Abstract
The causal relationship between chronic inflammation and cancer is widely accepted. Numerous investigations have identified nuclear factor-κB (NF-κB) as an important modulator in driving chronic inflammation to cancer. This study aimed to determine the prevalence of human papillomavirus (HPV) in penile cancer in Japanese patients and whether NF-κB is subsequently overexpressed in penile cancer. Thirty-four specimens of penile tissue (16 malignant and 18 benign cases) were examined to determine the association of HPV infection. An in situ hybridization (ISH) method was used to detect and localize HPV-DNA. A sensitive HPV polymerase chain reaction (PCR) procedure was used for the detection of HPV-DNA, and DNA sequencing was used to identify the HPV genotype. HPV-DNA was detected in 37.5 and 75% of cases of penile cancer, using ISH and PCR, respectively. Our efforts to detect HPV genotypes were unsuccessful as HPV-DNA could not be extracted from these materials. Using ISH, a prevalence of 68.2% of HPV infection was found in penile cancer in Kenyan patients in east Africa. In the present study, all 9 HPV-positive cases, (100%) were NF-κB-positive in the nucleus and/or cytoplasm. In contrast, of the 25 HPV-negative cases, 15 (60%) were NF-κB-positive in the nucleus and/or cytoplasm. Therefore, ISH is a method which is able to prove infection of a large quantity of HPV more effectively when compared with PCR. Thus, a large quantity of HPV infection leads to the activity of NF-κB. The most prevalent genotype was the HPV-22 found in 83.3% of the penile cancer cases. In addition, HPV-11 was found in 81.8% of the non-cancer cases. For cases with a high level of infection, the activity of NF-κB increased compared with those with a low level of HPV infection.
Keywords: human papillomavirus, penile cancer, nuclear factor-κB
Introduction
Genital human papillomavirus (HPV) is the most common sexually transmitted infection. In the 1970s, investigation on the possible role of HPV in cancer was postulated (1). Recently, it has been established that HPVs are important in human carcinogenesis. HPV infection can cause normal cells on infected mucous membranes and the skin to become abnormal. There are more than 40 HPV genotypes that can infect genital lesions of women and men in regions such as the vulva, vagina, cervix, penis, anus and rectum. Low-risk HPV types can cause genital warts in men and women. High-risk HPV types can cause cervical cancer and other less common cancers, including vulvar, vaginal, penile, anal and rectal (2). Although HPV-DNA can be detected in some penile cancer tissues, the presence of HPV may be insufficient by itself to establish full malignant transformation, and other factors must be considered to be important in the carcinogenic process. The synergistic action of HPV associated with poor hygienic conditions may explain the epidemiological evidence that many people are infected with HPV although only a small percentage progress to malignancy over a period of many years, often several decades (3,4).
Virchow suggested in the nineteenth century that chronic inflammation is linked to cancer, but this was not widely accepted before the discovery of nuclear factor-κB (NF-κB) (5,6). Many investigators reported a key role for the transcription factor NF-κB signaling pathway in controlling the initiation and progression of human cancer. NF-κB and associated regulatory proteins are activated downstream of numerous oncoproteins. There is abundant evidence for the activation of NF-κB-dependent target genes in malignant tumors (7,8). The proportion of total cancer deaths attributable to infectious agents is estimated to be 20–25% in developing countries and 7–10% in industrialized countries. A causal relationship between chronic infection and cancer has been widely accepted (9). In particular, there is a strong association between tumor viruses and the development of human cancers. Both DNA and RNA viruses have been reported to be capable of causing cancer. Epstain-Barr virus (EBV), HPV, hepatitis B virus (HBV) and Kaposi’s sarcoma-associated herpes virus (KSHV) are DNA viruses known to lead to the development of cancers. Cancer-inducing RNA viruses include hepatitis C virus (HCV) and human T lymphotropic virus type-1 (HTLV-1). EBV is associated with Burkitt’s lymphoma, nasopharyngeal carcinoma, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma. HBV and HCV viruses are associated with hepatocellular carcinoma, HTLV-1 with adult T-cell leukemia/lymphoma and HPV with cervical, penile, anal, oropharyngeal and skin cancers (2).
In the present study, the procedure of in situ hybridization (ISH) was used to determine the tissue localization of HPV-DNA. Polymerase chain reaction (PCR) was used for the detection of HPV genotypes in penile cancer. This study aimed to determine the prevalence of HPV in penile cancer and the subsequent overexpression of NF-κB.
Materials and methods
Tissue specimens
Thirty-four surgical specimens of penile tissue, obtained at Nagasaki University Hospital and Japanese Red Cross Nagasaki Hospital, Japan from 1972 to 2006, were used in this study. The specimens were fixed in 10% formalin and embedded in paraffin for histochemical, ISH and HPV-DNA sequence studies. Histological analysis was performed using tissue sections (3.5 μm) stained with hematoxylin and eosin. Penile cancer was classfied as i) keratinizing squamous cell carcinoma, ii) non-keratinizing squamous cell carcinoma and iii) verrucous carcinoma. Non-cancer penile tissue was classified as i) Bowen’s disease, ii) condyloma and iii) balanitis. Parallel sections were prepared for ISH and HPV-DNA sequences.
In situ hybridization
Paraffin-embedded tissue specimens were cut into 3.5-μm sections and collected on silane-coated glass slides. The ISH detection kit (Dako Co., Carpinteria, CA, USA) was used for the detection of HPV-DNA. Wide spectrum HPV-DNA probe (Dako), ISH-positive control probe, ISH-negative control probe and HPV-positive control slides were examined.
The steps involved in the ISH procedure using the HPV detection kit are: After hybridization with the probes, an alkaline phosphatase-conjugated antibody against digoxigenin was applied to the sections. The localization of HPV-DNA was detected using NBT/BCIP substrate and observed under a light microscope.
DNA isolation from paraffin-embedded tissue
DNA was isolated from sections of formalin-fixed, paraffin-embedded tissue by using the DNA isolator PS kit (Wako Pure Chemicals Industries, Ltd., Osaka, Japan), according to the manufacturer’s instructions. Briefly, two 5-μm sections were cut with a new blade and collected in a 1.5-ml tube, deparaffinized in xylene, washed with 70% ethanol and digested with protease. The DNA was precipitated with isopropanol and washed with 70% ethanol. The dried DNA was dissolved in 20 ml of TE buffer. A DNA aliquot of 2 ml was used for PCR.
General primer-mediated HPV-PCR and sequencing
General primer-mediated HPV-PCR was undertaken using SPF10 primers, which can detect at least 43 different HPV genotypes, as previously described (10). The PCR products were sequenced for the detection and typing of HPV-DNA. Using the primers 5′-CTTGACCAGCCTCTCTCATGC-3′ and 5′-TGCAGTCTTAGACCCCACCC-3′, PCR amplification of the C-reaction (CRP) gene was carried out in a separate reaction. This resulted in a 1,000-bp product using the SPF10 primers.
PCR and subsequent sequencing were carried out for the detection and typing of HPV-DNA (10). Samples were subjected to PCR using PureTaq Ready-to-Go PCR Beads (Amersham Biosciences Corp., Piscataway, NJ, USA) with 5-mM SPF primers located in the L1 open reading frame of the HPV gene. The PCR amplifications were carried out in a thermal cycler (Applied Biosystems, Foster, CA, USA) under the following conditions: 94°C for 9 min; 45 cycles of 30 sec at 94°C, 45 sec at 45°C and 45 sec at 72°C and a final extension of 5 min at 72°C. The amplified products were separated on a 3% agarose 21 gel (Wako) and detected with ethidium bromide. The 65-bp fragment of SPF was purified using a Qiaex II Gel Extraction Kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s instructions, subsequently cloned into the pGEM-T Easy Vector System (Promega Corp., Madison, WI, USA) and transformed into Escherichia DH5a competent cells (Promega). Plasmids were isolated from several independent colonies by using a Gene Elute Plasmid Miniprep Kit (Sigma-Aldrich Japan, Tokyo, Japan). The Genetic Analyzer (Applied Biosystems) was used for DNA sequencing. The sequences of 22-bp interprimer regions in the PCR products were compared with the GenBank database using the BLAST program.
Immunohistochemistry for NF-κB
Sections (3.5-μm) were placed on silane-coated glass slides. The slides were deparaffinized to remove embedded medium and then dehydrated. Slides were boiled in 0.01 mol/l citrate buffer, pH 7.0, at 98°C for 40 min for antigen retrieval, and then cooled at room temperature for 30 min. After the slides were rinsed in 0.01 mol/l phosphate-buffered saline (PBS), pH 7.4, the endogenous peroxidase activity was blocked with 3% H2O2 and abolute methanol for 10 min. The tissue sections were covered with a 1:50 dilution of mouse monoclonal anti-human NF-κB antibody (Cell Signaling Technology Inc., Beverly, MA, USA) or control serum at 37°C for 3 h. After being washed with PBS, the sections were covered with EnVision (Dako) at 37°C for 40 min, and rinsed in PBS. Antigenic sites on sections were demonstrated by reacting the sections with a mixture of 0.05% 33-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl buffer and 0.01% hydrogen peroxide for 10 min. The sections were then counterstained with methyl green for 10 min, dehydrated in ethanol, cleared in xylene and mounted.
Results
Clinicopathological findings
Penile specimens were diagnosed histologically as penile cancer (16 cases), Bowen’s disease (3 cases), condyloma (10 cases) and balanitis (5 cases). Sixteen cases of penile cancer were classified into 10 cases of keratinizing squamous cell carcinoma, 4 cases of non-keratinizing carcinoma and 2 cases of verrucous carcinoma. The age of penile cancer patients ranged from 38 to 77 years (mean 64 years).
Detection of HPV-DNA and typing
Table I shows the detection of HPV in cancer and non-cancer cases by ISH (Fig. 1) and PCR methods. A prevalence of 80% HPV-positive specimens was found in the cancer cases by PCR. The PCR method gave higher positive rates than the ISH method in both the cancer and non-cancer cases. Of the 16 cancer cases, 12 (75%) showed HPV-positivity. In contrast, of the 18 non-cancer cases, 11 (61.1%) showed HPV-positivity. The results of HPV genotype detection in the different histological categories are summarized in Table II. Among the 12 HPV-positive penile cancers there were 5 single and 7 multiple HPV infection cases. The single HPV-22 infection was the most prevalent (25%), and single and multiple HPV-22 infections (83.3%) were detected in all cancer HPV-positive cases. The single HPV-11 infection was the most prevalent (45.5%), and single and multiple HPV-11 infections (81.8%) were detected in all non-cancer HPV-positive cases.
Table I.
HPV-DNA detection by ISH and SPF-PCR.
| HPV-positive for ISH | HPV-positive for PCR | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Diagnosis | No. | No. | % | No. | % |
| Keratinizing SCC | 10 | 4 | 40.0 | 8 | 80.0 |
| Non-keratinizing SCC | 4 | 1 | 25.0 | 3 | 75.0 |
| Verrucous carcinoma | 2 | 1 | 50.0 | 1 | 50.0 |
| All cancer cases | 16 | 6 | 37.5 | 12 | 75.0 |
| Bowen’s disease | 3 | 0 | 0.0 | 2 | 66.7 |
| Condyloma | 10 | 3 | 30.0 | 9 | 90.0 |
| Balanitis | 5 | 0 | 0.0 | 1 | 20.0 |
| All non-cancer cases | 18 | 3 | 16.7 | 11 | 61.1 |
SCC, squamous cell carcinoma.
Figure 1.
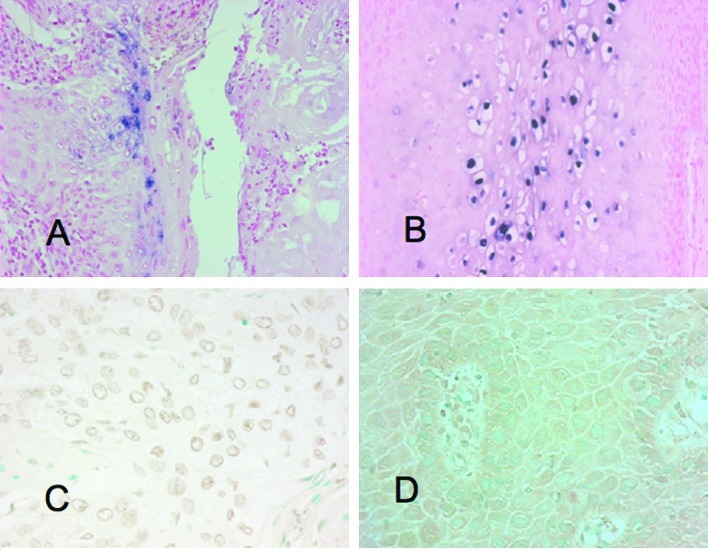
(A) ISH for the HPV signal exhibited in penile cancer. (B) ISH for the HPV signal exhibited in condyloma. (C) Immunohistochemical expression of NF-κB in the nucleus in penile cancer. (D) Immunohistochemical expression of NF-κB in the cytoplasm in penile cancer.
Table II.
HPV-DNA genotype detection in penile cancer and non-cancer cases.
| HPV-positive | Single-HPV-6 | Single-HPV-11 | Single-HPV-22 | Single-HPV-68 | Double-HPV-6,-11 | Double-HPV-11,-22 | Double-HPV-11,-70 | Double-HPV-22,-56 | Double-HPV-22,-68 | Triple-HPV-6,-11,-22 | Triple-HPV-18,-22,-56 | Triple-HPV-22,-56,-68 | Triple-HPV-45,-52,-96 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
| Diagnosis | No. | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
| Keratinizing SCC | 10 | 8 | 80.0 | 3 | 30.0 | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | ||||||||||||||
| Non-keratinizing SCC | 4 | 3 | 75.0 | 1 | 25.5 | 1 | 25.0 | 1 | 25.0 | ||||||||||||||||||||
| Verrucous carcinoma | 2 | 1 | 50.0 | 1 | 50.0 | ||||||||||||||||||||||||
| All cancer cases | 16 | 12 | 75.0 | 1 | 6.3 | 0 | 0.0 | 3 | 18.8 | 1 | 6.3 | 0 | 0.0 | 1 | 6.3 | 0 | 0.0 | 2 | 12.5 | 1 | 6.3 | 1 | 6.3 | 1 | 6.3 | 1 | 6.3 | 0 | 0.0 |
| Bowen’s disease | 3 | 2 | 66.7 | 1 | 33.3 | 1 | 33.3 | ||||||||||||||||||||||
| Condyloma | 10 | 9 | 90.0 | 5 | 50.0 | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | 1 | 10.0 | ||||||||||||||||
| Balanitis | 5 | 1 | 20.0 | 1 | 20.0 | ||||||||||||||||||||||||
| All non-cancer cases | 18 | 11 | 61.1 | 0 | 0.0 | 5 | 27.8 | 2 | 11.1 | 0 | 0.0 | 1 | 5.6 | 1 | 5.6 | 1 | 5.6 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 |
SCC, squamous cell carcinoma; low-risk HPVs, -6, -11, -70; high-risk HPVs, -18, -45, -52, -56, -68; unclassified-risk HPVs, -22, -96.
Immunohistochemistry for NF-κB
Immunohistochemical analyses for NF-κB were performed for all of the specimens (Fig. 1). The PCR method showed that of the 24 HPV-positive cases, 15 (62.5%) were NF-κB-positive in the nucleus and 8 (33.3%) were NF-κB-positive in the cytoplasm. Moreover, NF-κB was detected in 16 (66.7%) cases in the nucleus and/or cytoplasm. Of the 10 HPV-negative cases, 6 (60%) were NF-κB-positive in the nucleus, 3 (30%) were NF-κB-positive in the cytoplasm and 7 (70%) were NF-κB-positive in the nucleus and/or cytoplasm (Table III). The ISH method showed that of the 9 HPV-positive cases, 8 (88.9%) were NF-κB-positive in the nucleus and 2 (22.2%) were NF-κB-positive in the cytoplasm. Additionally, NF-κB was detected in all 9 (100%) cases in the nucleus and/or the cytoplasm. Of the 25 HPV-negative cases, 14 (56%) were NF-κB-positive in the nucleus, 7 (28%) were NF-κB-positive in the cytoplasm and 15 (60%) were NF-κB-positive in the nucleus and/or the cytoplasm (Table IV).
Table III.
HPV-DNA detected by PCR and correlation with NF-κB.
| HPV by PCR | NF-κB in nucleus | NF-κB in cytoplasm | NF-κB in nucleus and cytoplasm | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Diagnosis | No. | % | No. | % | No. | % | No. | % |
| HPV-positive cases | 24 | 70.6 | 15 | 62.5 | 8 | 33.3 | 16 | 66.7 |
| Keratinizing SCC | 8 | 23.5 | 7 | 87.5 | 3 | 37.5 | 7 | 87.5 |
| Non-keratinizing SCC | 3 | 8.8 | 2 | 66.7 | 0 | 0.0 | 2 | 66.7 |
| Verrucous carcinoma | 1 | 2.9 | 1 | 100.0 | 0 | 0.0 | 1 | 100.0 |
| Bowen’s disease | 2 | 5.9 | 2 | 100.0 | 2 | 100.0 | 2 | 100.0 |
| Condyloma | 9 | 26.5 | 3 | 33.3 | 3 | 33.3 | 4 | 44.4 |
| Balanitis | 1 | 2.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV-negative cases | 10 | 29.4 | 6 | 60.0 | 3 | 30.0 | 7 | 70.0 |
| Keratinizing SCC | 2 | 5.9 | 1 | 50.0 | 0 | 0.0 | 1 | 50.0 |
| Non-keratinizing SCC | 1 | 2.9 | 0 | 0.0 | 1 | 100.0 | 1 | 100.0 |
| Verrucous carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s disease | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Condyloma | 1 | 2.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Balanitis | 4 | 11.8 | 3 | 75.0 | 0 | 0.0 | 3 | 75.0 |
| Total | 34 | 100.0 | 20 | 58.8 | 11 | 32.4 | 23 | 67.6 |
SCC, squamous cell carcinoma.
Table IV.
HPV-DNA detected by ISH and correlation with NF-κB.
| HPV by ISH | NF-κB in nucleus | NF-κB in cytoplasm | NF-κB in nucleus and cytoplasm | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Diagnosis | No. | % | No. | % | No. | % | No. | % |
| HPV-positive cases | 9 | 26.5 | 8 | 88.9 | 2 | 22.2 | 9 | 100.0 |
| Keratinizing SCC | 4 | 11.8 | 4 | 100.0 | 1 | 0.0 | 4 | 100.0 |
| Non-keratinizing SCC | 1 | 2.9 | 1 | 100.0 | 0 | 0.0 | 1 | 100.0 |
| Verrucous carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s disease | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 100.0 |
| Condyloma | 3 | 8.8 | 2 | 66.7 | 0 | 0.0 | 3 | 22.2 |
| Balanitis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV-negative cases | 25 | 73.5 | 14 | 56.0 | 7 | 28.0 | 15 | 60.0 |
| Keratinizing SCC | 6 | 17.6 | 4 | 66.7 | 1 | 16.7 | 4 | 66.7 |
| Non-keratinizing SCC | 3 | 8.8 | 1 | 66.7 | 1 | 66.7 | 2 | 66.7 |
| Verrucous carcinoma | 1 | 2.9 | 1 | 100.0 | 1 | 100.0 | 1 | 100.0 |
| Bowen’s disease | 3 | 8.8 | 3 | 100.0 | 3 | 100.0 | 3 | 100.0 |
| Condyloma | 7 | 20.6 | 2 | 28.6 | 1 | 13.0 | 2 | 28.6 |
| Balanitis | 5 | 14.7 | 3 | 60.0 | 0 | 0.0 | 3 | 60.0 |
| Total | 34 | 100.0 | 22 | 64.7 | 9 | 26.5 | 24 | 70.6 |
SCC, squamous cell carcinoma.
Discussion
Human papillomavirus (HPV) infection is associated with a broad spectrum of benign and malignant neoplastic epithelial changes. The association of HPV with neoplastic transformation has been extensively investigated in lesions of the uterine cervix, and the role of HPV in malignant transformation of the cervical epithelium has been well established. Although several epidemiological, clinical and pathological studies have indicated that this virus is sexually transmitted, detailed information on the association of HPV with penile cancer is less clearly understood than that with cervical cancer. The virus can affect the squamous epithelium of the male genitalia in a similar way to the female genital tract. The prevalence of HPV in tumor tissue has been reported to vary considerably (2). The frequency of HPV-positive penile cancer (75%) reported in this study is similar to that reported by Picconi et al (71%) (11) and Senba et al (79%) (12). Higher rates were reported by Sarkar et al (82%) (13) and Tornesello et al (83%) (14). Lower rates were reported by Senba et al (68.2%) (15), Cupp et al (66%) (16), Tornesello et al (46%) (17), Rubin et al (42%) (18) and Cubilla et al (31%) (19).
HPVs can be classified into two groups depending on whether they cause benign or malignant tumors. The high-risk group includes HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -54, -56, -58, -59, -66, -68 and -69, and the low-risk group includes HPV-6, -11, -26, -30, -34, -40, -42, -43, -44, -53, -55, -57, -61, -62, -64, -67, -70, -71, -73, 74, -79, -81, -82, -83 and -84 (20). It is generally accepted that the malignant transformation of penile epidermis cells is associated with infection by high-risk HPV genotypes. Previous studies have shown that among the high-risk HPV genotypes, HPV-16 and -18 are ascendant types and are correlated with penile cancer, constituting 13–60 and 3–19% of infections, respectively (11,16,18,21). The low-risk genotypes are almost regularly encountered in benign lesions, and the predominance of HPV-6 and -11, which are ascendant types, is correlated with benign tumors. Notably, in this series, the high-risk HPV-18 was detected in only 1 case of penile cancer as part of a multiple infection with HPV-22 and -56. HPV-16 infections were not identified. The most prevalent genotype was the HPV-22 found in 83.3% of the penile cancer cases. In addition, HPV-11 was found in 81.8% of the non-cancer cases. Thus, in Japanese penile cancer patients, the HPV genotypes differed from those of patients from other countries.
Published data from recent studies suggest that NF-κB activation is a key component in inflammation-based cancer progression. This transcription factor is indispensable for the malignant progression of transformed cells associated with various inflammatory cells and a network of signaling molecules. The expression and function of numerous cytokines, chemokines, growth factors and survival factors are NF-κB-dependent. NF-κB activation has been implicated in a variety of processes related to transformation and oncogenesis (7,8,22). NF-κB activation is important in the early stages of damaged DNA-induced carcinogenesis. Increased NF-κB activity is associated with many types of cancers including those associated with viral infections. NF-κB activity is modulated for many different viruses, such as human immunodeficiency virus 1, human T-lymphotropic virus 1, Epstein-Barr virus, hepatitis B virus, adenoviruses and HPVs (23–28). NF-κB-dependent proliferation of cells and protection from apoptosis are likely to have significant effects on the oncogenesis associated with HPV infections. In this study, for the HPV-positive cases detected by ISH, NF-κB was detected in the nucleus and/or cytoplasm in 100% of the samples. However, NF-κB was detected in the nucleus and/or cytoplasm in only 60% of the HPV-negative cases. It is known that the PCR method, which amplifies the virus genome, is more sensitive than the ISH method. HPV-DNA was detected in 37.5 and 75% of cases of penile cancer, using ISH and PCR, respectively. These results suggest that ISH is a valid method for detecting HPV infection from cases that present with a high level of HPV infection. Moreover, a high level of HPV infection leads to a more active NF-κB. HPV oncoproteins E6 and E7 are essential factors for HPV oncogenesis. E7 and E6 react with the tumor-suppressor genes p53 and pRb in host cell protein, resulting in induced cellular immortalization, transformation and carcinogenesis, due to their interference with cell cycle and apoptosis control. E7- and E6-induced genetic instability leads to the activation of oncogenes and inactivation of tumor-suppressor genes (25). A fraction of the E7 protein is found in association with the IκB kinase complex and attenuates induced kinase activity of IκB kinase α (IKKα) and IKKβ, thus resulting in impaired IκBα phosphorylation and degradation. While E7 obviates IKK activation in the cytoplasm, the E6 protein reduces NF-κB p65-dependent transcriptional activity within the nucleus. It has been suggested that the HPV oncogene-mediated suppression of NF-κB activity contributes to HPV escape from the immune system (24). Therefore, HPV E6 and E7 oncoproteins are important regulatory proteins inside host cells and are associated with the transcriptional activity of NF-κB.
References
- 1.Zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;44:219–224. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Senba M, Mori N, Wada A. Oncogenesis of and the link between inflammation and cancer due to human papillomavirus (HPV) infectionand the development of vaccine control strategies. Cancer Res J. 2009;2:307–338. [Google Scholar]
- 3.Smith JS, Herrero R, Bosetti C, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604–1613. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- 4.Smith JS, Munoz N, Herrero R, et al. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J Infect Dis. 2002;185:324–331. doi: 10.1086/338569. [DOI] [PubMed] [Google Scholar]
- 5.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 6.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 8.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 9.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 10.Kleter B, Doorn LJ, Schegget J, et al. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;135:1731–1739. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picconi MA, Ejian AM, Distefano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65–69. doi: 10.1002/(sici)1096-9071(200005)61:1<65::aid-jmv10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Senba M, Kumatori A, Fujita S, et al. The prevalence of human papilomavirus genotypes in penile cancers from northern Thailand. J Med Virol. 2006;78:1341–1346. doi: 10.1002/jmv.20703. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar FH, Miles BJ, Plieth DH, Crissman JD. Detection of human papillomavirus in squamous neoplasm of the penis. J Urol. 1992;147:389–392. doi: 10.1016/s0022-5347(17)37245-2. [DOI] [PubMed] [Google Scholar]
- 14.Tornesello ML, Buonaguro FM, Beth-Giraldo E, Kyalwazi SK, Giraldo G. Human papillomavirus (HPV) DNA in penile carcinomas and two cell lines from high-incidence area for genital cancers in Africa. Int J Cancer. 1992;51:587–592. doi: 10.1002/ijc.2910510414. [DOI] [PubMed] [Google Scholar]
- 15.Senba M, Buziba N, Mori N, Wada A, Irie S, Toriyama K. Detection of human papillomavirus and cellular regulators p16INK4a, p53, and NF-κB in penile cancer cases in Kenya. Acta Virol. 2009;53:43–48. doi: 10.4149/av_2009_01_43. [DOI] [PubMed] [Google Scholar]
- 16.Cupp MR, Malek RS, Goellner JR, Smith TF, Espy MJ. The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol. 1995;154:1024–1029. [PubMed] [Google Scholar]
- 17.Tornesello ML, Duraturo ML, Losito S, et al. Human papillomavirus genotypes and HPV 16 variants in penile carcinoma. Int J Cancer. 2008;122:132–137. doi: 10.1002/ijc.23062. [DOI] [PubMed] [Google Scholar]
- 18.Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubilla AL, Reuter VE, Gregoire L, et al. Basaloid squamous cell carcinoma: a distinctive human papilloma virus-related penile neoplasm. A report of 20 cases. Am J Surg Pathol. 1998;22:755–761. doi: 10.1097/00000478-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasia and in genital warts. Med Microbiol Immunol. 2004;193:35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]
- 21.Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD. Preferential association of human papillomavirus with high-grade histological variants of penile invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87:1705–1709. doi: 10.1093/jnci/87.22.1705. [DOI] [PubMed] [Google Scholar]
- 22.Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-κB-dependent: studies using adenoviruses expressing the endogenous NF-κB inhibitor IκBα and kinase-defective form of the IκB kinase 2. J Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 23.Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes. J Virol. 2001;75:4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitkovsky D, Hehner SP, Hofmann TG, Moller A, Schmitz ML. The human papillomavirus oncoprotein E7 attenuates NF-κB activation by targeting IκB kinase complex. J Biol Chem. 2002;277:25576–25582. doi: 10.1074/jbc.M201884200. [DOI] [PubMed] [Google Scholar]
- 25.Havard L, Delvenne P, Frare P, Boniver J, Giannini SL. Differential production of cytokines and activation of NF-κB in HPV transformed keratinocytes. Virology. 2002;298:271–285. doi: 10.1006/viro.2002.1468. [DOI] [PubMed] [Google Scholar]
- 26.Havard L, Rahmouni S, Boniver J, Delvenne P. High levels of p105 (NFκB1) and p100 (NFκB2) proteins in HPV 16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology. 2005;331:357–366. doi: 10.1016/j.virol.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-κB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int J Cancer. 2006;119:2840–2850. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- 28.James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]


