Background: Nephrin is an immunoglobulin-like protein that facilitates insulin release by pancreatic beta cells.
Results: Nephrin phosphorylation at tyrosine residues responsible for SH2 domain binding is a Dynamin-dependent phenomenon, and it is necessary for glucose-stimulated insulin release in insulinoma cells and human islets.
Conclusion: Dynamin-dependent Nephrin phosphorylation is necessary for glucose-stimulated insulin secretion.
Significance: Pharmacological modulation of Nephrin phosphorylation may facilitate pancreatic beta cell function.
Keywords: Cell Culture, Diabetes, Insulin Secretion, Phosphotyrosine, Vesicles, Dynamin, Nephrin
Abstract
We have previously demonstrated a role for Nephrin in glucose stimulated insulin release (GSIR). We now hypothesize that Nephrin phosphorylation is required for GSIR and that Dynamin influences Nephrin phosphorylation and function. MIN6-C3 Nephrin-deficient pancreatic beta cells and human islets were transfected with WT-Nephrin or with a mutant Nephrin in which the tyrosine residues responsible for SH2 domain binding were substituted with phenylalanine (3YF-Nephrin). GSIR and live images of Nephrin and vesicle trafficking were studied. Immunoprecipitation experiments and overexpression of WT-Dynamin or dominant negative Dynamin mutant (K44A-Dynamin) in WT-Nephrin, 3YF-Nephrin, or Nephrin siRNA-transfected cells were utilized to study Nephrin-Dynamin interaction. In contrast to WT-Nephrin or to single tyrosine mutants, 3YF-Nephrin did not positively affect GSIR and led to impaired cell-cell contacts and vesicle trafficking. K44A-Dynamin prevented the effect of Nephrin on GSIR in the absence of protein-protein interaction between Nephrin and Dynamin. Nephrin gene silencing abolished the positive effects of WT-Dynamin on GSIR. The effects of protamine sulfate and vanadate on Nephrin phosphorylation and GSIR were studied in MIN6 cells and human islets. WT-Nephrin phosphorylation after glucose occurred at Tyr-1176/1193 and resulted in improved GSIR. On the contrary, protamine sulfate-induced phosphorylation at Tyr-1176/1193/1217 was associated with Nephrin degradation and impaired GSIR. Vanadate, which prevented Nephrin dephosphorylation after glucose stimulation, improved GSIR in human islets and MIN6 cells. In conclusion, Dynamin-dependent Nephrin phosphorylation occurs in response to glucose and is necessary for Nephrin-mediated augmentation of GSIR. Pharmacological modulation of Nephrin phosphorylation may thus facilitate pancreatic beta cell function.
Introduction
We have previously shown that Nephrin, an immunoglobulin-like transmembrane protein originally identified in kidney podocytes (1), is also expressed by pancreatic beta cells where it facilitates insulin release and vesicle formation (2). In the kidney, Nephrin modulates actin polymerization and cellular morphology of podocytes, which are highly specialized glomerular epithelial cells with numerous foot processes bridged by an extracellular structure known as the slit diaphragm (3, 4). At the slit diaphragm, Nephrin engages in homophilic interactions with other Nephrin molecules and in heterophilic interactions with other slit diaphragm proteins from neighboring podocytes (5). Because such interaction results in the modulation of Nephrin-dependent signaling (6), it is likely that Nephrin may contribute to cell-cell communication, an important feature of highly specialized cells such as pancreatic beta cell (7–9). An important role of Nephrin phosphorylation in governing cellular phase transitions (10) and in governing actin dynamics and lamellipodia formation (11, 12) has been recently reported. Upon homophilic or heterophilic interaction, tyrosine phosphorylation of Nephrin controls the interaction of Nephrin with the SH2-SH3 domain-containing adaptor proteins Nck1 and Nck2 (13, 14) and with PI3K (15–17), thus influencing actin polymerization and cellular morphology (18). Although Nephrin phosphorylation at different sites seems to have a major role in the formation of complexes that either facilitate or prevent Nephrin trafficking (19), the physiological role of Nephrin phosphorylation remains unclear. Inhibition of Nephrin phosphorylation by blockade of protein-tyrosine phosphatase 1B (PTP1b) has also been demonstrated to be detrimental to podocyte function (20). Although decreased Nephrin tyrosine phosphorylation has been described in both human and experimental proteinuria (21, 22), exogenous inducers of Nephrin phosphorylation can also cause proteinuria (23, 24). Nephrin phosphorylation can be experimentally induced by protamine sulfate (23), anti-Nephrin antibodies (24), podocin and the Src kinase Fyn (24–27), Nephrin interaction with Neph1 (28), or Nephrin homophilic interaction (29). However, the nature of physiological stimuli of Nephrin phosphorylation remains to be determined. The recent evidence that hyperglycemia causes a PKCα-dependent Nephrin internalization (30) suggests an important role of glucose as a stimulus for Nephrin phosphorylation and trafficking. If and how Nephrin phosphorylation affects pancreatic beta cell function remains to be determined.
Dynamin is a large guanosine triphosphatase (GTPase) that plays an important role in vesicle formation (31), actin remodeling (32–38), Nck interaction (39), glucose-stimulated ATP production (40), and insulin granule exocytosis (41). Furthermore, Dynamin dependent raft-mediated endocytosis is required for Nephrin internalization and proper signaling in podocytes (23). The similarities in Nephrin and Dynamin function, together with the evidence that Dynamin can affect Nephrin phosphorylation (23) have prompted us to investigate whether Nephrin and Dynamin share common functions in the process of glucose-stimulated insulin release (GSIR)2 in pancreatic beta cells. In particular, we studied the ability of Dynamin to influence Nephrin phosphorylation, Nephrin trafficking, and Nephrin augmentation of GSIR, and we investigated how the pharmacological modulation of Nephrin phosphorylation may affect GSIR in human islets and MIN6 cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Islet Culture
Both MIN6 cells and the Nephrin-deficient non-glucose-responsive C3 subclones (gift of Dr. A. Thomas) were cultured in 25 mm glucose DMEM (Invitrogen) (42). Human islets from cadaveric donors with research consent were obtained through the Islet Cell Resource Distribution system or were isolated at the local Human Cell Processing facility (43). Bright field microscopy at 20× magnification was utilized to screen the morphology of MIN6 cell lines transfected with wild type Nephrin and mutated Nephrin as described below. When indicated, human islets and MIN6 cells were incubated with a selective inhibitor of Src family tyrosine kinases (PP2, 1 μm; Sigma) overnight prior to glucose stimulation.
Western Blotting (WB) and Immunoprecipitation
For WB in MIN6 cells, a polyclonal guinea pig anti-Nephrin antibody (1:5000, C-terminal, Fitzgerald Laboratories, Concord, MA) and rabbit anti-phospho-Nephrin antibodies (1:2000, Tyr(P)-1176/1193 and 1:500 Tyr(P)-1217; Epitomics, Burlingame, CA) were utilized. For WB in human islets, 3.3K human islets (IEQ) were lysed in 0.5% CHAPS buffer (20 mm Tris, pH 7.5, 500 mm NaCl, and 0.5% CHAPS (w/v)). The lysates were run on 4–20% Mini-PROTEAN TGX precast polyacrylamide gels (Bio-Rad) and transferred to PVDF membranes (Millipore). The following antibodies were serially used: guinea pig anti-Nephrin (1:2000,1h,Fitzgerald), rabbit anti-guinea pig (1:3000, 1 h; Abcam), biotin goat anti-rabbit (1:3000, 1 h; Abcam) and streptavidin-peroxidase (1:1000, 15 min; Sigma). Washing buffer (TBS-T + 0.05% Tween 20) and blocking buffer (TBS-T + 5% milk or TBS-T + 1% BSA) were used. The biotin-blocking system (Dako) was also applied after incubation of rabbit anti-guinea pig. Co-immunoprecipitation studies were performed using HEK293 cells co-transfected with FLAG-Nephrin and GFP-tagged Nck1 or podocin. FLAG-Pin1 served as a negative control. Subconfluent cells grown on a 10-cm dish were transfected with 2 μg of cDNA using 6 μl of FuGENE 6 reagent (Roche Applied Science). The cells were incubated for 2 days at 37 °C after transfection. Protein lysates were collected in Triton buffer (50 mm Tris-HCl, 150 mm NaCl, and 1% Triton X-100), and FLAG fusion proteins were immunoprecipitated with 50 μl of FLAG-M2 beads (Sigma). Bound proteins were eluted with 50 μl of FLAG-peptide (Sigma), followed by standard SDS-polyacrylamide gel electrophoresis (Bio-Rad). For Western blot detection, rabbit anti-FLAG (1:50,000; Sigma), rabbit anti-GFP (1:1000; Clontech), and rabbit anti-Dynamin2 (1:40,000; Novus Biologicals, Littleton, CO) were used.
Cell Transfection, Infection, and siRNA
Fusion proteins of GFP with human WT-Nephrin or with mutated forms of human Nephrin were inserted into pcDNA3. In the mutated forms of Nephrin, the three tyrosine residues (at positions 1176, 1193, and 1217) responsible for SH2 domain binding were mutated to phenylalanine, either separately (Y1176F, Y1193F, and Y1217F) or all three at once (3YF-Nephrin). All of the constructs were used to generate stably transfected MIN6 cell lines using sterile sorting and antibiotic selection as described (2). The pcDNA3-GFP vector was used as negative control. Four separate clones were studied. For Nephrin gene silencing, a pool of four siRNAs (On Target Plus SMART pool; Dharmacon, Lafayette, CO) was transfected, and efficiency was determined as previously described (2). Adenovirus carrying WT-Dynamin or the Dynamin mutant K44A (K44A-Dynamin, a dominant negative mutant that lacks GTPase activity; gift of Dr. S. Sever) (44) was applied to MIN6 cells stably transfected with either WT-Nephrin, 3YF-Nephrin, Nephrin siRNA, or their respective controls. Nephrin overexpression was achieved by lentiviral transduction. GFP-tagged WT-Nephrin and 3YF-Nephrin were cloned into the VVPW lentiviral vector (gift of Dr. G. Luca Gusella). GFP alone in the VVPW vector was used as a control. 100% confluent HEK 293T cells were transfected with the VVPW and the packing and envelope plasmids, psPAX2 and pCMV-VSVG (Addgene, Cambridge, MA), in a ratio of 3:2:1 using FuGENE 6, according to the manufacturer's protocol. After 16 h, the medium was changed to Dulbecco's modified Eagle's medium and 10% fetal bovine serum containing penicillin and streptomycin. After 24 or 48 h of incubation, lentivirus-containing supernatant was harvested and stored at 4 °C. The pooled supernatant was centrifuged (600 × g/5 min), filtered through a 0.45-μm filter, and aliquoted at −80 °C. Viral titers were determined by serial dilution, Western blotting, and immunofluorescence. Isolated human islets (n = 4) were infected with the lentivirus for 16 h. At day 4, the infected islets were used for insulin secretion experiments.
Cell Viability
The cells expressing Nephrin wild-type or mutated constructs were trypsinized after 72 h in culture, stained with 7-amino-actinomycin D (Invitrogen) as a marker for cell death, and analyzed by flow cytometry, and the data were expressed as percentages of 7-amino-actinomycin D positive cells.
Confocal Images of Nephrin Localization and Trafficking
For immunocytochemistry, WT-Nephrin-overexpressing cells were fixed with 4% paraformaldehyde and counterstained with rhodamine-labeled phalloidin and DAPI (Invitrogen). The same cells were utilized to study WT-Nephrin and 3YF-Nephrin localization at base line and in response to different stimuli. Images of WT-Nephrin-overexpressing cells exposed to 11 mm glucose for 20 min at 37 °C, protamine sulfate (PS, 300 μg/ml for 20 min), and PP2 (1 μm) administered overnight prior to glucose stimulation were acquired with a Leica SP5-confocal-DMI6000 microscope. Staining of GFP-Nephrin-transfected cells with N-(3-Triethylammoniumpropyl)-4-(6-(4-(Diethylamino)Phenyl)Hexatrienyl)Pyridinium Dibromide (FM4-64) styryl dye (Invitrogen) was performed to quantify the percentage of FM positive plasma membrane that was also positive for GFP-Nephrin using the National Institutes of Health ImageJ software.
Live Images of Vesicle Trafficking
For live cell imaging, the cells were grown on 15-mm coverglasses and mounted in an open face chamber. An inflow and an outflow port connected to a pump allowed for the continuous perifusion 0.5 mm glucose in HBSS first, followed by stimulation with 11 mm glucose up to 20 min at 37 °C. The rate of vesicle endocytosis and exocytosis was determined based on an established method utilizing 1 μg/ml of the lipophilic styryl dye FM4-64 (45). Image acquisitions were performed using a Leica SP5 confocal DMI6000 microscope. For the quantification of FM dye intensity over time following glucose stimulation, an acquisition of 10 different cells per condition was performed, and images were analyzed by ImageJ.
Total Internal Reflection Fluorescence (TIRF) Microscopy
The cells were grown on 25-mm coverslips and incubated in HBSS with 0.5 mm glucose and analyzed by TIRF microscopy using a Zeiss Axiovert 200M microscope (Carl Zeiss, New York, NY) equipped with a αPlan-Fluar ×100/1.45 oil objective, a TIRF-slider, a LASOS 77 laser for excitation, and an AxioCamHS camera for image capture. For detection of GFP fluorescence, the following filter sets from Zeiss were used: excitation, 488/10 nm; dichroic, 492 nm; and emission, 520/35 nm. The cells were randomly selected and analyzed for 5 min to obtain the base-line change in fluorescence caused by a combination of photobleaching and Nephrin trafficking. New cells on the same coverslip were then imaged after addition of 16.7 mm glucose at 60 s. To mimic the effect of addition of fluid to the chamber, HBSS was added at 90 s for the untreated cells. Changes in fluorescence intensity were evaluated using ImageJ, and the data were expressed as f/f0 plot diagram of the means ± S.D. of three independent experiments.
Static Incubation and Perifusions
Analyses of insulin production at base line and after glucose stimulation were performed as previously described (46). Briefly, control, Nephrin siRNA, or Nephrin (WT and mutants) overexpressing MIN6 cells were utilized for the in vitro analysis of GSIR after stimulation with 11 mm glucose (expressed as the ratio of insulin secreted at 11 mm glucose to 0.5 mm glucose). Insulin secretion was studied by ELISA (Mercodia, SW) and normalized to DNA content (Quanti-iT PicoGreen dsDNA assay kit; Invitrogen). For perifusion studies, 110 human islets/condition were loaded on microcolumns connected to an inflow and an outflow port of a customized perifusion system (Biorep, Miami, FL). Islets were perifused with medium of defined composition (3 mm glucose, 11 mm glucose and 25 mm KCl), and the samples were collected every 2 min for insulin determination. For experiments with PS (30 and 300 μg/ml) or vanadate (0.1 or 1 mm), human islets and MIN6 cells were incubated for 30 min at low glucose concentration modified HEPES buffer (125 mm NaCl, 5.9 mm KCl, 2.56 mm CaCl2, 1.2 mm MgCl2, 25 mm HEPES, and 1 mg/ml BSA) with any of the above agents prior to glucose stimulation. For static incubation studies, insulin secretion was measured in 25 size-matched human islets isolated from four different donors at day 4 after viral infection. Groups of 25 size-matched infected islets of control, WT-Nephrin, or 3YF-Nephrin were serially incubated in 3 mm glucose for 1.5 h and then 11 mm glucose for 1.5 h. Insulin content was determined using an Insulin ELISA kit (Mercodia) and normalized to total cellular protein measured by the Bio-Rad DC protein assay kit.
Statistical Analysis
The results are represented as the means with standard deviation of three to eight independent experiments. When one-way analysis of variance showed statistical significance, the results were compared using t test after Tukey's correction for multiple comparisons (Graph Pad Prism software). Statistical significance was set at p < 0.05.
RESULTS
Glucose-induced Nephrin Phosphorylation Is Responsible for Nephrin Trafficking and Insulin Secretion
Because Nephrin trafficking plays an important role in GSIR (2), and Nephrin trafficking is phosphorylation-dependent and induced by hyperglycemia (30), we investigated whether 11 mm glucose can induce Nephrin phosphorylation when compared with 0.5 mm in MIN6-C3 overexpressing WT-Nephrin or 3YF-Nephrin. We were able to detect increased Nephrin phosphorylation at tyrosine residues 1176 and 1193 after exposure to high glucose (11 mm versus 0.5 mm) in WT-Nephrin-overexpressing cells (Fig. 1A). PS treatment (300 μg/ml for 20 min) utilized as positive control led to a significant induction of Nephrin phosphorylation at tyrosine residues 1176, 1193, and 1217. Because these three residues have been reported to mediate SH2 domain binding (13), we tested whether overnight incubation with 1 μm of the Src kinase inhibitor PP2 prior to exposure to 11 mm glucose would prevent Nephrin phosphorylation. We were able to demonstrate a complete prevention of Nephrin phosphorylation, confirming that Nephrin phosphorylation is mediated by Src. Nephrin phosphorylation at residue 1176, 1193, or 1217 did not occur in 3YF-Nephrin-overexpressing cells. To understand the role of Nephrin tyrosine phosphorylation in the process of GSIR, we investigated whether overexpression of mutated Nephrin containing individual tyrosine to phenylalanine substitutions at position 1176, 1193, or 1217 (Y1176F, Y1193F, or Y1217F) or combined (3YF) would affect GSIR. As expected, overexpression of WT-Nephrin resulted in the increase in GSIR (Fig. 1B). However, transfection of the cells with the single mutant forms of Nephrin was not sufficient to affect GSIR, whereas GSIR was impaired when the triple mutant form was utilized. These data indicate that Nephrin phosphorylation at more than one tyrosine residue is required for proper GSIR. Cells transfected with WT-Nephrin or with any of the mutants were viable when compared with control cells (Fig. 1C). Because we previously described an important role of Nephrin trafficking in response to glucose (2), we further investigated whether the pharmacological modulation of Nephrin phosphorylation would affect Nephrin trafficking in MIN6 cells. We were able to demonstrate that PS (300 μg/ml for 20 min) induced WT-Nephrin internalization similar to what we have described for glucose (1), whereas treatment with 1 μm PP2 overnight prior to stimulation with 11 mm glucose prevented such phenomenon (Fig. 1D). Interestingly, 3YF-Nephrin was primarily localized to intracellular compartments and did not traffic in response to any of the stimuli utilized. The localization of 3YF-Nephrin to intracellular compartments and not to the plasma membrane suggests that Nephrin phosphorylation may be essential for both Nephrin endocytosis and exocytosis. Because proper GSIR in pancreatic beta cells requires cell to cell contact, and Nephrin contributes to the formation of a slit diaphragm between two interdigitating podocytes in the kidney, we tested whether contacts between neighboring cells were affected by Nephrin. We compared WT-Nephrin to 3YF-Nephrin-overexpressing cells cultured in 0.5 mm glucose for 2 h. We were able to demonstrate that although WT-Nephrin-transfected cells establish side to side contacts, such contacts were compromised in cells overexpressing 3YF-Nephrin, which grew in a scattered fashion. Furthermore, 3YF-Nephrin-overexpressing cells lost the cortical actin distribution that is typical for WT-Nephrin-overexpressing cells (Fig. 1E). Overall, these data support a role for Nephrin and Nephrin phosphorylation in the modulation of pancreatic beta cell morphology, communication, and function. To validate our findings in human islets, islet cell preparations from four different donors were utilized for lentivirus-dependent transfection with either WT-Nephrin or 3YF-Nephrin or empty vector control, and static incubation experiments were performed to investigate insulin release at both 3 and 11 mm glucose. Although Nephrin transfection resulted in the expected augmentation of insulin release in response to 11 mm glucose, this phenomenon did not occur in 3YF-Nephrin-transfected human islets (Fig. 1F). WB analysis confirmed Nephrin transfection in human islets (Fig. 1G).
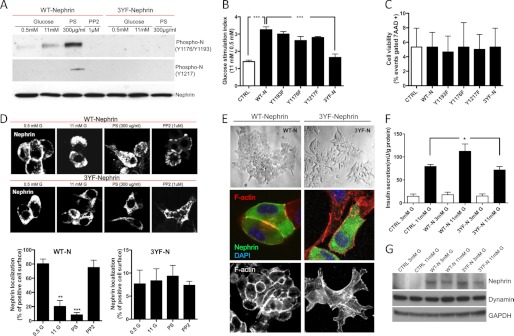
FIGURE 1.
Nephrin phosphorylation affects responsiveness to glucose and cell morphology. A, the degree of Nephrin phosphorylation at tyrosine residues 1176/1193 and 1217 was studied by WB in MIN6-C3 subclones (glucose unresponsive) overexpressing WT-Nephrin or 3YF-Nephrin. The cells were stimulated with glucose (11 mm), with PS (300 μg/ml) or PP2 (1 μm) in combination with 11 mm glucose. Although both glucose and PS induced phosphorylation of WT-Nephrin, PS induced phosphorylation of Nephrin only at residue Tyr-1217. PP2 and transfection with 3YF-Nephrin prevented Nephrin phosphorylation. B, static incubation experiments were performed in MIN6-C3 cells transfected with an empty vector (CTRL), WT-Nephrin (WT-N), single tyrosine residues mutants (Y1193F, Y1176F, and Y1217F), or 3YF-Nephrin (3YF-N) and demonstrated that Nephrin phosphorylation is essential for GSIR. ***, p < 0.001, n = 6. C, bar graph analysis of cell viability (% of 7-amino-actinomycin D (7AAD) positive cells) in MIN6-C3 cells transfected with an empty vector (CTRL), WT-Nephrin (WT-N), single tyrosine residues mutants (Y1193F, Y1176F, and Y1217F), or 3YF-Nephrin (3YF-N). No effects on cell viability were observed. D, Nephrin trafficking after 11 mm glucose, PS (300 μg/ml), and PP2 (1 μm) stimulation was studied in WT-Nephrin or 3YF-Nephrin-overexpressing cells. Although glucose and PS induced WT-Nephrin internalization, PP2 treatment resulted in a peripheral WT-Nephrin distribution. On the contrary, 3YF-Nephrin was localized in the intracellular compartments and was not affected by glucose, PS, or PP2. Bar graph analyses of the co-localization of WT-Nephrin and 3YF-Nephrin with the FM dye 4-64 utilized to stain plasma membranes are also shown. **, p < 0.01; ***, p < 0.001. E, WT-Nephrin-overexpressing cells presented with a normal morphology by light microscopy, whereas 3YF-Nephrin-overexpressing cells seemed more scattered and were characterized by impaired cell to cell contacts. Confocal images of WT-Nephrin-overexpressing cells showing a localization of WT-Nephrin primarily on the cell surface and at sites of cell to cell contact, whereas 3YF-Nephrin seemed to be primarily localized to intracellular compartments. 3YF-Nephrin-overexpressing cells did not show any cell to cell contacts. Phalloidin staining (bottom) was performed to demonstrate that cortical F-actin distribution seen in WT-Nephrin-overexpressing cells was impaired in 3YF-Nephrin-overexpressing cells. Three independent experiments with imaging of 5–10 cells each were performed. F, bar graph analysis for insulin secretion in human islets isolated from four different donors transfected with an empty vector (control, CTRL), WT-Nephrin (WT-N), or 3YF-Nephrin (3YF-N) exposed to either 3 or 11 mm glucose. *, p < 0.05. G, representative Western blot demonstrating efficiency of transfection of WT-Nephrin and 3YF-Nephrin in human islets.
Nephrin Phosphorylation Positively Influences Vesicle Formation and Secretion
We performed live cell imaging of WT- and 3YF-Nephrin-overexpressing cells stained with FM dye 4-64 to visualize endocytosis and exocytosis of vesicles. The relative level of vesicle endocytosis was measured by determining the total fluorescence achieved after complete vesicles exocytosis via a single stimulation with 25 mm KCl followed by incubation with 1 μg/ml of FM4-64 for 15 min as described (45). After three washes with calcium-free PBS, fluorescence images were acquired at base line and after subsequent stimulation with 11 mm glucose to assess vesicle exocytosis. Images were taken every 5 min for a total of 20 min, and the loss of fluorescence measured during stimulation was utilized to indicate the rate and amount of vesicle exocytosis. Although WT-Nephrin-overexpressing cells had a higher base-line fluorescence, suggesting a higher endocytotic activity, stimulation with glucose led to a more rapid loss of fluorescence in WT-Nephrin-overexpressing cells when compared with 3YF-Nephrin-overexpressing cells, where the fluorescence intensity remained mainly unchanged over time (Fig. 2A). Next, we performed TIRF microscopy to study WT-Nephrin and 3YF-Nephrin trafficking in response to glucose. The fluorescence signal obtained by TIRF imaging represents GFP-Nephrin within 150–200 nm of the coverslip, i.e., in or in close proximity to the plasma membrane. In untreated cells, the fluorescence signal decayed over time mostly because of photobleaching (Fig. 2B, untreated) independently of the expressed Nephrin form. Glucose stimulation reversed this decay and even led to a significant increase in TIRF-fluorescence in WT-Nephrin-overexpressing cells. However, this effect was absent in 3YF-Nephrin-overexpressing cells. The difference in TIRF between untreated and glucose-stimulated cells at the end of the experiment was 2.2-fold for WT-Nephrin cells and only 1.1-fold for 3YF-Nephrin, indicating that WT-Nephrin was recruited to the plasma membrane, whereas 3YF-Nephrin was not.
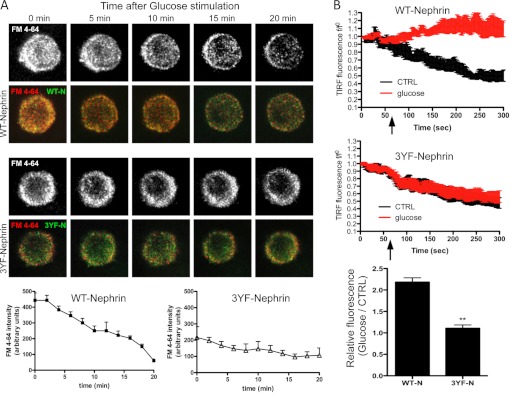
FIGURE 2.
Vesicles formation and secretion differs in WT-Nephrin and 3YF-Nephrin-overexpressing cells. A, cell clones were exposed to the FM dye 4-64 (red) to determine the degree of vesicle internalization at base line (time 0) and to determine the degree of vesicle secretion over time after glucose stimulation with 11 mm glucose. Although WT-Nephrin-overexpressing cells (WT-N) had higher vesicle content at time 0, red fluorescence over time was lost in WT-Nephrin clones upon glucose stimulation. In contrast, 3YF-Nephrin-overexpressing cells (3YF-N) demonstrated an impaired ability to secrete vesicles upon glucose stimulation. The graphic representation of the means ± S.D. of FM4-64 intensity measured every 2 min in four different WT-Nephrin clones when compared with four 3YF-Nephrin clones is shown. WT-Nephrin-overexpressing cells generated more vesicles at base line and were characterized by a more rapid loss of vesicles after glucose stimulation. B, WT-Nephrin- or 3YF-Nephrin-overexpressing cells were analyzed by TIRF microscopy. Changes in GFP-TIRF fluorescence intensity were monitored after either HBSS addition (CTRL) or a 16.7 mm glucose addition (glucose) at 60 s and plotted as the means ± S.D. f/f0 of three experiments. The time point of addition is marked with an arrow. The bar graph represents the ratio of relative TIRF fluorescence intensity at 300 s between glucose-treated and untreated cells. **, p < 0.001, n = 3.
Nephrin Is a Downstream Effector of Dynamin in Glucose-stimulated Insulin Release
Dynamin is a master regulator of vesicle trafficking and actin remodeling (24–32), and it promotes GSIR (33). Because those functions are similar to what we have described for Nephrin (1), we investigated whether a decrease in Nephrin expression in MIN6 cells could alter the effect of Dynamin on GSIR. Although WT-Dynamin induced the expected augmentation of GSIR (41), WT-Dynamin overexpression in Nephrin siRNA-treated MIN6 cells did not result in an augmentation of GSIR, similar to what was observed with the dominant negative Dynamin mutant (K44A-Dynamin; Fig. 3A). We also performed GSIR experiments in cells overexpressing WT-Dynamin or K44A-Dynamin mutants that were stably transfected with WT-Nephrin, 3YF-Nephrin, or GFP alone. WT-Nephrin did not overcome the negative effect of K44A-Dynamin on GSIR, suggesting that Dynamin is essential to promote Nephrin-mediated GSIR. Likewise, overexpression of WT-Dynamin was not sufficient to overcome the inhibitory effect of 3YF-Nephrin on GSIR (Fig. 3B). We therefore investigated whether Dynamin is essential to promote Nephrin phosphorylation. Although the induction of Nephrin phosphorylation by high glucose (11 mm) and PS (300 μg/ml) were conserved in WT-Dynamin-overexpressing cells, this effect was abolished in K44A-Dynamin-overexpressing cells (Fig. 3, C and D). Overall, these data suggest that Dynamin GTPase activity is necessary for Nephrin phosphorylation and function.
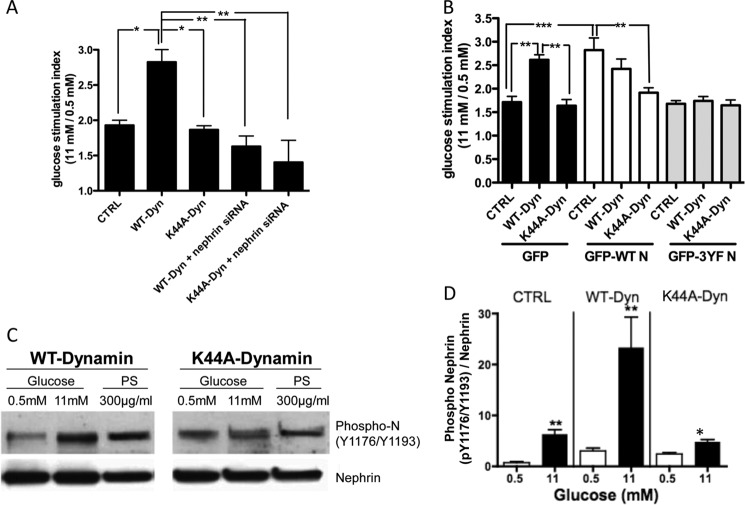
FIGURE 3.
Nephrin mediates the positive effect of Dynamin on insulin secretion. A, regular MIN6 cells that in contrast to MIN6-C3 express endogenous Nephrin were infected with WT-Dynamin or K44A-Dynamin mutants in the presence or absence of Nephrin siRNA. When Nephrin siRNA was present, the effect of Dynamin on insulin secretion was abolished. Likewise, no GSIR was observed in K44A-Dynamin-overexpressing cells in the presence of Nephrin siRNA. *, p < 0.05; **, p < 0.01, n = 4. B, MIN6 cells stably transfected with either empty vector control (CTRL), WT-Nephrin, or 3YF-Nephrin were infected with Dynamin constructs (WT-Dynamin or K44A-Dynamin). Glucose stimulation indices demonstrated that WT-Dynamin did not further augment the stimulation index observed in WT-Nephrin-overexpressing cells. In addition, the positive effect of WT-Dynamin on GSIR was completely abolished in 3YF-Nephrin-overexpressing cells. **, p < 0.01; ***, p < 0.001. C, Nephrin phosphorylation was determined in WT-Dynamin- and K44A-Dynamin-infected cells. Overexpression of K44A-Dynamin abolished the ability of glucose and PS to stimulate Nephrin phosphorylation. D, quantitative bar graph analysis of Nephrin phosphorylation in control cells (CTRL), WT-Dynamin-overexpressing cells (WT-Dyn), and K44A-Dynamin-overexpressing cells (K44A-Dyn) cultured in 0.5 or 11 mm glucose. *, p < 0.05; **, p < 0.01, n = 4.
Nephrin Trafficking Requires Dynamin GTPase Activity but Does Not Involve a Nephrin Dynamin Protein-Protein Interaction
WT-Nephrin-overexpressing cells were infected with either WT-Dynamin or K44A-Dynamin. WT-Dynamin-overexpressing cells conserved the ability of WT-Nephrin to internalize in response to glucose and PS (as shown for untransfected cells in Fig. 1D). On the contrary, K44A-Dynamin-overexpressing cells resulted in deficient WT-Nephrin trafficking from the plasma membrane to intracellular compartments in response to either stimulus (Fig. 4A). Given the presence of a functional interaction between Nephrin and Dynamin and based on the ability of Dynamin to bind several modulators of actin cytoskeleton remodeling (32–38), we tested whether Nephrin and Dynamin could physically interact. HEK293 cells, which express endogenous Dynamin (Fig. 4B), were transfected with FLAG-Nephrin or FLAG-Pin1. FLAG-Pin1 was used as a negative control. Co-transfections with GFP-Podocin or GFP-Nck1 were utilized as positive controls. After immunoprecipitation of FLAG fusion proteins, bound proteins were eluted with a FLAG peptide, and eluates were analyzed for the presence of FLAG and GFP proteins as well as Dynamin by Western blotting. Although FLAG-Nephrin was able to co-immunoprecipitate GFP-Nck1 and GFP-Podocin, FLAG-Nephrin did not interact with endogenous Dynamin. Overexpression of WT-Dynamin in FLAG-Nephrin expressing cells did also not result in the co-immunoprecipitation of both proteins (data not shown). As expected, FLAG-Pin1 did not bind GFP-Nck1, GFP-Podocin, or Dynamin (Fig. 4B), underlining the specificity of the binding assay. These results suggest that Nephrin is a downstream target of Dynamin function and that Dynamin-dependent Nephrin phosphorylation is not mediated by a direct protein-protein interaction but rather requires additional intermediates.
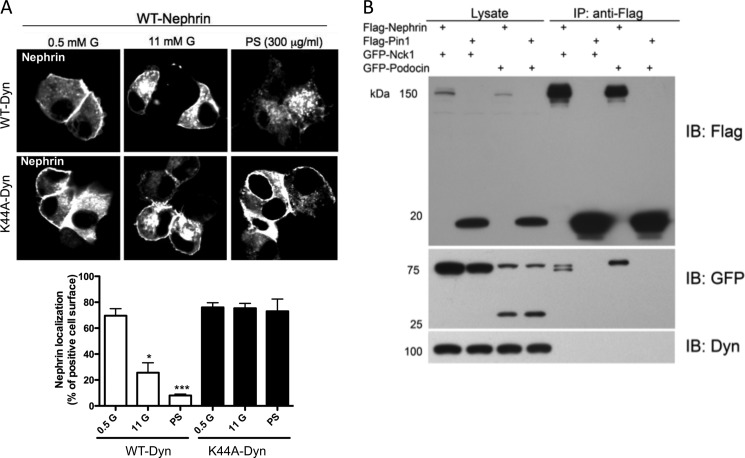
FIGURE 4.
Dynamin regulates Nephrin trafficking but does not physically interact with Nephrin. A, WT-Nephrin-transfected cells were utilized to visualize Nephrin trafficking upon stimulation with glucose or PS. Although WT-Dynamin overexpression did not impair Nephrin trafficking, K44A-Dynamin overexpression prevented Nephrin trafficking to intracellular compartments. The bottom panel shows bar graph analyses of WT-Nephrin and FM dye 4-64 co-localization in the presence of WT-Dynamin or K44A-Dynamin. FM dye 4-64 was utilized to stain plasma membranes. *, p < 0.05; ***, p < 0.001. B, immunoprecipitation experiments in FLAG-Nephrin-overexpressing cells were performed to determine whether Dynamin and Nephrin interact. FLAG-Pin1 was utilized as a negative control. Co-transfection of FLAG-Nephrin and GFP-Nck1 or GFP-Podocin was utilized as a positive control. Immunoprecipitation efficacy was verified by Western blot. A FLAG antibody was utilized to detect the FLAG epitope in eluates; the interaction with podocin and Nck1 was determined using a GFP antibody, and interaction with Dynamin was determined using a Dynamin-specific antibody. Western blot analysis indicates that endogenous Dynamin does not immunoprecipitates with FLAG-Nephrin. Six independent experiments were performed. IB, immunoblot.
Pharmacological Modulation of Nephrin Phosphorylation Influences Glucose-stimulated Insulin Release
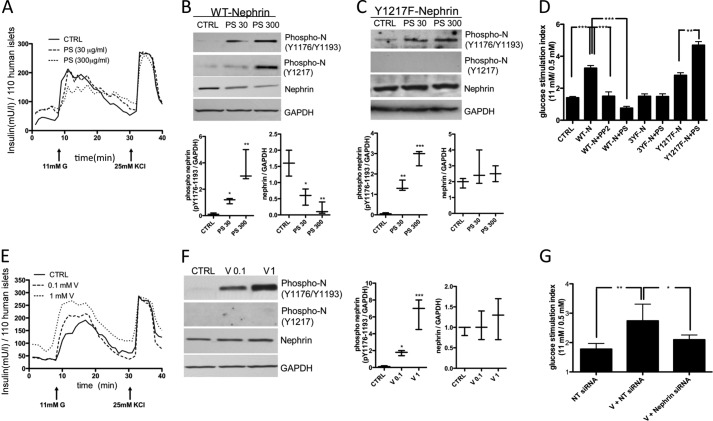
Given that Nephrin phosphorylation is essential for Nephrin trafficking and GSIR, we studied GSIR in human islets and MIN6 cells in the presence of an inducer of Nephrin phosphorylation (PS), an inhibitor of Src kinases involved in Nephrin phosphorylation (PP2), or a general tyrosine phosphatase inhibitor (sodium orthovanadate; Sigma). GSIR was impaired in human islets treated with 300 μg/ml PS (Fig. 5A). We furthermore observed that although PS caused a dose-dependent Nephrin phosphorylation in MIN6 cells at 20 min, this resulted in a reduction of total Nephrin protein levels at the same time (Fig. 5B). Because PS caused Nephrin phosphorylation at residue Tyr-1217 whereas glucose did not, we hypothesize that PS-induced Nephrin phosphorylation at Tyr-1217 is responsible for Nephrin degradation. To prove our hypothesis, we studied MIN6 cells infected with the Y1217F-Nephrin mutant, and we were able to demonstrate that PS failed to cause Nephrin degradation in these cells (Fig. 5C). Static incubation of WT-Nephrin-overexpressing cells confirmed that the addition of PS (300 μg/ml) resulted in a decreased stimulation index (primarily because of increased secretion of insulin at 0.5 mm glucose) when compared with control cells (Fig. 5D). However, PS had a positive effect on GSIR in cells transfected with the Y1217F-Nephrin mutant (Fig. 5D). Inhibition of Src kinase-dependent Nephrin phosphorylation achieved by incubation of WT-Nephrin-overexpressing cells with 1 μm PP2 abolished the positive effect of Nephrin on GSIR (Fig. 5D). Inhibition of tyrosine dephosphorylation by vanadate resulted in a dose-dependent increase of GSIR, although 1 mm vanadate also caused increased insulin secretion at low glucose concentration (Fig. 5E), suggesting that vanadate may affect the threshold for stimulus-secretion coupling. MIN6 cells exposed for 30 min to vanadate in the presence of 11 mm glucose showed a dose-dependent increase in Nephrin phosphorylation without affecting total Nephrin protein levels (Fig. 5F). Furthermore, vanadate treatment resulted in a significant augmentation of GSIR in MIN6 cells. This effect was partially prevented when Nephrin siRNA-treated cells were exposed to vanadate, suggesting that the positive effects of vanadate on GSIR may be partially mediated by the inhibition of Nephrin dephosphorylation (Fig. 5G). These data obtained in both MIN6 cells and human islets suggest that compounds capable of inducing Nephrin phosphorylation at Tyr-1176/1193 may improve stimulus-secretion coupling by pancreatic beta cells.
FIGURE 5.
PS reduces but vanadate increases insulin secretion in response to glucose in human islets. A, PS decreased insulin secretion in response to glucose (11 mm) in human islets. B, in MIN6 cells transfected with WT-Nephrin, PS (30 and 300 μg/ml) increased Nephrin phosphorylation (Tyr(P)-1176/Tyr-1193/Tyr-1217) in a dose-dependent manner, but this was accompanied by increased Nephrin degradation. *, p < 0.05; **, p < 0.01. C, in MIN6 cells transfected with Y1217F-Nephrin, PS (30 and 300 μg/ml) increased Nephrin phosphorylation (Tyr(P)-1176/Tyr-1193) in a dose-dependent manner without affecting phosphorylation of Nephrin at Tyr-1217, and this was accompanied by stable total Nephrin levels. **, p < 0.01; ***, p < 0.001. D, PS decreased GSIR in WT-Nephrin-transfected MIN6 cells, similar to what was observed with PP2. ***, p < 0.001. However, PS increased GSIR in Y1217F-transfected MIN6 cells. **, p < 0.01. E, a dose-dependent increase in insulin secretion was observed in human islets pretreated with vanadate prior to glucose stimulation. F, Western blot analysis of phosphorylated and total Nephrin revealed that vanadate induced Nephrin phosphorylation (Tyr(P)-1176/Tyr-1193) in a dose-dependent manner (0.1 and 1 mm) without affecting total Nephrin levels. *, p < 0.05; ***, p < 0.001. G, vanadate (1 mm) also improved GSIR in MIN6 cells, a phenomenon that was partially prevented in Nephrin siRNA-treated cells. Islets from three independent donors were utilized for each experiment. *, p < 0.05; **, p < 0.01. CTRL, control. NT siRNA is a pool of non-targeting siRNA as a control.
DISCUSSION
Stimulus-secretion coupling of pancreatic beta cells has been extensively studied to develop new therapeutic strategies for the improvement of pancreatic beta cell function, and several key molecular players have been identified. However, only a few of the commonly available drugs for the treatment of patients with diabetes mellitus target glucose-stimulated insulin release. Therefore, the identification and characterization of novel drug targets involved in glucose-stimulated insulin release represents a major gap in the translation of experimental findings into clinical applications. We have previously described an important role of Nephrin in GSIR in both human islets and MIN6 cells, a role that is fully dependent on the ability of Nephrin to undergo glucose- and cytoskeleton-dependent trafficking in pancreatic beta cells (2). To date, no natural ligand that can bind Nephrin and facilitate its function has been identified. Because others have recently described that Nephrin trafficking is essential for Nephrin function in podocytes and is regulated by phosphorylation, we investigated how Nephrin phosphorylation affects GSIR. Furthermore, we have tested endogenous and exogenous inducers of Nephrin phosphorylation and examined their role in GSIR in MIN6 cells and in human islets.
Because Nephrin tyrosine phosphorylation has been described to be primarily mediated by Src kinases such as Fyn (24–27), we developed several Nephrin mutant forms with tyrosine to phenylalanine substitutions at three major residues that have been shown to be crucial for Nephrin-mediated regulation of the actin cytoskeleton. Because single mutants did not significantly affect GSIR (Fig. 1B), we have primarily utilized a triple mutant form to demonstrate that glucose- and PS-dependent Nephrin phosphorylation can be abrogated by using this mutant form of Nephrin (3YF-Nephrin; Fig. 1A). MIN6 C3 clones overexpressing 3YF-Nephrin showed impaired GSIR and were not capable of forming cell-cell contacts that have been described to be essential for the augmentation of GSIR in cultured pancreatic beta cells (7–9). Furthermore, Nephrin trafficking from and to the plasma membrane was impaired in these cells (Fig. 1D) without affecting cell viability (Fig. 1C). The scattered distribution of 3YF-Nephrin-overexpressing cells with decreased cell to cell contacts and the reorganization of the actin cytoskeleton including the loss of cortical actin (Fig. 1E) supports a primary role of Nephrin as an active regulator of actin cytoskeleton remodeling in pancreatic beta cells, similar to what has been described in podocytes (13, 14). Human islets infected with 3YF-Nephrin mutants also did not experience increases in insulin release as normally observed after infection with WT-Nephrin (Fig. 1F). It has been assumed for over a decade since Nephrin has been discovered that Nephrin localizes exclusively to the plasma membrane where it fulfills its primary role in the formation of the slit diaphragm by engaging in homophilic interactions with adjacent Nephrin molecules (5, 24) and heterophilic interactions with other structural components of the S.D (16, 47–49). Instead, the more dynamic aspects of Nephrin trafficking are a relatively new concept. It has been reported that Nephrin endocytosis in podocytes occurs under pathological conditions that involve the impairment of the slit diaphragm integrity (19). We demonstrated herein that phosphorylation-dependent Nephrin trafficking is essential under physiological conditions to grant GSIR and to facilitate proper secretory vesicle formation and exocytosis in pancreatic beta cells (Fig. 2). This is in line with the evidence that a novel role of Nephrin in vesicular docking has been described in podocytes, where the C terminus of Nephrin interacts with the v-SNARE protein VAMP-2 (50), which is also found on the membrane of insulin secretory granules in pancreatic beta cells (51). In pancreatic beta cells, glucose transiently modulates the cortical actin organization and disrupts the interaction of F-actin with the t-SNARE complex at the plasma membrane to facilitate glucose-stimulated insulin secretion (52). However, the relationship between SNARE-mediated exocytosis and actin reorganization remains to be fully understood. The pathway accounting for the regulated targeting of vesicles to their cognate t-SNAREs has not been established, and a functional Nephrin/actin interaction could represent the molecular link between GTPases and SNAREs. Actin reorganization and actin binding to t- and v-SNARE proteins has been shown to be dependent on Dynamin-2 (33, 41), a large GTPase that is functionally coupled to insulin granule exocytosis and that facilitates GSIR through a yet unknown mechanism (41). Because Dynamin is essential for the maintenance of podocyte structure and function (44), and it has recently been demonstrated that Dynamin affects Nephrin internalization (23), we tested whether Dynamin could mediate Nephrin phosphorylation and function in pancreatic beta cells. Glucose-induced Nephrin phosphorylation and insulin release were impaired in cells overexpressing a dominant negative Dynamin mutant form (Fig. 3, B–D), and Nephrin deficiency abolished the positive effect of Dynamin on GSIR (Fig. 3A), suggesting that Dynamin is a key mediator in the signaling pathway linking glucose stimulation to Nephrin phosphorylation. Because Dynamin is degraded by Cathepsin L, a proteolytic enzyme that is elevated in diabetes (44), it may be interesting to determine whether Cathepsin L inhibitors may facilitate GSIR. Alternatively, identification of small molecules or ligands that can stimulate Nephrin phosphorylation could be tested as drugs for the augmentation of GSIR. However, our data with PS and vanadate suggest that the role of Nephrin phosphorylation is more complex than originally expected, because inducers of Nephrin phosphorylation such as PS may result in a reduction of total Nephrin and therefore negatively affect islet cell function (Fig. 5). It is therefore likely that the phosphorylation states of different tyrosine residues within the C-terminal portion of Nephrin are responsible for the formation of different signaling complexes, which may lead to either Nephrin degradation or to physiological Nephrin trafficking. In fact, a positive effect of PS on GSIR that is associated with preservation of total Nephrin is observed in cells transfected with the Y1217F-Nephrin mutant (Fig. 5, C and D), strongly supporting a key role of Nephrin phosphorylation at Tyr-1217 as a prerequisite for Nephrin degradation.
In conclusion, we have demonstrated an important role of Dynamin-dependent Nephrin phosphorylation in stimulus-secretion coupling in pancreatic beta cells. A definitive role for Nephrin in pancreatic beta cell function remains to be established through ongoing metabolic studies in patients with Nephrin mutations and through the phenotypic analysis of mice carrying a conditional deletion of the Nephrin gene in pancreatic beta cells. Nevertheless, our study offers the rationale to screen drug libraries for the identification of modifiers of Nephrin phosphorylation that might be pharmacologically used to improve pancreatic beta cell function.
Acknowledgment
We thank Dr. George W. Burke (Department of Surgery, University of Miami, Miami, FL) for careful review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK82636, DK70460, and U42 RR016603. This work was also supported by American Diabetes Association Grant 7-09-JF-23, the Forest County Potawatomi Community Foundation, the Max and Yetta Karasik Family Foundation, the Diabetes Research Institute Foundation, the Swedish Research Council, the Knut and Alice Wallenberg Foundation, Torsten and Ragnar Söderberg's Foundation, the Swedish Diabetes Association, the Family Erling-Persson Foundation, the Peggy and Harold Katz Family Foundation, and the City of Hope, Duarte, CA.
- GSIR
- glucose-stimulated insulin release
- K44A-Dynamin
- a dominant negative Dynamin mutant
- PS
- protamine sulfate
- TIRF
- total internal reflection fluorescence
- WB
- Western blotting.
REFERENCES
- 1. Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R., Kashtan C. E., Peltonen L., Holmberg C., Olsen A., Tryggvason K. (1998) Positionally cloned gene for a novel glomerular protein–Nephrin–is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 [DOI] [PubMed] [Google Scholar]
- 2. Fornoni A., Jeon J., Varona Santos J., Cobianchi L., Jauregui A., Inverardi L., Mandic S. A., Bark C., Johnson K., McNamara G., Pileggi A., Molano R. D., Reiser J., Tryggvason K., Kerjaschki D., Berggren P. O., Mundel P., Ricordi C. (2010) Nephrin is expressed on the surface of insulin vesicles and facilitates glucose-stimulated insulin release. Diabetes 59, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestilä M., Jalanko H., Holmberg C., Tryggvason K. (1999) Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. U.S.A. 96, 7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tryggvason K. (1999) Unraveling the mechanisms of glomerular ultrafiltration. Nephrin, a key component of the slit diaphragm. J. Am. Soc. Nephrol. 10, 2440–2445 [DOI] [PubMed] [Google Scholar]
- 5. Khoshnoodi J., Sigmundsson K., Ofverstedt L. G., Skoglund U., Obrink B., Wartiovaara J., Tryggvason K. (2003) Nephrin promotes cell-cell adhesion through homophilic interactions. Am. J. Pathol. 163, 2337–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heikkilä E., Ristola M., Havana M., Jones N., Holthöfer H., Lehtonen S. (2011) Trans-interaction of Nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of Nephrin. Biochem. J. 435, 619–628 [DOI] [PubMed] [Google Scholar]
- 7. Jaques F., Jousset H., Tomas A., Prost A. L., Wollheim C. B., Irminger J. C., Demaurex N., Halban P. A. (2008) Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology 149, 2494–2505 [DOI] [PubMed] [Google Scholar]
- 8. Hauge-Evans A. C., Squires P. E., Persaud S. J., Jones P. M. (1999) Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli. Enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 48, 1402–1408 [DOI] [PubMed] [Google Scholar]
- 9. Wojtusciszyn A., Armanet M., Morel P., Berney T., Bosco D. (2008) Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 51, 1843–1852 [DOI] [PubMed] [Google Scholar]
- 10. Li P., Banjade S., Cheng H. C., Kim S., Chen B., Guo L., Llaguno M., Hollingsworth J. V., King D. S., Banani S. F., Russo P. S., Jiang Q. X., Nixon B. T., Rosen M. K. (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. George B., Verma R., Soofi A. A., Garg P., Zhang J., Park T. J., Giardino L., Ryzhova L., Johnstone D. B., Wong H., Nihalani D., Salant D. J., Hanks S. K., Curran T., Rastaldi M. P., Holzman L. B. (2012) Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J. Clin. Invest. 122, 674–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venkatareddy M., Cook L., Abuarquob K., Verma R., Garg P. (2011) Nephrin regulates lamellipodia formation by assembling a protein complex that includes Ship2, filamin and lamellipodin. PLoS One 6, e28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones N., Blasutig I. M., Eremina V., Ruston J. M., Bladt F., Li H., Huang H., Larose L., Li S. S., Takano T., Quaggin S. E., Pawson T. (2006) Nck adaptor proteins link Nephrin to the actin cytoskeleton of kidney podocytes. Nature 440, 818–823 [DOI] [PubMed] [Google Scholar]
- 14. Verma R., Kovari I., Soofi A., Nihalani D., Patrie K., Holzman L. B. (2006) Nephrin ectodomain engagement results in Src kinase activation, Nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116, 1346–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu J., Sun N., Aoudjit L., Li H., Kawachi H., Lemay S., Takano T. (2008) Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 73, 556–566 [DOI] [PubMed] [Google Scholar]
- 16. Huber T. B., Hartleben B., Kim J., Schmidts M., Schermer B., Keil A., Egger L., Lecha R. L., Borner C., Pavenstädt H., Shaw A. S., Walz G., Benzing T. (2003) Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell Biol. 23, 4917–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simons M., Schwarz K., Kriz W., Miettinen A., Reiser J., Mundel P., Holthöfer H. (2001) Involvement of lipid rafts in Nephrin phosphorylation and organization of the glomerular slit diaphragm. Am. J. Pathol. 159, 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tryggvason K., Pikkarainen T., Patrakka J. (2006) Nck links Nephrin to actin in kidney podocytes. Cell 125, 221–224 [DOI] [PubMed] [Google Scholar]
- 19. Quack I., Rump L. C., Gerke P., Walther I., Vinke T., Vonend O., Grunwald T., Sellin L. (2006) β-Arrestin2 mediates Nephrin endocytosis and impairs slit diaphragm integrity. Proc. Natl. Acad. Sci. U.S.A. 103, 14110–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aoudjit L., Jiang R., Lee T. H., New L. A., Jones N., Takano T. (2011) Podocyte protein, Nephrin, is a substrate of protein tyrosine phosphatase 1B. J. Signal Transduct. 2011, 376543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uchida K., Suzuki K., Iwamoto M., Kawachi H., Ohno M., Horita S., Nitta K. (2008) Decreased tyrosine phosphorylation of Nephrin in rat and human nephrosis. Kidney Int. 73, 926–932 [DOI] [PubMed] [Google Scholar]
- 22. Fan Q., Xing Y., Ding J., Guan N. (2009) Reduction in VEGF protein and phosphorylated Nephrin associated with proteinuria in adriamycin nephropathy rats. Nephron. Exp. Nephrol. 111, e92-e102 [DOI] [PubMed] [Google Scholar]
- 23. Qin X. S., Tsukaguchi H., Shono A., Yamamoto A., Kurihara H., Doi T. (2009) Phosphorylation of Nephrin triggers its internalization by raft-mediated endocytosis. J. Am. Soc. Nephrol. 20, 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahdenperä J., Kilpeläinen P., Liu X. L., Pikkarainen T., Reponen P., Ruotsalainen V., Tryggvason K. (2003) Clustering-induced tyrosine phosphorylation of Nephrin by Src family kinases. Kidney Int. 64, 404–413 [DOI] [PubMed] [Google Scholar]
- 25. Li H., Lemay S., Aoudjit L., Kawachi H., Takano T. (2004) SRC-family kinase Fyn phosphorylates the cytoplasmic domain of Nephrin and modulates its interaction with podocin. J. Am. Soc. Nephrol. 15, 3006–3015 [DOI] [PubMed] [Google Scholar]
- 26. Verma R., Wharram B., Kovari I., Kunkel R., Nihalani D., Wary K. K., Wiggins R. C., Killen P., Holzman L. B. (2003) Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J. Biol. Chem. 278, 20716–20723 [DOI] [PubMed] [Google Scholar]
- 27. Liu X. L., Doné S. C., Yan K., Kilpeläinen P., Pikkarainen T., Tryggvason K. (2004) Defective trafficking of Nephrin missense mutants rescued by a chemical chaperone. J. Am. Soc. Nephrol. 15, 1731–1738 [DOI] [PubMed] [Google Scholar]
- 28. Garg P., Verma R., Nihalani D., Johnstone D. B., Holzman L. B. (2007) Neph1 cooperates with Nephrin to transduce a signal that induces actin polymerization. Mol. Cell Biol. 27, 8698–8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barletta G. M., Kovari I. A., Verma R. K., Kerjaschki D., Holzman L. B. (2003) Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J. Biol. Chem. 278, 19266–19271 [DOI] [PubMed] [Google Scholar]
- 30. Quack I., Woznowski M., Potthoff S. A., Palmer R., Königshausen E., Sivritas S., Schiffer M., Stegbauer J., Vonend O., Rump L. C., Sellin L. (2011) PKC α mediates β-arrestin2-dependent Nephrin endocytosis in hyperglycemia. J. Biol. Chem. 286, 12959–12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Praefcke G. J., McMahon H. T. (2004) The Dynamin superfamily. Universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 [DOI] [PubMed] [Google Scholar]
- 32. Krueger E. W., Orth J. D., Cao H., McNiven M. A. (2003) A Dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14, 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schafer D. A. (2004) Regulating actin dynamics at membranes. A focus on Dynamin. Traffic 5, 463–469 [DOI] [PubMed] [Google Scholar]
- 34. Merrifield C. J., Feldman M. E., Wan L., Almers W. (2002) Imaging actin and Dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4, 691–698 [DOI] [PubMed] [Google Scholar]
- 35. McNiven M. A., Kim L., Krueger E. W., Orth J. D., Cao H., Wong T. W. (2000) Regulated interactions between Dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 151, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orth J. D., Krueger E. W., Cao H., McNiven M. A. (2002) The large GTPase Dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee E., De Camilli P. (2002) Dynamin at actin tails. Proc. Natl. Acad. Sci. U.S.A. 99, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gu C., Yaddanapudi S., Weins A., Osborn T., Reiser J., Pollak M., Hartwig J., Sever S. (2010) Direct Dynamin-actin interactions regulate the actin cytoskeleton. EMBO J. 29, 3593–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim Y., Chang S. (2006) Ever-expanding network of Dynamin-interacting proteins. Mol. Neurobiol. 34, 129–136 [DOI] [PubMed] [Google Scholar]
- 40. Zhang Z., Wakabayashi N., Wakabayashi J., Tamura Y., Song W. J., Sereda S., Clerc P., Polster B. M., Aja S. M., Pletnikov M. V., Kensler T. W., Shirihai O. S., Iijima M., Hussain M. A., Sesaki H. (2011) The Dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol. Biol. Cell 22, 2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Min L., Leung Y. M., Tomas A., Watson R. T., Gaisano H. Y., Halban P. A., Pessin J. E., Hou J. C. (2007) Dynamin is functionally coupled to insulin granule exocytosis. J. Biol. Chem. 282, 33530–33536 [DOI] [PubMed] [Google Scholar]
- 42. Miyazaki J., Araki K., Yamato E., Ikegami H., Asano T., Shibasaki Y., Oka Y., Yamamura K. (1990) Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion. Special reference to expression of glucose transporter isoforms. Endocrinology 127, 126–132 [DOI] [PubMed] [Google Scholar]
- 43. Ricordi C., Strom T. B. (2004) Clinical islet transplantation. Advances and immunological challenges. Nat. Rev. Immunol. 4, 259–268 [DOI] [PubMed] [Google Scholar]
- 44. Sever S., Altintas M. M., Nankoe S. R., Möller C. C., Ko D., Wei C., Henderson J., del Re E. C., Hsing L., Erickson A., Cohen C. D., Kretzler M., Kerjaschki D., Rudensky A., Nikolic B., Reiser J. (2007) Proteolytic processing of Dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J. Clin. Invest. 117, 2095–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gaffield M. A., Betz W. J. (2006) Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat. Protoc. 1, 2916–2921 [DOI] [PubMed] [Google Scholar]
- 46. Fornoni A., Pileggi A., Molano R. D., Sanabria N. Y., Tejada T., Gonzalez-Quintana J., Ichii H., Inverardi L., Ricordi C., Pastori R. L. (2008) Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia 51, 298–308 [DOI] [PubMed] [Google Scholar]
- 47. Liu G., Kaw B., Kurfis J., Rahmanuddin S., Kanwar Y. S., Chugh S. S. (2003) Neph1 and Nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J. Clin. Invest. 112, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huber T. B., Kottgen M., Schilling B., Walz G., Benzing T. (2001) Interaction with podocin facilitates Nephrin signaling. J. Biol. Chem. 276, 41543–41546 [DOI] [PubMed] [Google Scholar]
- 49. Lehtonen S., Lehtonen E., Kudlicka K., Holthöfer H., Farquhar M. G. (2004) Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing Nephrin. Am. J. Pathol. 165, 923–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Coward R. J., Welsh G. I., Koziell A., Hussain S., Lennon R., Ni L., Tavaré J. M., Mathieson P. W., Saleem M. A. (2007) Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56, 1127–1135 [DOI] [PubMed] [Google Scholar]
- 51. Wheeler M. B., Sheu L., Ghai M., Bouquillon A., Grondin G., Weller U., Beaudoin A. R., Bennett M. K., Trimble W. S., Gaisano H. Y. (1996) Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology 137, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 52. Thurmond D. C., Gonelle-Gispert C., Furukawa M., Halban P. A., Pessin J. E. (2003) Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol. Endocrinol. 17, 732–742 [DOI] [PubMed] [Google Scholar]