Background: APC promotes β-catenin phosphorylation and degradation.
Results: APC is reversibly modified with K63-linked ubiquitin chains that are assembled or disassembled in concert with the assembly and disassembly of the β-catenin destruction complex.
Conclusion: Atypical ubiquitin chains are involved in β-catenin turnover.
Significance: This posttranslational modification on APC could lead to an understanding of its precise role in β-catenin degradation.
Keywords: Signal Transduction, Tumor Suppressor Gene, Ubiquitin, Ubiquitylation, Wnt Signaling, β-catenin Destruction Complex, APC, Axin, K63-linked Ubiquitin Chains
Abstract
The adenomatous polyposis coli (APC) tumor suppressor forms a complex with Axin and GSK3β to promote the phosphorylation and degradation of β-catenin, a key co-activator of Wnt-induced transcription. Here, we establish that APC is modified predominantly with K63-linked ubiquitin chains when it is bound to Axin in unstimulated HEK293 cells. Wnt3a stimulation induced a time-dependent loss of K63-polyubiquitin adducts from APC, an effect synchronous with the dissociation of Axin from APC and the stabilization of cytosolic β-catenin. RNAi-mediated depletion of Axin or β-catenin, which negated the association between APC and Axin, resulted in the absence of K63-adducts on APC. Overexpression of wild-type and phosphodegron-mutant β-catenin, combined with analysis of thirteen human cancer cell lines that harbor oncogenic mutations in APC, Axin, or β-catenin, support the hypothesis that a fully assembled APC-Axin-GSK3β-phospho-β-catenin complex is necessary for the K63-polyubiquitylation of APC. Intriguingly, the degree of this modification on APC appears to correlate inversely with the levels of β-catenin in cells. Together, our results indicate that K63-linked polyubiquitin adducts on APC regulate the assembly and/or efficiency of the β-catenin destruction complex.
Introduction
The APC3 tumor suppressor is a core negative component of the Wnt signaling pathway (1, 2). In unstimulated cells, APC associates with Axin and the kinases GSK3β and CK1 (the β-catenin destruction complex) to foster the phosphorylation of cytosolic β-catenin at specific serine/threonine residues creating a phosphodegron motif. Recognition of this phosphodegron by the SCF-β-TrCP ubiquitin ligase complex leads to the modification of β-catenin with degradative polyubiquitin, culminating in its proteasomal destruction (3–8). Inactivation of the β-catenin destruction complex occurs upon Wnt ligand binding to Frizzled and LRP5/6 transmembrane receptors at the cell surface, and involves the formation of Dishevelled (Dvl) protein assemblies and the recruitment of Axin-GSK3β to LRP6 signalosomes (9–11). The accumulation of β-catenin in the nucleus following Wnt stimulation allows it to bind and co-activate the TCF/LEF family of transcription factors thus mediating the expression of Wnt target genes (12).
Aberrant activation of the Wnt signaling pathway resulting in the accumulation and constitutive transcriptional activity of β-catenin is a recurring theme in tumorigenesis (13). The tumor suppressor activities of APC and Axin reside in their common ability to downregulate β-catenin (5, 14). Both proteins contain binding sites for β-catenin, but only Axin has dedicated regions for binding GSK3β and CK1. Thus, Axin functions as a scaffold for the recruitment of these kinases to catalyze essential phosphorylations of APC and β-catenin in the destruction complex (5, 15–20). That Axin does not require its interaction with APC to scaffold the phosphorylation of β-catenin (5, 16, 21) raises questions as to the precise function of APC in β-catenin degradation.
Genetic mutations that lead to the expression of a truncated APC polypeptide can be found in 85% of human colorectal cancers, and the majority of these mutant APC proteins have lost motifs required for Axin binding (13). The importance of this interaction is consistent with APC proposed role as an essential cofactor for the Axin-mediated assembly of a functional β-catenin destruction complex in Drosophila and human cells (22). Biochemical and structural studies have shown that the phosphorylation of APC enhances its affinity for β-catenin. This allows APC to compete with Axin for β-catenin and could be a mechanism that enables the removal of phosphorylated β-catenin from Axin for downstream processing (3, 18, 20, 23, 24). Compatible with this model is the demonstration that APC protects the β-catenin phosphodegron from the activity of protein phosphatase PP2A (25). Thus, APC might chaperone the interaction between phosphorylated β-catenin and β-TrCP to promote the ubiquitylation and proteasomal destruction of β-catenin. Indeed, constitutively high levels of β-catenin in APC-mutant cancer cells could be linked to the lack of this protective function of truncated APC proteins (25).
Ubiquitin adducts linked through lysine 48 (K48) of ubiquitin target protein substrates for proteasomal degradation (26). K48-linked adducts controls not only the stability of β-catenin, but also that of several Wnt pathway components including Axin, Dvl, and APC (27–30). Interestingly, Dvl and APC are also modified with K63-linked ubiquitin chains, as inferred from loss-of-function studies of the deubiquitylating enzymes CYLD and Trabid, respectively (31, 32). Distinct both structurally and functionally from K48-linked polyubiquitin, K63-polyubiquitin adducts are known to regulate the activity of proteins that function in DNA repair and NF-κB signaling, possibly by serving as platforms for the assembly of multi-protein complexes (33–35). Intriguingly, K63-polyubiquitin adducts on Dvl correlates with activation of Wnt signaling, whereas the same modification on APC correlates with repression of the pathway (31, 32). We therefore sought to characterize in depth the role of K63-linked ubiquitin chains in Wnt signaling, and in particular the possibility that these adducts are important for APC function.
EXPERIMENTAL PROCEDURES
Cells and Reagents
HEK293, HCT116, and 624mel cells were maintained in DMEM medium supplemented with 10% FBS and 2 mm l-glutamine (Invitrogen). Colo320, SW403, SW480, DLD1, Colo205, SW48, Ls174T, A427, SNU423, SNU475, RKO, Colo741, A549, A431, Kelly, and SJCRH30 cells were maintained in RPMI 1640 medium (Sigma) supplemented with 10% FBS and 2 mm l-glutamine. HepG2 and HeLa cells were maintained in EMEM medium (ATCC) supplemented with 10% FBS and 2 mm l-glutamine. HEK293 cells stably expressing Myc-hAxin1 was a generous gift from Bonnee Rubinfeld. Cells were grown at 37 °C with 5% CO2. HEK293 cells were transfected with siRNAs (50 nm total) using RNAiMAX or plasmid DNA using Lipofectamine 2000 as instructed (Invitrogen). Fugene 6 HD (Roche) was used for plasmid DNA transfection of HCT116 cells. Recombinant mouse Wnt3a/Wnt5a was purchased from R&D Systems, GSK3 inhibitor IX (BIO) from Calbiochem and MG132 from Tocris Bioscience.
Antibodies
Polyubiquitin linkage-specific antibodies have been described (36, 37). APC antibody clone ALi 12–28 (Santa Cruz Biotechnology) was used for immunoprecipitation and Western blotting unless indicated otherwise. Other commercial antibodies: anti-APC H-290 (Santa Cruz Biotechnology), anti-β-catenin, anti-GSK3 4G1E (Millipore), anti-Axin1 C95H11, anti-β-TrCP D13F10, anti-ubiquitin P4D1, anti-Dvl2 (Cell Signaling), anti-β-actin-HRP AC15 (Sigma), and anti-Myc 9E10 (Stratagene).
Immunoprecipitation with Polyubiquitin Linkage-specific Antibodies
The procedure was performed on ice or at 4 °C unless indicated otherwise. Cells in 100 mm poly-d-lysine-coated dishes (BD Biosciences) were washed twice with ice-cold PBS, and lysed with Nonidet P-40 lysis buffer (Nonidet P-40 LB: 50 mm Tris-HCl, pH 7.5, 120 mm NaCl, 1% Nonidet P-40, 1 mm EDTA) containing 6 m urea and complete protease inhibitor mixture tablets (Roche, 1 tablet per 10 ml of lysis buffer). Depending on degree of confluency, 0.6 to 1.0 ml of lysis buffer was added to each dish of cells. After centrifugation at 14,000 rpm for 10 min, the supernatant was transferred to a fresh tube and assayed for total protein (BCA protein assay, Pierce). Typically, 700 μg of total protein per sample was diluted to 3 m urea with an equal volume of Nonidet P-40 LB, then pre-cleared with 100 μl of protein G-Sepharose slurry (Invitrogen, washed and resuspended in Nonidet P-40 LB at 50% v/v) and 4 μg of anti-E25 (humanized IgG1 mAb, Xolair, Genentech) for 1 h with constant rotation. Pre-cleared lysates were immunoprecipitated with 4 μg of anti-E25 (IgG isotype-matched control) or 4 μg of the indicated polyubiquitin linkage-specific antibodies overnight with constant rotation. Antibody-antigen complexes were captured with 40 μl of protein-G-Sepharose slurry for 1 h. Beads were washed twice with Nonidet P-40 LB (without urea) and once with PBS (1 ml, 10 min per wash), then eluted with 25 μl of 2× LDS sample buffer (Invitrogen) at 65 °C for 5 min. Samples were resolved on 3–8% Tris-acetate NuPAGE gels and transferred to nitrocellulose membranes (Invitrogen). Co-immunoprecipitation of endogenous protein complexes was similarly performed, except that urea was omitted from the lysis buffer. Western blotting was performed according to standard procedures. SuperSignal West Pico chemiluminescent substrate (Pierce) was used to detect polyubiquitylated APC.
siRNAs
All are ON-TARGETplus individual siRNA duplexes purchased from Dharmacon. Control siRNA: non-targeting siRNA #1 (Cat No. D-001810-01-20). APC (only sense strand shown): GACAAGAGCUAGAAGAUAA; α-catenin: GGGCAAUGCUGGACGUAAA; β-catenin: UAAUGAGGACCUAUACUUA; γ-catenin: GCAACAACAGCAAGAACAA; β-TrCP: AGAUAAUACCAGAGAAGAA; Axin1: CGGGAAAGGUGUUGGCAUU; Axin2: GCGAUCUACAAAAGGUACA; GSK3α: GGACAAAGGUGUUCAAAUC; GSK3β: AGAAAGUAUUGCAGGACAA.
RESULTS
APC Is Modified Predominantly with K63-linked Polyubiquitin
To validate our previous finding that endogenous APC might be modified with polyubiquitin chains (31), we employed recently described polyubiquitin linkage-specific antibodies recognizing K11-, K48-, and K63-linked ubiquitin chains (36, 37). Immunoprecipitation of urea lysates from proteasome inhibitor (MG132)-treated HEK293 cells, and immunoblotting specifically for APC, revealed that APC is modified with polyubiquitin of all linkage types (Fig. 1A). Notably, the strongest signal for the APC smear was observed from the anti-K63 polyubiquitin immunoprecipitate, suggesting that APC is conjugated predominantly with K63-linked ubiquitin chains. This was supported by immunoblotting for total ubiquitin, which showed that the pool of K63-linked polyubiquitin adducts are the least abundant of the three examined, consistent with the relative abundance of K63-linked chains in the total cellular polyubiquitin pool as analyzed by quantitative mass spectrometry (38). These results indicate that endogenous APC is modified primarily with K63-linked ubiquitin chains.
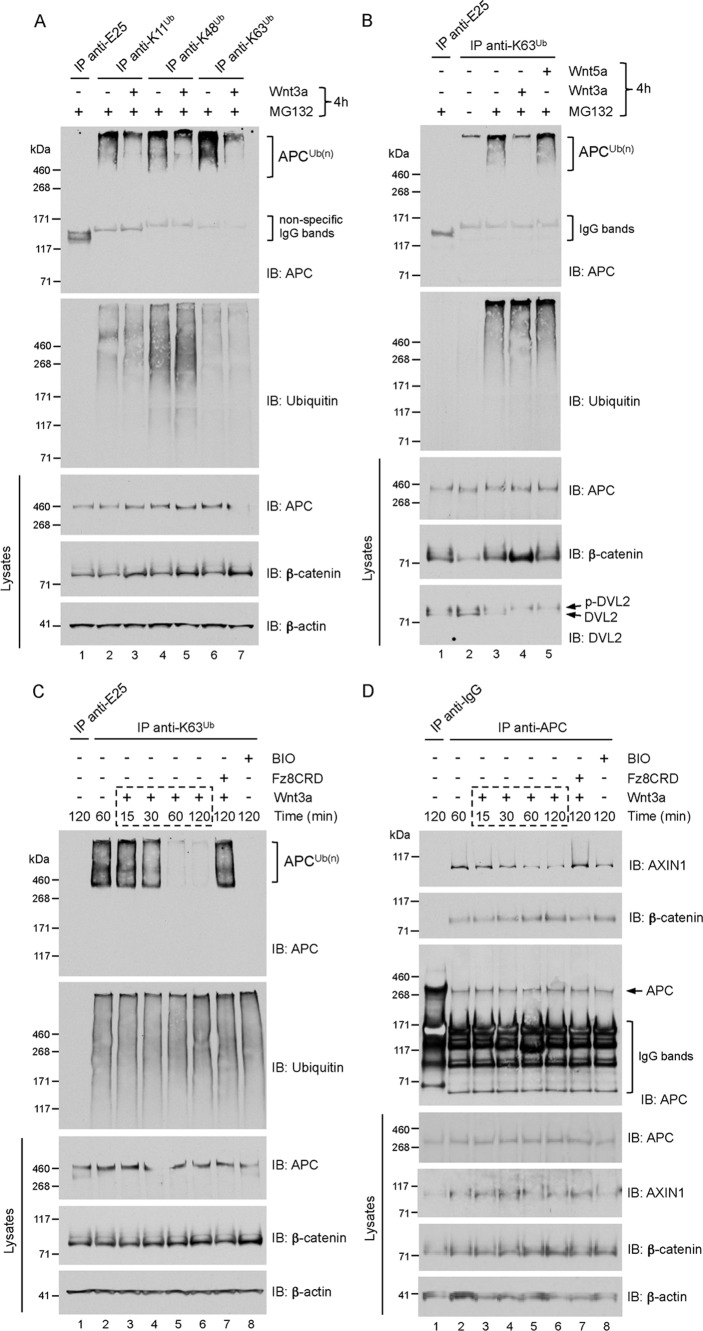
FIGURE 1.
A, APC is modified predominantly with K63-linked polyubiquitin. HEK293 cells were treated with 10 μm MG132 together with vehicle (PBS + 0.2% BSA, −) or recombinant mouse Wnt3a (200 ng/ml) for 4 h. Lysates were immunoprecipitated (IP) with the indicated polyubiquitin linkage-specific antibodies or an isotype-matched control antibody (anti-E25). Immunoprecipitates and lysates were analyzed by immunoblot (IB) as indicated. High-molecular-weight smears (APCUb(n)) indicate polyubiquitylated species of APC, and the absence of this smear in the immunoprecipitate from APC-depleted cells (see Fig. 3A) validates the specificity of the APC antibody (clone ALi 12–28) used throughout this study. B, Wnt3a, but not Wnt5a, inhibits the formation of K63-polyubiquitin adducts on APC. HEK293 cells were treated with vehicle (DMSO, −) or 10 μm MG132 together with Wnt3a or Wnt5a (200 ng/ml) for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies or anti-E25 control, and analyzed by immunoblot as indicated. C, Wnt3a induced a time-dependent loss of K63-polyubiquitin adducts from APC. HEK293 cells were pre-treated with 10 μm MG132 for 2 h, followed by treatment with vehicle (PBS + 0.2% BSA, −) or with Wnt3a (200 ng/ml), Fz8CRD-hFc (5 μg/ml), or BIO (GSK3 inhibitor, 5 μm) for the indicated times. Lysates were immunoprecipitated as in B and analyzed by immunoblot as indicated. D, Wnt3a induced a time-dependent decrease of APC-Axin association. HEK293 cells were treated as described in C. Lysates were immunoprecipitated with anti-APC or control anti-mouse IgG, and analyzed by immunoblot as indicated.
Wnt3a Inhibits the K63 Polyubiquitylation of APC
Because accumulation of polyubiquitin on APC correlated with a loss of Wnt signaling in cells (31), we asked if Wnt stimulation could affect the ubiquitylation status of APC. Stimulation of cells with Wnt3a significantly inhibited the formation of ubiquitin chains of all linkage types on APC, whereas the total pools of K11-, K48-, and K63-adducts were unaffected (Fig. 1A).
Because K63-polyubiquitin appears to represent the predominant adduct on APC, we chose to focus specifically on this modification. To demonstrate specificity for canonical Wnt signaling, we treated cells with Wnt5a followed by immunoprecipitation with K63 polyubiquitin linkage-specific antibodies. In contrast to Wnt3a, the non-canonical ligand Wnt5a, which does not stabilize β-catenin in HEK293 cells, did not induce loss of K63-adducts from APC (Fig. 1B). The use of MG132 enabled robust immunoprecipitation of polyubiquitin adducts from cells, suggesting dynamic turnover of ubiquitin chains, directly or indirectly, by the proteasome. Importantly, MG132 did not result in significant stabilization of total APC levels nor interfered with the Wnt3a-mediated stabilization of β-catenin. The time-dependent loss of K63-adducts from APC following Wnt3a treatment was evaluated, and we found significant loss occurring 60 min after Wnt stimulation (Fig. 1C). As a further test of specificity, we treated cells with both Wnt3a and the soluble Wnt antagonist Frizzled8CRD-hFc (39). This resulted in a complete block of the Wnt3a-induced loss of APC polyubiquitylation. We conclude that the modification of APC with K63-linked polyubiquitin is regulated by canonical Wnt signaling.
K63 Polyubiquitylation of APC Coincides with APC-Axin Association
A downstream effect of Wnt3a stimulation is the inhibition of GSK3β activity, which abrogates the phosphorylation of β-catenin, Axin, and APC (3, 16, 40, 62). To test the possibility that GSK3β activity is required for APC polyubiquitylation, we treated cells with a selective inhibitor of GSK3 (BIO). This treatment effectively eliminated K63-linked ubiquitin chain formation on APC (Fig. 1C). However, it is likely that BIO-treatment also negated the phosphorylation of Axin and β-catenin, which could have negative effects on APC polyubiquitylation (see below).
Phosphorylation of APC has been shown to be dependent upon APC association with Axin, which binds GSK3β (16, 18, 41). Therefore, we asked whether Wnt3a induces a dissociation of Axin from APC, and if so, whether this correlated with the loss of APC polyubiquitylation. We performed co-immunoprecipitation from Wnt3a-stimulated HEK293 cells, as described in experimental conditions for Fig. 1C, except that cells were lysed in the absence of urea to preserve protein-protein interactions. Immunoprecipitation of endogenous APC from these lysates revealed a time-dependent decrease of co-precipitated Axin (Fig. 1D). Significant disruption of the APC-Axin interaction occurred 60 min following Wnt3a treatment, coinciding with the loss of APC polyubiquitylation at this time point (Fig. 1C). We noted that complete disruption of the APC-Axin interaction was not observed, even in BIO-treated cells where the polyubiquitylation of APC was abolished. This suggests the possibility of more than one pool of APC-Axin complexes, and it is conceivable that a phosphorylated and polyubiquitylated APC-Axin pool represents the functional complex that targets β-catenin for degradation. Accordingly, the phosphorylation-dependent distribution of APC between different functional pools has been proposed (42), and the increased association between β-catenin and APC following Wnt3a stimulation (Fig. 1D) could represent an unphosphorylated APC-β-catenin complex that is targeted to peripheral membrane pools (3, 43–47). Notwithstanding, these results show that canonical Wnt pathway activation leads to the dissociation of Axin from APC, and indicate that K63-polyubiquitin adducts on APC coincide with APC-Axin association.
Wnt3a-mediated Loss of K63-polyubiquitin Adducts from APC Requires Its Dissociation from Axin
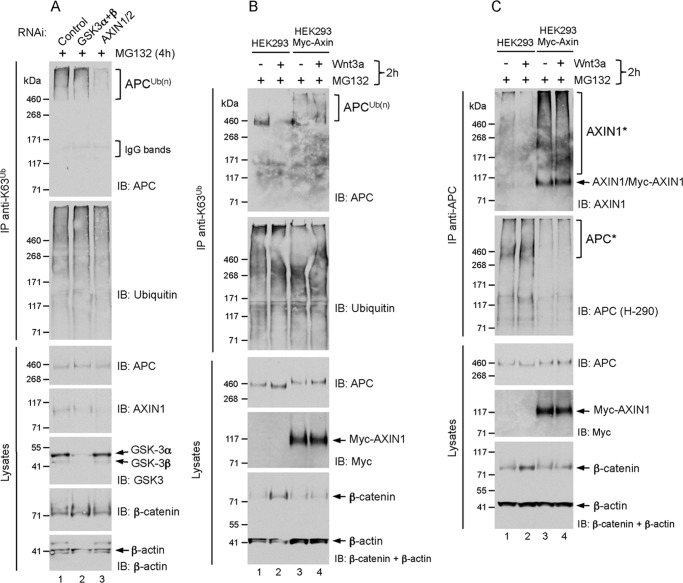
The results above predict that in the absence of Wnt stimulation, experimental disruption of the APC-Axin association might result in loss of K63-adducts from APC. We thus depleted Axin (both 1 and 2) by RNAi, and found that this resulted in the loss of K63-polyubiquitin from APC in unstimulated HEK293 cells (Fig. 2A). Although we achieved efficient knockdown of both GSK3 isoforms-α and -β (Fig. 2A), this did not lead to significant inhibition of APC polyubiquitylation as would be expected from the strong negative effect on this modification following GSK3 inhibitor treatment (Fig. 1C). Since Axin is a limiting component (48) and only 3–5% of total GSK3β is associated with Axin (49), it is conceivable that the small amount of GSK3β remaining following siRNA knockdown is sufficient for Axin-dependent phosphorylation of APC in the destruction complex, leading to its polyubiquitylation.
FIGURE 2.
A, RNAi-mediated depletion of Axin leads to loss of K63-polyubiquitin-modified APC. HEK293 cells were transfected with siRNA for 72 h as indicated, followed by treatment with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies, and analyzed by immunoblot as indicated. B and C, Wnt3a-induced loss of APC K63-adducts requires APC-Axin dissociation. HEK293 and HEK293-Myc-Axin1 cells were treated with 10 μm MG132 together with vehicle (−) or Wnt3a (200 ng/ml) for 2 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies (B) or anti-APC (C) and analyzed by immunoblot as indicated.
The requirement of Axin for APC polyubiquitylation suggested that its overexpression might enhance this modification on APC. To address this, we examined the polyubiquitylation of endogenous APC in HEK293 cells stably expressing Myc-tagged Axin1. Compared with unstimulated parental HEK293 cells, higher levels of K63-polyubiquitylated APC in unstimulated Myc-Axin-expressing cells were detected (Fig. 2B). Notably, in contrast to parental cells, the Wnt3a-mediated loss of APC polyubiquitylation and stabilization of β-catenin in the Myc-Axin cells was blocked (Fig. 2B).
Since APC polyubiquitylation correlates with APC-Axin association, we asked whether the polyubiquitin-modified pool of endogenous APC in Wnt-stimulated, Axin-overexpressing cells was a result of its failure to dissociate from Myc-Axin. Immunoprecipitation of endogenous APC from Myc-Axin cells revealed that it co-precipitated Myc-Axin in unstimulated cells, but also equally in cells stimulated with Wnt3a. In contrast, we found reduced association between APC and endogenous Axin in Wnt3a-stimulated parental cells (Fig. 2C, see also Fig. 1D). We suspect that the high-molecular-weight smears of co-precipitated endogenous and Myc-Axin (AXIN1*), could represent PARsylated/K48-polyubiquitylated populations that have accumulated in MG132-treated cells (28, 30). The total precipitated APC pool also resolved with a retarded electrophoretic mobility (APC*), as revealed by immunoblotting with a rabbit polyclonal APC antibody (clone H-290), but the reason for this is unclear (Fig. 2C). We noted that the ability to immunoprecipitate endogenous APC from Myc-Axin cells was significantly reduced, and believe the strong expression levels of Myc-Axin might lead to the formation of puncta that efficiently and stably titrate a large fraction of endogenous APC (21). This could account for the lack of dissociation between APC and Myc-Axin following Wnt-stimulation in these cells. Nevertheless, these results suggest that the loss of K63-polyubiquitin adducts from APC requires its dissociation from Axin.
β-Catenin Is Required for APC K63 Polyubiquitylation
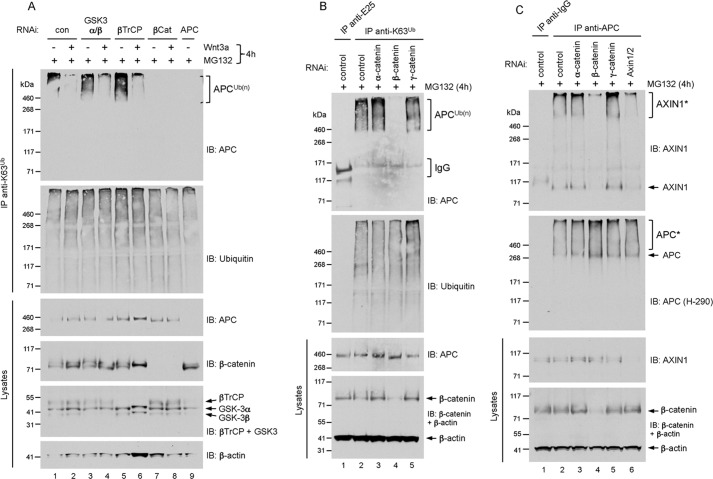
Because β-catenin is a component of the destruction complex, we tested its requirement for APC-Axin association and APC polyubiquitylation. RNAi-mediated depletion of β-catenin in HEK293 cells abolished the formation of K63-adducts on APC, whereas knockdown of β-TrCP, α-catenin, and γ-catenin did not inhibit APC polyubiquitylation (Fig. 3, A and B). We asked whether loss of APC K63-adducts correlated with loss of APC-Axin association in β-catenin-depleted cells. By co-immunoprecipitation, we discovered that knockdown of β-catenin, but not α- or γ-catenin, resulted in significant dissociation between APC and Axin (Fig. 3C). These results underscore the positive correlation between APC polyubiquitylation and APC-Axin association, and indicate that β-catenin stimulates the APC-Axin interaction and the polyubiquitylation of APC.
FIGURE 3.
A, RNAi-mediated depletion of β-catenin leads to loss of K63-polyubiquitin-modified APC. HEK293 cells were transfected with the indicated siRNAs for 72 h, followed by treatment with 10 μm MG132 together with vehicle (−) or Wnt3a (200 ng/ml) for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies, and analyzed by immunoblot as indicated. B, HEK293 cells were transfected with siRNA for 72 h as indicated, followed by treatment with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies, and analyzed by immunoblot as indicated. C, RNAi-mediated depletion of β-catenin leads to loss of APC-Axin interaction. HEK293 cells were transfected with the indicated siRNAs for 72 h, followed by treatment with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with anti-APC and analyzed by immunoblot as indicated.
Absence of APC K63 Polyubiquitylation in Cancer Cells Harboring Oncogenic Mutations in APC, Axin, and β-Catenin
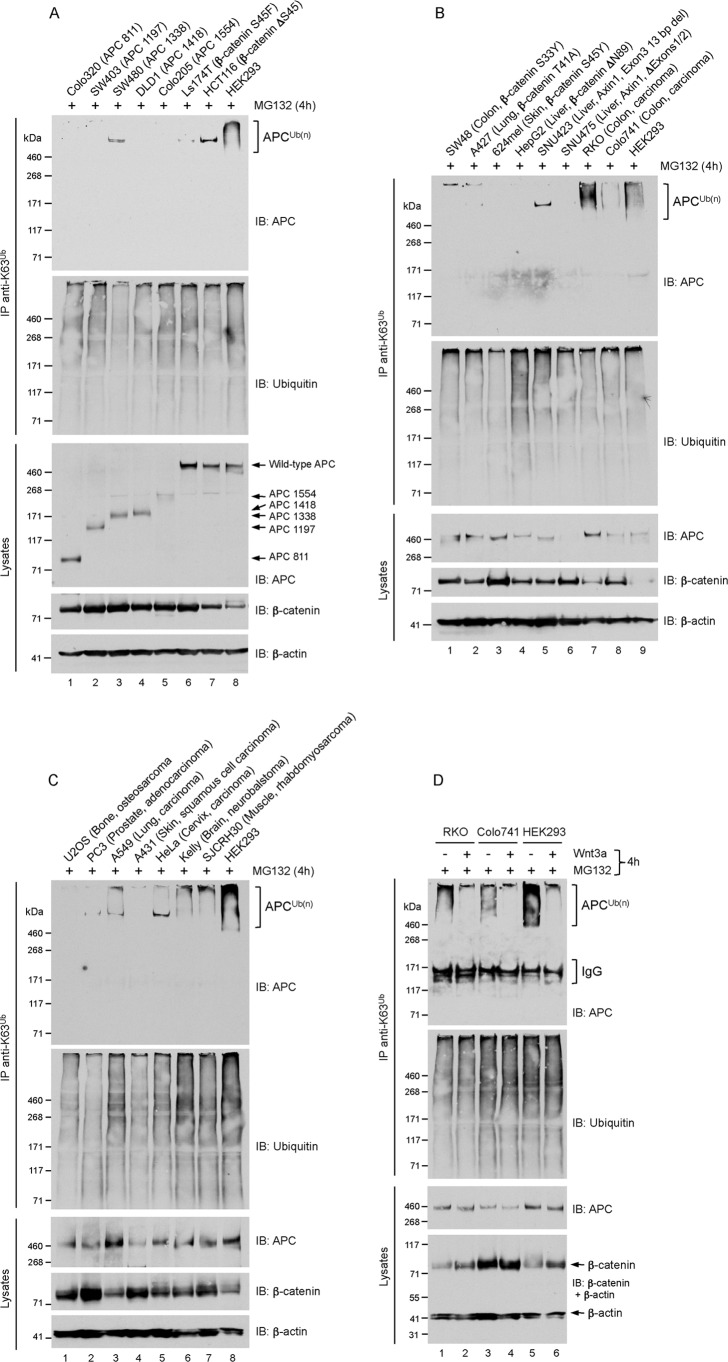
The evidence so far suggests that the modification of APC with K63-adducts coincide with the assembly of a functional β-catenin destruction complex. To consolidate this idea, we examined APC polyubiquitylation in cancer cells that harbor inactivating/activating mutations of APC, Axin, or β-catenin. Utilizing the anti-K63-polyubiquitin immunoprecipitation assay, we first examined five colorectal cancer cell lines that express truncated APC proteins at various positions between residues 811 (Colo320) and 1554 (Colo205). We found that none of these truncated APC proteins were conjugated with K63-linked adducts (Fig. 4A). That these mutants all lack the SAMP repeats required for Axin binding (50, 51) further support the proposition that APC-Axin association is required for APC polyubiquitylation. However, it is possible that APC is modified with K63-adducts at a lysine acceptor site(s) C-terminal to residue 1554, which would preclude detection of ubiquitylated forms of mutant APC in these cells, irrespective of their ability to bind Axin.
FIGURE 4.
A, B, and C, cancer cells harboring oncogenic mutations in APC, Axin and β-catenin lack K63-polyubiquitin-modified APC. Cell lines with known mutation in APC, Axin or β-catenin are indicated. Subconfluent cells were treated with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies, and analyzed by immunoblot as indicated. The single high-molecular-weight band detected prominently in some cell lines, including SW480 and HCT116 cells, was not observed in other experiments and is likely nonspecific (see Fig. 5A). D, cells were treated as described in Fig. 1A. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies and analyzed by immunoblot as indicated.
Because β-catenin depletion resulted in the striking loss of K63-adducts from APC (Fig. 3), we pondered whether cancer cells expressing stable mutant forms of β-catenin might show increased levels of this modification on APC. Surprisingly, of the six β-catenin mutant (serine/threonine phosphodegron substitutions or deletions (52)) cancer cell lines examined, none contained K63-polyubiquitin-modified APC, a population reproducibly detected in HEK293 cells (Fig. 4, A and B). We noted that total levels of wild-type APC in these cells were comparable to HEK293 levels. These results imply that the K63-polyubiquitylation of APC requires wild-type β-catenin.
To support prior evidence indicating a requirement of Axin and GSK3β activity for APC polyubiquitylation (Figs. 1 and 2), we monitored this modification of APC in SNU423 cells, which expresses a GSK3β-binding-deficient mutant of Axin1 or in SNU475 cells that does not express Axin1 (53). As anticipated, we did not detect APC K63-adducts in these cells (Fig. 4B). However, the lack of APC expression in SNU475 cells (for reasons unknown) does not permit us to draw any conclusions about the requirement of Axin1 for APC polyubiquitylation in these cells. Nevertheless, the absence of APC K63-adducts in SNU423 cells support the notion that modification of APC with K63-polyubiquitin requires the formation of an APC-Axin-GSK3β complex.
We also examined APC K63 polyubiquitylation in nine additional cancer cell lines without known mutations in APC, Axin, or β-catenin. These lines were derived from human tumors of different tissue origin, and in six, including RKO and Colo741 colorectal cancer cells the APC K63-polyubiquitylated pool was readily detected (Fig. 4, B and C). Consistent with observations in HEK293 cells, stimulation of RKO and Colo741 cells with Wnt3a inhibited the K63 polyubiquitylation of APC, and led to β-catenin stabilization in RKO cells demonstrating intact Wnt pathway activity (Fig. 4D). The high levels of β-catenin in Colo741 and many of the cancer cell lines (without mutations in Wnt components) suggest unusually high Wnt pathway activity, perhaps through autocrine overexpression of Wnt ligands and receptors (54). Regardless of the mechanism, we recognize a general inverse relationship between β-catenin levels and the degree of APC polyubiquitylation in these cells. This supports the notion that APC K63-adducts controls the efficiency of β-catenin degradation. Thus, monitoring the K63 polyubiquitylation of APC could have diagnostic utility in terms of evaluating Wnt pathway activity in cells that do not harbor mutations in Wnt pathway components.
Phosphorylated β-Catenin Is Required for APC K63 Polyubiquitylation
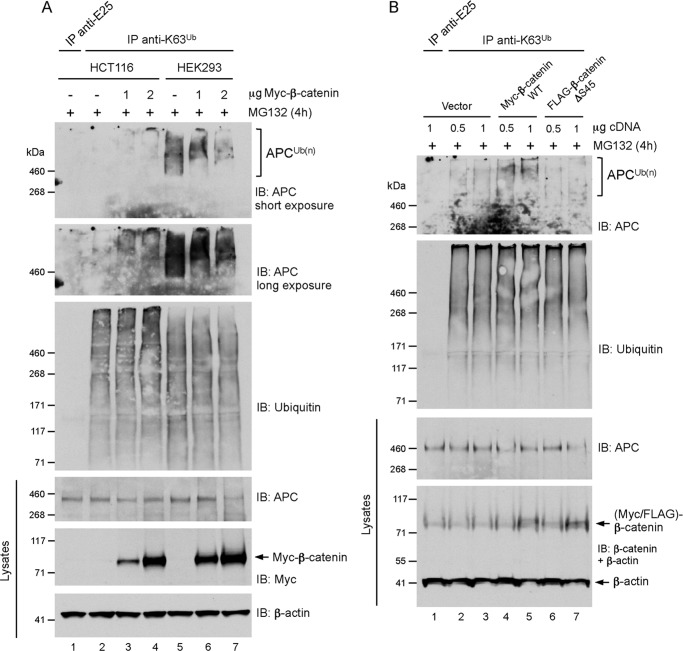
The absence of APC K63 polyubiquitylation in cancer cells expressing high levels of mutant β-catenin (Fig. 4) is intriguing considering that knockdown of β-catenin produced the same negative effect (Fig. 3). We postulated that the excess, non-phophorylatable β-catenin, for example in HCT116 cells (β-catenin ΔS45), might outcompete wild-type β-catenin in these cells for APC binding. This would prevent wild-type β-catenin from promoting the assembly of an APC-Axin-GSK3β complex to stimulate APC polyubiquitylation. We thus predicted that overexpression of wild-type β-catenin should restore APC polyubiquitylation in HCT116 cells. Ectopic expression of wild-type, Myc-tagged β-catenin at increasing levels partially restored the K63-polyubiquitylation of APC in HCT116 cells in a dose-dependent manner (Fig. 5A). It is possible that the ability to form a functional destruction complex in HCT116 cells is ineffective, explaining the weak effect. Overexpression of β-catenin in HEK293 cells also induced the formation of longer ubiquitin chains on a subpopulation of the polyubiquitin-modified APC pool, as indicated by the shift towards a higher-molecular-weight APC K63-polyubiquitylated smear (Fig. 5A). Finally, expression of wild-type, but not ΔS45-β-catenin, increased the K63-ubiquitylated APC pool in HEK293 cells (Fig. 5B), indicating that phosphorylation of β-catenin is important for the modification of APC with K63-adducts. Together, our evidence suggests that β-catenin regulates its own destruction by stimulating the assembly of an APC-Axin-GSK3β complex and the K63 polyubiquitylation of APC.
FIGURE 5.
A, ectopic expression of β-catenin increased the pool of K63-polyubiquitin-modified APC in HCT116 and HEK293 cells. Subconfluent cells were transfected with 2 μg of empty vector (−) or the indicated amounts of Myc-β-catenin expression vector for 48 h, followed by treatment with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies or anti-E25 control, and analyzed by immunoblot as indicated. In HEK293 cells transfected with 2 μg of Myc-β-catenin expression vector, total APC levels were significantly reduced for reasons that are unclear, but this likely explains the reduced intensity of the ubiquitylated APC smear (lane 7). B, ectopic expression of wild-type (WT), but not β-catenin-ΔS45, increased the pool of K63-polyubiquitin-modified APC in HEK293 cells. Subconfluent cells were transfected the indicated amounts of empty vector, Myc-β-catenin-WT or FLAG-β-catenin-ΔS45 expression vectors for 24 h, followed by treatment with 10 μm MG132 for 4 h. Lysates were immunoprecipitated with K63 polyubiquitin linkage-specific antibodies or anti-E25 control, and analyzed by immunoblot as indicated.
DISCUSSION
The precise role of APC in β-catenin degradation is not fully understood. The evidence presented here establishes K63-linked ubiquitin chains as an important regulatory and reversible posttranslational modification on APC. Our results suggest that these chains on APC are assembled or disassembled in concert with the assembly and disassembly of the β-catenin destruction complex. The analysis of APC K63 polyubiquitylation in 22 human cancer cell lines indicate that polyubiquitin-modified APC might enhance the efficiency of β-catenin degradation, implicating a new, non-scaffold function for APC in the β-catenin destruction complex.
The rapid disappearance of polyubiquitin from APC following Wnt3a stimulation (Fig. 1) suggested the involvement of a deubiquitylating enzyme (DUB) activity that can remove these chains from APC. We have previously characterized Trabid as a positive regulator of Wnt signaling and implicated this DUB in the cleavage of K63-adducts from APC (31). It was thus surprising to find, contrary to expectation, that depletion of Trabid did not block the Wnt3a-induced loss of these chains from APC (data not shown). However, this is consistent with the negligible effect of Trabid depletion on Wnt3a-induced β-catenin stabilization, and epistasis analysis that placed Trabid's function in Wnt signaling downstream of the β-catenin destruction complex (31). Moreover, although Trabid can cleave K63-linked ubiquitin chains, the increased polyubiquitylation of APC following Trabid depletion might reflect to a large degree the accumulation of K29- and/or K33-linked adducts, as these appear to be Trabid's preferred polyubiquitin substrates (55). Thus, either Wnt3a stimulation does not require DUB activity to remove K63-adducts from APC (inhibition of APC phosphorylation appears to suffice), or Wnt3a activated an unidentified DUB for the deubiquitylation of APC. It is conceivable that APC might be a substrate of Trabid in a different context, for example during serum-induced cell migration.4
The positive correlation between K63-adducts on APC and an assembled β-catenin destruction complex suggests the possibility that these chains might regulate the activity or assembly of this complex in unstimulated cells. That K63-polyubiquitin adducts can regulate the assembly of multi-protein complexes is not without precedent, as the conjugation of these chains to RIP or NEMO mediate the assembly of protein complexes that activate NF-κB-dependent signaling pathways (34, 35, 56). How might K63-linked chains on APC be required for assembly of the β-catenin destruction complex? One possibility is that Axin could harbor an uncharacterized domain that preferentially binds K63-linked polyubiquitin. This might enforce its interaction with polyubiquitylated APC to stabilize the destruction complex. This hypothesis is particularly intriguing since K63-adducts on APC and Dvl correlates with decreased or increased Wnt pathway activity, respectively (31, 32). Thus, in response to Wnt, a reciprocal loss and gain of K63-polyubiquitin on APC and Dvl, respectively, could shift the balance of Axin protein between these opposing effectors, leading to disruption of the APC-Axin interaction (Fig. 6, Interaction 1). Our observation that Wnt3a induced the dissociation between APC and Axin are consistent with recent findings (57) and suggests a new mechanism for inactivation of the β-catenin destruction complex.
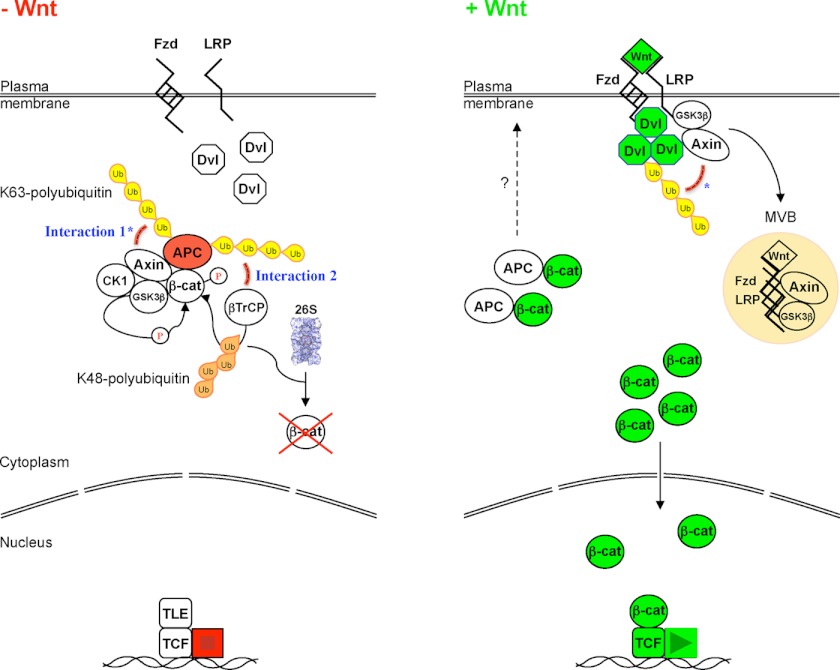
FIGURE 6.
Model. In unstimulated cells (−Wnt), APC associated with Axin in the β-catenin destruction complex is conjugated with K63-linked polyubiquitin. This modification could either enforce APC's interaction with Axin (Interaction 1*) or facilitate the recruitment of β-TrCP to phosphorylated β-catenin for its K48-ubiquitylation and 26S proteasomal degradation (Interaction 2). Following Wnt stimulation (+Wnt), a reciprocal loss and gain of K63-polyubiquitin on APC and Dvl, respectively, could facilitate the recruitment of Axin-GSK3β to Dvl/LRP signalosomes (*), and the signalosome complex may subsequently be internalized to multi-vesicular bodies (MVB) for processing (9, 32, 59, 60). Unphosphorylated β-catenin associated with APC might conceivably be targeted to peripheral membrane pools (3, 43–47), and the free β-catenin pool enters the nucleus to activate TCF-dependent transcription (61). Fzd, Frizzled; LRP, low-density lipoprotein receptor-related protein 5/6; TLE, transducin-like enhancer of split (transcriptional co-repressor).
As we explore the possibility that Axin might bind K63-linked polyubiquitin, we postulate alternative roles for the K63-adducts on APC. A recently ascribed function for APC in the destruction complex proposed that APC protects the β-catenin phosphodegron from the activity of protein phosphatase PP2A, thus enabling β-TrCP-mediated K48-ubiquitylation of β-catenin (25). This protective function of APC was dependent on its phosphorylation, a modification that appears to be coupled to its polyubiquitylation (Fig. 1), which led us to contemplate whether K63-linked chains on APC might contribute a protective role toward phosphorylated β-catenin in the destruction complex. The absence of these adducts on truncated APC proteins in SW480, DLD1, and HT29 colorectal cancer cells (Fig. 4A, data not shown), is consistent with this idea, as these APC mutants do not confer the protection of phosphorylated β-catenin from PP2A in these cells (25).
The requirement of β-catenin for APC-Axin association and APC polyubiquitylation was remarkable and unexpected (Fig. 3). This might indicate that β-catenin can promote the assembly of its own destruction complex. The finding that wild-type β-catenin (but not a non-phosphorylatable version) can stimulate the formation of K63-adducts on APC (Fig. 5) supports a model where phosphorylation of β-catenin in an assembled destruction complex promotes the phosphorylation and modification of APC with K63-polyubiquitin. This in turn fosters the recruitment of β-TrCP to bind phosphorylated β-catenin (Fig. 6, Interaction 2). Compatible with this hypothesis is the finding that β-catenin can stimulate the phosphorylation of APC by GSK3β in an Axin-dependent manner (41), and that β-TrCP co-immunoprecipitate efficiently only with a phosphorylated APC-β-catenin complex (7, 25, 58). However, our experiments have not allowed us to distinguish whether APC is conjugated with K63-polyubiquitin chains before or after assembly of the destruction complex. Understanding these sequence of events will help discriminate the importance of APC K63-adducts in either the assembly or efficiency of the β-catenin destruction complex. Identification of the E3 ubiquitin ligase responsible for catalyzing the formation of these atypical chains on APC should allow us to address this issue.
H. Tran and P. Polakis, unpublished data.
- APC
- adenomatous polyposis coli
- GSK
- glycogen synthase kinase.
REFERENCES
- 1. Polakis P. (1997) The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta 1332, F127–F147 [DOI] [PubMed] [Google Scholar]
- 2. Bienz M. (2002) The subcellular destinations of APC proteins. Nat. Rev. Mol. Cell Biol. 3, 328–338 [DOI] [PubMed] [Google Scholar]
- 3. Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272, 1023–1026 [DOI] [PubMed] [Google Scholar]
- 4. Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. (1997) β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16, 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behrens J., Jerchow B. A., Würtele M., Grimm J., Asbrand C., Wirtz R., Kühl M., Wedlich D., Birchmeier W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596–599 [DOI] [PubMed] [Google Scholar]
- 6. Winston J. T., Strack P., Beer-Romero P., Chu C. Y., Elledge S. J., Harper J. W. (1999) The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hart M., Concordet J. P., Lassot I., Albert I., del los Santos R., Durand H., Perret C., Rubinfeld B., Margottin F., Benarous R., Polakis P. (1999) The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–210 [DOI] [PubMed] [Google Scholar]
- 8. Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 9. Bilic J., Huang Y. L., Davidson G., Zimmermann T., Cruciat C. M., Bienz M., Niehrs C. (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619–1622 [DOI] [PubMed] [Google Scholar]
- 10. Schwarz-Romond T., Fiedler M., Shibata N., Butler P. J., Kikuchi A., Higuchi Y., Bienz M. (2007) The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14, 484–492 [DOI] [PubMed] [Google Scholar]
- 11. Fiedler M., Mendoza-Topaz C., Rutherford T. J., Mieszczanek J., Bienz M. (2011) Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc. Natl. Acad. Sci. U.S.A. 108, 1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosimann C., Hausmann G., Basler K. (2009) β-Catenin hits chromatin: regulation of Wnt target gene activation. Nat. Rev. Mol. Cell Biol. 10, 276–286 [DOI] [PubMed] [Google Scholar]
- 13. Polakis P. (2000) Wnt signaling and cancer. Genes Dev. 14, 1837–1851 [PubMed] [Google Scholar]
- 14. Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. (1995) Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 92, 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17, 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hart M. J., de los Santos R., Albert I. N., Rubinfeld B., Polakis P. (1998) Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr. Biol. 8, 573–581 [DOI] [PubMed] [Google Scholar]
- 17. von Kries J. P., Winbeck G., Asbrand C., Schwarz-Romond T., Sochnikova N., Dell'Oro A., Behrens J., Birchmeier W. (2000) Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat. Struct. Biol. 7, 800–807 [DOI] [PubMed] [Google Scholar]
- 18. Rubinfeld B., Tice D. A., Polakis P. (2001) Axin-dependent phosphorylation of the adenomatous polyposis coli protein mediated by casein kinase 1epsilon. J. Biol. Chem. 276, 39037–39045 [DOI] [PubMed] [Google Scholar]
- 19. Dajani R., Fraser E., Roe S. M., Yeo M., Good V. M., Thompson V., Dale T. C., Pearl L. H. (2003) Structural basis for recruitment of glycogen synthase kinase 3β to the axin-APC scaffold complex. EMBO J. 22, 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ha N. C., Tonozuka T., Stamos J. L., Choi H. J., Weis W. I. (2004) Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol. Cell 15, 511–521 [DOI] [PubMed] [Google Scholar]
- 21. Faux M. C., Coates J. L., Catimel B., Cody S., Clayton A. H., Layton M. J., Burgess A. W. (2008) Recruitment of adenomatous polyposis coli and β-catenin to axin-puncta. Oncogene 27, 5808–5820 [DOI] [PubMed] [Google Scholar]
- 22. Mendoza-Topaz C., Mieszczanek J., Bienz M. (2011) The Adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin's interaction with Dishevelled. Open. Biol. 1, 110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xing Y., Clements W. K., Kimelman D., Xu W. (2003) Crystal structure of a β-catenin/axin complex suggests a mechanism for the β-catenin destruction complex. Genes Dev. 17, 2753–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts D. M., Pronobis M. I., Poulton J. S., Waldmann J. D., Stephenson E. M., Hanna S., Peifer M. (2011) Deconstructing the ß-catenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol. Biol. Cell 22, 1845–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su Y., Fu C., Ishikawa S., Stella A., Kojima M., Shitoh K., Schreiber E. M., Day B. W., Liu B. (2008) APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol. Cell 32, 652–661 [DOI] [PubMed] [Google Scholar]
- 26. Weissman A. M., Shabek N., Ciechanover A. (2011) The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat. Rev. Mol. Cell Biol. 12, 605–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 28. Callow M. G., Tran H., Phu L., Lau T., Lee J., Sandoval W. N., Liu P. S., Bheddah S., Tao J., Lill J. R., Hongo J. A., Davis D., Kirkpatrick D. S., Polakis P., Costa M. (2011) Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One 6, e22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi J., Park S. Y., Costantini F., Jho E. H., Joo C. K. (2004) Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. J. Biol. Chem. 279, 49188–49198 [DOI] [PubMed] [Google Scholar]
- 30. Zhang Y., Liu S., Mickanin C., Feng Y., Charlat O., Michaud G. A., Schirle M., Shi X., Hild M., Bauer A., Myer V. E., Finan P. M., Porter J. A., Huang S. M., Cong F. (2011) RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nat. Cell Biol. 13, 623–629 [DOI] [PubMed] [Google Scholar]
- 31. Tran H., Hamada F., Schwarz-Romond T., Bienz M. (2008) Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 22, 528–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tauriello D. V., Haegebarth A., Kuper I., Edelmann M. J., Henraat M., Canninga-van Dijk M. R., Kessler B. M., Clevers H., Maurice M. M. (2010) Loss of the tumor suppressor CYLD enhances Wnt/β-catenin signaling through K63-linked ubiquitination of Dvl. Mol. Cell 37, 607–619 [DOI] [PubMed] [Google Scholar]
- 33. Hoege C., Pfander B., Moldovan G. L., Pyrowolakis G., Jentsch S. (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 [DOI] [PubMed] [Google Scholar]
- 34. Zhou H., Wertz I., O'Rourke K., Ultsch M., Seshagiri S., Eby M., Xiao W., Dixit V. M. (2004) Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 [DOI] [PubMed] [Google Scholar]
- 35. Chen Z. J. (2012) Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Newton K., Matsumoto M. L., Wertz I. E., Kirkpatrick D. S., Lill J. R., Tan J., Dugger D., Gordon N., Sidhu S. S., Fellouse F. A., Komuves L., French D. M., Ferrando R. E., Lam C., Compaan D., Yu C., Bosanac I., Hymowitz S. G., Kelley R. F., Dixit V. M. (2008) Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell 134, 668–678 [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto M. L., Wickliffe K. E., Dong K. C., Yu C., Bosanac I., Bustos D., Phu L., Kirkpatrick D. S., Hymowitz S. G., Rape M., Kelley R. F., Dixit V. M. (2010) K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol. Cell 39, 477–484 [DOI] [PubMed] [Google Scholar]
- 38. Phu L., Izrael-Tomasevic A., Matsumoto M. L., Bustos D., Dynek J. N., Fedorova A. V., Bakalarski C. E., Arnott D., Deshayes K., Dixit V. M., Kelley R. F., Vucic D., Kirkpatrick D. S. (2011) Improved quantitative mass spectrometry methods for characterizing complex ubiquitin signals. Mol. Cell. Proteomics 10, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeAlmeida V. I., Miao L., Ernst J. A., Koeppen H., Polakis P., Rubinfeld B. (2007) The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 67, 5371–5379 [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto H., Kishida S., Kishida M., Ikeda S., Takada S., Kikuchi A. (1999) Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J. Biol. Chem. 274, 10681–10684 [DOI] [PubMed] [Google Scholar]
- 41. Ikeda S., Kishida M., Matsuura Y., Usui H., Kikuchi A. (2000) GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by β-catenin and protein phosphatase 2A complexed with Axin. Oncogene 19, 537–545 [DOI] [PubMed] [Google Scholar]
- 42. Penman G. A., Leung L., Näthke I. S. (2005) The adenomatous polyposis coli protein (APC) exists in two distinct soluble complexes with different functions. J. Cell Sci. 118, 4741–4750 [DOI] [PubMed] [Google Scholar]
- 43. Näthke I. S., Adams C. L., Polakis P., Sellin J. H., Nelson W. J. (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134, 165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Papkoff J., Rubinfeld B., Schryver B., Polakis P. (1996) Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol. Cell Biol. 16, 2128–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barth A. I., Pollack A. L., Altschuler Y., Mostov K. E., Nelson W. J. (1997) NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J. Cell Biol. 136, 693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosin-Arbesfeld R., Ihrke G., Bienz M. (2001) Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J. 20, 5929–5939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Townsley F. M., Bienz M. (2000) Actin-dependent membrane association of a Drosophila epithelial APC protein and its effect on junctional Armadillo. Curr. Biol. 10, 1339–1348 [DOI] [PubMed] [Google Scholar]
- 48. Lee E., Salic A., Krüger R., Heinrich R., Kirschner M. W. (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS biology 1, E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ng S. S., Mahmoudi T., Danenberg E., Bejaoui I., de Lau W., Korswagen H. C., Schutte M., Clevers H. (2009) Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J. Biol. Chem. 284, 35308–35313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miyoshi Y., Nagase H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Human Mol. Genet. 1, 229–233 [DOI] [PubMed] [Google Scholar]
- 51. Spink K. E., Polakis P., Weis W. I. (2000) Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 19, 2270–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Polakis P. (1999) The oncogenic activation of β-catenin. Curr. Opin. Genet. Dev. 9, 15–21 [DOI] [PubMed] [Google Scholar]
- 53. Satoh S., Daigo Y., Furukawa Y., Kato T., Miwa N., Nishiwaki T., Kawasoe T., Ishiguro H., Fujita M., Tokino T., Sasaki Y., Imaoka S., Murata M., Shimano T., Yamaoka Y., Nakamura Y. (2000) AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24, 245–250 [DOI] [PubMed] [Google Scholar]
- 54. Benhaj K., Akcali K. C., Ozturk M. (2006) Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncology Rep. 15, 701–707 [PubMed] [Google Scholar]
- 55. Licchesi J. D., Mieszczanek J., Mevissen T. E., Rutherford T. J., Akutsu M., Virdee S., El Oualid F., Chin J. W., Ovaa H., Bienz M., Komander D. (2012) An ankyrin-repeat ubiquitin-binding domain determines TRABID's specificity for atypical ubiquitin chains. Nat. Struct. Mol. Biol. 19, 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wertz I. E., O'Rourke K. M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D. L., Ma A., Koonin E. V., Dixit V. M. (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signaling. Nature 430, 694–699 [DOI] [PubMed] [Google Scholar]
- 57. Valvezan A. J., Zhang F., Diehl J. A., Klein P. S. (2012) Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J. Biol. Chem. 287, 3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kitagawa M., Hatakeyama S., Shirane M., Matsumoto M., Ishida N., Hattori K., Nakamichi I., Kikuchi A., Nakayama K., Nakayama K. (1999) An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taelman V. F., Dobrowolski R., Plouhinec J. L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D., De Robertis E. M. (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Metcalfe C., Bienz M. (2011) Inhibition of GSK3 by Wnt signaling–two contrasting models. J. Cell Sci. 124, 3537–3544 [DOI] [PubMed] [Google Scholar]
- 61. Henderson B. R., Fagotto F. (2002) The ins and outs of APC and β-catenin nuclear transport. EMBO Rep. 3, 834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willert K., Shibamoto S., Nusse R. (1999) Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 14, 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]