Abstract
Human papillomavirus (HPV) E6 and E7 oncoproteins are essential factors for HPV oncogenesis. These E6 and E7 gene products play a central role in the induction of malignant transformation by interacting with several cellular regulatory proteins such as p16INK4a, p53 and nuclear factor κB (NF-κB). In the present study, conducted in northern Thailand, HPV-DNA was detected in penile cancer cases using an in situ hybridization procedure and p16INK4a, p53 and NF-κB were detected by immunohistochemistry. Using the cell cycle regulatory proteins p16INK4a (61.5%) and p53 (71.8%), it was found that of the 51 cases, 39 (76.5%) were HPV-DNA-positive in penile cancer. On the other hand, 25% p16INK4a and 75% p53, respectively, were found in HPV-negative cases. Prevalence of HPV infection (76.5%) was shown in penile cancer cases in northern Thailand. No difference was found between HPV-positive and HPV-negative cases with respect to the presence of the cell cycle regulatory protein p53. On the other hand, p16INK4a was found to be different between HPV-positive and HPV-negative cases. Inactivation of tumor suppressor genes, such as p16INK4a and p53, to genetic instability, cell immortalization, accumulation of mutations and cancer formation, with or without HPV and irrespective of HPV infection, is therefore suggested. Of the 39 HPV-positive cases, 35 (89.7%) were NF-κB-positive in the nucleus, 29 (74.4%) in the cytoplasm and 37 (94.9%) in the nucleus and/or cytoplasm. NF-κB was detected in 4 (33.3%) of the 12 HPV-negative cases. Therefore, we propose that penile cancer cases with HPV infection are more likely to activate NF-κB than those without HPV infection.
Keywords: human papillomavirus, p16INK4a, p53, NF-κB, penile cancer
Introduction
Human papillomavirus (HPV) infection is associated with a broad spectrum of benign and malignant neoplastic epithelial changes. The association of HPV with neoplastic transformation has been extensively investigated in lesions of the uterine cervix. Moreover, the role of HPV in malignant transformation of the cervical epithelium is well established. On the other hand, HPV-DNA has been found to be less common in penile cancer cases (1). The incidence of penile cancer is lower compared to cervical cancer. Although several epidemiological, clinical and pathological studies have indicated that this virus is sexually transmitted, detailed information on the association of HPV with penile cancer has yet to be sufficiently elucidated as compared to cervical cancer. HPV infection is the most common sexually transmitted disease, thus the risk factor increases with certain sexual behaviors. The highest prevalence of HPV infection is observed in sexually active adolescents and young adults. The virus affects the squamous epithelium of the male genitalia in a similar way to the female genital tract (1–3).
The HPV viral oncogenes, E6 and E7, are the main contributors to the development of HPV-induced cancers, probably due to the integration of the viral genes in the host cell genome. E6 and E7 react with the tumor suppressor gene products p53 and pRb in host cell proteins, resulting in induced cellular immortalization, transformation and carcinogenesis, due to their interference with cell cycle and apoptosis control (4). p16INK4a belongs to the inhibitors of cyclin-dependent kinase (CDK)-4 families (INK4a family) that decelerate the cell cycle by inactivating the cyclin-dependent kinase inhibitors. By interacting with CDK4 and CDK6, p16INK4a inhibits the formation of the cyclin D/CDK4 and CDK6 complex, which is a proliferation-stimulating protein. The p16INK4a protein prevents the phosphorylation of pRb family members. Normally, the overexpression of p16INK4a results in the inhibition of E2F-dependent transcription and of cell cycle progression at the G1 to S phase of the cell cycle (5). The nuclear and cytoplasmic overexpression of p16INK4a protein was previously reported for certain cancers (5–10).
NF-κB was first discovered as a protein bound to the κ immunoglobulin light chain gene enhancer in the nuclei of B cells (11). NF-κB transcription factors consist of five homologous subunits, such as RelA/p65, c-Rel, RelB, p50/NF-κB1 and p52/NF-κB2. IκB-bound NF-κB dimers are IκB kinase (IKK) complexes, comprising two catalytic (IKKα and IKKβ) and one regulatory (IKKγ/NEMO) subunit (12). The causal relationship between chronic inflammation and cancer is widely accepted. Numerous investigations have identified nuclear factor-κB (NF-κB) as an important modulator in driving chronic inflammation to cancer. This transcriptional factor is indispensable for the malignant progression of transformed cells associated with various inflammatory cells and a network of signaling molecules. Significant factors for cancer development include self-sufficiency in growth signals, insensitivity to growth inhibitors, evasion of apoptosis, limitless proliferation potential, tissue invasion and sustained angiogenesis. Experimental evidence revealing specific mechanisms by which NF-κB influences cancer initiation, promotion and progression has been reported (13,14). The expression and function of numerous cytokines, chemokines, growth factors and survival factors are NF-κB-dependent. NF-κB activation has been implicated in a variety of processes related to transformation and oncogenesis (15).
To the best of our knowledge, the immunohistochemical localization of NF-κB associated with HPV infection in penile cancer was previously reported using materials obtained from previous studies of Kenya, East Africa and Nagasaki, Japan (16,17). The purpose of this study was to determine the relationship among HPV infection, p16INK4a, p53 and NF-κB in penile cancer in northern Thailand.
Materials and methods
Tissue specimens
Penile tissue used in this study, was obtained from 51 surgical cases. Surgery was performed at Chiang Mai University Hospital in northern Thailand. The specimens were fixed in 10% formalin and embedded in paraffin for histochemical, in situ hybridization (ISH) and HPV-DNA sequence studies. Histological analysis was performed using 3.5-μm tissue sections stained with hematoxylin and eosin. Penile cancer was classfied as i) keratinizing and ii) non-keratinizing squamous cell carcinoma.
In situ hybridization
Paraffin-embedded tissue specimens were cut at 3.5-μm sections and collected on silane-coated glass slides. To detect HPV-DNA, the ISH detection kit (Dako, Carpinteria, CA, USA) was used. Wide spectrum HPV-DNA (Dako), ISH-positive control and ISH-negative control probes, as well as HPV-positive control slides were examined.
The ISH procedure using the HPV detection kit was performed as follows: following hybridization with the probes, alkaline phosphatase conjugated antibody against digoxigenin was applied to the sections. The localization of HPV-DNA was detected by using NBT/BCIP substrate and observed under a light microscope.
Immunohistochemistry for NF-κB
Sections of 3.5 μm were placed on silane-coated glass slides. The slides were deparaffinized to remove embedded medium and dehydrated. They were then boiled in 0.01 mol/l citrate buffer (pH 7.0) at 98˚C for 40 min for antigen retrieval and cooled at room temperature for 30 min. After rinsing the slides in 0.01 M phosphate-buffered saline (PBS) (pH 7.4), the endogenous peroxidase activity was blocked with 3% H2O2 and absolute methanol for 10 min. The tissue sections were covered with 1:50 dilution of mouse monoclonal anti-human NF-κB antibody (Cell Signaling Technology Inc., Beverly, MA, USA) or control serum at 37˚C for 3 h. After being washed with PBS, the sections were covered with EnVision (Dako) at 37˚C for 40 min and rinsed in PBS. Antigenic sites on sections were demonstrated by reacting these sections with a mixture of 0.05% 3,3′-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl buffer and 0.01% hydrogen peroxide for 10 min. The sections were then counterstained with methyl green for 10 min, dehydrated in ethanol, cleared in xylene and mounted.
Results
Clinicopathological findings
The age of penile cancer patients ranged from 29 to 76 years (mean 56.9). Of the 51 cases of penile cancer included in the study, 42 cases were classified as keratinizing (Fig. 1A) and 9 cases as non-keratinizing squamous cell carcinoma.
Figure 1.
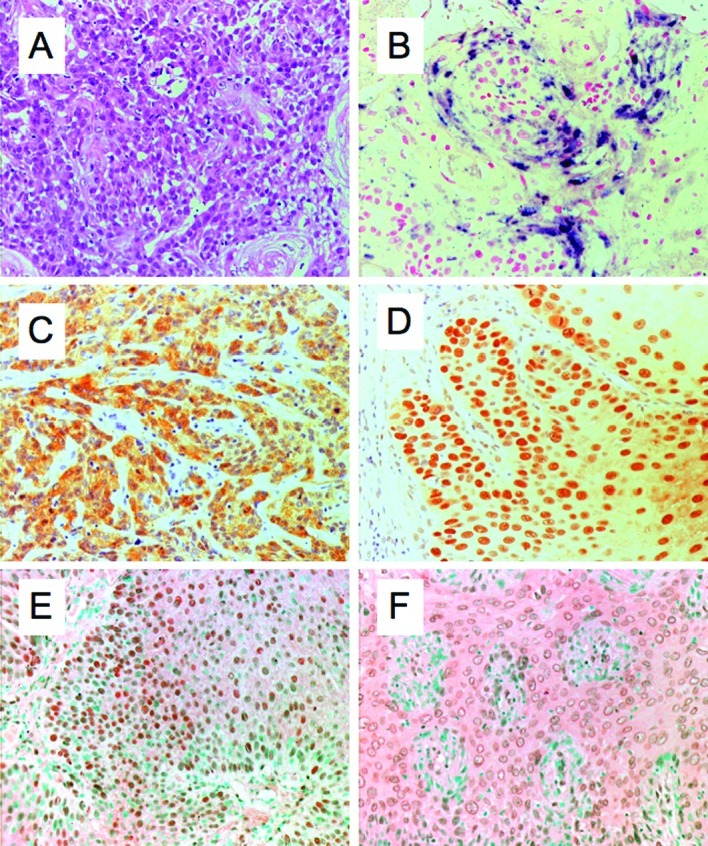
(A) Hematoxylin and eosin staining of squamous cell carcinoma is shown. (B) ISH procedure used to detect HPV-DNA in penile cancer. Immunohistochemical expression of (C) p16INK4a; (D) p53; (E) NF-κB in the nucleus; and (F) NF-κB in the cytoplasm.
Detection of HPV-DNA, p16INK4a, p53 and NF-κB
Of the 51 cases, 39 (76.5%) were HPV-DNA-positive in penile cancer, as determined by the ISH procedure (Fig. 1B). In the keratinizing squamous cell carcinoma type, 32 (76.2%) out of 42 cases were HPV-DNA-positive. On the other hand, in the non-keratinizing squamous cell carcinoma type, 7 (77.8%) out of 9 cases were HPV-DNA-positive.
Immunohistochemical analysis for p16INK4a, p53 and NF-κB was performed for all of the specimens. The results in relation to HPV are summarized in Table I. Of the 51 cases, 39 (76.5%) were HPV-positive and 12 (23.5%) were HPV-negative. Of the 39 HPV-positive cases, 24 (61.5) were p16INK4a-positive (Fig. 1C) and 28 (71.8%) were p53-positive (Fig. 1D). Of the 12 HPV-negative cases, 3 (25%) were p16INK4a-positive and 9 (75%) were p53-positive. Of the 39 HPV-positive cases, 29 (74.4%) were NF-κB-positive in the cytoplasm (Fig. 1E) and 35 (73.3%) in the nucleus (Fig. 1F). NF-κB was detected in the nucleus and/or cytoplasm in 37 (94.9%) of the 39 HPV-positive cases. Of the 12 HPV-negative cases, 4 (33.3%) were NF-κB-positive in both the nucleus and cytoplasm.
Table I.
Detection of HPV-DNA, p16INK4a, p53 and NF-κB in penile carcinoma.
| Cases | HPV DNA | p16INK4a | p53 | NF-κB in nucleus | NF-κB in cytoplasm | NF-κB in nucleus and/or cytoplasm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| HPV-positive cases | 39 | 76.5 | 24 | 61.5 | 28 | 71.8 | 35 | 89.7 | 29 | 74.4 | 37 | 94.9 |
| Keratinizing SCC | 32 | 62.7 | 18 | 56.3 | 24 | 75.0 | 28 | 87.5 | 23 | 71.2 | 30 | 93.8 |
| Non-keratinizing SCC | 7 | 13.7 | 6 | 85.7 | 4 | 57.1 | 7 | 100.0 | 6 | 85.7 | 7 | 100.0 |
| HPV-negative cases | 12 | 23.5 | 3 | 25.0 | 9 | 75.0 | 4 | 33.3 | 4 | 33.3 | 4 | 33.3 |
| Keratinizing SCC | 10 | 19.6 | 2 | 20.0 | 8 | 80.0 | 4 | 40.0 | 4 | 40.0 | 4 | 40.0 |
| Non-keratinizing SCC | 2 | 3.9 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 51 | 100.0 | 27 | 52.9 | 37 | 72.5 | 39 | 76.5 | 33 | 64.7 | 41 | 80.4 |
HPV, human papillomavirus; NF-κB, nuclear factor κB; SCC, squamous cell carcinoma.
Discussion
Penile cancer is a relatively rare disease in Europe and the United States, with incidence rates varying from 0.5–1.5 per 100,000 men. On the other hand, in developing countries, it is a more common type of cancer, with an incidence of 2–5 per 100,000 men. The highest frequency of penile cancer occurs in Asia, Africa and Latin America (1).
HPV is a large family of DNA viruses with more than 100 different HPVs, and with approximately 40 HPVs infecting the genital mucosa following sexual transmission. HPVs are classified into two groups depending on whether it causes benign or malignant tumors. The high-risk group includes HPV-16, -18, -31, -33, -35, -39, -45, -51, -54, -56, -58, -59, -66, -68 and -69; and the low-risk group includes HPV-6, -11, -26, -30, -34, -40, -42, -43, -44, -53, -55, -57, -61, -62, -64, -67, -70, -71, -73, -74, -79, -81, -82, -83 and -84 (2). The most common oncogenic HPV genotypes causing cervical cancer are HPV-16 and-18. HPV-16 is the most prevalent high-risk type and is found in cervical cancer, with HPV-18 being the second most prevalent. However, in our investigation, HPV-18 was found to be the most prevalent type in penile cancer in northern Thailand (18). HPVs are believed to be the primary causal agents for the development of benign and malignant tumors in mucosal and skin lesions, and high-risk types such as HPV-16 and -18 are associated with more than 90% of all cervical cancers. HPV-6 and-11 are usually associated with common genital condyloma. However, we cannot exclude the possibility of cancer formation from low-risk HPV types. The prevalence of HPV-DNA is significantly greater in cancer of the genital organs than in other organs. HPV-related disease is well documented in lesions of the female genital organs, and results have been obtained using a wide range of epidemiological, clinical and molecular techniques. The prevalence of HPV in the tumor tissue has been reported to vary considerably. The frequency of HPV-positive (Fig. 1B) penile cancer (76.5%) reported in this study (Table I) is similar to that reported by Picconi et al (71%) (19) and Cupp et al (66%) (20). Higher expression rates were reported by Senba et al (79%) (18), Sarkar et al (82%) (21) and Tornesello et al (83%) (22). Lower expression rates were reported by Tornesello et al (46%) (1), Rubin et al (42%) (23) and Cubilla et al (31%) (24).
HPV is a DNA tumor virus whose genome is organized in three regions, including the early gene (E1 to E7), the late gene (L1 and L2) regions and the upper regulatory region. The nuclear protein in E6 and E7 of HPV is considered to be one of the two major proteins involved in malignant transformation that is consistently transcribed in HPV-positive cervical cancers. HPV E6 and E7 oncoproteins are essential factors for HPV oncogenesis. Inactivation of tumor suppressor p53 and pRb is a common event in the carcinogenesis of human cells. E7- and E6-induced genetic instability leads to the activation of oncogenes and inactivation of tumor suppressor genes. In HPV infection, significant interactions are noted between pRb and p53 proteins, which are important molecules in the cell cycle and apoptosis control. Notably, pRb and p53 proteins are mutated in many human types of cancer. Both E6 and E7 oncogenes interact with pRb and p53, which inhibit the activities of these tumor suppressors. The cell cycle in the S phase normally leads to apoptosis via pRb activity. However, in HPV-infected cells, this process is counteracted by the viral E6 protein, which targets p53 for proteolytic degradation (4). The E7 protein interacts with pRb, an important negative point of entry into the S phase of the cell division cycle. In the hypophosphorylated state, a combination of E7 and pRb activates the E2F transcription factors that trigger the expression of proteins necessary for DNA replication and cell cycle progression. Phosphorylation of pRb by G1 cyclin-dependent kinases releases E2F, leading to cell cycle progression in the S phase. Since E7 is able to bind to unphosphorylated pRb, it may prematurely induce cells to enter the S phase by disrupting pRb-E2F complexes (25). The loss of p53 function is implicated in the pathogenesis of tumors as well as in the prognosis of many neoplasms. The mutant protein accumulates in the nucleus of tumor cells and is identified by immunohistochemical reaction. The frequency of the overexpression of the p53 protein product was reported to be 41–89% in penile squamous cell carcinomas (8,26,27). Our data showed that overexpression of the p53 protein product was detected in 37 (72.5%) of 51 penile cancer cases with and without HPV infection.
The p16INK4a is a cyclin-dependent kinase inhibitor that prevents the phosphorylation of pRb family members. Normally, the overexpression of p16INK4a results in the inhibition of E2F-dependent transcription and of cell cycle progression at the G1 to S checkpoint (5). The repression of the p16INK4a gene expression by hypermethylation or mutation is a common occurrence in cancer. There is a close association between p16INK4a overexpression and high-risk HPV infection. Therefore, overexpression of p16INK4a is suggested to be a useful marker for evaluating HPV activity in cancer lesions and its precursors (6,9). The overexpression frequency of p16INK4a was reported to be 55–97% in cervical squamous cell carcinoma (6–8) and 48–80% in cervical adenocarcinoma (28,29). The overexpression frequency of p16INK4a was previously reported to be 29–50% in penile squamous cell carcinoma (9,10). Klaes and co-workers reported that p16INK4a is a specific biomarker used to identify dysplastic cervical epithelia in sections of cervical biopsy samples or cervical smears (7). As shown in Table I, overexpression of the p16INK4a protein product was observed in 24 (61.5%) of 39 HPV-positive cases, and in 3 (25.0%) of 12 HPV-negative cases. Therefore, overexpression of p16INK4a was higher in HPV-positive vs. HPV-negative cases. Moreover, overexpression of p16INK4a was found to have a higher prevalence in cervical vs. penile cancer (30,31). In our data, no significant association of a p16INK4a (Fig. 1C) and p53 (Fig. 1D) abnormality with HPV-DNA was noted in the penile cancer.
Two pathways are identified in NF-κB signaling. One depends on NEMO, IKKβ activation, nuclear localization of RelA/p50 dimers, and is associated with inflammation. The other depends on IKKα activation probably via the upstream kinase NIK and nuclear localization of p52/RelB dimers. The two pathways involved in the activation of NF-κB are implicated in oncogenesis (32). Activation of NF-κB was observed in many types of cancer, including melanoma, lung cancer, colon cancer, multiple myeloma, pancreatic cancer, esophageal adenocarcinoma, leukemia and lymphoma. Increased NF-κB activity is associated with viral infections. NF-κB activity is modulated for many different viruses, such as HIV-1, HTLV-1, EBV, HBV, adenovirus and HPV (33–38). NF-κB-dependent proliferation and protection from apoptosis are likely to have significant effects on the oncogenesis of HPV associated with cancers. As shown in Table I and Fig. 1E and F, NF-κB was detected in the HPV-positive (94.9%) and HPV-negative cases (33.3%) in the nucleus and/or cytoplasm, respectively. HPV E6- and E7-positive cells have shown IL-1b-induced NF-κB activation and elevated levels of NF-κB components (35). E6 as opposed to E7 expression was found to be associated with the nuclear location of these components. It is frequently reported that HPV-encoded E6 and E7 oncoproteins are important regulatory proteins in host cells, which are associated with the transcriptional activity of NF-κB (33). A fraction of the E7 protein is found in association with the IκB kinase complex and attenuates the induced kinase activity of IκB kinase α (IKKα) and IKKβ, resulting in impaired IκBα phosphorylation and degradation. While E7 obviates IKK activation in the cytoplasm, the E6 protein reduces NF-κB p65-dependent transcriptional activity within the nucleus. It is suggested that the HPV oncogene-mediated suppression of NF-κB activity contributes to HPV escaping from the immune system (34).
References
- 1.Tornesello ML, Duraturo ML, Losito S, et al. Human papillomavirus genotypes and HPV 16 variants in penile carcinoma. Int J Cancer. 2008;122:132–137. doi: 10.1002/ijc.23062. [DOI] [PubMed] [Google Scholar]
- 2.Gross G, Pfister H. Role of human papillomavirus in penile cancer, penile intraepithelial squamous cell neoplasias and in genital warts. Med Microbiol Immunol. 2004;193:35–44. doi: 10.1007/s00430-003-0181-2. [DOI] [PubMed] [Google Scholar]
- 3.Senba M, Mori N, Wada A. Oncogenesis and the link between inflammation and cancer due to human papillomavirus (HPV) infection, and the development of vaccine control strategies. Cancer Res J. 2009;2:307–338. [Google Scholar]
- 4.Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HS, Postigo AA, Dean DC. Active transcriptional repression by the Rb-E2F complex mediates G1 arrest triggered by p16INK4a, TGFb, and contact inhibition. Cell. 1999;97:53–61. doi: 10.1016/s0092-8674(00)80714-x. [DOI] [PubMed] [Google Scholar]
- 6.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16INK4a as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 8.Humbey O, Cairey-Remonnay S, Guerrini JS, et al. Detection of the human papillomavirus and analysis of the TP53 polymorphism of exon 4 at codon 72 in penile squamous cell carcinoma. Eur J Cancer. 2003;39:684–690. doi: 10.1016/s0959-8049(02)00835-3. [DOI] [PubMed] [Google Scholar]
- 9.Ferreux E, Lont AP, Horenblas S, et al. Evidence for at least three alternative mechanisms targeting the p16INK4a/cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J Pathol. 2003;201:109–118. doi: 10.1002/path.1394. [DOI] [PubMed] [Google Scholar]
- 10.Prowse DM, Ktori EN, Chandrasekaran D, Prapa A, Baithun S. Human papillomavirus-associated increase in p16INK4a expression in penile lichen sclerosus and squamous cell carcinoma. Br J Dermatol. 2008;158:261–265. doi: 10.1111/j.1365-2133.2007.08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 12.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 13.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 14.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 15.Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-κB-dependent: studies using adenoviruses expressing the endogenous NF-κB inhibitor IkkappaBalpha and a kinase-defective form of the IkkappaB kinase 2. J Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 16.Senba M, Buziba N, Mori N, Wada A, Irie S, Toriyama K. Detection of human papillomavirus and cellular regulators p16INK4A, p53, and NF-κB in penile cancer cases in Kenya. Acta Virol. 2009;53:43–48. doi: 10.4149/av_2009_01_43. [DOI] [PubMed] [Google Scholar]
- 17.Senba M, Mori N, Wada A, et al. Human papillomavirus genotypes in penile cancers from Japanese patients and HPV-induced NF-κB activation. Oncol Lett. 2010;1:267–272. doi: 10.3892/ol_00000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senba M, Kumatori A, Fujita S, et al. The prevalence of human papillomavirus genotypes in penile cancers from northern Thailand. J Med Virol. 2006;78:1341–1346. doi: 10.1002/jmv.20703. [DOI] [PubMed] [Google Scholar]
- 19.Picconi MA, Eijan AM, Distefano AL, et al. Human papillomavirus (HPV) DNA in penile carcinomas in Argentina: analysis of primary tumors and lymph nodes. J Med Virol. 2000;61:65–69. doi: 10.1002/(sici)1096-9071(200005)61:1<65::aid-jmv10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Cupp MR, Malek RS, Goellner JR, Smith TF, Espy MJ. The detection of human papillomavirus deoxyribonucleic acid in intraepithelial, in situ, verrucous and invasive carcinoma of the penis. J Urol. 1995;154:1024–1029. [PubMed] [Google Scholar]
- 21.Sarkar FH, Miles BJ, Plieth DH, Crissman JD. Detection of human papillomavirus in squamous neoplasm of the penis. J Urol. 1992;147:389–392. doi: 10.1016/s0022-5347(17)37245-2. [DOI] [PubMed] [Google Scholar]
- 22.Tornesello ML, Buonaguro FM, Beth-Giraldo E, Kyalwazi SK, Giraldo G. Human papillomavirus (HPV) DNA in penile carcinomas and in two cell lines from high-incidence areas for genital cancers in Africa. Int J Cancer. 1992;51:587–592. doi: 10.1002/ijc.2910510414. [DOI] [PubMed] [Google Scholar]
- 23.Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–1218. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cubilla AL, Reuter VE, Gregoire L, et al. Basaloid squamous cell carcinoma: a distinctive human papilloma virus-related penile neoplasm. A report of 20 cases. Am J Surg Pathol. 1998;22:755–761. doi: 10.1097/00000478-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DX, Nguyen MM, Lee D, Griep AE, Lambert PF. Human papillomavirus type 16 E7 maintains elevated levels of the cdk25A tyrosine phosphatase during deregulation of cell cycle arrest. J Virol. 2002;77:619–632. doi: 10.1128/JVI.76.2.619-632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam KY, Chan KW. Molecular pathology and clinicopathologic features of penile tumors: with special reference to analyses of p21 and p53 expression and unusual histologic features. Arch Pathol Lab Med. 1999;123:895–904. doi: 10.5858/1999-123-0895-MPACFO. [DOI] [PubMed] [Google Scholar]
- 27.Lopes A, Bezerra AL, Pino CA, Serrano SV, Mello CA, Villa LL. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol. 2002;168:81–86. [PubMed] [Google Scholar]
- 28.Milde-Langosch K, Riethdorf S, Kraus-Poppinghaus A, Riethdorf L, Loning T. Expression of cyclin-dependent kinase inhibitors p16MTS1, p21WAF1, and p27KIP1 in HPV-positive and HPV-negative cervical adenocarcinomas. Virch Arch. 2001;439:55–61. doi: 10.1007/s004280100439. [DOI] [PubMed] [Google Scholar]
- 29.Zielinski GD, Snijders PJ, Rozendaal L, et al. The presence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to high-risk HPV as the main causative agent for adenocarcinoma in situ and adenocarcinoma of the cervix. J Pathol. 2003;201:535–543. doi: 10.1002/path.1480. [DOI] [PubMed] [Google Scholar]
- 30.Kalof AN, Evans MF, Simmons-Arnold L, Beatty BG, Cooper K. p16INK4a immunoexpression and HPV in situ hybridization signal patterns: potential markers of high-grade cervical intraepithelial neoplasia. Am J Surg Pathol. 2005;29:674–679. doi: 10.1097/01.pas.0000155164.78785.c2. [DOI] [PubMed] [Google Scholar]
- 31.Dehn D, Torkko KC, Shroyer KR. Human papillomavirus testing and molecular markers of cervical dysplasia and carcinoma. Cancer. 2007;111:1–14. doi: 10.1002/cncr.22425. [DOI] [PubMed] [Google Scholar]
- 32.Naugler WE, Karin M. NF-κB and cancer – identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-κB-responsive genes in cervical keratinocytes. J Virol. 2001;75:4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitkovsky D, Hehner SP, Hofmann TG, Moller A, Schmitz ML. The human papillomavirus oncoprotein E7 attenuates NF-κB activation by targeting IκB kinase complex. J Bio Chem. 2002;277:25576–25582. doi: 10.1074/jbc.M201884200. [DOI] [PubMed] [Google Scholar]
- 35.Havard L, Delvenne P, Frare P, Boniver J, Giannini SL. Differential production of cytokines and activation of NF-κB in HPV transformed keratinocytes. Virology. 2002;298:271–285. doi: 10.1006/viro.2002.1468. [DOI] [PubMed] [Google Scholar]
- 36.Havard L, Rahmouni S, Boniver J, Delvenne P. High levels of p105 (NF-κB1) and p100 (NF-κB2) proteins in HPV 16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology. 2005;331:357–366. doi: 10.1016/j.virol.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. Differential expression and activation of NF-κB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int J Cancer. 2006;119:2840–2850. doi: 10.1002/ijc.22262. [DOI] [PubMed] [Google Scholar]
- 38.James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]


