Background: An unusual bacterium GFAJ-1 grows in +As/−P medium and was purported to substitute arsenic for phosphorus in cell macromolecules.
Results: E. coli also grows in +As/−P medium and is due to massive ribosome degradation and arsenate tolerance.
Conclusion: Growth in +As/−P medium can be explained without invoking arsenic incorporation into biological macromolecules.
Significance: It is unlikely that strain GFAJ-1 represents a novel arsenic-based life form.
Keywords: Bacteria, Bacterial Metabolism, Ribosomal RNA (rRNA), Ribosome Assembly, Stress, Arsenate, Arsenate Resistance
Abstract
A recent study (Wolfe-Simon, F., Switzer Blum, J., Kulp, T. R., Gordon, G. W., Hoeft, S. E., Pett-Ridge, J., Stolz, J. F., Webb, S. M., Weber, P. K., Davies, P. C., Anbar, A. D., and Oremland, R. S. (2011) Science 332, 1163–1166) described the isolation of a special bacterial strain, GFAJ-1, that could grow in medium containing arsenate, but lacking phosphate, and that supposedly could substitute arsenic for phosphorus in its biological macromolecules. Here, we provide an alternative explanation for these observations and show that they can be reproduced in a laboratory strain of Escherichia coli. We find that arsenate induces massive ribosome degradation, which provides a source of phosphate. A small number of arsenate-tolerant cells arise during the long lag period prior to initiation of growth in +As/−P medium, and it is this population that undergoes the very slow, limited growth observed for both E. coli and GFAJ-1. These results provide a simple explanation for the reported growth of GFAJ-1 in arsenate without invoking replacement of phosphorus by arsenic in biological macromolecules.
Introduction
A great deal of excitement has been generated by the recent report of the isolation of a bacterial strain that can grow in the presence of arsenate in a medium apparently lacking any source of phosphate (+As/−P) (1). Based on this information, the authors concluded that arsenic substitutes for phosphorus in the phosphorus-containing molecules of this organism. Although justifiable skepticism has arisen over whether arsenic can actually substitute for phosphorus in DNA, RNA, and other molecules (Ref. 2 and references therein), the fact remains that the addition of arsenate was found to stimulate growth of Halomonadaceae GFAJ-1 cells in a Pi-free medium (1). Thus, although arsenic cannot replace phosphorus in biological material, based on recent evidence (3, 4), how growth could occur in +As/−P medium is still an open question. Despite considerable speculation, no clear explanation has been provided for this observation.
Wolfe-Simon et al. (1) found that GFAJ-1 cells do not grow in medium lacking both arsenate and phosphate, but that growth ensues after a long lag of ∼80 h upon the addition of 40 mm arsenate. Growth is extremely slow and limited, amounting to a 20-fold increase in cell number over a period of 6 days. Nevertheless, growth does occur and was shown not to be due to phosphate impurities in the growth medium as there is no growth in the absence of added arsenate. However, no explanation was given for the long lag period prior to commencement of growth or for why growth is so limited despite the presence of a high concentration of arsenate.
In this study, we provide a detailed explanation for this phenomenon and show that it can be completely reproduced in a laboratory strain of Escherichia coli. We find that the presence of arsenate induces massive ribosome degradation in the bacterial cell population. This degradation, which releases free bases from RNA, provides sufficient phosphate to allow limited growth of the small number of cells that are selected for arsenate tolerance during the long lag period prior to initiation of growth. These findings provide a simple explanation for the growth of GFAJ-1 cells in arsenate with no need to invoke the replacement of phosphorus by arsenic in biological macromolecules.
EXPERIMENTAL PROCEDURES
Bacterial Strains
E. coli strain MG1655 (seq)* I− was used in this study. MG1655 (seq)* is an rph+ derivative of MG1655, constructed by Donald Court (NCI, National Institutes of Health, Bethesda, MD) and provided by Kenneth Rudd (University of Miami). The RNase I− derivative was constructed by recombineering (5, 6) and confirmed by PCR and direct assay for RNase I.
In Vivo Assay for Ribosome Degradation
Ribosome breakdown was measured by a variation of a previously described method (7, 8). Fifty ml of Tris-buffered salts (100 mm Tris-HCl, pH 7.6, 50 mm NaCl, 18.8 mm NH4Cl) supplemented with 0.1 mm KH2PO4 and 0.2% glucose, 0.1 mm uridine, and 0.2 μCi/ml [3H]uridine (GE Healthcare) were inoculated with a small volume of overnight culture to an initial A600 of 0.01. Cultures were grown to an A600 of ∼0.2. The culture was split into two equal volumes, and cells were collected by centrifugation for 15 min in a Sorvall SS34 rotor. The cell pellets were washed twice in Tris-buffered salts and resuspended in 25 ml of the same solution containing either 0 mm or 1.5 mm KH2PO4 and 0.2% glucose. Each sample was divided into two 12-ml cultures, of which one was supplemented with 40 mm Na2HAsO4, and incubated with shaking at 37 °C. Five hundred-μl portions were collected from each of the four cultures prior to and at various times throughout the incubation period and immediately stored at −80 °C for further analysis of the acid-soluble material. A600 readings were taken to monitor cell growth. In addition, cultures lacking radioactivity were followed to analyze cell growth in the presence of arsenate.
Analysis of Acid-soluble Material
Stored culture samples were thawed, mixed with 250 μl of 4 m formic acid, and incubated on ice for 15 min. After incubation, samples were centrifuged at maximum speed for 20 min in a Fisher bench-top microcentrifuge at 4 °C. Half of the supernatant fraction was removed and added to 5 ml of scintillation fluid. Samples were counted in a scintillation counter to determine acid-soluble radioactivity. The remaining acid-soluble fraction was neutralized with 1 m Tris and further analyzed by separation on 3MM paper in a tert-amyl alcohol:formic acid:H2O (6:4:1) solvent. After chromatography, the paper was cut into 1-cm strips, and radioactivity was determined.
Isolation of Arsenate-tolerant Cells
To measure growth of cells after exposure to arsenate, cells were grown with shaking at 37 °C in medium containing 0.1 mm phosphate in the presence or absence of 40 mm arsenate. After a 250-h incubation, which allowed for generation of arsenate-tolerant cells, portions of culture were diluted (1:25) into fresh medium either in the presence or in the absence of 40 mm arsenate. Cell growth was monitored by A600 readings.
RESULTS
Arsenate Induces Increased Ribosome Degradation
A major clue as to how cells are able to grow in +As/−P medium came from data already in the Wolfe-Simon et al. study (1). The gel presented in Fig. 2A of that study reveals the disappearance of two intense bands in the +As/−P culture when compared with cells grown in −As/+P. Based on the intensity of the bands relative to DNA, we speculated that these bands are 23 S and 16 S rRNA. Interestingly, Wolfe-Simon et al. (1) did not comment on these two bands or on their disappearance.
FIGURE 2.
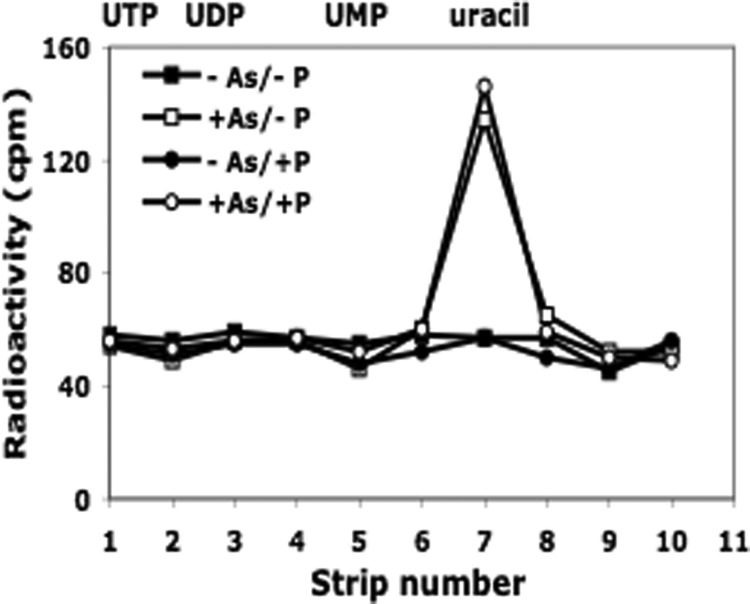
Analysis of acid-soluble material released during ribosome degradation. A portion of the acid-soluble material obtained in Fig. 1 was analyzed by paper chromatography as described under “Experimental Procedures.” The migration positions of standards are shown at the top of the figure.
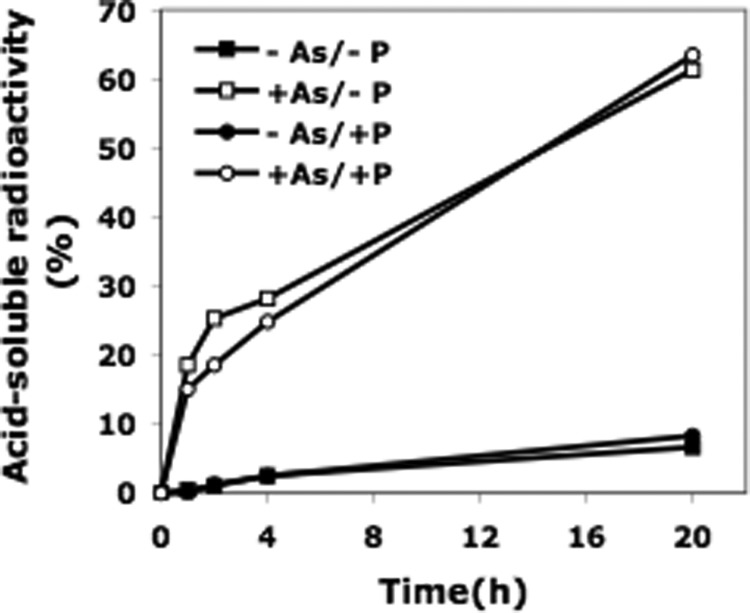
It is known that nutrient deprivation, such as carbon or Pi starvation, can lead to the breakdown of ribosomes (9). To assess directly whether the presence of arsenate might affect ribosome degradation, we made use of assays previously developed in our laboratory to measure this process (7, 8). Cells growing in low phosphate medium (0.1 mm Pi) were prelabeled with [3H]uridine, which primarily labels ribosomes, and were then washed to remove unincorporated uridine and Pi. Cells were then placed in various media under conditions described by Wolfe-Simon et al. (1), the release of acid-soluble radioactive products was measured, and the products identified. This assay is superior to a gel-based assay in that it affords a quantitative measure of ribosome breakdown (7, 8). The efficiency of the wash protocol was indicated by the absence of growth in medium lacking both phosphate and arsenate (see Fig. 3).
FIGURE 3.
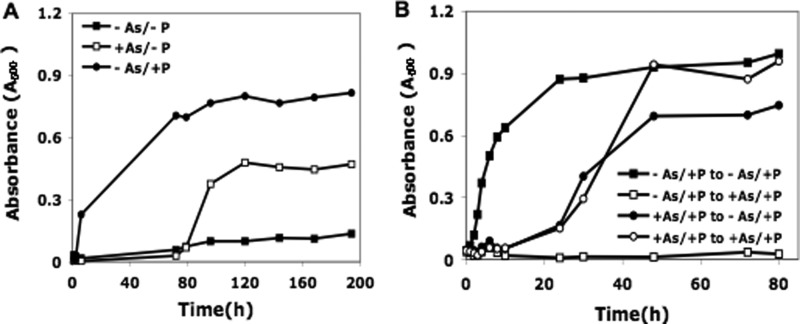
A, growth of E. coli cells in media containing arsenate. Cultures were centrifuged, washed in Tris-buffered salts, and resuspended in the same solution containing 0.2% glucose plus either no addition or additions of 1.5 mm KH2PO4 or 40 mm arsenate. Cultures were incubated with shaking at 37 °C. A600 readings were taken to monitor cell growth. B, growth of E. coli cells pre-exposed to arsenate. Cells were initially grown with shaking at 37 °C in Tris-buffered salts/glucose medium containing 0.1 mm phosphate in the presence or absence of 40 mm arsenate. After 250 h of incubation to select for arsenate-tolerant cells, two portions were diluted into fresh medium containing 0.1 mm phosphate in the presence or absence of 40 mm arsenate. Cell growth was monitored by A600 readings.
As is shown in Fig. 1, a low level of RNA degradation (less than 10% in 20 h) was observed when cells were placed in medium lacking Pi or containing only a low level of Pi (1.5 mm). However, in the presence of 40 mm arsenate, almost 70% of the total radioactivity originally present in the cells was recovered in the acid-soluble fraction after 20 h, indicating massive ribosome degradation. This number actually represented the minimum amount of ribosome degradation because, based on earlier work (8), some portion of 16 S and 23 S rRNA may still be present as fragments that are too large to be in the acid-soluble fraction. Most importantly, these results reproduced in E. coli what appeared likely from the data in Wolfe-Simon et al. (1), i.e. that ribosomes largely disappear in the presence of 40 mm arsenate.
FIGURE 1.
Ribosome degradation during growth of E. coli in media lacking or containing arsenate. Samples from each of four cultures prelabeled with [3H]uridine were analyzed for acid-soluble material at the indicated times as described under “Experimental Procedures.”
Additional analysis by paper chromatography of the acid-soluble radioactive material generated revealed a peak that migrated at the same position as uracil (Fig. 2), indicating that the radioactive nucleotides produced during rRNA degradation were acted upon further to release the free base, thereby also providing a source of Pi. Uracil was also the final product of ribosome degradation induced by carbon starvation.2
Growth of E. coli in the Presence of Arsenate
To further examine the relation between our findings with E. coli and the results of Wolfe-Simon et al. (1) with strain GFAJ-1, we monitored growth of E. coli in media containing arsenate. Initially, we examined growth during the experiment presented in Fig. 1. In this experiment, growth was observed only in the −As/+P medium. There was no growth in the −As/−P medium or in either of the media containing 40 mm arsenate during the 20-h incubation (data not shown).
To more closely recapitulate the growth experiments of Wolfe-Simon et al. (1), unlabeled cell cultures were monitored for more extended periods of time. One representative experiment is presented in Fig. 3A. E. coli did not grow in the absence of both phosphate and arsenate, whereas growth began immediately in a medium containing 1.5 mm phosphate. In +As/−P medium, cells initially died. Based on viable cell count, >99% of cells were eliminated in 24 h; however, a small population survived, and eventually, the culture grew, but only after a long lag, amounting to ∼80 h in this experiment. The time of growth initiation varied in different experiments, and we propose that is due to the variable time required for outgrowth of the small population of arsenate-tolerant cells (see below). The growth pattern closely reproduced the observations of Wolfe-Simon et al. (1) and indicated that the ability to grow in arsenate is not a special property of GFAJ-1 cells.
Selection of Arsenate-tolerant Cells
To test the hypothesis that arsenate-tolerant cells arise during the long lag period prior to the initiation of growth in arsenate-containing media, cells were first pre-exposed to arsenate and were then placed in medium containing either 0.1 mm phosphate or 0.1 mm phosphate supplemented with 40 mm arsenate. These two cultures initially lagged due to the many dead cells present, but then displayed essentially identical growth curves (Fig. 3B). These data show that after pre-exposure to arsenate, the presence of arsenate is no longer inhibitory, confirming that arsenate-tolerant cells were selected. In contrast, cells that were not previously exposed to arsenate did not grow in its presence during the 80-h period of this experiment, whereas they grew normally in −As/+P medium (Fig. 3B). In another experiment carried out for 125 h, cells not previously exposed to arsenate eventually did grow in arsenate-containing medium, as expected for the variable nature of development of arsenate tolerance (data not shown). The significance of the long lag period prior to growth in arsenate was not examined or discussed by Wolfe-Simon et al. (1).
DISCUSSION
The studies described here provide a simple, alternative explanation for the recent findings of Wolfe-Simon et al. (1). These authors proposed that arsenic is incorporated into biological molecules in an “unusual microbe, strain GFAJ-1, that exceptionally can vary the elemental composition of its basic biomolecules” to explain growth in +As/−P medium, whereas we argue that their findings can be explained by known mechanisms and can be reproduced in a laboratory strain of E. coli. Thus, although we agree with the experimental observations of Wolfe-Simon et al. (1), we disagree with their interpretation of the data based on our following observations: (a) arsenate induces massive ribosome degradation, thereby providing a source of phosphate (Fig. 1); and (b) placing cells in medium containing arsenate leads to a population of cells that are arsenate-tolerant and can grow in 40 mm arsenate (Fig. 3).
Ribosome degradation under conditions of stress is a well known phenomenon (9). Although the effect of arsenate on this process has not previously been examined, its dramatic stimulation of the process is not surprising. Arsenate affects energy metabolism and, therefore, would be expected to inhibit protein synthesis. We have already shown that inhibition of protein synthesis stimulates ribosome degradation (7). The large amount of ribosome degradation provides a straightforward explanation for the observation of Wolfe-Simon et al. (1) in which the two most intense bands on their gel of isolated nucleic acids essentially disappear after growth in +As/−P medium, which we confirmed by a completely different method.
The ability of E. coli to develop arsenate resistance is also well known, and a large literature exists that describes this phenomenon (e.g. Ref. 10). Resistance may arise through a number of mechanisms including transport or conversion to less damaging chemical species. Arsenic resistance genes may be chromosomal or plasmid-enclosed. Detailed description of arsenate resistance is beyond the scope of this study, but as we have shown, it is responsible for the ability of E. coli to grow in the presence of arsenate in our experiments, and it explains the long lag we observed prior to initiation of growth, which was also seen with GFAJ-1 (1).
Finally, we may inquire whether sufficient phosphate could be released from ribosome degradation to permit the growth observed in arsenate. Both in our experiments and in those of Wolfe-Simon et al. (1), growth is extremely limited, amounting to 4–5 doublings from the original cell number. Moreover, growth is extremely slow in +As/−P medium, with doubling times ranging from ∼10 h in our experiments to ∼40 h for GFAJ-1. The ribosome content of cells is closely related to their growth rate. For example, reducing the growth rate of E. coli from 24 to 100 min reduces their ribosome content >10-fold (11). Assuming that a similar relationship holds at the extremely low growth rates examined here, we would expect cells in +As/−P medium to contain only ∼10% as many ribosomes as the cells originally growing in the −As/+P medium because they grow ∼5-fold more slowly. In addition, because <1% of the original cells survive in arsenate, and it is these cells that grow out, the extensive ribosome degradation observed provides more than sufficient phosphate to support the limited 20-fold increase in cell number reported by Wolfe-Simon et al. (1) and the similar increase in our experiments.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grant GM16317 (to M. P. D.).
This article was selected as a Paper of the Week.
G. N. Basturea and M. P. Deutscher, unpublished observation.
REFERENCES
- 1. Wolfe-Simon F., Switzer Blum J., Kulp T. R., Gordon G. W., Hoeft S. E., Pett-Ridge J., Stolz J. F., Webb S. M., Weber P. K., Davies P. C., Anbar A. D., Oremland R. S. (2011) A bacterium that can grow by using arsenic instead of phosphorus. Science 332, 1163–1166 [DOI] [PubMed] [Google Scholar]
- 2. Hayden E. C. (2011) Will you take the “arsenic-life” test? Nature 474, 19. [DOI] [PubMed] [Google Scholar]
- 3. Reaves M. L., Sinha S., Rabinowitz J. D., Kruglyak L., Redfield R. J. (July 8, 2012) Absence of detectable arsenate in DNA from arsenate-grown GFAJ-1 cells. Science 10.1126/science.1219861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erb T. J., Kiefer P., Hattendorf B., Gunther D., Vorholt J. A. (July 8, 2012) GFAJ-1 is an arsenate-resistant, phosphate-dependent organism. Science DOI: 10.1126/science.1218455 [DOI] [PubMed] [Google Scholar]
- 5. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Datta S., Costantino N., Court D. L. (2006) A set of recombineering plasmids for gram-negative bacteria. Gene 379, 109–115 [DOI] [PubMed] [Google Scholar]
- 7. Zundel M. A., Basturea G. N., Deutscher M. P. (2009) Initiation of ribosome degradation during starvation in Escherichia coli. RNA 15, 977–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basturea G. N., Zundel M. A., Deutscher M. P. (2011) Degradation of ribosomal RNA during starvation: comparison with quality control during steady-state growth and a role for RNase PH. RNA 17, 338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deutscher M. P. (2003) Degradation of stable RNA in bacteria. J. Biol. Chem. 278, 45041–45044 [DOI] [PubMed] [Google Scholar]
- 10. Stolz J. F., Basu P., Santini J. M., Oremland R. S. (2006) Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol 60, 107–130 [DOI] [PubMed] [Google Scholar]
- 11. Bremer H., Dennis P. P. (1996) Modulation of chemical composition and other parameters of the cell by growth rate in Escherichia coli and Salmonella (Neidhardt F. C., ed) pp. 1553–1569, ASM Press, Washington, D. C [Google Scholar]