Background: LacdiNAc (GalNAcβ1,4GlcNAc) is present on O- and N-linked carbohydrate moieties of pro-opiomelanocortin.
Results: β1,4-N-acetylgalactosaminyltransferases β4GalNAc-T3 and β4GalNAc-T4 mediate peptide-specific transfer of GalNAc to O-linked structures in vivo and in vitro.
Conclusion: β4GalNAc-T3 and β4GalNAc-T4 can account for LacdiNAc sequences on O-linked structures on specific glycoproteins.
Significance: The protein-specific addition of LacdiNAc to O-linked carbohydrates generates a family of unique structures recognized by carbohydrate-specific receptors.
Keywords: Carbohydrate Biosynthesis, Glycobiology, Glycoprotein Biosynthesis, Glycoprotein Hormones, Glycosyltransferases, LacdiNAc, O-Linked
Abstract
N- and O-linked oligosaccharides on pro-opiomelanocortin both bear the unique terminal sequence SO4-4-GalNAcβ1,4GlcNAcβ. We previously demonstrated that protein-specific transfer of GalNAc to N-linked oligosaccharides on glycoprotein substrates is dependent on the presence of both an oligosaccharide acceptor and a peptide recognition motif consisting of a cluster of basic amino acids. We characterized how two β1,4-N-acetylgalactosaminyltransferases, β4GalNAc-T3 and β4GalNAc-T4, require the presence of both the peptide recognition motif and the N-linked oligosaccharide acceptors to transfer GalNAc in β1,4-linkage to GlcNAc in vivo and in vitro. We now show that β4GalNAc-T3 and β4GalNAc-T4 are able to utilize the same peptide motif to selectively add GalNAc to β1,6-linked GlcNAc in core 2 O-linked oligosaccharide structures to form Galβ1,3(GalNAcβ1,4GlcNAcβ1,6)GalNAcαSer/Thr. The β1,4-linked GalNAc can be further modified with 4-linked sulfate by either GalNAc-4-sulfotransferase 1 (GalNAc-4-ST1) (CHST8) or GalNAc-4-ST2 (CHST9) or with α2,6-linked N-acetylneuraminic acid by α2,6-sialyltransferase 1 (ST6Gal1), thus generating a family of unique GalNAcβ1,4GlcNAcβ (LacdiNAc)-containing structures on specific glycoproteins.
Introduction
The LacdiNAc2 sequence, GalNAcβ1,4GlcNAcβ, is found on O-linked as well as N-linked oligosaccharides. The synthetic pathway leading to LacdiNAc-containing structures on O-linked oligosaccharides is illustrated in Fig. 1. Following conversion of the core 1 structure (see structure denoted 1) to a core 2 structure (see structure 4) by the addition of β1,6-linked GlcNAc, either β1,4-linked Gal or β1,4-linked GalNAc can be added to form structure 5 or the LacdiNAc-containing structure 7 in Fig. 1. The LacdiNAc can be further modified with either SO4 (see structures 10 and 11) or α2,6-linked sialic acid (see structure 9). Pro-opiomelanocortin (POMC) was the first glycoprotein reported to bear O-linked structures with the LacdiNAc sequence (1). The presence of LacdiNAc-modified N-linked oligosaccharides on murine POMC (2, 3) raised the possibility that the addition of β1,4-linked GalNAc to the β1,6-linked GlcNAc in O-linked oligosaccharides with a core 2 structure (see Fig. 1, structure 4) is mediated by the same β1,4-N-acetylgalactosaminyltransferases (β4GalNAc-T) that we have reported to account for protein-specific transfer of GalNAc to N-linked oligosaccharides on glycoproteins such as the luteinizing hormone (LH) (4–9) and carbonic anhydrase-6 (CA6) (10). Here we have addressed this possibility with chimeric glycoprotein substrates that can be used to examine GalNAc transfer in vivo and in vitro. We now demonstrate that β4GalNAc-T3 (B4GALNT3, GenBankTM AB089940 and AB114826) (11) and β4GalNAc-T4 β4GalNAc-T4 (B4GALNT4, GenBank AB089939 and AB114827) (12) mediate the protein-specific addition of β1,4-linked GalNAc to core 2 O-linked structures both in vivo and in vitro. In addition, GalNAc-4-sulfotransferase 1 (GalNAc-4-ST1, CHST8) (13), GalNAc-4-sulfotransferase 2 (GalNAc-4-ST2, CHST9) (14, 15), and α2,6-sialyltransferase (ST6Gal1) (16, 17) are able to add 4-linked sulfate and α2,6-linked NeuNAc to the LacdiNAc structures on core 2 O-linked structures, generating a family of unique LacdiNAc-bearing structures. N-Linked oligosaccharides terminating LacdiNAc modified with sulfate or NeuNAc are recognized by the mannose receptor (18–23) and the asialoglycoprotein receptor (24, 25), respectively, and regulate the circulatory half-lives of glycoprotein hormones bearing these structures in vivo. LacdiNAc termini modified with sulfate or NeuNAc on O-linked structures may be recognized by the same and/or additional receptors and also have important biological consequences in vivo.
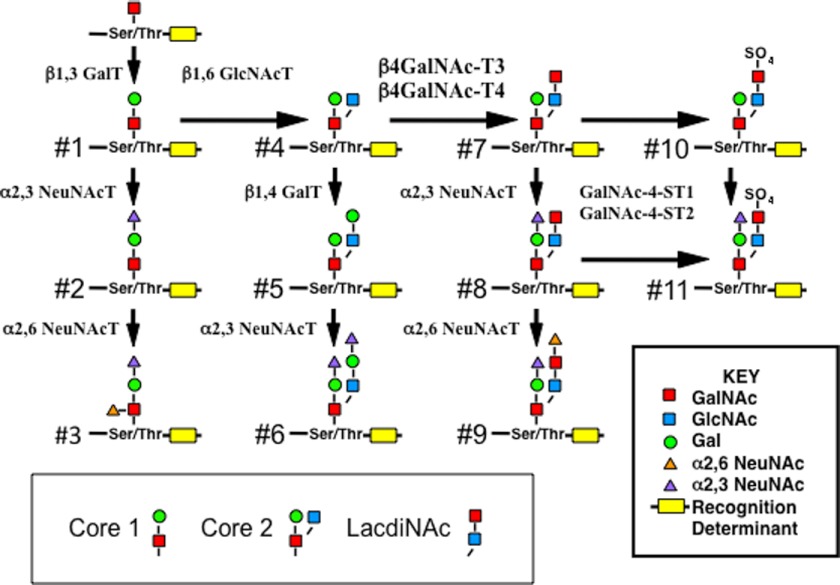
FIGURE 1.
Synthetic pathway for Ser/Thr O-linked LacdiNAc-containing structures on core 2 oligosaccharides. O-Linked oligosaccharides with a core 2 structure containing a GlcNAc-linked β1,6 to the O-linked GalNAc (structure 4) can be further modified by the addition of either β1,4-linked Gal or GalNAc to generate structures 5 and 7, respectively. Structure 7 can be further modified to generate structures 8, 9, 10, and 11. CHO cells do not express the β1,6GlcNAc transferase C2GnT1 and are not able to synthesize core 2 O-linked structures. β1,3 GalT, β1,3Galactosyltransferase; β4GalNAc-T, β1,4-N-Acetylgalactosaminyltransferase; NeuNAcT, NeuNAc transferase.
EXPERIMENTAL PROCEDURES
Reagents and Assays
Soluble, secreted forms of β4GalNAc-T3-F and β4GalNAc-T4-F were obtained by transfection of HEK 293T cells as described (26). pcDNAI-C2GnT1 (27) was provided by Dr. Minoru Fukuda (Burnham Institute for Medical Research, La Jolla, CA), and a plasmid for expression of α2,6NeuNAc-transferase (17) was provided by Dr. Karen Colley (University of Illinois, Chicago, IL). pcDNA3.1-GalNAc-4-ST1 and pcDNA3.1-GalNAc-4-ST2 were described previously (13, 14). Diplococcal β-galactosidase (DP β-galactosidase) was prepared by affinity chromatography (28). Arthrobacter ureafaciens neuraminidase was purchased from Roche Applied Science.
Quantitation of GalNAc incorporated either in vitro or in vivo into Gaussia luciferase (GLuc)-containing chimeric glycoproteins was carried out as described (26). The pH of the medium was adjusted to pH 5.0 with sodium acetate for digestion with A. ureafaciens neuraminidase and DP β-galactosidase at 37 °C. The digestions were stopped by heating.
pCMV-GlucCG-CTP-CA(1–19)Myc-His and pCMV-GlucCG-CTP-CA(1–2)Myc-His
pCMV-GlucCG-CTP-CA(1–19)Myc-His was prepared from pCMV-GLuc-αCA(1–19)Myc-His and a plasmid, pcDNA1/Amp-hCGβ, containing hCGβ. An acceptor plasmid was prepared by subjecting pCMV-GLuc-αCA(1–19)Myc-His to 10 cycles of PCR using Klentaq Long and Accurate DNA polymerase (29, 30) and the primers Gaussia-3′ASr: GTCACCACCGGCCCCCTTGAT(ribo)c and LMYC-S: GAGGGCCCGAACAAAAACTCATCT(ribo)c. The sequence containing the carboxyl terminal 28-amino acid peptide sequence from hCG (CTP) was prepared by PCR by performing five cycles of PCR with CAVI-LMYCb: CAAAGAGGAAAAAAGAAAAGTATTGGCCGTCTAGAGGGCCCGAACAAAAACTCATCTC plus LYMC-AS: GAGATGAGTTTTTGTTCGGGCCCT(ribo)c followed by five additional cycles with 19CAVIb: TTGAGAAGATTTATTGAACAGAAGATAACAAAGAGGAAAAAAGAAAAGTATTGGCCG. GAU-CTP AS-BA: GGAGGGGCCTTTGAGGAAGAGGAGTCACCACCGGCCCCCTTGATC plus Gaussia-3′Sr: GATCAAGGGGGCCGGTGGTGA(ribo)c and pcDNAI/AMP-CGβ were added, and 15 additional cycles of PCR were performed. The 159-bp product and the plasmid acceptor were digested with ribonuclease and ligated to generate pCMV-GlucCG-CTP-CA(1–19)Myc-His. pCMV-GlucCG-CTP-CA(1–2)Myc-His was generated from pCMV-GlucCG-CTP-CA(1–19)Myc-His by PCR using CTP-CA1–2-BA-S: CTCGGACACCCCGATCCTCCCACAAAGCTTGTCTAGAGGGCCCGAACAAAAACTCATCTC and LYMC-AS plus LYMC-S. All constructs were confirmed by sequencing.
Preparation of β4GT3/CHO and β4GT4/CHO
Flp-InTM Chinese hamster ovary (CHO) cell lines (Invitrogen) expressing β4GalNAc-T3 (β4GT3/CHO) or β4GalNAc-T4 (β4GT4/CHO) were prepared using protocols provided by the manufacturer. Murine β4GalNAc-T3 and β4GalNAc-T4 were amplified using Klentaq Long and Accurate DNA polymerase and ligated into pEF5/FRT/V5-D-TOPO using the manufacturer's protocols. DNA sequencing confirmed that the sequence was correct. The constructs were co-transfected with pOG44 Flp recombinase into Flp-InTM CHO cells and placed under selection with hygromycin. Hygromycin-resistant clones were selected that expressed β4GalNAc-T3 and β4GalNAc-T4 based on immunostaining for the V5 epitope and the presence of β1,4-linked GalNAc on glycoproteins expressed at the cell surface by immunostaining with FITC-Wisteria floribunda agglutinin, a lectin specific for terminal β1,4-linked GalNAc (31, 32). After subcloning, the expression of β4GalNAc-T3 and β4GalNAc-T4 was confirmed by Western blot using anti-V5-HRP antibody (Invitrogen). Expression levels, under control of the EF-1α promotor, were similar for β4GalNAc-T3 and β4GalNAc-T4 based on Western blot analysis.
RESULTS
GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His
A limited number of glycoproteins bearing either N-linked or O-linked oligosaccharides containing the LacdiNAc sequence have been described in vertebrates, suggesting potential distinctive functions for this carbohydrate moiety. As illustrated in Fig. 1 for O-linked structures, GalNAc is added to the β1,6-linked GlcNAc moiety of core 2 type structures generating structure 7 that can be further modified with 4-linked SO4 or α2,6-linked NeuNAc (structures 9 and 10). The addition of β1,4-linked GalNAc to N-linked oligosaccharides to form LacdiNAc sequences on glycoproteins such as LH and CA6 is mediated by protein-specific β1,4GalNAc transferases that recognize a peptide motif as well as the oligosaccharide acceptor (8, 9, 26). We previously utilized chimeric glycoproteins consisting of a secreted form of luciferase, GLuc (33, 34) followed by a glycoprotein of interest and an epitope tag, Myc-His, to define the protein-specific addition of GalNAc to N-linked oligosaccharides by β4GalNAc-T3 and β4GalNAc-T4 in vivo and in vitro (26). We have now taken a similar approach to determine whether the same β4GalNAc-T3 and β4GalNAc-T4 enzymes can account for the protein-specific addition of GalNAc to core 2 O-linked oligosaccharides.
The substrates used for these studies are illustrated schematically in Fig. 2. The hCG β subunit has a CTP sequence containing four Ser residues that become O-glycosylated when this protein is expressed in CHO cells (35). The CTP sequence was added to the carboxyl terminus of GLuc (Gaussia luciferase) followed by the 19-amino acid sequence from CA6 (CA1–19) that we have shown is recognized by β4GalNAc-T3 and β4GalNAc-T4 followed by the Myc-His epitope tag. The same construct containing only the first 2 amino acids of the CA1–19 sequence (CA1–2) was also prepared. Because GLuc is not glycosylated, the only carbohydrate moieties present on GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His are the O-linked structures in the CTP.
FIGURE 2.

Chimeric glycoprotein substrates used for characterization of GalNAc transfer in vitro and in vivo. Plasmids pCMV-GlucCG-CTP-CA(1–19)Myc-His and pCMV-GlucCG-CTP-CA(1–2)Myc-His encoding chimeric proteins consisting of GLuc, the CTP sequence, the CA1–19 sequence, or the CA1–2 sequence, respectively, and the epitope tag Myc-His were prepared. The Ser residues in the CTP that have been shown to be modified with O-linked GalNAc when expressed in CHO cells are indicated by asterisks. The sequence of the 19-amino acid carboxyl terminal peptide from CA6 is shown, and the residues deleted from the sequence to generate CA(1–2) are blocked out with a black line.
Transfer of GalNAc to GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His in Vivo
Flp-In CHO, β4GT3/CHO, and β4GT4/CHO cells were transfected with pCMV-GLuc-CTP-CA(1–19)Myc-His and pCMV-GLuc-CTP-CA(1–2)Myc-His. Because CHO cells do not express the β1,6GlcNAc transferase, C2GnT1, that is responsible for synthesis of the core 2 structure (Fig. 1) (27), the CHO cells were co-transfected with pcDNAI-C2GnT1. Based on the levels of GLuc activity found in the medium and on Western blot analysis following SDS-PAGE (Fig. 3A), GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His were highly expressed. The reduced mobility of both GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His when C2GnT was present reflects the increased size of the core 2 when compared with the core 1 structures (Fig. 1) and indicated that the core 1 structures on the CTP sequence were being modified by C2GnT1. Each of the products was examined for the presence of terminal β-linked GalNAc by comparing the amount of GLuc activity captured by WFA, a lectin specific for β-linked GalNAc (31, 32), onto 96-well plates where each well had the identical input (Fig. 3B). CHO cells that did not express either β4GalNAc-T3 or β4GalNAc-T4 showed little or no evidence of terminal GalNAc on GLuc-CTP-CA(1–19)Myc-His or GLuc-CTP-CA(1–2)Myc-His in either the presence or the absence of C2GnT1 (Fig. 3B). In contrast, β4GT3/CHO and β4GT4/CHO both transferred GalNAc to GLuc-CTP-CA(1–19)Myc-His but only when C2GnT1 was also present. GalNAc was also transferred to GLuc-CTP-CA(1–2)Myc-His when it was expressed in β4GT3/CHO and β4GT4/CHO in the presence but not the absence of C2GnT1. However, GLuc-CTP-CA(1–2)Myc-His was less extensively modified with GalNAc by either β4GalNAc-T3 or β4GalNAc-T4 than GLuc-CTP-CA(1–19)Myc-His in the Flp-In CHO cells stably expressing these enzymes.
FIGURE 3.
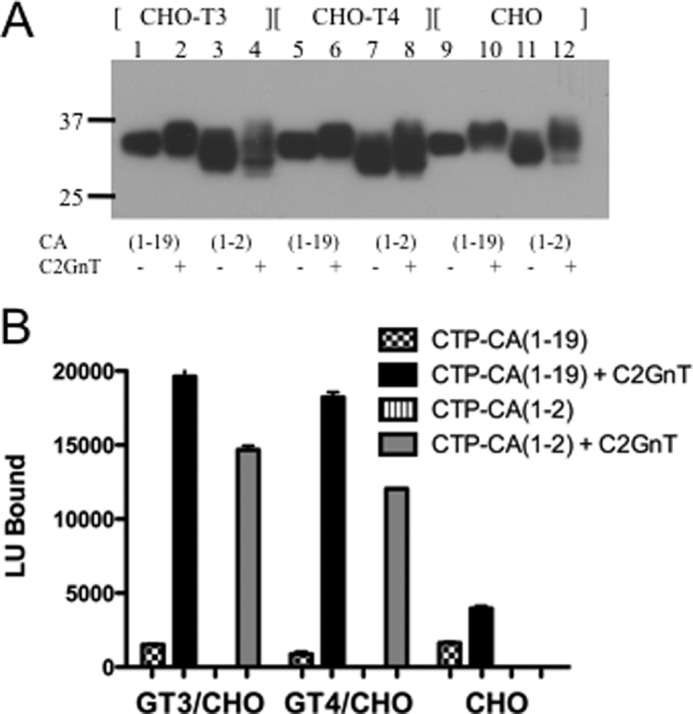
Transfer of GalNAc to GLuc-CTP constructs expressed in β4GT3/CHO, β4GT4/CHO, and CHO cells. A, Flp-In CHO cells expressing β4GalNAc-T3 (β4GT3/CHO), β4GalNAc-T4 (β4GT4/CHO), or nothing (CHO) were co-transfected with pCMV-GlucCG-CTP-CA(1–19)Myc-His or pCMV-GlucCG-CTP-CA(1–2)Myc-His and either control plasmid (odd numbered lanes) or pcDNAI-C2GnT1 (even numbered lanes). The media were collected and analyzed by SDS-PAGE. Following electrophoretic transfer to PVDF, the bands were visualized using anti-His. B, the amount of GalNAc transferred to the products of transfection of β4GT3/CHO, β4GT4/CHO, and CHO cells with pCMV-GlucCG-CTP-CA(1–19)Myc-His or pCMV-GlucCG-CTP-CA(1–2)Myc-His and either control plasmid or pcDNAI-C2GnT1 was compared by determining the amount of GLuc activity captured onto 96-well plates coated with WFA, a GalNAc-specific carbohydrate-binding protein. In all cases, the amount of GLuc activity loaded into each well during the binding phase was identical. All assays were done in quadruplicate. Error bars indicate S.E. LU, light units.
The difference in the extent of modification with GalNAc seen for GLuc-CTP-CA(1–19)Myc-His versus GLuc-CTP-CA(1–2)Myc-His when expressed in β4GT3/CHO or β4GT4/CHO cells was modest when compared with the 10-fold difference seen previously in our studies of N-linked glycosylation of GLuc-TrfCA6(1–19) versus GLucTrf when they were expressed in either β4GT3/CHO or β4GT 4/CHO (9). In the present experiments, high levels of β4GalNAc-T3 and β4GalNAc-T4 expression in β4GT3/CHO and β4GT4/CHO may have obscured or reduced any dependence on recognition of the peptide motif for GalNAc addition to O-linked structures. A single copy of β4GalNAc-T3 or β4GalNAc-T4 under the regulation of the EF-1α promotor was incorporated at the FRT site of Flp-In CHO cells to generate these β4GT3/CHO and β4GT4/CHO cell lines. Although EF-1α is a weak promoter, the levels of β4GalNAc-T expression were high when compared with the levels of expression seen in cell lines such as HEK 293T that express endogenous β4GalNAc-T3 and β4GalNAc-T4 (11, 12). Steady state β4GalNAc-T3 and β4GalNAc-T4 mRNA levels were 7- and 55-fold greater per μg of RNA in β4GT3/CHO and β4GT4/CHO than in HEK 293T cells, respectively. Furthermore, using the in vitro assay we have described for transfer of GalNAc to N-linked glycans on GLuc-α(PLRSKK)CA(1–19) (26), we determined that cell extracts from β4GT3/CHO and β4GT4/CHO had 40- and 58-fold more β4GalNAc-T activity per 106 cells, respectively, than HEK 293T cells (not shown). The high levels of GalNAc-T expression in β4GT3/CHO and β4GT4/CHO likely account for GalNAc transfer to glycoproteins that do not contain a peptide recognition determinant. We therefore expressed GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His in HEK 293T cells to see whether there would be a greater dependence on the presence of the peptide recognition motif than was seen in β4GT3/CHO or β4GT4/CHO.
GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His were expressed in HEK 293T cells in the absence or presence of additional C2GnT1 to ensure that production of core 2 structures would not be limiting. Because HEK 293T cells also express endogenous α2,6NeuNAc transferase activity, the products were digested with neuraminidase to expose any GalNAc that had been modified with α2,6-linked NeuNAc. The products were also digested with DP β-galactosidase to remove any β1,4-linked Gal that had been transferred to the β1,6-linked GlcNAc to yield structure 5. Western blot analyses of GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His and their digestion products are shown in Fig. 4A. GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His both expressed well and were modified with NeuNAc as indicated by the shift in mobility following neuraminidase digestion. GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His that were expressed in the presence of exogenous C2GnT1 migrated at a slightly higher molecular weight following digestion with either neuraminidase alone or neuraminidase plus DP β-galactosidase than their counterparts expressed in the absence of exogenous C2GnT1. The slower mobility seen with exogenous C2GnT1 was consistent with the presence of additional core 2 structures being synthesized, suggesting that the endogenous levels of C2GnT are limiting in the HEK 293T cells.
FIGURE 4.
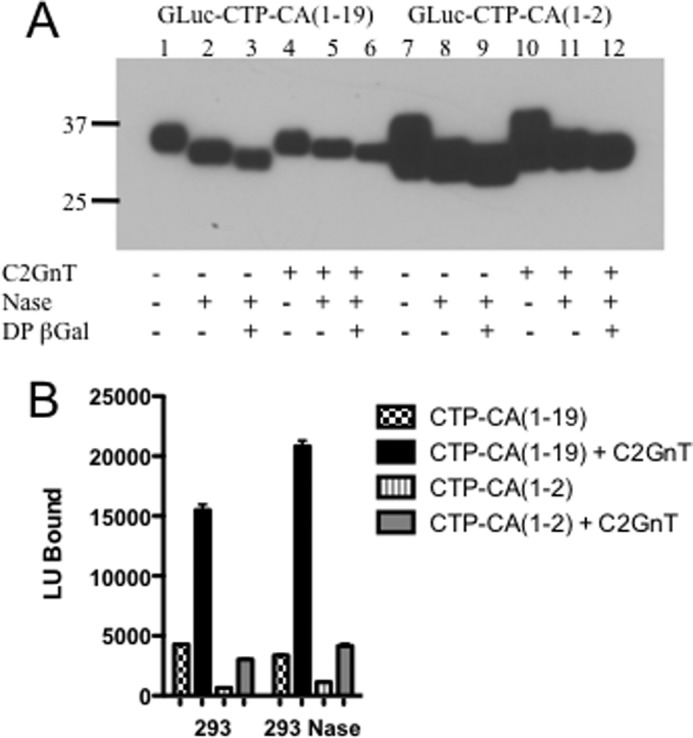
Transfer of GalNAc to GLuc-CTP constructs expressed in HEK 293T cells. A, HEK 293T cells were co-transfected with pCMV-GlucCG-CTP-CA(1–19)Myc-His or pCMV-GlucCG-CTP-CA(1–2)Myc-His and either control plasmid (lanes 1–3 and 7–9) or pcDNAI-C2GnT1 (lanes 4–6 and 10–12). The media were collected, and the products were digested with neuraminidase (Nase) or neuraminidase plus DP β-galactosidase as indicated. Following separation by SDS-PAGE and electrophoretic transfer to PVDF, the bands were visualized using anti-His. B, the amount of GalNAc transferred to the products of transfection of HEK 293T cells with pCMV-GlucCG-CTP-CA(1–19)Myc-His or pCMV-GlucCG-CTP-CA(1–2)Myc-His and either control plasmid or pcDNAI-C2GnT1 was compared by determining the amount of GLuc activity captured onto 96-well plates coated with WFA. The same samples were digested with neuraminidase to expose any GalNAc modified with α2,6-neuraminic acid. All assays were done in quadruplicate. Error bars indicate S.E. LU, light units.
In contrast to what we observed in β4GT3/CHO or β4GT4/CHO cells, a significant fraction of the GLuc-CTP-CA(1–19)Myc-His expressed in HEK 293T cells was modified with GalNAc when it was expressed in the absence of exogenous C2GnT1 (Fig. 4B versus Fig. 3B). Co-expression of exogenous C2GnT1 in HEK 293T cells increased the amount of GLuc-CTP-CA(1–19)Myc-His modified with GalNAc by 3.5-fold (Fig. 4B), indicating that C2GnT activity rather than β4GalNAc-T activity was limiting in HEK 293T cells. Notably, 5-fold more GLuc-CTP-CA(1–19)Myc-His was modified with GalNAc in either the presence or the absence of exogenous C2GnT1 than was GLuc-CTP-CA(1–2)Myc-His. This enhancement of GalNAc transfer by the full-length peptide recognition determinant indicated that the endogenous β4GalNAc-T activity in HEK 293T was displaying the expected protein specificity. Digestion with neuraminidase increased the amount of GLuc-CTP-CA(1–19)Myc-His or GLuc-CTP-CA(1–2)Myc-His that could be bound by WFA by only 25% (Fig. 4B), indicating that a minor fraction of the added GalNAc was further modified with α2,6-linked NeuNAc when these proteins were expressed in HEK 293T cells.
Transfer of GalNAc to GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His in Vitro
GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His were expressed in CHO cells in the absence or presence of C2GnT1 to generate substrates for in vitro transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4. GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His expressed in the presence of C2GnT1 migrated at a higher molecular weight than GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His expressed in the absence of C2GnT1 when examined by Western blot following SDS-PAGE (Fig. 5A). The slower migration reflected the larger size of the core 2 structures (Fig. 1, structures 4, 5, and 6) when compared with the core 1 structures (Fig. 1, structures 1 and 2) synthesized by CHO cells in the presence of C2GnT. The CHO products were digested with neuraminidase plus DP β-galactosidase, which removes β1,4-linked but not β1,3-linked Gal on the core 2 structure, to convert structures 5 and 6 to structure 4 in Fig. 1, the acceptor for GalNAc addition by either β4GalNAc-T3 or β4GalNAc-T4. Following these glycosidase digestions, GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His expressed in the presence of C2GnT1 continued to migrate at a slightly higher molecular weight than GLuc-CTP-CA(1–19)Myc-His and GLuc-CTP-CA(1–2)Myc-His expressed in the absence of C2GnT1, reflecting the additional β1,6-linked GlcNAc in the core 2 structure.
FIGURE 5.
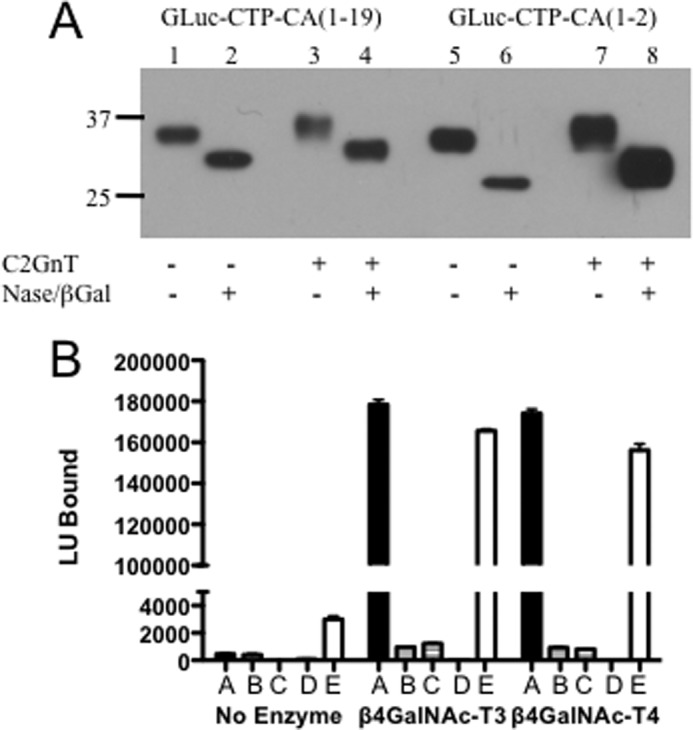
Requirements for transfer of GalNAc to O-linked oligosaccharides by β4GalNAc-T3 and β4GalNAc-T4 in vitro. A, GLuc-CTP-CA(1–19)-Myc-His (lanes 1–4) and GLuc-CTP-CA(1–2)-Myc-His (lanes 5–8) were expressed in CHO Flp-In cells in the absence (lanes 1, 2, 5, and 6) or presence (lanes 3, 4, 7, and 8) of C2GnT1. The media were collected, and the products were digested with neuraminidase (Nase) plus β-galactosidase (lanes 2, 4, 6, and 8) to generate the core 2 structure 4 in Fig. 1. Following separation by SDS-PAGE and electrophoretic transfer to PVDF, the products were visualized using anti-His. DP β-Gal, Diplococcal β-galactosidase. B, equal light units (LU) of GLuc-CTP-CA(1–19)-Myc-His and GLuc-CTP-CA(1–2)-Myc-His expressed in CHO-Flp-In cells and digested with neuraminidase and β-galactosidase were incubated with no additions, β4GalNAc-T3, or β4GalNAc-T4. The amount of GalNAc-modified product was determined by capture onto WFA-coated 96-well plates. GLuc-α(PLRSKK)CA1–19 expressed in Lec8 CHO cells was used as a positive control containing the same recognition determinant. Bars are marked as follows: A, GLuc-CTP-CA(1–19)Myc-His co-expressed with C2GnT1. B, GLuc-CTP-CA(1–19)Myc-His. C, GLuc-CTP-CA(1–2)Myc-His co-expressed with C2GnT1. D, GLuc-CTP-CA(1–2)Myc-His. E, GLuc-α(PLRSKK)CA1–19-Myc-His. Error bars indicate S.E.
The neuraminidase- plus DP β-galactosidase-digested products were used as substrates for the in vitro assay. β4GalNAc-T3-F and β4GalNAc-T4-F both transferred GalNAc to GLuc-CTP-CA(1–19)Myc-His bearing core 2 O-linked structures (Figs. 1 and 5B, bars marked A) but not to GLuc-CTP-CA(1–19)Myc-His bearing core 1 (Galβ1,3GalNAcα) structures (Fig. 1) (Fig. 5B, bars marked B). Furthermore, neither β4GalNAc-T3-F nor β4GalNAc-T4-F transferred GalNAc to either core 2 (Fig. 5B, bars marked C) or core 1 (Fig. 5B, bars marked D) structures on GLuc-CTP-CA(1–2)Myc-His. The extent of GalNAc addition to GLuc-CTP-CA(1–19)Myc-His (Fig. 5B, bars marked A) was similar to that seen for GLuc-α(PLRSKK)CA1–19 (Fig. 5B, bars marked E), which bears N-linked rather than O-linked structures.
Addition of Sulfate and NeuNAc to O-Linked LacdiNAc-containing Structures in Vivo
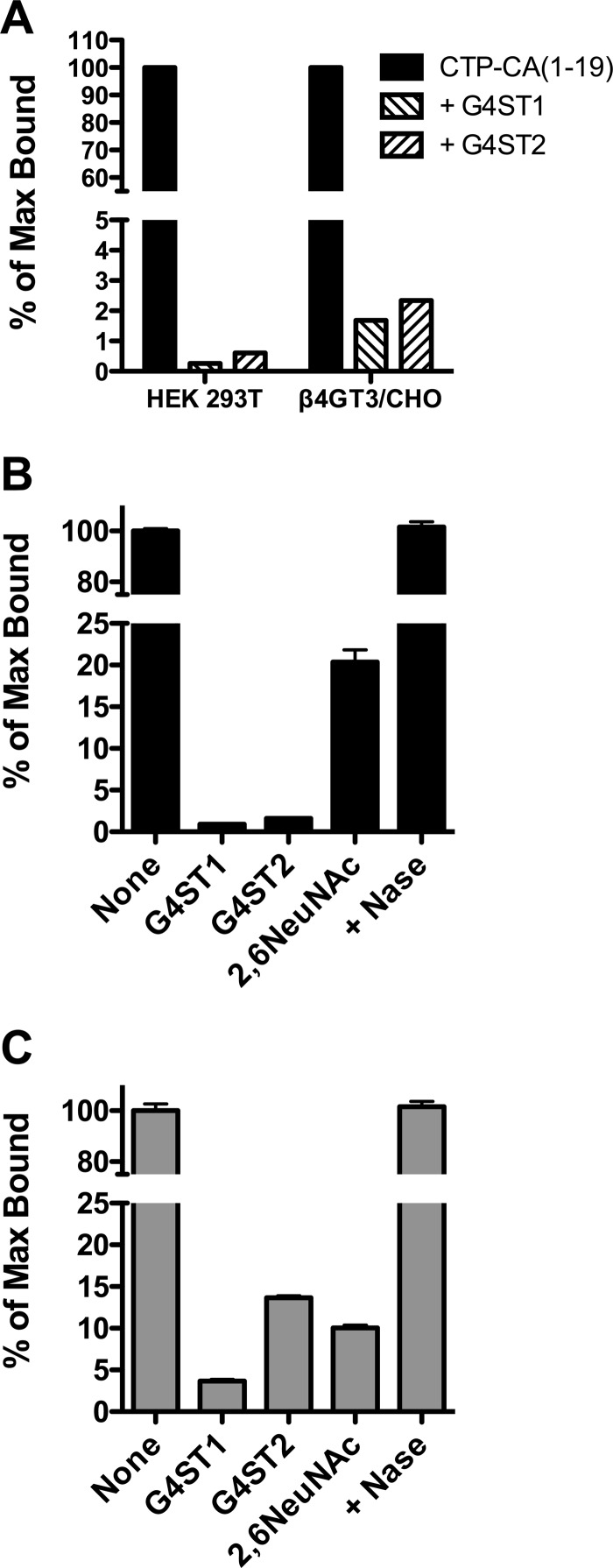
The terminal GalNAc of LacdiNAc structures on N-linked oligosaccharides can be further modified with either 4-linked SO4 or α2,6-linked NeuNAc. Either of these additional modifications prevents binding by WFA. We previously identified two GalNAc-4-sulfotransferases, GalNAc-4-ST1 (CHST8) (13) and GalNAc-4-ST2 (CHST9) (14), that specifically add SO4 to the terminal LacdiNAc sequence on N-linked oligosaccharides. Expression of either GalNAc-4-ST1 or GalNAc-4-ST2 in either HEK 293T or β4GT3/CHO (Fig. 6A) expressing GLuc-CTP-CA(1–19)Myc-His and C2GnT1 abolished binding to WFA. Expression of α2,6NeuNAc-transferase in β4GT3/CHO also reduced binding of GLuc-CTP-CA(1–19)Myc-His to WFA but was not as effective as either GalNAc-4-ST1 or GalNAc-4-ST2 (Fig. 6B). Expression of GalNAc-4-ST1, GalNAc-4-ST2, or α2,6NeuNAc-transferase along with GLuc-α(PLRSKK)CA1–19 in β4GT3/CHO cells also largely abolished binding of GLuc-α(PLRSKK)CA1–19 by WFA (Fig. 6C); however, GalNAc-4-ST2 and α2,6NeuNAc-transferase were not as effective as GalNAc-4-ST1. Digestion of α2,6NeuNAc-modified GLuc-CTP-CA(1–19)Myc-His (Fig. 6B) or GLuc-α(PLRSKK)CA1–19 (Fig. 6C) with neuraminidase restored WFA binding. Thus, the LacdiNAc structures on either the O-linked oligosaccharides or the N-linked oligosaccharides can be efficiently modified with 4-linked SO4 or α2,6-linked NeuNAc by GalNAc-4-ST1, GalNAc-4-ST2, and α2,6NeuNAc-transferase, respectively, in vivo to produce structures 10, 11, and 9 in Fig. 1.
FIGURE 6.
LacdiNAc on 0-linked core 2 structures can be modified with SO4 or NeuNAc. A, GLuc-CTP-CA(1–19)Myc-His and C2GnT1 were co-expressed in HEK-293T cells or β4GT3/CHO cells alone or in the presence of GalNAc-4-ST1 (G4ST1) or GalNAc-4-ST2 (G4ST2). B, GLuc-CTP-CA(1–19)Myc-His and C2GnT1 were co-expressed in β4GT3/CHO cells alone or in the presence of GalNAc-4-ST1 (G4ST1), GalNAc-4-ST2 (G4ST2), or α2,6-NeuNAc-transferase. GLuc-CTP-CA(1–19)Myc-His that had been co-expressed with α2,6-NeuNAc-transferase was digested with neuraminidase (Nase). C, GLuc-α(PLRSKK)CA1–19 and C2GnT1 were co-expressed in β4GT3/CHO cells alone or in the presence of GalNAc-4-ST1 (G4ST1), GalNAc-4-ST2 (G4ST2), or α2,6-NeuNAc-transferase. GLuc-α(PLRSKK)CA1–19 that had been co-expressed with α2,6-NeuNAc-transferase was digested with neuraminidase. Media were collected from transfected cells, and the amount of terminal β1,4-linked GalNAc that could be bound by immobilized WFA for the identical input of GLuc activity was determined. The amount of GLuc-CTP-CA(1–19)Myc-His (panel B) and α(PLRSKK)CA1–19 (panel C) that had been co-expressed with α2,6-NeuNAc-transferase that could be bound by WFA was determined before and after digestion with neuraminidase. The results are expressed as the percentage of maximum bound (% of Max Bound) for GLuc-CTP-CA(1–19)Myc-His co-expressed with C2GnT1 in panels A and B and for α(PLRSKK)CA1–19 co-expressed with C2GnT1 in panel C. Error bars in panels B and C indicate S.E.
DISCUSSION
Our current studies expand the role of protein-specific synthesis of LacdiNAc structures by β4GalNAc-T3 and β4GalNAc-T4 to O-linked oligosaccharides. The subsequent addition of SO4, α2,6-linked NeuNAc, or other substituents can produce a family of unique carbohydrate structures that may have important biological roles as we have defined for N-linked oligosaccharides modified with LacdiNAc on glycoprotein hormones such as LH (8, 36–38). We can now attribute the presence of both N-linked and O-linked oligosaccharides containing the LacdiNAc sequence on POMC to the same enzymes β4GalNAc-T3 and/or β4GalNAc-T4. Although a number of glycoproteins bearing N-linked structures containing LacdiNAc have been described since we originally reported this structure on the glycoprotein hormone LH (51), POMC (1) and zona pellucida 3 (39) have to date remained the only glycoproteins reported to bear core 2 O-linked structures with the LacdiNAc sequence in vertebrates. Thus, the addition of GalNAc to core 2 O-linked structures may also be restricted to glycoproteins bearing a peptide motif such as the sequences we have described on the glycoprotein hormone α subunit (6, 7) and CA6 (9, 26), which are recognized by β4GalNAc-T3 and β4GalNAc-T4.
The carboxyl terminal amino acid sequence of the hCGβ subunit contains four Ser residues that are modified with O-linked oligosaccharide structures when expressed in CHO cells (35). Adding this sequence to the carboxyl terminus of GLuc produced a substrate that contained only O-linked oligosaccharides and was efficiently secreted into the medium of cells following transfection. We have demonstrated that the carboxyl-terminal 19 amino acid sequence found on CA6 is recognized by β4GalNAc-T3 and β4GalNAc-T4 and can account for the protein-specific addition of GalNAc to N-linked oligosaccharides both in vitro and in vivo (26). Adding either the CA1–19 sequence or alternatively just the CA1–2 sequence to the carboxyl terminus of the CTP from hCGβ yielded chimeric glycoproteins that did and did not contain a determinant recognized by β4GalNAc-T3 and β4GalNAc-T4, respectively, and could be utilized to define transfer of GalNAc in vitro as well as in vivo. We have used similar constructs to show that efficient transfer of GalNAc to N-linked glycans is dependent on the presence of a peptide recognition determinant such as CA1–19 (26). Constructs containing portions of the CA1–19 sequence were not modified as efficiently as those containing the full CA1–19 sequence. The construct GLuc-α(PLESEE)CA1–10, which contains only the first 10 amino acids of the CA1–19 sequence, was a poor substrate for GalNAc transfer by either β4GalNAc-T3 or β4GalNAc-T4 when compared with GLuc-α(PLESEE)CA1–19 (26). The independence of peptide recognition and GalNAc transfer to N-linked oligosaccharides suggests that the peptide requirements will be similar, if not identical, for transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4 to O-linked structures.
The results presented above demonstrate the following. 1) Transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4 to O-linked oligosaccharides is absolutely dependent on the presence of the β1,6-linked GlcNAc in the core 2 structure in vivo and in vitro. 2) Transfer of GalNAc to core 2 O-linked oligosaccharides by β4GalNAc-T3 and β4GalNAc-T4 in vitro is inefficient in the absence of a recognized peptide determinant such as CA1–19. 3) High levels of β4GalNAc-T3 and β4GalNAc-T4 expression in cells such as β4GT3/CHO and β4GT4/CHO can result in transfer of GalNAc to core 2 structures in the absence of the peptide recognition determinant. However, endogenous levels of β4GalNAc-T3 and β4GalNAc-T4 expression found in HEK 293T require the presence of a peptide recognition determinant for efficient transfer of GalNAc to core 2 structures. 4) GalNAc-4-ST1 (CHST8) and GalNAc-4-ST2 (CHST9) are able to quantitatively modify the LacdiNAc structures on both O-linked and N-linked oligosaccharides with SO4 when expressed in CHO cells. 5) α2,6-NeuNAc transferase is able to modify the LacdiNAc structures on O-linked and N-linked oligosaccharides with α2,6-linked NeuNAc when expressed in CHO cells. Therefore the same transferases that account for the modification of N-linked structures with LacdiNAc, SO4-4-GalNAcβ1,4GlcNAcβ, and NeuNAcα2,6GalNAcβ1,4GlcNAcβ can account for protein-specific synthesis of this family of unique carbohydrate structures on O-linked structures.
Three isoforms of the core 2 β1,6-N-acetylglucosaminyltransferase, C2GnT1 (27), C2GnT2 (40), and C2GnT3 (41), have been identified and cloned. An O-glycomic analysis of wild type mice and mice deficient in individual C2GnTs or all three C2GnTs was recently published (42). Core 2 structures (see Table 1, m/z 1024, in Ref. 42) that contain the LacdiNAc sequence were present although not abundant in the colon of the wild type mice but not in the colon of C2GnT-deficient mice. β4GalNAc-T3 transcripts have been detected in human stomach and colon (11). Furthermore, LacdiNAc structures were reported to be present on surface mucous cells of the human stomach based on WFA staining (43). The distribution of β4GalNAc-T3 transcripts and LacdiNAc bearing core 2 structures in the colon and stomach of mice and humans suggests that additional O-linked glycans bearing LacdiNAc will be identified in the future. The LacdiNAc sequence may, however, be confined to only those core 2 structures that also have an accessible recognition motif for β4GalNAc-T3.
O-Glycosylation of Ser and Thr residues with α-linked GalNAc is an abundant form of glycosylation. As is illustrated by the O-glycomic analysis done by Ismail et al. (42), the structures produced are complex. As many as 20 distinct isoenzymes have been identified that mediate the site-specific addition of GalNAc (44, 45). An additional repertoire of transferases serves to build complex oligosaccharide structures on the O-linked GalNAc. Recently developed approaches have identified a rapidly growing number of glycoproteins that are O-glycosylated at specific sites that in some cases serve to modulate critical biological processes (46). The selective addition of LacdiNAc sequences to O-linked structures that have an associated recognition motif for either β4GalNAc-T3 or β4GalNAc-T4 and their subsequent modification with sulfate or NeuNAc provides a mechanism to produce highly unique structures at very specific locations in glycoproteins with O-linked oligosaccharides.
β4GalNAc-Ts that are either not protein-specific or have a specificity that differs from that of β4GalNAc-T3 and β4GalNAc-T4 have been identified using in vitro assays (47–49). Furthermore, not all glycoproteins bearing N- or O-linked oligosaccharides modified with LacdiNAc have readily identifiable recognition motifs similar to those we have described on α and CA6. Although it was possible to detect β4GalNAc-T activity in vitro in CHO cells, no evidence of LacdiNAc addition to glycoproteins expressed in CHO cells was obtained (48). However, expression of a β1,4GalNAcT cloned from Caenorhabditis elegans in CHO Lec8 cells resulted in LacdiNAc synthesis on multiple endogenous glycoproteins as well as the glycoprotein hormone α subunit (50). Until other β4GalNAc-Ts can be identified or cloned, it will be difficult to assess whether they are protein-specific; however, the approach we have taken using chimeric glycoprotein acceptors makes this a more approachable problem in the future. More examples of glycoproteins bearing O-linked structure modified with LacdiNAc will be required to assess whether they are confined to specific glycoproteins and whether β4GalNAc-T3 and/or β4GalNAc-T4 are responsible.
The presence of the LacdiNAc sequence on N-linked oligosaccharides of glycoprotein hormones such as LH is of critical importance for their biology. In the case of LH, the structural features of the LacdiNAc determine the circulatory half-life of the hormone following its release into the circulation, and as a result, its potency in vivo (22, 23, 36). SO4-4-GalNAcβ1,4GlcNAcβ is recognized by the N-terminal Cys-rich domain of the mannose receptor in its dimeric form (18, 19, 21, 23), whereas NeuNAcα2,6GalNAcβ1,4GlcNAcβ is recognized by the asialoglycoprotein receptor (24). The mannose receptor and the asialoglycoprotein receptor are highly abundant endocytic receptors that reside in endothelial cells and parenchymal cells of the liver, respectively. Glycoproteins bearing multiple O-linked structures terminating with LacdiNAc, SO4-4-GalNAcβ1,4GlcNAcβ, or NeuNAcα2,6GalNAcβ1,4GlcNAcβ may interact with the mannose receptor and the asialoglycoprotein receptor differently from glycoproteins bearing N-linked oligosaccharides with the same termini. In addition, it is quite possible that the LacdiNAc-containing O-linked structures may be recognized by other receptors and have quite different functions in vivo such as mediating cell or matrix recognition.
Efficient transfer of GalNAc to O- and N-linked structures on specific glycoproteins by β4GalNAc-T3 and β4GalNAc-T4 is dependent on the presence of a peptide motif that is recognized by these transferases and the presence of the appropriate acceptor structure. Further modification of the LacdiNAc sequence by the addition of sulfate or NeuNAc also reflects the repertoire of GalNAc-4-STs and α2,6NeuNAc transferases being expressed. As a consequence, a unique family of LacdiNAc-containing structures can be added to specific glycoproteins bearing either O-linked or N-linked carbohydrates. We expect that additional glycoproteins bearing O-linked glycans with LacdiNAc structures will be identified in the future. The protein-specific synthesis of this unique family of O-linked structures makes it highly likely that like their N-linked counterparts, they well be recognized by specific receptors with functional consequences.
Acknowledgment
We thank Nancy L. Baenziger for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HD058474 and R01-CA21923 (to J. U. B.).
- LacdiNAc
- GalNAcβ1,4GlcNAcβ
- CA
- carbonic anhydrase
- CTP
- carboxyl-terminal peptide from hCG
- hCG
- human chorionic gonadotropin
- GLuc
- Gaussia luciferase
- CG
- chorionic gonadotropin
- Trf
- transferrin
- PMOC
- pro-opiomelanocortin
- WFA
- W. floribunda agglutinin
- β4GalNAc-T
- β1,4-N-acetylgalactosaminyltransferase
- GalNAc-4-ST
- N-acetylgalactosamine-4-sulfotransferase
- NeuNAc
- N-acetylneuraminic acid
- PCR
- polymerase chain reaction
- C2GnT
- core 2 β1,6-N-acetylgluctosaminyltransferase
- LH
- luteinizing hormone
- DP β-galactosidase
- Diplococcal β-galactosidase.
REFERENCES
- 1. Siciliano R. A., Morris H. R., Bennett H. P., Dell A. (1994) O-Glycosylation mimics N-glycosylation in the 16-kDa fragment of bovine pro-opiomelanocortin: the major O-glycan attached to Thr-45 carries SO4-4GalNAcβ1,4GlcNAcβ1-, which is the archetypal non-reducing epitope in the N-glycans of pituitary glycohormones. J. Biol. Chem. 269, 910–920 [PubMed] [Google Scholar]
- 2. Siciliano R. A., Morris H. R., McDowell R. A., Azadi P., Rogers M. E., Bennett H. P., Dell A. (1993) The Lewis x epitope is a major nonreducing structure in the sulfated N-glycans attached to Asn-65 of bovine pro-opiomelanocortin. Glycobiology 3, 225–239 [DOI] [PubMed] [Google Scholar]
- 3. Skelton T. P., Kumar S., Smith P. L., Beranek M. C., Baenziger J. U. (1992) Pro-opiomelanocortin synthesized by corticotrophs bears asparagine-linked oligosaccharides terminating with SO4-4GalNAc β1,4GlcNAc β1,2Manα. J. Biol. Chem. 267, 12998–13006 [PubMed] [Google Scholar]
- 4. Smith P. L., Baenziger J. U. (1988) A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science 242, 930–933 [DOI] [PubMed] [Google Scholar]
- 5. Smith P. L., Baenziger J. U. (1990) Recognition by the glycoprotein hormone-specific N-acetylgalactosaminetransferase is independent of hormone native conformation. Proc. Natl. Acad. Sci. U.S.A. 87, 7275–7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith P. L., Baenziger J. U. (1992) Molecular basis of recognition by the glycoprotein hormone-specific N-acetylgalactosamine-transferase. Proc. Natl. Acad. Sci. U.S.A. 89, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mengeling B. J., Manzella S. M., Baenziger J. U. (1995) A cluster of basic amino acids within an α helix is essential for α subunit recognition by the glycoprotein hormone N-acetylgalactosaminyltransferase. Proc. Natl. Acad. Sci. U.S.A. 92, 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzella S. M., Hooper L. V., Baenziger J. U. (1996) Oligosaccharides containing β1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 271, 12117–12120 [DOI] [PubMed] [Google Scholar]
- 9. Miller E., Fiete D., Blake N. M., Beranek M., Oates E. L., Mi Y., Roseman D. S., Baenziger J. U. (2008) A necessary and sufficient determinant for protein-selective glycosylation in vivo. J. Biol. Chem. 283, 1985–1991 [DOI] [PubMed] [Google Scholar]
- 10. Hooper L. V., Beranek M. C., Manzella S. M., Baenziger J. U. (1995) Differential expression of GalNAc-4-sulfotransferase and GalNAc-transferase results in distinct glycoforms of carbonic anhydrase VI in parotid and submaxillary glands. J. Biol. Chem. 270, 5985–5993 [DOI] [PubMed] [Google Scholar]
- 11. Sato T., Gotoh M., Kiyohara K., Kameyama A., Kubota T., Kikuchi N., Ishizuka Y., Iwasaki H., Togayachi A., Kudo T., Ohkura T., Nakanishi H., Narimatsu H. (2003) Molecular cloning and characterization of a novel human β1,4-N-acetylgalactosaminyltransferase, β4GalNAc-T3, responsible for the synthesis of N,N′-diacetyllactosediamine, GalNAc β1-4GlcNAc. J. Biol. Chem. 278, 47534–47544 [DOI] [PubMed] [Google Scholar]
- 12. Gotoh M., Sato T., Kiyohara K., Kameyama A., Kikuchi N., Kwon Y. D., Ishizuka Y., Iwai T., Nakanishi H., Narimatsu H. (2004) Molecular cloning and characterization of β1,4-N-acetylgalactosaminyltransferases IV synthesizing N,N′-diacetyllactosediamine. FEBS Lett. 562, 134–140 [DOI] [PubMed] [Google Scholar]
- 13. Xia G., Evers M. R., Kang H. G., Schachner M., Baenziger J. U. (2000) Molecular cloning and expression of the pituitary glycoprotein hormone N-acetylgalactosamine-4-O-sulfotransferase. J. Biol. Chem. 275, 38402–38409 [DOI] [PubMed] [Google Scholar]
- 14. Kang H. G., Evers M. R., Xia G., Baenziger J. U., Schachner M. (2001) Molecular cloning and expression of an N-acetylgalactosamine-4-O-sulfotransferase that transfers sulfate to terminal and nonterminal β1,4-linked N-acetylgalactosamine. J. Biol. Chem. 276, 10861–10869 [DOI] [PubMed] [Google Scholar]
- 15. Hiraoka N., Misra A., Belot F., Hindsgaul O., Fukuda M. (2001) Molecular cloning and expression of two distinct human N-acetylgalactosamine 4-O-sulfotransferases that transfer sulfate to GalNAc β1,4GlcNAc β1,R in both N- and O-glycans. Glycobiology 11, 495–504 [DOI] [PubMed] [Google Scholar]
- 16. Weinstein J., Lee E. U., McEntee K., Lai P. H., Paulson J. C. (1987) Primary structure of β-galactoside α2,6-sialyltransferase: conversion of membrane-bound enzyme to soluble forms by cleavage of the NH2-terminal signal anchor. J. Biol. Chem. 262, 17735–17743 [PubMed] [Google Scholar]
- 17. Ma J., Qian R., Rausa F. M., 3rd, Colley K. J. (1997) Two naturally occurring α2,6-sialyltransferase forms with a single amino acid change in the catalytic domain differ in their catalytic activity and proteolytic processing. J. Biol. Chem. 272, 672–679 [DOI] [PubMed] [Google Scholar]
- 18. Roseman D. S., Baenziger J. U. (2000) Molecular basis of lutropin recognition by the mannose/GalNAc-4-SO4 receptor. Proc. Natl. Acad. Sci. U.S.A. 97, 9949–9954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiete D. J., Beranek M. C., Baenziger J. U. (1998) A cysteine-rich domain of the “mannose” receptor mediates GalNAc-4-SO4 binding. Proc. Natl. Acad. Sci. U.S.A. 95, 2089–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiete D., Beranek M. C., Baenziger J. U. (1997) The macrophage/endothelial cell mannose receptor cDNA encodes a protein that binds oligosaccharides terminating with SO4-4-GalNAcβ1,4GlcNAcβ, or Man at independent sites. Proc. Natl. Acad. Sci. U.S.A. 94, 11256–11261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fiete D., Baenziger J. U. (1997) Isolation of the SO4-4-GalNAcβ1,4GlcNAcβ1,2Manα-specific receptor from rat liver. J. Biol. Chem. 272, 14629–14637 [DOI] [PubMed] [Google Scholar]
- 22. Baenziger J. U., Kumar S., Brodbeck R. M., Smith P. L., Beranek M. C. (1992) Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc. Natl. Acad. Sci. U.S.A. 89, 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiete D., Srivastava V., Hindsgaul O., Baenziger J. U. (1991) A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAc β1,4GlcNAc β1,2Manα that mediates rapid clearance of lutropin. Cell 67, 1103–1110 [DOI] [PubMed] [Google Scholar]
- 24. Park E. I., Mi Y., Unverzagt C., Gabius H. J., Baenziger J. U. (2005) The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid α2,6GalNAc. Proc. Natl. Acad. Sci. U.S.A. 102, 17125–17129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park E. I., Manzella S. M., Baenziger J. U. (2003) Rapid clearance of sialylated glycoproteins by the asialoglycoprotein receptor. J. Biol. Chem. 278, 4597–4602 [DOI] [PubMed] [Google Scholar]
- 26. Fiete D., Beranek M., Baenziger J. U. (June 21, 2012) Molecular Basis for protein-specific transfer of N-acetylgalactosamine to N-linked glycans by the glycosyltransferases β1,4-N-acetylgalactosaminyl transferase 3 (β4GalNAc-T3) and β4GalNAc-T4. J. Biol. Chem. 287, 29194–29203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bierhuizen M. F., Fukuda M. (1992) Expression cloning of a cDNA encoding UDP-GlcNAc:Gal β1–3-GalNAc-R (GlcNAc to GalNAc) β1–6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc. Natl. Acad. Sci. U.S.A. 89, 9326–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glasgow L. R., Paulson J. C., Hill R. L. (1977) Systematic purification of five glycosidases from Streptococcus (Diplococcus) pneumoniae. J. Biol. Chem. 252, 8615–8623 [PubMed] [Google Scholar]
- 29. Barnes W. M. (1994) PCR amplification of up to 35-kb DNA with high fidelity and high yield from λ bacteriophage templates. Proc. Natl. Acad. Sci. U.S.A. 91, 2216–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng S., Fockler C., Barnes W. M., Higuchi R. (1994) Effective amplification of long targets from cloned inserts and human genomic DNA. Proc. Natl. Acad. Sci. U.S.A. 91, 5695–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mengeling B. J., Smith P. L., Stults N. L., Smith D. F., Baenziger J. U. (1991) A microplate assay for analysis of solution-phase glycosyltransferase reactions: determination of kinetic constants. Anal. Biochem. 199, 286–292 [DOI] [PubMed] [Google Scholar]
- 32. Torres B. V., McCrumb D. K., Smith D. F. (1988) Glycolipid-lectin interactions: reactivity of lectins from Helix pomatia, Wisteria floribunda, and Dolichos biflorus with glycolipids containing N-acetylgalactosamine. Arch. Biochem. Biophys. 262, 1–11 [DOI] [PubMed] [Google Scholar]
- 33. Verhaegent M., Christopoulos T. K. (2002) Recombinant Gaussia luciferase: Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 74, 4378–4385 [DOI] [PubMed] [Google Scholar]
- 34. Tannous B. A., Kim D. E., Fernandez J. L., Weissleder R., Breakefield X. O. (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 11, 435–443 [DOI] [PubMed] [Google Scholar]
- 35. Sugahara T., Pixley M. R., Fares F., Boime I. (1996) Characterization of the O-glycosylation sites in the chorionic gonadotropin β subunit in vivo using site-directed mutagenesis and gene transfer. J. Biol. Chem. 271, 20797–20804 [DOI] [PubMed] [Google Scholar]
- 36. Mi Y., Fiete D., Baenziger J. U. (2008) Ablation of GalNAc-4-sulfotransferase-1 enhances reproduction by altering the carbohydrate structures of luteinizing hormone in mice. J. Clin. Invest. 118, 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baenziger J. U. (2003) Glycoprotein hormone GalNAc-4-sulfotransferase. Biochem. Soc. Trans. 31, 326–330 [DOI] [PubMed] [Google Scholar]
- 38. Mi Y., Shapiro S. D., Baenziger J. U. (2002) Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4-SO4 receptor is critical for implantation in vivo. J. Clin. Invest. 109, 269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dell A., Chalabi S., Easton R. L., Haslam S. M., Sutton-Smith M., Patankar M. S., Lattanzio F., Panico M., Morris H. R., Clark G. F. (2003) Murine and human zona pellucida 3 derived from mouse eggs express identical O-glycans. Proc. Natl. Acad. Sci. U.S.A. 100, 15631–15636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yeh J. C., Ong E., Fukuda M. (1999) Molecular cloning and expression of a novel β-1,6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J. Biol. Chem. 274, 3215–3221 [DOI] [PubMed] [Google Scholar]
- 41. Schwientek T., Yeh J. C., Levery S. B., Keck B., Merkx G., van Kessel A. G., Fukuda M., Clausen H. (2000) Control of O-glycan branch formation: molecular cloning and characterization of a novel thymus-associated core 2 β1,6-N-acetylglucosaminyltransferase. J. Biol. Chem. 275, 11106–11113 [DOI] [PubMed] [Google Scholar]
- 42. Ismail M. N., Stone E. L., Panico M., Lee S. H., Luu Y., Ramirez K., Ho S. B., Fukuda M., Marth J. D., Haslam S. M., Dell A. (2011) High-sensitivity O-glycomic analysis of mice deficient in core 2 β1,6-N-acetylglucosaminyltransferases. Glycobiology 21, 82–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ikehara Y., Sato T., Niwa T., Nakamura S., Gotoh M., Ikehara S. K., Kiyohara K., Aoki C., Iwai T., Nakanishi H., Hirabayashi J., Tatematsu M., Narimatsu H. (2006) Apical Golgi localization of N,N′-diacetyllactosediamine synthase, β4GalNAc-T3, is responsible for LacdiNAc expression on gastric mucosa. Glycobiology 16, 777–785 [DOI] [PubMed] [Google Scholar]
- 44. Gill D. J., Clausen H., Bard F. (2011) Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–158 [DOI] [PubMed] [Google Scholar]
- 45. Hassan H., Bennett E. P., Mandel U., Hollingsworth M. A., Clausen H. (2000) Control of mucin-type O-glycosylation: O-glycan occupancy is directed by substrate specificities of polypeptide GalNAc transferases. in Carbohydrates in Chemistry and Biology: A Comprehensive Handbook. (Ernst B., Hart G. W., Sinay P., eds)pp. 273–292, Wiley-VCH, New York [Google Scholar]
- 46. Steentoft C., Vakhrushev S. Y., Vester-Christensen M. B., Schjoldager K. T., Kong Y., Bennett E. P., Mandel U., Wandall H., Levery S. B., Clausen H. (2011) Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 [DOI] [PubMed] [Google Scholar]
- 47. Dharmesh S. M., Skelton T. P., Baenziger J. U. (1993) Co-ordinate and restricted expression of the ProXaaArg/Lys-specific GalNAc-transferase and the GalNAc β1,4GlcNAc β1,2Man α-4-sulfotransferase. J. Biol. Chem. 268, 17096–17102 [PubMed] [Google Scholar]
- 48. Do K. Y., Do S. I., Cummings R. D. (1997) Differential expression of LacdiNAc sequences (GalNAc β1-4GlcNAc-R) in glycoproteins synthesized by Chinese hamster ovary and human 293 cells. Glycobiology 7, 183–194 [DOI] [PubMed] [Google Scholar]
- 49. Van den Eijnden D. H., Neeleman A. P., Van der Knaap W. P., Bakker H., Agterberg M., Van Die I. (1995) Novel glycosylation routes for glycoproteins: the lacdiNAc pathway. Biochem. Soc. Trans. 23, 175–179 [DOI] [PubMed] [Google Scholar]
- 50. Kawar Z. S., Haslam S. M., Morris H. R., Dell A., Cummings R. D. (2005) Novel poly-GalNAcβ1-4GlcNAc (LacdiNAc) and fucosylated poly-LacdiNAc N-glycans from mammalian cells expressing β1,4-N-acetylgalactosaminyltransferase and α1,3-fucosyltransferase. J. Biol. Chem. 280, 12810–12819 [DOI] [PubMed] [Google Scholar]
- 51. Green E. D., van Halbeek H., Boime I., Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 [PubMed] [Google Scholar]