The complex architecture of the inner ear, aptly named the labyrinth by early anatomists, houses the senses of both hearing and balance. The cochlea distributes pressure variations derived from sound along the length of its coils, in a frequency-dependent manner. As these variations in fluid pressure move the basilar membrane that divides the cochlea longitudinally, they deform the stereocilia of sensory cells known as hair cells in the attached epithelium. After amplification of basilar membrane motion by the mechanically active outer hair cells, the inner hair cells convert the stimulus into neuronal impulses via afferent synapses with the dendrites of primary auditory neurons. The hair cells and neurons are the most vulnerable elements in the cochlea, and damage to them is the most common cause of permanent hearing loss (Fig. 1). The paper by Duan et al. (1) in this issue of PNAS advances the possibility, emerging over the past few years, that damage to hair cells and neurons can be prevented. Moreover, it does so in a surprising and unexpected manner.
Figure 1.
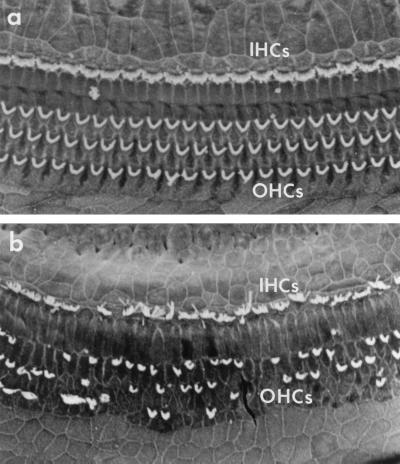
Scanning electron micrographs of the normal (a) and damaged (b) cochlear sensory epithelium. In the normal cochlea, the stereocilia of a single row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) are present in an orderly array. In the damaged cochlea, hair cells are missing, and stereocilia are abnormal, leading to hearing loss. Duan et al. (1) present data indicating that hair cell damage can be prevented by protecting the postsynaptic structures of their associated sensory neurons. (Micrographs are courtesy of Elizabeth M. Keithley.)
Sensorineural hearing loss (SNHL) can result from a variety of causes, including genetic disorders, infectious disease, overexposure to intense sound, and certain drugs. By far the most prevalent cause of the loss of sensorineural elements from the cochlea is aging, and many individuals experience noticeable difficulty with hearing later in life. Mild SNHL can produce difficulty in extracting signals from noise, as in the “cocktail party effect.” At the other end of the spectrum is severe deafness, which can produce devastating isolation.
Hearing loss caused by sensorineural damage has been recognized for over a century, and experiments to understand the phenomenon dates from the early 1900s. This research and clinical experience has shown that SNHL is often progressive, and always irreversible. Cochlear hair cells of mammals, unlike those of fish and birds, do not regenerate. Moreover, even mild-to-moderate SNHL cannot be restored to normal with amplification. This is because SNHL causes significant distortion of neuronal output from the cochlea, in addition to loss of sensitivity.
Until recently, damage to cochlear hair cells and neurons has been regarded as an inevitable consequence of genetic conditions, age, or exposure to certain environmental stimuli. This made avoidance of potentially harmful stimuli the primary means of protecting sensorineural structures and, consequently, hearing. Unfortunately, many causes of SNHL, including age and genetics, cannot be avoided. In addition, despite decades of research, the underlying mechanisms responsible for damage to auditory hair cells and neurons remained largely obscure.
During the past several years, however, significant progress in understanding SNHL has been made. Intracellular events that mediate aspects of damage to hair cells have been discovered. For example, production of free radicals has been found to be an important factor (see, e.g., ref. 2). Moreover, the death of hair cells appears to occur via an apoptotic process (3). These observations have led to the demonstration that hair cell loss caused by various potentially damaging stimuli can be prevented by the application of endogenous substances that interfere with the mechanism leading to cell damage, or that interfere in apoptotic processes (see, e.g., refs. 4–6). It has also been shown that hair cells can be protected from damage by the exposure to certain non-neurotrophic growth factors (7, 8), indicating that hair cells can be influenced by the activation of the same cell survival programs that operate in other tissues.
With respect to cochlear neurons, swelling of the spiral ganglion dendrites because of intense noise and ischemia had been noted for decades, as was the fact that hair cell loss can lead to anterograde degeneration of primary auditory neurons, with little or no understanding of the underlying mechanisms. However, by the early 1990s, it became clear that neurotransmission between the hair cell and the spiral ganglion neuron was mediated by glutamate receptors (9, 10) and that dendritic damage could be ameliorated by glutamate antagonists (11). In a separate series of significant advances, it was found that hair cells produce neurotrophins and other neuronal survival factors (12), and that absence of these factors can result in anterograde degeneration of primary sensory neurons (13, 14). This in turn led to the demonstration that neuronal loss, secondary to hair cell loss, could be reduced by administration of neurotrophins (see, e.g., ref. 15).
The observations of Duan et al. (1) add significantly to these prior findings. In one experiment, they found that the NMDA receptor antagonist MK801 provides modest protection of hearing from noise or the ototoxic antibiotic amikacin. This observation resembles the previous work of Basile et al. (16), who observed protection of both hair cells and spiral ganglion neurons from ototoxicity by glutamate antagonists. Duan et al. then combined MK801 with the neurotrophin NT-3. The authors perfused the guinea pig cochlea with a dosage of amikacin that produces near-total loss of hair cells, 90% loss of primary neurons, and severe (>70 dB) deafness. Perfusion of NT-3 and MK801 in combination with amikacin resulted in protection of approximately 50% of hair cells, and all cochlear neurons. Moreover, by preserving hearing to within about 20 dB of preamikacin thresholds, the authors maintained their animals close to the normal range of hearing sensitivity. Although the protection achieved by Duan et al. is impressive, some other investigators have obtained similar levels. It is the manner in which such protection was achieved that is intriguing.
The data of Duan et al. (1) demonstrate a strong protective interaction between MK801 and NT-3. With respect to spiral ganglion neurons, this interaction might well be expected because protective factors often act synergistically. However, NT-3 alone has little or no effect on hair cell loss caused by amikacin (15), not surprising because these sensory cells do not appear to express receptors for neurotrophins (12). Therefore, the striking synergy of protection produced in combination with MK801 is both unexpected and revealing. This finding suggests that protection of spiral ganglion neurons can be beneficial to hair cells and, conversely, that damage to spiral ganglion neurons may be deleterious to hair cells. Neuronal damage may in fact amplify the effects of stimuli that damage hair cells. Such transcellular damage cannot be the sole means of hair cell injury because hair cells can be damaged by ototoxins in culture without neurons present (7). However, the data of Duan et al. (1) suggest that a process involving cochlear neurons may make a substantial contribution to hair cell damage in vivo.
Because hair cells survive quite well without spiral ganglion neurons, for example, in organotypic culture (7), neurons presumably do not supply trophic factors that are required for hair cell survival. Moreover, cochlear excitotoxicity alone, produced by kainate, does not appear to damage hair cells (17). Thus, transcellular injury of hair cells, of the nature implied by data of Duan et al. (1), is presumably specific to postsynaptic excitotoxicity in combination with an insult to hair cells. What process might mediate this potentiation of hair cell damage is not directly addressed by Duan et al. (1). However, they suggest the second messenger nitric oxide (NO) as a potential candidate. Although they present no direct evidence, NO is known to be generated in excitotoxicity (18), the dendrites of spiral ganglion neurons are richly endowed with NO synthase (19), and NO readily diffuses between cells, which all argue in support of this possibility. Future experiments will be required to test the hypothesis. However, regardless of mechanism, the potential for damage to postsynaptic structures to in turn damage hair cells provides an additional target for hair cell pharmacotherapy.
It should be noted that there are potential alternative explanations for the observations of Duan et al. (1). There is little evidence to suggest the presence of either NMDA or neurotrophin receptors on hair cells. However, as Duan et al. note, it is possible that a small number of receptors that have a disproportionate effect on hair cell survival have been overlooked. Another possibility is direct physical interaction of NT-3 and/or MK801 with amikacin before their contact with the hair cell. Duan et al. found that amikacin lost none of its bactericidal effect in the presence of these two factors but, because the ototoxic and bactericidal effects may be separate, this possibility cannot be ruled out.
As noted above, the results of Duan et al. (1) are the latest in a series of studies demonstrating that damage to sensory cells and neurons can be ameliorated by intervention in a variety of biological pathways. Although Duan et al. achieved a high degree of protection, we can be confident that improved amelioration of cochlear sensorineural damage can be achieved. This will be based on a more thorough understanding of the cellular basis for damage to these cells. Continuing and rapid advances in cell signaling within and between cochlear cells should drive this process.
Several hurdles must be overcome before the application of protective strategies similar to those used by Duan et al. (1) can be applied to humans. Individuals receiving ototoxic drugs for the treatment of life-threatening illnesses are ideal candidates for protection of sensorineural structures mediated by systemic or local delivery of protectants. Moreover, strategies and devices for delivery to the cochlea are under active development. However, the agents used must be safe as well as efficacious. Germane to this issue, there is evidence that the antioxidant and iron chelating effects of salicylate can attenuate aminoglycside ototoxicity (20). It is also possible that protection effective for the acute exposure to ototoxins typically used in animal experiments such as the Duan et al. study might not be effective for the longer-term exposures often required for patients. Finally, developing protection that might benefit even more chronic causes of hearing loss, such as aging or late-onset inherited deafness, may be even more challenging. However, vector-based (8, 21) and germ-line (22) gene therapy have been shown to provide protection of both hair cells and auditory neurons in animals and have the potential for long-term delivery of protective substances.
Acknowledgments
I thank Dr. Elizabeth M. Keithley for her insightful comments and Ms. Julie Lightner for editorial assistance. This paper was supported by Grant DC00139 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders and by the Research Service of the Veterans Affairs Hospitals.
Footnotes
See companion article on page 7597.
References
- 1.Duan M, Agerman K, Ernfors P, Canlon B. Proc Natl Acad Sci USA. 2000;97:7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman D W, Whitworth C A, Jones K L, Rybak L P. Hearing Res. 1987;31:217–222. doi: 10.1016/0378-5955(87)90190-0. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Nevill G, Forge A. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- 4.Otto W C, Brown R D, Gage-White L, Kupetz S, Anniko M, Penny J E, Henley C M. Hearing Res. 1988;35:79–85. doi: 10.1016/0378-5955(88)90042-1. [DOI] [PubMed] [Google Scholar]
- 5.Garetz S L, Altschuler R A, Schacht J. Hearing Res. 1994;77:81–87. doi: 10.1016/0378-5955(94)90255-0. [DOI] [PubMed] [Google Scholar]
- 6.Song B B, Sha S H, Schacht J. Free Radical Biol Med. 1998;25:189–195. doi: 10.1016/s0891-5849(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 7.Low W, Dazert S, Baird A, Ryan A F. J Cell Physiol. 1996;167:443–450. doi: 10.1002/(SICI)1097-4652(199606)167:3<443::AID-JCP8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Yagi M, Magal E, Sheng Z, Ang K A, Raphael Y. Hum Gene Ther. 1999;10:813–823. doi: 10.1089/10430349950018562. [DOI] [PubMed] [Google Scholar]
- 9.Ryan A F, Brumm D, Kraft M. NeuroReport. 1991;2:543–646. doi: 10.1097/00001756-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Eybalin M. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- 11.Pujol R, Puel J L, Eybalin M. NeuroReport. 1992;3:299–302. doi: 10.1097/00001756-199204000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumäe U, Saarma M. Proc Natl Acad Sci USA. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernfors P, Lee K-F, Kucera J, Jaenisch R. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 14.Farinas I, Jones K R, Backus C, Wang X-Y, Reichardt L F. Nature (London) 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 15.Ernfors P, Duan M L, Elshamy W M, Canlon B. Nat Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 16.Basile A S, Huang J M, Xie C, Webster D, Berlin C, Skolnick P. Nat Med. 1996;2:1338–1343. doi: 10.1038/nm1296-1338. [DOI] [PubMed] [Google Scholar]
- 17.Lerner-Natoli M, Ladrech S, Renard N, Puel J L, Eybalin M, Pujol R. Brain Res. 1997;749:109–119. doi: 10.1016/s0006-8993(96)01306-6. [DOI] [PubMed] [Google Scholar]
- 18.Lynch D R, Dawson T M. Curr Opin Neurol. 1994;7:510–516. doi: 10.1097/00019052-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Fessenden J D, Coling D E, Schacht J. Brain Res. 1994;668:9–15. doi: 10.1016/0006-8993(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 20.Sha S H, Schacht J. Lab Invest. 1999;79:807–813. [PubMed] [Google Scholar]
- 21.Staecker H, Gabaizadeh R, Federoff H, Van De Water T R. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- 22.Dulon D, Ryan A F. NeuroReport. 1999;10:1189–1193. doi: 10.1097/00001756-199904260-00006. [DOI] [PubMed] [Google Scholar]