidy | <0.001 | |||||||
| Diploid | 10 (100.0) | 4 (80.0) | 9 (56.3) | 0 (0.0) | 17 (70.8) | 0 (0.0) | 0 (0.0) | |
| Aneuploid | 0 (0.0) | 1 (20.0) | 7 (43.8) | 7 (100.0) | 7 (29.2) | 23 (100.0) | 3 (100.0) | |
| 5cER | <0.001 | |||||||
| Without | 8 (80.0) | 4 (80.0) | 9 (56.3) | 0 (0.0) | 19 (79.2) | 2 (8.7) | 0 (0.0) | |
| With | 2 (20.0) | 1 (20.0) | 7 (43.8) | 7 (100.0) | 5 (20.8) | 21 (91.3) | 3 (100.0) | |
| p53 (positivity) | ||||||||
| mean value | 10 (0.0) | 6 (20.7) | 17 (35.5) | 7 (37.4) | 34 (36.0) | 26 (44.0) | 4 (38.9) | <0.001 |
| Ki-67 (positivity) | ||||||||
| mean value | 10 (0.0) | 6 (16.8) | 17 (23.5) | 7 (29.3) | 34 (23.3) | 26 (34.0) | 4 (23.9) | <0.001 |
Normal, morphological normal urothelium; HYP, hyperplasia; DYS, dysplasia; CIS, carcinoma in situ; LGP, low-grade papillary urothelial carcinoma; HGP, high-grade papillary urothelial carcinoma; INV, invasive urothelial carcinoma.
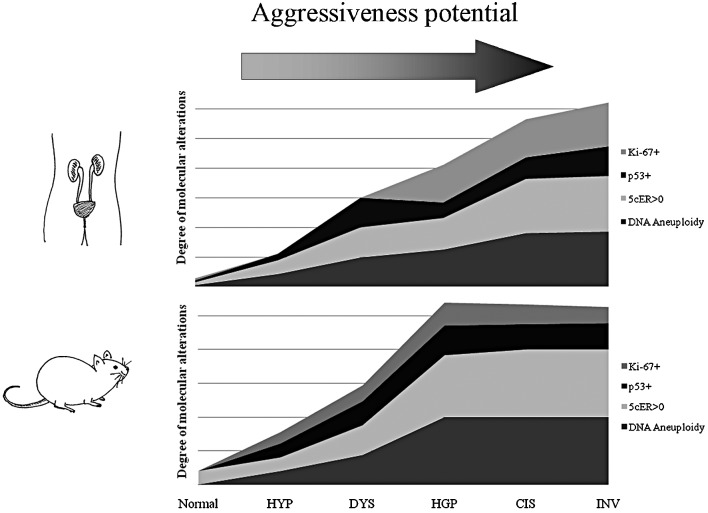
The comparison between the two cancer models, in regards to the molecular alterations found in each histological pattern, is illustrated in Fig. 1. High similarities were observed between the rodent urothelium carcinogenesis process and the corresponding process in humans, in regards to histopathological features and the profile of biological alterations: DNA aneuploidy, p53 overexpression and high proliferative index measured by Ki-67 immunoexpression. Despite these similarities, a higher frequency of alterations was observed in earlier stages in rat chemical-induced carcinogenesis, namely in 5c aneuploid cells, p53 overexpression and higher Ki-67 labelling index.
Figure 1.
Frequency of molecular alterations for each histological type observed in the human and rodent models. Normal, morphological normal urothelium; HYP, hyperplasia; DYS, dysplasia; HGP, high-grade papillary urothelial carcinoma; CIS, carcinoma in situ; INV, invasive urothelial carcinoma.
Discussion
Cancer involves abnormal cellular growth and appears to have a common molecular basis. Comparing the genetic signatures of human and murine bladder carcinogenesis, William and colleagues (7) found that cell cycle-related genes are involved in the two processes, supporting our options to select DNA content, p53 alterations and Ki-67 labelling index as surrogate markers of bladder carcinogenesis.
The present study found compelling similarities between the rodent urothelium carcinogenesis process and the corresponding process in humans, in regards to histopathological features and the profile of biological alterations: DNA aneuploidy, p53 overexpression and high proliferative index measured by Ki-67 immunoexpression.
The histopathological alterations observed by our group in rat chemical-induced bladder carcinogenesis, namely hyperplasia, dysplasia, low- and high-grade papillary urothelial cell carcinoma, CIS and INV were also observed in natural human bladder carcinogenesis (14,15).
The biological history of bladder cancer shows that DNA aneuploidy is a reflection of genomic instability and chromosomal derangement, and a high aneuploidy level was observed in human advanced bladder cancer (17). TP53 genetic alterations are associated with tumour stage and grade, and there is a significant association with patient outcome (16,18,19). A high proliferative index is now accepted as a prognostic factor in human bladder cancer improving identification of patients who are at increased risk for disease progression after radical cystectomy (20).
In the experimental orthotopic model described herein, we observed the entire spectrum of lesions that is described in the dual-track pathway of human bladder carcinogenesis: the papillary and non-papillary pathways (21). We also observed that the variation in the frequency of genetic alterations was similar between the rodent (rat) and human cancer models. Thus, the rate of DNA aneuploidy, p53 immunoexpression and Ki-67 labelling index was higher in more aggressive lesions. However, a biological profile similar to that in human invasive tumours was observed in early tumour stages in rats suggesting that this murine model is a beneficial model with which to study the invasive carcinogenesis pathway. William and colleagues also observed that induced murine tumours exhibited more similarities in gene expression to human muscle invasive tumours than non-invasive tumours. Thus, rodent (rat) tumours provide an accurate mechanistic study of genes putatively involved in invasive and metastatic bladder cancer (7).
We also observed a high frequency of biological alterations in high-risk non-muscle invasive tumours, similar to those that occur in human counterparts. This result indicates that this model is also suitable for the study of these particular tumours.
The present and previous results obtained by our group (10,11) emphasize that we now have a technically suitable and highly reproducible model of bladder urothelial carcinogenesis. This model resembles human disease both histologically and in biological behaviour, particularly for high-risk non-muscle invasive and invasive urothelial bladder cancer. Therefore, it is a reliable model with which to identify new molecular targets and evaluate the feasibility and tumour response of new cytotoxic drugs or drug combinations in the field of urologic oncology.
Acknowledgements
This research study was supported by a grant from Associação Portuguesa de Urologia, 2008. The authors are also grateful to Eng. D. Sanders, Portuguese Institute of Oncology, for the English version.
References
- 1.Höglund M. Bladder cancer, a two phased disease? Semin Cancer Biol. 2007;17:225–232. doi: 10.1016/j.semcancer.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Crallan RA, Georgopoulos NT, Southgate J. Experimental models of human bladder carcinogenesis. Carcinogenesis. 2006;27:374–381. doi: 10.1093/carcin/bgi266. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira PA, Colaço A, De la Cruz PLF, Lopes C. Experimental bladder carcinogenesis-rodent models. Exp Oncol. 2006;28:2–11. [PubMed] [Google Scholar]
- 4.Arentsen HC, Hendricksen K, Oosterwijk E, Witjes JÁ. Experimental rat bladder urothelial cell carcinoma models. World J Urol. 2009;27:313–317. doi: 10.1007/s00345-009-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GF, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–5602. [PubMed] [Google Scholar]
- 6.Oliveira PA, Palmeira C, Lourenço LM, Lopes CA. Evaluation of DNA content in preneoplastic changes of mouse urinary bladder induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. J Exp Clin Cancer Res. 2005;24:609–616. [PubMed] [Google Scholar]
- 7.Williams PD, Lee JK, Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druckrey H, Preussmann R, Ivankovic S, Schmidt CH, Mennel HD, Stahl KW. Selective induction of bladder cancer in rats by dibutyl-and N-butyl-n-(4-hydroxybutyl)nitrosamine. Z Krebsforsch. 1964;66:280–290. [PubMed] [Google Scholar]
- 9.Kunze E, Schauer A, Schatt S. Stages of transformation in the development of N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced transitional cell carcinomas in the urinary bladder of rats. Z Krebsforch Klin Onkol Cancer Res Clin Oncol. 1976;87:139–160. doi: 10.1007/BF00284372. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira PA, Palmeira C, Colaço A, De La Cruz PLF, Lopes C. DNA content analysis, expression of Ki-67 and p53 in rat urothelial lesions induced by N-butyl-N-(4-hydroxybutyl) nitrosamine and treated with mitomycin C and bacillus Calmette-Guérin. Anticancer Res. 2006;26:2995–3004. [PubMed] [Google Scholar]
- 11.Palmeira C, Oliveira PA, Arantes-Rodrigues R, Colaço A, De la Cruz PLF, Lopes C, Santos L. DNA cytometry and kinetics of rat urothelial lesions during chemical carcinogenesis. Oncol Rep. 2009;21:247–252. [PubMed] [Google Scholar]
- 12.Palmeira C, Lameiras C, Amaro T, Lima L, Kock A, Lopes C, Oliveira PA, Santos L. CIS is a surrogate marker of genetic instability and field carcinogenesis in the urothelial mucosa. Urol Oncol. 2009 Oct 23; doi: 10.1016/j.urolonc.2009.07.022. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th edition. Springer Verlag; New York: 2002. [Google Scholar]
- 14.Sauter G, Algaba F, Amin M, et al. Tumours of the urinary system: non-invasive urothelial neoplasias. In: Eble JN, Sauter G, Epstein JL, Sesterhenn I, editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. IARCC Press; Lyon: 2004. pp. 110–123. [Google Scholar]
- 15.Lopez-Beltran A, Sauter G, Gasser T, et al. Tumours of the urinary system: infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstein JL, Sesterhenn I, editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. IARCC Press; Lyon: 2004. pp. 93–109. [Google Scholar]
- 16.Santos L, Amaro T, Costa C, Pereira S, Bento MJ, Lopes P, Oliveira J, Criado B, Lopes C. Ki-67 index enhances the prognostic accuracy of the urothelial supeficial bladder carcinoma risk group classification. Int J Cancer. 2003;105:267–272. doi: 10.1002/ijc.11049. [DOI] [PubMed] [Google Scholar]
- 17.Palmeira CA, Oliveira PA, Seixas F, Pires MA, Lopes C, Santos L. DNA image cytometry in bladder cancer: state of the art. Anticancer Res. 2008;28:443–450. [PubMed] [Google Scholar]
- 18.Cordon-Cardo C. Molecular alterations associated with bladder cancer initiation and progression. Scand J Urol Nephrol. 2008;42:S154–S165. doi: 10.1080/03008880802291915. [DOI] [PubMed] [Google Scholar]
- 19.Lacy S, Lopez-Beltran A, MacLennan GT, Foster SR, Montironi R, Cheng L. Molecular pathogenesis of urothelial carcinoma – the clinical utility of emerging new biomarkers and future molecular classification of bladder cancer. Anal Quant Cytol Histol. 2009;31:5–16. [PubMed] [Google Scholar]
- 20.Margulis V, Lotan Y, Karakiewicz PI, et al. Multi-institutional validation of the predictive value of Ki-67 labeling index in patients with urinary bladder cancer. J Natl Cancer Inst. 2009;101:114–119. doi: 10.1093/jnci/djn451. [DOI] [PubMed] [Google Scholar]
- 21.Spiess PE, Czerniak B. Dual-track pathway of bladder carcinogenesis. Practical implications. Arch Pathol Lab Med. 2006;130:844–852. doi: 10.5858/2006-130-844-DPOBCP. [DOI] [PubMed] [Google Scholar]