Background: Luteinizing hormone and carbonic anhydrase-6 bear unique N-linked oligosaccharides terminating with LacdiNAc (GalNAcβ1,4GlcNAc).
Results: Two related β1,4-N-acetylgalactosaminyltransferases have distinct specificities for peptide motifs on luteinizing hormone and carbonic anhydrase-6.
Conclusion: Affinity for the peptide motif determines the efficiency of GalNAc transfer to oligosaccharide acceptors.
Significance: Kinetic parameters define the basis for protein-specific synthesis of carbohydrate structures in vivo.
Keywords: Carbohydrate Biosynthesis, Glycobiology, Glycoprotein Biosynthesis, Glycoprotein Hormones, Glycosyltransferases, N-Acetylgalactosamine
Abstract
Two closely related β1,4-N-acetylgalactosaminyltransferases, β4GalNAc-T3 and β4GalNAc-T4, are thought to account for the protein-specific addition of β1,4-linked GalNAc to Asn-linked oligosaccharides on a number of glycoproteins including the glycoprotein hormone luteinizing hormone and carbonic anhydrase-6 (CA6). We have utilized soluble, secreted forms of β4GalNAc-T3 and β4GalNAc-T4 to define the basis for protein-specific GalNAc transfer in vitro to chimeric substrates consisting of Gaussia luciferase followed by a glycoprotein substrate. Transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4 to terminal GlcNAc is divalent cation-dependent. Transfer of GalNAc to glycoprotein acceptors that contain a peptide recognition determinant is maximal between 0.5 and 1.0 mm MnCl2; however, transfer is increasingly inhibited by concentrations of MnCl2 above 1 mm and by anion concentrations above 15 mm. In contrast, transfer of GalNAc to the simple sugar acceptor N-acetylglucosamine-β-p-nitrophenol (GlcNAcβ-pNP) is not inhibited by concentrations of MnCl2 or anions that would inhibit transfer to glycoprotein acceptors by >90%. This finding indicates that interaction with the peptide recognition determinant in the substrate is sensitive to the anion concentration. β4GalNAc-T3 and β4GalNAc-T4 have similar but distinct specificities, resulting in a 42-fold difference in the IC50 for transfer of GalNAc to chimeric glycoprotein substrates by agalacto human chorionic gonadotropin, comprising 29 nm for β4GalNAc-T3 and 1.2 μm for β4GalNAc-T4. Our in vitro analysis indicates that enzymatic recognition of the peptide determinant and the oligosaccharide acceptor are independent events.
Introduction
Glycoprotein hormones including luteinizing hormone and thyroid-stimulating hormone (TSH) that are synthesized in the anterior lobe of the pituitary bear N-linked oligosaccharides that terminate with the sequence SO4-4-GalNAcβ1,4GlcNAcβ1,2Man (1). The addition of β1,4-linked GalNAc to N-linked oligosaccharides on the glycoprotein hormones (1, 2) and other glycoproteins such as the prolactin-like proteins (3), carbonic anhydrase-6 (CA6)2 (4), and SorLA/LR11 (5) reflects the action of one or more protein-specific β1,4-N-acetylgalactosaminyl-transferases (β4GalNAc-T). Two β4GalNAc-Ts have been cloned that are able to transfer GalNAc to β-linked GlcNAc, β4GalNAc-T3 (B4GALNT3, GenBankTM AB089940, AB114826) (6), and β4GalNAc-T4 (B4GALNT4, GenBankTM AB089939, AB114827) (7). We previously reported that β4GalNAc-T3 and β4GalNAc-T4 expressed in Chinese hamster ovary (CHO) cells exhibit protein-specific transfer of GalNAc to the N-linked oligosaccharides on the glycoprotein hormone α subunit and those on CA6 but not to those on transferrin (Trf) (8). We found that a 19-amino acid peptide sequence located at the carboxyl terminus of CA6 confers the protein-specific addition of β1,4-linked GalNAc to N-linked oligosaccharides on CA6 by CHO cells expressing either β4GalNAc-T3 or β4GalNAc-T4. The addition of this 19-amino acid sequence, CA(1–19), to the carboxyl terminus of Trf confers GalNAc addition to the N-linked oligosaccharides on Trf in CHO cells expressing either β4GalNAc-T3 and β4GalNAc-T4.
Transcript levels for β4GalNAc-T3 and β4GalNAc-T4 differ in tissues known to express β1,4-N-acetylgalactosaminyltransferase activity (9). β4GalNAc-T3 transcripts are abundant in stomach, colon, and, testis, whereas β4GalNAc-T4 transcripts are abundant in ovary, brain, colon, and uterus (7). We found transcripts for both β4GalNAc-T3 and β4GalNAc-T4 in mouse pituitaries; however, β4GalNAc-T3 transcripts are more abundant than β4GalNAc-T4 transcripts.3 A key question is whether β4GalNAc-T3 and β4GalNAc-T4 are simply redundant or display differences in specificity or catalytic properties. Why are two closely related transferases produced? Do β4GalNAc-T3 and β4GalNAc-T4 modify different substrates in vivo? Examination of these questions requires in vitro analysis of the specificity and properties of β4GalNAc-T3 and β4GalNAc-T4.
We have now examined protein-specific transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4 to N-linked oligosaccharides on various chimeric glycoprotein substrates in vitro. We previously defined the peptide recognition determinant itself in in vivo studies in CHO cells expressing β4GalNAc-T3 and β4GalNAc-T4. Our present studies in the in vitro system complement the in vivo findings, revealing that β4GalNAc-T3 and β4GalNAc-T4 display similar but distinct specificities. In vitro analysis has further allowed us to separate enzymatic recognition of the peptide determinant and the oligosaccharide acceptor as two independent events.
EXPERIMENTAL PROCEDURES
Preparation of Soluble Forms of β4GalNAc-T3 and β4GalNAc-T4
Constructs in which the cytosolic and transmembrane domains of murine β4GalNAc-T3 and β4GalNAc-T4 were replaced by the preprotrypsin leader followed by the FLAG epitope were generated using pFLAG-CMV-1 (Sigma) as was described for human and murine β4GalNAc-T3 and β4GalNAc-T4 (6, 7) and are referred to as β4GalNAc-T3-F and β4GalNAc-T4-F. Soluble β4GalNAc-T3-F and β4GalNAc-T4-F were obtained by transfection of HEK 293-T cells grown in serum-free Pro293A-CDM plus 4.1 mm l-glutamine medium (Lonza 12-764Q) using Xfect (Clontech) per the manufacturer's protocol. β4GalNAc-T3-F and β4GalNAc-T4-F were purified from the medium by binding to anti-FLAG M-1 affinity gel (Sigma 4596) and elution with FLAG peptide (Sigma 3290). The eluted transferases were stored at −80 °C after the addition of glycerol to a final concentration of 10%.
Preparation of Substrates
Chimeric forms of the glycoprotein hormone α subunit and Trf (see Fig. 1) were prepared as described (8). pCMV-GLuc was obtained from New England Biolabs. The Gaussia luciferase (GLuc) constructs in each case consist of GLuc followed by the α subunit or Trf and the epitope Myc-His. GLuc chimeras were expressed in Lec8/CHO cells (10) grown in serum-free Ultra CHO medium (Lonza 12-724Q) using Lipofectamine and Lipofectamine Plus (Invitrogen) per the manufacturer's transfection protocol. Media containing the chimeric proteins were harvested and purified using nickel-nitrilotriacetic acid-agarose (Qiagen). The bound proteins were eluted using 250 mm imidazole, 50 mm monobasic sodium phosphate, 300 mm sodium chloride, 0.05% Tween 20, pH 8.0. The purified substrates were exchanged into 25 mm HEPES buffer, pH 7.5, containing 0.1% BSA and stored at −80 °C after the addition of glycerol to a final concentration of 10%.
FIGURE 1.
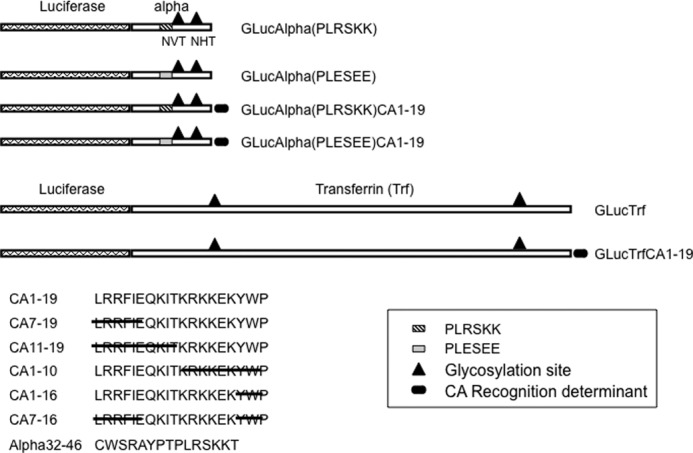
Schematic representation of chimeric glycoprotein substrates. Plasmids encoding GLuc followed by the glycoprotein hormone α subunit (Alpha) or Trf were prepared by ribocloning (8). Locations of Asn glycosylation sites are indicated by the ▴. The location of the sequence PLRSKK that was mutated to PLESEE is indicated for the α subunit. The carboxyl-terminal 19 amino acids from CA6 (CA1–19) were added to the carboxyl terminus of α and the carboxyl terminus of Trf and are indicated by the filled oval. Sequences of CA1–19 and of peptides with partial sequences are shown. All chimeric glycoprotein substrates contained a Myc epitope and His6 residues. The sequence of the synthetic peptide from the α subunit that contains the key residues required for recognition, α32–46, is also shown.
Quantitation of GalNAc Incorporation into Gaussia Luciferase Containing Chimeric Glycoproteins
In vitro transfer of GalNAc to GLuc chimeric glycoproteins was carried out in a 50-μl reaction containing: 25 mm Hepes buffer, pH 7.5, 10% glycerol, 0.05% Triton X-100, 5 mm KCl, 0.5 or 1.0 mm MnCl2, 1 mg/ml BSA, 0.24 mm UDP-GalNAc, and the affinity-purified GLuc substrate. The affinity-purified substrate was diluted sufficiently to maintain the final concentration of the imidazole below 5 mm. Following the addition of either β4GalNAc-T3-F or β4GalNAc-T4-F, transfer of GalNAc was allowed to proceed for 20 min at 25 °C. Transfer was terminated by the addition of 450 μl of ice-cold 2 mm EDTA, pH 7.5.
The amount of GalNAc transferred to GLuc substrates was determined using a microplate assay. B&W Isoplate-96 HB (PerkinElmer Life Sciences 6005580) 96-well plates were coated with Wisteria floribunda agglutinin (WFA) by adding 100 μl of 25 mm sodium carbonate buffer, pH 8.5, containing 1.25 μg of WFA (Sigma L8258) per well and incubating overnight at 4 °C. Plates were washed with 0.1% BSA/PBS using a Bio-Rad Immunowash microplate washer and blocked with 5% BSA/PBS. The light units of GLuc activity present following GalNAc transfer were determined for each in vitro assay by adding 5–8 × 105 light units of each transferase reaction in 100 μl of 15.6 μg/ml histone, 0.1% BSA, PBS (positive signal) or in 100 μl of 15.6 μg/ml histone, 0.1% BSA/PBS, containing 50 mm GalNAc (background signal) to WFA-coated wells. After incubation overnight at 4 °C, unbound GLuc substrate was removed by washing, and the amount of bound GLuc substrate was determined using a Wallac Victor2 luminometer using the Renilla luciferase assay system (Promega E2820). Fifty mm GalNAc inhibits binding to immobilized WFA. The difference between the light units of GLuc substrate bound in the absence and presence of GalNAc reflects the amount of GLuc substrate that is modified with β1,4-linked GalNAc. In the absence of added β4GalNAc-T3 or β4GalNAc-T4, the amount of GLuc substrate bound in the absence and presence of GalNAc was identical. All assays were preformed in quadruplicate.
Transfer of UDP-[3H]GalNAc to N-Acetylglucosamine-β p-Nitrophenol
Transfer of GalNAc to β-linked GlcNAc in the absence of peptide recognition was assayed using N-acetylglucosamine-β-p-nitrophenol (GlcNAcβ-pNP) as a substrate. Unless otherwise indicated, the assay was carried out in a final volume of 50 μl containing: 100 mm HEPES buffer, pH 7.5, 0.2% Triton X-100, 20 mm MnCl2, 2 mm ATP,1 mm GlcNAcβ-pNP (Sigma N9376), and 0.4 mm UDP-GalNAc plus 150,000 dpm of UDP-[3H]GalNAc. Transfer was initiated by the addition of either purified β4GalNAc-T3-F or purified β4GalNAc-T4-F and incubating at 37 °C for 2 h. The reaction was terminated by the addition of 950 μl of ice-cold 2 mm EDTA, pH 7.0. The reaction was loaded onto a 500-mg Sep-Pak C-18 cartridge (Waters) previously activated with 2 ml of ethanol and equilibrated with 10 ml of H2O. The Sep-Pak was washed with 15 ml of H2O, and bound [3H]GalNAc-GlcNAcβ-pNP was eluted with 2 ml of 1-butanol. The amount of [3H]GalNAc eluted was determined by liquid scintillation counting in 10 ml of Ultima Gold counting mixture (PerkinElmer Life Sciences).
Peptides
The 19-amino acid peptide CA1–19 with and without an amino-terminal biotin moiety was synthesized and purified by Biomolecules Midwest, St. Louis, MO. The 15-amino acid peptide α32–46, glycoprotein hormone α(32–46)amide, was purchased from Bachem Biochemicals.
RESULTS
Secreted Forms of β4GalNAc-T3 and β4GalNAc-T4 Mediate Protein-specific Transfer of GalNAc in Vitro
Substrate constructs encoding chimeric glycoproteins consisting of GLuc, a secreted form of luciferase (11, 12), followed by either the glycoprotein hormone α subunit or Trf, were generated as described (8). The chimeric substrate GLuc glycoproteins shown in schematic form in Fig. 1 were epitope-tagged at their carboxyl terminus with sequences for Myc, and 6 residues of His that were utilized for purification by chelate affinity chromatography. The GLuc chimeras were expressed in Lec8/CHO cells, which are not able to transport UDP-Gal into the Golgi (10, 13) and thus do not add Gal to N-linked oligosaccharides on secreted glycoproteins. The fusion protein expressed in Lec8/CHO cells bears N-linked complex type oligosaccharides terminating with β-linked GlcNAc that acts as the substrate for GalNAc addition by either β4GalNAc-T3 or β4GalNAc-T4 in vitro. Equal light units of purified GLuc substrates were incubated with either affinity-purified β4GalNAc-T3-F or β4GalNAc-T4-F (secreted FLAG-tagged forms, see “Experimental Procedures”) and UDP-GalNAc. Following termination of the transferase reaction, equal amounts of each GLuc substrate were incubated in 96-well plates coated with the GalNAc-specific lectin WFA (14). After removing unbound substrate, the amount of GLuc substrate bound by the WFA was then determined by injection of coelenterazine.
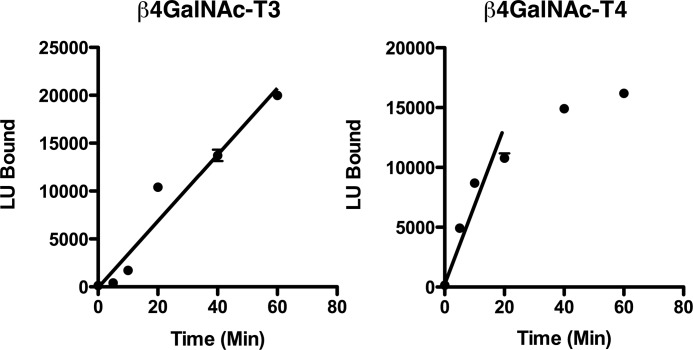
Conditions were established to ensure that both the transferase assay itself and the plate assay for bound GalNAc-modified GLuc substrates remained in a linear range. The incorporation of GalNAc into the N-linked oligosaccharides of GLucα(PLRSKK) and GLucα(PLRSKK)CA1–19 increased linearly for at least 15 min for both β4GalNAc-T3-F and β4GalNAc-T4-F (Fig. 2). The incorporation of GalNAc was proportional to the amount of β4GalNAc-T3-F and β4GalNAc-T4-F added (not shown).
FIGURE 2.
Time course for GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F in vitro. β4GalNAc-T3-F (left panel) and β4GalNAc-T4-F (right panel) were incubated with GLucα(PLRSKK)CA1–19 and UDP-GalNAc at 25 °C for the times indicated. The amount of GalNAc incorporated into GLucα(PLRSKK)CA1–19 was determined by capturing GalNAc-modified GLucα(PLRSKK)CA1–19 onto 96-well plates coated with the lectin WFA that binds terminal β1,4-linked GalNAc. The input of luciferase activity was identical for each well. The amount of bound luciferase activity was determined using the Renilla luciferase assay (Promega) as described. The S.E. is shown for all data points, which were performed in triplicate, but is obscured by the data points. Incorporation of GalNAc increased linearly for both β4GalNAc-T3-F and β4GalNAc-T4-F for a minimum of 20 min. LU, light units.
Anion and Cation Requirements for Optimal Transfer of GalNAc by β4GalNAc-T3-F and β4GalNAc-T4-F in Vitro
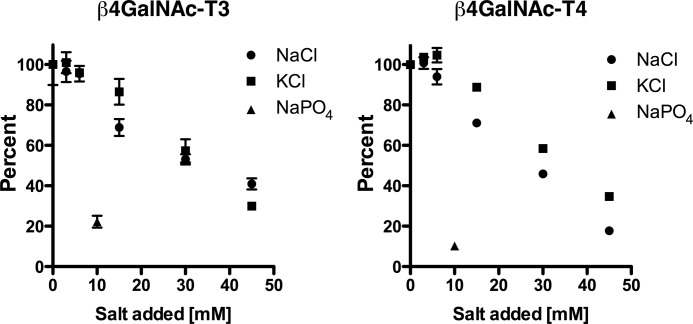
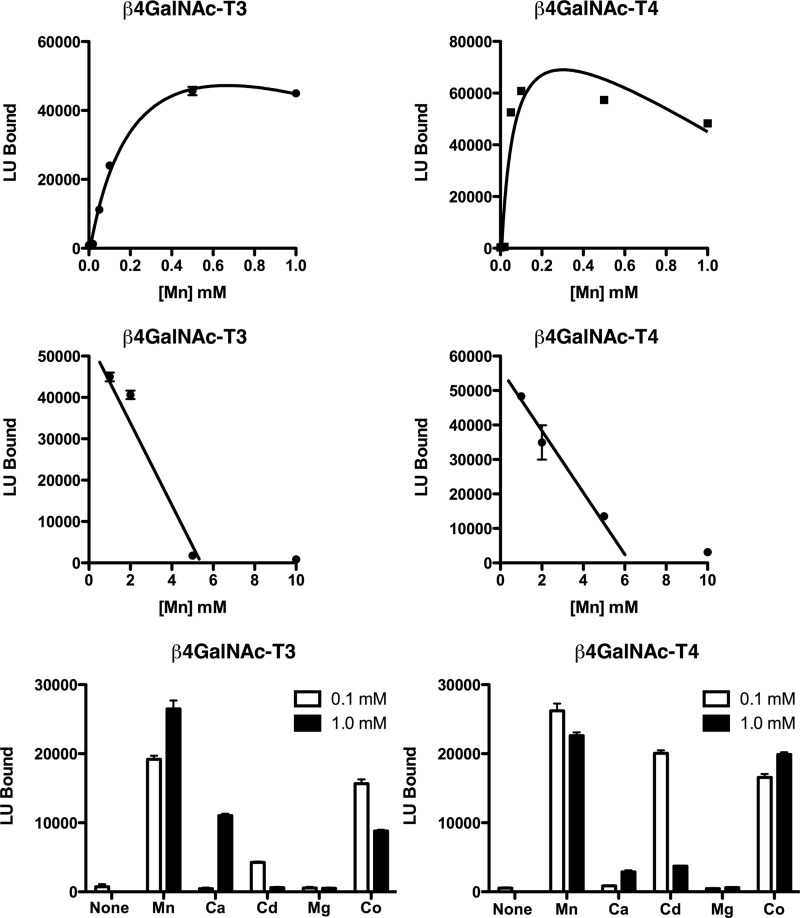
Optimization of enzymatic reaction conditions revealed that transfer of GalNAc to GLucα(PLRSKK) by either β4GalNAc-T3-F or β4GalNAc-T4-F was progressively inhibited by concentrations of NaCl and KCl in excess of 10–15 mm (Fig. 3). NaPO4 reduced transfer of GalNAc by 80–90% at a concentration of 10 mm. The addition of a divalent cation such as Mn2+ was essential for transfer; maximal rates of transfer were achieved for both β4GalNAc-T3-F and β4GalNAc-T4-F at 0.5 mm Mn2+ (Fig. 4). The apparent kd for Mn2+ binding based on GalNAc transfer was 0.25 mm for β4GalNAc-T3-F and 0.06 mm for β4GalNAc-T4-F. Concentrations of Mn2+ greater than 1 mm inhibited GalNAc transfer (Fig. 4), likely reflecting the free Cl-concentrations. Thus, maintenance of a low anion concentration was essential for optimal transfer of GalNAc to glycoprotein substrates.
FIGURE 3.
The impact of salt on UDP-GalNAc transfer to GLucα(PLRSKK)CA1–19. Increasing amounts of NaCl, KCl, or NaPO4 were added to the transferase assay, which contains 25 mm HEPES, 5 mm KCl, and 1 mm MnCl2, at the concentrations shown. All assays were performed in triplicate, and the error bars (S.E.) are shown. The amount of GalNAc transferred is expressed as a percentage of the amount of transfer obtained in the absence of additional salt.
FIGURE 4.
Divalent cation dependence for GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F. No transfer of GalNAc by either β4GalNAc-T3-F or β4GalNAc-T4-F to GLucα(PLRSKK)CA1–19 was observed in the presence of 50 μm EDTA. Upper panels, increasing amounts of MnCl2 were added to bring the free concentration of Mn2+ to the levels indicated for the transfer reaction. Middle panels, concentrations of Mn2+ above 1 mm inhibited transfer of GalNAc by both β4GalNAc-T3-F and β4GalNAc-T4-F. Lower panels, the ability of Mn2+, Ca2+, Cd2+, Mg2+, and Co2+ to support GalNAc transfer was examined at final concentrations of 0.1 and 1.0 mm using β4GalNAc-T3-F and β4GalNAc-T4-F that had been treated with 50 μm EDTA. LU, light units. Error bars indicate S.E.
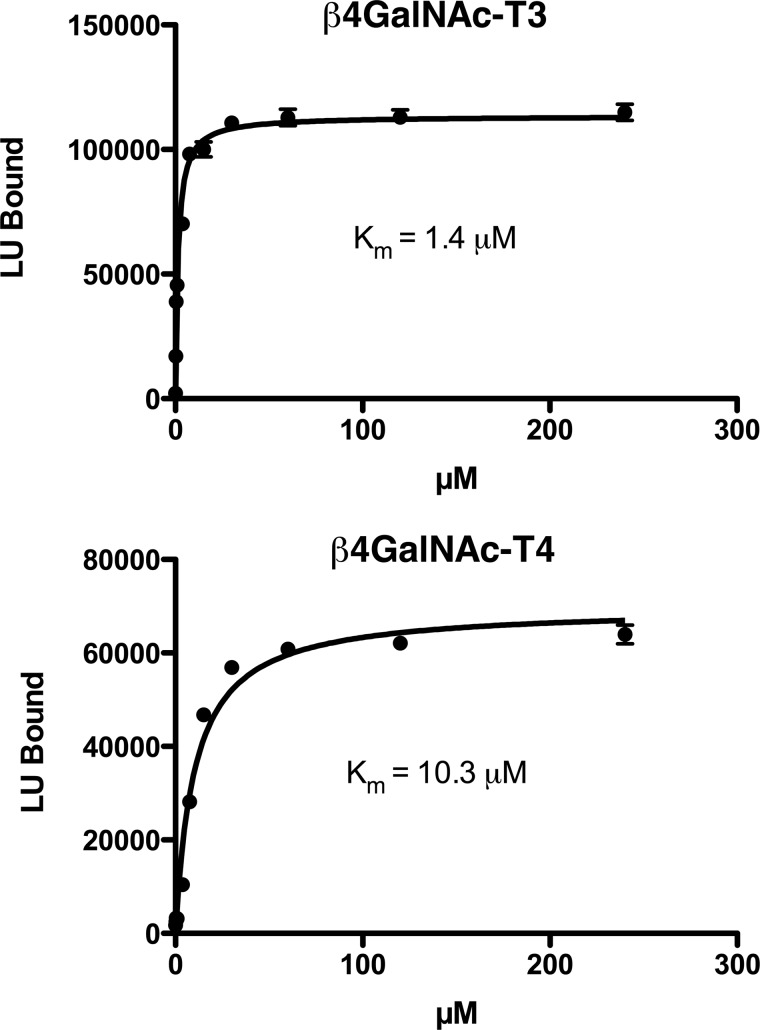
The divalent cations Co2+, Cd2+, and Ca2+ were also able to support transfer of GalNAc to GLucα, whereas Mg2+ was not (Fig. 4). However, the order of cation effectiveness for the two transferases differed; for β4GalNAc-T3-F, the order was Mn2+ > Co2+ > Ca2+ > Cd2+, whereas for β4GalNAc-T4-F, this order was Mn2+ > Co2+ = Cd2+ ≫ Ca2+. Thus, the enzymatic properties of β4GalNAc-T3-F and β4GalNAc-T4-F display discernable differences in divalent cation binding. Using GLucα(PLRSKK)CA1–19 as the substrate, we determined an apparent Km for UDP-GalNAc of 1.4 μm for β4GalNAc-T3-F and 10 μm for β4GalNAc-T4-F (Fig. 5).
FIGURE 5.
The apparent Km for UDP-GalNAc incorporation by β4GalNAc-T3-F and β4GalNAc-T4-F. β4GalNAc-T3-F and β4GalNAc-T4-F were incubated with increasing amounts of UDP-GalNAc for 15 min, and the amount of GalNAc incorporated into GLucα(PLRSKK)CA1–19 was determined. Nonlinear regression analysis assuming a single binding site for UDP-GalNAc provided the best fit and yielded an apparent Km of 1.4 μm for β4GalNAc-T3-F and 10.3 μm for β4GalNAc-T4-F. Error bars indicate S.E. LU, light units.
The reduced rate of GalNAc transfer by both β4GalNAc-T3 and β4GalNAc-T4 in the presence of increasing concentrations of anions could reflect an impact on peptide recognition, saccharide recognition, or both. We therefore determined the rate of [3H]GalNAc transfer to GlcNAcβ-pNP, a small substrate that is devoid of peptide components, by β4GalNAc-T3-F under different conditions (Table 1). In the presence of 0.2 mm Mn2+, the same amount of GalNAc was transferred to GlcNAcβ-pNP in 25 mm HEPES versus 100 mm HEPES. Furthermore, transfer of GalNAc increased 3-fold in the presence of 20 mm Mn2+ and 100 mm HEPES, conditions that would have markedly inhibited transfer to the peptide substrate GLucα(PLRSKK) bearing a recognition motif. Thus, it is peptide recognition rather than saccharide recognition that is sensitive to inhibition by anions. In addition, the apparent Km for UDP-GalNAc is not sensitive to anion concentrations used for these assays (not shown).
TABLE 1.
Transfer of [3H]GalNAc to GlcNAcβ-p-nitrophenol by β4GalNAc-T3-F
Reactions were carried out for 2 h at 37° C in HEPES buffer, pH 7.5, containing 0.2% Triton X-100, 2 mm ATP, 1 mm GlcNAcβ-pNP, 0.4 mm UDP-GalNAc plus 75,000 dpm UDP-[3H]GalNAc with the concentrations of Mn2+ and HEPES indicated. The results shown are representative of multiple experiments performed with different Mn2+ and HEPES concentrations.
| 0.2 mm Mn2+ and 25 mm HEPES | 0.2 mm Mn2+ and 100 mm HEPES | 20 mm Mn2+ and 100 mm HEPES | |
|---|---|---|---|
| Condition | |||
| No Enzyme | 96 | 91 | 94 |
| β4GalNAc-T3-F | 1684 | 1568 | 5094 |
β4GalNAc-T3-F and β4GalNAc-T4-F Are Protein-specific
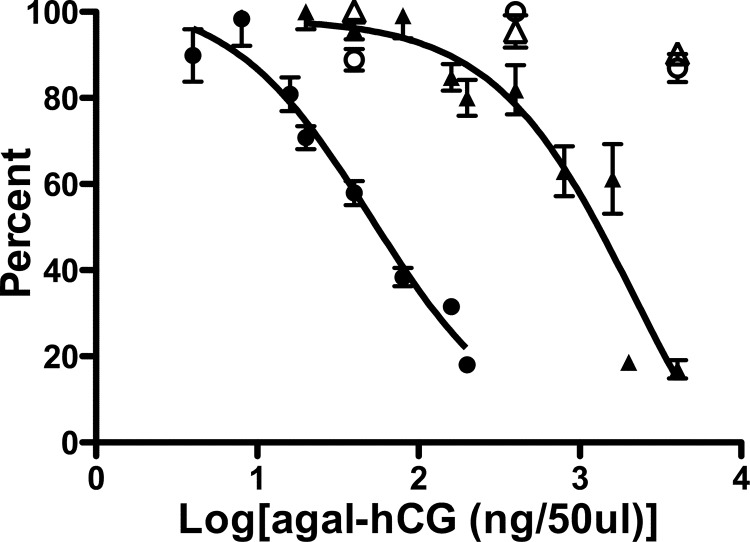
We previously demonstrated that hCG, but not Trf, that has had terminal Sia and Gal removed from its N-linked oligosaccharide structures is specifically modified with GalNAc by one or more β1,4GalNAc transferases present in the pituitary (2). We established an in vitro competition-type assay to examine the impact of different peptide recognition motifs on transfer of GalNAc by β4GalNAc-T3-F and β4GalNAc-T4-F. The addition of increasing amounts of agalacto-hCG to the transferase assay reduced the incorporation of GalNAc into GLucα(PLRSKK) by both β4GalNAc-T3-F and β4GalNAc-T4-F (Fig. 6). In contrast to the observed competition with agalacto-hCG, the highest concentration of agalacto-Trf examined, 1.05 μm, did not reduce the incorporation of GalNAc into GLucα(PLRSKK) by either β4GalNAc-T3-F or β4GalNAc-T4-F (Fig. 6). The IC50 for agalacto-hCG inhibition of GalNAc incorporation into GLucα(PLRSKK)CA1–19 was 29 nm for β4GalNAc-T3-F and 1.23 μm for β4GalNAc-T4-F. The 42-fold difference in the IC50 for agalacto-hCG competition indicates differences in the peptide specificities of β4GalNAc-T3-F as compared with β4GalNAc-T4-F.
FIGURE 6.
Comparison of agalacto-hCG and agalacto-Trf as inhibitors of GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F in vitro. Transfer of GalNAc to GLucα(PLRSKK)CA1–19 by β4GalNAc-T3-F (●, ○) and β4GalNAc-T4-F (△, ▴) was monitored in the presence of increasing amounts of agalacto-hCG (agal-hGC). For β4GalNAc-T3-F (●), between 0 and 200 ng of agalacto-hCG was added per 50-μl assay, and for β4GalNAc-T4-F (▴), between 0 and 4026 ng of agalacto-hCG was added per 50-μl assay to obtain optimal inhibition. The best fit of the data was obtained using nonlinear transforms and assuming a single binding site. agalacto-hCG inhibited transfer of GalNAc to GLucα(PLRSKK)CA1–19 with an IC50 of 29 nm for β4GalNAc-T3-F and 1.23 μm for β4GalNAc-T4-F. The addition of 40, 400, or 4000 ng of agalacto-Trf per 50-μl assay (open symbols) did not inhibit transfer of GalNAc to GLucα(PLRSKK)CA1–19 by either β4GalNAc-T3-F (○) or β4GalNAc-T4-F (△). Error bars indicate S.E.
Peptide Inhibition of Protein-specific Transfer of GalNAc by β4GalNAc-T3-F and β4GalNAc-T4-F
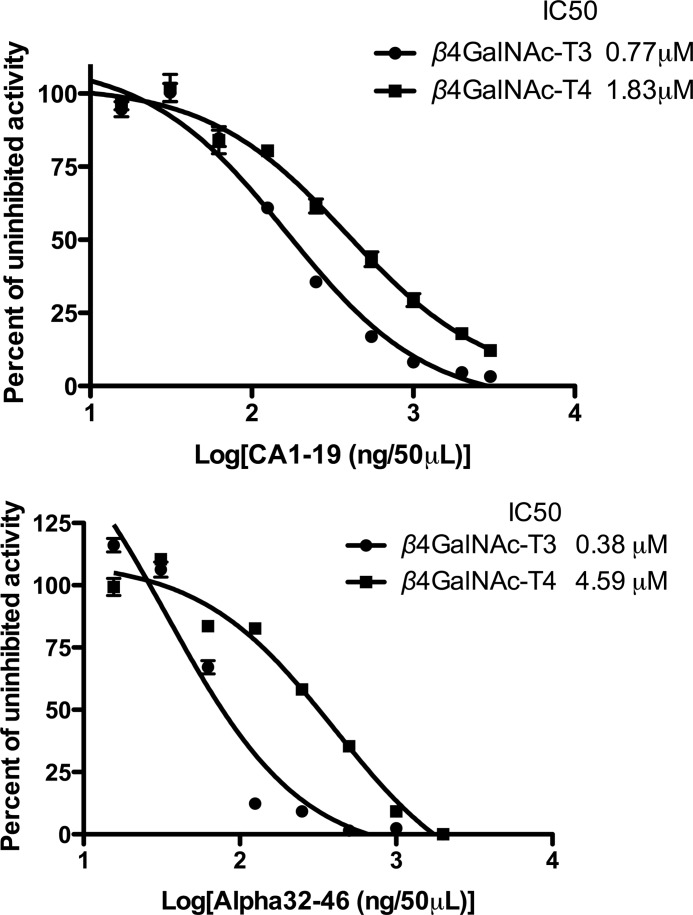
Like the N-linked oligosaccharides on luteinizing hormone, the N-linked oligosaccharides on a secreted form of carbonic anhydrase, CA6, are also modified with β1,4-linked GalNAc (15). When either RLucα(PLRSKK) or RLucCA6 is synthesized in CHO cells stably expressing β4GalNAc-T3 or β4GalNAc-T4, the basic residues within the sequence PTPLRSKK that is present in the glycoprotein hormone α subunit and the 19 amino acids located at the carboxyl terminus of CA6, respectively, increase the efficiency of GalNAc addition to their N-linked oligosaccharides 3–5-fold as compared with a substrate such as RLucTrf that does not contain a recognition determinant (8). We tested both the peptide CA1–19, consisting of the 19-amino acid sequence located at the carboxyl terminus of CA6, and the peptide α32–46 (Fig. 1) that contains the PTPLRSKK sequence as competitive inhibitors of GalNAc transfer to GLucα(PLRSKK)CA1–19 by β4GalNAc-T3-F and β4GalNAc-T4-F (Fig. 7). The peptide CA1–19 inhibited transfer of GalNAc to GLucα(PLRSKK)CA1–19 with similar IC50 values of 0.77 μm for β4GalNAc-T3-F and 1.83 μm β4GalNAc-T4-F. In contrast, α32–46 inhibited transfer of GalNAc with an IC50 of 0.38 μm for β4GalNAc-T3-F and 4.59 μm β4GalNAc-T4-F. The 12-fold difference in the IC50 for α32–46 agrees with the difference in specificity seen above for β4GalNAc-T3-F and β4GalNAc-T4-F using agalacto-hCG.
FIGURE 7.
Inhibition of GalNAc transfer by peptides CA1–19 and α32–46. Upper panel, the peptide CA1–19 inhibited transfer of GalNAc to GLucα(PLRSKK)CA1–19 with an IC50 of 0.77 μm for β4GalNAc-T3-F and 1.83 μm for β4GalNAc-T4-F. Lower panel, the peptide α32–46 inhibited transfer of GalNAc to GLucα(PLRSKK) with an IC50 of 0.38 μm for β4GalNAc-T3-F and 4.59 μm for β4GalNAc-T4-F. The best fit of the data for inhibition with CA1–19 and α32–46 was obtained using nonlinear transforms and assuming a single binding site. Error bars indicate S.E.
The Presence of a Peptide Recognition Motif Is Essential for Efficient Protein-specific Transfer of GalNAc by β4GalNAc-T3-F and β4GalNAc-T4-F
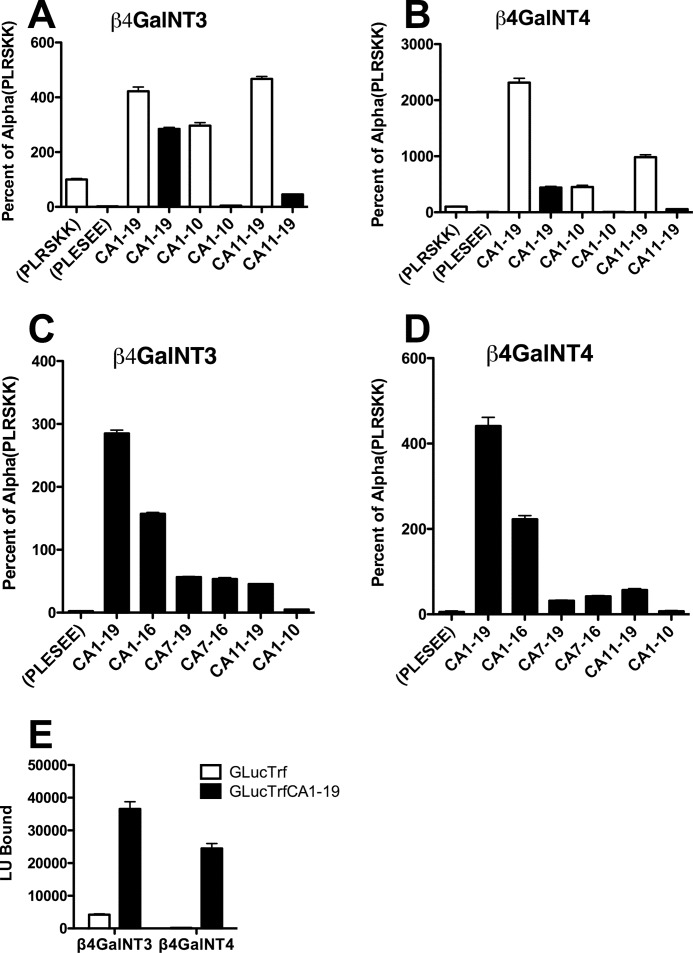
We next examined GalNAc addition in vitro by β4GalNAc-T3-F and β4GalNAc-T4-F to GLuc substrates that did or did not contain the sequences that we had proposed are critical for recognition in vivo. The constructs we examined are shown in Fig. 1. Mutation of the basic residues in the sequence PLRSKK of α subunit to acidic residues, i.e. PLESEE, reduced GalNAc transfer to GLucα 42-fold by β4GalNAc-T3-F and 19-fold by β4GalNAc-T4-F (Fig. 8, A and B), confirming that the basic residues within the sequence PLRSKK are essential for substrate recognition by both β4GalNAc-T3-F and β4GalNAc-T4-F. The minimal amount of GalNAc transferred to GLucα(PLESEE) is similar to that transferred to GLucTrf. Trf is a glycoprotein that we have previously shown is not recognized by the pituitary β1,4GalNAc transferase (2). Adding the CA1–19 peptide sequence to the carboxyl terminus of GLucTrf increased the amount of GalNAc transferred to this substrate 9-fold for β4GalNAc-T3-F and 135-fold for β4GalNAc-T4-F (Fig. 8E), thus converting GLucTrf from a poor to a highly effective substrate that is recognized by both β4GalNAc-T3-F and β4GalNAc-T4-F. Similarly adding the CA1–19 peptide sequence to the carboxyl terminus of GLucα(PLESEE) restored the acceptor activity of GLucα(PLESEE)CA1–19 for both β4GalNAc-T3-F and β4GalNAc-T4-F. In fact GLucα(PLESEE)CA1–19 was modified to a greater extent than GLucα(PLRSKK) by both β4GalNAc-T3-F and β4GalNAc-T4-F. Adding CA1–19 to GLucα(PLRSKK) enhanced GalNAc transfer to the α subunit oligosaccharides 4.2-fold for β4GalNAc-T3-F and 23-fold for β4GalNAc-T4-F, consistent with our observation above that β4GalNAc-T4-F has a stronger affinity for CA1–19 than for α32–46.
FIGURE 8.
Peptide recognition determinant requirements for transfer of GalNAc by β4GalNAc-T3-F and β4GalNAc-T4-F in vitro. The chimeric glycoprotein acceptors illustrated schematically in Fig. 1 were incubated with either β4GalNAc-T3-F or β4GalNAc-T4-F. The relative amount of GalNAc incorporated was determined by incubating aliquots containing the identical amount of GLuc activity with WFA-coated 96-well plates. The amount of transfer to each of the constructs shown was normalized to the amount of transfer to GLucα(PLRSKK), which was set as equal to 100%. In A and B, open bars are constructs based on GLucα(PLRSKK), and black bars are constructs based on GLucα(PLESEE). Error bars indicate S.E. LU, light units.
Minimal Requirements for Recognition of the Peptide Motif by β4GalNAc-T3-F and β4GalNAc-T4-F
The effectiveness of the CA1–19 recognition motif at promoting GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F when this motif was added to the carboxyl terminus of multiple glycoprotein substrates suggested that the context of the sequence was not critical and that the in vitro assay could be used to determine the minimal requirements for recognition by β4GalNAc-T3-F and β4GalNAc-T4-F. We therefore appended different regions of the CA1–19 peptide sequence to the carboxyl terminus of GLucα(PLESEE) and compared these constructs as substrates for β4GalNAc-T3-F and β4GalNAc-T4-F (Fig. 8). The full-length sequence CA1–19 was the best substrate for both β4GalNAc-T3-F (panel C) and β4GalNAc-T4-F (panel D). Removing the carboxyl-terminal 3 amino acids to form CA1–16 reduced the extent of modification by 50% for both enzymes. The smallest region that was recognized by both β4GalNAc-T3-F and β4GalNAc-T4-F was CA7–16, which has the sequence QKITKRKKEK. The carboxyl-terminal region consisting of CA11–19 was recognized, whereas the amino-terminal half of the sequence consisting of CA1–10 was not. Thus, residues outside of the critical region CA7–16 enhance recognition but are not themselves essential.
Western Blot Analysis of β4GalNAc-T3-F and β4GalNAc-T4-F to Compare Specific Activities
The substrate GLucα(PLRSKK) was used for our initial characterization of β4GalNAc-T3-F and β4GalNAc-T4-F. Significantly more β4GalNAc-T4-F than β4GalNAc-T3-F was required to transfer the same amount of GalNAc to GLucα(PLRSKK), suggesting that β4GalNAc-T4-F was not as active an enzyme as β4GalNAc-T3-F. In contrast, transfer of GalNAc to GLucα(PLRSKK)CA1–19 suggested that β4GalNAc-T3-F and β4GalNAc-T4-F have similar specific activities. To resolve this paradox, we utilized Western blots to compare the amount of β4GalNAc-T3-F and β4GalNAc-T4-F that was required to transfer equal amounts of GalNAc to GLucα(PLRSKK) versus GLucα(PLRSKK)CA1–19 as substrates.
Western blot analysis of β4GalNAc-T3-F and β4GalNAc-T4-F under nonreducing (Fig. 9A) and reducing (Fig. 9B) conditions using an antibody directed at the FLAG epitope revealed proteolytic cleavage of the major fraction of both transferases. The two fragments produced were covalently associated via one or more disulfide bonds because in the absence of reducing agents, both β4GalNAc-T3-F and β4GalNAc-T4-F migrated as doublets with Mr 140,000–160,000. Equal amounts of enzyme activity based on transfer of GalNAc to GLucα(PLRSKK) and to GLucα(PLRSKK)CA1–19, respectively, were subjected to quantitation by Western blot following separation by SDS-PAGE using an antibody directed at the FLAG epitope (Fig. 9C). In the case of β4GalNAc-T3-F, the same amount of β4GalNAc-T3-F protein was detected by immunoblotting whether the amount of activity examined was based on transfer to GLucα(PLRSKK) or GLucα(PLRSKK)CA1–19 (compare the GT3 Alpha lane and the GT3 Alpha(CA1–19) lane in panel C). The amounts of β4GalNAc-T3-F and β4GalNAc-T4-F were also similar by Western blot when the amount of activity examined was based on transfer of GalNAc to GLucα(PLRSKK)CA1–19 (compare the GT3 Alpha lane and the GT4 Alpha(CA1–19) lane in panel C). In marked contrast, significantly more β4GalNAc-T4-F was required to transfer the same amount of GalNAc to GLucα(PLRSKK) than to GLucα(PLRSKK)CA1–19 (compare the GT4 Alpha lane with the GT4 Alpha(CA1–19) lane in panel C). Thus, the specific activity for transfer of GalNAc associated with β4GalNAc-T3-F and β4GalNAc-T4-F is similar for transfer to GLucα(PLRSKK)CA1–19, whereas the specific activity associated with β4GalNAc-T4-F for transfer of GalNAc to GLucα(PLRSKK) is significantly lower than for transfer to GLucα(PLRSKK)CA1–19.
FIGURE 9.
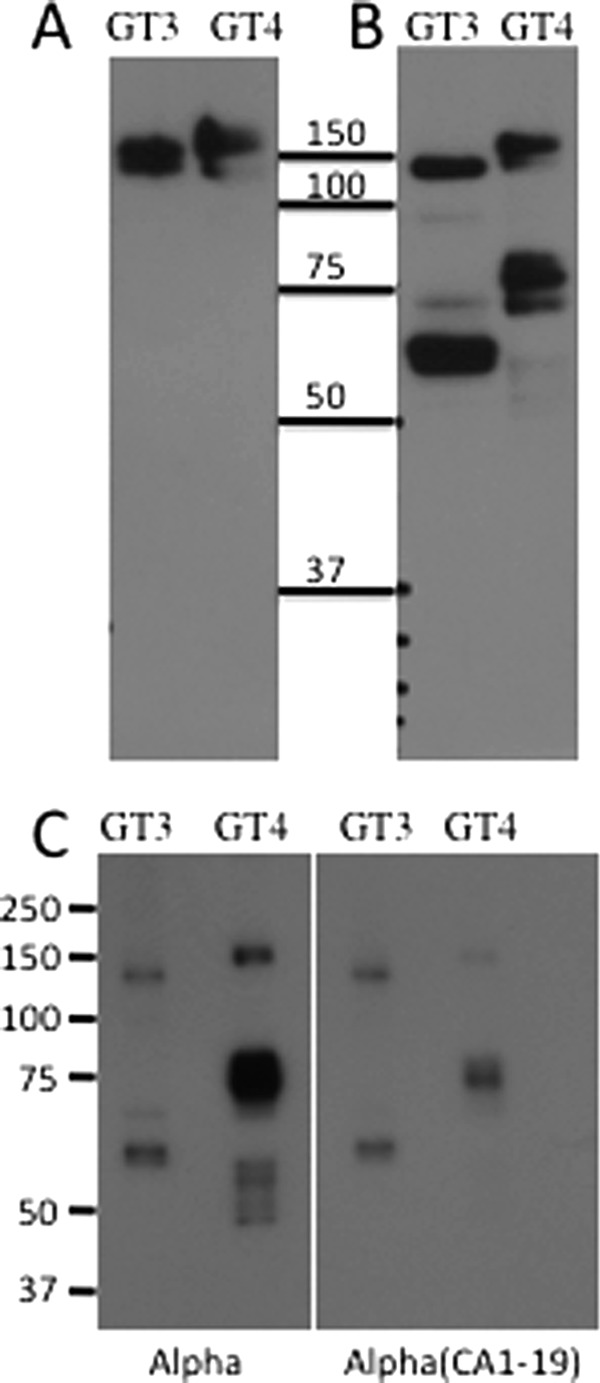
Western blot analysis of affinity-purified β4GalNAc-T3-F and β4GalNAc-T4-F. β4GalNAc-T3-F and β4GalNAc-T4-F were affinity-purified from medium using anti-FLAG-agarose and elution with FLAG peptide. The affinity-purified enzymes were examined by SDS-PAGE in the absence (A) or presence (B and C) reducing agent. Following electrophoresis, the proteins were electrophoretically transferred to a PVDF membrane, and proteins containing the FLAG epitope were visualized using anti-FLAG. In C, identical light units of activity using either GLucα(PLRSKK), indicated as Alpha, or GLucα(PLRSKK)CA1–19, indicated as Alpha(CA1–19), were loaded onto the gel. In the absence of reducing agents, β4GalNAc-T3-F and β4GalNAc-T4-F both migrate as closely spaced doublets reflecting the presence of the uncleaved form and the disulfide-linked cleaved form.
Clearly more β4GalNAc-T4-F was required to transfer the same amount of GalNAc to GLucα(PLRSKK) than to GLucα(PLRSKK)CA1–19. Furthermore, the specific activities of β4GalNAc-T3-F and β4GalNAc-T4-F were similar for transfer of GalNAc when GLucα(PLRSKK)CA1–19 was used as the substrate. Thus, the difference in the affinity of β4GalNAc-T4-F for the recognition motif found in the α subunit as compared with the motif in CA6 accounts for this apparent difference in activity.
DISCUSSION
Unique oligosaccharide structures occur on a number of glycoproteins, serving as information-bearing signposts whose recognition is utilized for intracellular trafficking and/or extracellular clearance of these molecular species. For example, β1,4-linked GalNAc-4-SO4 and Siaα2,6GalNAc on glycoprotein hormones (16–18) are recognized by receptors that mediate clearance of these hormones from the blood, and Man-6-PO4 is recognized by receptors that direct enzymes and other glycoproteins bearing these structures to lysosomes (19). However, much remains to be learned about how these specific oligosaccharide structures are added to individual glycoproteins and, in some instances, how individual glycosylation sites are chosen. We recently described a system that provides unique opportunities to identify those parameters that are critical for synthesis of unique carbohydrate structures in vivo (8). This strategy, based on chimeric proteins consisting of GLuc followed by the glycoprotein of interest and an epitope tag, has defined the requirements for the protein-specific addition of β1,4-linked GalNAc to N-linked oligosaccharides using CHO cells expressing β4GalNAc-T3 or β4GalNAc-T4 (8). In the current studies, we have used the identical GLuc chimeras to examine kinetic parameters for GalNAc addition by β4GalNAc-T3 and β4GalNAc-T4 to glycoprotein substrates in vitro.
Our in vitro studies demonstrate that the conditions optimal for transfer of GalNAc to glycoprotein substrates containing a particular recognition determinant surprisingly are not typical of the conditions traditionally utilized for in vitro characterization of glycosyltransferase properties. Transfer of GalNAc to glycoprotein acceptors by both β4GalNAc-T3-F and β4GalNAc-T4-F is progressively inhibited by increasing Cl− concentrations above 10 mm. The actual concentration of ions to which these transferases would be exposed in the cellular milieu is thus an interesting question. The concentration of Mn2+ has not been directly measured in the Golgi. However, culturing cells in medium containing 100 μm Mn2+ induces transport of a cis-Golgi localized protein GPP130 to multivesicular bodies (20). Furthermore, culturing cells in the presence of 1 mm Mn2+ reduces cell viability (21). Such studies suggest that the actual concentration of Mn2+ is likely less than 200 μm in the Golgi where β4GalNAc-T3 and β4GalNAc-T4 reside and carry out their functional activities; this would be consistent with their respective affinities for Mn2+ of 0.25 and 0.06 mm.
The behavior of GalNAc transfer to a small-molecule acceptor that does not contain a peptide recognition determinant stands in sharp contrast to the transfer of GalNAc to GLucα(PLRSKK)CA1–19. In the former case, the transfer of GalNAc to GlcNAcβ-pNP is not inhibited by 20 mm MnCl2. Furthermore, the IC50 values for inhibition of GalNAc addition to GLucα(PLRSKK)CA1–19 by agalacto-hCG and the peptide CA1–19 are shifted to higher concentrations in the presence of 5 mm MnCl2, suggesting that it is peptide recognition that is sensitive to the concentration of salt rather than the catalytic transfer of GalNAc from UDP-GalNAc to the oligosaccharide acceptor.
Both β4GalNAc-T3-F and β4GalNAc-T4-F require that a divalent cation be present for transfer of GalNAc from UDP-GalNAc to the glycoprotein oligosaccharide. They do, however, differ in the order of their preference for divalent cations, suggesting that although the sequences of the catalytic domains are 67% identical, there are structural features that differentiate their catalytic properties. These same differences may also account for the difference in the affinity for Mn2+.
In previous studies using detergent extracts of bovine pituitary, we reported that GalNAc is transferred to the synthetic intermediate GlcNAc2Man3GlcNAc2Asn on hCG with an apparent Km of 4.4 μm and to glycopeptides bearing the same synthetic intermediate with an apparent Km of 1.2–2.6 mm (22). The >270-fold difference in the Km for hCG versus glycopeptides can account for the ability of the β4GalNAc transferase present in pituitary extracts to add GalNAc to oligosaccharides on substrate glycoproteins such as hCG that contain a recognition determinant but not to the same oligosaccharide structures on glycoproteins such as Trf that do not contain a recognition determinant (2). hCG is a dimeric glycoprotein consisting of two noncovalently associated subunits, each of which has two Asn-linked oligosaccharides. The α and β subunits each contain a recognition determinant that can mediate the selective addition of GalNAc to the separated subunits. We used an in vitro assay to identify the basic residues in the sequence PLRSKK as being essential components of the glycoprotein hormone α subunit recognition determinant (23). Both β4GalNAc-T3 and β4GalNAc-T4 display the predicted specificity for the glycoprotein hormone α subunit versus Trf when expressed in CHO cells (8). Among those glycoproteins identified as bearing N-linked oligosaccharides with β1,4-linked GalNAc, we have demonstrated that regions with a recognition determinant of clustered basic residues are critical for the protein-specific addition of GalNAc by β4GalNAc-T3 and β4GalNAc-T4 to the α subunit, to CA6 (8, 15), and to SorLA/LR11 (5).
Our in vitro studies reveal significant differences in the specificity of β4GalNAc-T3 versus β4GalNAc-T4 for the recognition motifs in the α subunit and CA6. Transfer of GalNAc to GLucα(PLRSKK)CA1–19, which contains both recognition motifs, is inhibited by agalacto-hCG but not by agalacto-Trf, indicating that the peptide recognition motif is required for inhibition of transfer of GalNAc. However, inhibition of GalNAc transfer to GLucα(PLRSKK)CA1–19 by β4GalNAc-T4-F requires a 42-fold higher concentration of agalacto-hCG (IC50 = 1.23 μm) than does inhibition of GalNAc transfer by β4GalNAc-T3-F (IC50 = 28.8 nm). The difference in IC50 indicates that β4GalNAc-T3-F has a significantly stronger affinity for the recognition motif in agalacto-hCG than does β4GalNAc-T4-F.
Further support for the role of peptide recognition was obtained from inhibition studies. Peptides containing a recognition motif fully inhibited transfer of GalNAc to glycoprotein substrates containing a recognition motif but had no effect on transfer to GlcNAcβ-pNP. Peptide recognition and transfer of GalNAc appear to be independent events. The IC50 values of 0.38 and 4.59 μm obtained for α32–46 inhibition of GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F, respectively, indicate that binding of the peptide recognition motif accounts for the majority of the affinity for glycoprotein substrates and that β4GalNAc-T3-F has a stronger affinity for the α subunit recognition motif than does β4GalNAc-T4-F.
High levels of β4GalNAc-T3 and β4GalNAc-T4 expression in stably transformed CHO cells had prevented a definitive assessment of the degree to which the presence of a recognition determinant could increase modification of N-linked oligosaccharides with GalNAc in vivo (8). Our in vitro analysis demonstrated that mutation of the basic residues within the recognition motif of the α subunit from PLRSKK to PLESEE reduced the amount of GalNAc transferred to GLucα by β4GalNAc-T3-F and β4GalNAc-T4-F by 42- and 19-fold, respectively. Adding the CA1–19 sequence to GLucTrf increased the amount of GalNAc transferred to GLucTrf(CA1–19) 9- and 135-fold for β4GalNAc-T3-F and β4GalNAc-T4-F, respectively. Adding the CA1–19 sequence to the carboxyl terminus of GLucα(PLESEE) yielded a substrate, GLucα(PLESEE)CA1–19, that was more efficiently modified with GalNAc than GLucα(PLRSKK) by both β4GalNAc-T3-F and β4GalNAc-T4-F. Thus, the CA1–19 sequence was recognized more efficiently than the PLRSKK sequence. Furthermore, the location of the recognition determinant within the linear sequence of the peptide was not critical because the PLRSKK motif precedes the two glycosylated Asn residues in the α subunit, whereas the CA1–19 sequence has been added to the carboxyl terminus of the α subunit. However, in spatial terms, accessibility of the peptide motif within the three-dimensional structure of the substrate relative to the oligosaccharide structure is likely critical. Based on the crystal structure of Trf (24), the CA1–19 added to the carboxyl terminus of Trf is predicted to be in close proximity to both N-linked oligosaccharides on Trf. Deletion of either glycosylation site on GLucTrfCA1–19 did not prevent modification with GalNAc (not shown), indicating that both sites can be modified in the presence of CA1–19.
The entire sequence of CA1–19 was not required for recognition by either β4GalNAc-T3-F or β4GalNAc-T4-F. Residues 7–16, QKITKRKKEK, were essential for recognition, whereas residues 1–6 and 17–19 appeared not to be essential but did enhance recognition. Given our previous insights on the importance of clustered basic residues (5, 8, 23), the bolded residues QKITKRKKEK within CA1–19 and PTPLRSKK within the α32–46 sequence may be the most critical for recognition by β4GalNAc-T3 and β4GalNAc-T4, whereas the other residues contribute to presentation of these basic residues to the transferase binding site.
The difference in substrate preference exhibited by β4GalNAc-T3 versus β4GalNAc-T4 was confirmed at the protein level. Western blot analysis with anti-FLAG demonstrated that a much greater quantity of β4GalNAc-T4-F than β4GalNAc-T3-F was required to provide the same light units of transferase activity based on GalNAc transfer to GLucα(PLRSKK). In contrast, when equal light units of transferase activity based on GalNAc transfer to GLucα(PLRSKK)CA1–19 were examined, the amounts of β4GalNAc-T3-F and β4GalNAc-T4-F present were similar. Thus, β4GalNAc-T3-F and β4GalNAc-T4-F have similar specific activities when using GLucα(PLRSKK)CA1–19 as the substrate.
The in vitro approach here expands our understanding of the contribution of peptide recognition determinants to the protein-specific transfer of GalNAc by β4GalNAc-T3 and β4GalNAc-T4. β4GalNAc-T3 and β4GalNAc-T4 are closely related structurally; however, they differ in their substrate preferences with β4GalNAc-T4 showing a strong preference for the recognition determinant represented by CA1–19 as compared with that present in the α subunit. There is a single peptide binding site in both β4GalNAc-T3 and β4GalNAc-T4 because the peptides CA1–19 and α32–46 are both able to completely inhibit transfer of GalNAc to GLucα(PLRSKK)CA1–19. The IC50 values for CA1–19 and α32–46 fall in the range of 0.38–4.59 μm, whereas the apparent Km for transfer to a glycopeptide acceptor devoid of any recognition determinant is 1.2–2.6 mm. Thus, as is seen with the phosphotransferase that adds GlcNAc-PO4 to oligomannose structures on lysosomal enzymes (25), the affinity for the peptide recognition determinant is much stronger than for the oligosaccharide acceptor.
The kinetics for GalNAc transfer by β4GalNAc-T3-F and β4GalNAc-T4-F in vitro indicate that both transferases are highly protein-specific. Multiple lines of evidence indicate that β4GalNAc-T3 has stronger affinity for the recognition determinant in the hormone α subunit than does β4GalNAc-T4 and, as a result, β4GalNAc-T3-F transfers GalNAc to the α subunit oligosaccharides more efficiently than β4GalNAc-T4-F under the conditions of the in vitro assay. Nonetheless, β4GalNAc-T3 and β4GalNAc-T4 have similar specificities, and either or both could contribute to the modification of the reproductive glycoprotein hormones in vivo. Supporting this conclusion is the marked decrease in GalNAc transfer by both β4GalNAc-T3-F and β4GalNAc-T4-F in vitro when the PLRSKK sequence in GLucα is mutated to PLESEE. Gotoh et al. (7) have reported that β4GalNAc-T4 transcripts are abundantly expressed in mouse brain, whereas β4GalNAc-T3 transcripts are not. In contrast, we have found that both β4GalNAc-T3 and β4GalNAc-T4 transcripts are present in pituitaries from mice.3 Thus, in the pituitary, either or both transferases may contribute to the modification of oligosaccharides on the glycoprotein hormones with GalNAc. It is possible that β4GalNAc-T3 and β4GalNAc-T4 are both required for efficient modification of the oligosaccharides on hormones such as luteinizing hormone.
Our in vitro studies indicate that it is now possible to generate glycoprotein substrates that differ in their affinity for β4GalNAc-T3 and β4GalNAc-T4. Expression of substrates with different affinities for β4GalNAc-T3 and β4GalNAc-T4 in cells that express one or both transferases offers the opportunity to examine how differences in location, affinity for the substrate, and relative levels of transferases determine the structural outcome in vivo. Our ability to use the identical substrates for in vitro and in vivo studies provides a unique opportunity to examine how different parameters affect the pattern and extent of glycosylation within the milieu of the Golgi.
Acknowledgment
We thank Nancy L. Baenziger for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-HD058474 and R01-CA21923 (to J. U. B.).
Y. Mi and J. U. Baenziger, unpublished observation.
- CA6
- carbonic anhydrase 6
- GLuc
- Gaussia luciferase
- RLuc
- Renilla luciferase
- Trf
- transferrin
- WFA
- W. floribunda agglutinin
- β4GalNAc-T
- β1,4-N-acetylgalactosaminyl transferase
- pNP
- p-nitrophenol
- hCG
- human chorionic gonadotropin.
REFERENCES
- 1. Baenziger J. U., Green E. D. (1991) Structure, synthesis, and function of the asparagine-linked oligosaccharides on pituitary glycoprotein hormones, in Biology of Carbohydrates, Volume 3 (Ginsberg V., Robbins P. W., eds.) pp. 1–46, JAI Press Ltd., London [Google Scholar]
- 2. Smith P. L., Baenziger J. U. (1988) A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science 242, 930–933 [DOI] [PubMed] [Google Scholar]
- 3. Manzella S. M., Dharmesh S. M., Cohick C. B., Soares M. J., Baenziger J. U. (1997) Developmental regulation of a pregnancy-specific oligosaccharide structure, NeuAcα2,6GalNAcβ1,4GlcNAc, on select members of the rat placental prolactin family. J. Biol. Chem. 272, 4775–4782 [DOI] [PubMed] [Google Scholar]
- 4. Hooper L. V., Hindsgaul O., Baenziger J. U. (1995) Purification and characterization of the GalNAc-4-sulfotransferase responsible for sulfation of GalNAc β1,4GlcNAc-bearing oligosaccharides. J. Biol. Chem. 270, 16327–16332 [DOI] [PubMed] [Google Scholar]
- 5. Fiete D., Mi Y., Oats E. L., Beranek M. C., Baenziger J. U. (2007) N-Linked oligosaccharides on the low density lipoprotein receptor homolog SorLA/LR11 are modified with terminal GalNAc-4-SO4 in kidney and brain. J. Biol. Chem. 282, 1873–1881 [DOI] [PubMed] [Google Scholar]
- 6. Sato T., Gotoh M., Kiyohara K., Kameyama A., Kubota T., Kikuchi N., Ishizuka Y., Iwasaki H., Togayachi A., Kudo T., Ohkura T., Nakanishi H., Narimatsu H. (2003) Molecular cloning and characterization of a novel human β1,4-N-acetylgalactosaminyltransferase, β4GalNAc-T3, responsible for the synthesis of N,N′-diacetyllactosediamine, GalNAcβ1–4GlcNAc. J. Biol. Chem. 278, 47534–47544 [DOI] [PubMed] [Google Scholar]
- 7. Gotoh M., Sato T., Kiyohara K., Kameyama A., Kikuchi N., Kwon Y. D., Ishizuka Y., Iwai T., Nakanishi H., Narimatsu H. (2004) Molecular cloning and characterization of β1,4-N-acetylgalactosaminyltransferases IV synthesizing N,N′-diacetyllactosediamine. FEBS Lett. 562, 134–140 [DOI] [PubMed] [Google Scholar]
- 8. Miller E., Fiete D., Blake N. M., Beranek M., Oates E. L., Mi Y., Roseman D. S., Baenziger J. U. (2008) A necessary and sufficient determinant for protein-selective glycosylation in vivo. J. Biol. Chem. 283, 1985–1991 [DOI] [PubMed] [Google Scholar]
- 9. Dharmesh S. M., Skelton T. P., Baenziger J. U. (1993) Coordinate and restricted expression of the ProXaaArg/Lys-specific GalNAc-transferase and the GalNAc β1,4GlcNAc β1,2Man α-4-sulfotransferase. J. Biol. Chem. 268, 17096–17102 [PubMed] [Google Scholar]
- 10. Patnaik S. K., Stanley P. (2006) Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 416, 159–182 [DOI] [PubMed] [Google Scholar]
- 11. Verhaegent M., Christopoulos T. K. (2002) Recombinant Gaussia luciferase: overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal Chem. 74, 4378–4385 [DOI] [PubMed] [Google Scholar]
- 12. Tannous B. A., Kim D. E., Fernandez J. L., Weissleder R., Breakefield X. O. (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 11, 435–443 [DOI] [PubMed] [Google Scholar]
- 13. Oelmann S., Stanley P., Gerardy-Schahn R. (2001) Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J. Biol. Chem. 276, 26291–26300 [DOI] [PubMed] [Google Scholar]
- 14. Nyame K., Smith D. F., Damian R. T., Cummings R. D. (1989) Complex-type asparagine-linked oligosaccharides in glycoproteins synthesized by Schistosoma mansoni adult males contain terminal β-linked N-acetylgalactosamine. J. Biol. Chem. 264, 3235–3243 [PubMed] [Google Scholar]
- 15. Hooper L. V., Beranek M. C., Manzella S. M., Baenziger J. U. (1995) Differential expression of GalNAc-4-sulfotransferase and GalNAc-transferase results in distinct glycoforms of carbonic anhydrase VI in parotid and submaxillary glands. J. Biol. Chem. 270, 5985–5993 [DOI] [PubMed] [Google Scholar]
- 16. Manzella S. M., Hooper L. V., Baenziger J. U. (1996) Oligosaccharides containing β1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 271, 12117–12120 [DOI] [PubMed] [Google Scholar]
- 17. Mi Y., Fiete D., Baenziger J. U. (2008) Ablation of GalNAc-4-sulfotransferase-1 enhances reproduction by altering the carbohydrate structures of luteinizing hormone in mice. J. Clin. Invest. 118, 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park E. I., Mi Y., Unverzagt C., Gabius H. J., Baenziger J. U. (2005) The asialoglycoprotein receptor clears glycoconjugates terminating with sialic acid α2,6GalNAc. Proc. Natl. Acad. Sci. U.S.A. 102, 17125–17129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornfeld S. (1986) Trafficking of lysosomal enzymes in normal and disease states. J. Clin. Invest. 77, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mukhopadhyay S., Bachert C., Smith D. R., Linstedt A. D. (2010) Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol. Biol. Cell 21, 1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mukhopadhyay S., Linstedt A. D. (2011) Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. U.S.A. 108, 858–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith P. L., Baenziger J. U. (1990) Recognition by the glycoprotein hormone-specific N-acetylgalactosaminetransferase is independent of hormone native conformation. Proc. Natl. Acad. Sci. U.S.A. 87, 7275–7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mengeling B. J., Manzella S. M., Baenziger J. U. (1995) A cluster of basic amino acids within an α-helix is essential for α subunit recognition by the glycoprotein hormone N-acetylgalactosaminyltransferase. Proc. Natl. Acad. Sci. U.S.A. 92, 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wally J., Halbrooks P. J., Vonrhein C., Rould M. A., Everse S. J., Mason A. B., Buchanan S. K. (2006) The crystal structure of iron-free human serum transferrin provides insight into interlobe communication and receptor binding. J. Biol. Chem. 281, 24934–24944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reitman M. L., Lang L., Kornfeld S. (1984) UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. Methods Enzymol. 107, 163–172 [DOI] [PubMed] [Google Scholar]