Abstract
Background:
Cocaine- and amphetamine-regulated transcript (CART) is a hypothalamic anorectic neuropeptide that controls feeding behaviour and body weight. The study objective was to investigate the association of the CART prepropeptide gene (CARTPT) rs2239670 variant with obesity and its related anthropometric indicators among patients of a Malaysian health clinic in Kampar, Perak, Malaysia.
Methods:
A total of 300 Malay/Peninsular Bumiputera, Chinese, and Indian subjects (115 males, 185 females; 163 non-obese, 137 obese) were recruited by convenience sampling, and anthropometric measurements, blood pressures, and pulse rate were taken. Genotyping was performed using AvaII polymerase chain reaction–restriction fragment length polymorphism.
Results:
Genotyping revealed 203 (67.7%), 90 (30.0%), and 7 (2.3%) subjects with the GG, GA, and AA genotypes, respectively, with a minor allele (A) frequency of 0.17. No significant difference in the CARTPT rs2239670 genotype and allele distribution was found between obese and non-obese subjects, and logistic regression showed no association between the mutated genotypes (GA, AA) and allele (A) with obesity, even after adjusting for age, gender, and ethnicity. Furthermore, the measurements did not differ significantly between the genotypes and alleles. No significant difference in the genotype and allele distribution was found among genders, but they were significantly different among ethnicities (P = 0.030 and P = 0.019, respectively).
Conclusion:
CARTPT rs2239670 is not a predictor for obesity among the Malaysian subjects in this study.
Keywords: cocaine- and amphetamine-regulated transcript protein, anthropometry, genetic association study, obesity, single nucleotide polymorphism, Malaysia
Introduction
Malaysia has experienced a rapid phase of industrialisation and urbanisation throughout the last few decades; thus, the nation’s lifestyle has also changed, leading to an increase in the incidence of obesity (1). This increasing trend has become a threat in developing countries including Malaysia, where the prevalence of the overweight and obese population has markedly increased from 16.6% to 29.1% and 4.4% to 14.0%, respectively, as reported by the Malaysian National Health and Morbidity Survey (NHMS) II (2) and NHMS III (3). Because the pathogenesis of obesity is multifactorial, the investigation of obesity-related genes involved in energy metabolism regulation, feeding behaviour, and body homeostasis has become a vital project (4).
The cocaine- and amphetamine-regulated transcript prepropeptide gene (CARTPT, Gene ID: 9607) encodes for the cocaine- and amphetamine-regulated transcript (CART) protein, which is one of the neuroendocrine modulators of energy balance present in the anorectic pro-opiomelanocortin (POMC)/CART neurons in the arcuate nucleus of the hypothalamus (5). The CARTPT is located at chromosome 5q13-14, and it produces long and short peptide fragments in rats; however, only the short form is found in humans, with 95% amino acid homology (6,7). The short peptide is believed to be a neuropeptide that regulates many physiological processes, especially those controlling feeding behaviour and body weight, and its mRNA expression is known to be induced with increased leptin levels (8). Also, CART is able to overcome the increase in appetite and feeding behaviour induced by neuropeptide Y, and vice versa (7).
Genetic variations in CARTPT may influence expression and/or function of the peptide and may associate with diseases/disorders. A missense mutation of G729C in CARTPT resulting in the substitution of leucine by phenylalanine at codon 34 has been identified in an early-onset obese patient (9), while the -156G/A variant of the 5’ flanking region of CARTPT has been associated with obesity among Japanese subjects (10). However, 3 polymorphisms in the 3’-untranslated region (UTR), namely +1361 A>G (A1475G), 1457delA (rs5868607), and C1442G (rs1800926) that have been identified in Danish, Pima Indian, British, and Italian subjects showed no associations with variations in body mass index (BMI) (9,11–13). In addition to obesity, 3 previous studies have examined polymorphisms in CARTPT and their association with addictive behaviours. Jung et al. (14) showed association of the rs2239670 SNP (G5514A located in the first intron of CARTPT) with alcoholism in Korean subjects, but no association was found with bipolar disorder or schizophrenia. Furthermore, no association of rs35862863 and rs2239670 was found with methamphetamine dependence in Japanese subjects (15) nor was there an association of rs6894758, rs11575893, or rs17358300 with cocaine dependence in African– American subjects (16). Because neurobiological research has shown commonalities in brain reward processes between obesity and substance abuse disorders (17), CARTPT variants associated with substance abuse might also be associated with obesity.
Due to the lack of data on the association between the CARTPT rs2239670 variant and obesity in the published literature, it is of interest to investigate this association in the Malaysian population. Therefore, the objectives of this study were to determine the prevalence of the CARTPT rs2239670 variant among the patients of a health clinic in Kampar, in the state of Perak, Malaysia, and to collect anthropometric measurements, blood pressures, and pulse rate as indicators of obesity in order to determine if there is an association between this gene variant and obesity and its related indicators.
Subjects and Methods
Subject recruitment and anthropometric measurements
A total of 300 subjects were recruited by convenience sampling from April to December 2010. They were visitors of the Kampar Health Clinic who had fasted overnight and were waiting for their blood samples to be collected by nurses for various biochemical tests ordered by the resident physicians. This cross-sectional study was registered under the National Medical Research Registry of Malaysia (NMRR-09-826-4266), and the protocol was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia. Informed consent was obtained from all the respondents in this study, and the blood samples were taken in accordance with the World Medical Association Declaration of Helsinki (as revised in Seoul, 2008). Each subject’s socio-demographic information including age, gender, ethnicity, marital status, occupation, monthly household income, and educational status were obtained. The ethnicities of the subjects were self-identified in 3 given choices: Malay/Peninsular Bumiputera (Orang Asli), Chinese, or Indian. The systolic blood pressure (SBP), diastolic blood pressures (DBP), and pulse rate were determined by using SEM-1 Model automatic blood pressure monitor (Omron, Japan) after the subjects had rested for 10 min. Height, waist circumference (WC), and hip circumference (HC) of subjects were measured with a measuring tape to the nearest 0.1 cm, and their waist-to-hip ratio (WHR) was calculated by dividing the WC by the HC. A bio-impedence body fat weighing scale, Model HBF-362 KaradaScan body composition monitor with scale (Omron, Japan), was used to determine both weight and body composition such as percentage of skeletal muscle (SM), total body fat (TBF), visceral fat level (VFL), and subcutaneous fat (SF). BMI and resting metabolism (RM) were also obtained with the weighing scale. Subjects with a BMI of 27 kg/m2 or above were considered obese (18).
Genotyping
Blood samples of approximately 5 mL were collected in EDTA anticoagulant tubes by medical practitioners. Genomic DNA isolation was conducted using the Wizard Genomic DNA Purification Kit (Promega, Madison, Wisconsin, US) according to its technical manual and the amount of DNA isolated was quantified with a NanoPhotometer (Implen, Munich, Germany).
Polymerase chain reaction (PCR) was performed using MyCycler thermal cycler system with gradient option (BioRad, California, US) by adapting the conditions and reagent concentrations from the study by Jung et al. (14). The forward and reverse primers were 5’-CCCGAGCCCTGGACATCTACT-3’ and 5’-CCGGACCCACACGCACTCT-3’, respectively, and PCR was carried out under the following conditions: denaturation at 94 °C for 30 s, annealing at 69 °C for 30 s, and extension at 72 °C for 45 s for 35 cycles. The reaction cycles were hot-started with a single cycle of denaturation at 94 °C for 5 min and ended with a single cycle of extension at 72 °C for 10 min. Final concentrations of reagents for a single reaction was 1× PCR buffer without Mg2+ (Fermentas, Lithuania), 1 mM MgCl2, 0.2 mM dNTP (RBC Bioscience, Taiwan), 1 μM of each CARTPT forward and reverse primers, 1 U/μL Taq DNA Polymerase (Fermentas, Lithuania), and 20 ng of DNA template. The restriction fragment length polymorphism (RFLP) analysis for the rs2239670 variant was performed by incubating the PCR products (approximately 394 bp) with AvaII (Fermentas, Lithuania) at 37 °C for at least 2 h. Both PCR and RFLP products were electrophoresed on 3% agarose gels in TBE running buffer and subsequently detected with ethidium bromide under ultraviolet illumination. The CARTPT rs2239670 wild-type G allele should be detected as an uncut products at 388 and 6 bp, and the mutant allele should yield bands at 278, 110, and 6 bp. The heterozygous subjects have both the G and A alleles, and should yield 4 bands at 388, 278, 110, and 6 bp. illustrates these 3 genotypes; however, the lowest band (6 bp) was not detected as it might have migrated out of the gel.
Figure 1:
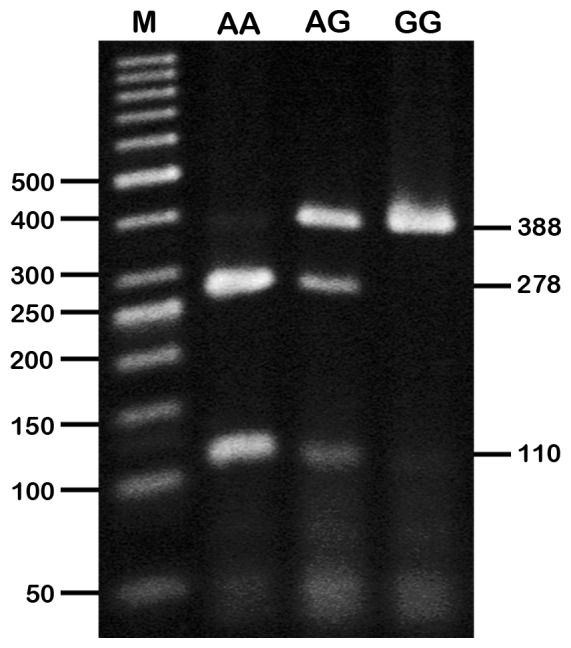
Genotyping of CARTPT rs2239670 variant by AvaII PCR-RFLP analysis. AA = homozygous mutated (278 and 110 bp), GA = heterozygous mutated (388, 278, and 110 bp), GG = homozygous wild type (388 bp).
Statistical analysis
The data obtained from the questionnaire and AvaII PCR-RFLP genotyping were analysed using SPSS version 17.0 (SPSS Inc., Chicago, Illinois, US). Because a majority of the subjects had the CARTPT rs2239670 GG genotype and only a few had the AA genotype, the mutated genotypes (GA+AA) were combined with the wild-type genotype (GG) for Pearson’s chi-square test. Then, the genotype and allele frequencies of CARTPT rs2239670 with respect to BMI status, gender, and ethnicity were assessed for association using Pearson’s chi-square test. Associations between the genotype as well as the allele and obesity were examined using logistic regression (enter method; unadjusted and adjusted with age, gender, and ethnicity). Anthropometric measurements, blood pressures and pulse rates for each genotype and allele were checked for normality using the Shapiro–Wilk test and were compared using one way analysis of variance (ANOVA) and Student’s t test for normally-distributed data (DBP and WHR) or Kruskal–Wallis and Mann–Whitney U tests for non–normally distributed data. A P value of less than 0.05 was considered statistically significant.
Results
The socio-demographic information collected from the 300 subjects ranging from 21 to 80 years old is shown in Table 1. The non-obese subjects had a mean age of 53.18 years (SD 15.85), while the obese subjects had a mean age of 51.46 years (SD 12.40). Overall, there were more non-obese than obese subjects, and more females than males. The majority of the subjects were Chinese (47.0%), followed by Malays/ Peninsular Bumiputeras (32.7%), and Indians (20.3%), which is reflective of the Kampar population. Additionally, more than half of the subjects were retired or not working (54.7%), or had a monthly household income of lower than RM1000 (54.0%). A third of the subjects had at least a primary-level education. According to this study, the prevalence of obesity was higher among females, Malay/Peninsular Bumiputeras, subjects 51–60 years of age, and those having a monthly household income of less than RM1000.
Table 1:
Frequencies of the mtDNA 10398 variant in the Malay population.
| Variables | Non-obese(n =163) | Obese(n=137) |
|---|---|---|
| Sex | ||
| Male | 68 (41.7) | 47 (34.3) |
| Female | 95 (58.3) | 90 (65.7) |
| Race | ||
| Malay/Peninsular Bumiputera | 37 (22.7) | 61 (44.5) |
| Chinese | 93 (57.1) | 48 (35.0) |
| Indian | 33 (20.2) | 28 (20.4) |
| Age groups (years) | ||
| 21–30 | 22 (13.5) | 9 (6.6) |
| 31–40 | 8 (4.9) | 16 (11.7) |
| 41–50 | 32 (19.6) | 33 (24.1) |
| 51–60 | 37 (22.7) | 47 (34.3) |
| 61–70 | 44 (27.0) | 27 (19.7) |
| 71–80 | 20 (12.3) | 5 (3.6) |
| Occupation | ||
| Professional | 3 (1.8) | 6 (4.4) |
| White-collar | 9 (5.5) | 8 (5.8) |
| Blue-collar | 23 (14.1) | 25 (18.2) |
| Retired/not working | 89 (54.6) | 75 (54.7) |
| Own/others | 39 (23.9) | 23 (16.8) |
| Monthly household income (RM) | ||
| Below 1000 | 91 (55.8) | 71 (51.8) |
| 1001–3000 | 56 (34.4) | 55 (40.1) |
| 3001–5000 | 10 (6.1) | 9 (6.6) |
| Above 5000 | 6 (3.7) | 2 (1.5) |
| Educational status | ||
| No formal education | 27 (16.6) | 15 (10.9) |
| Primary | 55 (33.7) | 44 (32.1) |
| Lower secondary | 27 (16.6) | 30 (21.9) |
| Upper secondary | 27 (16.6) | 32 (23.4) |
| Pre-university | 7 (4.3) | 5 (3.6) |
| University | 20 (12.3) | 11 (8.0) |
Numbers in parentheses are percentage of total non-obese or obese.
A significant difference in the distribution between genotypes and alleles among ethnicities but not obesity status groups and gender was found and is reported in Table 2. Anthropometric measurements, blood pressures, and pulse rates were grouped according to genotypes, and alleles to determine the effect of having the mutated genotype or allele on these obesity indicators. However, none of these anthropometric measurements were significantly different between the genotypes and alleles.
Table 2:
Obesity status, ethnicity and gender frequencies, and body measurement values of the Kampar Health Clinic subjects according to their CARTPT rs2239670 genotypes and alleles
| Allele | Genotype | P value | Allele | P value | |||
|---|---|---|---|---|---|---|---|
| GG | GA | AA | G | A | |||
| Obesity status | |||||||
| Non-obese | 111 (68.1) | 48 (29.4) | 4 (2.5) | 0.862a | 270 (82.8) | 56 (17.2) | 0.913 |
| Obese | 92 (67.2) | 42 (30.7) | 3 (2.2) | 227 (82.8) | 47 (17.2) | ||
| Ethnicity | |||||||
| Malay/Peninsular Bumiputera | 71 (72.4) | 26 (26.5) | 1 (1.1) | 0.030a | 158 (84.9) | 28 (15.1) | 0.019 |
| Chinese | 85 (60.3) | 50 (35.5) | 6 (4.3) | 220 (78.0) | 62 (22.0) | ||
| Indian | 47 (77.0) | 14 (23.0) | 0 (0.0) | 108 (88.5) | 14 (11.5) | ||
| Gender | |||||||
| Male | 77 (67.0) | 36 (31.3) | 2 (1.7) | 0.836a | 190 (82.6) | 40 (17.4) | 0.976 |
| Female | 126 (68.1) | 54 (29.2) | 5 (2.7) | 306 (82.7) | 64 (17.3) | ||
| Measurements | |||||||
| SBP (mmHg)b | 138.33 (21.43) | 140.43 (22.11) | 135.00 (20.07) | 0.731 | 138.71 (21.52) | 139.70 (21.75) | 0.602 |
| DBP (mmHg)c | 80.81 (11.13) | 82.98 (10.26) | 74.86 (8.90) | 0.504 | 81.20 (10.98) | 81.88 (10.39) | 0.642 |
| Pulse rate (mmHg)b | 73.51 (14.58) | 73.09 (13.38) | 74.71 (14.51) | 0.923 | 73.44 (14.34) | 73.31 (13.40) | 0.905 |
| WC (cm)b | 90.41 (11.34) | 91.05 (11.18) | 86.10 (12.79) | 0.469 | 90.52 (11.29) | 90.38 (11.40) | 0.876 |
| WHRc | 0.89 (0.08) | 0.90 (0.09) | 0.87 (0.08) | 0.640 | 0.89 (0.08) | 0.89 (0.09) | 0.128 |
| Height (cm)b | 159.50 (8.91) | 158.23 (18.02) | 156.64 (12.10) | 0.590 | 159.27 (11.11) | 158.01 (17.26) | 0.755 |
| Weight (cm)b | 72.51 (58.81) | 69.35 (13.43) | 66.47 (15.68) | 0.716 | 71.94 (53.44) | 68.96 (13.62) | 0.744 |
| BMI (kg/m2)b | 26.90 (5.06) | 27.17 (4.91) | 27.04 (5.35) | 0.945 | 26.95 (5.02) | 27.15 (4.91) | 0.756 |
| TBF (%)b | 33.44 (7.08) | 33.44 (6.72) | 33.29 (6.86) | 0.961 | 33.44 (7.00) | 33.32 (6.67) | 0.824 |
| SF (%)b | 27.34 (8.30) | 27.23 (8.18) | 28.27 (9.07) | 0.949 | 27.32 (8.27) | 27.37 (8.22) | 0.891 |
| VFL (%)b | 11.82 (6.15) | 12.52 (6.22) | 11.71 (6.75) | 0.603 | 11.95 (6.15) | 12.41 (6.23) | 0.418 |
| RM (kcal)b | 1431.58 (255.53) | 1451.04 (243.07) | 1392.43 (287.82) | 0.596 | 1435.11 (252.93) | 1443.15 (247.20) | 0.653 |
| SM (%)b | 24.91 (4.17) | 24.82 (3.90) | 24.80 (4.42) | 0.993 | 24.90 (4.11) | 24.82 (3.93) | 0.973 |
Values for obesity status, ethnicity, and gender are in number of subjects (percentage). P values were determined by Pearson’s chi-square test, significant at P < 0.05. a Pearson’s chi-square test was performed for wild-type and combined mutant genotypes (GG and GA+AA) as some AA frequencies had values of less than 5.
Values for measurements are in mean (SD). P values were determined by b Mann–Whitney U or Kruskal–Wallis tests for nonnormally distributed data and c Student’s t test or ANOVA for normally distributed data, significant at P < 0.05.
Abbreviations: SBP = systolic blood pressure, DBP = diastolic blood pressure, WC = waist circumference, WHR = waist-to-hip ratio, BMI = body mass index, TBF = total body fat, SF = subcutaneous fat, VFL = visceral fat level, RM = resting metabolism, SM = skeletal muscle.
To further test for the direct association between CARTPT rs2239670 genotypes and alleles with obesity, logistic regression (enter method) was performed, with and without adjustment for other confounders of obesity such as age, gender, and ethnicity (Table 3). Having a mutant genotype (GA or AA) or a mutant allele (A) did not appear to affect the obesity status of the subjects (all P > 0.05), with all odds ratios near 1.000. This result indicates that the subjects having the mutant genotypes or allele had nearly an equal chance of being obese compared with the wild-type genotype or allele.
Table 3.
Association studies of CARTPT rs2239670 genotypes and alleles with obesity in the Kampar Health Clinic subjects
| CARTPT rs2239670 | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Genotype | ||||
| GG | 1.000 | |||
| GA | 1.105 (0.241, 5.064) | 0.898 | 0.702 (0.136, 3.624) | 0.702 |
| AA | 1.167 (0.247, 5.514) | 0.846 | 0.898 (0.169, 4.763) | 0.898 |
| Allele | ||||
| G | 1.000 | |||
| A | 0.977 (0.639, 1.492) | 0.913 | 0.809 (0.511, 1.280) | 0.365 |
aAdjusted for age, gender, and ethnicity.
Discussion
Based on the socio-demographic data reported herein, there were more female obese subjects compared with males. More specifically, the prevalence of obesity in Malaysian women was greater than that in Malaysian men (19), which is in accordance with one of the national studies on obesity among Malaysians that reported a significantly higher prevalence of obesity in females (13.8%) compared with males (9.6%) (2). This higher prevalence may be due to a majority of the females in the study being housewives, married, or having a lower educational level. Hence, there may be increased susceptibility to a sedentary lifestyle and reduced health literacy (19). Among the ethnic groups, obesity was more prevalent among Malays, as supported by the nationwide study by Rampal et al. (2). There was an increased prevalence of obesity in the 21–30 and the 51–60 age groups and a decreased prevalence in the 61–70 and the 71–80 age groups. These observations may be due to a shift towards a sedentary lifestyle as age increases. Meanwhile, there was a higher prevalence of obesity in subjects that were retired or not working compared with other occupational groups. This higher prevalence may be due to the sedentary lifestyles and increased accessibility to food for those who were not working. Among the household income categories, the prevalence of obesity was the highest in subjects with an income below RM1000. There was also an increased prevalence of obesity in subjects with only primary-level education. According to Kee et al. (19), there is an inverse relationship between the prevalence of obesity with the level of education possibly because high educational attainment can affect a person’s attitude towards body weight control, dietary pattern, and healthier lifestyle.
The CARTPT rs2239670 variant has shown a positive association with alcoholism in the Korean population (14), and because substance abuse and obesity share a common neurobiological basis (17), we sought to investigate the association of this common variant with obesity in our study. To the best of our knowledge, this study is the first to examine the prevalence of CARTPT rs2239670 and its association with obesity in the Malaysian population. According to our results, the genotype and allele distribution of this gene variant was similar to the study reported in another Asian population in Korea (14) where the GG genotype showed the highest frequency and the AA genotype had the lowest frequency; however, the minor A allele frequency in this study was approximately 2-fold lower (0.17 versus 0.30). The Korean study population of 877 subjects included patients with alcoholism, bipolar disorder, schizophrenia, and the respective controls, and the differences in study population number, demographics, and disease/disorder conditions might explain this discrepancy in the minor allele frequency. Both rs2239670 variant genotypes and alleles were not associated with gender, but their distributions were significantly different among the ethnic groups. Both chi-square analysis and logistic regression analysis (adjusted for age, gender, and ethnicity) showed no association between the CARTPT rs2239670 variant genotypes and alleles with obesity. In addition, the anthropometric measurements, blood pressures, and pulse rate were not significantly different among the genotypes and alleles. Taken together, the CARTPT rs2239670 variant may not be a predictor for obesity and its related body indicators in the Malaysian population, at least among patients attending the Kampar Health Clinic. This variant falls within the non-coding region, and the potential effects on obesity may be eliminated during mRNA splicing; however, it is not possible to conclude at present whether the observed molecular variant rs2239670 directly affects CART function or whether there is a functional variant that may have a direct effect on obesity and other related metabolic syndromes.
There have been a few association studies between other CARTPT polymorphisms with obesity or diabetes. Two variants in the 3’-UTR were first identified in the Caucasian population (1457delA and A1475G); however, these variants were not associated with obesity (11). Also, 1457delA (rs5868607) and another variant in the 3’-UTR, C1442G (rs1800926) were not associated with type 2 diabetes in Chinese (20) and obesity in Pima Indians (12), respectively. However, Yamada et al. (10) showed that the A-156G polymorphism in the promoter region was associated with obesity in the Japanese population. In exon 2 of the CARTPT coding region, Challis et al. (13) found that the Ser66Thr (serine to threonine substitution at codon 66) mutation was detected in 1 obese subject, but not in 100 unrelated Caucasian controls, while del Giudice et al. (9) reported that the Leu34Phe (leucine to phenylalanine substitution at codon 34) was associated with reduced resting energy expenditure as an obesity phenotype in a large family. All of these inconsistencies in findings from different populations warrant further association studies in the Malaysian population, not only involving obesity and metabolic syndrome, but also neuropsychiatric disorders (14) and substance abuse or addictive disorders such as alcohol (14,21), methamphetamine (15), cocaine (16), and nicotine (21) dependence.
There were several limitations in present study. First, there was not an equal distribution of subjects across age groups, as most patients of the clinic were elderly. Indeed, age could be one of the predictors of obesity. Moreover, the nonprobability convenience sampling could have a risk of bias, where the target population may not be sufficiently generalised to assess the reliability of the data. In addition, the study population was heterogeneous because different ethnic groups were included. A larger number of subjects with investigations based on separate ethnicities would help to define the effects of variants on obesity and allow us to compare the results with other populations.
Conclusion
The rs2239670 variant of the CARTPT gene was not associated with obesity and its related indicators among the patients of the Kampar Health Clinic. This variant was neither a risk factor nor a predictor for obesity. Also, there was no significant difference in the genotype and allele distributions among genders, but they were significantly different among ethnicities. This finding suggests that other genes or lifestyle and dietary factors may contribute to obesity among the Kampar Health Clinic patients.
Acknowledgments
This project was funded by the Universiti Tunku Abdul Rahman Research Fund (IPSR/RMC/UTARRF/C109/S1). We would like to extend our deepest gratitude to the Kinta District Health Office for granting us permission to carry out this study at the Kampar Health Clinic, to the nurses who assisted with the blood sampling, and to all the respondents who had volunteered to participate in this study. We also thank Ms Kavitha Subramaniam of Universiti Tunku Abdul Rahman for her assistance in the statistical analysis.
Footnotes
Authors’ contributions
Conception and design: SHF, YHS
Obtaining of funding, critical revision and final approval of the article: YHS
Collection and assembly of the data: LY, SHF
Analysis and interpretation of the data: LY, SHF, YHS
Drafting of the article: LY, YHS
References
- 1.Ismail MN, Chee SS, Nawawi H, Yusoff K, Lim TO, James WP. Obesity in Malaysia. Obes Rev. 2002;3(3):203–208. doi: 10.1046/j.1467-789x.2002.00074.x. [DOI] [PubMed] [Google Scholar]
- 2.Rampal L, Rampal S, Khor GL, Zain AM, Ooyub SB, Rahmat RB, et al. A national study on the prevalence of obesity among 16127 Malaysians. Asia Pac J Clin Nutr. 2007;16(3):561–566. [PubMed] [Google Scholar]
- 3.Suzana S, Kee CC, Jamaludin AR, Noor Safiza MN, Khor GL, Jamaiyah H, et al. The Third National Health and Morbidity Survey: Prevalence of obesity, and abdominal obesity among the Malaysian elderly population. Asia Pac J Public Health. doi: 10.1177/1010539510380736. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: Perception and integration. Nat Rev Genet. 2002;3(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 5.Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: A cocaineand amphetamine-regulated transcript. Gene. 1996;169(2):241–245. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- 6.Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Targets CNS Neurol Disord. 2003;2(3):201–205. doi: 10.2174/1568007033482896. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic. 2005;4(2):95–111. doi: 10.1093/bfgp/4.2.95. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 9.Del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: Identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and co segregating with obesity phenotype in a large family. Diabetes. 2001;50(9):2157–2160. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- 10.Yamada K, Yuan X, Otabe S, Koyanagi A, Koyama W, Makita Z. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulatedtranscript gene and identification of polymorphic sites associated with obesity. Int J Obes Relat Metab Disord. 2002;26(1):132–136. doi: 10.1038/sj.ijo.0801848. [DOI] [PubMed] [Google Scholar]
- 11.Echwald SM, Sorensen TI, Andersen T, Hansen C, Tommerup N, Pedersen O. Sequence variants in the human cocaine and amphetamine-regulated transcript (CART) gene in subjects with early onset obesity. Obes Res. 1999;7(6):532–536. doi: 10.1002/j.1550-8528.1999.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Walder K, Morris C, Ravussin E. A polymorphism in the gene encoding CART is not associated with obesity in Pima Indians. Int J Obes Relat Metab Disord. 2000;24(4):520–521. doi: 10.1038/sj.ijo.0801196. [DOI] [PubMed] [Google Scholar]
- 13.Challis BG, Yeo GS, Farooqi IS, Luan J, Aminian S, Halsall DJ, et al. The CART gene and human obesity: Mutational analysis and population genetics. Diabetes. 2000;49(5):872–875. doi: 10.2337/diabetes.49.5.872. [DOI] [PubMed] [Google Scholar]
- 14.Jung SK, Hong MS, Suh GJ, Jin SY, Lee HJ, Kim BS, et al. Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett. 2004;365(1):54–57. doi: 10.1016/j.neulet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Morio A, Ujike H, Nomura A, Tanaka Y, Morita Y, Otani K, et al. No association between CART (cocaineand amphetamine-regulated transcript) gene and methamphetamine dependence. Ann N Y Acad Sci. 2006;1074:411–417. doi: 10.1196/annals.1369.041. [DOI] [PubMed] [Google Scholar]
- 16.Lohoff FW, Bloch PJ, Weller AE, Nall AH, Doyle GA, Buono RJ, et al. Genetic variants in the cocaineand amphetamine-regulated transcript gene (CARTPT) and cocaine dependence. Neurosci Lett. 2008;440(3):280–283. doi: 10.1016/j.neulet.2008.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson GT. Eating disorders, obesity and addiction. Eur Eat Disord Rev. 2010;18(5):341–351. doi: 10.1002/erv.1048. [DOI] [PubMed] [Google Scholar]
- 18.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24(8):1011–1017. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 19.Kee CC, Jamaiyah H, Noor Safiza MN, Geeta A, Khor GL, Suzana S, et al. Abdominal obesity in Malaysian adults: National Health and Morbidity Survey III (NHMS III, 2006) Mal J Nutr. 2008;14(2):125–135. [PubMed] [Google Scholar]
- 20.Fu M, Cheng H, Chen L, Wu B, Cai M, Xie D, et al. Association of the cocaine and amphetamineregulated transcript gene with type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi. 2002;41(12):805–808. [PubMed] [Google Scholar]
- 21.Busto A, Souza RP, Lobo DS, Shaikh SA, Zawertailo LA, Busto UE, et al. Cocaine and amphetamine regulated transcript (CART) gene in the comorbidity of schizophrenia with alcohol use disorder and nicotine dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):834–836. doi: 10.1016/j.pnpbp.2010.03.030. [DOI] [PubMed] [Google Scholar]


