Abstract
Erlotinib treatment in combination with gemcitabine is a standard therapy for patients with locally advanced pancreatic cancer in many countries, including the US and the EU. Since mutations of the K-ras oncogene (KRAS) occur in approximately 90% of pancreatic cancers, we examined the antitumor activity of erlotinib in combination with gemcitabine in KRAS-mutated pancreatic cancer cell lines, HPAC and Capan-1, which have the KRAS mutation G12D and G12V, respectively. We analyzed the mode of inhibition of in vitro tumor cell proliferation by means of a combination index and found that a combination treatment of erlotinib plus gemcitabine had an additive effect in the two cell lines. We then examined the effect of erlotinib and gemcitabine on the phosphorylation of epidermal growth factor receptor (EGFR). Erlotinib strongly suppressed, while gemcitabine augmented the phosphorylation of EGFR, which was completely blocked by erlotinib in the two cell lines. An in vivo tumor growth inhibition test was then performed using the HPAC tumor xenograft model. The combination therapy of erlotinib and gemcitabine resulted in a significant inhibition of tumor growth compared with erlotinib or gemcitabine monotherapy. To the best of our knowledge, this is the first study to show the combination effect of erlotinib and gemcitabine in vivo using a xenograft model of a KRAS-mutated pancreatic cancer cell line.
Keywords: epidermal growth factor receptor inhibitor, pancreatic cancer, erlotinib, gemcitabine, KRAS mutation
Introduction
Pancreatic cancer is a particularly lethal disease with an annual incidence rate almost identical to the mortality rate. At the time of diagnosis, more than 80% of patients usually display either locally advanced or metastatic disease. The median survival of patients with locally advanced or metastatic disease is 9 or 4 months, respectively, and the 5-year survival rate is 1–4% (1). In the past 10 years, gemcitabine has replaced 5-fluorouracil as a standard therapy for advanced disease; however, the median survival has only modestly improved from 4.4 to 5.6 months (2). Since then, phase III trials of novel cytotoxic or biologic agents combined with gemcitabine have failed to show any survival improvement compared with gemcitabine alone (3). A recent phase III trial (PA.3 study) compared gemcitabine plus erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, with gemcitabine alone. The findings showed a significant improvement, with a median survival of 6.2 vs. 5.9 months, as well as a 1-year survival of 23 vs. 17%, respectively (4). As a result, erlotinib in combination with gemcitabine was approved by the U.S. Food and Drug Administration in 2005 for the treatment of locally advanced or metastatic chemonaive pancreatic cancer.
Human EGFR is a member of the ERBB family of receptor tyrosine kinases, consisting of four closely related members: EGFR (ERBB1), HER2 (ERBB2), HER3 (ERBB3) and HER4 (ERBB4). On binding to EGFR, ligands, such as epidermal growth factor (EGF) or transforming growth factor α, cause receptor dimerization with one of the ERBB family molecules. Dimerization of receptors activates the tyrosine kinase located at the intracellular domain of the receptor molecules, leading to receptor autophosphorylation and the initiation of signal-transduction cascades involving RAS/RAF/MAPK and PI3K/AKT, culminating in cell proliferation and survival (5). The EGFR signal network, one of the important processes involved in tumor progression including cell proliferation, inhibition of apoptosis, metastasis and angiogenesis, is often dysregulated in cancer cells (6).
Overexpression of EGFR occurs in more than 50% of pancreatic cancers and correlates with poor prognosis and disease progression (7). The frequency of the EGFR mutation is only 1.5% in pancreatic cancers (8), but 59% in lung cancers (9). On the other hand, pancreatic cancer shows the highest frequency of K-ras oncogene (KRAS) mutations among human cancers (10). KRAS mutations, which constitutively activate RAF/MAPK signaling, are detected in up to 90% of pancreatic cancers (11). In the PA.3 study, no significant correlations were found between outcome and KRAS mutations, which were detected in 79% of the erlotinib arm, although a favorable trend for erlotinib in patients that carry the wild-type KRAS has been observed (12).
Erlotinib is an orally active, reversible, competitive inhibitor of the EGFR tyrosine kinase ATP-binding site and blocks the downstream signal transduction of the EGFR associated with cancer progression (13). Erlotinib reportedly enhances the antitumor activity of gemcitabine in pancreatic cancer cell lines with or without a KRAS mutation (14). However, there is no in vivo evidence regarding the improvement of antitumor activity of a combination therapy of erlotinib and gemcitabine in KRAS-mutated pancreatic cancer cell lines (14,15). Therefore, the present study aimed to determine whether a combination treatment of erlotinib and gemcitabine produces an enhanced antitumor activity in a xenograft model using a KRAS-mutated pancreatic cancer cell line.
Materials and methods
Chemicals
Erlotinib was provided by F. Hoffman-La Roche Ltd. (Basel, Switzerland) and dissolved in DMSO for the in vitro study, and in 6% Captisol® (Cydex Inc., Lenexa, KS, USA) solution for the in vivo study. Captisol and gemcitabine (Eli Lilly, Tokyo, Japan) were dissolved in distilled water and saline, respectively.
Tumors
The KRAS-mutated human pancreatic cancer cell lines, HPAC and Capan-1, were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in ATCC-recommended medium at 37°C in 5% CO2. The HPAC cell lines were maintained in DMEM/F12 (Invitrogen, Carlsbad, CA, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS; Japan Bioserum, Hiroshima, Japan), 0.005 mg/ml transfferin (Invitrogen), 40 ng/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 10 ng/ml EGF (Invitrogen) and 0.002 mg/ml insulin (Sigma-Aldrich). The Capan-1 cells were maintained in IMDM (Sigma-Aldrich) supplemented with 20% FBS.
In vitro cell proliferation-inhibition assays
To evaluate cell proliferation inhibition, the tetrazolium dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT) (Dojindo, Tokyo, Japan) assay was performed. Cells were precultured overnight at 37°C in 96-well clear plates, and the drugs were added alone or in combination. After treatment for 4 (HPAC cells) or 6 days (Capan-1 cells) at 37°C, 10 ml of MTT was added to each well and incubated for 2–5 h at 37°C. The optical density of each well was measured at 450 and 600 nm with a Benchmark Plus microplate reader (Bio-Rad, Hercules, CA, USA). Each experiment was performed in duplicate or triplicate for each drug concentration and was independently performed two or three times. The percentage of cell proliferation was calculated as: [(mean absorbance of drug-treated wells - mean absorbance of cell-free wells)/(mean absorbance of vehicle wells − mean absorbance of cell-free wells)] × 100. The effects of the erlotinib and gemcitabine combination were evaluated using a combination index (CI) interpreted as: <1.0, synergistic; 1.0, additive and >1.0, antagonistic (16). The CI for each fraction-affected value representing the percentage of proliferation inhibited by a drug was calculated using the Chou-Talalay method [the isobologram equation was used mutually non-exclusive (α=1)] (16,17). The fraction-affected value (Fa)/CI plots for the cell lines were constructed in Excel 2003.
Immunoblotting
Cultured cells were washed twice with ice-cold PBS, scraped and pelleted by centrifugation at 650 × g for 2 min at 4°C. The pellets were lysed in lysis buffer (Invitrogen) supplemented with 1 mM PMSF, 1 mM NaF and 1 mg/ml aprotinin (all from Sigma-Aldrich) for 5 min on ice, followed by sonication. Supernatants were collected by centrifugation at 16,000 × g for 10 min at 4°C. Protein concentrations were determined using DC protein assay reagent (Bio-rad). Samples of the proteins (50 mg) were diluted with Laemmli sample buffer (Sigma-Aldrich) and applied to 7.5% XV Pantera gel (DRC, Tokyo, Japan). Separated proteins were electrophoretically transferred to 0.22-mm Immobilon membranes (Millipore, Tokyo, Japan) and blocked for 1 h at room temperature in Superblock blocking buffer (Thermo Scientific, Yokohama, Japan). Membranes were incubated overnight at 4°C with antibodies recognizing phospho-EGFR (Y1068, and Y845), EGFR and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then washed with TTBS (Thermo Scientific) and probed with horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody (Santa Cruz Biotechnology) for 2 h at room temperature. After three additional washes with TTBS, the membranes were incubated in Enhanced Chemiluminescence Plus reagent (Amersham Biosciences, Piscataway, NJ, USA) and detected by an ImageQuant Imager (GE Healthcare Bio-Sciences, Tokyo, Japan).
Human cancer xenograft models
Male 5-week-old BALB-nu/nu (CAnN.Cg-Foxn1<nu>/CrlCrlj nu/nu) mice were purchased from Charles River Japan, Inc. (Yokohama, Japan). The animals were housed in a pathogen-free environment under controlled conditions (temperature 20–26°C, humidity 40–70%, light-dark cycle 12–12 h). Chlorinated water and irradiated food were provided ad libitum. The animals were allowed to acclimatize and recover from shipping-related stress for one week prior to the study. The health of the mice was monitored daily.
A cell suspension of HPAC cells (106 viable cells/mouse) was subcutaneously inoculated into the right flank of each mouse. Fifteen days after the tumor cell inoculation, the mice were randomly divided into four groups of eight mice and administered either oral erlotinib (50 mg/kg/day) on days 1–21 or intravenous gemcitabine (20 mg/kg) on days 1, 8 and 15. In the combination therapy, erlotinib and gemcitabine were administered at the same dose and schedule as each drug alone. Captisol (6%) or saline was administered as the control. The drugs were administered following the same schedule as the clinical setting (4). Tumor diameter was measured twice a week using calipers, and tumor volume was calculated as: ab2/2 mm3, where a is the length and b is the width of the tumor. Day 1 denotes the first day of treatment and day 22, the day on which the drug effects were estimated.
The protocol was reviewed by the Institutional Animal Care and Use Committee of Chugai Pharmaceutical Co., Ltd. Animal experiments were performed in accordance with the ‘Guidelines for the Accommodation and Care of Laboratory Animals’ of Chugai Pharmaceutical Co., Ltd.
Statistical analysis
The Wilcoxon test was used to detect the statistical differences in tumor volume. Probability values <0.05 were considered to be significant. Statistical analysis was performed using an SAS preclinical package (version 8.2; SAS Institute Inc., Cary, NC, USA).
Results
In vitro anti-proliferative activity of erlotinib in combination with gemcitabine in the HPAC and Capan-1 pancreatic cancer cell lines
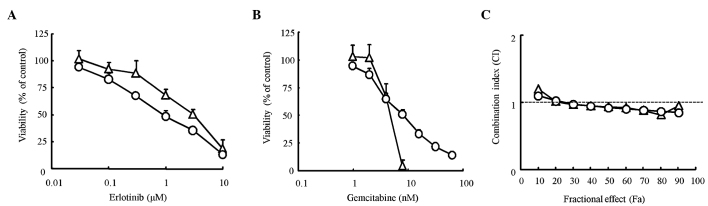
We first determined the proliferation inhibitory effect of erlotinib and gemcitabine alone on pancreatic cancer cell lines using the MTT assay. The IC50 value for erlotinib was 1.1 μM for HPAC cells and 3.0 μM for Capan-1 cells. The IC50 value for gemcitabine was 7.8 nM for HPAC cells and 4.4 nM for Capan-1 cells (Fig. 1A and B). The difference between the IC50 values found for the two cell lines was only slight for both erlotinib and gemcitabine. To evaluate the combination effect of erlotinib and gemcitabine, a combination index (CI) was determined using the Chou-Talalay method. The CI value was nearly equal to 1 for every dose in the two cell lines, suggesting that the combination effects of erlotinib and gemcitabine were ‘additive’ in the two cell lines (Fig. 1C). Thus, the effects of erlotinib in combination with gemcitabine were considered additive in KRAS-mutated pancreatic cancer cells.
Figure 1.
Effect of erlotinib and gemcitabine on the proliferation of pancreatic cancer cell lines in vitro. The cells were treated with the indicated concentrations of erlotinib (A) or gemcitabine (B) alone for 4 days (HPAC cells, open circles) or 6 days (Capan-1 cells, open triangles), and the proliferation of HPAC and Capan-1 cells were determined by the MTT assay. Each point represents the mean ± SD of triplicates. (C) The cells were treated either alone or in combination with the indicated concentrations of erlotinib and gemcitabine at a fixed molar ratio for 4 days, erlotinib:gemcitabine = 160:1 (HPAC cells, open circles), 80:1 (Capan-1 cells, open triangles), and the viabilities of the cells were determined by the MTT assay. The combination index (CI) for each fraction-affected value (Fa) was calculated using the Chou-Talalay method.
In vitro effects of erlotinib in combination with gemcitabine on EGFR signaling in the HPAC pancreatic cancer cell line
Fig. 2 shows that 10 μM erlotinib inhibited EGFR phosphorylation at the Y845 (Src-dependent phosphorylation) and Y1068 (auto-phosphorylation) sites. Gemcitabine (100 nM) augmented the phosphorylation levels at Y845 and Y1068 of EGFR, and these phosphorylations were completely blocked by erlotinib (Fig. 2).
Figure 2.
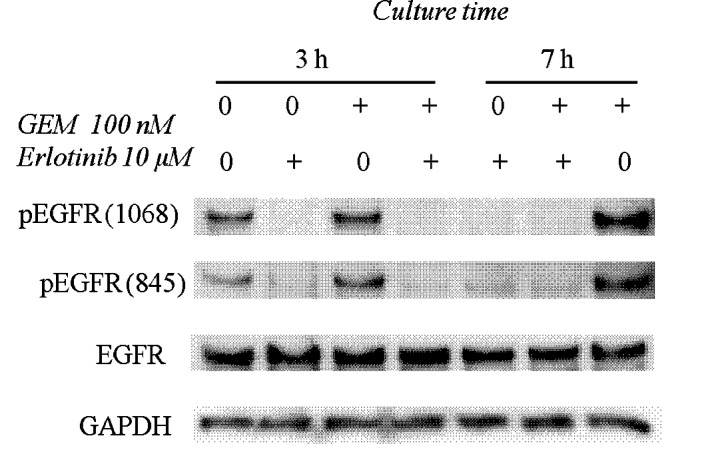
Effects of erlotinib in combination with gemcitabine on EGFR signaling in the HPAC pancreatic cancer cell line. HPAC cells were treated with 10 mM erlotinib and/or 100 nM gemcitabine (GEM) for 3 or 7 h. The cells were harvested and immunoblotted for the indicated proteins as described in Materials and methods.
Antitumor effects of erlotinib in combination with gemcitabine in the HPAC xenograft model
To evaluate the combination effect of erlotinib and gemcitabine on in vivo tumor growth inhibition, we conducted xenograft model experiments using the HPAC cell line. HPAC tumor growth was significantly inhibited by erlotinib and gemcitabine monotherapy (P<0.05, Fig. 3). The combination treatment with erlotinib and gemcitabine resulted in significantly stronger tumor growth inhibition compared to each drug alone (P<0.05) (Fig. 3). Tumor volume on day 22 (mean ± SD) was 371±28 mm3 in the combination treatment group, 634±58 mm3 in the erlotinib group, 549±66 mm3 in the gemcitabine group and 938±122 mm3 in the vehicle group. Significant body weight loss was not observed in any treatment group throughout the experiment (Table I). These results showed that erlotinib enhanced the antitumor activity of gemcitabine without additional toxicity of normal tissue in the KRAS-mutated pancreatic cancer xenograft model.
Figure 3.
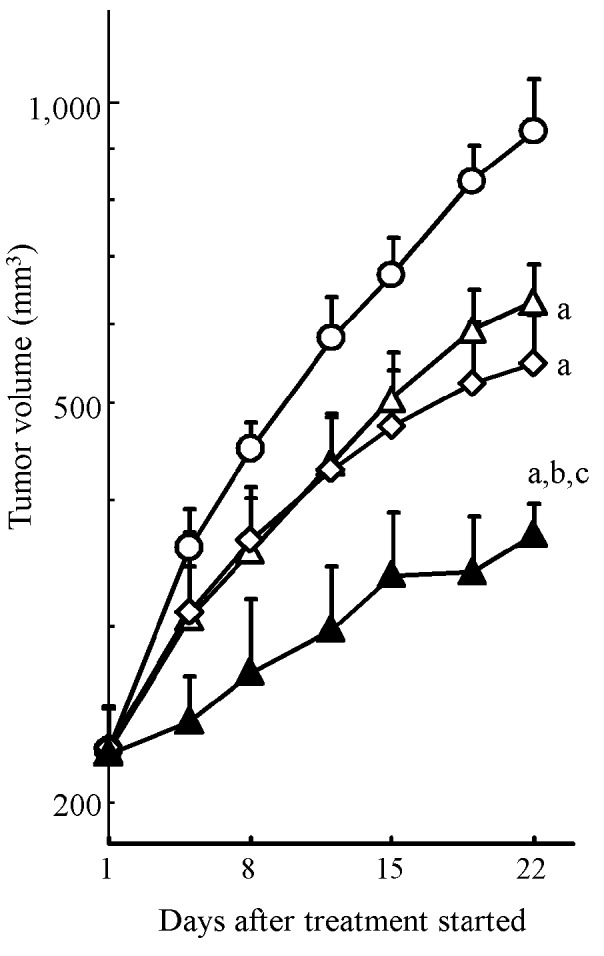
Antitumor activity of erlotinib in combination with gemcitabine in the HPAC tumor xenograft model. Nude mice bearing (s.c.) HPAC xenografts were treated with 50 mg/kg of erlotinib daily (open triangles), 20 mg/kg of gemcitabine once a week for 3 weeks (open diamonds), a combination of the two drugs (closed triangles) and vehicle (open circles). Each point represents the mean ± SD of the tumor volume (n=8 per group). Statistical differences (P<0.05) on day 22 are shown vs. the control (a), erlotinib (b) and gemcitabine (c) (Wilcoxon test).
Table I.
Body weight of mice treated with erlotinib and/or gemcitabine in the HPAC tumor xenograft model.
| Group | Body weight (g) | |
|---|---|---|
|
|
||
| Day 1 | Day 22 | |
| Vehicle (control) | 26.6±1.1 | 27.0±1.8 |
| Erlotinib (50 mg/kg) | 26.8±1.0 | 26.1±0.8 |
| Gemcitabine (20 mg/kg) | 26.5±1.1 | 26.3±1.5 |
| Erlotinib (50 mg/kg) + gemcitabine (20 mg/kg) | 27.0±1.0 | 26.4±1.5 |
Values represent the mean ± SD of body weight (n=8 per group) on days 1 and 22.
Discussion
No clinical trial thus far, using combination agents such as oxaliplatin, cisplatin, irinotecan, 5-fluorouracil, marimastat (matrix metalloproteinase inhibitor) or tipifarnib (farnesyltransferase inhibitor) with gemcitabine, has produced significant survival improvement over gemcitabine alone (3). Erlotinib is the first agent to produce a significant improvement in survival in combination with gemcitabine compared with gemcitabine alone (4).
Erlotinib, an EGFR-targeting drug, has been approved in many countries for the treatment of non-small-cell lung cancer (NSCLC) patients who previously received chemotherapy. Recently, the combination therapy of erlotinib with gemcitabine has been approved for the treatment of locally advanced or metastatic chemonaive pancreatic cancer. In a pre-clinical study, Morgan et al demonstrated the therapeutic effect of erlotinib in combination with gemcitabine in a mouse xenograft model using the KRAS wild-type human pancreatic cancer cell line BxPC-3 (14). However, in the clinical setting, the frequency of mutations in the KRAS oncogene can reach 90% in pancreatic ductal adenocarcinomas (11). Therefore, we conducted in vivo experiments using KRAS-mutated pancreatic cancer cell lines. The present study showed that erlotinib significantly enhanced the antitumor activity of gemcitabine in a xenograft model inoculated with the KRAS-mutated pancreatic cancer cell line, HPAC. Our results, together with those of Morgan et al, are consistent with the results of the PA.3 clinical trial (4) and indicate the clinical benefit of a combination therapy of erlotinib and gemcitabine for the treatment of pancreatic cancer regardless of KRAS status.
The present in vitro and in vivo experiments showed that erlotinib enhanced the antitumor activity of gemcitabine against KRAS-mutated cells. However, the mechanism involved in the effects of the combination of erlotinib and gemcitabine remains unclear. An increase in apoptosis with a combination treatment compared with each agent alone in head and neck carcinoma has been reported (18). Changes in cell cycle distributions may explain the mechanism involved in the increase of apoptosis of cancer cells treated with a combination of an EGFR inhibitor and gemcitabine. Gemcitabine treatment induces an accumulation of the S-phase population, which is considered sensitive to erlotinib; thus, the combination treatment may enhance antitumor activity.
We found that gemcitabine increased the level of EGFR phosphorylation, which was entirely blocked by erlotinib in the KRAS-mutated pancreatic cancer cells. EGFR expression and its phosphorylation are one of the significant factors determining the sensitivity of cells to erlotinib-induced growth inhibition (19). Therefore, the increase in EGFR phosphorylation by gemcitabine may render cancer cells more sensitive to erlotinib. These results suggest that EGFR activation may be a survival response in HPAC pancreatic cancer cells treated with gemcitabine. Furthermore, the inhibition of the gemcitabine-induced phosphorylation of EGFR by erlotinib may block this initial survival response and promote cytotoxicity from gemcitabine. Phosphorylation of EGFR at Y845 in response to gemcitabine was previously shown in several pancreatic and head and neck cancer cells (14,18). Although the mechanism involved in the increase in phosho-EGFR from gemcitabine remains unclear, it is plausible that the gemcitabine-mediated degradation of Cdc25A phosphatase, which is known to directly regulate EGFR phosphorylation levels, is involved (20). Numerous studies suggest that EGFR activation induced by cellular stress, such as H2O2, UV and chemotherapeutic agents including cisplatin, 5-fluorouracil, paclitaxel, doxorubicin and irinotecan (14), promotes an anti-apoptotic survival response through the activation of the MAPK or Akt signal pathways (21,22). Therefore, gemcitabine may also be an agent of cellular stress that induces EGFR activation.
Recently, the sequence-specific interactions of erlotinib and chemotherapeutic drug combinations were found to influence the efficacy of regimens in NSCLC (23). The treatment schedule of erlotinib and gemcitabine also affects the combination effects. Gemcitabine followed by erlotinib enhances the antitumor effects of each drug alone, while erlotinib followed by gemcitabine has antagonistic effects (14,18). In the present study, erlotinib and gemcitabine were administered, not sequentially, but concurrently in both in vitro and in vivo experiments, consistent with the drug treatment schedule of the PA.3 clinical trial. The difference in the effects between sequential treatment with gemcitabine followed by erlotinib and concurrent treatment was not determined in our experiments. However, it is plausible that sequential treatment may be more effective compared to concurrent treatment, since erlotinib immediately increases the G1-phase population of the cell cycle (24) leading to a reduction in S-phase entry, which is crucial for gemcitabine-mediated cytotoxicity.
In conclusion, we demonstrated that the treatment of erlotinib in combination with gemcitabine exerted antitumor activity superior to each drug as a monotherapy in the KRAS-mutated pancreatic cancer model. Our results confirm that the combination of erlotinib and gemcitabine has potential therapeutic benefits against pancreatic cancers. Our findings are useful in the investigation of molecular agents targeted to pathways other than EGFR signal cascade in pancreatic cancer treated with gemcitabine, as well as in the exploration of new combination regimens including erlotinib and gemcitabine for more efficacious therapies against pancreatic cancer.
Acknowledgements
We thank Ms. F. Ford for the editorial assistance.
References
- 1.Wanebo HJ, Vezeridis MP. Pancreatic carcinoma in perspective. A continuing challenge. Cancer. 1996;78:580–591. doi: 10.1002/(SICI)1097-0142(19960801)78:3<580::AID-CNCR38>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Verslype C, Grusenmeyer PA. Lessons learned in the management of advanced pancreatic cancer. J Clin Oncol. 2007;25:1949–1952. doi: 10.1200/JCO.2006.09.4664. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a Phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 6.Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov. 2003;2:296–313. doi: 10.1038/nrd1066. [DOI] [PubMed] [Google Scholar]
- 7.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Jang K-T, Ki C-S, Lim T, Park YS, Lim HY, Choi D-W, Kang WK, Park K, Park JO. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–1569. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 9.Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 10.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 11.Lohr M, Kloppel G, Maisonneuve P, Lowenfels AB, Luttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore MJ, da Cunha Santos G, Kamel-Reid S, Chin K, Tu D, Parulekar W, Ludkovski O, Squire J, Richardson F, Tsao M. The relationship of K-ras mutations and EGFR gene copy number to outcome in patients treated with erlotinib in the National Cancer Institute of Canada Clinical Trials Group trial study PA.3. Proc ASCO. 2007;25:abs 4521. [Google Scholar]
- 13.Dowell J, Minna JD, Kirkpatrick P. Erlotinib hydrochloride. Nat Rev Drug Discov. 2005;4:13–14. doi: 10.1038/nrd1612. [DOI] [PubMed] [Google Scholar]
- 14.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777–783. [PubMed] [Google Scholar]
- 16.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Koizumi F, Kanzawa F, Ueda Y, Koh Y, Tsukiyama S, Taguchi F, Tamura T, Saijo N, Nishio K. Synergistic interaction between the EGFR tyrosine kinase inhibitor gefitinib (‘Iressa’) and the DNA topoisomerase I inhibitor CPT-11 (irinotecan) in human colorectal cancer cells. Int J Cancer. 2004;108:464–472. doi: 10.1002/ijc.11539. [DOI] [PubMed] [Google Scholar]
- 18.Chun PY, Feng FY, Scheurer AM, Davis MA, Lawrence TS, Nyati MK. Synergistic effects of gemcitabine and gefitinib in the treatment of head and neck carcinoma. Cancer Res. 2006;66:981–988. doi: 10.1158/0008-5472.CAN-05-2665. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65:6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki Y, Hiraoka S, Tsutsui S, Kitamura S, Shinomura Y, Matsuzawa Y. Epidermal growth factor receptor mediates stress-induced expression of its ligands in rat gastric epithelial cells. Gastroenterology. 2001;120:108–116. doi: 10.1053/gast.2001.20950. [DOI] [PubMed] [Google Scholar]
- 23.Mahaffey CM, Davies AM, Lara PN, Jr, Pryde B, Holland W, Mack PC, Gumerlock PH, Gandara DR. Schedule-dependent apoptosis in K-ras mutant non-small cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clin Lung Cancer. 2007;8:548–553. doi: 10.3816/clc.2007.n.041. [DOI] [PubMed] [Google Scholar]
- 24.Ling YH, Li T, Yuan Z, Haigentz M, Jr, Weber TK, Perez-Soler R. Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small cell lung cancer cell lines. Mol Pharmacol. 2007;72:248–258. doi: 10.1124/mol.107.034827. [DOI] [PubMed] [Google Scholar]