Abstract
Mammary tumors were developed by intraperitoneal injection of N-methyl-N-nitrosourea (MNU) in 21-day-old, sexually immature female Wistar rats. Injection of MNU was repeated 14 weeks after the first one. When palpable tumors were evident in all of the rats, various dietary treatments were initiated for a period of 8 weeks. The treatments were designed to provide 30 mg green tea extract either alone or as a nutrient mixture (E). E was then expanded to include either a nutrient supplement (N), quercetin (Q) or both (N+Q). At the end of the treatment, tumor size/rat measured in the live rats was significantly lower in the groups receiving E, E+Q, E+N and E+N+Q than in the positive control (PC) group which did not receive any dietary treatment. Tumor number/rat, tumor volume/rat and tumor weight/rat were evaluated after sacrificing the rats on the 60th day. The rats receiving E+N+Q showed significantly lower values for the three parameters as compared to the PC group. The PC group showed 24 carcinomas mostly of grade III severity, while the E+N+Q group had only 6 carcinomas, all of which were of grade II severity.
Keywords: mammary carcinogenesis, green tea extract, regression, N-methyl-N-nitrosourea
Introduction
N-methyl-N-nitrosourea (MNU)-induced mammary carcinogenesis in female rats is frequently used as an animal model for the investigation of breast carcinogenesis and the treatment of breast cancer in humans (1–12). In our previous studies with MNU-induced rat mammary carcinogenesis, we observed that the specific formulation of nutrient supplements containing ascorbic acid, L-lysine, L-proline, L-arginine, N-acetyl cysteine selenium, copper and manganese along with green tea extract (GTE) fed to the animals reduced the incidence, number and weights of the tumors (12) measured 30 weeks post-MNU. The combination was designated by the authors as ‘Nutrient Synergy’ (NS) to underscore the synergistic action of various constituents. The beneficial results obtained in the studies were mainly attributed to epigallocatechin-3-gallate (EGCG), the principal anticancer agent present in the GTE in the NS (13,14). Our previous in vitro studies with cancer cell lines demonstrated that an increase in the concentration of EGCG in the cell culture media resulted in increased anticancer activity. These findings suggested that when the plasma levels of EGCG are increased, this increase is reflected in the elevated anticancer activity. Our recent investigation (15) showed that the plasma level of EGCG in rats was elevated by as much as 25% when a small amount of quercetin (Q) was administered along with GTE as a nutrient mixture (E) + nutrient supplement (N). It was therefore hypothesized that the anticancer activity of E is enhanced when Q is fed in combination with E+N.
Our previous MNU study (12), based on previous investigations, was conducted for as extensive a period as 30 weeks following the MNU injection. Various studies by Thompson and associates (6,8,16) indicated that the prolonged period of experimentation of such studies can be considerably reduced when MNU is administered to 21-day-old sexually immature female rats rather than to 50-day-old female rats. They attributed the decrease in the period needed for the development of tumors to the rapid induction of pre-malignant and malignant stages of mammary carcinogenesis by MNU in sexually immature rats. Therefore, we conducted the proposed study by injecting MNU into the rats at 21 days of age. In our previous MNU study, the animals were exposed to treatments for an extensive period prior to the tumors reaching a palpable size (12). There are reservations in recommending this approach for human cancer clinical studies as patients generally seek treatment after the cancer is established. In view of this consideration, the present study was designed with the following objectives: a) to accurately mimic the intervention approach by allowing the tumors to grow in size before starting different oral treatments; b) to study the anticancer effect of E administration on well-established tumors; c) to study whether the addition of N to E improves the anticancer activity and plasma concentration of EGCG in rats; and d) to study whether further expansion of the treatment to include Q results in a further increase in the anticancer effect and plasma concentration of EGCG.
Materials and methods
Chemicals and other agents used
Green tea was supplied by Vita Tech International (Tusin, CA, USA). E was made available as Epican Forte™ by Matthias Rath, Inc. (Santa Clara, CA, USA). Its composition is included in the product packaging. The 405 mg of N that was used included 202 mg ascorbic acid, 155 mg lysine HCl, 12 mg proline and 36 mg citrus flavonoids. N was prepared by mixing calculated quantities of the relevant products supplied by Matthias Rath, Inc. Other reagents used were of the the highest purity and were obtained from Sigma Aldrich (Mumbai, India).
Animals
The study was initiated after approval by the Institutional Animal Ethics Committee. Wistar female rats aged 18 days were obtained from the University Animal House. The rats were housed in an environmentally controlled animal laboratory, maintained at 22±2°C with a 12-h light/dark cycle. Diet and water were provided ad libitum. At 21 days of age the rats were weighed. Fifty-six rats were given MNU intraperitoneally at a rate of 50 mg/kg body weight. The solution of MNU (10 mg/ml) was prepared in sterile acidified normal saline (pH 4.0). The solution was filter sterilized prior to administration. The 8 rats that were not given MNU continued in the study as the negative control (NC).
Commencing 2 weeks after MNU injection, the body weights of the individual rats and food consumption, as well as water intake per cage were monitored every week. The mammary glands of the rats were palpated every week for the presence of tumors, from the 6th week after the MNU administration. Since no tumors were palpable until the 14th week post-MNU, a second injection of MNU (50 mg/kg) was administered on the 100th day after the first MNU injection. Palpable tumors measuring ≥5 mm in one dimension were detected in all MNU-administered rats on the 18th week after the first MNU injection. At this time, the length and width of palpable tumors were measured using digital calipers. All of the rats were then divided into seven experimental groups of 8 rats each on the basis of tumor size so that average tumor size (length × breadth × 0.5) in the different groups did not differ by >0.15 units. Seven experimental treatments were randomly assigned to the seven groups (Table I). The ingredients used in each treatment were suspended in 1 ml normal saline and were administered individually to the animals. The material sticking to the syringe was washed down with an additional 0.5 ml of saline. The animals in the PC and NC groups were administered only 1.5 ml of normal saline. The mammary glands of the animals were palpated, and tumor size was measured every week.
Table I.
Details of the dietary treatmentsa used.
| Group | Treatment |
|---|---|
| NC | Negative control; only saline (no carcinogen, no treatment given) |
| PC | Positive control; MNU (carcinogen, no treatment given) |
| GTE | Green tea extract (30 mg) |
| Eb | Epican Forte (130 mg) |
| E+Nc | Epican Forte (130 mg) + nutrient mixture (405 mg) |
| E+Q | Epican Forte (130 mg) + quercetin (0.3 mg) |
| E+N+Q | Epican Forte (130 mg) + nutrient mixture (405 mg) + quercetin (0.3 mg) |
All of the rats in the study, except those in the NC group, received an MNU injection (i.p.) on the 21st and 100th day of age.
E (130 mg) contained 30 mg GTE.
N (405 mg) supplied 202 mg ascorbic acid, 155 mg lysine-HCl, 12 mg proline and 36 mg citrus flavonoids.
Some rats in the PC group showed ulceration and necrosis of tumors on the 26th week after the first MNU injection. The studies were therefore terminated after that week.
At the end of the experiment, the rats were sacrificed by carbon dioxide asphyxiation and skinned partially to expose the tumors of the mammary glands. Grossly visible tumors were excised, and a detailed necropsy was performed on each rat. Location, number, volume (length × breadth × 0.5) and weight of the excised mammary tumors were recorded.
Estimation of EGCG in rat plasma
Blood samples from 5 randomly selected rats from each group were collected for the determination of EGCG. EGCG from rat plasma was processed and separated as previously described (17) and estimated by the method described by Graham (18).
Processing of tumors
Tumors were found in both the abdominal-inguinal mammary and cervical-thoracic mammary gland chains. The lesions in the mammary gland chains were processed as described by Thompson et al (8). In brief, the tumors along with the mammary gland chains were carefully excised and fixed in 10% neutral-buffered formalin for 18–24 h, dehydrated in increasing concentrations of ethanol, and embedded in paraffin. The specimens were then sectioned (5 μm), and the sections were stained using the standard hematoxylin and eosin protocol. Tumors were assayed for their pathological status using the criteria described by Russo and coworkers (19). Grading of the tumors was carried out by the modified Bloom-Richardson (B-R) scheme (20–22). The scoring of the lesions was conducted independently by two trained oncopathologists. No difference was noted in the grades assigned to various tumors by the two oncopathologists.
Statistical analysis
The results were analyzed by analysis of variance and the Student’s t-test using Analyse-It 2.21 for Microsoft Excel (23).
Results and Discussion
Body weight, food and water intake during oral administration to rats
The average body weights of the rats of the different treatment groups did not differ significantly. The food and water consumption were more or less the same in all of the groups.
Tumor incidence and size as measured in live rats
The dietary treatments commenced when every rat in the study had developed at least one palpable tumor. At the start of the experiment, each group had 8 rats. During the early course of the experiment, 2 rats from the PC group and 1 rat from each of the other groups that were given MNU died. There was no mortality in the NC group. Necropsy of the dead rats did not indicate that the mortality was related to the treatments. The number of palpable tumors in the various dietary groups at the start of the treatments ranged between 11 and 12. The NC group mice did not develop any tumors. Total number of palpable tumors in the live animals at various times during the study increased in the PC group from 9 to 16 (Fig. 1). In the E+N+Q group the number increased from an initial value of 11 to 12. One of the tumors regressed by the 60th day. In the E and E+Q groups the tumor number increased from 9 to 12 and from 10 to 14, respectively. In the remaining groups the number of tumors increased by 1 or 2.
Figure 1.
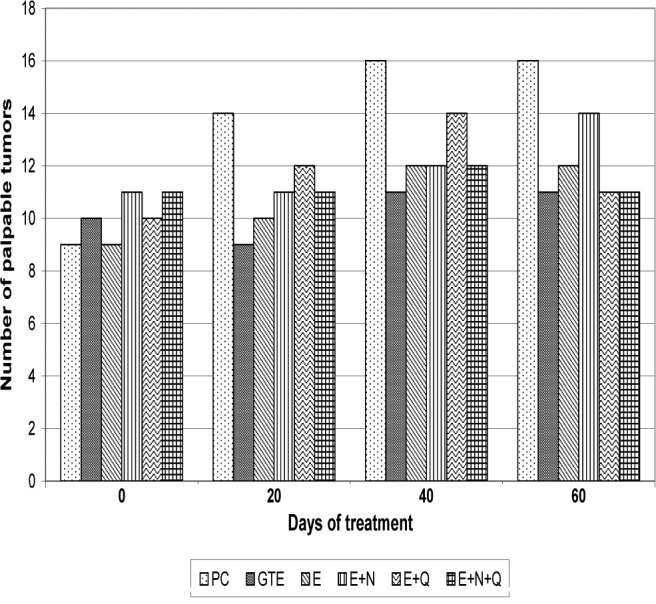
Number of palpable tumors detected at various intervals in live rats.
The average size of the palpable tumors/rat in different groups did not differ significantly at the beginning of the study. During the course of the study the tumors in the PC group grew at a faster rate. By the end of the study (60th day) the tumor size/rat was 3.38 units. The GTE group reached a tumor size of 2.17 units. Notably, the tumor size in the E+N+Q group was only 0.574 units. Despite such a large variation in values, the differences failed to attain significance. When the average sizes of the palpable tumors/rat for the different groups were compared with the PC group, the values for the E, E+N+Q and E+Q groups were found to be lower than that for the PC group (data not shown).
Tumor number, weight and volume after necropsy
The number of tumors dissected from each group was higher than the number of tumors palpated in the live rats (data not shown). Most of the additional tumors detected after sacrificing the animals were of a smaller size. Histopathological examinations revealed that some of the tumors were benign (Fig. 2). The PC group had 24 carcinomas, while the E+N+Q group had only 6 carcinomas. The values for the other groups ranged between 9 and 14. The rats in the PC group had ≥1 carcinomas per rat. In the E+Q group, 2 rats did not have any carcinoma. In the remaining groups the number of rats free from any carcinoma varied from 3 to 4.
Figure 2.
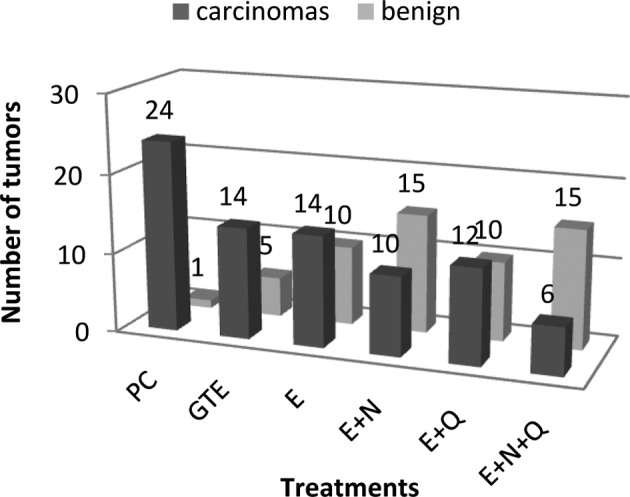
Number of carcinomas and benign tumors detected in the rats after necropsy.
The tumor number, volume and weight per rat, as determined after sacrificing the animals, varied widely among the groups. Despite such a variation, these values failed to achieve significance (Table II). The values for all of the groups were therefore independently compared with those of the PC using the Student’s t-test using one-tailed P-values to determine significance (P<0.05). The tumor number, size and weight per rat for the E+N+Q group were significantly lower than the corresponding values for the PC group. Percentage inhibitions with respect to tumor number, size and weight per rat in the E+N+Q group were 76, 74 and 76%, respectively. Among the remaining groups, only the E+N and E+Q groups had significantly smaller numbers of tumors/rat than the value for the PC group. However, their tumor volume and weight per rat did not differ significantly from the PC group. The GTE and E groups did not differ from the PC group in any of the parameters indicated above.
Table II.
Average number, size and weight of malignant tumors per rat as determined after sacrifice of the rats and histopathological examination of the tumors.
| Treatment | Number of tumors/rat | Size of tumors/rat (cc) | Weight of tumors/rat (g) |
|---|---|---|---|
| PC | 4.0±0.89 | 10.36±6.00 | 5.71±2.39 |
| GTE | 2.0±1.09 | 7.27±6.32 | 5.33±4.72 |
| E | 2.0±1.05 | 4.13±2.74 | 1.70±1.11 |
| E+N | 1.4±0.81a | 3.39±2.42 | 1.71±1.34 |
| E+Q | 1.4±0.95a | 3.52±2.94 | 1.59±1.25 |
| E+N+Q | 1.0±0.53a | 2.69±1.76a | 1.35±0.93a |
Significantly differed from positive control group values (p<0.05) by Student’s t-test.
Histopathological analysis of the tumors
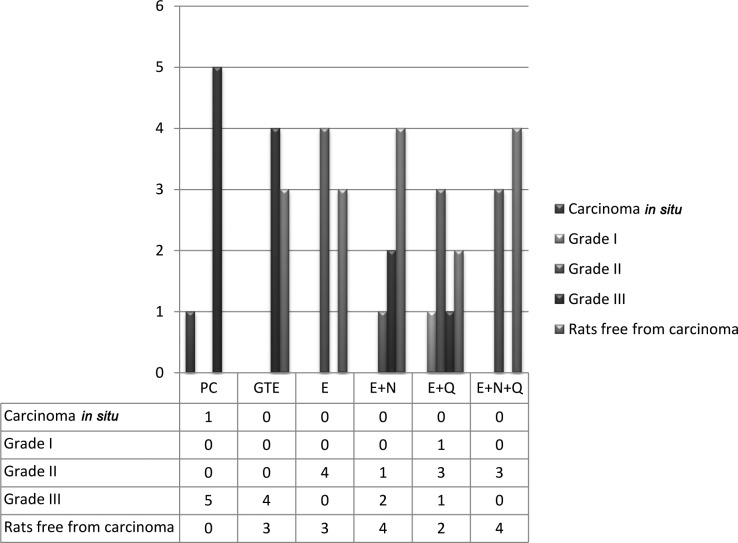
Tumors were assayed for grades of malignancy using the criteria described by Russo et al (19). The severity of the cancerous tumors was graded as detailed in the modified Bloom-Richardson (B-R) scheme (20). The modified B-R scheme is a semi-quantitative grading method based on three morphologic features of tumors: degree of tubal tumor formation, tumor mitotic activity and nuclear pleomorphism of the tumor cells. In the B-R scheme, seven possible scores are condensed into the three B-R grades of low, intermediate and high. Out of 6 rats in the PC group, 5 rats had grade III papillary carcinomas, while 1 rat had carcinoma in situ. In the other groups, 2–4 rats out of 7 were free from carcinomas (Fig. 3); these rats had consistently shown palpable tumors. In the GTE group, 3 rats were free from any carcinomas. The remaining 4 rats had grade III carcinomas. In contrast, the E+N+Q group had 4 rats that did not have any carcinomas. The remaining 3 rats in the group had only grade II carcinomas. The other groups had a variation of grade II and III carcinomas, except for the E+Q group which had 1 grade I carcinoma, along with 3 grade II and 1 grade III carcinomas.
Figure 3.
Number of rats detected with various grades of carcinoma in the different treatment groups.
Plasma concentration of EGCG
The plasma concentration of EGCG was very low (<2 ng/ml) in the PC group (Table III). The relationship between the plasma concentration of EGCG and the number of carcinomas in the rats of the different treatment groups is shown in Fig. 4.
Table III.
Average plasma concentration of EGCG (mean ± SE) determined 1 h after the administration of various dietary treatments.
| Treatment | Plasma concentration of EGCG (ng/ml) mean ± SE |
|---|---|
| PC | 1.99±0.81a |
| GTE | 35.94±1.23b |
| E | 42.60±1.26c |
| E+N | 45.81±2.49d |
| E+Q | 61.94±0.83d |
| E+N+Q | 65.94±1.23d |
The values with different superscripts differed significantly from each other (p<0.05) by Student’s t-test.
Figure 4.
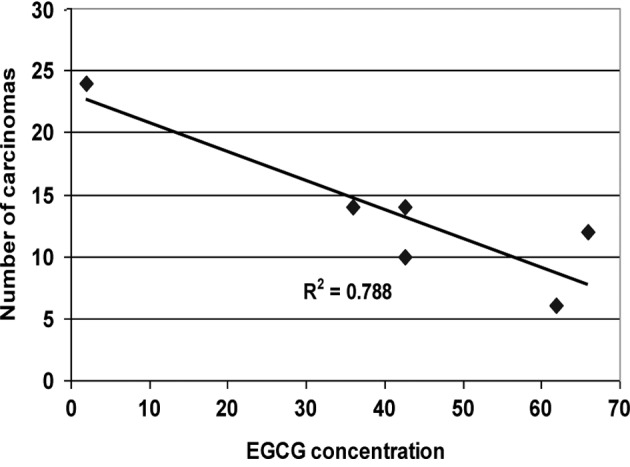
Relation of plasma concentration of EGCG with the number of carcinomas in rats receiving the different treatments.
In conclusion, the results indicate that intervention of E alone may not be as efficient as it occurs in the inhibition of carcinogenesis. E must be supplemented with a nutrient mixture as used in this study along with Q in order to increase the plasma level of EGCG for significant anticancer activity. Furthermore, supplementation of E with the nutrient mixture alone caused a significant decrease in the number of tumors per rat. Doubling the quantity of E did not offer any advantage even when it was given with Q and the plasma level was considerably elevated.
Acknowledgements
The authors are grateful to oncopathologists Dr Girish Moghe and Dr Sachin Mopkar for the histopathological evaluation and for the determination of grades for the cancerous lesions. A.K. and S.G. conducted the experiments, S.K. and S.N. designed and analyzed the data and drafted the manuscript; W.R., V.I., M.R. and A.N. supervised the work and helped in the final drafting of the manuscript.
Abbreviations
- MNU
N-methyl-N-nitrosourea
- GTE
green tea extract
- EGCG
epigallocatechin-3-gallate
References
- 1.Gullino PM, Pettigrew HM, Grantham FH. N-Nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975;54:401–414. [PubMed] [Google Scholar]
- 2.Moon RC, Grubbs CJ, Sporn MB, Goodman DG. Retinyl acetate inhibits mammary carcinogenesis induced by N-methyl-N-nitrosourea. Nature. 1977;267:620–621. doi: 10.1038/267620a0. [DOI] [PubMed] [Google Scholar]
- 3.McCormick DL, Adamoski CB, Fiks A, et al. Lifetime dose-response relationships for mammary tumor induction by a single administration of N-methyl-N-nitrosourea. Cancer Res. 1981;41:1690–1694. [PubMed] [Google Scholar]
- 4.Thompson HJ, Meeke LD. Induction of mammary gland carcinomas by subcutaneous injection of 1-methyl-1-nitrosourea. Cancer Res. 1983;41:1628–1629. [PubMed] [Google Scholar]
- 5.Thomson HJ, Adlakha H. Dose-response induction of mammary gland carcinomas by intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res. 1991;51:3411–3415. [PubMed] [Google Scholar]
- 6.Thompson HJ, McGinley JN, Rothhammer K, et al. Rapid induction of mammary intraductal proliferation, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16:2407–2411. doi: 10.1093/carcin/16.10.2407. [DOI] [PubMed] [Google Scholar]
- 7.Green A, Shilkaitis A, Christov K. 4-(Hydroxyphenyl) retinamide selectively inhibits the development and progression of ductal hyperplastic lesions and carcinoma in situ in mammary gland. Carcinogenesis. 1999;20:1535–1540. doi: 10.1093/carcin/20.8.1535. [DOI] [PubMed] [Google Scholar]
- 8.Thompson HJ, Singh M, McGinley J. Classification of premalignant and malignant lesions developing in the rat mammary gland after injection of sexually immature rats with 1-methyl-1-nitrosourea. J Mammary Gland Biol Neoplasia. 2000;5:201–210. doi: 10.1023/a:1026495322596. [DOI] [PubMed] [Google Scholar]
- 9.Kotsopoulos J, Sohn KJ, Martin R, et al. Dietary folate deficiency suppresses N-methyl-N-nitrosourea-induced mammary tumorigenesis in rats. Carcinogenesis. 2003;24:937–944. doi: 10.1093/carcin/bgg036. [DOI] [PubMed] [Google Scholar]
- 10.Kotsopoulos J, Medline A, Renlund R, et al. Effects of dietary folate on the development and progression of mammary tumors in rats. Carcinogenesis. 2005;20:1603–1612. doi: 10.1093/carcin/bgi117. [DOI] [PubMed] [Google Scholar]
- 11.Melancon K, Cheng Q, Kiefer TL, et al. Regression of MNU-induced mammary tumors with combination of melatonin and 9-cis-retinoic acid. Cancer Lett. 2005;227:39–48. doi: 10.1016/j.canlet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Roomi MW, Roomi NW, Ivanov V, et al. Modulation of N-methyl-N-nitrosourea induced mammary tumors in Sprague-Dawley rats by combination of lysine, proline, arginine, ascorbic acid and green tea extract. Br Cancer Res. 2005;7:R291–R295. doi: 10.1186/bcr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roomi MW, Ivanov V, Kalinovsky T, et al. In vivo and in vitro antitumiorigenic activity of a mixture of lysine, proline and green tea extract on human breast cancer cell lines MDA-MB-231 and MCF-7. Med Oncol. 2005;22:129–138. doi: 10.1385/MO:22:2:129. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad N, Feyes AL, Nieminen R, et al. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 15.Kale A, Gawande S, Kotwal S, et al. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallet from green tea extract. Phytother Res. 2009 Jul 7; doi: 10.1002/ptr.2899. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Thompson HJ, McGinley JN, Wolfe P, et al. Temporal sequence of mammary intraductal proliferation, ductal carcinomas in situ and adenocarcinomas induced by 1-methyl-1-nitrosourea in rats. Carcinogenesis. 1998;19:2181–2185. doi: 10.1093/carcin/19.12.2181. [DOI] [PubMed] [Google Scholar]
- 17.Gawande S, Kale A, Kotwal S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (−) epigallocatechin-3-gallate from green tea extract: a human study. Phytotherapy Res. 2008;22:802–806. doi: 10.1002/ptr.2372. [DOI] [PubMed] [Google Scholar]
- 18.Graham HD. Stabilization of prussian blue color in the determination of polyphenols. J Agric Food Chem. 1992;40:801–805. [Google Scholar]
- 19.Russo J, Gusterson BA, Rogers AE, et al. Comparative study of human and rat tumorigenesis. Lab Invest. 1990;62:244–278. [PubMed] [Google Scholar]
- 20.Bloom HJG, Richardson WW. Histological grading and prognosis in breast cancer. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton LW, Dage DL, Dupont WD. Histologic grading of breast carcinoma: a reproducibility study. Cancer. 1994;73:2765–2770. doi: 10.1002/1097-0142(19940601)73:11<2765::aid-cncr2820731119>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 22.Elston CW. Grading of invasive carcinoma of the breast. In: Page DL, Anderson TJ, editors. Diagnostic Histopathology of the Breast. Edinburgh Churchill Livingston; New York: 1987. pp. 300–311. [Google Scholar]
- 23.Analyse-it for Microsoft Excel (version 2.20) Analyse-it Software Ltd; 2009. http://www.analyse-it.com/ [Google Scholar]
- 24.Ahlers I, Solar P, Buresova A, et al. Very low sensitivity of Wistar:Han female rats to chemocarcinogens in mammary carcinogenesis induction. Neoplasma. 1998;45:373–376. [PubMed] [Google Scholar]
- 25.Bojkova B, Ahlers I, Kubatka P, et al. Repeated administration of carcinogen in critical developmental periods increases the susceptibility of female Wistar:Han rats to mammary carcinogenesis induction. Neoplasma. 2000;47:230–233. [PubMed] [Google Scholar]