Background: P2X receptors are localized both to the cell surface and within intracellular vacuoles. Mechanisms activating intracellular receptors are unclear.
Results: The P2X receptor ATP binding site faces into the vacuole lumen. ATP translocation triggers P2X receptor-dependent release of calcium.
Conclusion: Vacuolar P2X receptors are luminal ATP sensors releasing stored calcium in response to luminal ATP accumulation.
Significance: Intracellular P2X receptors are calcium release channels.
Keywords: ATP, Calcium Intracellular Release, Dictyostelium, Ion Channels, Subcellular Organelles
Abstract
P2X receptors (P2XRs) are ATP-activated calcium-permeable ligand-gated ion channels traditionally viewed as sensors of extracellular ATP during diverse physiological processes including pain, inflammation, and taste. However, in addition to a cell surface residency P2XRs also populate the membranes of intracellular compartments, including mammalian lysosomes, phagosomes, and the contractile vacuole (CV) of the amoeba Dictyostelium. The function of intracellular P2XRs is unclear and represents a major gap in our understanding of ATP signaling. Here, we exploit the genetic versatility of Dictyostelium to investigate the effects of physiological concentrations of ATP on calcium signaling in isolated CVs. Within the CV, an acidic calcium store, P2XRs are orientated to sense luminal ATP. Application of ATP to isolated vacuoles leads to luminal translocation of ATP and release of calcium. Mechanisms of luminal ATP translocation and ATP-evoked calcium release share common pharmacology, suggesting that they are linked processes. The ability of ATP to mobilize stored calcium is reduced in vacuoles isolated from P2XAR knock-out amoeba and ablated in cells devoid of P2XRs. Pharmacological inhibition of luminal ATP translocation or depletion of CV calcium attenuates CV function in vivo, manifesting as a loss of regulatory cell volume decrease following osmotic swelling. We propose that intracellular P2XRs regulate vacuole activity by acting as calcium release channels, activated by translocation of ATP into the vacuole lumen.
Introduction
P2X receptors (P2XRs)2 comprise a family of calcium-permeable cation channels directly activated by adenosine 5′-triphosphate (ATP). P2XRs are evolutionarily conserved among mammals (1), trematode (2), amoeba (3), and single-celled algae (4), yet absent in higher plants, yeast, fruit flies, and nematodes (5). A traditional view of P2XRs is that of cell surface sensors for extracellular ATP, sensing ATP released in physiological processes including pain, inflammation, taste, mechanical stress, and tissue necrosis (6). However, P2XRs also populate membranes of intracellular compartments in mammalian and other eukaryotic cells, including lysosomes (7–9), phagosomes (10), and the contractile vacuole (CV) of the amoeba Dictyostelium discoideum (3, 11). The best evidence that intracellular P2XRs are functional within the cell comes from studying P2XRs in Dictyostelium amoeba. In Dictyostelium, the genome encodes five receptors (P2XA–P2XE) of which P2XA, P2XB, and P2XE form functional receptors when heterologously expressed in mammalian cells (3, 11). Contrary to mammalian cells, Dictyostelium P2XRs are exclusively intracellular, populating the membranes of the CV, the major osmoregulatory organelle in protists (3, 11). Genetic disruption of P2XRs in Dictyostelium causes an aberration in regulatory cell volume decrease (RCVD) following osmotic swelling (3, 11), although the severity of this phenotype shows some strain variance. Genetic disruption of P2XA in AX4 strain amoeba causes a loss of RCVD, described previously in our own study (3). However, disruption of all five receptors in AX2 strain amoeba causes a significant delay in RCVD but not a loss (11). The reason for this difference in phenotype between strains is unknown, although aberration in RCVD observed in both strains suggests that P2XR knock-out does impair CV function though the underlying mechanism is not described (3, 11). Genetic variation in laboratory strains of Dictyostelium is widespread (12), and phenotypic differences between AX2 and AX4 strains are apparent from previous studies (13–15). Despite this difference, AX4 Dictyostelium remains the best genetically amenable model organism with which to investigate intracellular P2XR function and their mechanism of activation.
The CV is an acidic calcium store closely related to acidocalcisomes found in animal cells, possessing both bafilomycin-sensitive vacuolar proton pumps (V-H+-ATPase) and vanadate-sensitive Ca2+ pumps (Ca2+-ATPase) (16). The CV is decorated with calcium-sensitive signaling proteins, including calmodulin (3, 17), and there is some evidence that the CV participates in receptor-mediated calcium influx (18). Voiding of the CV is not via a conventional exocytosis, rather the CV membranes are recycled after emptying (19). During voiding the CV and plasma membrane are transiently connected by a channel permitting ejection of the CV content without exchange of plasma and CV membranes (19–21). This has important implications for potential mechanisms of intracellular P2XR activation in Dictyostelium, suggesting that receptors are not exposed to the extracellular face of the cell and extracellular ATP is not a source of agonist. In addition, Ludlow et al. (11) provide some evidence that the postulated ATP binding site is orientated within the CV lumen and not the cytoplasm. We here use Dictytostelium as a model to test the hypothesis that intracellular P2XRs operate as calcium release channels and investigate how intracellular P2X receptors are activated.
EXPERIMENTAL PROCEDURES
Cell Culture and Transformation
AX4 strain amoebae were cultured in shaking flasks containing HL5 medium with glucose at 22 °C. Amoeba were transformed with plasmids encoding P2XA-GFP, dajumin-GFP (Prof. Gunther Gerisch, Max-Plank Institute for Biochemistry), or calreticulin-GFP (Dictybase depository), and selected with G418. P2XA knock-out amoeba were generated as described previously (3) using a targeting vector conferring blasticidin resistance (Dr. Steve Ennion, University of Leicester). Quintuple P2X receptor knock-out AX2 strain was generously provided by Dr. Steve Ennion.
Fluorescence Microscopy and Protease Protection Assay
Amoeba expressing a P2XA C-terminal GFP fusion protein were seeded on glass and washed with phosphate-buffered saline (PBS). Endosomes were labeled with TRITC-dextran (1 mg/ml, 30 min; Invitrogen) and lysosomes labeled with LysoTracker Red DND-99 (500 nm, 45 min; Invitrogen). When performing the protease protection assay, amoeba expressing either P2XA-GFP or calreticulin-GFP were seeded on glass and washed with PBS containing 2 mm MgCl2. Selective permeabilization of the plasma membrane was achieved by incubating cells with 20 μm digitonin for 2 min at room temperature. Cells were washed with PBS and then exposed to 5 mm trypsin (22). Fluorescence intensity was captured at 15-s intervals using a time lapse IX71 Olympus microscope equipped with a Hamamatsu digital camera. Images were captured using Simple PCI software (Digital Pixel).
Isolation of Vacuoles
For isolation of calcium-loaded vacuoles, 2.5 × 109 amoeba were sedimented, washed with PBS, and resuspended in ice-cold lysis buffer (125 mm sucrose, 50 mm KCl, 0.5 mm EDTA, 5 mm dithiothreitol, and 20 mm HEPES, pH 7.2) and protease inhibitor mixture. For isolation of calcium-depleted vacuoles, amoebae were shaken in Sorenson's phosphate buffer containing 5 mm EGTA for 5 h prior to sedimentation, washing, and lysis. All cells were lysed by repetitive vortexing with glass beads (Sigma) followed by clarification of lysate at 1,500 × g for 10 min at 4 °C. Pellets were resuspended in lysis buffer and homogenized using a 22-gauge needle. The homogenate was fractionated on a discontinuous iodixanol gradient (Optiprep; Sigma). The discontinuous gradient was centrifuged at 50,000 × g for 1 h at 4 °C, followed by collection of 40 1-ml fractions. Estimations of protein concentration in fractions and whole cell lysates were by Bradford assay.
Estimation of Fraction Purity
The enzymatic activity of marker enzymes and the distribution of GFP-tagged organelle markers were used to establish successful purification of intact contractile vacuole. Marker enzyme assays for acid phosphatase (lysosome), alkaline phosphatase (ALP, contractile vacuole), and succinate dehydrogenase (mitochondria) were carried out on all fractions. Reaction volumes were 250 μl and initiated by addition of a 12.5-μl fraction. Acid phosphatase and ALP reactions contained 5 mm p-nitrophenol phosphate in 250 mm glycine-HCl, pH 3.0, for acid phosphatase assay or in 100 mm Tris, 100 mm NaCl, 1 mm MgCl2, pH 9.5, for ALP assay. Reactions were incubated at 25 °C for 1 h or 15 min for ALP and acid phosphatase assay, respectively, and terminated by the addition of 1 ml of 1 m NaOH. Liberation of p-nitrophenol was measured at 405 nm. Succinate dehydrogenase assay reaction buffer contained 100 mm sodium phosphate, pH 9.5, 20 mm sodium succinate, and 0.6 mm nitro blue tetrazolium. Reactions were incubated at 25 °C for 30 min and terminated by addition of 1 ml of 2% SDS. Assays were measured at 630 nm. Enzymatic activity was expressed as a percentage of total cellular activity, as determined from whole cell lysates. Latent enzymatic activity, determined in the presence of Triton X-100, was calculated in an effort to demonstrate the intactness of the contractile vacuole and other organelles.
ATP Translocation Assay
Fractions 1–3 were pooled and diluted 1:3 with transport buffer (50 mm KCl, 2 mm MgCl2, 3% sucrose, 6 μg/ml antimycin A, 6 μg/ml oligomycin, 100 μg/ml NaN3, and 10 mm HEPES, pH 7.2) and centrifuged at 18,500 × g for 30 min at 4 °C; the pellet was resuspended in transport buffer. 1-ml reactions contained 200 μg/ml protein fraction and were incubated at 23 °C for 15 min. Reactions were initiated by the addition of 4 mm ATP and quenched at stipulated time intervals by the addition of 1 ml of ice-cold transport buffer followed by immediate centrifugation at 18,500 × g for 10 min at 4 °C. Pellets were washed three times in 2 ml of ice-cold transport buffer. 100 μl of supernatant from the final spin was collected to estimate background ATP, and the pellet was resuspended in 100 μl of transport buffer. Samples were briefly heated at 105 °C for 2 min immediately prior to assay. ATP was quantified using the luciferase-luciferin assay (Roche Applied Science), and protein was estimated by the Bradford assay. Background ATP readings were subtracted from pellets, and measurements at time zero were subtracted from all time points. For experiments including ATP regeneration, 4 μg/ml creatine kinase (CK) and 5 mm creatine phosphate (CP) (Roche Applied Science) were added, and reactions were initiated by the addition of ATP.
Identification of Vacuolar Nucleotides
Nucleotide extraction was performed following the methodology of zur Nedden et al. (23) using fractions 1–3. 200-μl fractions were diluted 1:3 with ice-cold transport buffer and centrifuged at 18,500 × g for 30 min at 4 °C. Pellets were resuspended in either luciferase buffer for quantitation by the luciferase-luciferin assay (Roche Applied Science) or in homogenization buffer for HPLC analysis. Perchloric acid (5% final concentration) was added and mixed thoroughly followed by centrifugation at 16,060 × g for 2 min at 4 °C. Supernatants were precipitated by the addition of 400 μl of tri-n-octylamine in 1,1,2-trichlorotrifluoroethane (1:1 v/v) and incubating on ice for 10 min. After centrifugation at 12,100 × g for 2 min at 4 °C, organic extraction was repeated twice with the upper aqueous phase. The aqueous phase was mixed 1:1 with Buffer A and incubated on ice for 15 min. For quantification of ATP, 10 μl of nucleotide extract was added to 90 μl of luciferase assay buffer and 100 μl of luciferase reagent (Roche Applied Science). Bioluminescence for measured in a Modulus luminometer (Turner Biosystems). For ion-pair reverse phase HPLC, the mobile phase consisted of Buffer A (65 mm potassium phosphate, 39 mm K2H phosphate, 26 mm KH2 phosphate, and 4 mm tetrabutylammonium hydrogen sulfate, pH 6) and Buffer B (65 mm potassium phosphate, 39 mm K2H phosphate, 26 mm KH2 phosphate, and 25% methanol, pH 6). Buffers were prepared in deionized water and filtered through a 0.4-μm filter. A Supercosil LC-18-T column (Sigma) was equilibrated with 10 column volumes of Buffer B and 30 column volumes of Buffer A at a flow rate of 1 ml/min. Analytical samples were injected after two blank injections and compared with nucleotide standards.
Measurement of Vacuole Calcium Release
For preparation of calcium-depleted vacuoles, amoeba were cultured in PBS containing 5 mm EGTA for 4 h prior to fractionation. Fractions were diluted 1:3 with transport buffer and centrifuged at 18,500 × g for 30 min at 4 °C, and pellets were resuspended in transport buffer. 200 μg/ml protein fractions were assayed for calcium release in transport buffer contained 0.5 μm fluo-3 (Invitrogen). 1.6-ml reactions were incubated for 15 min at 23 °C in a quartz cuvette with constant mixing in a fluorescence spectrophotometer (Hitachi F-2000), 505-nm excitation and 526-nm emission. Reactions were initiated by injection of 2 mm ATP. Competing nucleotides were added at 1 mm.
Real-time Measurement of Cell Size
Changes in cell size were monitored by right-angled light scattering using a Hitachi F-2000 spectrophotometer. 1 × 106 cells/ml were exposed to hypotonic stress by complete replacement of HL5 medium with distilled water. Scattered light was collected at 600 nm. For depletion of CV calcium, cells were preincubated in Sorenson's medium containing 10 mm EGTA for 4 h prior to experimentation.
Statistical Analysis
Average results are expressed as the mean ± S.E. from the number experiments indicated (minimum of three for all). Data are analyzed by an unpaired two-tailed Student's t test to determine significant differences between data groups.
RESULTS
Intracellular P2XRs Are Orientated to Sense Changes in Luminal ATP
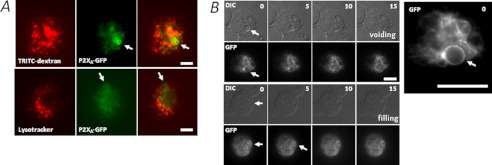
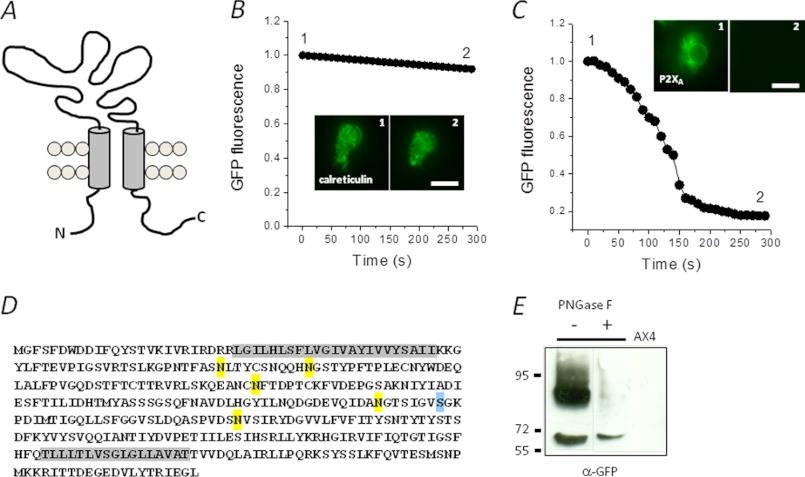
We reported previously that P2XA localizes to the CV in Dictyostelium. However, whether this is an exclusive localization or a dynamic association remains unclear. The nature of P2XA localization is important when considering its involvement in regulating CV function, as some proteins associate with both CV and endosomal compartments in Dictyostelium, including V-H+-ATPase (20) and rab4-like GTPase (24). To this end, we examined the association of P2XA-GFP with lysosomes and endosomes using fluorescent marker dyes. The use of GFP as a tag has been used extensively to localize proteins in Dictyostelium (3, 11, 19, 21). P2XA-GFP labeled both the bladder and tubular network of the CV, showing no overlap with LysoTracker dye (lysosomes) or TRITC-dextran (endosome)-labeled compartments (Fig. 1A). P2XA remained associated with the CV network during both filling and voiding of the vacuole (Fig. 1B). A supplemental video shows P2XA-GFP movement. Cell surface P2XRs are orientated to sense extracellular ATP as the ATP-binding ectodomain faces outward (25), with the receptor N and C termini residing within the cytoplasm (Fig. 2A). We used two independent techniques to determine whether P2XA is orientated to sense cytoplasmic or luminal ATP. First, we sought to determine the localization of the receptor C terminus by performing a fluorescence protease protection assay (22) with amoeba expressing a C-terminal GFP fusion of P2XA (3). P2XA-GFP- or calreticulin-GFP- (a membrane-bound endoplasmic reticulum (ER) marker) expressing cells were briefly exposed to digitonin to enable selective permeabilization of the plasma membrane, followed by addition of trypsin. Calreticulin-GFP fluorescence showed no significant depreciation with upon trypsin addition (Fig. 2B). In stark contrast, P2XA-GFP fluorescence decayed rapidly (Fig. 2C). Calreticulin-GFP became trypsin-sensitive only after prolonged permeabilization (data not shown). The susceptibility of the P2XA receptor C terminus to proteolytic degradation by trypsin demonstrates that the C terminus resides within the cytoplasm and is not membrane-bound within the cell. Second, the P2XA receptor has six predicted glycan acceptor sites (five N-linked and one O-linked), all of which are within the ectodomain (Fig. 2D). Immunodetection of P2XA-GFP from enriched vacuole fractions revealed two distinct bands: a lighter band (∼70 kDa) corresponding to the predicted mass of unmodified P2XA-GFP and a heavier more diffuse band sensitive to degradation by PNGase F endoglycosylidase (Fig. 2E). Anti-GFP immunoreactivity was not detected in parental AX4 amoeba (Fig. 2E). These data suggest that the P2XA receptor is modified by N-linked glycosylation. As the only N-linked glycan sites are predicted to be within the ectodomain (Fig. 2A), this indirectly suggests that the receptor ectodomain is exposed to the Golgi lumen during maturation, placing the ectodomain with the CV lumen by definition of the biosynthetic pathway of membrane proteins in eukaryotes. These data are in good agreement with the receptor orientation as determined by Ludlow et al. (11) and suggest that the receptor may serve to sense vacuolar ATP.
FIGURE 1.
P2XA is a permanent contractile vacuole resident. A, absence of P2XA from lysosomes and endosomes. Live P2XA-GFP expressing AX4 amoeba labeled with TRITC-dextran (endosomes; upper panels) or Lysotracker (lysosomes; lower panels). Arrows indicate contractile vacuole localization. Scale bars, 5 μm. B, association of P2XA with the contractile vacuole during voiding and filling. Overlapping differential inference contrast (DIC) and fluorescence (GFP) images were taken from a time lapse video (supplemental Video) showing P2XA-GFP association with the contractile vacuole during both voiding (top panels) and filling (bottom panels) phases. Time is in seconds (top right). Scale bars, 5 μm. P2XA-GFP localization is shown in the expanded view (inset). Arrows indicate CV.
FIGURE 2.
P2X receptor is orientated to sense luminal ATP. A, schematic representation of P2XR topology. B and C, fluorescence protease protection performed using cells expressing calreticulin or P2XA both with C-terminal GFP tags. Relative fluorescence from representative experiments was captured following trypsin addition to permeabilized cells. Trypsin was added a time 0. Inset, representative images captured at time points 1 and 2. Scale bar, 10 μm. D, peptide sequence of Dictyostelium P2XA showing predicted N-linked (yellow) and O-linked (blue) glycan acceptor sites. Transmembranes are highlighted in gray. E, immunoblot for P2XA-GFP showing anti-GFP immunoreactivity probed in lysates treated with PNGase F (+) or control lysates (−). No anti-GFP immunoreactivity is detected in parental AX4 cells (AX4). Molecular masses are in kDa.
Identification of Receptor Ligand within Vacuole Lumen
If intracellular P2XRs are activated by luminal ATP, then ATP must be present within the CV lumen. To test this we adopted a strategy to isolate intact CV from cultures and ensure freedom from contaminated intact mitochondria, ER, and lysosomes. Only the latent activity of biochemical markers was taken into account to determine isolation of intact organelles. Importantly, because ATP is a major cytoplasmic component we opted to liberate the water-soluble components of CVs by organic extraction (23) only after extensive washing to remove any trace of contaminating bound cytoplasmic ATP. The distribution of intact CVs was determined by assaying ALP activity, which is present with the CV (26). More than 40 fractions peak latent ALP activity were detectable in the most buyont fractions, with ∼50% total cellular latent ALP activity recovered in fractions 1–3 (Fig. 3A). Peak succinate dehydrogenase activity peaked at fraction 21 and represented <1.5% total cellular activity in fractions 1–3 (Fig. 3B). No significant latent acid phosphatase activity (lysosomes) was detectable in fractions 1–16, although nonlatent activity did show a nonuniform distribution across fractions (supplemental Fig. S1). Nonlatent acid phosphatase activity was ∼4% total activity cellular activity in fractions 1–3. These data reveal that lysosomes isolated using this protocol are not intact and therefore negated as a source of ATP in fractions 1–3.
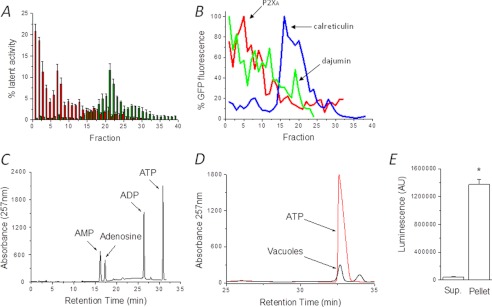
FIGURE 3.
Detection of P2X receptor ligand in vacuole lumen. A, purification of intact contractile vacuoles by subcellular fractionation. Latent activity of ALP (CV, red) and succinate dehydrogenase (mitochondria, green) is shown and is expressed as percentage total cellular activity. n = 4 fractionations. B, distribution of GFP-tagged organelle markers across different fractions. Markers are for contractile vacuole (P2XA and dajumin) and ER (calreticulin). GFP fluorescence is expressed as percentage peak fluorescence. Representative traces of four independent fractions are shown. C and D, ion-pair reverse phase HPLC analysis of ATP in isolated vacuoles. Separation of adenine nucleotide standards (1 μm each) (C) or water-soluble contents liberated from isolated vacuoles (D) are plotted. ATP standard is given as a reference. Representative traces are from four independent experiments. E, detection of ATP liberated from vacuole pellet versus supernatant as determined by luciferase-luciferin assay. n = 4; *, p < 0.01; error bars, S.D.
Because the purity of the CV preparation is critical to determination of luminal ATP, we further demonstrate the purity of isolated CVs by determining the distribution of several GFP-tagged organelle markers across isolated fractions. The distribution of CV markers P2XA-GFP and dajumin-GFP (19) correlated well with the distribution of latent ALP activity (Fig. 3B). The peak ER fraction, as labeled by calreticulin-GFP, was distinct from that of the buoyant CV-enriched fractions peaking at fraction 18 (Fig. 3B). These data demonstrated that fractions 1–3 do not contain intact mitochondria, lysosomes, or ER, and intact CVs are successfully purified. Following pooling, sedimentation, and extensive washing of fractions 1–3, organic extraction liberated ATP detectable by both HPLC (Fig. 3, C and D) and luciferase-luciferin assay (Fig. 2E). Importantly, nominal amounts of ATP were detectable in the supernatant following sedimentation of vacuoles (Fig. 3E), suggesting that washing had successfully removed any cytoplasmic ATP and that any liberated ATP is derived from inside isolated vacuoles.
ATP Translocation Triggers ATP-evoked Calcium Release in Isolated Vacuoles
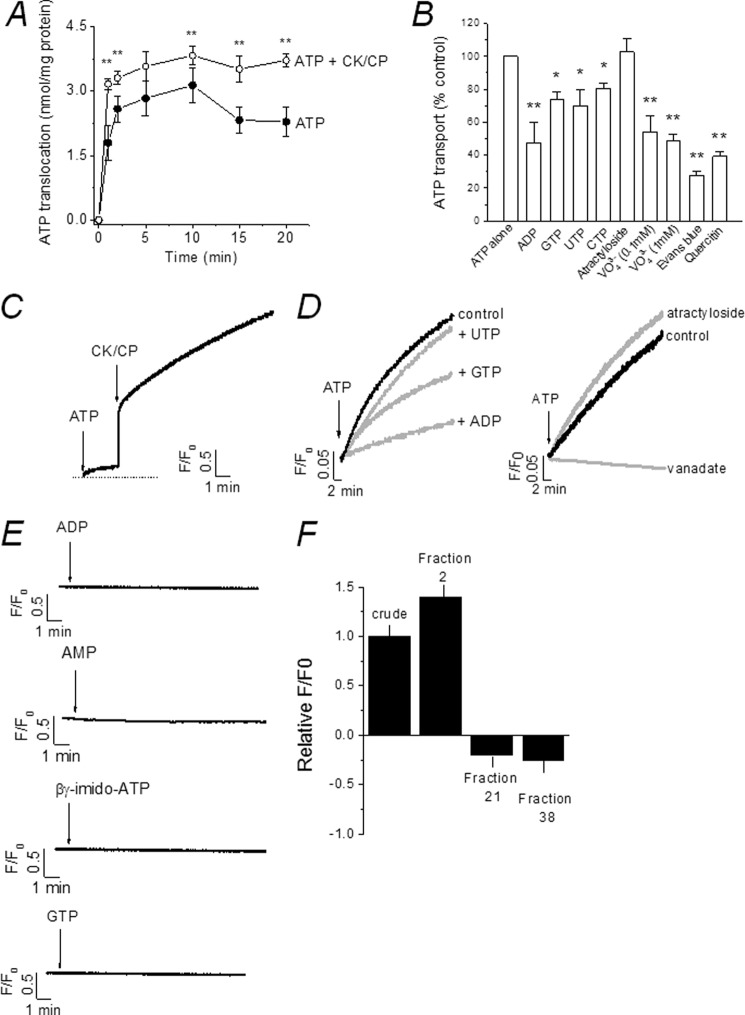
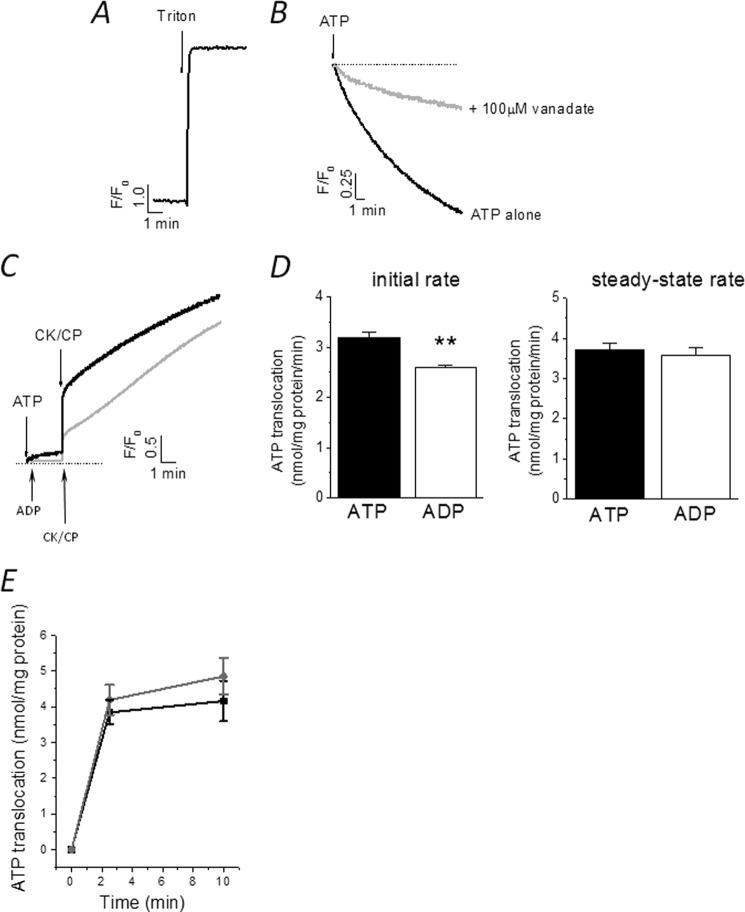
Millimolar quantities of free cytoplasmic ATP establish a large chemical gradient across the membranes of organelles, yet ATP is a strong anion at physiological pH and unable to diffuse passively. The detection of ATP within the CV lumen suggests the presence of a mechanism to facilitate ATP transport (27) or a mechanism for ATP synthesis de novo (10). HPLC analysis of vacuole nucleotides failed to detect any significant amounts of AMP or ADP (Fig. 3D), suggesting that substrates necessary for ATP synthesis are not present or are present at levels below the detection limit of HPLC. To this end, we tested for the presence of an ATP-translocating mechanism. Using 4 mm ATP to mimic cytoplasmic conditions, purified vacuoles accumulated ATP in a time-dependent manner at an initial rate of 1.8 ± 0.4 nmol/mg per min (n = 3) (Fig. 4A). The translocation of ATP was initially very rapid, reaching a plateau after 2 min (Fig. 4A). ATP regeneration using a CK-CP system significantly increased both the initial rate of ATP translocation (3.2 ± 0.1 nmol/mg of protein per min; n = 5, p < 0.01) and the total amount of ATP transported at steady state with respect to ATP alone (Fig. 4A). These data suggest that ATP may be hydrolyzed to ADP upon addition to isolated vacuoles and that CK-CP increases the amount of free ATP for transport. Not surprisingly, ATP is hydrolyzed rapidly upon addition to vacuoles (supplemental Fig. S2). Translocation of ATP was attenuated by the addition of other nucleotides to varying degrees, with ADP causing the greatest inhibition (Fig. 4B). HPLC analysis of luminal ATP revealed transport of ADP, although GTP and UTP were not transported (supplemental Fig. S3). These data are indicative of adenine nucleotide-selective transport and suggest that CK-CP addition may facilitate ATP transport (Fig. 4A) by depleting the pool of ADP, derived from ATP hydrolysis, available to attenuate ATP transport. ATP transport was inhibited by Evans Blue, quercetin, and vanadate, but insensitive to the mitochondrial ATP/ADP exchanger antagonist atractyloside (Fig. 4B).
FIGURE 4.
Luminal ATP translocation triggers release of stored calcium. A, time-dependent luminal ATP translocation in the presence of 4 mm ATP alone or ATP with CK-CP. n = 4; **, p < 0.01. Error bars, S.D. B, effect of nucleotides (2 mm each), vanadate, atractyloside (100 μm), Evans Blue (1 μm), and quercetin (100 μm) on luminal ATP translocation. n = 3–4; *, p < 0.05; **, p < 0.01. C, ATP-evoked calcium release in isolated vacuoles. Representative traces of six or seven independent experiments show calcium release in response to ATP alone with subsequent (CK-CP) addition. D, effect of various inhibitors of luminal ATP translocation on ATP-evoked calcium release. Concentrations are as in B. E, β,γ-imido-ATP, ADP, AMP, or GTP cannot mimic ATP-evoked calcium release. F, specificity and enrichment of ATP-evoked calcium release in contractile vacuole fractions. Magnitude of ATP-evoked calcium release in crude lysate, CV-enriched fraction (fraction 2), mitochondrial/ER fraction (fraction 21), or dense fraction (fraction 38) is shown.
The Dictyostelium P2XA receptor, like other P2XRs, is highly permeable to calcium (3). Moreover, the combination of the receptor orientation and the presence of an ATP-translocating mechanism suggest that ATP translocation into the vacuole lumen may be a means to activate vacuolar P2XRs and trigger release of stored calcium. To test this hypothesis we mimicked the experimental conditions used to observe ATP translocation (Fig. 4A) but instead measured extravacuolar calcium using the membrane-impermeable indicator fluo-3. Strikingly, addition of ATP to vacuoles evoked a release in calcium which progressed over several minutes (Fig. 4C). Subsequent addition of CK-CP caused a rapid rise in calcium release which slowed but progressed over several minutes (Fig. 4C). Addition of ATP or CK-CP caused no rise in fluo-3 fluorescence in the absence of vacuoles, nor could creatine or inorganic phosphate substitute for CP in evoking a calcium release in the presence of vacuoles (data not shown). ATP-evoked calcium release was inhibited by ADP (76.4 ± 2.4%; n = 4), GTP (44.2 ± 1.2%; n = 3), and UTP (15.4 ± 1.2%; n = 3) (Fig. 4D). Vanadate ablated ATP-evoked calcium release (95 ± 5.2%; n = 4), but atractyloside was ineffective (1.2 ± 3.2%; n = 3) (Fig. 4D). These data demonstrate common pharmacology between the ATP translocation and ATP-stimulated calcium release. We found that Evans Blue and quercetin directly quenched fluo-3 fluorescence at concentrations that inhibit ATP translocation (Fig. 4B) and therefore could not be used to probe calcium release. ADP, AMP, and GTP, which do not activate recombinant Dictyostelium P2XRs (3, 11), did not evoke calcium release, suggesting an effect specific to ATP (Fig. 4E). β,γ-Imido-ATP also could not evoke calcium release (Fig. 4E). To rule out the possibility that ATP-evoked calcium release is not specific to CV, we tested the ability of ATP to mobilize calcium in fractions enriched with other organelles. ATP evoked a calcium release in crude whole cell lystates (Fig. 4F), although the magnitude of the response was greater in CV-enriched fractions (Figs. 3, A and B, and 4F). ATP did not mobilize calcium in fractions enriched in the ER and mitochondria (fraction 21) or denser fractions (fraction 38) (Fig. 4F); indeed, addition of ATP caused a decrease in fluo-3 fluorescence in these fractions indicative of ATPase-dependent calcium loading (16).
The Ability of ATP to Mobilize Calcium Is Dependent on the Calcium State of the Store
Detergent solubilization of vacuoles used in this study releases calcium ∼30-fold that of the magnitude of the ATP-stimulated release, demonstrating that the vacuoles are preloaded with calcium (Fig. 5A). Previous studies examining movement of calcium in isolated CVs from Dictyostelium have used CVs isolated following deletion of cellular calcium for several hours with EGTA (18, 28) to allow Ca2+-ATPase-dependent loading stimulated by ATP addition. We confirm that the effect of ATP addition on vacuolar calcium is opposite in CVs from cultures following EGTA buffering. Similar to previous studies (16, 18) robust vanadate-sensitive uptake of calcium is observed upon ATP addition in calcium-depleted vacuoles (Fig. 5B). Interestingly, these data suggest that the net movement of calcium across the vacuole membrane evoked by ATP is dependent upon the calcium state of the vacuole. Although ADP alone could not evoke calcium (Fig. 4E), ADP in the presence of CK-CP could (Fig. 5C). In these experiments creatine could not replace CP (data not shown), suggesting that it is the conversion of ADP to ATP that stimulates release (Fig. 5C). The initial rapid calcium release evoked by ADP plus CK-CP was significantly less than that evoked by ATP plus CK-CP (Fig. 5C), but the magnitude of calcium was similar after 10 min (Fig. 5C). In good correlation, the magnitude of luminal ATP translocation at steady state was not significantly different if ATP or ADP was the ATP source, yet the initial rate of ATP translocation was significantly less if ADP was the source and not ATP (Fig. 5D). An explanation for these data is that the processes of luminal ATP translocation and ATP-evoked calcium release are coupled. A direct comparison of ATP translocation in vacuoles isolated from wild-type and P2XA knock-out cells revealed no significant difference (Fig. 5E).
FIGURE 5.
Net movement of vacuolar calcium evoked by ATP is dependent upon the calcium state of the store and the initial rate of luminal ATP translocation. A, vacuoles isolated from cultures without EGTA buffering a loaded with calcium. Release of calcium was evoked by solubilization of vacuoles with 0.1% Triton X-100. B, ATP causing vanadate-sensitive loading of vacuoles isolated from calcium-depleted cultures. C, paired experiment showing that ADP alone does not evoke calcium release. ATP synthesis from ADP using CK-CP can evoke a release, yet the initial rise is less but approaches that of ATP plus CK-CP after 10 min. D, comparison of initial rates and steady-state rates of luminal ATP translocation in the presence of ATP plus CK-CP or ADP plus CK-CP. n = 4; **, p < 0.01. E, comparison of luminal ATP translocation in vacuoles isolated from WT (black) and P2XA KO (red) amoeba (n = 4).
ATP-evoked Calcium Release Is Dependent upon P2XRs
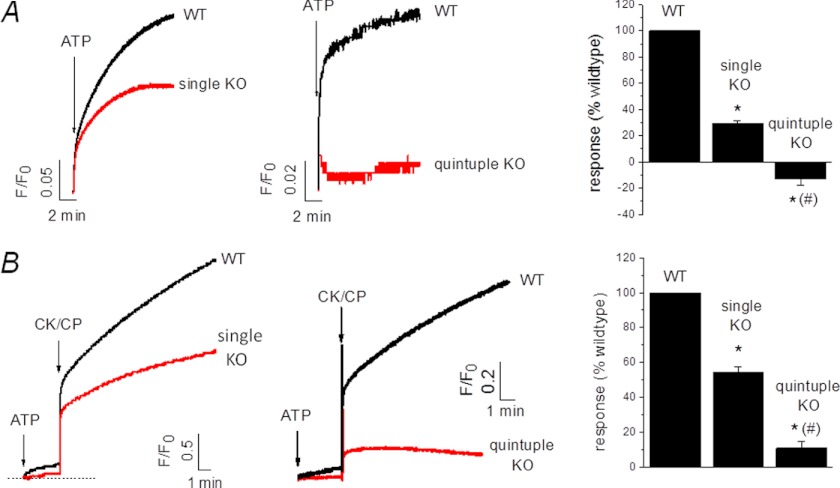
To test for an involvement of P2XRs in mediating ATP-evoked calcium release, we compared the magnitude of release in wild-type AX4 and P2XA knock-out amoeba (3). The magnitude of calcium release stimulated by ATP was reduced by 72% (F/F0 0.17 ± 0.02 versus 0.05 ± 0.01 AX4 wild-type versus P2XA KO cells; n = 6–7, p < 0.01) in knock-out cells (Fig. 6, A and B). ATP plus CK-CP could also evoke a calcium release in P2XA knock-out cells but with a 42% reduced response (F/F0 2.76 ± 0.18 versus 1.5 ± 0.12 wild type versus KO; n = 7, p < 0.01) (Fig. 6, A and B). The latent activity of ALP in CV-enriched fractions (pooled fraction 1–3) was not significantly different in wild-type versus P2XA knock-out cells (supplemental Fig. S4A). Moreover, the specific activity of ALP was not significantly different between wild-type and knock-out cells (supplemental Fig. S4B), demonstrating that the amount of vacuole per mass of protein used to assay calcium release was not significantly different between the two cell types and cannot account for the decreased magnitude in P2XA knock-out cells. One possible reason underlying the reduced ATP-stimulated calcium release in P2XA knock-out cells is that ATP translocation is also reduced in these cells versus parental cells. These data can be explained if the P2XA receptor mediates some of the ATP-stimulated calcium release. To test whether the residual ATP-evoked calcium release was dependent upon other P2X receptor subtypes present in the vacuole (11), we used a quintuple knock-out strain devoid of P2XA–E. Strikingly, ATP mobilization of CV calcium was ablated in the quintuple knock-out strain (F/F0 0.06 ± 0.001 versus −0.02 ± 0.01 wild-type versus KO cells; n = 5, p < 0.01) (Fig. 6A). Indeed, a significant decrease in extravesicular calcium is observed upon addition of ATP in P2XR-null cells, indicative of calcium loading (Fig. 6A). Release of calcium evoked by the addition of ATP plus CK/CP was inhibited by 78% (F/F0 0.491 ± 0.08 versus 0.107 ± 0.05 wild type versus KO; n = 5, p < 0.01) in P2XR-null cells (Fig. 6B). ATP-evoked calcium mobilization was significantly smaller in AX2 versus AX4 strains.
FIGURE 6.
P2X receptors mediate ATP-evoked calcium mobilization. Representative traces show the magnitude vacuolar calcium release evoked by ATP alone (A) or ATP plus CK-CP (B) in AX4 wild-type (WT) versus P2XA receptor knock-out (KO) cells and AX2 WT versus P2XR-null (quintuple KO) cells. Right, average peak responses. n = 4–5; *, p < 0.01 KO versus WT equivalent; #, p < 0.01 quintuple versus single KO.
Antagonists of Luminal ATP Translocation and ATP-evoked Calcium Release Impair Osmoregulation
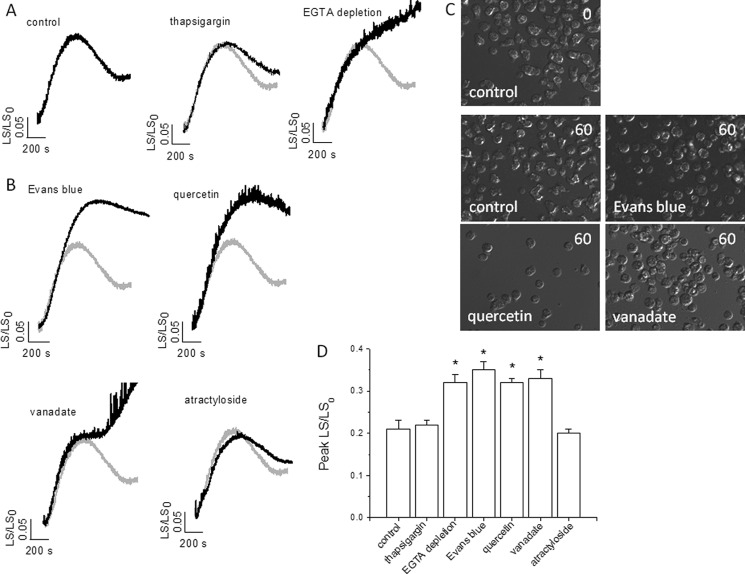
In Dictyostelium RCVD following osmotic swelling is achieved by the expulsion of water from the CV. In an effort to link the information gained from purified vacuoles with cellular function, we sought to test the requirement of normal osmoregulation for ATP-evoked calcium release from the CV using antagonists that block luminal ATP translocation and ATP-evoked calcium release. Exposure of Dictyostelium to hypotonic stress caused a robust cell swelling followed by RCVD of ∼50% in control experiments (Fig. 7, A and C). Culturing cells in the presence of EGTA to deplete cellular CV calcium (Fig. 5B) abolished RCVD (Fig. 7, A and C). The effect of EGTA was not likely to be due to a perturbation of general intracellular calcium signaling as inhibition of the ER by thapsigargin had no effect on RCVD (Fig. 7A). Moreover, cells treated with Evans Blue, quercetin, or vanadate all attenuated RCVD in response to osmotic swelling (Fig. 7, B and C). RCVD was insensitive to atractyloside (Fig. 7B). These data suggest that common antagonist pharmacology is shared by luminal ATP translocation, P2X receptor-dependent CV calcium release, and RCVD.
FIGURE 7.
Inhibitors of luminal ATP translocation or depletion of CV calcium impairs recovery from osmotic swelling. A and B, measurement of real-time changes in cell size by right-angled light scattering. A, cell swelling and recovery in response to hypotonic stress in control cells or cells with depleted ER calcium (thapsigargin; 10 μm, 30 min) or depleted CV calcium (EGTA depletion). B, effect of luminal ATP translocation inhibitors on cell swelling and recovery. Control traces are superimposed (gray) for comparison. C, images of control cells immediately after exposure to distilled water (top right time 0) and following 60- min exposure to water with and without (control) inhibitors (bottom left). D, average data on peak swelling. n = 4; *, p < 0.05.
DISCUSSION
In this study we demonstrate that translocation of ATP into the vacuole lumen is a mechanism by which intracellular P2XRs can be activated. This mode of activation is atypical among other calcium release channels which are activated by ligands, including nucleotides. Activation of the inositol 1,4,5-trisphosphate receptor, ADP-ribose-activated TRPM2 channels and presumably NAADP activation TPC channels is via interaction with ligand on the cytoplasmic face of calcium stores (29–31). Our evidence for the orientation of intracellular P2XRs in Dictyostelium with the ATP-binding site facing in the CV lumen is consistent with previous work (11) and also consistent with the postulated orientation of the P2X4 receptor in mammalian lysosomes (7). Unlike the ATP-binding site of P2X4 which can become exposed to the extracellular environment following lysosome exocytosis (7–8), this study and the study of others (19, 32) argue that the membranes of CV and plasma membrane do not mix upon exocytosis and hence that intracellular P2XRs in Dictyostelium would never be exposed to the extracellular space. The P2XA receptor would be a rather ineffective sensor of cytoplasmic ATP as one would predict the receptor to exist in a permanently desensitized state because of the millimolar amounts of ATP in the cytoplasm (3). Despite the detection of ATP we have not determined the absolute concentration of ATP within the lumen of a single vacuole, nor can we estimate such from our assays carried out on populations of vacuoles. This is for a number of reasons: (i) the number of vacuoles varies per cell, and the absolute number of vacuoles assayed is not known; and (ii) the volume of CV is dynamic and changes with time due to swelling by water accumulation. Whatever the absolute concentration of ATP, translocation of ATP in isolated vacuoles is capable of raising the luminal ATP concentration high enough to activate P2XA (EC50 ∼170 μm) (3). The observation that ATP-stimulated calcium release is significantly attenuated but not abolished in P2XA knock-out cells is suggestive of a level of redundancy between vacuolar P2XRs. Indeed, P2XB and P2XE have recently been shown to form functional receptors, although ATP is 3–5-fold less potent at activating B and E receptor subtypes compared with P2XA (3, 11). It is therefore interesting to observe that the calcium release evoked during ATP regeneration is only reduced by 42% in P2XA KO cells compared with the 72% reduced in calcium released by ATP alone. Our data suggest that the rate and steady-state amount of ATP translocated are significantly greater with ATP regeneration than without and that during these conditions there is less dependence on P2XA for calcium release. One possible explanation for this observation is that different receptor subtypes, with higher ATP EC50, e.g. P2XB and P2XE (11), contribute to calcium release at higher initial and steady-state rates of luminal ATP translocation. This may allow the vacuole to fine tune a response to ATP, as observed for the complex repertoire of cell surface P2XRs in mammalian cells (1). Experiments with P2XR-null cells clearly demonstrate that ATP-evoked calcium mobilization is entirely dependent upon P2XRs. In combination with experiments with P2XA, these data support a role for multiple receptor subtypes in releasing calcium in response to ATP. Intriguingly, the response to ATP plus CK/CP is heavily suppressed (78%) but not abolished in P2XR-null cells. It should be noted that the quasi-instantaneous release observed upon addition of ATP plus CK/CP is only observed in the presence of vacuoles and therefore not an artifactual effect of CK/CP on fluo-3 fluorescence. The molecular basis for the residual rapid component is unclear at present, but P2XR activation represents the major component.
Our data show that luminal ATP translocation increases ∼25% in the presence of an ATP-regenerating system than without, yet ATP regeneration triggers an immediate calcium release of some ∼500%, although these data do demonstrate that increased ATP translocation increases the magnitude of calcium release. It is more difficult to explain the differences observed in the magnitudes of responses without knowing the absolute change in luminal ATP concentration which results from a 25% enhancement of ATP translocation. The relationship between ATP concentration and receptor activation is not linear and steep for Dictyostelium P2XRs (3, 11), and it is therefore possible that small changes in luminal ATP could result in large real-term changes in receptor activity. The rapid release of calcium stimulated by ATP regeneration is not observed in the absence of vacuoles or if creatine phosphate is substituted for creatine, suggesting that vacuoles and ATP regeneration are required. More importantly, the magnitude of calcium release stimulated by ATP regeneration is dependent upon P2XA.
One outstanding issue is the molecular correlate of the vacuolar ATP translocator. Vesicular adenine nucleotide transporters have been identified in diverse organisms (33, 34) and are found in secretory vesicles of neurons (35), chromaffin cells (27), and pancreas (36). The ATP translocator identified in this study was inhibited by micromolar Evans Blue in common with some mammalian ATP transporters (27, 35, 36). One interesting observation of this current study is that the translocation of ATP may be dependent upon ATP hydrolysis. Vanadate sensitivity suggests that ATP hydrolysis may be required. In addition, the nonhydrolyzable ATP analog β,γ-imido-ATP is 10-fold more potent that ATP at activating P2XA (3) and ∼3-fold more potent at activating P2XB (11). Despite this, β,γ-imido-ATP does not evoke calcium release. Our data demonstrate that this transporter is capable of transporting ATP and ADP, but not GTP or UTP. Recently, putative ABC transporters have been identified in a proteomic analysis of CV from Trypansoma cruzi (37). Indeed, MRP-type ABC transporters are responsible for the accumulation of ADP in secretory granules of platelets (38). The Dictyostelium genome encodes a plethora of ABC transporters, including some homologous to mammalian MRP-type transporters (39). The subcellular localization of ABC transporters in Dictyostelium is not well defined, but they remain potential candidates underlying the vacuolar transportation of ATP described here. We have revealed that ATP controls the net movement of calcium across the vacuole membrane by two counteracting mechanisms: (i) ATP hydrolysis to drive calcium uptake and (ii) ATP activation of P2XRs to release calcium. Although this may initially appear counterintuitive, it should be noted we have studied this phenomenon on a macroscopic scale. Signaling events on a local level may be different, and indeed Ca2+-ATPases and the ATP translocator may utilize different pools of ATP (40). A more refined view will come from further investigation into the modulation of this signaling system.
In this study we tested the hypothesis that intracellular P2XRs can act as vacuolar calcium release channels in vitro in an attempt to understand why genetic disruption of P2XA impairs CV activity in AX4 Dictyostelium in vivo (3). Although we have not presented direct evidence that hypotonic stress triggers a calcium release from the CV in vivo there are some excellent examples from other studies which hypothesize that calcium signaling is important for normal CV function. These include (i) the CV is an acidic calcium store (16, 18), (ii) the CV is decorated with calcium-responsive signaling proteins (3, 17), (iii) calcium-permeable ion channels are exclusive CV residents (3, 11), and (iv) CV calcium release and signaling necessitate osmoprotection and CV function in other protists (41, 42). In this study we suggest that P2XR-dependent release of calcium triggered by vacuolar ATP translocation is necessary for normal CV function and this may explain the impairment of CV function following disruption of the P2XA gene (3). We demonstrate that conditions that deplete CV calcium lead to loss of RCVD, which is not mimicked by depletion of ER calcium by thapsigargin. We have provided evidence that the mechanisms we have described in purified vacuoles are important for cellular function and that pharmacological inhibitors of vacuolar ATP translocation and ATP-evoked release phenocopy the osmotic swelling observed in P2XA knock-out cells (3). It is difficult to ascertain whether the compounds that we have used to inhibit RCVD are selective for ATP transport and ATP-evoked release, for example vanadate is a generic inhibitor of ATPase function. However, the observation that thapsigargin and atractyloside, inhibitors of other major ER and mitochondrial calcium pools, respectively, do not impair RCVD gives some degree of confidence of specificity. A pharmacological approach remains the only option until the molecular correlate of the CV ATP transporter is elucidated, allowing a genetic approach to be taken in future studies.
The identification of intracellular P2XRs as calcium release channels in the model eukaryote Dictyostelium makes it is tempting to speculate that a similar role exists for P2XRs that reside in intracellular compartments of mammalian cells. A potential candidate for this is the mammalian P2X4 receptor which targets to lysosomes (7–9). The receptor is protected from degradation in this compartment, which is suggestive of a requirement for function. P2X4 receptors are regulated by pH (43), and lysosomes can accumulate ATP (44). Our observations further suggest that the compartmentalization of ATP by translocation allows ATP to act as a discrete signaling molecule, either by its release into the extracellular space or shuttling into an intracellular compartment as described here. The evolutionary conservation of the intracellular residency of P2XRs between amoeba and mammals is highly suggestive of a conserved function. This study also suggests that cell signaling by the compartmentalization of ATP has early evolutionary beginnings (5) and may have evolved to include plasma membrane events. Further work is required to elucidate any functional role for intracellular P2X receptors in mammalian cells.
Supplementary Material
Acknowledgments
We thank Dr. Steve Ennion (University of Leicester) for the P2X knockout constructs and AX2 cells, Professor Antony Galione (University of Oxford) for useful comments and constructive feedback on the manuscript, and Dr. Iain Manfield (Centre for Biomolecular Interactions, University of Leeds) and Dr. Charles Brealey (University of East Anglia) for technical and scientific assistance with nucleotide analysis by HPLC.
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) through a BBSRC David Phillips Fellowship personal award (to S. J. F.).

This article contains supplemental Figs. S1–S4 and a video.
- P2XR
- P2X receptor
- ALP
- alkaline phosphatase
- CK
- creatine kinase
- CP
- creatine phosphate
- CV
- contractile vacuole
- ER
- endoplasmic reticulum
- RCVD
- regulatory cell volume decrease.
REFERENCES
- 1. North R. A. (2002) Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 2. Agboh K. C., Webb T. E., Evans R. J., Ennion S. J. (2004) Functional characterization of a P2X receptor from Schistosoma mansoni. J. Biol. Chem. 279, 41650–41657 [DOI] [PubMed] [Google Scholar]
- 3. Fountain S. J., Parkinson K., Young M. T., Cao L., Thompson C. R., North R. A. (2007) An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature 448, 200–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fountain S. J., Cao L., Young M. T., North R. A. (2008) Permeation properties of a P2X receptor in the green algae Ostreococcus tauri. J. Biol. Chem. 283, 15122–15126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fountain S. J., Burnstock G. (2009) An evolutionary history of P2X receptors. Purinergic Signal. 5, 269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khakh B. S., North R. A. (2006) P2X receptors as cell surface ATP sensors in health and disease. Nature 442, 527–532 [DOI] [PubMed] [Google Scholar]
- 7. Qureshi O. S., Paramasivam A., Yu J. C., Murrell-Lagnado R. D. (2007) Regulation of P2X4 receptors by lysosomal targeting, glycan protection, and exocytosis. J. Cell Sci. 120, 3838–3849 [DOI] [PubMed] [Google Scholar]
- 8. Stokes L., Surprenant A. (2009) Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur. J. Immunol. 39, 986–995 [DOI] [PubMed] [Google Scholar]
- 9. Toulme E., Garcia A., Samways D., Egan T. M., Carson M. J., Khakh B. S. (2010) P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J. Gen. Physiol. 135, 333–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuehnel M. P., Rybin V., Anand P. K., Anes E., Griffiths G. (2009) Lipids regulate P2X7-receptor-dependent actin assembly by phagosomes via ADP translocation and ATP synthesis in the phagosome lumen. J. Cell Sci. 122, 499–504 [DOI] [PubMed] [Google Scholar]
- 11. Ludlow M. J., Durai L., Ennion S. J. (2009) Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J. Biol. Chem. 284, 35227–35239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloomfield G., Tanaka Y., Skelton J., Ivens A., Kay R. R. (2008) Widespread duplications in the genomes of laboratory stocks of Dictyostelium discoideum. Genome Biol. 9, R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. May T., Blusch J., Sachse A., Nellen W. (1991) A cis-acting element responsible for early gene induction by extracellular cAMP in Dictyostelium discoideum. Mech. Dev. 33, 147–155 [DOI] [PubMed] [Google Scholar]
- 14. Jain R., Gomer R. H. (1994) A developmentally regulated cell surface receptor for a density-sensing factor in Dictyostelium. J. Biol. Chem. 269, 9128–9136 [PubMed] [Google Scholar]
- 15. Deery W. J., Gao T., Ammann R., Gomer R. H. (2002) A single cell density-sensing factor stimulates distinct signal transduction pathways through two different receptors. J. Biol. Chem. 277, 31972–31979 [DOI] [PubMed] [Google Scholar]
- 16. Rooney E. K., Gross J. D., Satre M. (1994) Characterisation of an intracellular Ca2+ pump in Dictyostelium. Cell Calcium 16, 509–522 [DOI] [PubMed] [Google Scholar]
- 17. Zhu Q., Clarke M. (1992) Association of calmodulin and an unconventional myosin with the contractile vacuole complex of Dictyostelium discoideum. J. Cell Biol. 118, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malchow D., Lusche D. F., Schlatterer C., De Lozanne A., Müller-Taubenberger A. (2006) The contractile vacuole in Ca2+-regulation in Dictyostelium: its essential function for cAMP-induced Ca2+-influx. BMC Dev. Biol. 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabriel D., Hacker U., Köhler J., Müller-Taubenberger A., Schwartz J. M., Westphal M., Gerisch G. (1999) The contractile vacuole network of Dictyostelium as a distinct organelle: its dynamics visualized by a GFP marker protein. J. Cell Sci. 112, 3995–4005 [DOI] [PubMed] [Google Scholar]
- 20. Heuser J., Zhu Q., Clarke M. (1993) Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121, 1311–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker M., Matzner M., Gerisch G. (1999) Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. EMBO J. 18, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorenz H., Hailey D. W., Wunder C., Lippincott-Schwartz J. (2006) The fluorescence protease protection (FPP) assay to determine protein localization and membrane topology. Nat. Protoc. 1, 276–279 [DOI] [PubMed] [Google Scholar]
- 23. zur Nedden S., Eason R., Doney A. S., Frenguelli B. G. (2009) An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze-thaw degradation of ATP. Anal. Biochem. 388,108–114 [DOI] [PubMed] [Google Scholar]
- 24. Bush J., Temesvari L., Rodriguez-Paris J., Buczynski G., Cardelli J. (1996) A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol. Biol. Cell 7, 1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts J. A., Evans R. J. (2007) Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2X receptors. J. Neurosci. 27, 4072–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nolta K. V., Steck T. L. (1994) Isolation and initial characterization of the bipartite contractile vacuole complex from Dictyostelium discoideum. J. Biol. Chem. 269, 2225–2233 [PubMed] [Google Scholar]
- 27. Sawada K., Echigo N., Juge N., Miyaji T., Otsuka M., Omote H., Yamamoto A., Moriyama Y. (2008) Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. U.S.A. 105, 5683–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malchow D., Lusche D. F., De Lozanne A., Schlatterer C. (2008) A fast Ca2+-induced Ca2+-release mechanism in Dictyostelium discoideum. Cell Calcium 43, 521–530 [DOI] [PubMed] [Google Scholar]
- 29. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 30. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lange I., Yamamoto S., Partida-Sanchez S., Mori Y., Fleig A., Penner R. (2009) TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2, ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heuser J. (2006) Evidence for recycling of contractile vacuole membrane during osmoregulation in Dictyostelium amoebae: a tribute to Günther Gerisch. Eur. J. Cell Biol. 85, 859–871 [DOI] [PubMed] [Google Scholar]
- 33. Palmieri L., Rottensteiner H., Girzalsky W., Scarcia P., Palmieri F., Erdmann R. (2001) Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 20, 5049–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leroch M., Neuhaus H. E., Kirchberger S., Zimmermann S., Melzer M., Gerhold J., Tjaden J. (2008) Identification of a novel adenine nucleotide transporter in the endoplasmic reticulum of Arabidopsis. Plant Cell 20, 438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gualix J., Alvarez A. M., Pintor J., Miras-Portugal M. T. (1999) Studies of chromaffin granule functioning by flow cytometry: transport of fluorescent ϵ-ATP and granular size increase induced by ATP. Receptors Channels 6, 449–461 [PubMed] [Google Scholar]
- 36. Haanes K. A., Novak I. (2010) ATP storage and uptake by isolated pancreatic zymogen granules. Biochem. J. 429, 303–311 [DOI] [PubMed] [Google Scholar]
- 37. Ulrich P. N., Jimenez V., Park M., Martins V. P., Atwood J., 3rd, Moles K., Collins D., Rohloff P., Tarleton R., Moreno S. N., Orlando R., Docampo R. (2011) Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS One 6, e18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jedlitschky G., Tirschmann K., Lubenow L. E., Nieuwenhuis H. K., Akkerman J. W., Greinacher A., Kroemer H. K. (2004) The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 104, 3603–3610 [DOI] [PubMed] [Google Scholar]
- 39. Anjard C., Loomis W. F. (2002) Dictyostelium sequencing consortium. Eukaryot. Cell 1, 643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yegutkin G. G., Mikhailov A., Samburski S. S., Jalkanen S. (2006) The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol. Biol. Cell 17, 3378–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergquist B. L. (1989) Trans. Am. Microsc. Soc. 108, 369–379 [Google Scholar]
- 42. Loukin S., Zhou X., Kung C., Saimi Y. (2008) A genome-wide survey suggests an osmoprotective role for vacuolar Ca2+ release in cell wall-compromised yeast. FASEB J. 22, 2405–2415 [DOI] [PubMed] [Google Scholar]
- 43. Clarke C. E., Benham C. D., Bridges A., George A. R., Meadows H. J. (2000) Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J. Physiol. 523, 697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., Wang W., Gu X. S., Duan S. (2007) Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.