Background: Anoikis, a detachment-free cell death, and pathways involving anoikis are not well understood.
Results: Diindolylmethane and cyclopamine induce anoikis in vitro and in vivo by inhibiting Gli1.
Conclusion: Gli1 suppresses anoikis resistance and inhibits tumor formation ability of ovarian cancer cells.
Significance: This is the first report demonstrating the role of Gli1 in anoikis. Gli1 inhibitors can be used clinically to inhibit metastasis.
Keywords: Adhesion, Apoptosis, Hedgehog, Metastasis, Ovarian Cancer, Signal Transduction, Anoikis, Gli1, Tumor Model
Abstract
Anoikis is a cell death that occurs due to detachment of a cell from the extracellular matrix (ECM). Resistance to anoikis is a primary feature of a cell that undergoes metastasis. In this study for the first time, we demonstrated the potential role of Gli1 in anoikis resistance. Treatment of various ovarian cancer cells by different concentrations of diindolylmethane (DIM), an active ingredient of cruciferous vegetables, reduced the anoikis resistance in a concentration-dependent manner. Reduction in anoikis resistance was associated with a decrease in the expression of Gli1 and an increase in the cleavage of poly(ADP-ribose) polymerase (PARP). Sonic hedgehog (Shh) treatment not only increased the expression of Gli1, but also blocked anoikis induced by DIM and abrogated the change in the expression of Gli1 and cleaved PARP by DIM. To confirm the role of Gli1, hedgehog inhibitor cyclopamine, Gli1 siRNA and Gli1−/− mouse embryonic fibroblasts (MEFs) were used. Cyclopamine treatment alone significantly reduced anoikis resistance in A2780 and OVCAR-429 cells. Cyclopamine-mediated reduction in anoikis resistance was associated with reduced expression of Gli1 and induction of cleaved PARP. Shh treatment blocked cyclopamine-induced anoikis. Silencing Gli1 expression induced anoikis and cleavage of PARP in A2780 and OVCAR-429 cells. Furthermore, Gli1−/− MEFs were more sensitive to anoikis compared with Gli1+/+ MEFs. Our in vivo studies established that DIM- or cyclopamine-treated ovarian cancer cells under suspension culture conditions drastically lost their ability of tumor formation in vivo in mice. Taken together, our results establish that Gli1 is a critical player in anoikis resistance in ovarian cancer.
Introduction
Extracellular matrix (ECM)2 serves as a basement membrane for the growth of cells (1). Epithelial cells depend on proper cell-cell and cell-matrix interactions for proper differentiation and survival (2). However, transformed epithelial cells acquire the ability to grow in anchorage-independent growth conditions, making it a critical feature of metastasis (3). Anoikis, meaning homeless in Greek, is apoptosis induced by lack of proper ECM attachment (4). Both normal and immortalized cells undergo anoikis. For the tumor cells to become metastatic, cells must survive detachment from the ECM and resist the apoptotic stimuli associated with invasion, migration, and metastasis by activating survival mechanisms (5). Hence, cells that are resistant to anoikis can lodge in distant sites, leading to metastasis (5). Anoikis is relatively a new concept, and many study groups are trying to evaluate new pathways that are involved in anoikis resistance. It is important to note that there is no common mechanism of anoikis resistance among all cancer types (6).
Hedgehog signaling was first identified in Drosophila as a pathway that directs patterning and is crucial for proper development (7). Subsequent studies identified three members of this family which include sonic hedgehog (Shh), desert hedgehog (Dhh), and Indian hedgehog (Ihh) (8). Among these three proteins, Shh is widely studied due to its role in cancer. At the molecular level, when Shh ligand binds to Ptch receptor, it releases Smo, which becomes active and initiates a signaling cascade that results in the activation of Gli1, a transcription factor that translocates to nucleus, binds to DNA, and causes the activation of several genes (9). Distinct tissues require specific levels of hedgehog signaling for proper function, and an increase or decrease of pathway activity results in severe defects, including cancer (10). Molecules of hedgehog signaling such as Gli1 are aberrantly expressed in various cancers including ovarian cancer (10–12). Accumulating evidence suggests that hedgehog signaling through Gli1 plays a role in cell cycle progression, antiapoptosis, angiogenesis, metastasis, and EMT (7, 13–18). However, the role of Gli1 in anoikis is not yet known.
Ovarian cancer is a leading gynecological malignancy in the United States with 15,500 deaths and 22,280 new cases diagnosed in 2011 (19). Ovarian cancer is one of the difficult cancers to detect before it metastasizes despite several daring attempts for early detection. Ovarian tumor cells are most sensitive to metastasis because the ovaries are exposed to the peritoneum (20). The most common sites of ovarian cancer metastasis are liver, lungs, and stomach, where cells travel through the peritoneum (21). Ovarian epithelial tumor cells that are resistant to anoikis can find their metastatic sites easily (5). Thus, ovarian cancer makes a very attractive and important model to study anoikis (22, 23). Several recent studies reported that hedgehog signaling plays a crucial role in ovarian tumorigenesis (11, 12). Hence, in this study we sought to determine whether hedgehog signaling plays any role in anoikis resistance in ovarian cancer cells.
Diindolylmethane (DIM) is an active constituent of cruciferous vegetables. Anticancer effects of DIM have been shown against prostate, breast, colon, and pancreatic cancers (24–27). Previously published studies from our laboratory indicated that DIM treatment suppresses the growth of ovarian cancer cells in vitro and in vivo (28–31). In addition, DIM inhibits angiogenesis and blocks invasion of ovarian cancer cells (32). In the current study, we investigated the role of DIM-induced anoikis in ovarian cancer cells.
MATERIALS AND METHODS
Chemicals
Cyclopamine was purchased from Enzo Life Sciences (Farmingdale, NY). Gli siRNA was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). BR-DIM is a kind gift from Dr. Michael Zeligs (Bio Response, Boulder, CO). Poly(2-hydroxyethyl methacrylate) (poly-HEMA), sulforhodamine B, MCDB105 medium, medium 199, and antibody against actin were obtained from Sigma-Aldrich. Antibodies against Gli1 and Cl-PARP were obtained from Cell Signaling Technology. Shh was obtained from R&D Systems. Transfection reagent siPORT was obtained from Applied Biosystems (Carlsbad, CA). RPMI and McCoy 5A were purchased from Mediatech (Manassas, VA). DMEM was procured from ATCC (Manassas, VA).
Cell Culture
A2780, OVCAR-429, SKOV-3, and TOV-21G were procured and cultured as described previously (32). Gli1+/+ and Gli1−/− MEFs, a kind gift from Dr. Bushman (University of Wisconsin, Madison, WI), were maintained in DMEM as explained previously (33).
Poly-HEMA Coating
Poly-HEMA coating was applied to culture plates to culture regular adherent cells in a suspension form to simulate extracellular matrix detachment growth conditions. Poly-HEMA was dissolved in absolute ethanol at a final concentration of 1 mg/ml by incubating it on a shaker at 60 °C overnight. Immediately, low adherent culture plates (catalog no. 351029) were coated with poly-HEMA and allowed to dry. After poly-HEMA coating was uniformly applied, plates were washed with sterile PBS and used for different anoikis assays.
Anoikis Assay
Anoikis assay was performed as described previously with few modifications (22, 34). Briefly, 1 × 106 cells were plated in poly-HEMA-coated plates. DMSO was added to control plates whereas other plates were exposed to various concentrations of DIM or cyclopamine. After the desired time, cells were centrifuged at 1000 rpm for 5 min and uniformly distributed in a 24-well plate and incubated at 37 °C in an incubator. After 8 h, plates were processed for Sulforhodamine B (SRB) assay as described previously by us (29). The average control value was used to divide the average value of treated sample and multiplied by 100. Anoikis was represented as a graph plotted as percentage anoikis resistance on the y axis against drug concentration on the x axis.
Western Blotting
A2780 or OVCAR-429 cells were exposed to varying concentrations of DIM or cyclopamine under suspension culture conditions for the desired time. Cells were collected, lysed, and approximately 10–60 μg of protein was subjected to SDS-gel electrophoresis followed by immunoblotting as previously described by us (28).
Gli Knockdown
Briefly, 0.3 × 106 A2780 or OVCAR-429 cells were plated in OPTI-MEM without antibiotics and reverse-transfected with siRNA. Complexes were prepared by incubating 100 nm Gli1 siRNA with 8 μl of siPORT transfection reagent in 200 μl of OPTI-MEM without serum or antibiotic for 1 h. These complexes were then added to the cells. Six hours after transfection, the medium was replaced by regular medium. After 48 h of transfection, cells were grown under matrix-free conditions for 24 h and processed for anoikis assay and Western blotting as described above.
In Vivo Anoikis Experiment
An in vivo anoikis experiment was performed as described previously with few modifications (35, 36). Four- to 6-week-old female athymic nude mice were purchased from Charles River Laboratories (Wilmington, MA). The use of mice and their treatment were approved by Institutional Animal Care and Use Committee (IACUC), Texas Tech University Health Sciences Center, and all of the experiments were carried out in strict compliance with regulations. Mice were fed with an antioxidant-free AIN-76A special diet for a week before starting the experiment. A2780 or OVCAR-429 cells were treated with DMSO or cyclopamine or DIM for 20 h under suspension culture conditions. The treated cells were washed thoroughly three times with Hanks' buffer to remove the free drug. Viable cells were counted by trypan blue dye exclusion assay. Approximately 5 × 106 viable cells from each group were resuspended in a 1:1 mixture of Matrigel and Hanks' buffer and inoculated subcutaneously into the flanks of nude mice using a Hamilton syringe. Once palpable tumors were formed, the tumor volume was measured every other day using digital vernier calipers as described previously by us (37). Tumor volume was calculated as volume = (length × width2)/2. The experiment was terminated after 28 days. In another experiment, A2780 cells grown under suspension culture conditions were injected as described above. After a palpable tumor was formed, either scrambled siRNA or 0.5 μm Gli1 siRNA was administered intratumorally once every 3 days until the experiment was terminated on day 23.
Statistical Analysis
All the statistical analysis was performed using Prism 5.0 (GraphPad Software Inc., San Diego, CA). The data represent mean ± S.D. or S.E. Student's t test was used to compare the control and treated groups. In experiments involving more than three groups, nonparametric analysis of variance followed by Bonferroni's post hoc multiple comparison test was used. All statistical tests were two-sided. Differences were considered statistically significant when the p value was <0.05.
RESULTS
DIM Reverses Anoikis Resistance by Inhibiting Gli1 in Ovarian Cancer Cells
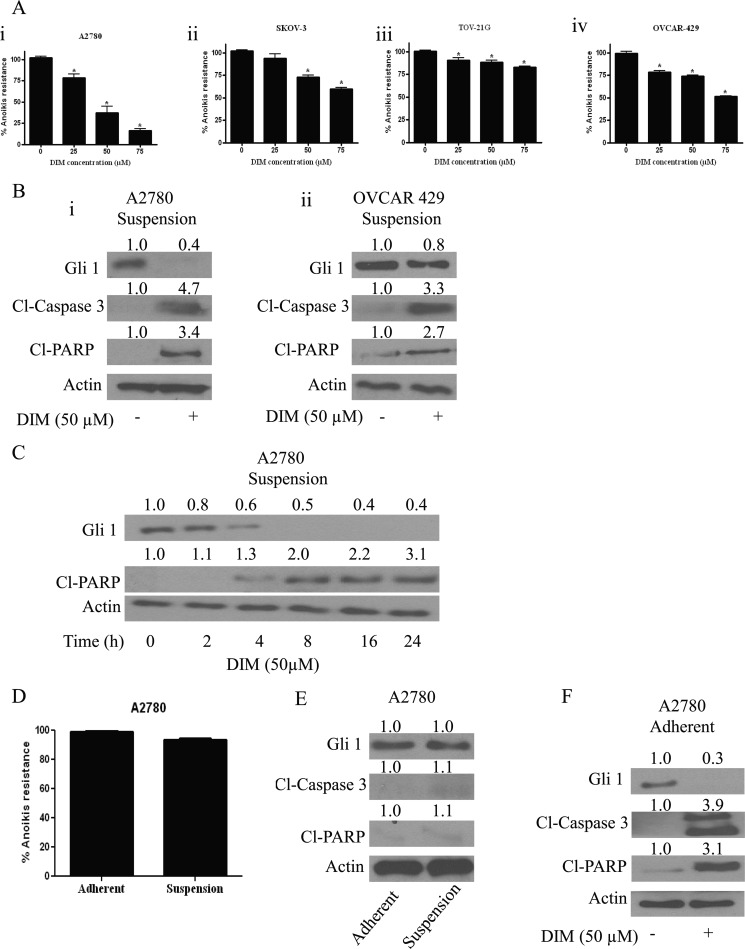
We have demonstrated previously that DIM induces apoptosis and exerts antiangiogenic effects in ovarian cancer cells (32). However, the mechanism by which DIM mediates these properties was not clear. Recent studies indicated that malignant cells acquire metastatic potential only after overcoming anoikis, a type of cell death which is triggered by detachment of cells from the ECM. To determine whether or not DIM overcomes anoikis resistance, A2780, SKOV-3, TOV-21G, and OVCAR-429 ovarian cancer cell lines were cultured as a suspension culture in poly-HEMA-coated plates and treated with varying concentrations of DIM for 24 h. The treated cells were recultured on an adherent plate where only anoikis-resistant cells attach and grow. Our results indicated that DIM treatment reduced the anoikis resistance in various ovarian cancer cell lines in a concentration-dependent manner. Reduction in the survival is represented as a percentage decrease in anoikis resistance. Anoikis resistance by 25–75 μm DIM treatment was reduced by 25–75% in A2780 cells (Fig. 1Ai). Similar concentrations of DIM reduced anoikis resistance by 15–40% in SKOV-3, 10–30% in TOV-21G, and 20–50% in OVCAR-429 cells (Fig. 1Aii–iv). These results clearly establish that DIM reduces anoikis resistance in ovarian cancer cells in a concentration-dependent manner.
FIGURE 1.
DIM reverses anoikis resistance in ovarian cancer cells. Ai, A2780; Aii, SKOV-3; Aiii, TOV-21G; and Aiv, OVCAR-429 cells were grown in poly-HEMA-coated plates as suspension culture and treated with DMSO or various concentrations of DIM. After 24 h, cells were replated in an adherent 24-well plate and allowed to attach overnight. Viable cells were analyzed by Sulforhodamine B (SRB) assay. Representative bar graphs show the percentage anoikis resistance in different treatment conditions. *, p < 0.05 compared with control. Error bars, S.D. B, blots are representative of Gli1, Cl-caspase3, and Cl-PARP from lysates collected from A2780 (i) and OVCAR-429 cells (ii) grown under suspension culture conditions and treated with or without 50 μm DIM. Actin was used as loading control. C, representative blot of Gli1 and Cl-PARP from lysates collected from A2780 cells grown under suspension culture conditions and treated with 50 μm DIM for different time points are shown. Actin was used as a loading control. D, A2780 cells were grown for 24 h under adherent and suspension culture conditions. The cells were trypsinized and replated in a 24-well plate and allowed to attach overnight. Viable cells were analyzed by SRB assay. Representative bar graphs show the percentage anoikis resistance under different growth conditions. E, representative Western blots are shown of Gli1, Cl-caspase3, and Cl-PARP from lysates collected from A2780 cells grown under adherent and suspension culture conditions. F, representative blots of Gli1, Cl-caspase3, and Cl-PARP from lysates collected from A2780 cells grown under adherent culture conditions and treated with 50 μm DIM are shown. Actin was used as a loading control.
Because anoikis is a form of apoptosis, the results were confirmed by Western blotting. Our Western blot analysis revealed that DIM treatment induces cleavage of caspase3 and PARP in A2780 and OVCAR-429 cells, strengthening our above observations with regard to anoikis (Fig. 1B).
Hedgehog signaling transcription factor Gli1 plays an important role in cancer progression. Furthermore, hedgehog signaling is aberrantly expressed in ovarian cancer. However, whether Gli1 is a target of DIM and whether hedgehog signaling plays any role in anoikis were not known. To test the role of hedgehog/Gli1 in DIM-induced anoikis, A2780 or OVCAR-429 ovarian cancer cells were treated with DIM under suspension culture conditions. Our Western blot analysis demonstrates that DIM treatment significantly reduced the expression of Gli1 in both cell lines tested (Fig. 1B). The decrease in the expression of Gli1 was approximately 60–70% in both the cell lines.
To determine whether the Gli1 down-regulation was the cause or effect of anoikis, we evaluated the time-dependent effect of DIM in A2780 cells grown under suspension culture conditions. We observed that Gli1 suppression by DIM in A2780 cells was as early as 2 h whereas the cleavage of PARP was observed only at 4 h (Fig. 1C), suggesting that Gli1 down-regulation was associated with the induction of anoikis in A2780 ovarian cancer cells.
DIM Down-regulates Gli1 under Adherent Culture Conditions
Next, we questioned whether there is any anoikis induction in A2780 cells grown under suspension culture conditions compared with adherent cells. Our results indicated a very modest decrease in the survival of A2780 cells under suspension culture compared with those in adherent culture conditions (Fig. 1D). Accordingly, our Western blot analysis revealed no change in the expression of Gli1 when A2780 cells were grown under adherent or suspension cell culture conditions (Fig. 1E).
Because we observed a decrease in Gli1 expression in cells grown under suspension culture conditions in response to DIM treatment, we asked ourselves whether DIM down-regulates Gli1 in adherent culture conditions as well. To answer this question, we treated A2780 cells grown under adherent conditions with DIM. Exposure of A2780 cells to 50 μm DIM resulted in the down-regulation of Gli1 in adherent cells (Fig. 1F). These results suggest Gli1 as a target of DIM in both adherent as well as suspension culture conditions.
Shh Pretreatment Reverses Anoikis Sensitization by DIM
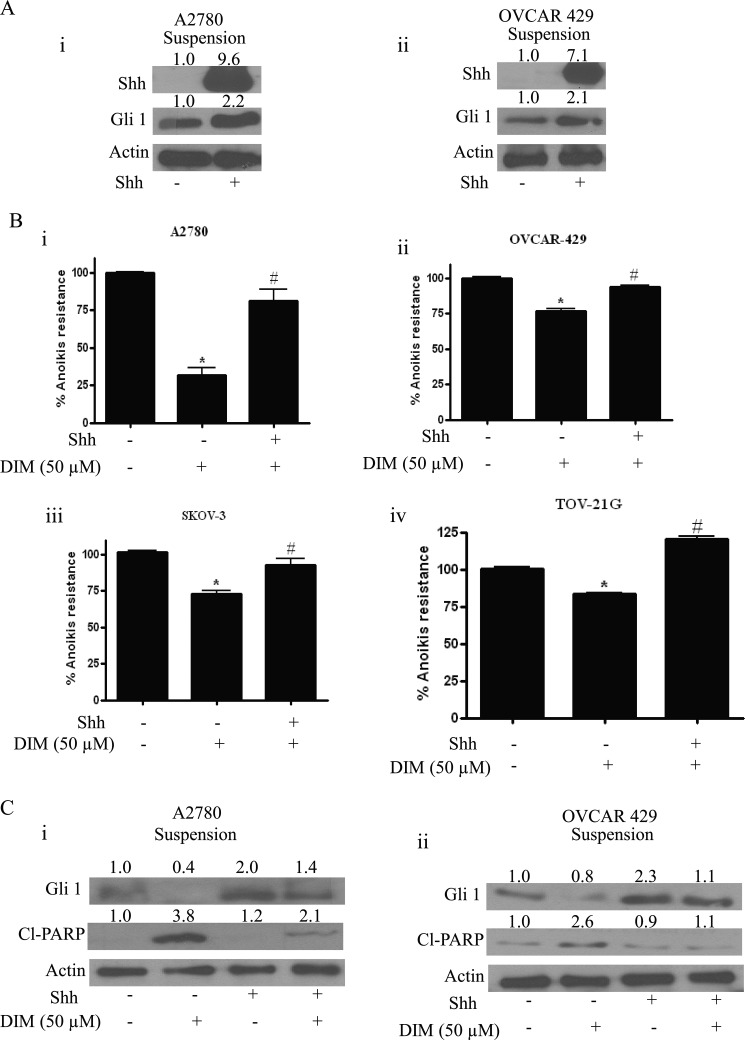
To strengthen the role of Gli1 in anoikis resistance, Shh was used to increase the expression of Gli1. Shh is a 45-kDa precursor protein that is cleaved into a 20-kDa N-terminal Shh peptide and 35-kDa C-terminal peptide (38). The N-terminal peptide is responsible for retaining signaling capabilities and the C-terminal for intramolecular precursor processing, acting as cholesterol transferase (38). Together, they play an important role in hedgehog signal generation (39). Gli1 translocates to nucleus and binds to specific regions of DNA leading to the increase in the expression of genes including Gli1 itself. As shown in Fig. 2A, treatment of A2780 or OVCAR-429 cells with 10 ng/ml Shh resulted in an enormous increase in the expression of Shh. Similarly, a >2-fold increase in the expression of Gli1 was observed in both cell lines in response to Shh treatment.
FIGURE 2.
Shh reverses anoikis induced by DIM. A, representative blots of Shh and Gli1 from lysates collected from A2780 (i) and OVCAR-429 cells (ii) exposed to 10 ng/ml Shh for 24 h under suspension culture conditions. B, representative bar graphs showing percentage anoikis resistance in cells treated under suspension culture conditions with DMSO, 50 μm DIM alone, or in combination with Shh in A2780 (i), OVCAR-429 (ii), SKOV-3 (iii), and TOV-21G cells (iv). *, p < 0.05 compared with control; #, p < 0.05 compared with DIM treatment. Error bars, S.D. C, representative blots of Gli1 and Cl-PARP from lysates collected from cells treated under suspension culture conditions with DMSO or DIM in the presence or absence of Shh. Actin was used as loading control.
Because our results indicated the involvement of Gli1 in DIM-induced anoikis, we hypothesized that Shh treatment would block DIM-induced anoikis. As expected, our results showed that Shh substantially abrogated the reduction in the anoikis resistance by DIM in four different ovarian cancer cell lines. For example, A2780 cells treated with 50 μm DIM showed a 70% reduction in the anoikis resistance (Fig. 2B). However, Shh treatment blocked DIM-induced anoikis by almost 40% (Fig. 2B). Similarly, significant reduction in anoikis resistance by DIM in OVCAR-429, SKOV-3, and TOV-21G cells was completely blocked by Shh pretreatment, confirming our hypothesis (Fig. 2B).
In support of the above observations, our Western blot analysis also revealed that substantial reduction in the expression of Gli1 by DIM was significantly blocked by Shh pretreatment (Fig. 2C). Similarly, Shh treatment substantially decreased the cleavage of PARP induced by DIM in A2780 and OVCAR-429 cells (Fig. 2C). These results clearly establish that DIM induces anoikis by inhibiting Gli1 in ovarian cancer cells.
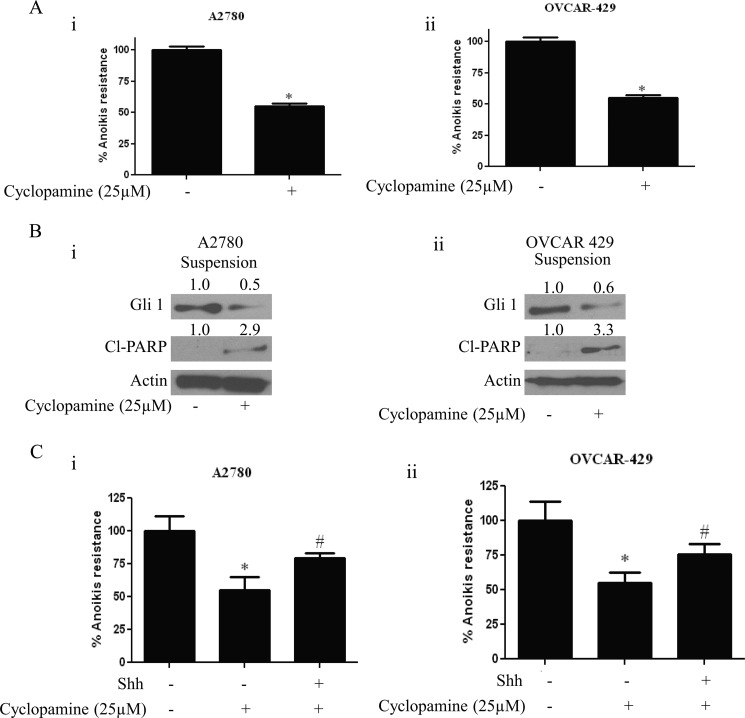
Cyclopamine Reduces Anoikis Resistance in Ovarian Cancer Cells by Inhibiting Gli1
Because induction of anoikis by DIM was mediated by Gli1 inhibition, we questioned whether hedgehog inhibitor can induce anoikis in ovarian cancer cells. To answer our question, we used cyclopamine, a well known Shh inhibitor. Cyclopamine acts as an antagonist to Shh and blocks smoothened (Smo) receptor on cell membrane there by blocking Gli1. A2780 or OVCAR-429 cells were grown in poly-HEMA-coated plates as described above and treated with 25 μm cyclopamine for 24 h. Our results demonstrate that cyclopamine reduces anoikis resistance in ovarian cancer cells (Fig. 3A). Decrease in the anoikis resistance was ∼40% in A2780 cells, and the reduction in anoikis resistance in OVCAR-429 cells was approximately 35% (Fig. 3A). In accordance with these data, our Western blotting data indicated that cyclopamine treatment reduced the expression of Gli1 in both A2780 and OVCAR-429 cell lines. Reduction in the Gli1 expression corresponded with increase in the cleavage of PARP, indicating that anchorage-independent cell death is induced by cyclopamine in ovarian cancer cells (Fig. 3B). Moreover, treating A2780 or OVCAR-429 cells with Shh prior to cyclopamine restored the anoikis resistance that was reduced by cyclopamine treatment (Fig. 3C). These results further strengthen our hypothesis that inhibiting hedgehog signaling reverses anoikis resistance in ovarian cancer cells.
FIGURE 3.
Cyclopamine induces anoikis in ovarian cancer cells. A, bar graphs representing percentage anoikis resistance in A2780 (i) and OVCAR-429 cells (ii) treated with 25 μm cyclopamine for 24 h under suspension culture conditions. *, p < 0.05 compared with control. Error bars, S.D. B, representative blots of Gli1 and Cl-PARP from lysates collected from A2780 (i) and OVCAR-429 cells (ii) treated with or without 25 μm cyclopamine for 24 h under suspension culture conditions. Actin was used as loading control. C, representative bar graphs showing percentage anoikis resistance in A2780 (i) and OVCAR-429 cells (ii) treated with 25 μm cyclopamine for 24 h in the presence or absence of 10 ng/ml Shh in suspension culture conditions. *, p < 0.05 compared with control; #, p < 0.05 compared with cyclopamine treatment.
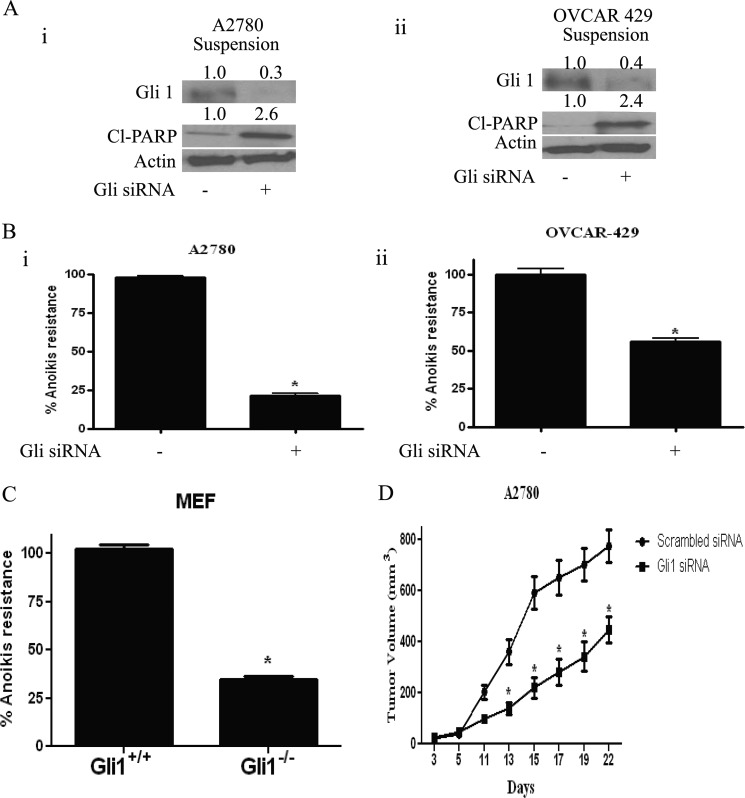
Silencing Gli1 Using siRNA Induces Anoikis in Ovarian Cancer Cells
To further confirm the role of Gli1 in inducing anoikis in our model, Gli1 was transiently silenced using specific Gli1 siRNA in A2780 and OVCAR 429 ovarian cancer cells. As expected, Gli1 siRNA completely silenced the expression of Gli1 (Fig. 4A). Interestingly, the decrease in the expression of Gli1 was associated with the increase in the cleavage of PARP (Fig. 4A). Our results also confirmed that inhibiting the expression of Gli1 sensitizes ovarian cancer cells to anoikis (Fig. 4B). Gli1 knockdown resulted in 70% decrease in anoikis resistance in A2780 cells and approximately 50% in OVCAR-429 cells (Fig. 4B).
FIGURE 4.
Cells without Gli1 expression are more sensitive to anoikis. A, representative blots of Gli1 and Cl-PARP in A2780 (i) and OVCAR-429 cells (ii) grown in suspension culture condition for 24 h after transfecting Gli1 siRNA in adherent cells for 48 h. Actin was used as loading control. B, bar graphs representing percentage anoikis resistance in A2780 (i) and OVCAR-429 cells (ii) transfected with Gli1 siRNA. *, p < 0.05 compared with control. Error bars, S.D. C, bar graphs representing percentage anoikis resistance in Gli1+/+ and Gli1−/− MEF cells grown in suspension culture conditions for 24 h. D, effect of control and Gli1 siRNA on the growth of A2780 ovarian tumor xenografts. *, p < 0.05 compared with control.
As a proof of principle, Gli1 wild-type and knock-out MEFs were used to test the role of Gli1 in anoikis. Both Gli1+/+ and Gli1−/− cells were grown under suspension conditions, and anoikis resistance was determined. As expected, our results indicate that anoikis resistance was 60% lesser in Gli1−/− MEFs compared with Gli1+/+ MEFs (Fig. 4C). Taken together, these results establish the role of Gli1 and thereby hedgehog signaling in anoikis in ovarian cancer cells.
Because we observed that silencing Gli1 expression using Gli1 siRNA resulted in induction of anoikis in ovarian cancer cells, we hypothesized that administering Gli1 siRNA would retard the tumor formation in vivo. To test our hypothesis, we implanted A2780 tumor xenografts by injecting 5 × 106 cells grown under suspension culture conditions for 24 h, in the flanks of female athymic nude mice. After palpable tumor mass was formed, control siRNA was injected intratumorally in the tumors of control mice whereas mice from the treated group were injected with Gli1 siRNA once every 3 days. Tumor volume was measured using vernier calipers. Our results demonstrated that intratumoral injection of Gli1 siRNA substantially retarded the growth of A2780 tumors compared with control tumors (Fig. 4D), suggesting that Gli1 suppression reduces the formation of ovarian tumors in vivo.
Effect of DIM and Cyclopamine on Ovarian Tumor Formation in Vivo
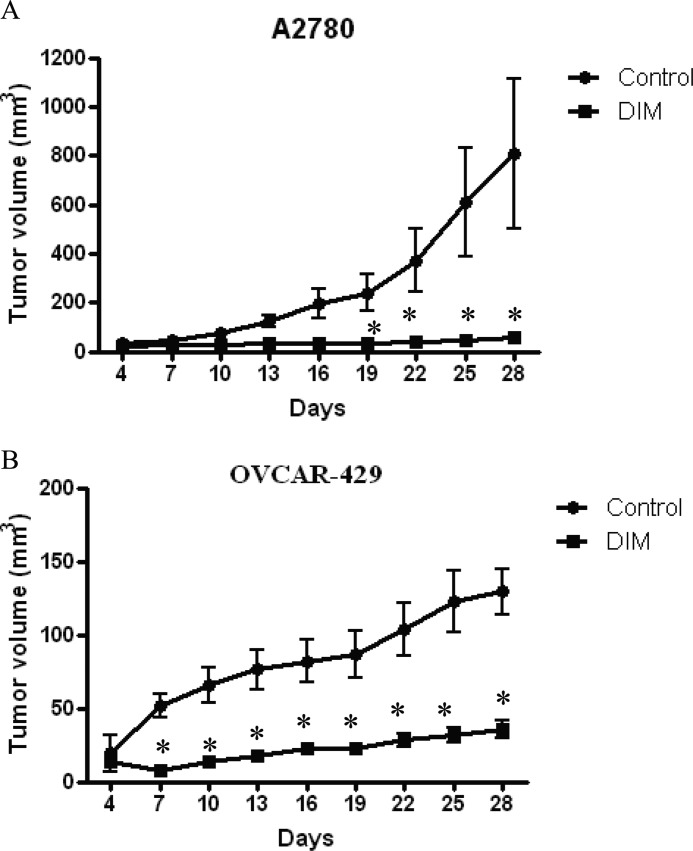
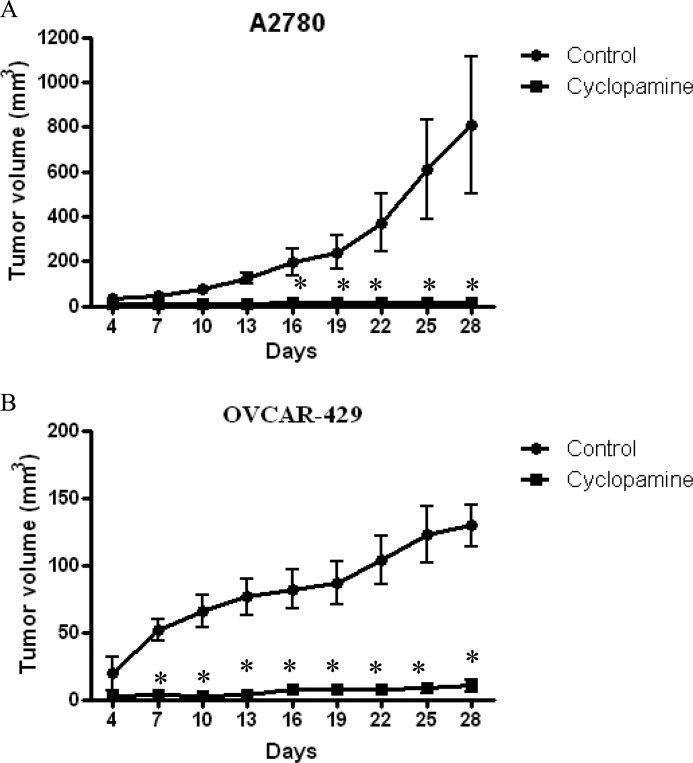
Several previous studies established that cells that are sensitive to anoikis, lose tumor formation ability in mice. Because we observed that both DIM and cyclopamine reduce the anoikis resistance, we sought to determine the tumor formation ability of ovarian cancer cells treated with DIM or cyclopamine. A2780 or OVCAR-429 cells were treated with DMSO, 10 μm cyclopamine, or 75 μm DIM for 20 h in suspension culture conditions. After 20 h of treatment, viable cells were counted using trypan blue. About 5 × 106 viable cells were injected in flanks of female athymic nude mice to determine the tumor formation ability of cells. Once palpable tumors were formed, tumor measurements were noted using vernier calipers every 2 days. Our results indicate that both DIM and cyclopamine almost completely suppressed the tumor formation ability of ovarian cancer cells in vivo. For example, on day 28 the average tumor volume formed by A2780 cells treated with cyclopamine was around 20 mm3, and those treated by DIM was 30 mm3, which was substantially lesser compared with an average tumor volume of 700 mm3 formed by control cells treated with only DMSO, demonstrating approximately 95% tumor growth inhibition (Figs. 5A and 6A). Similarly, in OVCAR-429 cells, the mean tumor volume formed by control OVCAR-429 cells was ∼130 mm3, whereas cyclopamine- and DIM-treated cells formed a tumor that accounted for 20 mm3 and 40 mm3, respectively (Figs. 5B and 6B). Taken together, these results establish that DIM or cyclopamine substantially inhibits the tumor formation ability of ovarian cancer cells in vivo.
FIGURE 5.
DIM treatment inhibits tumor formation potential of ovarian cancer in athymic nude mice. A2780 (A) or OVCAR-429 cells (B) were treated with DMSO or 75 μm DIM under suspension culture conditions for 20 h after which 5 × 106 viable cells counted by trypan blue assay were injected in flanks of female athymic nude mice. Once each mouse had a palpable tumor, tumor volume was measured every other day using vernier calipers as described under “Materials and Methods.” Effect of DIM on A2780 cells (A) and OVCAR-429 cells (B) on tumor formation ability is represented by graphs plotted as tumor volume (mm3) against number of days. *, p < 0.05 compared with control.
FIGURE 6.
Cyclopamine treatment inhibits tumor formation potential of ovarian cancer in athymic nude mice. A2780 (A) or OVCAR-429 cells (B) were treated with DMSO or 10 μm cyclopamine under suspension culture conditions for 20 h after which 5 × 106 viable cells counted by trypan blue assay were injected in flanks of female athymic nude mice. Once each mouse had a palpable tumor, tumor volume was measured every other day using vernier calipers as described under “Materials and Methods.” Effect of cyclopamine on A2780 cells (A) and OVCAR-429 cells (B) on tumor formation ability is represented by graphs plotted as tumor volume (mm3) against number of days. *, p < 0.05 compared with control. Error bars, S.D.
DISCUSSION
To best of our knowledge, this is the first report showing the role of hedgehog/Gli1 in relation to anoikis. The key findings from our study are that hedgehog/Gli1 signaling plays an important role in anoikis resistance, and disrupting Gli1 induces anoikis in ovarian cancer cells. Our study shows evidence that inhibiting Gli1 by DIM or hedgehog signaling inhibitor cyclopamine reverses anoikis resistance in ovarian cancer cells. Silencing Gli1 using siRNA also sensitized ovarian cancer cells to anoikis. Furthermore, Gli1−/− MEFs were more susceptible to anoikis compared with Gli1+/+ MEFs. As a proof of concept, we showed that both DIM and cyclopamine reduced the tumor formation capability of ovarian cancer cells when treated under ECM-free conditions.
One of the key reasons for the high mortality rate of ovarian cancer is because it is usually detected in late stages after it has metastasized to organs around peritoneum. Malignant cells metastasize only after acquiring resistance to anoikis (40). Because ovaries are exposed to the peritoneum, cancer cells from the ovarian surface epithelium can easily lodge into metastatic sites. The ovarian cancer cells detach from the primary tumor and subsequently invade the peritoneum, leading to the accumulation of large amounts of ascites containing tumor cells (34). A recent report suggested that intraperitoneally injected cancer cells take 5 days to adhere to the peritoneum, indicating that these cells acquire resistance to anoikis and thereby gain metastatic potential (41). Hence, understanding anoikis and factors that play a role in anoikis resistance is of prime importance in ovarian cancer.
In this study, we identified Gli1 as a novel mediator regulating DIM-induced anoikis in ovarian cancer cells. Gli1 is a major transcription factor of hedgehog signaling (14). Several recent studies showed that hedgehog plays a role in metastasis of various cancers (16). Furthermore, it is reported that expression of hedgehog/Gli1 proteins increased stepwise in benign, borderline, and malignant neoplasms (12, 42). Hence, there is every reason for this pathway to play a major role in anoikis resistance. Cyclopamine is a well known hedgehog inhibitor and blocks Gli1 expression. Our results showing that cyclopamine-mediated anoikis was associated with Gli1 suppression were in support with recent studies showing that cyclopamine inhibits Gli1 (42). Gli1 plays a major role in transcribing several prosurvival genes and aid in angiogenesis, Epithelial to Mesenchymal Transition (EMT), and metastasis (43). Silencing Gli1 using siRNA in our model blocked anoikis resistance, confirming the role of Gli1 in anoikis resistance. We observed significant anoikis in cells that were devoid of Gli1 expression. Our results demonstrated that silencing Gli1 induces cleavage of PARP in ovarian cancer cells. Because there was no induction of cleaved PARP in A2780 cells cultured under suspension conditions for 24 h, it is possible that the Gli1 suppression might be leading to onset of apoptosis in A2780 suspension cells. Importantly, our results are in agreement with two recently published studies which demonstrated that silencing Gli1 expression using Gli1 siRNA induces apoptosis (42, 44). These observations were also confirmed by Gli1 knock-out MEFs. Approximately 50% reduced anoikis resistance was observed in untreated Gli−/− MEFs compared with their wild-type counterparts. All of these data confirm that sonic hedgehog/Gli1 plays a major role in conferring anoikis resistance and promoting abdominal dissemination of ovarian cancer. Hence, our studies to some extent support the accumulating clinical and pathologic evidence on the role of hedgehog in the aggressiveness of various cancers.
DIM is a phytochemical present in cruciferous vegetables. Several studies including those from our laboratory demonstrated the anticancer potential of DIM against various cancers (28, 32, 45). DIM is also currently in clinical trials to treat several cancers (45). Our studies on DIM led us to two new findings: (i) DIM is able to induce anoikis and (ii) hedgehog/Gli1 is a therapeutic target of DIM. DIM treatment reduced the growth of various ovarian cancer cells in ECM-free culture conditions. Importantly, Gli1 expression was significantly reduced by DIM treatment. Furthermore, Shh not only substantially blocked anoikis induced by DIM, but also blocked the down-regulation of Gli1 by DIM, suggesting that Gli1 is a target of DIM. Our studies are in agreement with two other reports which showed that phytochemicals such as curcumin and EGCG inhibit hedgehog/Gli1 signaling in meduloblastoma and prostate cancer cells (46, 47). We observed that Gli1 expression varied in various ovarian cancer cells (supplemental Fig. 1). Gli1 expression was highest in SKOV-3 cells followed by A2780, TOV-21G, and OVCAR-429 cells. No correlation was observed between the expression of Gli1 and suppression of anoikis resistance by DIM in these cancer cells. However, involvement of other signaling pathways in DIM induced anoikis in ovarian cancer cells cannot be ruled out.
Several studies showed that cells that are sensitive to anoikis fail to form tumors in vivo (35, 36). Our results showed that exposing the cells to cyclopamine under suspension culture condition inhibited the A2780 cell growth and tumor formation in nude mice. This supported a potential role of Gli1 in the growth of ovarian tumors in vivo. These in vivo observations were further confirmed using another ovarian cancer cell line, OVCAR-429. Similarly, exposure of A2780 or OVCAR-429 to DIM under suspension condition prior to tumor inoculation significantly reduced the tumor formation ability and delayed ovarian tumor appearance. Furthermore, intratumoral injection of Gli1 siRNA substantially reduced the A2780 tumor xenografts compared with control tumors. Our studies along with several previous studies suggest that hedgehog/Gli1 is a good target for anticancer therapy in ovarian cancer and advocate the use of hedgehog inhibitors clinically. Two very recent reports indicated that hedgehog signaling is aberrantly expressed in patients with various tumors including ovarian tumors (11, 12, 42). It is noteworthy that a clinical trial using hedgehog inhibitor GDC-0049 against ovarian cancer was recently concluded in November 2011 (48); however, the results of these studies have not been published yet. Our studies would further strengthen the hypothesis that hedgehog inhibitors may play a significant role in ovarian cancer treatment.
In summary, we report here novel characteristics of Gli1 in promoting resistance to anoikis. These results strongly suggest that DIM, cyclopamine, or any other hedgehog pathway inhibitor can be used as a rational therapeutic strategy for preventing and/or inhibiting metastasis in ovarian cancer.
Supplementary Material
Acknowledgments
We thank Dr. Wade Bushman (University of Wisconsin, Madison, WI) for providing Gli1+/+ and Gli1−/− MEF cells, Dr. Thomas Hamilton (Fox Chase Cancer Center, PA) for providing A2780 cells, Dr. Laurie Hudson (University of New Mexico, NM) for providing OVCAR-429 cells, and Dr. Michael Zeligs (Bio Response, CO) for providing BR-DIM for our studies. We greatly appreciate the technical help of Srinivas Reddy Boreddy with the in vivo experiment and Dr. Xiao Ping He (Mayo Clinic, FL) for technical help.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA106953 and CA129038 (to S. K. S.) through the NCI.

This article contains supplemental Fig. 1.
- ECM
- extracellular matrix
- DIM
- diindolylmethane
- DMSO
- dimethyl sulfoxide
- MEF
- mouse embryonic fibroblast
- PARP
- poly(ADP-ribose) polymerase
- poly-HEMA
- poly(2-hydroxyethyl methacrylate)
- Shh
- sonic hedgehog.
REFERENCES
- 1. Gilmore A. P. (2005) Anoikis. Cell Death Differ. 12, 1473–1477 [DOI] [PubMed] [Google Scholar]
- 2. Frisch S. M., Screaton R. A. (2001) Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562 [DOI] [PubMed] [Google Scholar]
- 3. Nagaprashantha L. D., Vatsyayan R., Lelsani P. C., Awasthi S., Singhal S. S. (2011) The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int. J. Cancer 128, 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grossmann J. (2002) Molecular mechanisms of “detachment-induced apoptosis: anoikis.” Apoptosis 7, 247–260 [DOI] [PubMed] [Google Scholar]
- 5. Simpson C. D., Anyiwe K., Schimmer A. D. (2008) Anoikis resistance and tumor metastasis. Cancer Lett. 272, 177–185 [DOI] [PubMed] [Google Scholar]
- 6. Coates J. M., Galante J. M., Bold R. J. (2010) Cancer therapy beyond apoptosis: autophagy and anoikis as mechanisms of cell death. J. Surg. Res. 164, 301–308 [DOI] [PubMed] [Google Scholar]
- 7. Nüsslein-Volhard C., Wieschaus E. (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 [DOI] [PubMed] [Google Scholar]
- 8. Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 9. Kalderon D. (2000) Transducing the hedgehog signal. Cell 103, 371–374 [DOI] [PubMed] [Google Scholar]
- 10. Pasca di Magliano M., Hebrok M. (2003) Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 3, 903–911 [DOI] [PubMed] [Google Scholar]
- 11. Bhattacharya R., Kwon J., Ali B., Wang E., Patra S., Shridhar V., Mukherjee P. (2008) Role of hedgehog signaling in ovarian cancer. Clin. Cancer Res. 14, 7659–7666 [DOI] [PubMed] [Google Scholar]
- 12. Liao X., Siu M. K., Au C. W., Wong E. S., Chan H. Y., Ip P. P., Ngan H. Y., Cheung A. N. (2009) Aberrant activation of hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion, and differentiation. Carcinogenesis 30, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vestergaard J., Lind-Thomsen A., Pedersen M. W., Jarmer H. O., Bak M., Hasholt L., Tommerup N., Tümer Z., Larsen L. A. (2008) GLI1 is involved in cell cycle regulation and proliferation of NT2 embryonal carcinoma stem cells. DNA Cell Biol. 27, 251–256 [DOI] [PubMed] [Google Scholar]
- 14. Duman-Scheel M., Weng L., Xin S., Du W. (2002) Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature 417, 299–304 [DOI] [PubMed] [Google Scholar]
- 15. Hu W. G., Liu T., Xiong J. X., Wang C. Y. (2007) Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol. Sin. 28, 1224–1230 [DOI] [PubMed] [Google Scholar]
- 16. Yoo Y. A., Kang M. H., Lee H. J., Kim B. H., Park J. K., Kim H. K., Kim J. S., Oh S. C. (2011) Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 71, 7061–7070 [DOI] [PubMed] [Google Scholar]
- 17. Donahue J. K. (2006) Gene therapy, angiogenesis, sonic hedgehog: sonic the hedgehog to the rescue? Gene Ther. 13, 998–999 [DOI] [PubMed] [Google Scholar]
- 18. Ruiz i Altaba A. (2011) Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci. Signal. 4, pt9. [DOI] [PubMed] [Google Scholar]
- 19. Siegel R., Ward E., Brawley O., Jemal A. (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 61, 212–236 [DOI] [PubMed] [Google Scholar]
- 20. Naora H., Montell D. J. (2005) Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat. Rev. Cancer 5, 355–366 [DOI] [PubMed] [Google Scholar]
- 21. Kyriazi S., Kaye S. B., deSouza N. M. (2010) Imaging ovarian cancer and peritoneal metastases: current and emerging techniques. Nat. Rev. Clin. Oncol. 7, 381–393 [DOI] [PubMed] [Google Scholar]
- 22. He X., Ota T., Liu P., Su C., Chien J., Shridhar V. (2010) Down-regulation of HtrA1 promotes resistance to anoikis and peritoneal dissemination of ovarian cancer cells. Cancer Res. 70, 3109–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sood A. K., Armaiz-Pena G. N., Halder J., Nick A. M., Stone R. L., Hu W., Carroll A. R., Spannuth W. A., Deavers M. T., Allen J. K., Han L. Y., Kamat A. A., Shahzad M. M., McIntyre B. W., Diaz-Montero C. M., Jennings N. B., Lin Y. G., Merritt W. M., DeGeest K., Vivas-Mejia P. E., Lopez-Berestein G., Schaller M. D., Cole S. W., Lutgendorf S. K. (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Invest. 120, 1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho H. J., Park S. Y., Kim E. J., Kim J. K., Park J. H. (2011) 3,3′-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. Mol. Carcinog. 50, 100–112 [DOI] [PubMed] [Google Scholar]
- 25. Jin Y. (2011) 3,3′-Diindolylmethane inhibits breast cancer cell growth via miR-21-mediated Cdc25A degradation. Mol. Cell. Biochem. 358, 345–354 [DOI] [PubMed] [Google Scholar]
- 26. Bhatnagar N., Li X., Chen Y., Zhou X., Garrett S. H., Guo B. (2009) 3,3′-Diindolylmethane enhances the efficacy of butyrate in colon cancer prevention through down-regulation of survivin. Cancer Prevent. Res. 2, 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee S., Wang Z., Kong D., Sarkar F. H. (2009) 3,3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 69, 5592–5600 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Kandala P. K., Srivastava S. K. (2010) Activation of checkpoint kinase 2 by 3,3′-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells. Mol. Pharmacol. 78, 297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kandala P. K., Wright S. E., Srivastava S. K. (2012) Blocking epidermal growth factor receptor activation by 3,3′-diindolylmethane suppresses ovarian tumor growth in vitro and in vivo. J. Pharmacol. Exp. Ther. 341, 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandala P. K., Srivastava S. K. (2012) Regulation of macroautophagy in ovarian cancer cells in vitro and in vivo by controlling glucose regulatory protein 78 and AMPK. Oncotarget 3, 435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kandala P. K., Srivastava S. K. (2012) Regulation of Janus-activated kinase-2 (JAK2) by diindolylmethane in ovarian cancer in vitro and in vivo. Drug Discoveries Therapeutics 6, 94–101 [PubMed] [Google Scholar]
- 32. Kandala P. K., Srivastava S. K. (2012) Diindolylmethane suppresses ovarian cancer growth and potentiates the effect of cisplatin in tumor mouse model by targeting signal transducer and activator of transcription 3 (STAT3). BMC Med. 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lipinski R. J., Bijlsma M. F., Gipp J. J., Podhaizer D. J., Bushman W. (2008) Establishment and characterization of immortalized Gli-null mouse embryonic fibroblast cell lines. BMC Cell Biol. 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chien J., Aletti G., Baldi A., Catalano V., Muretto P., Keeney G. L., Kalli K. R., Staub J., Ehrmann M., Cliby W. A., Lee Y. K., Bible K. C., Hartmann L. C., Kaufmann S. H., Shridhar V. (2006) Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J. Clin. Invest. 116, 1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lui V. W., Yau D. M., Wong E. Y., Ng Y. K., Lau C. P., Ho Y., Chan J. P., Hong B., Ho K., Cheung C. S., Tsang C. M., Tsao S. W., Chan A. T. (2009) Cucurbitacin I elicits anoikis sensitization, inhibits cellular invasion and in vivo tumor formation ability of nasopharyngeal carcinoma cells. Carcinogenesis 30, 2085–2094 [DOI] [PubMed] [Google Scholar]
- 36. Mawji I. A., Simpson C. D., Hurren R., Gronda M., Williams M. A., Filmus J., Jonkman J., Da Costa R. S., Wilson B. C., Thomas M. P., Reed J. C., Glinsky G. V., Schimmer A. D. (2007) Critical role for Fas-associated death domain-like interleukin-1-converting enzyme-like inhibitory protein in anoikis resistance and distant tumor formation. J.N.C.I. 99, 811–822 [DOI] [PubMed] [Google Scholar]
- 37. Boreddy S. R., Pramanik K. C., Srivastava S. K. (2011) Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin. Cancer Res. 17, 1784–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Porter J. A., Young K. E., Beachy P. A. (1996) Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–259 [DOI] [PubMed] [Google Scholar]
- 39. Incardona J. P., Eaton S. (2000) Cholesterol in signal transduction. Curr. Opin. Cell Biol. 12, 193–203 [DOI] [PubMed] [Google Scholar]
- 40. Eccles S. A., Welch D. R. (2007) Metastasis: recent discoveries and novel treatment strategies. Lancet 369, 1742–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buck R. C. (1973) Walker 256 tumor implantation in normal and injured peritoneum studied by electron microscopy, scanning electron microscopy, and autoradiography. Cancer Res. 33, 3181–3188 [PubMed] [Google Scholar]
- 42. Chen X., Horiuchi A., Kikuchi N., Osada R., Yoshida J., Shiozawa T., Konishi I. (2007) Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: its inhibition leads to growth suppression and apoptosis. Cancer Sci. 98, 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaur M., Agarwal R., Agarwal C. (2006) Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol. Cancer Ther. 5, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 44. Chen X. L., Cao L. Q., She M. R., Wang Q., Huang X. H., Fu X. H. (2008) Gli-1 siRNA induced apoptosis in Huh7 cells. World J. Gastroenterol. 14, 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Banerjee S., Kong D., Wang Z., Bao B., Hillman G. G., Sarkar F. H. (2011) Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): from bench to clinic. Mutat. Res. 728, 47–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elamin M. H., Shinwari Z., Hendrayani S. F., Al-Hindi H., Al-Shail E., Khafaga Y., Al-Kofide A., Aboussekhra A. (2010) Curcumin inhibits the sonic hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol. Carcinog. 49, 302–314 [DOI] [PubMed] [Google Scholar]
- 47. Slusarz A., Shenouda N. S., Sakla M. S., Drenkhahn S. K., Narula A. S., MacDonald R. S., Besch-Williford C. L., Lubahn D. B. (2010) Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 70, 3382–3390 [DOI] [PubMed] [Google Scholar]
- 48. Low J. A., de Sauvage F. J. (2010) Clinical experience with hedgehog pathway inhibitors. J. Clin. Oncol. 28, 5321–5326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.