Background: Ubiquitination is essential for regulation of MHC class II molecules.
Results: Multiple elements in the transmembrane region of HLA-DR influence interaction with MARCH8.
Conclusion: Sequences in the TM region of MHC class II molecules control recognition by different MARCH E3 ligases.
Significance: MHC class II isotypes are subject to differential post-translational regulation by different MARCH E3 ligases.
Keywords: Antigen Presentation, Immunology, Protein Targeting, Protein Turnover, Ubiquitination, MARCH E3 Ligase, MHC Class II, Transmembrane Domain
Abstract
MARCH E3 ligases play a key role in controlling MHC class II surface expression by regulated ubiquitination of a lysine residue in the β-chain. Little is known concerning how these enzymes target their specific substrates. Here we show that recognition of HLA-DR by MARCH proteins is complex. Several features associated with the transmembrane domain and bordering regions influence the overall efficiency of receptor internalization. A cluster of residues at the interface of the lipid bilayer and the cytosol plays the most important role in MARCH8 recognition of HLA-DRβ. Variation in this sequence also determines specificity of MARCH9 for HLA-DQ. Residues located in helical face four of HLA-DRβ together with a charged residue at the boundary with the stalk region also contribute significantly to recognition. Truncation analysis suggested that a dileucine-like motif in the DRβ cytoplasmic tail influences the efficiency of co-localization of HLA-DR with MARCH8. The DRβ-encoded acceptor lysine functioned optimally when placed in its natural location relative to the bilayer. In the DRα/DRβ dimer most other amino acids in the cytoplasmic tail could be substituted for alanine with minimal influence on function. Our data support a model whereby multiple features of HLA-DR are involved in substrate recognition by MARCH8. The single most important region is located at the interface between the transmembrane domain and the cytosol. Variation in sequence in this location between different class II isotypes controls efficiency of recognition by different MARCH E3 ligases.
Introduction
Professional antigen-presenting cells respond rapidly to invading pathogens by presenting peptides derived from the infecting organism to T cells. Post-translational control of class II molecules by ubiquitination facilitates this (1–4). In dendritic cells (DCs)2 ubiquitination of MHC class II by MARCH E3 ligases provides control over the cell surface expression of peptide-loaded class II molecule complexes, and regulation of this process through pattern recognition receptor signaling allows cells to respond rapidly in the face of infection. This enables the immune system to focus its response by providing a “snap-shot” of antigens present at the time of DC activation.
The first MARCH family members to be described were K3 (MIR1) and K5 (MIR2), two virally encoded proteins identified due to their ability to down-regulate surface MHC class I molecules (5–7). These E3 ligases are integral membrane proteins that span the membrane twice and have both N and C termini located in the cytoplasm. They possess a conserved variant RING domain at the C terminus but share little homology throughout the rest of the molecule, including their TM domains (8). Human homologues with similar domain structures have been identified and are referred to as MARCH (membrane-associated RING-CH) proteins. They function through association with a ubiquitin-modified E2 conjugating enzyme, and the E2-E3 complex then facilitates ubiquitin transfer to the substrate. MARCH1 is important for regulated ubiquitination in DCs, monocytes, and B cells (3, 9). The closely related homologue, MARCH8, also regulates MHC-II in a number of cell types but is more widely expressed and shows little down-regulation upon DC maturation. Whereas MARCH1 and MARCH8 interact with HLA-DP, -DQ, and -DR, a third ligase MARCH9 shows specificity for only HLA-DQ (10).
MHC class II molecules play a central role in controlling adaptive immune responses. The process of peptide acquisition and assembly is complex and involves a number of dedicated chaperones and accessory molecules. Briefly, class II α/β dimers initially assemble in the endoplasmic reticulum together with a third component, invariant chain (Ii). Upon egress from the endoplasmic reticulum, Ii targets the complex into the endocytic pathway using dileucine-based sorting signals (11). Here Ii undergoes proteolysis, leaving a small peptide fragment, CLIP, occupying the peptide-binding groove. Under the control of HLA-DM, a nonclassical class II molecule, CLIP is removed and exchanged for peptides derived from antigens that have entered the endocytic pathway (12–14). These peptide-loaded class II molecules then traffic to the cell surface for presentation to CD4-restricted T cells. Class II molecules associate closely via their TM domains, and these regions alone are sufficient to force strong association in reporter systems (15). The interaction is mediated by Gly-Xaa-Xaa-Xaa-Gly motifs present in both the α- and β-chains (16). The class II dimer also associates with the invariant chain via TM domain interactions (16). However, immature DRα-DRβ-Ii complexes are not subject to MARCH-induced ubiquitination (2, 3, 17).
Very little is known about what determines MARCH ligase substrate specificity. Whereas some MARCH proteins affect a wide range of targets, others are more limited. The two viral proteins, K3 and K5, for example, target MHC class I molecules and a number of other surface receptors. They share some targets such as HLA-A and HLA-B, but then differ in their ability to down-regulate other closely related molecules such as HLA-E and HFE (5, 18). MARCH proteins can target substrates that have no discernable common binding motif, other than the presence of a ubiquitinatable amino acid, usually a lysine, but sometimes cysteine or serine (19). A widely accepted model is that MARCH proteins recognize their substrates through TM domain interactions (10, 19–24). Here, we have analyzed in detail the requirements for HLA-DR recognition by MARCH8.
MATERIALS AND METHODS
Cell Culture and Transfection
Human embryonic kidney (HEK 293T) cells were maintained in Dulbecco's modified Eagle's medium, and Raji cells were cultured in RPMI medium 1640. Media were supplemented with 10% fetal calf serum, 10 mm HEPES, 10 mm sodium pyruvate, 10 mm nonessential amino acids, and 2 mm l-glutamine. 293T cells were transiently transfected using 25-kDa linear polyethyleneimine (Sigma-Aldrich) as described by Ehrhardt et al. (25). For lentivirus production, 293T cells were transfected with pCMV8.91, pMD-G, and pHR'SIN (−) carrying EGFP-MARCH8 or 9. After 48 h, supernatants containing lentiviral particles were used directly to transduce Raji cells.
Antibodies
Antibodies were from ATCC (anti-CD8α; clone OKT8), Thermo Scientific (rabbit anti-mouse IgG-Fc RPE), ImmunoTools (IgG isotype controls), Santa Cruz Biotechnolology (anti-ubiquitin-HRP, clone P4D1), Cancer Research Technology (anti HLA-DQα, clone L2DQ; anti HLA-DP, clone B7/21), and anti HLA-DR dimer antibody was from clone L243.
Plasmid Constructs
CD8-DRα and β reporter constructs were based on the human CD8α luminal domain (amino acids 1–176) and the TM domains and cytoplasmic tails of DRα (DRA*0101) and DRβ (DRB4*0101) as described previously (26). There is limited polymorphism in the cytoplasmic tails of DRB alleles. In particular, the DRB4*0101 has a dileucine (235LL236) sequence that is shared with mouse and rat I-Aβ and I-Eβ and HLA-DQβ. In other HLA-DRβ molecules this sequence is 235FL236. Mutant CD8-DRβ constructs were synthesized by PCR using KOD HiFi polymerase (Calbiochem) and sequence alterations generated using overlapping primer pairs. PCR products were digested and ligated into pcDNA3.1/Zeo+ or/Neo− (Invitrogen). HLA-DRα and HLA-DRβ were amplified from their parental constructs (DRA*0101; DRB1*0303) and cloned into pcDNA3.1 Zeo+ and Hygro+, respectively. The lysine residues in the HLA-DRα cytoplasmic tail were substituted with arginines. To generate the HLA-DRβwt and Lys−1 to Lys7 constructs, a unique ClaI site was introduced in the stalk region substituting residues 191RS192 for SI. The TM regions and cytoplasmic tails of the relevant CD8-DRβ reporters were then inserted into HLA-DRβ. HLA-DQβ cDNA was generated from HeLa-CIITA cells (allele DQB1*0502) using SuperScriptII reverse transcriptase (Invitrogen). All constructs were verified by DNA sequencing and primers listed in supplemental Table 1. pEGFPc1-MARCH 9 as well as pCMV8.91 and pMD-G were kindly provided by Paul Lehner (Cambridge, UK). pTracer-GFP/MARCH8 and MARCH8 mutant (mut) constructs were kind gifts from Satoshi Ishido (Yokohama, Japan). pEGFPc2-MARCH8wt and mut were created by PCR subcloning of human MARCH8-V5-Hiswt and mut in-frame into pEGFP-c2. pHR'SIN(−)-EGFP-MARCH8 and MARCH9 have been described elsewhere (27).
Flow Cytometry
24–36 h after transfection, 293T cells were harvested and stained on ice with primary and secondary antibodies for 30 min in FACS buffer (4% FCS, 2 mm EDTA in PBS) and then fixed in 3% formaldehyde in PBS for acquisition. Raji cells were transduced using lentiviral supernatants and grown for 48 h in RPMI 1640 medium in which glutamine had been replaced by 2 mm alanine-glutamine dipeptide to ensure normal class II processing (27) before they were collected and stained as above. Data acquisition was performed on a FACScan flow cytometer (BD Bioscience) and analyzed using Summit v4.3 software. Expression of surface antigens was calculated as the proportion of mean fluorescent intensity (MFI) in the presence of catalytically active MARCH E3 ligase (GFP expression was used as a marker for ligase expression) compared with MFI in the presence of MARCHmut (or MFI in untransduced cells in the case of Raji): Surface expression (%) = (MFI cells transfected with MARCHwt × 100)/(MFI cells transfected with MARCHmut). Statistical analysis was performed using Microsoft EXEL software (two-tailed t test: paired samples, unequal variances, α = 0.05). Differences of means were classed as not significant (NS), significant (*, p < 0.05), and highly significant (**, p < 0.01). Representation of the importance of individual residues in the form of a heat map was calculated using Matrix2png software (28). Two independent alanine scans of the DRβ TM domain were performed, one scan of the linear sequence and one of the helical faces (see Fig. 4). The average effects on targeting by MARCH8 for each individual residue were determined by calculating the arithmetic mean of the expression of the two constructs in which the residues had been substituted with alanine. The numerical values were converted into a scale ranging from white (not important for recognition by MARCH8) to black (important for recognition) using Matrix2png software (28). The heat map was then mapped onto a projection of the DRβ TM domain α-helix (see Fig. 4C). A color version of the heat map is shown in supplemental Fig. 1.
FIGURE 4.
Residues in the TM region of DRβ are important for MARCH8 recognition. Alanine scanning mutagenesis of the seven helical faces of DRβ and of linear groups of residues in the TM region is shown. A, table showing the amino acid composition of constructs used. B, graph summarizing the surface down-regulation of CD8-DRβ reporter constructs in the presence of MARCH8. Surface CD8-DR was measured by FACS using anti-CD8 mAb OKT8. The percentage down-regulation was calculated as MFI in the presence of MARCH8wt × 100/MFI in the presence of MARCH8mut. Statistical significance was calculated using a paired t test. C, summary of the importance of individual amino acids in MARCH8-regulated receptor internalization. Average contribution of each amino acid to down-regulation was calculated as detailed under “Materials and Methods” and is expressed as a heat map on a black/white scale ranging from 7 to 32% remaining at the cell surface. The darker the infill the greater the influence of the residue upon internalization. Lined infills denote residues bordering the TM domain but not included in the analysis. The position of the wild type is marked. Data were analyzed and heat maps generated using Matrix2png software (28).
Immunoprecipitation and Western Blotting
293T transfectants were washed with cold PBS with 5 mm EDTA and lysed in lysis buffer (150 mm NaCl, 1% Nonidet P-40 substitute (Fluka), 50 mm Tris (pH 7.5), 5 mm EDTA, protease inhibitor mixture (Roche Applied Science), and 5 mm iodoacetamide) for 20 min at 4 °C. Debris and nuclei were removed by centrifugation at 14,000 rpm, and CD8 reporters were precipitated using OKT8 antibody and protein A-agarose (Sigma) according to standard protocols. Precipitates were separated by SDS-PAGE, and ubiquitination was analyzed by Western blotting with directly conjugated anti-ubiquitin-HRP antibody using rapid immunodetection according to the manufacturer's instructions and chemiluminescence using ECL+ reagent (GE Healthcare).
RESULTS
Multiple Sequences in the Cytoplasmic Tail of DRβ Influence MARCH8-induced Down-regulation
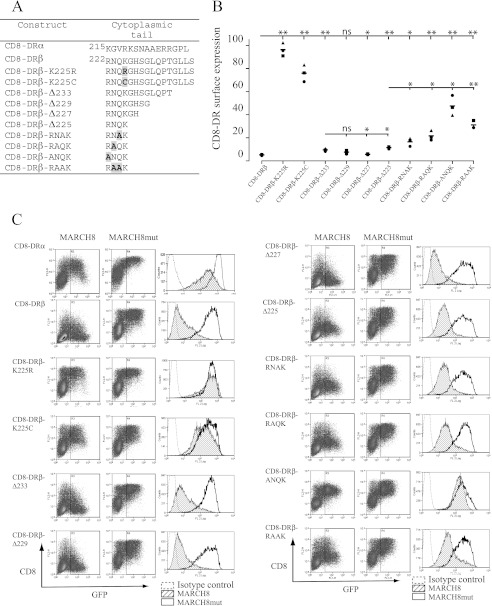
To investigate features of HLA-DR important for MARCH8 interaction, we generated CD8-reporter molecules encoding the TM and cytoplasmic tails of DRα and DRβ attached to the extracellular regions of CD8 (Fig. 1A). This reporter system has been used extensively as a proxy for monitoring ubiquitination-induced internalization in mammalian cells (21). The reporter constructs were transiently expressed in 293T cells together with either MARCH8 or a catalytically inactive mutant (MARCH8mut). The CD8-DRβ-reporter showed a much greater reduction (>90%) in surface expression in the presence of MARCH8 compared with the corresponding CD8-DRα-reporter (66%) (Fig. 1C). We therefore initially focused our studies on CD8-DRβ. We confirmed that DRβ-K225 was essential for MARCH8 induced down-regulation and showed that this residue cannot be substituted by cysteine, a potential target for unconventional ubiquitination (Fig. 1, B and C) (19). Deletion of residues 234GLLS237 (CD8-DRβ-Δ233) caused a small but significant reduction in MARCH8-induced down-regulation. Further deletions up to Lys225 (CD8-DRβ-Δ225) were not significantly different from deletion of residues 234GLLS237 alone (Fig. 1B). Therefore, residues Arg222 to Lys225 in the DRβ cytoplasmic tail were sufficient to mediate efficient MARCH8-induced internalization of CD8-DRβ. We then examined the importance of residues 222RNQK225 by alanine scanning mutagenesis. Substitution of Arg222 or Asn223 for alanine resulted in a small but statistically significant reduction in MARCH8-induced internalization compared with the CD8-DRβ-Δ225 construct (Fig. 1B). Substitution of Gln224 for Ala was not significantly different. From these studies we find that residues Arg222, Asn223, and 234GLLS237 all play a role in MARCH8-induced down-regulation, but Lys225 has by far the greatest impact.
FIGURE 1.
Residues in the cytoplasmic tail of DRβ influence MARCH8 recognition. CD8-DRβ reporter constructs were expressed together with either GFP/MARCH8 or GFP/MARCH8mut in 293T cells. A, table showing the amino acid composition of the cytoplasmic tail domain of CD8 reporter constructs. Single amino acid code is used. Substituted residues are highlighted and shown in bold. B, graph showing the percentage down-regulation of various CD8-DR reporter constructs. Symbols represent data sets obtained in different experiments. Surface CD8 was measured by FACS using anti-CD8 mAb OKT8. The percentage down-regulation was calculated as MFI in the presence of MARCH8 × 100/MFI in the presence of MARCH8mut. Statistical significance was calculated using a paired t test. C, representative FACS dot plots from data shown in B. Histograms show surface CD8-reporter expression in the presence of MARCH8 (hatched) or MARCH8mut (open), dotted line shows the antibody isotype control.
Recognition of DRβ by MARCH8 Is Optimal When the Ubiquitinated Lysine Residue Is Placed in Its Naturally Occurring Position
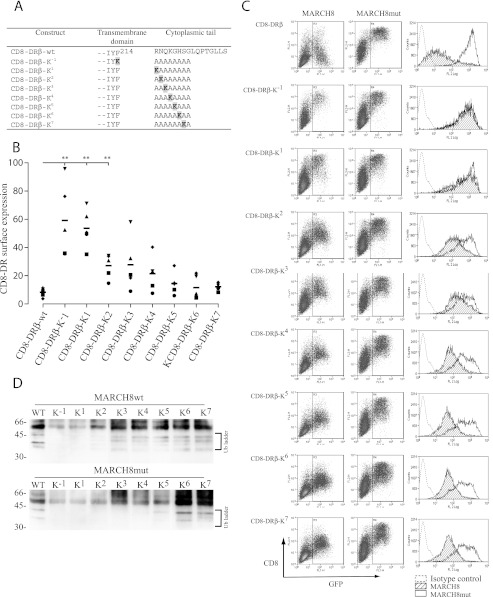
The position of Lys225 is conserved in all class II molecules. We considered whether this was important for MARCH8 recognition. Precisely defining the boundaries of a TM region in a protein is a significant problem, even for solved structures. We delineated the TM domain as lying between Lys198 and Arg222. This would allow for a TM domain of 22 amino acids, placing Arg222 at position one of the cytoplasmic tail. As in Cadwell and Coscoy (19), we placed the lysine residue at various positions along an artificial CD8-DRβ-polyalanine cytoplasmic domain. We limited this investigation to 8 residues because cytoplasmic tails of this length are a natural feature of some class II molecules (see Fig. 6A). A significant reduction in surface down-regulation was observed when the lysine was placed in positions +3 to −1, and this was most pronounced in the +1 and −1 positions (Fig. 2, A and B). Locating the lysine at positions Lys4 to Lys7 was not statistically different from wild-type and had only a minor influence on MARCH8-induced down-regulation. A ubiquitin ladder was clearly associated with constructs CD8-DRβ-K2 to CD8-DRβ-K7 but not with constructs CD8-DRβ-K−1 or CD8-DRβ-K1 (Fig. 2D). In the presence of MARCH8mut, ubiquitination by endogenous E3 ligases was also seen in constructs CD8-DRβ-K4 to -K7. These results demonstrate that MARCH8 did not efficiently ubiquitinate lysine residues in the −1 or +1 position.
FIGURE 6.
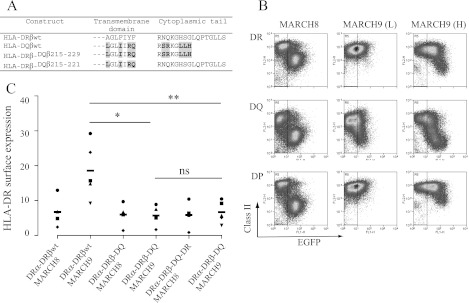
Selectivity of MARCH9 for HLA-DQ can be transferred to HLA-DR by a cluster of residues in the TM region of HLA-DQβ. Residues differing between HLA-DRβ and HLA-DQβ were exchanged in chimeric DRβ/DQβ constructs. A, table showing the amino acid composition of constructs used. B, FACS analysis of Raji cells transduced with MARCH8-EGFP and MARCH9-EGFP shown as dot plots. Surface HLA-DP, -DQ, and -DR was measured using mAbs B7/21, L2DQ (33), and L243, respectively. At low transduction efficiency (L), MARCH9 is specific for HLA-DQ. At higher transduction efficiency (H), MARCH9 also recognizes HLA-DP and -DR, but shows greater down-regulation of HLA-DQ. C, graph summarizing the surface down-regulation of chimeric DRβ/DQβ constructs in the presence of MARCH9. Chimeric HLA-DQβ/DRβ constructs, DRα, and MARCH8-EGFP or MARCH9-EGFP were expressed in 293T cells. Surface HLA-DR was measured by FACS using anti-DR mAb L243, and percentage down-regulation was calculated as MFI in the presence of MARCH8wt × 100/MFI in the presence of MARCH8mut. Down-regulation of chimeric constructs HLA-DRβ-DQβ215–229 and HLA-DRβ-DQβ215–221 by MARCH9 was significantly enhanced compared with wild-type-DR. MARCH8 recognition of the same constructs showed no significant differences.
FIGURE 2.
Spacing of the lysine reside influences MARCH8-induced CD8-DRβ internalization. CD8-DRβ reporter constructs were transfected into 293T cells together with either GFP-MARCH8 or GFP-MARCH8mut. After 24 h cells were harvested and processed for FACS analysis or immunoprecipitation. A, positioning of the lysine residue within the TM and cytoplasmic regions of the various CD8-DRβ reporter constructs. Single letter amino acid code is used. B, summary of surface down-regulation of CD8-DRβ reporter constructs in the presence of MARCH8. Surface CD8 was measured by FACS using anti-CD8 mAb OKT8. The percentage down-regulation was calculated as MFI in the presence of MARCH8wt × 100/MFI in the presence of MARCH8mut. Statistical significance was calculated using a paired t test. Constructs CD8-DRβ-K3 to -K7 were either not statistically different from, or showed only a very small change from CD8-DRβ-wt. Different symbols represent independent data sets. C, FACS analysis of surface CD8 down-regulation using anti-CD8 mAb OKT8. GFP expression identifies GFP/MARCH8- or GFP/MARCH8mut-expressing cells. Histograms show surface expression in the presence of GFP/MARCH8 (hatched) and GFP/MARCH8mut (open). D, immunoprecipitation of CD8-reporter constructs in the presence of GFP/MARCH8 (upper) or GFP/MARCH8mut (lower). Blots were probed with anti-ubiquitin antibody P4D1-HRP. The position of a clear ubiquitin ladder is shown.
Upon close examination of Fig. 2C we noted that the expression level from constructs CD8-DRβ-K2 to CD8-DRβ-K7 in the presence of MARCH8mut was lower than CD8-DRβ. This was also evident in transfectants expressing DRβ-ANQK (Fig. 1C). This was also seen when these constructs were expressed in the absence of MARCH8 or MARCH8mut (data not shown). A common feature of these constructs is the loss of Arg222, a residue that potentially forms part of a stop transfer signal. This complicated our interpretation of the positional effects of the Lys residue in the CD8-DRβ reporter background because mutation of this signal may interfere with efficient membrane insertion and hence influence protein expression in ways that are unrelated to MARCH8-induced ubiquitination.
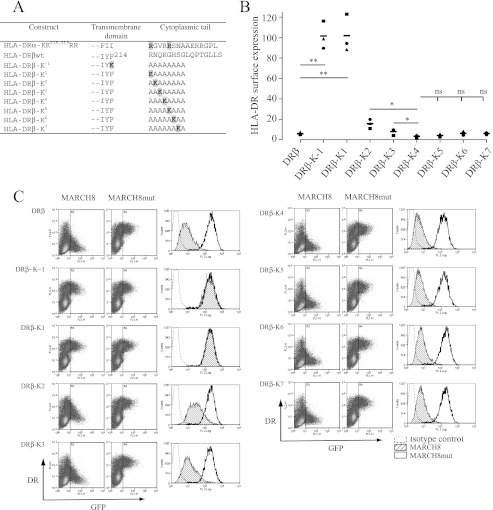
To circumvent these problems we performed similar studies using DRα/β dimer constructs. Here we predicted that close association of DRα/DRβ would facilitate efficient membrane insertion of DRβ, even in the absence of Arg222. To eliminate ubiquitination of DRα (26), both lysine residues present in this chain were substituted for arginine. All constructs expressed well, demonstrating that loss of Arg222 was not a problem for the DRα/DRβ dimer (Fig. 3C). Efficient down-regulation of DRα/β was observed when the lysine residue was placed between positions +7 and +2 of DRβ (Fig. 3). In closer proximity to the TM domain (DRβ position +1 or −1) MARCH8-induced down-regulation was entirely abrogated. Optimal MARCH8-induced down-regulation was observed with the lysine in its natural +4 location, and moving further from the TM region had no significant beneficial or detrimental effect.
FIGURE 3.
MARCH8 recognition of HLA-DRβ is influenced by the location of the lysine residue. The DRβ-encoded lysine residue was placed at different positions relative to the TM domain in an artificial polyalanine tail. Surface down-regulation in the presence of MARCH8 was used as a proxy to measure efficiency of recognition. A, table showing the amino acid composition of the cytoplasmic tail region of the DRβ-polyalanine reporter constructs. Single amino acid code is used, substituted residues are highlighted. B, summary of surface down-regulation of DRα/DRβ reporter constructs in the presence of MARCH8. Surface DR was measured by FACS using anti-DR mAb L243. The percentage down-regulation was calculated as MFI in the presence of MARCH8wt × 100/MFI in the presence of MARCH8mut. Statistical significance was calculated using a paired t test. Optimal surface down-regulation was seen with the lysine in the +4 position (DRβ-K4); beyond this no significant changes were observed. Symbols represent independent data sets. C, representative FACS dot plots from data shown in B. Histograms show surface DR-polyalanine expression in the presence of MARCH8 (hatched) or MARCH8mut (open), dotted line shows the antibody isotype control.
Sequences in the TM Domain of DRβ Play a Major Role in MARCH8 Recognition
We next examined whether sequences in the TM region of DRβ influenced MARCH8 interactions. Transmembrane domains usually adopt an α-helical structure within lipid bilayers, where 3.6 amino acids are found per turn and every 7th residue is stacked sequentially below. This heptad arrangement results in the presence of 7 helical faces around each TM domain. Using alanine-scanning mutagenesis we determined that mutations to the 4th helical face impaired the ability of MARCH8 to down-regulate the CD8 reporter (Fig. 4). Mutations to other helical faces were not significantly different from wild type. We also performed a linear scan of the DRβ TM domain by substituting blocks of 5 or 6 amino acids for alanine. Substitution of the C-terminal 217LFIYF221 region significantly impaired CD8 reporter down-regulation (Fig. 4B). These mutagenesis studies are summarized in a heat map that visually displays the relative importance of individual DRβ residues with respect to MARCH8-induced down-regulation (Fig. 4C). By far the most important sequence in the TM region was the C-terminal 217LFIYF221 motif.
MARCH8 Shows a Preference for Charged Residues at Intracellular and Extracellular Juxtamembrane Positions
Finally, we considered the importance of sequences located in the membrane-proximal regions of DRβ. Substitution of extracellular residues 197SK198 or 194SAQSK198 for alanine resulted in reduced down-regulation, suggesting a role for Ser197 and or Lys198 in MARCH8 recognition (Fig. 5, A and B). Substitution of the positively charged residue Lys198 for a negatively charged residue (Glu198) restored efficient down-regulation. This suggests that MARCH8 requires charged residues in the extracellular membrane proximal region, but these can be either positively or negatively charged. Substitutions involving Arg222 also indicated that charged residues at the TM/cytoplasmic tail interface may be important (Fig. 5C). However, it was difficult to disentangle effects that may involve mutation of the stop transfer signal from requirements for MARCH8 recognition. Taken together, the data show that MARCH8 substrate recognition is complex and involves residues located in helical face 4 of the DRβ TM domain, a cluster of residues proximal to the TM/cytoplasmic domain interface, and a charged residue at the extracellular (and possibly intracellular) juxtamembrane location.
FIGURE 5.
MARCH8 recognition of HLA-DR is sensitive to charged amino acids flanking the N- and C-terminal regions of the TM domain. Regions flanking the TM domain of DRβ were examined by alanine scanning mutagenesis for involvement in MARCH8-induced receptor ubiquitination. A, table showing the amino acid composition of constructs used. Single amino acid code is used. B, graph summarizing the surface down-regulation of CD8-DRβ reporter constructs in the presence of MARCH8. Surface CD8-DR was measured by FACS using anti-CD8 mAb OKT8. The percentage down-regulation was calculated as MFI in the presence of MARCH8wt × 100/MFI in the presence of MARCH8mut. Data demonstrate the importance of Lys198 and Arg222 for MARCH8 recognition. Statistical significance was calculated using a paired t test. C, representative FACS dot plots from data shown in B. Histograms show surface CD8-DRβ reporter construct expression in the presence of MARCH8 (hatched) or MARCH8mut (open); dotted line shows the antibody isotype control.
Specificity of Different MARCH Proteins for MHC Class II Is Determined by Sequences Proximal to the TM/Cytoplasmic Tail Interface
MARCH9, a MARCH protein with the same domain structure as MARCH8, has been shown to target HLA-DQ but not HLA-DR (10). We used this information to help identify sequences important for MHC class II isotype-specific recognition by MARCH E3 ligases. We established that transduction of Raji cells with MARCH8 led to similar reductions in all three class II isotypes, HLA-DP, -DQ, and -DR (Fig. 6B). In contrast, MARCH9 specifically targeted HLA-DQ and showed little down-regulation of HLA-DP or -DR. At high MARCH9 transduction levels, down-regulation of HLA-DP and -DR became evident, but HLA-DQβ was much more efficiently targeted (Fig. 6B). HLA-DRβ and HLA-DQβ differ in their cytoplasmic tails but have very similar TM sequences, apart from a cluster of residues proximal to the TM/cytoplasmic tail boundary (Fig. 6A) (10). We therefore investigated whether MARCH9 specificity for HLA-DQ was determined by these sequence differences. We generated chimeric constructs which exchanged sequences C-terminal of DRβ Ala215 for those present in DQβ (HLA-DRβ-DQβ215–229) or exchanged residues located between positions 215 and 221 of DRβ for those present in DQβ (HLA-DRβ-DQβ215–221). MARCH8 induced similar levels of down-regulation in DRβwt and both DRβ chimeric constructs (Fig. 6C). In contrast, MARCH9 down-regulated the HLA-DRβ-DQβ215–229 and HLA-DRβ-DQβ215–221 constructs more efficiently than DRβwt. Therefore, enhanced recognition by MARCH9 could be transferred to HLA-DRβ by the 215LGLIIRQ221 sequence motif present in the C-terminal region of HLA-DQβ. Together, our data show the importance of the sequence 217LFIYF221 for recognition of HLA-DR by MARCH8 and that variation in this sequence motif explains the preferential recognition of HLA-DQ by MARCH9.
DISCUSSION
Individual MARCH proteins target substrates that have no discernable common binding motif. The only common feature appears to be the presence of a ubiquitinable target amino acid in the cytoplasmic tail, usually a lysine but sometimes cysteine or serine (19). Here we investigated the interaction of MARCH proteins with MHC class II. Our findings demonstrate that multiple features contribute to the overall efficiency of MARCH8-induced HLA-DR internalization. One of the most important components is the sequence 217LFIYF221, located at the C-terminal region of TM domain of DRβ. Variation in this sequence in HLA-DQ also explains the preference of MARCH9 for this class II isotype over HLA-DP and -DR. Residues located in helical face four of the DRβ TM domain had a significant, albeit less dramatic, influence upon recognition. Charged amino acids at the extracellular/TM boundary also had a major impact upon MARCH8 recognition, but there was no absolute requirement for a particular residue, with both positively and negatively charged residues functioning equally well. Sequence elements at the TM/cytoplasmic tail boundary may also contribute. However, in this location it is difficult to disentangle effects that may be due to interruption of the stop transfer signal from those that may be due to direct receptor recognition. Finally, residues 234GLLS237 also influenced MARCH-induced receptor internalization possibly as the sequence encodes a dileucine-like motif (29). These results are summarized in a model that illustrates features of DRβ important for MARCH8-mediated ubiquitination (Fig. 7).
FIGURE 7.
Model of features important for MARCH8 recognition of HLA-DR. Interaction between MARCH8 and HLA-DR is mediated by the sequence LFIYF, situated at the interfacial region between the TM hydrophobic core region and the cytosol (large arrow). Residues located in helical face 4 of DRβ (S, L, and T (small arrows)) are also important for MARCH8 interaction and are likely situated within the hydrophobic core. A significant interaction between a positively charged lysine on the exoplasmic side of the membrane is also indicated (large arrow). Close packing of the TM domains between DRα and DRβ is shown to involve glycine residues (16). Ubiquitination (Ub) occurs on a single lysine residue in the DRβ cytoplasmic tail (K). The dileucine motif (LL) at the C-terminal region of the DRβ cytoplasmic tail influences the efficiency of MARCH-mediated ubiquitination, probably by enhancing interactions with MARCK8 by internalization of HLA-DR molecules.
MARCH8/cMIR was initially identified as a functional and structural mammalian homologue of K3 and K5. These virally encoded E3 ligases are twin TM proteins with cytoplasmic variant-RING (RINGv) catalytic domains. They modulate immune function by targeting receptors such as MHC class I, CD1d, ICAM, and B7.2. A number of studies have reported that the TM and cytoplasmic tails of receptors are required for recognition by viral or mammalian MARCH family members (10, 19–23). In our studies of DRβ we observed that truncation of the C-terminal four amino acids of the cytoplasmic tail influenced MARCH8-induced down-regulation. A probable explanation for this effect is that the sequence encodes a dileucine-like internalization motif, the removal of which reduces the rate of reentry of mature class II into the endocytic pathway (29). This could influence the efficiency of interaction of class II with MARCH8 resident in endosomal compartments. There is limited polymorphism in this region of DRβ (233Thr/Arg) but it is not known whether this impacts on the function of the adjacent 234GLLS237 sequence. We suggest that apart from the key lysine residue other amino acids in the cytoplasmic tail play only a minor, if any, role in MARCH8 recognition. In support of this, truncations that left a minimal 4 amino acid tail (RNQK) had only a minor effect on the ability of MARCH8 to down-regulate a CD8-DRβ reporter. Also, in the context of a DRα/DRβ dimer an 8-residue polyalanine tail is an effective substitute for the entire DRβ cytoplasmic tail. These observations are consistent with studies involving the viral MIR ligases where a polyalanine/polyglycine tail effectively substituted for the entire cytoplasmic tail of HLA-B7 (19).
Our analysis of the cytoplasmic tail/TM boundary was of interest. In the CD8 reporter background substitution of either Arg222 or Asn223 reduced MARCH8-induced down-regulation. However, in the context of a DRα/DRβ dimer both residues could be substituted for alanine without any apparent negative effect (Fig. 3). In type I TM proteins positively charged residues are commonly located in this location and constitute part of a stop transfer signal that halts translocation and plays a major role in determining membrane topology by the “positive-inside” rule (30, 31). Substitution of Arg222 for Ala in CD8-DRβ removes this signal and may cause unpredictable effects, for example by allowing “pullback” into the lipid bilayer thereby disturbing presentation of TM and cytoplasmic sequences to MARCH8. Use of the DRα/DRβ dimer is a novel way to circumvent this problem. These chains form strong TM interactions within the lipid bilayer where close packing is fostered by the presence of conserved glycine residues in both DRα and DRβ (15, 16). In the context of the DRα/DRβ dimer sequence alterations involving the stop transfer signal of DRβ were well tolerated. In fact, the entire DRβ cytoplasmic tail, including the Arg222 stop transfer signal, could be replaced by a polyalanine sequence (DRα/DRβ-K4; Fig. 3) without any apparent loss in MARCH8-related interaction. We conclude that specific sequences in the membrane-proximal region of the cytoplasmic tail do not contribute significantly to MARCH8 recognition.
Previous studies showed that the position of the lysine residue in the cytoplasmic tail is important for MIR interaction (20). MIR2/K5 targets a wide range of immunoreceptors for down-regulation and interacts with lysine residues at or near stop transfer sequences. MIR1/K3 predominantly targets MHC I and shows preference for a more distal lysine localization. We find that MARCH8 is similar to MIR2 in this respect. Optimal MARCH8-induced down-regulation was observed with the lysine in its natural +4 location, and no detrimental effect was observed when it was positioned up to 8 residues from the stop transfer signal. We limited our study to a cytoplasmic tail of this size because this is the natural length for some MHC II molecules. This spatial arrangement is presumably important as it is absolutely conserved among classical HLA-DP, -DQ, and -DR molecules as well as nonclassical HLA-DO. We are confident that analysis performed with the DRα/DRβ dimer accurately reflects true spatial positioning requirements. Complementary studies using the CD8-DRβ reporter showed the same general trend but generated more variable results, likely due to complications involving disturbance of the stop transfer signal, as discussed above.
A critical region of DRβ required for MARCH recognition and specificity is the linear cluster of five amino acids 217LFIYF221, located in the TM domain at the interface between the phospholipid bilayer and cytoplasm. Two lines of evidence point to its significance. First, a major reduction in receptor down-regulation was seen when the sequence was replaced by alanine. Second, substitution of the DR-encoded sequence for the analogous sequence from HLA-DQ transferred properties of MARCH9-mediated down-regulation to the chimeric DR/DQ molecule. This region is likely important for recognition by MARCH proteins in general. Domain swapping between MIR1/K3 and MIR2/K5 shows that the two TM regions of K5 confer specificity for B7.2 (24). It seems likely therefore that sequences present in the N-terminal region of TM domain one and the C-terminal region of domain two of MARCH8 and MARCH9 will be particularly important for MHC class II recognition.
Sequences in the inter-TM region of MARCH8 probably interact with residues in the juxtamembrane stalk region of DRβ. In particular a charged residue in DRβ appears important. This is naturally a positively charged lysine but can be substituted for a negatively charged amino acid without loss of MARCH8-induced receptor internalization. It seems likely that this lysine residue has additional functions because it is absolutely conserved among classical and nonclassical class II molecules and may interact with the conserved glutamate residue present in the analogous position of class II α-chains. MARCH8 encodes the sequence Asp-Arg at the C-terminal region of TM domain one and could therefore potentially interact with either positively or negatively charged residues in DRβ. Interestingly, in a thorough study by Kajikawa et al. (32), interaction between residues in the inter-TM region of MIR2 and an aspartate residue in the juxtamembrane region of B7-2 was identified as important, but there was no fixed requirement for conservation of particular residues in B7-2. We conclude that sequences in the juxtamembrane region play a role in MARCH8-induced receptor internalization, but the requirement for specific residues in DRβ is not absolute.
Supplementary Material
Acknowledgments
We thank Paul Lehner, Satoshi Ishido, and Yasuhiro Ikeda for plasmid constructs.
This work was supported by the Wellcome Trust, the Medical Research Council, and the National Institute of Health Research Cambridge Biomedical Research Centre.

This article contains supplemental Fig. 1 and Table 1.
- DC
- dendritic cell
- Ii
- invariant chain
- MARCH
- membrane-associated RING-CH
- MFI
- mean fluorescent intensity
- mut
- mutant
- TM
- transmembrane
- MIR
- modulator of immune function
- HFE
- high iron Fe.
REFERENCES
- 1. Shin J. S., Ebersold M., Pypaert M., Delamarre L., Hartley A., Mellman I. (2006) Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444, 115–118 [DOI] [PubMed] [Google Scholar]
- 2. van Niel G., Wubbolts R., Ten Broeke T., Buschow S. I., Ossendorp F. A., Melief C. J., Raposo G., van Balkom B. W., Stoorvogel W. (2006) Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25, 885–894 [DOI] [PubMed] [Google Scholar]
- 3. De Gassart A., Camosseto V., Thibodeau J., Ceppi M., Catalan N., Pierre P., Gatti E. (2008) MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl. Acad. Sci. U.S.A. 105, 3491–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young L. J., Wilson N. S., Schnorrer P., Proietto A., ten Broeke T., Matsuki Y., Mount A. M., Belz G. T., O'Keeffe M., Ohmura-Hoshino M., Ishido S., Stoorvogel W., Heath W. R., Shortman K., Villadangos J. A. (2008) Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat. Immunol. 9, 1244–1252 [DOI] [PubMed] [Google Scholar]
- 5. Ishido S., Wang C., Lee B. S., Cohen G. B., Jung J. U. (2000) Down-regulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74, 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coscoy L., Ganem D. (2000) Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. U.S.A. 97, 8051–8056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevenson P. G., Efstathiou S., Doherty P. C., Lehner P. J. (2000) Inhibition of MHC class I-restricted antigen presentation by γ2-herpesviruses. Proc. Natl. Acad. Sci. U.S.A. 97, 8455–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohmura-Hoshino M., Goto E., Matsuki Y., Aoki M., Mito M., Uematsu M., Hotta H., Ishido S. (2006) A novel family of membrane-bound E3 ubiquitin ligases. J. Biochem. 140, 147–154 [DOI] [PubMed] [Google Scholar]
- 9. Matsuki Y., Ohmura-Hoshino M., Goto E., Aoki M., Mito-Yoshida M., Uematsu M., Hasegawa T., Koseki H., Ohara O., Nakayama M., Toyooka K., Matsuoka K., Hotta H., Yamamoto A., Ishido S. (2007) Novel regulation of MHC class II function in B cells. EMBO J. 26, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hör S., Ziv T., Admon A., Lehner P. J. (2009) Stable isotope labeling by amino acids in cell culture and differential plasma membrane proteome quantitation identify new substrates for the MARCH9 transmembrane E3 ligase. Mol. Cell Proteomics 8, 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakke O., Dobberstein B. (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63, 707–716 [DOI] [PubMed] [Google Scholar]
- 12. Denzin L. K., Cresswell P. (1995) HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell 82, 155–165 [DOI] [PubMed] [Google Scholar]
- 13. Sherman M. A., Weber D. A., Jensen P. E. (1995) DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity 3, 197–205 [DOI] [PubMed] [Google Scholar]
- 14. Sloan V. S., Cameron P., Porter G., Gammon M., Amaya M., Mellins E., Zaller D. M. (1995) Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature 375, 802–806 [DOI] [PubMed] [Google Scholar]
- 15. Cosson P., Bonifacino J. S. (1992) Role of transmembrane domain interactions in the assembly of class II MHC molecules. Science 258, 659–662 [DOI] [PubMed] [Google Scholar]
- 16. King G., Dixon A. M. (2010) Evidence for role of transmembrane helix-helix interactions in the assembly of the class II major histocompatibility complex. Mol. Biosyst. 6, 1650–1661 [DOI] [PubMed] [Google Scholar]
- 17. ten Broeke T., de Graaff A., van't Veld E. M., Wauben M. H., Stoorvogel W., Wubbolts R. (2010) Trafficking of MHC class II in dendritic cells is dependent on but not regulated by degradation of its associated invariant chain. Traffic 11, 324–331 [DOI] [PubMed] [Google Scholar]
- 18. Rhodes D. A., Boyle L. H., Boname J. M., Lehner P. J., Trowsdale J. (2010) Ubiquitination of lysine 331 by Kaposi's sarcoma-associated herpesvirus protein K5 targets HFE for lysosomal degradation. Proc. Natl. Acad. Sci. U.S.A. 107, 16240–16245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cadwell K., Coscoy L. (2005) Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309, 127–130 [DOI] [PubMed] [Google Scholar]
- 20. Cadwell K., Coscoy L. (2008) The specificities of Kaposi's sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets. J. Virol. 82, 4184–4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goto E., Mito-Yoshida M., Uematsu M., Aoki M., Matsuki Y., Ohmura-Hoshino M., Hotta H., Miyagishi M., Ishido S. (2008) An excellent monitoring system for surface ubiquitination-induced internalization in mammals. PLoS ONE 3, e1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goto E., Ishido S., Sato Y., Ohgimoto S., Ohgimoto K., Nagano-Fujii M., Hotta H. (2003) c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J. Biol. Chem. 278, 14657–14668 [DOI] [PubMed] [Google Scholar]
- 23. Coscoy L., Sanchez D. J., Ganem D. (2001) A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez D. J., Coscoy L., Ganem D. (2002) Functional organization of MIR2, a novel viral regulator of selective endocytosis. J. Biol. Chem. 277, 6124–6130 [DOI] [PubMed] [Google Scholar]
- 25. Ehrhardt C., Schmolke M., Matzke A., Knoblauch A., Will C., Wixler V., Ludwig S. (2006) Polyethylenimine, a cost-effective transfection reagent. Signal Transduction 6, 179–184 [Google Scholar]
- 26. Lapaque N., Jahnke M., Trowsdale J., Kelly A. P. (2009) The HLA-DRα chain is modified by polyubiquitination. J. Biol. Chem. 284, 7007–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jahnke M., Trowsdale J., Kelly A. P. (2012) Ubiquitination of human leukocyte antigen (HLA)-DM by different membrane-associated RING-CH (MARCH) protein family E3 ligases targets different endocytic pathways. J. Biol. Chem. 287, 7256–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pavlidis P., Noble W. S. (2003) Matrix2png: a utility for visualizing matrix data. Bioinformatics 19, 295–296 [DOI] [PubMed] [Google Scholar]
- 29. Zhong G., Romagnoli P., Germain R. N. (1997) Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J. Exp. Med. 185, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansson M., Nilsson I., von Heijne G. (1993) Positively charged amino acids placed next to a signal sequence block protein translocation more efficiently in Escherichia coli than in mammalian microsomes. Mol. Gen. Genet. 239, 251–256 [DOI] [PubMed] [Google Scholar]
- 31. von Heijne G. (1992) Membrane protein structure prediction: hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225, 487–494 [DOI] [PubMed] [Google Scholar]
- 32. Kajikawa M., Li P. C., Goto E., Miyashita N., Aoki-Kawasumi M., Mito-Yoshida M., Ikegaya M., Sugita Y., Ishido S. (2012) The intertransmembrane region of Kaposi's sarcoma-associated herpesvirus modulator of immune recognition 2 contributes to B7–2 down-regulation. J. Virol. 86, 5288–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fermand J. P., Chevalier A., Brouet J. C. (1986) Characterization of a monoclonal antibody recognizing a monomorphic determinant of the α-chain of class II DQ antigens. Scand. J. Immunol. 24, 313–319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.