Abstract
The collagen gel droplet embedded culture-drug sensitivity test (CD-DST) is an anticancer drug sensitivity test developed about 10 years ago. This study reports the application of this test in the choice of neoadjuvant chemotherapy for the treatment of one patient with a large synovial sarcoma in the right shank. A 28-year old man presented at our hospital with a large mass in his right shank which had a hard texture and an obscure boundary. The histopathological diagnosis of excisional biopsy specimens was synovial sarcoma with low differentiation. Theprubicin/Cisplatin (THP/CDDP) neoadjuvant chemotherapy was selected based on the results obtained from the CD-DST. After three courses, a computed tomography (CT) scan was performed which indicated that the volume of the tumor had decreased significantly. Additionally, tumor necrosis, as well as the clinical response, showed complete response. The histopathological diagnosis of wide excision specimens indicated a grade III chemotherapy response. The patient was alive and without recurrence after a follow-up of 16 months. Our results indicated that the CD-DST is a useful tool for selecting neoadjuvant chemotherapeutic drugs for patients with synovial sarcoma.
Keywords: synovial sarcoma, collagen gel droplet embedded culture-drug sensitivity test, neoadjuvant chemotherapy
Introduction
Synovial sarcoma is a type of malignant soft-tissue tumor that usually occurs in 80% of cases and affects knee and ankle joints in young adults. It also occurs in shoulder, elbow and hip regions. This neoplasm develops close to the joints, tendon sheaths and bursae, but it is rare for it to involve the synovial membrane. For the treatment of these tumors, the mainstay of curative therapy is the complete surgical resection of such tumor manifestations with negative histological margins. However, up to 50% of patients will develop distant metastases during the course of their disease (1). Regional radiotherapy or adjuvant chemotherapy following surgery is therefore crucial. In certain cases, surgery is difficult to perform due to the large volume of the tumor, and then neoadjuvant chemotherapy is considered. A screening method for the selection of effective anticancer drugs for individual patients may be useful, particularly for patients who have large tumors and need neoadjuvant chemotherapy to decrease the volume of the tumor for local excision. The collagen gel droplet embedded culutre-drug sensitivity test (CD-DST) is an anticancer drug sensitivity test that requires only a small number of cells (3×103 per droplet) (2–4). The clinical usefulness of CD-DST for colon (5), breast (6) and gastric (7) cancers and even certain rare cases of solid tumors, such as ovarian mature cystic teratoma with malignant transformation to adenocarcinoma (8), have been reported. Yabushita et al (9) found a strong correlation between clinical outcome and CD-DST results for anticancer drugs. This is a study of a case of a large synovial sarcoma in the right shank, for which neoadjuvant chemotherapy was investigated using CD-DST.
Materials and methods
Anticancer drugs
Isophosphamide (IFO) (10,11) and anthracycline anti-tumor drugs (11,12), including therarubicin (THP), adriamycin (ADM) and epirubicin (EPI), are the first choice in the chemotherapy of soft tissue tumors. Cisplatin (CDDP) (13), vincristine (VCR) (13) and 5-fluorouracil (5-FU) (14,15) have also been reported to be effective against soft tissue tumors. CDDP, THP, VCR and 5-FU were examined in CD-DST. IFO has no effect in in vitro tests, and since it was necessary for IFO to be activated by phosphamidase in vivo, it was used as a negative control.
Preparation of the tumor cell suspension
The excisional specimen was minced finely using scissors, suspended in Hank's balanced saline solution (HBSS, Gibco), treated with Dispersion Enzyme Cocktail EZ (including 1.0% collagenase; Nitta Gelatin, Inc., Osaka, Japan) and digested at 37°C for 1 h. The dispersed tumor cells were collected by centrifugation at 1000 rpm for 3 min, filtered through a 308 nm nylon mesh, washed in HBSS, suspended in PCM-1 medium (Nitta Gelatin) and then incubated in a collagen gel-coated flask (CG-flask, Nitta Gelatin) in a CO2 incubator at 37°C for 24 h. The collagen gel in the CG-flask was dissolved in a cell dispersion using EZ, and only viable cells that adhered to the collagen gel were collected and used for the sensitivity test.
Collagen gel droplet embedded culture-drug sensitivity test
Type I collagen, 10X F-12 medium and reconstitution buffer (Cellmatrix Type CD, Nitta Gelatin) were combined in an ice bath at a ratio of 8:1:1. The prepared tumor cell suspension was added to a collagen solution (1:10, v:v) at a final density of 2×105 cells/ml. A total of three drops of the collagen-cell mixture (30 μl/drop) were placed in each well of a 6-well multiplate on ice and allowed to gel at 37°C in a CO2 incubator; the final concentration was ~3×103 cells/droplet. One hour later, each well was overlaid with 3 ml DMEM/F 12 medium (Gibco) containing 10% fetal bovine serum (Gibco, Canada), incubated in a CO2 incubator at 37°C overnight. CDDP, THP, VCR and 5-FU were then added to the final concentrations of 0.2 and 2.0 μg/ml, 0.02 and 0.2 μg/ml, 0.01 and 0.1 μg/ml and 1.0 and 10 μg/ml, respectively, followed by further incubation for 24 h. IFO (1 or 10 μg/ml) was added as a negative control.
In vitro chemosensitivity test
After removal of the medium containing the anticancer drugs, each well was rinsed twice with 3 ml HBSS, overlaid with 4 ml PCM-2 medium (Serum Free Medium, Nitta Gelatin) and incubated for an additional 7 days. On the fourth day of incubation, the medium was changed. At the end of the incubation, neutral red (Nitta Gelatin) was added to each well at a final concentration of 50 μg/ml, and cells in the collagen gel droplets were stained for 2 h. Each collagen droplet was fixed with 10% neutral formalin buffer, washed in double-distilled water, air-dried and quantified using image analysis. The in vitro sensitivity was expressed as the percentage of the T/C ratio, where T is the total volume in the treated group and C is the total volume in the control group. When the T/C ratio was ≤50%, the in vitro drug sensitivity was regarded as effective. A T/C ratio of >50 and ≤60% was considered borderline, and a T/C ratio of >60% was considered to indicate a lack of efficacy (2). Clinical responses were assessed according to World Health Organization criteria, whereby tumors demonstrating a complete response or partial response are considered clinically responsive. The results of the CD-DST are shown in Fig. 4.
Figure 4.
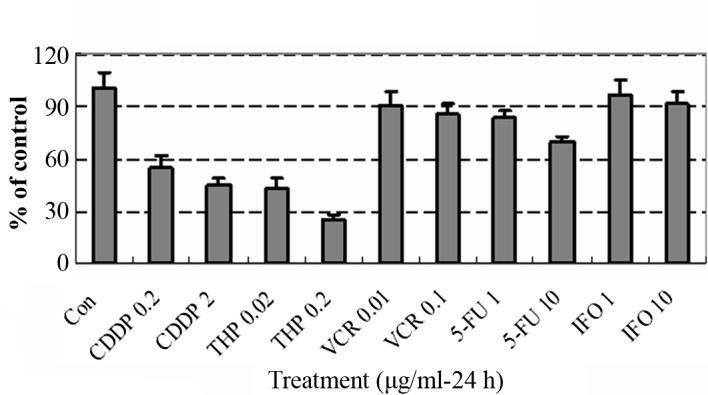
The survival rate of tumor cells and results of the CD-DST. CDDP and THP have a significant effect on the growth of tumor cells, while VCR and 5-FU show no significant effects. IFO was used as a negative control. Based on these results, CDDP/THP neoadjuvant chemotherapy was selected.
Results
The patient was a 28-year-old male who presented with a large mass in the right shank. The perimeter of the right shank was 46.5 cm, while the left side was 37 cm (Fig. 1A). Magnetic resonance imaging (MRI) revealed a 15×10×9 cm mass in the right shank (Fig. 2A and B), and a B-type Ultrasound scan showed a 1.0×0.7×0.5 cm intumesce in the right inguinal fold. The histopathological diagnosis of excisional biopsy specimens was synovial sarcoma with low differentiation (Fig. 3A).
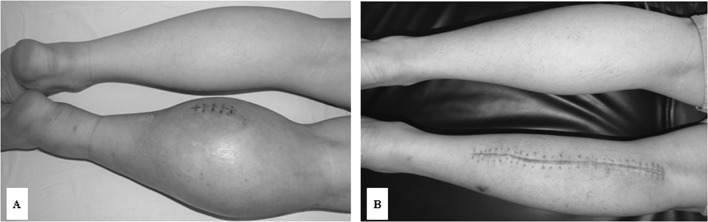
Figure 1.
A large synovial sarcoma in the right shank. (A) Prior to and (B) after neoadjuvant chemotherapy and local tumor excision.
Figure 2.
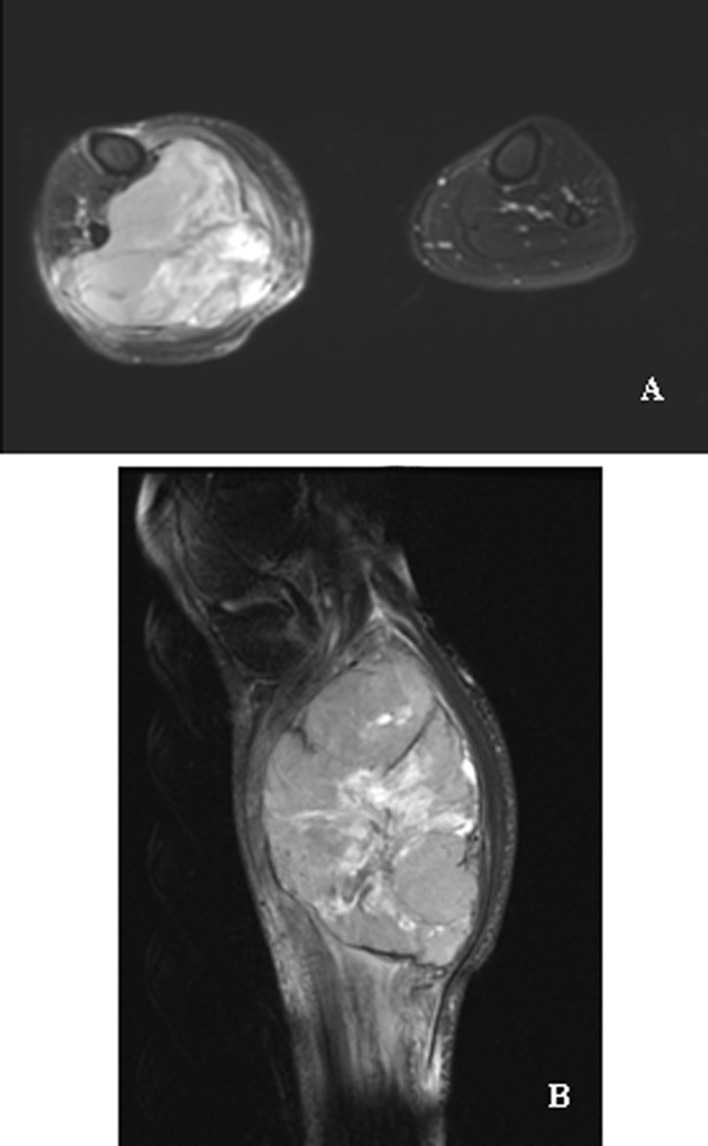
MRI of synovial sarcoma in the right shank prior to neoadjuvant chemotherapy. The sarcoma is 15×10×9 cm, with an obscure boundary and uneven texture and without any destruction of the bone. (A) Axial plane. (B) Sagital plane.
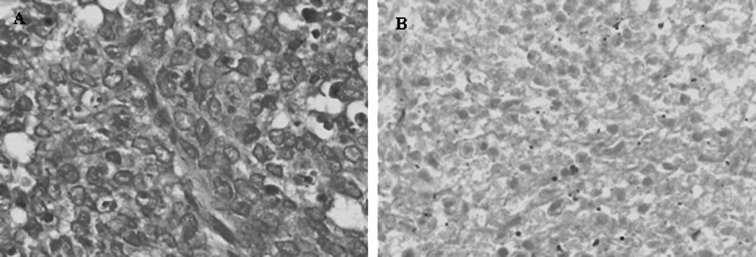
Figure 3.
(A) H&E staining of excisional biopsy specimens prior to neoadjuvant chemotherapy (magnification, ×200). (B) H&E staining of local tumor excision specimens after neoadjuvant chemotherapy. Fibroblast cell proliferation and tumor cell necrosis, grade III chemotherapy response (magnification, ×200).
Although the tumor was extremely large for local excision, the patient refused to accept amputation of the right extremity. Neoadjuvant chemotherapy was considered in order to decrease the volume of the tumor to allow for a local excision. A sample of 0.5 g of the excisional specimen was used for the CD-DST test.
Neoadjuvant THP/CDDP chemotherapy was selected based on the results of the CD-DST (Fig. 4), and administration of THP (30 mg/m2, d1-d2) and CDDP (40 mg/m2, d1-d2) was initiated. Upon completion of three courses of THP/CDDP chemotherapy, the perimeter of the right shank decreased from 46.5 to 42.5 cm, and its texture became much more supple. A precontrast CT scan showed that the volume of the tumor had decreased to 11×8.2 cm, while a contrast scan indicated tumor necrosis (Fig. 5A and B) and a B-type Ultrasound scan showed that the intumesce in the right inguinal fold had disappeared. Since the neoadjuvant THP/CDDP chemotherapy was successful, a local tumor excision was performed (Fig. 1B). The pathodiagnosis indicated fibroblast cell proliferation and tumor cell necrosis as well as a grade III chemotherapy response (Fig. 3B). Post-operatively, the patient received further adjuvant chemotherapy of THP/CDDP, and he was alive and without recurrence at a 16-month follow-up.
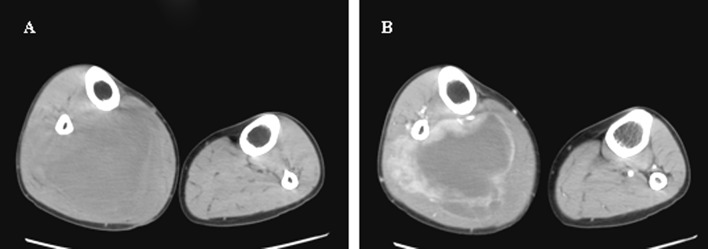
Figure 5.
A CT scan of a synovial sarcoma in the right shank after neoadjuvant chemotherapy. (A) A precontrast scan showed that the volume of the sarcoma had decreased to 11×8.2 cm, and (B) a contrast scan revealed tumor necrosis.
Discussion
The first choice for the treatment of synovial sarcoma is local excision with wide margins of normal tissue, followed by regional radiotherapy or adjuvant chemotherapy (16,17). However, in some cases surgery is difficult to perform due to the large volume of the tumor, thus neoadjuvant chemotherapy is considered. In the case we reported, the tumor was extremely large and we were unable to perform a local excision. The patient involved was young and refused to accept amputation of the extremity. Neoadjuvant chemotherapy was therefore considered in order to decrease the volume of the tumor. IFO (10,11) and anthracycline anti-tumor drugs (11,12), including THP, ADM and EPI, are the first choice in the chemotherapy of soft tissue tumors. CDDP (13), VCR (13) and 5-FU (14,15) are also reported to be effective against soft tissue tumors. In this case, we used the system of CD-DST described by Kobayashi et al (2–4) to check the sensitivity of tumor cells to THP, VCR, CDDP and 5-FU. Since IFO should be activated by phosphamidase in vivo, we used it as the negative control. Based on the results of the CD-DST, THP/CDDP neoadjuvant chemotherapy was administered. Upon completion of three courses of THP/CDDP chemotherapy, the perimeter of the right shank decreased from 46.5 to 42.5 cm, the texture became much more supple, and a CT scan showed that the volume of the tumor had decreased from 15×10 to 11×8.2 cm and that the tumor necrosis had disappeared. A B-type Ultrasound scan showed that the intumesce in the right inguinal fold had also disappeared. The pathodiagnosis of the local excision specimen indicated fibroblast cell proliferation and tumor cell necrosis, as well as a grade III chemotherapy response (Fig. 3B). The patient was alive and without recurrence at a 16-month follow-up evaluation. This clinical course therefore correlated successfully with the results of the CD-DST. Our results show that CD-DST can be used to evaluate anticancer drug sensitivity. Further investigation of CD-DST is required as a test for the prediction of anticancer drug sensitivity in cancer patients. CD-DST may therefore be particularly useful for selecting chemotherapeutic drugs for patients with synovial sarcoma, particularly for those with large tumors who require neoadjuvant chemotherapy to decrease the volume of the tumor prior to local excision surgery.
References
- 1.Schmitt T, Kasper B. New medical treatment options and strategies to assess clinical outcome in soft-tissue sarcoma. Expert Rev Anticancer Ther. 2009;9:1159–1167. doi: 10.1586/era.09.64. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Higashiyama M, Minamigawa K, Tanisaka K, Takano T, Yokouchi H, Kodama K, Hata T. Examination of in vitro chemosensitivity test using collagen gel droplet culture method with colorimetric endpoint quantification. Jpn J Cancer Res. 2001;92:203–210. doi: 10.1111/j.1349-7006.2001.tb01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res. 2003;161:48–61. doi: 10.1007/978-3-642-19022-3_5. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H. Collagen gel droplet culture method to examine in vitro chemosensitivity. Methods Mol Med. 2005;110:59–67. doi: 10.1385/1-59259-869-2:059. [DOI] [PubMed] [Google Scholar]
- 5.Araki Y, Isomoto H, Matsumoto A, Kaibara A, Yasunaga M, Hayashi K, Yatsugi H, Yamauchi K. An in vitro chemosensitivity test for colorectal cancer using collagen-gel droplet embedded cultures. Kurume Med J. 1999;46:163–166. doi: 10.2739/kurumemedj.46.163. [DOI] [PubMed] [Google Scholar]
- 6.Tamura Y, Kobayashi H, Taguchi T, Motoyama K, Inaji H, Noguchi S. Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancers. Int J Cancer. 2002;98:450–455. doi: 10.1002/ijc.10208. [DOI] [PubMed] [Google Scholar]
- 7.Hanatani Y, Kobayashi H, Kodaira S, Takami H, Asagoe T, Kaneshiro E. An in vitro chemosensitivity test for gastric cancer using collagen gel droplet embedded culture. Oncol Rep. 2000;7:1027–1033. doi: 10.3892/or.7.5.1027. [DOI] [PubMed] [Google Scholar]
- 8.Yamagami W, Banno K, Kawaguchi M, Yanokura M, Kuwabara Y, Hirao N, Susumu N, Tsukazaki K, Aoki D. Use of the collagen gel droplet embedded drug sensitivity test to determine drug sensitivity against ovarian mature cystic teratoma with malignant transformation to adenocarcinoma: a case report. Chemotherapy. 2007;53:137–141. doi: 10.1159/000099985. [DOI] [PubMed] [Google Scholar]
- 9.Yabushita H, Ohnishi M, Komiyama M, Mori T, Noguchi M, Kishida T, Noguchi Y, Sawaguchi K, Noguchi M. Usefulness of collagen gel droplet embedded culture drug sensitivity testing in ovarian cancer. Oncol Rep. 2004;12:307–311. [PubMed] [Google Scholar]
- 10.Macdermed DM, Miller LL, Peabody TD, Simon MA, Luu HH, Haydon RC, Montag AG, Undevia SD, Connell PP. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;15:1147–1153. doi: 10.1016/j.ijrobp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp HG, Patel S, Brücher B, Hartmann JT. Potential combination chemotherapy approaches for advanced adult-type soft-tissue sarcoma. Am J Clin Dermatol. 2008;9:207–217. doi: 10.2165/00128071-200809040-00001. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Keohan ML, Saif MW, Rushing D, Baez L, Feit K, DeJager R, Anderson S. Phase II study of intravenous TZT-1027 in patients with advanced or metastatic soft-tissue sarcomas with prior exposure to anthracycline-based chemotherapy. Cancer. 2006;107:2881–2887. doi: 10.1002/cncr.22334. [DOI] [PubMed] [Google Scholar]
- 13.Goto T, Kosaku H, Kobayashi H, Hozumi T, Kondo T. Soft tissue sarcoma: postoperative chemotherapy. (In Japanese) Gan To Kagaku Ryoho. 2004;31:1324–1330. [PubMed] [Google Scholar]
- 14.Wada Y, Hirayama Y, Seki R, Konuma Y, Kohda K, Yoshida M, Nakamura Y, Obata M, Ando M. Long-term remission survival with a case of rectal carcinoid tumor with metastasis in the soft tissue effectively treated with the combination therapy of irinotecan/5-fluorouracil/levofolinate followed by resection. (In Japanese) Nippon Naika Gakkai Zasshi. 2007;96:2513–2515. doi: 10.2169/naika.96.2513. [DOI] [PubMed] [Google Scholar]
- 15.Marchal JA, Boulaiz H, Rodríguez-Serrano F, Peran M, Carrillo E, Vélez C, Domínguez J, Gómez-Vidal JA, Campos J, Gallo MA, Espinosa A, Aránega A. 5-Fluorouracil derivatives induce differentiation mediated by tubulin and HLA class I modulation. Med Chem. 2007;3:233–239. doi: 10.2174/157340607780620671. [DOI] [PubMed] [Google Scholar]
- 16.Kopp HG, Patel S, Brucher B, Hartmann JT. Potential combination chemotherapy approaches for advanced adult-type soft-tissue sarcoma. Am J Clin Dermatol. 2008;9:207–217. doi: 10.2165/00128071-200809040-00001. [DOI] [PubMed] [Google Scholar]
- 17.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trails of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]