Background: Osteoclasts and osteoblasts are major players in bone metabolism.
Results: TRAF family member-associated NF-κB activator (TANK) is induced during osteoclastogenesis and osteoblastogenesis. RANKL-induced osteoclastogenesis was increased in TANK-deficient cells. Osteoblastogenesis was also increased in TANK-deficient mice.
Conclusion: TANK is a negative feedback regulator of osteoclastogenesis and osteoblastogenesis.
Significance: TANK is a novel suppressor of bone degradation and formation.
Keywords: Bone, Gene Knockout, NF-κB (NF-κB), Osteoclast, Signal Transduction
Abstract
The differentiation of bone-resorbing osteoclasts is induced by RANKL signaling, and leads to the activation of NF-κB via TRAF6 activation. TRAF family member-associated NF-κB activator (TANK) acts as a negative regulator of Toll-like receptors (TLRs) and B-cell receptor (BCR) signaling by inhibiting TRAF6 activation. Tank−/− mice spontaneously develop autoimmune glomerular nephritis in an IL-6-dependent manner. Despite its importance in the TCRs and BCR-activated TRAF6 inhibition, the involvement of TANK in RANKL signaling is poorly understood. Here, we report that TANK is a negative regulator of osteoclast differentiation. The expression levels of TANK mRNA and protein were up-regulated during RANKL-induced osteoclastogenesis, and overexpression of TANK in vitro led to a decrease in osteoclast formation. The in vitro osteoclastogenesis of Tank−/− cells was significantly increased, accompanied by increased ubiquitination of TRAF6 and enhanced canonical NF-κB activation in response to RANKL stimulation. Tank−/− mice showed severe trabecular bone loss, but increased cortical bone mineral density, because of enhanced bone erosion and formation. TANK mRNA expression was induced during osteoblast differentiation and Tank−/− osteoblasts exhibited enhaced NF-κB activation, IL-11 expression, and bone nodule formation than wild-type control cells. Finally, wild-type mice transplanted with bone marrow cells from Tank−/− mice showed trabecular bone loss analogous to that in Tank−/− mice. These findings demonstrate that TANK is critical for osteoclastogenesis by regulating NF-κB, and is also important for proper bone remodeling.
Introduction
Bone mass and structure are strictly regulated by the delicate balance between bone resorption and formation. Bone-forming osteoblasts are derived from mesenchymal cells and express M-CSF and receptor activator of NF-κB (RANK)3 ligand (RANKL). Runt-related transcription factor 2 (Runx2) is known to a key transcription factor for osteoblast differentiation. On the other hand, bone-resorbing osteoclasts are giant, multinucleated, tartrate-resistant acid phosphatase (TRAP)-positive cells derived from the monocyte/macrophage lineage (1). When M-CSF and RANKL stimulate their receptors c-fms and RANK on osteoclast precursors, respectively, transcription factors such as NFATc1 (2), c-Fos (3), and NF-κB (4) are activated in the cells. NFATc1 is the master regulator of osteoclasts, and NF-κB has been implicated in the induction of NFATc1 (5). The RANKL-induced NF-κB pathways include both canonical and non-canonical pathways. The canonical NF-κB pathway activates heterodimers of RelA/p65 and p50, which is generated from the NF-κB1/p105 fragment, while the non-canonical pathway induces processing of the NF-κB2/p100 fragment into active p52. Following the activation of such transcription factors, osteoclast differentiation is induced by the induction of osteoclastic genes, such as TRAP, calcitonin R, and DC-STAMP. Among these osteoclastic genes, DC-STAMP is known to be involved in cell-cell fusion, because osteoclast and IL-3 plus IL-4-induced multinucleated giant cell formation by macrophage cell fusion is abrogated in DC-STAMP-deficient mice (6, 7). Mice lacking both NF-κB1 and NF-κB2 develop typical osteopetrosis through defective development of osteoclasts (8), thereby confirming the importance of NF-κB signaling in controlling bone homeostasis.
RANKL is a major NF-κB activator in osteoclastogenesis. RANKL binds to its receptor RANK, which belongs to the TNF receptor superfamily. Upon activation, RANK recruits TRAF family E3 ubiquitin ligases, such as TRAF2, TRAF5, and TRAF6 (9). Among the TRAF family members, TRAF6 plays a critical role in RANK signaling toward NF-κB, because its deficiency leads to severe osteopetrosis similar to that in RANKL- and RANK-deficient mice (10–12). Collectively, it has been proposed that RANKL-RANK interactions and the subsequent signaling via TRAF6 are important for the differentiation of osteoclasts.
Various cytokines and cellular stresses are known to activate intracellular signaling pathways leading to NF-κB activation. This transcription factor is well known to control the expressions of proinflammatory cytokines downstream of Toll-like receptors (TLRs) and IL-1 receptor (IL-1R). TLRs directly sense the presence of microbial components and trigger intracellular signaling pathways that evoke innate immune responses against pathogens. Activation of TLRs leads to the recruitment of the adaptor molecule MyD88, which in turn activates IL-1R-associated kinases (IRAKs). TRAF6 is ubiquitinated downstream of IRAK-1/-2, and activates NF-κB by generating unconjugated polyubiquitin chains and activating TAK1 (13, 14). The importance of TRAF6 in TLR/IL-1R signaling has been established by the observation that NF-κB activation in response to LPS and IL-1 fails in TRAF6-deficient mice (11). In acquired immunity, the antigen receptor signaling pathways emanating from TCR and BCR are known to activate NF-κB via TRAF proteins.
TRAF family member-associated NF-κB activator (TANK; also known as TRAF-interacting protein) binds to TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6 (15–18), and acts as a regulator of TRAF-mediated signaling. Macrophages and B cells from TANK-deficient (Tank−/−) mice exhibit more enhanced NF-κB and AP-1 activation in response to stimulation of TLRs and B-cell receptor (BCR) (19). TLR and BCR-induced ubiquitination of TRAF6 is up-regulated in Tank−/− macrophages, suggesting that TANK inhibits TRAF6 activation downstream of TLRs (19). While TANK has been implicated in the type I IFN responses against virus infection by binding to TANK-binding kinase 1 (20), type I IFNs are produced normally in Tank−/− cells in response to RNA virus infection (19). Furthermore, Tank−/− mice spontaneously develop lupus-like autoimmune nephritis through IL-6 expression (19). MyD88 and the intestinal flora contribute to the development of autoantibody production in Tank−/− mice (19), suggesting that spontaneous activation of TLRs by intestinal bacteria leads to IL-6 production that causes autoimmunity.
Despite the importance of TRAF6 in the RANKL signaling, the involvement of TANK in RANKL-induced osteoclast differentiation and bone homeostasis is poorly understood. In this study, we found that TANK was induced during RANKL-mediated osteoclastogenesis, and overexpression of TANK led to a decrease in osteoclast formation. Tank−/− macrophages underwent increased osteoclastogenesis mediated by enhanced ubiquitination of TRAF6, the canonical NF-κB pathway, and c-Fos and NFATc1 activation. Tank−/− mice showed trabecular bone loss, but increased cortical bone mineral density (BMD) and resistance to bone fracture, because of enhanced bone erosion and formation. Interestingly, TANK was also induced in osteoblasts and Tank−/− osteoblasts formed more bone nodules. These findings provide unexpected insights into the roles of TANK in bone remodeling.
EXPERIMENTAL PROCEDURES
Mice
Tank−/− mice on a C57BL/6 background were generated as previously described (19) and maintained under specific pathogen-free conditions. All animal experiments were carried out under approval from the Animal Research Committee of the Research Institute for Microbial Diseases (Osaka University, Osaka, Japan).
In Vitro Osteoclast Culture and Functional Evaluation
For stromal cell-free in vitro osteoclast cultures, M-CSF-derived macrophages (MDMs) were used as osteoclast precursors. Bone marrow cells were flushed from femurs or tibias and cultured for 6 h in α-MEM containing 10% FCS. Non-adherent cells were collected and plated in 48-well plates at a density of 1.2 × 105 cells/well with 25 ng/ml M-CSF (PeproTech, Rocky Hill, NJ). After 3 days, the cultures were washed with PBS and adherent cells were used as MDMs. The MDMs were induced to differentiate into osteoclasts in the presence of 25 ng/ml M-CSF and various concentrations of RANKL (R&D Systems, Minneapolis, MN). After 3 days, the adherent cells were fixed with 4% paraformaldehyde, treated with ethanol/acetone (50:50, v/v), and stained for TRAP using a TRAP/ALP staining kit (Wako, Tokyo, Japan). The numbers of TRAP-positive multinucleated cells were counted. The osteoclast area (percentage of TRAP-positive multinucleated cells relative to the total area) was measured using the software ImageJ (software from NIH). For pit assays, ivory dentine slices (Wako, Tokyo, Japan) were placed in 48-well plates. MDMs were cultured on the dentine slices, and then cultured in 25 ng/ml M-CSF and 50 ng/ml RANKL to induce osteoclast differentiation. After 5 days, the dentine slices were immersed in 1 m NH4OH for 3 h and sonicated to remove the cells. The resorption pits were observed by scanning electron microscopy (MiniScope TM-1000; Hitachi, Tokyo, Japan). Quantitative analyses of the resorption pits were performed using ImageJ.
Retroviral Gene Transfer
A murine TANK cDNA was cloned into the pLZR-IRES/GFP retroviral vector (21). The construct was transfected into the packaging cell line PlatE, and viral supernatants were collected. Bone marrow cells from C57BL/6 mice were cultured for 6 h in α-MEM. Subsequently, non-adherent cells were collected and plated in 24-well plates at a density of 1 × 106 cells/well with 25 ng/ml M-CSF. After 24 h, the cells were transduced with a retroviral supernatant in the presence of 8 μg/ml polybrene for 8 h. Following culture for 48 h in the presence of 25 ng/ml M-CSF, the cells were cultured with 25 ng/ml M-CSF and 50 ng/ml RANKL. After 3 days, the numbers of TRAP-positive multinucleated cells were counted.
Immunoblotting, Immunoprecipitation, and Ubiquitination Assays
MDMs (5 × 106) were stimulated with 50 ng/ml LPS, TNF-α, or RANKL for various times. The cells were then lysed in a lysis buffer comprising 20 mm Tris-HCl, 1 mm EDTA, 1.0% Nonidet P40, 150 mm NaCl, and a protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). The cell lysates were separated by SDS-PAGE and transferred onto polyvinylfluoride membranes. The membranes were incubated with anti-TANK (H-300; Santa Cruz Biotechnology, Santa Cruz, CA), anti-p100/p52 (#4882; Cell Signaling), anti-TRAF6 (sc-7221; Santa Cruz Biotechnology), anti-Ub (P4D1; Santa Cruz Biotechnology), and anti-actin (C-11; Santa Cruz Biotechnology) antibodies. After the incubation, membranes were washed and incubated with HRP-linked anti-rabbit IgG (NA934V; GE Healthcare) or HRP-linked anti-mouse IgG (NA931V; GE Healthcare). For chemiluminescence, membranes were incubated with fresh Western lightning Plus-ECL reagents (Perkin Elmer). TRAF6 immunoprecipitation and detection of its ubiquitination were performed as described previously (19).
Proliferation and Apoptosis Assays
Cells from wild-type and Tank−/− mice were stained with trypan blue, and the numbers of viable cells were counted under a light microscope. Apoptotic cells were determined using a Poly Caspase Assay Kit, Green FLICA (ImmunoChemistry Technologies, Bloomington, MN).
DNA Binding Activity of NF-κB p65
MDMs (1 × 106) were stimulated with 350 ng/ml RANKL for 30 min. Nuclear extracts were purified from the cells and the DNA binding activity of NF-κB p65 was analyzed using a TransAM Transcription Factor Assay Kit (Active Motive, Carlsbad, CA).
Osteoblast Culture and Functional Assays
Primary osteoblasts were isolated from the calvariae of 2-day-old mice. The calvariae were digested in α-MEM containing 0.1% collagenase and 0.2% dispase at 37 °C for 10 min. The cells were then expanded in α-MEM containing 10% FCS. For induction of osteoblastogenesis, primary osteoblasts were plated in 48-well plates at a density of 3 × 104 cells/well. After 1 day, the medium was supplemented with an Osteoblast-Inducer Reagent (Takara, Tokyo, Japan) containing ascorbic acid, hydrocortisone, and glycerophosphate. In some experiment, 10 μg/ml neutralizing antibodies to IL-6 or IL-11 (R&D) were added to the medium. The medium was changed every 3 days. After 9 days, calcified nodules and ALP were stained using a Calcified nodule Staining kit (AK-21; Primary Cell, Hokkaido, Japan) and TRAP/ALP staining kit (Wako, Tokyo, Japan), respectively. In some experiments, 5 mm NF-κB inhibitor BAY117085 (Sigma) was added at day 9 for 24 h, and mRNA was harvested.
Osteoclast and Osteoblast Coculture
Bone marrow cells (3 × 105) and primary calvarial osteoblasts (5 × 105) were isolated and cultured in 24-well plates containing α-MEM supplemented with 10% FCS for 10 days in the presence of 10 nm 1α,25(OH)2D3. After the culture, the cells were fixed and stained for TRAP to evaluate the numbers of osteoclasts.
Quantitative PCR Analysis
RNA was extracted from cultured cells using TRIzol (Invitrogen, Carlsbad, CA) and subjected to reverse transcription using ReverTra Ace (Toyobo, Tokyo, Japan). Quantitative PCR was performed with an ABI PRISM 7500 using TaqMan Assays-on-Demand primers and probes (Applied Biosystems) for TANK, TRAP, calcitonin R, c-Fos, NFATc1, DC-STAMP, RANK, Runx2, IL-11, OncostatinM, IL-6, RANKL, and osteoprotegerin (OPG). 18S control reagents were used for normalization of the cDNAs.
Bone Phenotype Analyses
Double calcein labeling was carried out to measure bone formation. Ten-week-old Tank−/− and age-matched control wild-type female mice were administered calcein intraperitoneally at 16 mg/kg body weight at 4 and 1 days before euthanasia. After euthanasia, the femurs are removed, fixed with 70% ethanol, and subjected to histomorphometric analyses. Three-dimensional μCT analysis of the femurs was conducted using Scan-Xmate RB080SS110 (Comscan Techno Co. Ltd., Kanagawa, Japan) and TRI/3D-Bon (Ratoc System Engineering Co. Ltd., Tokyo, Japan) software. The cortical BMD and cortical area were measured by Dual Energy x-ray Absorptiometry DCS-600EX-IIIR (Aloka, Tokyo, Japan). To evaluate the cortical bone mechanical properties, the right femoral mid-shaft was tested by three-point bending using a mechanical testing machine (MZ-500S; Maruto, Tokyo, Japan). To test the bone mechanical properties, each femur was placed on two lower supports that were 6 mm apart and tested with a displacement rate of 2 mm/min until failure. The whole bone mechanical properties of maximum load (N), stiffness (N/mm), post-yield deflection (mm), and work to failure (N.mm) were measured. The bone microarchitectural parameters were analyzed in the trabecular regions from 0.1 to 1.5 mm away from the chondro-osseous junction. For bone histomorphometric analyses, the tibias or femurs were fixed with 70% ethanol, subjected to Villanueva bone staining, and embedded in methyl methacrylate. Serial longitudinal sections (6 mm in thickness) of proximal tibias and cross sections of proximal one-third of the right shaft of femurs were prepared using an RM2255 microtome (Leica, Jena, Germany). Metaphysis areas of tibias and endosteum/periosteum areas of femurs were analyzed using a Histometry RT Camera (System Supply Co. Ltd., Nagano, Japan).
Generation of Bone Marrow Chimeric Mice
Donor bone marrow cells were collected from 6-week-old Tank−/− and age-matched control wild-type female mice. After suspension in PBS, 1 × 107 cells were intravenously injected into lethally irradiated 4-week-old wild-type recipient mice (C57BL/6 background). The chimeric mice were given neomycin and ampicillin in their drinking water for 4 weeks. The mice were analyzed at 7 weeks after the bone marrow transplantation.
ALP Activity Assay
Cultured calvarial osteoblasts were homogenized with 0.1% Triton X-100, and the alkaline phosphatase (ALP) activity was measured using a kit (LabAssay; Wako). The serum ALP levels were also measured using the same kit.
ELISA
The serum levels of TRACP5b were measured using a TRACP5b ELISA Kit (Immunodiagnostic Systems, Bolden, UK). Serum levels of Cross Linked C-Telopeptide of Type I collagen (CTX-I) were measured by ELISA Kit (USCN Life Science, Wuhan, China). All assays were performed according to the corresponding manufacturer's protocols.
Statistical Analysis
The significance of differences between values was analyzed by Student's t test. Values of p < 0.05 were accepted as statistically significant.
RESULTS
TANK Expression Is Induced by RANKL Stimulation
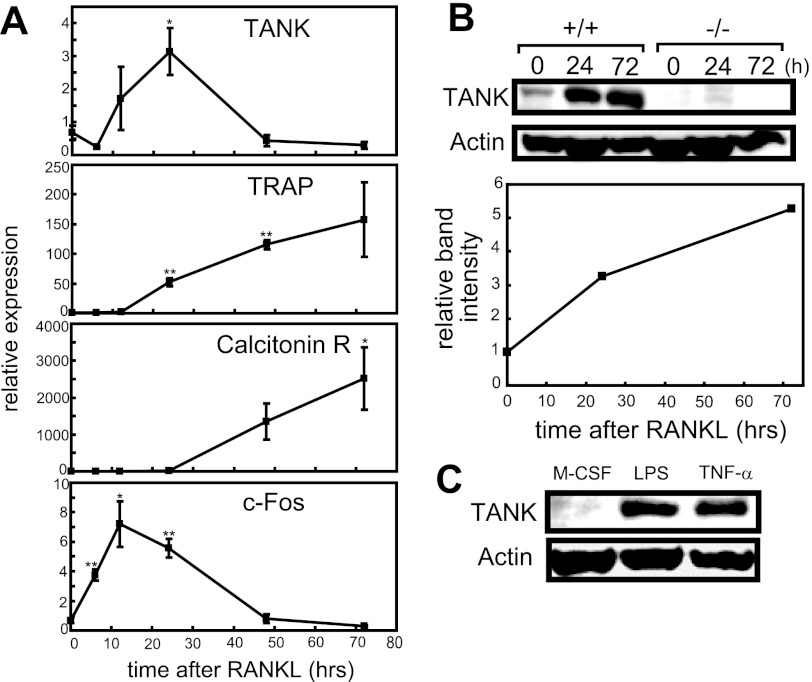
First, we examined the expression pattern of TANK during the course of RANKL-induced osteoclastogenesis. TANK mRNA expression was markedly increased after 24 h of RANKL stimulation in MDMs, and then decreased to the pre-stimulation level by 48 h of RANKL treatment (Fig. 1A). TANK protein expression gradually increased during osteoclastogenesis, and its expression level reached nearly 5-fold after 72 h of RANKL stimulation (Fig. 1B). Furthermore, not only RANKL but also LPS and TNF induced TANK expression in MDMs (Fig. 1C). These findings prompted us to explore the role of TANK during osteoclastogenesis.
FIGURE 1.
TANK is induced by RANKL. A, MDMs from wild-type mice were treated with M-CSF plus 25 ng/ml RANKL for 0, 6, 12, 24, 48, and 72 h. The mRNA levels of TANK and osteoclastogenic markers such as TRAP, Calcitonin receptor (Calcitonin R), and c-Fos were measured by quantitative PCR. Error bars: S.E. (n = 3). *, p < 0.05, **, p < 0.01, versus 0 h. B, MDMs from wild-type and Tank−/− mice were treated with M-CSF alone (0 h) or 50 ng/ml RANKL plus M-CSF (24 and 72 h) and the levels of TANK expression were analyzed by Western blotting. The relative band intensities of wild-type MDMs were measured using ImageJ and normalized by actin expression. C, MDMs from wild-type and Tank−/− mice were treated with M-CSF alone (M-CSF), 50 ng/ml LPS plus M-CSF (LPS), or 50 ng/ml TNF-α plus M-CSF for 24 h and the levels of TANK expression was analyzed by Western blotting. The data shown are representative of three independent experiments.
Increased Osteoclast Differentiation and Bone Resorption Activity in Tank−/− MDMs
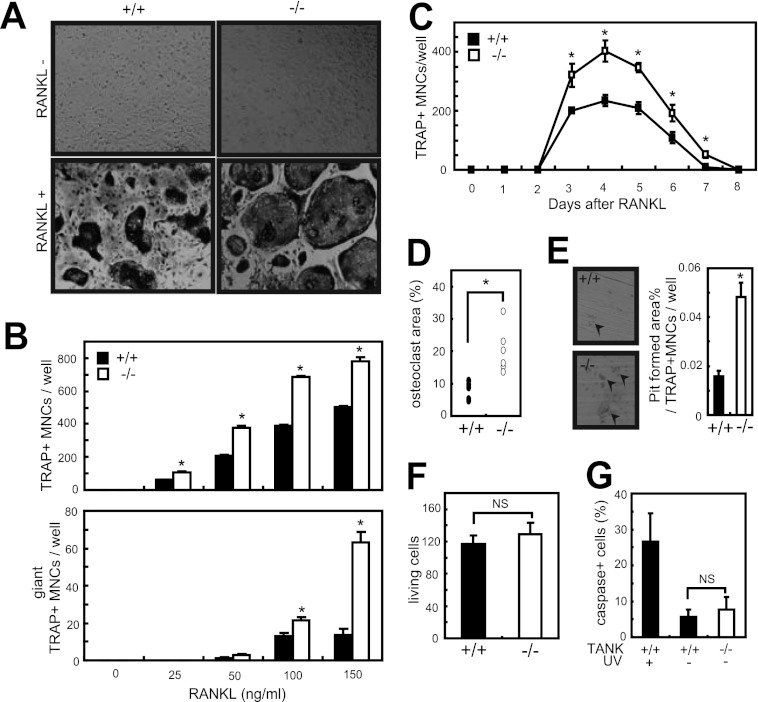
To investigate the role of TANK deficiency in osteoclastogenesis, we analyzed in vitro osteoclastogenesis using wild-type and Tank−/− MDMs. We treated the MDMs with increasing concentrations of RANKL, and TRAP-positive multinucleated cells were identified as mature osteoclasts after 3 days. RANKL-induced osteoclastogenesis was augmented in Tank−/− cells compared with wild-type cells, and the number of osteoclasts was increased by ∼60%. Furthermore, Tank−/− osteoclasts had increased numbers of nuclei and were larger in size (Fig. 2, A–D). TANK deficiency did not alter the proliferation or apoptosis of MDMs (Fig. 2, F and G).
FIGURE 2.
Osteoclast differentiation is increased in Tank−/− MDMs. A, MDMs from wild-type and Tank−/− mice were cultured in the presence of 100 ng/ml RANKL. After 3 days, TRAP staining was performed. B, numbers of TRAP-positive multinucleated (>3 nuclei/cell identified in a ×10 field) cells and giant TRAP-positive multinucleated (>20 nuclei/cell) cells were counted. Error bars: S.E. (n = 3). *, p < 0.05 versus wild-type. C, sequential TRAP staining to count the numbers of osteoclasts from wild-type and Tank−/− MDMs (RANKL, 50 ng/ml). Error bars: S.E. (n = 3). *, p < 0.05 versus 0 h. D, osteoclast areas (percentages of TRAP-positive multinucleated cells relative to the total area) were measured using ImageJ (RANKL, 100 ng/ml) and normalized by osteoclast number. Error bars: S.E. (n = 6). *, p < 0.05. E, formation of resorption pits by osteoclasts induced from wild-type or Tank−/− mice (RANKL, 50 ng/ml). Error bars: S.E. (n = 3). *, p < 0.05. F, MDMs were stained with trypan blue, and the numbers of viable cells were determined under a light microscope. G, apoptosis of wild-type and Tank−/− MDMs. Caspases were stained using a Poly Caspase Assay Kit, and the numbers of caspase-positive cells were counted under a fluorescence microscope. The data shown are representative of three (A, B, D, F, and G) and two (C and E) independent experiments.
Next, we compared the bone resorption activities of osteoclasts from wild-type and Tank−/− MDMs using pit assays. When wild-type and Tank−/− MDMs were cultured on dentine slices with RANKL for 5 days, they formed resorption pits. However, the total bone resorption pit area for Tank−/− osteoclasts normalized by osteoclast number was greater than that for wild-type MDMs (Fig. 2E), indicating that TANK inhibits the bone resorption activity of bone marrow-derived osteoclasts.
To further substantiate the effect of TANK deficiency on osteoclastogenesis, we examined the expression levels of mRNAs encoding osteoclast-related genes after 0, 24, and 72 h of RANKL stimulation. Consistent with the findings for osteoclastogenesis, the expression profiles revealed that osteoclast-associated genes, including NFATc1, c-Fos, TRAP, calcitonin R, and DC-STAMP, were significantly increased in Tank−/− cells compared with wild-type cells (Fig. 3, A–E). Collectively, these findings suggest that TANK negatively regulates osteoclast differentiation in cultured bone marrow cells.
FIGURE 3.

TANK negatively regulates the expressions of osteoclastogenic genes. A–E, quantitative PCR analyses of osteoclastogenic marker genes in wild-type and Tank−/− cells cultured with M-CSF alone (0 h) or 50 ng/ml RANKL plus M-CSF (24 and 72 h). Error bars: S.D. (n = 2). Filled bars: wild-type; open bars: knock-out. The data shown are representative of three independent experiments.
TANK Negatively Regulates RANKL Signaling
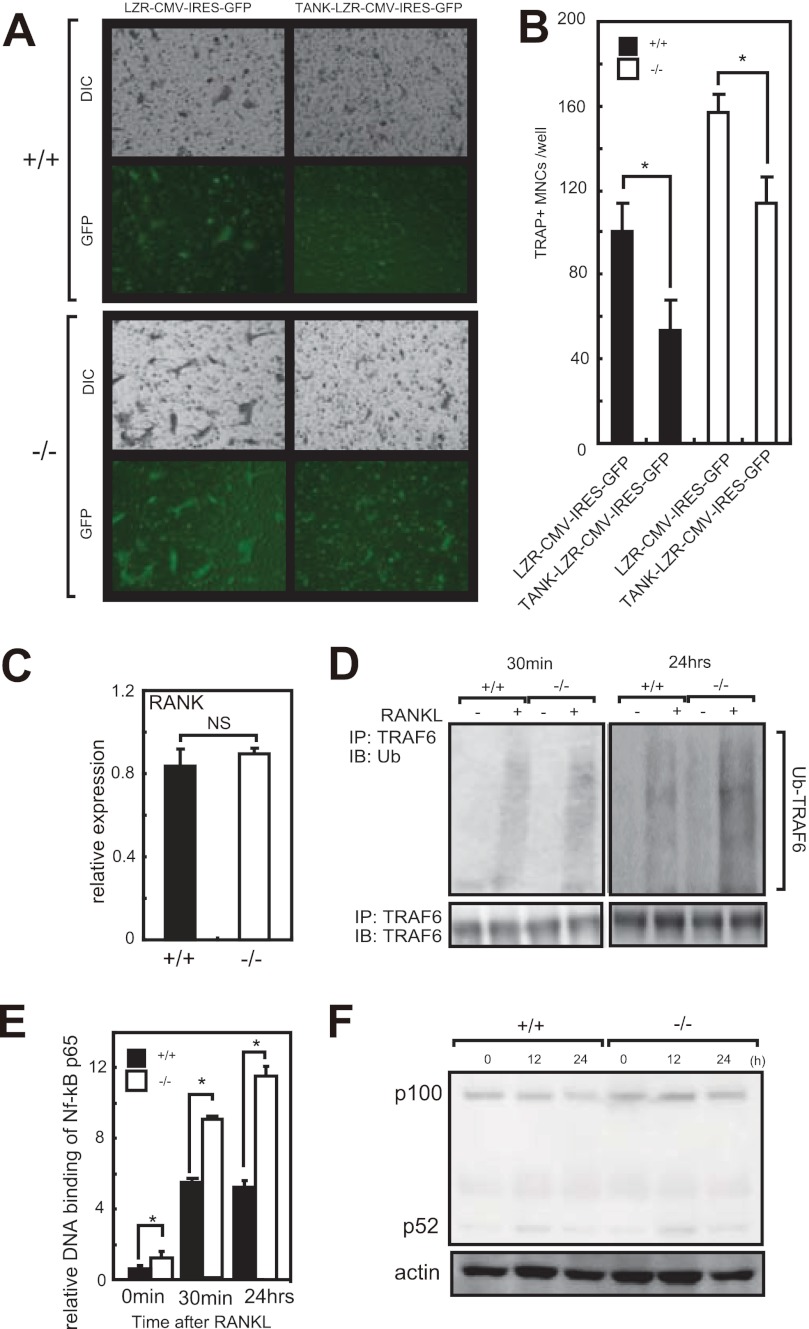
To explore the effect of gain of function of TANK in osteoclastogenesis, we overexpressed TANK in MDMs using a retroviral vector. As shown in Fig. 4A, wild-type MDMs overexpressing TANK did not sufficiently differentiate into osteoclasts, and the number of TRAP-positive multinucleated cells (Fig. 4, A and B). Further, retrovirus reconstitution of TANK in Tank−/− MDMs suppressed RANKL induced osteoclastogenesis to the same level as wild-type (Fig. 4, A and B). These findings indicate that the level of TANK expression is negatively correlated with osteoclastogenesis induced by RANKL treatment.
FIGURE 4.
TANK negatively regulates TRAF6 ubiquitination and NF-κB p65 activation, and its overexpression attenuates osteoclast differentiation. A, MDMs from wild-type Tank−/− mice were retrovirally transfected with empty vector (LZR-CMV-IRES-GFP) or vector expressing TANK (TANK-LZR-CMV-IRES-GFP). After 3 days of culture with 25 ng/ml M-CSF plus 50 ng/ml RANKL, GFP-positive cells were identified by fluorescence microscopy (GFP) and the cells were stained for TRAP (DIC). C, mRNA levels of RANK in MDMs were measured by quantitative PCR (n = 3). D, immunoblotting analysis of anti-TRAF6 antibody immunoprecipitates from MDMs stimulated with RANKL (350 ng/ml) for indicated times probed with an anti-Ub antibody. As a loading control, immunoblotting analysis of TRAF6 was performed (bottom). E, DNA binding activity of NF-κB p65 in response to RANKL was measured using a TransAM Transcription Factor Assay Kit. Error bars: S.E. (n = 3). *, p < 0.05. F, MDMs were treated with RANKL for the indicated time and analyzed by Western blotting using anti-p100/p52 antibodies. Actin was evaluated as a loading control. The data shown are representative of three independent experiments.
To rule out the possibility that differential RANK expression is responsible for the augmented osteoclastogenesis under TANK deficiency, we examined the levels of RANK in MDMs. As shown in Fig. 4C, the RANK mRNA levels were comparable between wild-type and Tank−/− MDMs. FACS analyses revealed that the RANK+CD11b+ osteoclast precursor cell populations in the spleen were also comparable between wild-type and Tank−/− mice (supplemental Fig. S1). Next, we checked whether the loss of TANK promoted TRAF6 ubiquitination and NF-κB pathway activation in response to RANKL stimulation. TRAF6 ubiquitination in response to RANKL stimulation was increased in Tank−/− MDMs compared with wild-type controls (Fig. 4D). Furthermore, RANKL-induced DNA binding of the NF-κB p65 subunit was significantly elevated in Tank−/− MDMs (Fig. 4E). In contrast, p100 cleavage to p52, which indicates activation of the non-canonical NF-κB pathway, did not differ between wild-type and Tank−/− MDMs (Fig. 4F). These findings indicate that TANK negatively affects the RANKL-mediated canonical NF-κB pathway by suppressing TRAF6 activation. TANK may act as a negative feedback regulator of osteoclastogenesis.
Tank−/− Mice Exhibit Increased Cortical BMD and Severe Trabecular Bone Loss
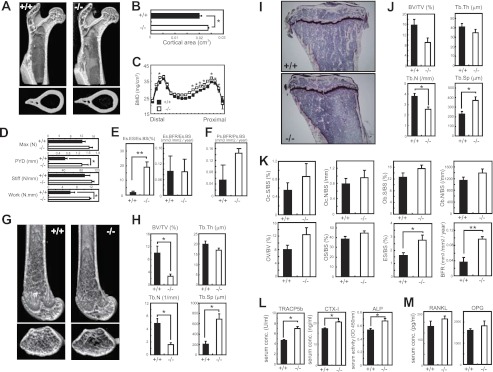
Next, we analyzed the in vivo bone phenotypes of wild-type and Tank−/− mice by μCT. First, we investigated the proximal femurs, and found no differences in the bone structures (Fig. 5A). However, the cortical area in the mid-shaft of the femurs was slightly increased in Tank−/− mice (Fig. 5B). This finding was further supported by increased BMD in the proximal end to mid-shaft in the femurs from Tank−/− mice (Fig. 5C). To determine whether the differences in BMD influenced the mechanical strength of the femur, we performed a three-point bending test on the shaft of the femurs from wild-type and Tank−/− mice. As expected, the maximum load and stiffness showed slight, but not significant, increases in the femurs from Tank−/− mice. Furthermore, the femurs from Tank−/− mice showed 2-fold increases in the post-yield deflection and work to failure compared with the femurs from wild-type mice (Fig. 5D). These findings indicate that the femurs of Tank−/− mice are more ductile than those of wild-type mice. Next, we cut the proximal one-third of the right shaft of femurs and cross sections were made. We performed the histomorphometric analysis and detected dramatically increased eroded surface/bone surface in endosteum (Fig. 5E). Furthermore, bone forming rate in periosteum was increased, but not significant (Fig. 5F). Additionally, endosteum surface was comparable (this means bone marrow cavity perimeter was comparable) but cortical thickness was significantly increased in TANK-deficient mice (supplemental Fig. S2).
FIGURE 5.
Tank−/− mice exhibit severe trabecular bone loss, but increased cortical BMD. A, representative images of proximal femurs from 10-week-old wild-type and Tank−/− mice (upper: longitudinal view; lower: axial view of the region of the third trochanter). B, cortical areas of the mid-shaft section in the femurs. Error bars: S.E. (n = 3). *, p < 0.05. C, BMDs in 20 longitudinal divisions of the femurs. Error bars: S.E. (n = 3). *, p < 0.05. D, mechanical properties of the femur shafts were measured by a three-point bending test. The whole bone mechanical properties of maximum load (N), stiffness (N/mm), post-yield deflection (mm), and work to failure (N.mm) were measured. Error bars: S.E. (n = 3). *, p < 0.05. E, bone histomorphometric analyses of the endosteum portion of cross section from proximal one-third of femur. Es.ES/Es.BS, endosteum eroded surface per endosteum bone surface; Es.BFR/Es.BS, endosteum bone formation rate per endosteum bone surface. Error bars: S.E. (n = 3). *, p < 0.05. F, bone histomorphometric analyses of the periosteum portion of cross section from proximal one-third of femur. Ps.BFR/Ps.BS, periosteum bone formation rate per periosteum bone surface. Error bars: S.E. (n = 3). *, p < 0.05. G, representative images of distal femurs from 10-week-old wild-type and Tank−/− mice (upper: longitudinal view; lower: axial view of the metaphyseal region). H, bone morphometric analyses of distal femurs from wild-type and Tank−/− mice using μCT. Error bars: S.E. (n = 4). *, p < 0.05. I, representative images of the metaphyseal portion of tibias from wild-type and Tank−/− mice (Villanueva bone staining). J and K, bone histomorphometric analyses of the metaphyseal portion of tibias from wild-type and Tank−/− mice. Error bars: S.E. (n = 4). *, p < 0.05, **, p < 0.01. L, serum concentrations of TRACP5b, CTX-I and ALP in wild-type and Tank−/− mice. Error bars: S.E. (n = 4) *, p < 0.05. M, serum concentrations of RANKL and OPG. Error bars: S.E. (n = 4) *, p < 0.05. BV/TV, bone volume per tissue volume; Tb.Th, trabecular bone thickness; Tb.N, trabecular bone number; Tb.Sp, trabecular bone spacing; Oc.S/BS, osteoclast surface per bone surface; Oc.N/BS, osteoclast number per bone surface; Ob.S/BS, osteoblast surface per bone surface; Ob.N/BS, osteoblast number per bone surface; OV/BV, osteoid volume per bone volume; OS/BS, osteoid surface per bone surface; ES/BS, eroded surface per bone surface; BFR, bone formation rate.
In contrast to the increased BMD and cortical area in the proximal to mid-shaft portion, the distal portion of the femurs from Tank−/− mice exhibited severe trabecular bone loss, as observed in three-dimensional images (Fig. 5F). Morphometric analyses of the distal femurs from Tank−/− mice revealed pronounced reductions in the trabecular bone volume per tissue volume and trabecular bone number (Fig. 5G). Analyses of the proximal tibias from Tank−/− mice also indicated a decrease in the trabecular bone number, but no significant decrease in the trabecular bone thickness, compared with wild-type mice (Fig. 5, H and I). Histomorphometric analyses revealed that the eroded surface per bone surface and bone formation rate of the proximal tibias from Tank−/− mice were significantly increased. Furthermore, comparable numbers of osteoclasts and osteoblasts were observed for wild-type and Tank−/− mice (Fig. 5J), suggesting enhancement of the osteoclast function in Tank−/− mice. Consistent with the increased bone eroded surface and bone formation, the serum levels of the bone resorption marker TRACP5, cross-linked C-Telopeptide of Type I collagen (CTX-I), and bone formation marker ALP were higher in Tank−/− mice than in wild-type mice (Fig. 5K). In contrast, serum RANKL and OPG levels were comparable (Fig. 5L). These findings suggest that Tank−/− mice exhibit increased cortical BMD in the proximal to mid-shaft femur, but severe trabecular bone loss in the distal femur and proximal tibia.
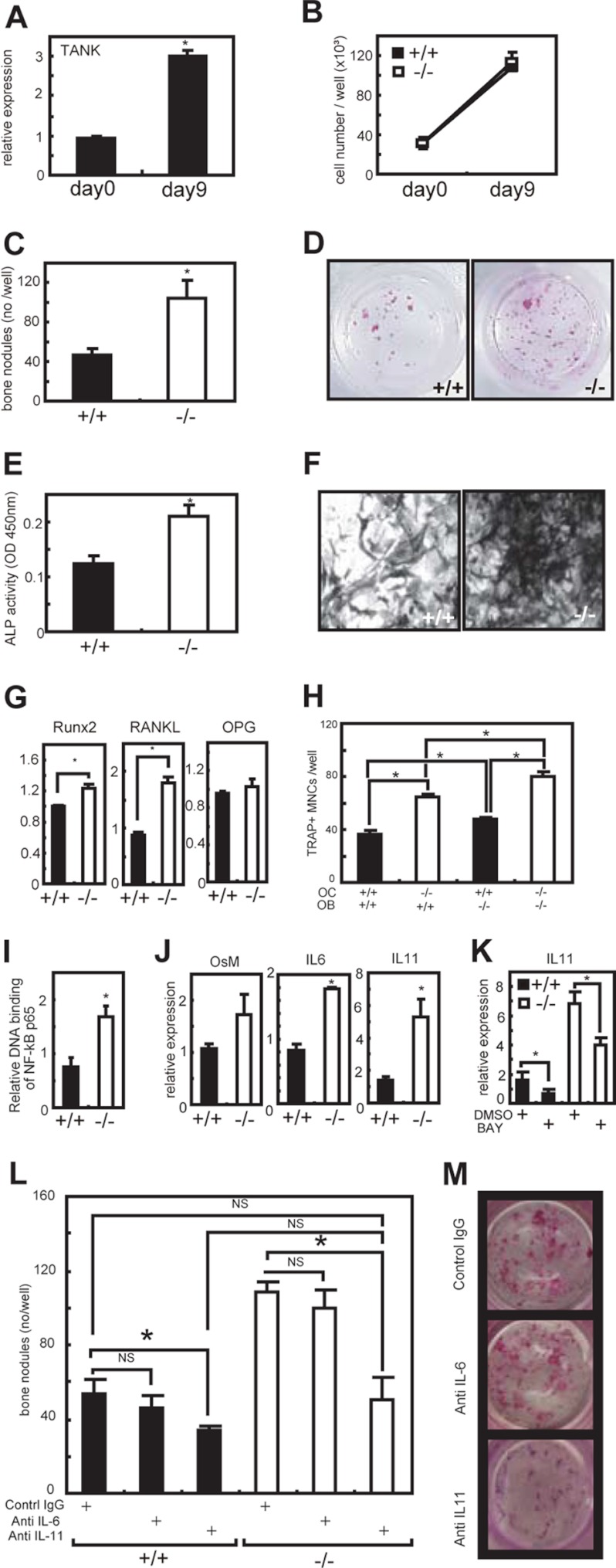
Increased Bone Nodule Formation in Tank−/− Osteoblasts
Because the increased osteoclastogenesis contradicted the increased BMD, we examined the function of calvarial osteoblasts from Tank−/− mice. The expression of TANK mRNA was up-regulated during in vitro osteoblast differentiation (Fig. 6A). During the differentiation, there were no significant differences in the cell numbers between wild-type and Tank−/− osteoblasts (Fig. 6B). Next, to analyze the bone nodule formation by osteoblasts, the cultured cells were stained with alizarin red. Interestingly, Tank−/− osteoblasts showed increased bone nodule formation (Fig. 6, C and D). The ALP activity, as a phenotypic marker of osteoblasts, was also elevated in Tank−/− osteoblasts (Fig. 6, E and F). To search for the osteoblastic factor responsible for the osteoclastogenesis, we examined the expression levels of Runx2, RANKL, and OPG in osteoblasts, and found that the expression levels of Runx2 and RANKL, but not OPG, were significantly up-regulated in Tank−/− cells (Fig. 6G). Osteoclast-osteoblast coculture assays indicated that the increased RANKL expression in Tank−/− osteoblasts potentiate osteoclastogenesis in vitro (Fig. 6H). To reveal the mechanisms of increased osteoblast functions in Tank−/− mice, we checked the activation of DNA binding activity of NF-κB p65 in osteoblasts, and observed the 2-fold increase of NF-κB activation in TANK-deficient osteoblast compared with wild-type control (Fig. 6I). It has been known that increased NF-κB activation leads to induce IL-6-type cytokines such as IL-6, IL-11 and OncostatinM. Importantly, previous reports have established that IL-6-type cytokines play a pivotal role in bone metabolism (22). Thus, we next checked the expression levels of IL-6-type cytokines in TANK-deficient osteoblasts (Fig. 6J). Intriguingly, mRNA levels of IL-6 and IL-11 (especially IL-11) are significantly increased in TANK-deficient osteoblasts compared with wild-type controls (Fig. 6J). When we cultured osteoblasts with NF-κB inhibitor BAY117085, IL-11 mRNA level was significantly inhibited in TANK-deficient osteoblast (Fig. 6K). IL-6-type cytokines are potent stimulators of the development of osteoblastogenesis. Thus, our findings above prompted us to explore the effect of IL-6 and IL-11 neutralizing antibodies on in vitro bone nodule formation in TANK-deficient osteoblasts (Fig. 6, L and M). IL-6 neutralizing antibody had no effect to bone nodule formation in TANK-deficient osteoblasts (Fig. 6L). In contrast, IL-11 neutralizing antibody significantly suppressed the bone nodule formation in TANK-deficient osteobalsts to the same level as wild-type controls (Fig. 6, L and M). These findings suggest that increased IL-11 expression is at least partially attributed to increased NF-κB activation and such enhanced IL-11 production promotes the bone formation in TANK-deficient osteoblasts. Collectively, these findings suggest that TANK is a negative regulator of osteoblast bone formation and IL-11 is a critical target of TANK in osteoblasts.
FIGURE 6.
Increased bone formation by Tank−/− calvarial osteoblasts. A, calvariae from wild-type mice were treated with an osteoblast-inducing reagent for 9 days. The mRNA levels of TANK were measured by quantitative PCR. Error bars: S.E. (n = 3). *, p < 0.05 versus d 0. B, numbers of osteoblasts in culture. C, osteoblasts (after a 9-day culture of calvariae) were stained with a calcified nodule staining kit, and the numbers of bone nodules were counted. Error bars: S.E. (n = 3). *, p < 0.05 versus wild-type. D, representative images of calcified nodules in C. E, ALP activities in homogenates of the osteoblasts. Error bars: S.E. (n = 3). *, p < 0.05 versus wild-type. F, representative images of ALP staining in osteoblasts. G, mRNA levels of Runx2, RANKL, and OPG were measured by quantitative PCR. Error bars: S.E. (n = 3). *, p < 0.05, versus wild-type. H, bone marrow cells (OC) and calvariae (OB) were cocultured in the presence of 1α,25(OH)2D3 for 10 days. The numbers of TRAP-positive multinucleated cells were counted. Error bars: S.E. (n = 3). *, p < 0.05. I, DNA binding activity of NF-κB p65 in osteoblasts (after a 9-day culture of calvariae) was measured using a TransAM Transcription Factor Assay Kit. Error bars: S.E. (n = 3). *, p < 0.05. J, quantitative PCR analyses OncostatinM (OsM), IL-6, and IL-11 in osteobalsts. Error bars: S.E. (n = 3). *, p < 0.05. K, 5 mm NF-κB inhibitor BAY117085 was added to the osteoblast culture for 24 h and IL-11 mRNA level was quantified. Error bars: S.E. (n = 3). *, p < 0.05. L, calvarial osteoblasts were cultured with neutralizing antibodies of IL-6 and IL-11. After a 9-day culture, cells were stained with a calcified nodule staining kit, and the numbers of bone nodules were counted. Error bars: S.E. (n = 3). *, p < 0.05. M, representative images of bone nodules formed by Tank−/− calvarial osteoblasts in L.
Trabecular Bone Loss in Tank−/− Mice Is Dependent on Hematopoietic Cells
To examine whether the severe trabecular bone loss in Tank−/− mice could be explained by TANK deficiency in osteoclasts, we generated bone marrow chimeric mice. TANK expression in splenocytes and protein in bone marrow cells from wild-type mice engrafted with bone marrow from Tank−/− mice (−/− → WT mice) was scarcely detected (Fig. 7, A and D). CD11b+RANK+ osteoclast precursor populations in splenocytes were comparable between −/− → WT and +/+ → WT mice (supplemental Fig. S3). MDMs from −/− → WT mice efficiently differentiated into osteoclasts in vitro (Fig. 7B) rather than MDMs from wild-type mice engrafted with bone marrow from wild-type mice (+/+ → WT mice). Consistent with the Tank−/− mice, −/− → WT mice exhibited severe trabecular bone loss in their femurs and tibias compared with +/+ → WT mice (Fig. 7, C and F). Morphometric analyses of the femurs from −/− → WT mice revealed severe reductions in the trabecular bone volume per tissue volume and trabecular bone number (Fig. 7E). Analyses of the proximal tibias from −/− → WT mice also indicated decreases in the trabecular bone volume per tissue volume and trabecular bone number compared with +/+ → WT mice (Fig. 7G). The osteoblast numbers were comparable between both types of chimeric mice, although the number was slightly, but not significantly, increased in the proximal end of the tibias from −/− → WT mice (Fig. 7H). Additionally, cortical thickness was decreased (Fig. 7I) and eroded surface/bone surface was drastically increased in endosteum from −/− → WT mice (Fig. 7J). Further, the serum levels of the bone resorption marker TRACP5b were higher in −/− → WT mice than in +/+ → WT mice but ALP levels were comparable (Fig. 7K). The trabecular bone structural analogy between Tank−/− mice and −/− → WT mice demonstrates that the trabecular bone loss in Tank−/− mice is caused by hematopoietic cells including osteoclasts.
FIGURE 7.
Wild-type mice engrafted with Tank−/− bone marrow exhibit severe bone loss. A, expression levels of TANK in splenocytes from wild-type mice engrafted with wild-type marrow (+/+ → WT) and Tank−/− marrow (−/− → WT) specimens were measured by quantitative PCR. B, MDMs from +/+ → WT and −/− → WT specimens were treated with 50 ng/ml RANKL for 3 days and the numbers of osteoclasts were counted. C, representative images of distal femurs from +/+ → WT and −/− → WT specimens. D, TANK protein expression levels in bone marrow cells from +/+ → WT and −/− → WT mice. E, bone morphometric analyses of distal femurs from +/+ → WT and −/− → WT specimens using μCT. F, representative photographs of the metaphyseal portion of tibias from +/+ → WT and −/− → WT specimens (Villanueva bone staining). G and H, bone histomorphometric analyses of tibias from +/+ → WT and −/− → WT specimens. I, cortical bone thickness in the portion of cross section from proximal one-third of femur. Error bars: S.E. (n = 3). *, p < 0.05. J, bone histomorphometric analyses of the endosteum portion of cross section from proximal one-third of femur. Error bars: S.E. (n = 3). *, p < 0.05. K, serum concentrations of TRACP5b and ALP in +/+ → WT and −/− → WT mice. Error bars: S.E. (n = 3). *, p < 0.05. BV/TV, bone volume per tissue volume; Tb.Th, trabecular bone thickness; Tb.N, trabecular bone number; Tb.Sp, trabecular bone spacing; Oc.N/BS, osteoclast number per bone surface; Ob.N/BS, osteoblast number per bone surface. Es.ES/Es.BS, endosteum eroded surface per endosteum bone surface; Es.BFR/Es.BS, endosteum bone formation rate per endosteum bone surface.
DISCUSSION
In the present study, we have shown that TANK is a novel regulator of osteoclastogenesis and bone formation. TANK deficiency caused significant trabecular bone loss in the femur and tibia. In vitro osteoclastogenesis of Tank−/− bone marrow cells was enhanced because of increased RANK signaling. Consistently, in vivo bone erosion by osteoclasts was significantly increased, which may explain the trabecular bone loss phenotype under TANK deficiency. However, we also found that lack of TANK led to increased bone formation and BMD in the proximal cortical portion of the femur. Bone nodule formation by in vitro cultured calvarial osteoblasts from TANK-deficient mice was also up-regulated. Collectively, these findings suggest that TANK negatively controls the functions of bone formation and erosion.
We have shown that osteoclasts lacking TANK exhibit increased bone-resorbing function both in vitro and in vivo. Expression of TANK was significantly increased during RANKL-induced osteoclastogenesis, while overexpression of TANK led to suppressed osteoclastogenesis. These observations support the idea that TANK acts as a negative regulator of osteoclastogenesis. Our previous report clarified that macrophages and B cells from Tank−/− mice exhibit enhanced canonical NF-κB and AP-1 activation in response to stimulation of TLRs and BCR (19). The RANKL-induced NF-κB pathways include canonical and non-canonical pathways involving the NF-κB precursor proteins p105 and p100, respectively. Previous reports have suggested that mice lacking both NF-κB subunits p50 and p52 develop osteopetrosis, accompanied by a reduction in osteoclastogenesis (4) (8). In these mice, both the canonical and non-canonical NF-κB pathways are inhibited. Alymphoplasia mice, in which processing of p100 to p52 does not occur owing to an inactive form of NIK (23), show mild osteopetrosis caused by significantly reduced osteoclastogenesis (24). These reports strongly suggest that both the canonical and non-canonical NF-κB pathways are important for efficient osteoclastogenesis. NFATc1 is a master regulator of osteoclastogenesis, and we found that the level of NFATc1 expression during osteoclastogenesis was increased in Tank−/− cells. Since NF-κB has been implicated in the induction of NFATc1 in RANKL signaling, we examined whether the loss of TANK also promoted NF-κB activation in response to RANKL, and found that canonical NF-κB pathway activation was significantly elevated in Tank−/− macrophages. In contrast, the non-canonical NF-κB pathway was not altered, suggesting that TANK negatively regulates canonical NF-κB pathway activation in response to RANKL, synonymous with TLR and BCR stimulation. These findings suggest that augmentation of canonical NF-κB activation alone is sufficient for controlling osteoclastogenesis. Several molecules have been characterized to play inhibitory roles in osteoclastogenesis. IRAK-M, a negative regulator of TLR/IL-1R signaling, acts as an inhibitor of osteoclast differentiation and activation, probably by suppressing IL-1R signaling in inflammation (25). Another example is Src homology 2-containing inositol-5-phosphatase (SHIP), which limits osteoclast precursor survival and differentiation by suppressing PI3-kinase (26). On the other hand, the regulation of RANKL-mediated signaling is less well characterized, except for CYLD. This molecule is a deubiquitinating enzyme induced by RANKL that suppresses RANK signaling by inhibiting TRAF6 ubiquitination (27). In this study, we found that TANK specifically inhibited RANKL-induced osteoclastogenesis under physiological conditions, synonymous with CYLD, and suggest a model whereby negative feedback regulation of RANKL signaling by TANK contributes to proper osteoclast differentiation. Information on the relationship between TANK and CYLD would be important for the future study. Although we observed enhanced TRAF6 ubiquitination in TANK−/− cells in response to RANKL stimulation, the effect of TANK deficiency was relatively modest. Thus, it is possible that a mechanism other than TRAF6 polyubiquitination also contribute to TANK-mediated control of osteoclastogenesis. Further detailed studies are needed to explore the role of TANK in osteoclasts.
Abnormal bone turnover is observed in several human diseases. Rheumatoid arthritis is a major high-bone turnover osteoporotic disease (28). Paget disease is also characterized by accelerated bone resorption by IL-6-producing giant osteoclasts and enhanced bone formation by osteoblasts, resulting in increased BMD and bone fragility (29). Schnitzler's syndrome is another high-bone turnover disorder, and is characterized by osteosclerosis, chronic urticaria, lymphadenopathy, IgM gammopathy, and joint pain (30, 31). Our previous report indicated that lymphadenopathy and increased basal serum concentrations of IgM were the major features in Tank−/− mice (19). Since TANK deficiency closely resembles some of the traits of the human high-bone turnover diseases described above, the involvement of TANK in these disorders must be clarified in the future.
As observed in the in vivo bone analyses, TANK suppresses both osteoclasts and osteoblasts. Bone formation and degradation are often manipulated simultaneously in the same direction, which is termed coupling (32). Such coupling events are crucial for the maintenance of bone quality. Understanding of coupling is important, but its molecular mechanisms are largely unknown. As TANK has a dual suppressive effect on both cell types, it may play a role, at least in part, in supporting the speed of such coupling. Intriguingly, Tank−/− mice seem to suffer from osteosclerosis and osteoporosis in the cortical and trabecular bone areas, respectively. In other words, Tank−/− bone has an abnormal morphology. Our detailed cellular studies in both osteoclasts and osteoblasts in vitro, Tank−/− mice have intrinsic abnormalities in the functions of both cell types. It is still unclear how TANK inhibits bone formation in osteoblasts. The ALP activity and expression level of Runx2, an essential transcription factor for the regulation of osteoblast differentiation (33), were slightly increased, suggesting that TANK may partially regulate osteoblast differentiation. Our data also indicated that increased IL-11 expression is at least partially attributed to increased NF-κB activation and such enhanced IL-11 production promotes the bone formation in TANK-deficient osteoblasts. Further detailed studies are needed to explore the role of TANK in osteoblast function, but our observations suggest for the first time that IL-11 is a critical target of TANK in osteoblasts.
Finally, considering the Tank−/− bone phenotype and suppressive functions of TANK toward both osteoclasts and osteoblasts, modulation of TANK will potentially result in distinct prophylactic effects, depending on the trabecular and cortical portions. Further, generation of methods for the inhibition and stimulation of TANK in osteoblasts and osteoclasts, respectively, may be a reasonable strategy to manipulate bone-destructive diseases.
Supplementary Material
Acknowledgments
We thank J. Kikuta, M. Ishii, H. Iwasaki, T. Satoh, and D. Ori for technical advice, E. Kamada and M. Kageyama for secretarial assistance, Y. Fujiwara, M. Kumagai, and N. Umano for technical assistance, and T. Imamura and S. Tartey for participating in fruitful discussions.
This work was supported by the Special Coordination Funds of the Japanese Ministry of Education, Culture, Sports, Science, and Technology, grants from the Ministry of Health, Labour, and Welfare in Japan, the Japan Society for the Promotion of Science (JSPS) through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), and a research fellowship from the JSPS for the Promotion of Science for Young Scientists.

This article contains supplemental Figs. S1–S3.
- RANK
- receptor activator of NF-κB
- OPG
- osteoprotegerin
- MDMs
- M-CSF-derived macrophages
- μCT
- microcomputed tomography
- TRAP
- tartrate-resistant acid phosphatase
- BMD
- bone mineral density
- ALP
- alkaline phosphatase
- Runx2
- runt-related transcription factor 2
- TANK
- TRAF family-associated NF-κB activator
- SHIP
- Src homology 2-containing inositol-5-phosphatase.
REFERENCES
- 1. Karsenty G., Wagner E. F. (2002) Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 [DOI] [PubMed] [Google Scholar]
- 2. Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., Saiura A., Isobe M., Yokochi T., Inoue J., Wagner E. F., Mak T. W., Kodama T., Taniguchi T. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 [DOI] [PubMed] [Google Scholar]
- 3. Grigoriadis A. E., Wang Z. Q., Cecchini M. G., Hofstetter W., Felix R., Fleisch H. A., Wagner E. F. (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 [DOI] [PubMed] [Google Scholar]
- 4. Franzoso G., Carlson L., Xing L., Poljak L., Shores E. W., Brown K. D., Leonardi A., Tran T., Boyce B. F., Siebenlist U. (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 11, 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamashita T., Yao Z., Li F., Zhang Q., Badell I. R., Schwarz E. M., Takeshita S., Wagner E. F., Noda M., Matsuo K., Xing L., Boyce B. F. (2007) NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 282, 18245–18253 [DOI] [PubMed] [Google Scholar]
- 6. Yagi M., Miyamoto T., Sawatani Y., Iwamoto K., Hosogane N., Fujita N., Morita K., Ninomiya K., Suzuki T., Miyamoto K., Oike Y., Takeya M., Toyama Y., Suda T. (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yagi M., Ninomiya K., Fujita N., Suzuki T., Iwasaki R., Morita K., Hosogane N., Matsuo K., Toyama Y., Suda T., Miyamoto T. (2007) Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner Res. 22, 992–1001 [DOI] [PubMed] [Google Scholar]
- 8. Iotsova V., Caamaño J., Loy J., Yang Y., Lewin A., Bravo R. (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 3, 1285–1289 [DOI] [PubMed] [Google Scholar]
- 9. Leibbrandt A., Penninger J. M. (2008) RANK/RANKL: regulators of immune responses and bone physiology. Ann. N.Y. Acad. Sci. 1143, 123–150 [DOI] [PubMed] [Google Scholar]
- 10. Naito A., Azuma S., Tanaka S., Miyazaki T., Takaki S., Takatsu K., Nakao K., Nakamura K., Katsuki M., Yamamoto T., Inoue J. (1999) Severe osteopetrosis, defective interleukin-1 signaling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4, 353–362 [DOI] [PubMed] [Google Scholar]
- 11. Lomaga M. A., Yeh W. C., Sarosi I., Duncan G. S., Furlonger C., Ho A., Morony S., Capparelli C., Van G., Kaufman S., van der Heiden A., Itie A., Wakeham A., Khoo W., Sasaki T., Cao Z., Penninger J. M., Paige C. J., Lacey D. L., Dunstan C. R., Boyle W. J., Goeddel D. V., Mak T. W. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi T., Walsh P. T., Walsh M. C., Speirs K. M., Chiffoleau E., King C. G., Hancock W. W., Caamano J. H., Hunter C. A., Scott P., Turka L. A., Choi Y. (2003) TRAF6 is a critical factor for dendritic cell maturation and development. Immunity 19, 353–363 [DOI] [PubMed] [Google Scholar]
- 13. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi O., Akira S. (2010) Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 15. Cheng G., Baltimore D. (1996) TANK, a co-inducer with TRAF2 of TNF- and CD 40L-mediated NF-kappaB activation. Genes Dev. 10, 963–973 [DOI] [PubMed] [Google Scholar]
- 16. Rothe M., Xiong J., Shu H. B., Williamson K., Goddard A., Goeddel D. V. (1996) I-TRAF is a novel TRAF-interacting protein that regulates TRAF-mediated signal transduction. Proc. Natl. Acad. Sci. U.S.A. 93, 8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chin A. I., Shu J., Shan Shi C., Yao Z., Kehrl J. H., Cheng G. (1999) TANK potentiates tumor necrosis factor receptor-associated factor-mediated c-Jun N-terminal kinase/stress-activated protein kinase activation through the germinal center kinase pathway. Mol. Cell Biol. 19, 6665–6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pomerantz J. L., Baltimore D. (1999) NF-κB activation by a signaling complex containing TRAF2, TANK, and TBK1, a novel IKK-related kinase. EMBO J. 18, 6694–6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawagoe T., Takeuchi O., Takabatake Y., Kato H., Isaka Y., Tsujimura T., Akira S. (2009) TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat. Immunol. 10, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo B., Cheng G. (2007) Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J. Biol. Chem. 282, 11817–11826 [DOI] [PubMed] [Google Scholar]
- 21. Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taguchi Y., Yamamoto M., Yamate T., Lin S. C., Mocharla H., DeTogni P., Nakayama N., Boyce B. F., Abe E., Manolagas S. C. (1998) Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc. Assoc. Am. Physicians 110, 559–574 [PubMed] [Google Scholar]
- 23. Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. (1999) Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κB-inducing kinase. Nat. Genet. 22, 74–77 [DOI] [PubMed] [Google Scholar]
- 24. Maruyama T., Fukushima H., Nakao K., Shin M., Yasuda H., Weih F., Doi T., Aoki K., Alles N., Ohya K., Hosokawa R., Jimi E. (2010) J. Bone Miner Res. 25, 1058–1067 [DOI] [PubMed] [Google Scholar]
- 25. Li H., Cuartas E., Cui W., Choi Y., Crawford T. D., Ke H. Z., Kobayashi K. S., Flavell R. A., Vignery A. (2005) IL-1 receptor-associated kinase M is a central regulator of osteoclast differentiation and activation. J. Exp. Med. 201, 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takeshita S., Namba N., Zhao J. J., Jiang Y., Genant H. K., Silva M. J., Brodt M. D., Helgason C. D., Kalesnikoff J., Rauh M. J., Humphries R. K., Krystal G., Teitelbaum S. L., Ross F. P. (2002) SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat. Med. 8, 943–949 [DOI] [PubMed] [Google Scholar]
- 27. Jin W., Chang M., Paul E. M., Babu G., Lee A. J., Reiley W., Wright A., Zhang M., You J., Sun S. C. (2008) Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J. Clin. Invest. 118, 1858–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris E. D., Jr. (1990) Rheumatoid arthritis. Pathophysiology and implications for therapy. N. Engl. J. Med. 322, 1277–1289 [DOI] [PubMed] [Google Scholar]
- 29. Roodman G. D., Windle J. J. (2005) Paget disease of bone. J. Clin. Invest. 115, 200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Koning H. D., Bodar E. J., van der Meer J. W., Simon A., and Schnitzler Syndrome Study, G. (2007) Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin. Arthritis Rheum. 37, 137–148 [DOI] [PubMed] [Google Scholar]
- 31. Almerigogna F., Giudizi M. G., Cappelli F., Romagnani S. (2002) Schnitzler's syndrome: what's new? J. Eur. Acad. Dermatol. Venereol 16, 214–219 [DOI] [PubMed] [Google Scholar]
- 32. Feng X., McDonald J. M. (2011) Annu. Rev. Pathol. 6, 121–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.