Abstract
Background
Behavioral research to improve lifestyle in broadly defined type 2 diabetic (T2DM) patient populations is limited.
Objective
Evaluate a behavioral intervention featuring technology-based self-monitoring on biophysiologic outcomes of glycemic control and markers of cardiovascular risk.
Design
In this single site randomized clinical trial, participants were stratified by good and poor glycemic control (A1c < or ≥ 8%) and absence or presence of kidney disease, (eGFR ≥ or < 60 ml/min) and randomized within strata. Measurements were obtained at 0, 3, and 6 mos.
Participants/setting
Self-referred, community-dwelling adults with T2DM.
Intervention
Intervention Group received Social Cognitive Theory-based counseling paired with technology-based self-monitoring, and compared to an Attention Control Group.
Main outcome measures
A1c, fasting serum glucose, lipids, blood pressure, weight, body mass index, and waist circumference were evaluated.
Statistical analyses performed
Mean differences within and between randomization groups were compared over time. Intervention effects over time were estimated using random intercept models.
Results
296 were randomized, 256 (86.5%) completed 3-mo and 246 (83.1%) completed 6-mo assessments. A1c was reduced in the Intervention Group by 0.5% at 3 mos and 0.6% at 6 mos (p<0.001 for each), and the Control Group by 0.3% (p<0.001) at 3 mos and 0.2% (p<0.05) at 6 mos; but between group differences were not significant . In those with baseline A1c≥8% and eGFR≥60 ml/min, A1c was reduced in the Intervention Group by 1.5% at 3 mos and 1.8% at 6 mos (p<0.001 for each), and the Control Group by 0.9% (p<0.001) at 3 mos and 0.8% (p<0.05) at 6 mos; but between group differences were not significant. In random intercept models, the estimated reduction in A1c of 0.29% was not significant.
Conclusions
Two behavioral approaches to improving general lifestyle management in T2DM were effective in improving glycemic control, but no significant between group differences were observed.
Keywords: diabetes mellitus, type 2; chronic kidney disease; computers, hand-held; personal digital assistant; randomized clinical trial; behavioral research
Introduction
Type 2 diabetes (T2DM) is a highly prevalent disorder in the US and a major cause of cardiovascular disease (CVD).1 The majority of adults with T2DM are overweight or obese, and have comorbidities that increase the risk of CVD (e.g., hyperlipidemia and/or hypertension). Those with chronic kidney disease (CKD), a common complication of diabetes,2 are at even higher risk of developing CVD.3,4 Chronic hyperglycemia is widely known to increase the risk of CVD.
To address CVD risk, the American Diabetes Association recommends the following biophysiologic targets for the majority of patients with T2DM: glycosylated hemoglobin (A1c) <7%; fasting lipid targets (e.g., low-density lipoprotein or LDL cholesterol < 100 mg/dl, high-density lipoprotein or HDL cholesterol > 50 mg/dl, and triglycerides < 150 mg/dl); systolic blood pressure ≤ 130 mmHg and diastolic blood pressure ≤ 80 mmHg; and weight loss for those who are overweight or obese.5
Recently reported trials of aggressive medication management of glycemia, blood pressure, and lipidemia in T2DM have not demonstrated significant differences in CVD outcomes, including the most recently completed Action to Control Cardiovascular Risk in Diabetes (ACCORD) study.6-9 ACCORD evaluated aggressive medication management in adults with established T2DM who were at especially high risk of CVD. Following the negative results of the ACCORD trial, the American College of Cardiology issued press releases stressing the importance of lifestyle modification to reduce CVD risk in those with T2DM.10,11 While behavior is an important target for CVD risk reduction, few published studies have evaluate behavioral approaches for engaging patients with multiple comorbidities and complex self-management regimens in a healthier lifestyle to reduce CVD risk.
The purpose of the Enhancing Adherence in Type 2 Diabetes (ENHANCE) study was to evaluate a behavioral intervention to enhance lifestyle behavior change within the context of a complex diabetes self-management regimen in a broadly defined clinical population with few exclusion criteria. The behavioral intervention was paired with personal digital assistant (PDA)-based self-monitoring of diet and physical activity. In this report the authors focus on biophysiologic outcomes and examine the hypotheses that, compared to those randomized to the Attention Control Group, Intervention Group participants would demonstrate improved glycemia, serum lipids, blood pressure, weight, body mass index (BMI), and waist circumference.
Methods
A. Design
The ENHANCE study was a single center, randomized controlled trial of adults with T2DM. Participants were stratified according to the baseline level of kidney function (i.e., decreased, defined as estimated glomerular filtration rate (eGFR) < 60 ml/min, or normal, defined as eGFR ≥ 60 ml/min), and good or poor baseline glycemic control (i.e., A1c of < 8% or ≥ 8%). Participants were randomized within these a priori defined A1c/eGFR strata to either the Intervention or Attention Control Groups using computer-generated permuted blocks. Because of the nature of behavioral interventions, neither participants nor investigators could be blinded to group assignment. The study was approved by the University of Pittsburgh Institutional Review Board. All participants provided written consent prior to baseline assessment.
B. Study population
Participants were recruited into the study between September 2004 and December 2008. Participants self-referred to the study in response to newspaper advertisements, mass transit (bus) advertisements, exhibits at two local health fairs, posters placed throughout the University of Pittsburgh Medical Center and liberal arts campuses, direct mailings, Audix voicemail (targeting University of Pittsburgh employees), word-of-mouth, or the ClinicalTrials.gov website.
Adults aged ≥ 18 years with a self-reported diagnosis of T2DM were eligible for this study. Exclusion criteria included hypoglycemic coma/seizure within the last 12 months; hypoglycemia requiring 3rd party assistance within the last 3 months; unwillingness or inability to self-monitor capillary blood glucose (CBG) or to participate in scheduled group sessions; history of type 1 diabetes; current receipt of renal dialysis or expectation of dialysis treatment before the conclusion of the 6-month intervention period; history of dementia, alcohol, substance abuse or other issues likely to interfere with adherence to the study protocol; intention to move outside of the study region within the study period; lack of support from the participant's primary health care provider; or participation in another clinical study.
C. Intervention and Attention Control Group Conditions
Participants of both groups received training in use of a study-provided glucose meter and sufficient supplies to perform ≥ 2 CBG measures per day. All participants also were given a pedometer with instructions for use and a target level of physical activity of 10,000 steps per day.
The Intervention Group was exposed to group counseling sessions guided by Social Cognitive Theory, which focused on building self-efficacy or sense of perceived mastery over the diabetes self-management regimen.12 Intervention Group participants were provided with a PalmOne Tungsten/E2 PDA (PalmOne, Inc.; Milpitas, CA) with a dietary self-monitoring program containing 4,300 foods, with nutrient composition derived from the United States Department of Agriculture.13 The software was programmed to permit entry of 3 meals and 1 snack daily. Calorie targets were based on each participant's resting metabolic rate, estimated from bioelectrical impedance analysis, (Tanita Body Composition Analyzer TBF-300A; Tanita Corporation of America, Inc.; Arlington Heights, IL) and the expected energy expenditure from usual activities. Unless the participant was underweight, calorie targets were set to allow a weight loss of no more than 4.54 kilograms (kgs; about 10 pounds) over 6 months, with 55% of calories derived from carbohydrates, 30% from fats (10% from saturated fats), and 15% from protein. Dietary prescriptions were reviewed by a study dietitian who was also a certified diabetes educator. Participants were shown how to use the PDA data to stay within their daily calorie target, distribute their carbohydrate intake throughout the day, and balance their intake of proteins, fats, and carbohydrates. Participants also were advised to pay attention to the connections between glycemic control and their PDA records of carbohydrate and fat intake, medication management, and physical activity. PDA use was intended to enhance self-management self-efficacy by: (1) enabling vigilance to diet and physical activity without unduly burdening the participant, (2) providing immediate meal-by-meal feedback regarding achievement of dietary and energy expenditure goals, (3) permitting straight-forward integration of information regarding calorie intake and energy expenditures, and (4) allowing participants to make real-time connections between carbohydrate and saturated fat intake (which increases insulin resistance14) and success in controlling glycemia.
Intervention Group sessions were held weekly in months 1-2; biweekly in months 3-4, and monthly in months 5-6. The frequency of group sessions was gradually decreased to permit the participant to develop independence in self-management. CBG results and PDA dietary and physical activity data were uploaded at each meeting and printed as reports to participants, with written and verbal feedback provided by two clinical diabetes educators (one dietitian and one nurse). Participants were instructed how to interpret these reports and use them to develop explicit diabetes self-management goals to achieve before the next meeting. As reported elsewhere,15 Intervention Group participants entered an average of 11 meals/week in the first 2 months of the study, 7 meals/week in months 3 and 4, and 4 meals/week in the final two months of the study. By the end of the study, approximately 20% of the participants continued to enter more than half of the expected meals consumed/week (i.e. 11 meals/week; assuming they consumed at least 21 meals per week).
The Attention Control Group had monthly contact with the study team. In months 1, 3, and 5 they attended group seminars, including general diabetes education and stress management instruction as well as an executive chef demonstration. In months 2, 4, and 6 they received a lay diabetes magazine. Additional details regarding the Intervention and Attention Control Group activities are reported elsewhere.16
D. Measurements
Data were collected at the Clinical and Translational Research Center at the University of Pittsburgh. Measurements were obtained at baseline, 3, and 6 months by a trained research assistant. BMI was computed from height (measured at baseline only, with a stadiometer) and weight (measured in light clothing, bare feet, using the Tanita scale). Waist circumference was obtained with a Gulick Tape measure (Power Systems, Inc,; Knoxville TN) on bare skin, at the natural waist with the abdomen relaxed. A1c and lipids were evaluated from blood, obtained from a venipuncture performed by a phlebotomist, after an 8-hour fast. Blood samples were collected, spun, refrigerated, batched and sent for processing in the CLIA-certified University of Pittsburgh Medical Center laboratories by personnel blinded to treatment assignment. Coefficients of variation for A1c and lipids were all <10%. Upon completion of each measurement visit, participants were compensated for their time with a $20 grocery store gift certificate.
E. Statistical Analysis
This study was designed to provide at least 80% power to detect a small to medium effect size (f=0.14) suggested by Cohen,17 based on a 2 degree of freedom (df) test of the group by time interaction in a repeated measures analysis of variance with a 2-sided 0.01 level test (to allow for multiple comparisons). The required sample size of 240 participants provided at least 80% power to detect medium (Cohen's d=0.40) treatment differences in time-specific means, using 2-sided 0.01 level tests. No interim analyses were planned or conducted. The sample size estimation allowed for 17% attrition; the primary analysis included all randomized participants who had at least one follow-up visit. Analyses were conducted using Stata version 11.18
Baseline characteristics in Table 1 were compared using Chi-squared statistics. In descriptive analyses, time-specific means, differences within each treatment group over time, and differential change over time between the two treatment groups were compared using t-tests with bootstrap standard errors. Glycemia outcomes (i.e., A1c and fasting serum glucose) within the 3 eGFR/A1c strata were also described.
Table 1.
Baseline characteristics of the ENHANCE participants overall, and by treatment arm
| Characteristic | Overall | Technology-Supported Behavioral Intervention (n=131) | Attention Control (n=132) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (in years) | 0.88 | ||||||
| 25-34 | 11 | 4.2 | 6 | 4.6 | 5 | 3.8 | |
| 35-44 | 27 | 10.3 | 16 | 12.2 | 11 | 8.3 | |
| 45-54 | 76 | 28.9 | 36 | 27.5 | 40 | 30.3 | |
| 55-64 | 100 | 38.0 | 47 | 35.9 | 53 | 40.2 | |
| 65-74 | 38 | 14.4 | 20 | 15.3 | 18 | 13.6 | |
| >= 75 | 11 | 4.2 | 6 | 4.6 | 5 | 3.8 | |
| Female | 179 | 68.1 | 93 | 71.0 | 86 | 65.2 | 0.31 |
| White | 184 | 70.0 | 90 | 68.7 | 94 | 71.2 | 0.66 |
| Married or living with partner | 137 | 52.3 | 67 | 51.5 | 70 | 53.0 | 0.81 |
| Health care Insurance | 244 | 93.5 | 121 | 93.1 | 123 | 93.9 | 0.79 |
| Employed | 153 | 58.4 | 76 | 58.5 | 77 | 58.3 | 0.98 |
| Any post-high school education | 181 | 69.1 | 87 | 66.9 | 94 | 71.2 | 0.45 |
| Duration of diabetes | 0.41 | ||||||
| Less than a year | 57 | 21.8 | 28 | 21.4 | 29 | 22.1 | |
| 1-5 years | 107 | 40.8 | 49 | 37.4 | 58 | 44.3 | |
| More than 5 years | 98 | 37.4 | 54 | 41.2 | 44 | 33.6 | |
| A1c & eGFR | 0.67 | ||||||
| A1c < 8%, eGFR ≥ 60 ml/min | 157 | 59.7 | 75 | 57.3 | 82 | 62.1 | |
| A1c ≥ 8%, eGFR ≥ 60 ml/min | 74 | 28.1 | 40 | 30.5 | 34 | 25.8 | |
| eGFR < 60 ml/min | 32 | 12.2 | 16 | 12.2 | 16 | 12.1 | |
| Diabetes medicationsa | 226 | 90.4 | 115 | 91.3 | 111 | 89.5 | 0.64 |
| Antihypertensive medicationsa | 173 | 69.2 | 85 | 67.5 | 88 | 71.0 | 0.55 |
| Lipid lowering medicationsa | 141 | 56.4 | 72 | 57.1 | 69 | 55.6 | 0.81 |
Medication data missing for 5 Technology-Supported Behavioral Intervention and 8 Attention Control participants
p-values computed using Pearson Chi-square test
Each outcome over time was modeled separately using a random intercept model that allowed each participant to have his/her own mean value. Fixed effects included time, treatment group, stratum, and interactions of these variables. The primary treatment effect in these models was a 2 parameter treatment by time interaction; the intervention effects at 3 and 6 months were estimated using linear contrasts. Preliminary analysis indicated that drop-out varied by age and marital status, so these variables were included as fixed effects in random intercept models that were estimated using maximum likelihood; this approach provides unbiased parameter estimates when data are assumed to be missing at random (i.e., in this study the missing data are assumed to depend on age and marital status but not on other variables). The statistical significance of main effects and interactions was assessed using Wald statistics.
Results
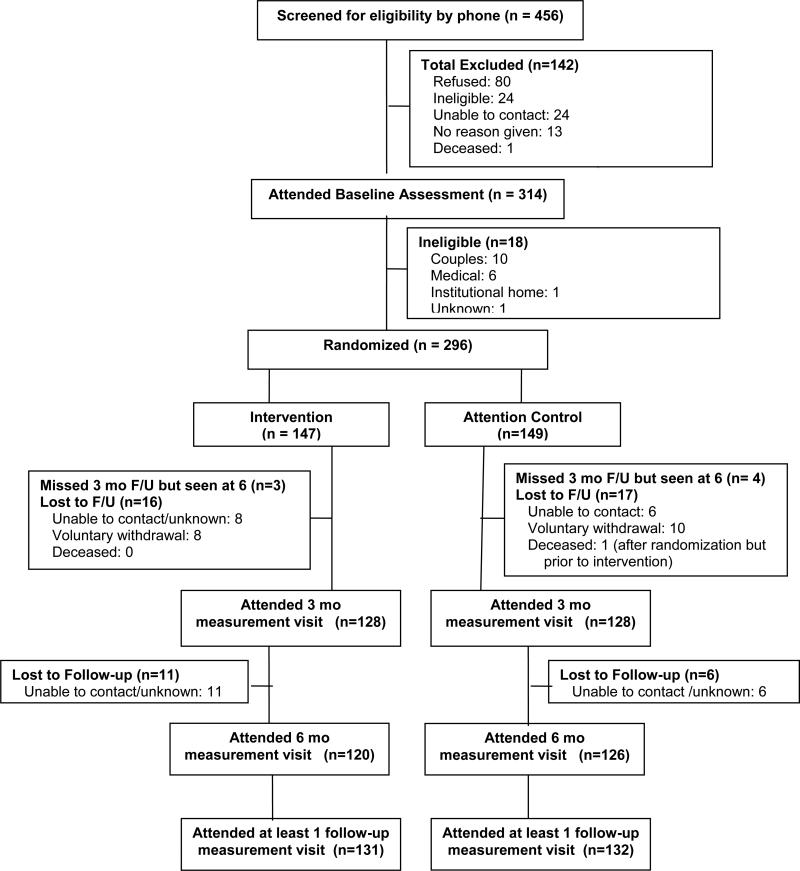
A CONSORT19 diagram describes study recruitment, enrollment, and retention (Figure 1). Of the 296 participants randomized to the study, 256 (86.5%) completed the 3-month assessment and 246 (83.1%) completed the 6-month assessment. Participants who were lost to follow-up were younger and less likely to be married than those who completed the 6-month assessment (p<0.01 for each).
Figure 1.
Summary of ENHANCE Study screening, enrollment, randomization, and follow-up.
Table 1 shows the baseline characteristics of the 263 participants having any follow-up data. The sample tended to be middle-aged; female, and white, insured, employed, and well educated. A majority of individuals (59.7% overall) had good baseline glycemic control (A1c< 8%) and normal kidney function (eGFR ≥ 60 ml/min); 28.1% had poor baseline glycemic control and normal eGFR. Only 12.2% of participants had decreased kidney function, so those with eGFR<60 having good and poor glycemic control were combined for subgroup analyses. The Intervention and Attention Control Groups did not differ significantly on any of these baseline characteristics.
At baseline, 90.4% of participants were prescribed one or more diabetes medications; 69.2% were taking antihypertensive medications; and 56.4% were taking lipid lowering medications (Table 1). Baseline medication regimens did not differ significantly by treatment group (p>0.54 for each).
Table 2 summarizes time-specific mean physiologic outcomes by treatment group. No significant differences were observed in any of these measures at baseline, 3, or 6 months (p>0.06 for each).
Table 2.
Time-specific physiologic outcomes, and within- and between- treatment group mean differences over time (i.e., baseline-3 months and baseline-6 months) in ENHANCE Study participants
| Physiologic Outcome | Time-specific outcomes | Within and between treatment group differences over time | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within group change from baselinec | Between group change from baselined | ||||||||||||||
| Technology Supported Behavioral Intervention (n=131a) | Attention Control (n=132a) | p-valueb | Time Interval | Technology Supported Behavioral Intervention (n=131a) | Attention Control (n=132a) | DiffINT-DiffControl | |||||||||
| Mean | SD | Mean | SD | DiffINT | SD | DiffControl | SD | Mean | 95% CI | p-value | |||||
| A1c (%) | Baseline | 7.7 | 2.2 | 7.5 | 1.7 | 0.49 | |||||||||
| 3m | 7.1 | 1.5 | 7.2 | 1.5 | 0.94 | Base-3m | 0.5*** | 1.5 | 0.3*** | 0.9 | 0.2 | -0.1 | 0.5 | 0.26 | |
| 6m | 7.1 | 1.3 | 7.3 | 1.6 | 0.31 | Base-6m | 0.6*** | 1.8 | 0.2* | 1.3 | 0.4 | 0.01 | 0.8 | 0.046 | |
| Fasting Serum Glucose (mg/dl) | Baseline | 145.3 | 58.1 | 143.1 | 58.1 | 0.78 | |||||||||
| 3m | 131.4 | 47.9 | 136.2 | 49.7 | 0.43 | Base-3m | 14.7** | 52.7 | 6.4 | 46.4 | 8.3 | -4.2 | 20.7 | 0.20 | |
| 6m | 132.6 | 49.1 | 135.2 | 52.1 | 0.69 | Base-6m | 13.2* | 61.0 | 8.0* | 44.9 | 5.2 | -8.7 | 19.0 | 0.46 | |
| Fasting LDL (mg/dl) | Baseline | 107.4 | 40.7 | 108.6 | 34.3 | 0.81 | |||||||||
| 3m | 104.2 | 37.4 | 108.5 | 37.1 | 0.37 | Base-3m | 5.0 | 33.9 | -0.03 | 28.8 | 5.0 | -3.3 | 13.3 | 0.23 | |
| 6m | 102.1 | 32.1 | 106.7 | 37.4 | 0.34 | Base-6m | 3.5 | 27.1 | 0.4 | 30.5 | 3.0 | -4.7 | 10.8 | 0.44 | |
| Fasting HDL (mg/dl) | Baseline | 49.0 | 15.4 | 46.1 | 13.5 | 0.11 | |||||||||
| 3m | 48.2 | 15.0 | 46.8 | 13.5 | 0.43 | Base-3m | 0.8 | 7.7 | -0.4 | 9.4 | 1.3 | -0.9 | 3.4 | 0.26 | |
| 6m | 50.0 | 15.4 | 46.6 | 13.8 | 0.08 | Base-6m | -1.1 | 9.2 | -0.3 | 9.1 | -0.7 | -3.0 | 1.6 | 0.54 | |
| Fasting Triglycerides (mg/dl) | Baseline | 155.0 | 111.6 | 153.7 | 91.7 | 0.92 | |||||||||
| 3m | 142.7 | 81.9 | 149.5 | 79.7 | 0.50 | Base-3m | 14.9* | 83.7 | 1.7 | 60.7 | 13.2 | -4.8 | 31.2 | 0.15 | |
| 6m | 143.7 | 101.8 | 149.4 | 89.5 | 0.64 | Base-6m | 6.3 | 78.8 | 4.4 | 69.2 | 2.0 | -16.9 | 20.8 | 0.84 | |
| Systolic BP (mmHg) | Baseline | 135.2 | 18.7 | 138.7 | 18.9 | 0.14 | |||||||||
| 3m | 133.2 | 20.9 | 136.0 | 19.1 | 0.26 | Base-3m | 2.1 | 17.0 | 3.2+ | 18.7 | -1.2 | -5.5 | 3.2 | 0.60 | |
| 6m | 134.2 | 19.0 | 136.8 | 20.1 | 0.29 | Base-6m | 0.8 | 19.9 | 2.4 | 21.7 | -1.7 | -7.0 | 3.7 | 0.54 | |
| Diastolic BP (mmHg) | Baseline | 75.6 | 9.8 | 75.8 | 10.0 | 0.83 | |||||||||
| 3m | 73.4 | 9.8 | 74.1 | 9.7 | 0.55 | Base-3m | 2.1** | 8.6 | 1.5 | 10.1 | 0.7 | -1.6 | 2.9 | 0.56 | |
| 6m | 74.0 | 10.1 | 74.5 | 10.6 | 0.73 | Base-6m | 1.2 | 10.8 | 1.2 | 10.9 | -0.004 | -2.8 | 2.8 | 0.99 | |
| Weight (kg) | Baseline | 95.1 | 21.7 | 98.3 | 21.5 | 0.24 | |||||||||
| 3m | 93.7 | 20.4 | 97.7 | 21.6 | 0.12 | Base-3m | 0.9** | 3.4 | 0.7** | 2.7 | 0.2 | -0.5 | 1.0 | 0.54 | |
| 6m | 94.3 | 22.7 | 97.3 | 21.3 | 0.26 | Base-6m | 0.6 | 4.6 | 0.8* | 3.8 | -0.1 | -1.2 | 0.9 | 0.78 | |
| Body Mass Index (kg/m2) | Baseline | 34.0 | 7.3 | 35.1 | 7.7 | 0.21 | |||||||||
| 3m | 33.4 | 6.7 | 34.9 | 7.7 | 0.11 | Base-3m | 0.3** | 1.2 | 0.3** | 1.0 | 0.1 | -0.2 | 0.3 | 0.61 | |
| 6m | 33.5 | 7.6 | 34.8 | 7.5 | 0.21 | Base-6m | 0.2 | 1.5 | 0.3* | 1.4 | -0.1 | -0.4 | 0.3 | 0.73 | |
| Waist Circumference (in) | Baseline | 43.6 | 5.8 | 44.8 | 6.9 | 0.12 | |||||||||
| 3m | 43.3 | 5.7 | 44.4 | 7.6 | 0.20 | Base-3m | 0.2 | 2.7 | 0.04 | 2.8 | 0.2 | -0.5 | 0.9 | 0.62 | |
| 6m | 42.9 | 5.8 | 44.3 | 6.1 | 0.07 | Base-6m | 0.5 | 2.8 | 0.5 | 3.3 | -0.03 | -0.8 | 0.8 | 0.94 | |
p=0.05
p<0.05
p<0.01
p<0.001
Assessments at 3 months are missing for 3 Technology Supported Behavioral Intervention and 4 Attention Control Group participants; Assessments at 6 months are missing for 11 Technology Supported Behavioral Intervention and 6 Attention Control Group participants. Other intermittent missing data are rare, except for 8.4% missing waist circumference in the Technology Supported Behavioral Intervention.
Two sample t-tests with bootstrap standard errors
One sample t-tests of difference scores with bootstrap standard errors
Two sample t-tests of difference scores with bootstrap standard errors
Table 2 also summarizes mean differences across time within and between treatment groups. Relative to baseline, the Intervention Group experienced a significant within-group reduction in A1c of 0.5% at 3 and 0.6% at 6 months, and the Attention Control Group experienced a significant within-group reduction in A1c of 0.3% at 3 months (p<0.001 for each). Statistically significant within-group reductions in fasting serum glucose, and diastolic blood pressure were observed in the Intervention Group at 3 months; and significant overall within-group reductions in weight and BMI were observed in both the Intervention and the Control Groups at 3 months. There were no statistically significant between-group differences over time for any of the main outcome variables.
Table 3 summarizes the stratum-specific analyses for the glycemia outcomes (A1c and fasting serum glucose). The greatest reductions from baseline A1c were observed in the stratum with poor baseline glycemic control and normal eGFR, with within-group reductions of 1.5% at 3 months and 1.8% at 6 months in the Intervention participants, and 0.9% at 3 months in the Attention Control participants (p<0.001 for each). At 6 months, fasting serum glucose also decreased significantly from baseline (by 44.7 mg/dl) among Intervention participants with poor baseline glycemic control and normal eGFR. However, none of the corresponding between-group differences over time approached statistical significance.
Table 3.
ENHANCE Study stratum-specific within- and between- group treatment differences in glycemia over time, where strata are defined by baseline A1c and eGFR status. A total of 157 participants had A1c <8% and eGFR ≥ 60 ml/min, 74 had A1c ≥ 8% and eGFR ≥ 60 ml/min, and 32 had eGFR < 60 ml/min.
| Within group change from baselineb | Between group change from baselinec | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physiologic Outcome | Stratum | Time Interval | Technology Supported Behavioral Intervention (n=131a) | Attention Control (n=132a) | DiffINT-DiffControl | |||||
| DiffINT | SD | DiffControl | SD | Mean | 95% CI | p-value | ||||
| A1c (%) | A1c<8%, normal eGFR | Base-3m | -0.003 | 0.8 | 0.1 | 0.6 | -0.1 | -0.3 | 0.1 | 0.55 |
| Base-6m | 0.1 | 0.6 | -0.04 | 0.8 | 0.1 | -0.1 | 0.4 | 0.28 | ||
| A1c≥8%, normal eGFR | Base-3m | 1.5** | 2.2 | 0.9** | 1.4 | 0.6 | -0.2 | 1.4 | 0.15 | |
| Base-6m | 1.8** | 2.9 | 0.8* | 1.9 | 1.0 | -0.2 | 2.1 | 0.10 | ||
| eGFR <60ml/min | Base-3m | 0.5** | 0.8 | 0.5* | 0.9 | -0.01 | -0.6 | 0.6 | 0.97 | |
| Base-6m | 0.5** | 0.7 | 0.5 | 1.0 | 0.002 | -0.6 | 0.6 | 0.99 | ||
| Fasting Serum Glucose (mg/dl) | A1c<8%, normal eGFR | Base-3m | 5.7 | 29.4 | 2.7 | 24.8 | 3.0 | -5.7 | 11.8 | 0.50 |
| Base-6m | -1.7 | 33.8 | 3.1 | 28.0 | -4.8 | -14.7 | 5.1 | 0.34 | ||
| A1c≥8%, normal eGFR | Base-3m | 34.8* | 85.2 | 21.4 | 75.9 | 13.4 | -23.9 | 50.7 | 0.48 | |
| Base-6m | 44.7** | 96.5 | 21.2 | 72.3 | 23.5 | -18.6 | 65.6 | 0.28 | ||
| eGFR <60ml/min | Base-3m | 9.5* | 15.1 | -5.1 | 47.4 | 14.7 | -10.0 | 39.4 | 0.25 | |
| Base-6m | 10.6 | 24.3 | 4.3 | 33.5 | 6.4 | -14.5 | 27.3 | 0.55 | ||
p<0.05
p<0.01
p<0.001
Assessments at 3 months are missing for 3 Technology Supported Behavioral Intervention and 4 Attention Control Group participants; Assessments at 6 months are missing for 11 Technology Supported Behavioral Intervention and 6 Attention Control Group participants.
One sample t-tests of difference scores with bootstrap standard errors
Two sample t-tests of difference scores with bootstrap standard errors
In the random intercept models, the interaction of stratum and treatment was not statistically significant for any of the physiologic outcomes (Table 4), so this term was dropped from the models. The remaining fixed effects were age group, time, treatment, stratum, stratum by time interaction, and treatment by time interaction. The estimated mean differences between otherwise similar participants in the Intervention and Attention Control Groups at baseline, 3 and 6 months are summarized in Table 4. No significant differences were observed at baseline. The estimated mean difference in A1c of 0.29% at 6 months (i.e., A1c decreased an average of 0.29% more in intervention participants than in the Attention Control group) was not significant.
Table 4.
Estimated mean differences in the ENHANCE Study physiologic outcomes between Technology Supported Behavioral Intervention (INT; n= 131) and Attention Control (n=132) participants at baseline, 3 and 6 months, based on random intercept models adjusted for A1c/eGFR status, age group and marital statusa
| Physiologic Outcome | Estimated mean difference at baseline | Estimated mean difference at 3 months | Estimated mean difference at 6 months | Treatment × Time interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| INT-Control | 95% Confidence Interval | p-value | INT-Control | 95% Confidence Interval | p-value | INT-Control | 95% Confidence Interval | p-value | p-value | |
| A1c (%) | 0.01 | -0.27, 0.29 | 0.94 | -0.12 | -0.40, 0.17 | 0.42 | -0.29 | -0.58, 0.0002 | 0.05 | 0.15 |
| Fasting glucose (mg/dl) | -0.56 | -11.07, 9.95 | 0.92 | -8.26 | -18.89, 2.36 | 0.13 | -3.96 | -14.72, 6.81 | 0.47 | 0.42 |
| LDL (mg/dl) | -1.43 | -10.42, 7.57 | 0.76 | -6.02 | -14.92, 2.88 | 0.19 | -5.55 | -14.56, 3.46 | 0.23 | 0.42 |
| HDL (mg/dl) | 3.28 | -0.19, 6.76 | 0.06 | 1.96 | -1.53, 5.44 | 0.27 | 3.77 | 0.26, 7.27 | 0.04 | 0.25 |
| Triglycerides (mg/dl) | 2.21 | -20.73, 25.15 | 0.85 | -10.23 | -33.27, 12.81 | 0.38 | -2.12 | -25.37, 21.12 | 0.86 | 0.38 |
| Systolic BP (mmHg) | -3.55 | -8.19, 1.10 | 0.14 | -2.88 | -7.56, 1.81 | 0.23 | -1.86 | -6.62, 2.90 | 0.44 | 0.79 |
| Diastolic BP (mmHg) | -0.26 | -2.70, 2.19 | 0.84 | -1.00 | -3.47, 1.46 | 0.42 | -0.23 | -2.73, 2.27 | 0.86 | 0.79 |
| Weight (kg) | -3.42 | -8.46, 1.62 | 0.18 | -3.66 | -8.70, 1.38 | 0.16 | -3.26 | -8.31, 1.78 | 0.21 | 0.65 |
| Body Mass Index(kg/m2) | -1.26 | -2.99, 0.47 | 0.16 | -1.33 | -3.06, 0.40 | 0.13 | -1.19 | -2.92, 0.54 | 0.18 | 0.65 |
| Waist Circumference (in) | -0.92 | -2.53, 0.69 | 0.26 | -1.01 | -2.62, 0.60 | 0.22 | -0.80 | -2.42, 0.82 | 0.33 | 0.86 |
Random intercept model with fixed effects of time, treatment group, stratum, and interactions of fixed effects, adjusting for age group and marital status. Statistical significance of main effects and treatment by time interaction assess using Wald statistic. Mean differences (INT-Control) at 3 and 6 months were estimated using linear contrasts.
The data safety and monitoring protocol included prospective assessment of self-reported hypoglycemic episodes requiring 3rd party assistance. Four (2.7%) Intervention Group participants had such an event, compared to 5 (3.4%) Attention Control Group participants (p>0.99). Three (2.5%) Intervention Group participants and 1 (0.8%) Attention Control Group participant gained more than 10 kg (p=0.36).
Discussion
The observed differential reduction of 0.4 in A1c at 6 months was not significant at the a prior-defined level of p<0.01. The investigators returned to the literature to explore the extent to which the size of the reduction is consistent with similar studies. In a meta-analysis of 12 randomized clinical trials of psychological interventions in people with Type 2 diabetes in which glycosylated hemoglobin was reported as an outcome, Ismail et al.24 found an pooled mean difference of -0.32% (95% CI: -0.57 to -0.07). It is important to note that over the years several methods have been developed for the measurement of glycosylated hemoglobin and a single sample can produce widely varying results among methods and laboratories used.25 While caution must be used in drawing firm conclusions about the comparability of ENHANCE A1c reductions to the pooled mean difference found by Ismail et al., the size of the effects observed in ENHANCE is roughly consistent. However, in 11 of the 12 studies reported by Ismail, the mean baseline glycosylated hemoglobin exceed 8%24 while the majority of ENHANCE participants had good baseline glycemic control.
ENHANCE's inability to demonstrate a significant improvement appears to be due to improved glycemia in the Attention Control Group. The Attention Control experience was designed to maintain participant interest in the study rather than promote a healthier lifestyle. Provision of a glucose meter, testing supplies, a pedometer with advice to increase number of steps per day, and 3 educational seminars are not typical of routine diabetes care. In particular, CBG testing strips are expensive and not universally covered under health insurance plans. While the importance of CBG checks is somewhat controversial, 3 recent meta-analyses show such checks to be associated with improved glycemia.20-22 This level of lifestyle intervention may encourage compliance when patients are provided with the tools to achieve self-management goals. Additionally, improvements in glycemic could have resulted from a study reactivity (i.e. Hawthorne) effect. Finally, it is possible that any level of intervention in a predominantly white, female, employed, well-educated, and insured sample of individuals with T2DM who are sufficiently motivated to refer themselves to a lifestyle management study would improve glycemic control.
Stratum-specific analyses showed that both Intervention and Attention Control Group participants with suboptimal baseline glycemic control and normal kidney function experienced significant within-group reductions in A1c, although these did not differ significantly between treatment groups. Regression-to-the-mean likely accounts for some of the observed within-group reductions. Nevertheless, the size of the within-group A1c reductions in this subgroup is somewhat surprising, as they are comparable to those achieved with intensive medication management in the glycemia arm of the ACCORD study, where an absolute A1c reduction of 1.4% was observed at 4 months and 1.7% at one year.26 The ENHANCE study reductions of 1.5% and 1.8% at 3- and 6-mos, respectively, were achieved without the risks for hypoglycemia or weight gain that would be expected with aggressive medication management (which were observed in ACCORD).
There are several limitations to this study. ENHANCE evaluated markers of CVD risk rather than actual CVD outcomes. These self-referred study participants may have been more motivated to manage their disease than the general population of individuals with T2DM. The study was not designed to estimate the separate effects of PDA self-monitoring-only or group sessions-only on the study outcomes. Future researchers may wish to evaluate the efficacy of an intervention that involves self-monitoring alone. It may be useful to evaluate a similar intervention approach in T2DM patients selected on the basis of poor glycemic control, and to compare the intervention group to a control condition that more closely resembles routine care. Finally, PDAs have become an outmoded technology, so future versions of this technology-based behavioral intervention would require adaptation to newer technologies, such as smart phones or tablet PCs with Web-based self-monitoring.
Conclusions
Both behavior-based intervention approaches resulted in within-group improvements in glycemia, weight, and BMI but the between-group differences were not statistically significant. Incorporating newer technologies and comparing subsequent interventions to a control condition that more closely resembles routine care might demonstrate stronger intervention effects.
Acknowledgments
The work of this paper was supported by the following grants: NIH/NINR/NRR01008792, NIH/NCRR/CTSA-UL1-RR024153, NIH/NCRR/GCRC-M01- RR000056, and NIH/NIDDK/DK-046204.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 2.United States Renal Data System . USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda: 2009. [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among Estimated Glomerular Filtration Rate, Proteinuria, and Adverse Cardiovascular Outcomes. Clin J Am Soc Nephrol. 2011;6(6):1418–1426. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standard of Medical Care in Diabetes – 2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dluhy RG, McMahon GT. Intensive Glycemic Control in the ACCORD and ADVANCE Trials. N Engl J Med. 2008;358(24):2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.The ACCORD Study Group Effects of Intensive Blood Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ACCORD Study Group The Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effects of Combination Lipid Therapy on Cardiovascular Events in Type 2 Diabetes Mellitus: The ACCORD Lipid Study. Press release video by Henry Ginsberg at the American College of Cardiology. March 14, 2010. http://www.cardiosource.org/News-Media/Meeting-Coverage/ACC/ACC-2010.aspx. (Accessed February 21, 2012).
- 11.Effects of Intensive Blood Pressure Control on Cardiovascular Events in Type 2 Diabetes Mellitus: The ACCORD Blood Pressure Trial. Press release video by William C. Cushman at the American College of Cardiology. March 14, 2010. http://www.cardiosource.org/News-Media/Meeting-Coverage/ACC/ACC-2010.aspx. (Accessed February 21, 2012).
- 12.Bandura A. Self Efficacy: The Exercise of Control. Freeman and Co.; New York: 1997. [Google Scholar]
- 13.USDA National Nutrient Database for Standard Reference. http://ndb.nal.usda.gov/. (Accessed February 21, 2012).
- 14.Vessby, Uusitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU study. Diabetologia. 2001;44(3):312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 15.Sevick MA, Stone RA, Zickmund S, Wang Y, Korytkowski M, Burke LE. Factors Associated with probability of personal digital assistant-based dietary self-monitoring in those with type 2 diabetes. J Behav Med. 2010;33(4):315–325. doi: 10.1007/s10865-010-9257-9. [DOI] [PubMed] [Google Scholar]
- 16.Sevick MA, Zickmund S, Korytkowski M, et al. Design, feasibility, and acceptability of an intervention using personal digital assistant–based self-monitoring in managing type 2 diabetes. Contemp Clin Trials. 2008;29(3):396–409. doi: 10.1016/j.cct.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 18.StataCorp . Stata: Release 11. Statistical Software. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 19.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. J Clin Epidemiol. 2010;63(8):813–814. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 20.St. John A, Davis WA, Price CP, Davis TM. The value of self-monitoring of blood glucose: a review of recent evidence. J Diabetes Complications. 2010;24(2):129–141. doi: 10.1016/j.jdiacomp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Alleman S, Houriet C, Diem P, Stettler C. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: a systematic review and meta-analysis. Curr Med Res Opin. 2009;25(12):2903–2913. doi: 10.1185/03007990903364665. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch G, Derry S, Moore RA. Self-monitoring of blood glucose in type-2 diabetes: what is the evidence? Diabetes Metab Res Rev. 2007;23(6):423–440. doi: 10.1002/dmrr.749. [DOI] [PubMed] [Google Scholar]
- 23.Estimated Average Glucose (eAG) [January 6, 2012]; http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/estimated-average-glucose.html?&utm_source=offline&utm_medium=print&utm_campaign=eag112009.
- 24.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-Management Education for Adults With Type 2 Diabetes: A meta-analysis of the effect on glycemic control. Diabetes Care. 2001;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 25.Little RR, Rohlfing CL. HbA1c Standardization: Background, Progress and Current Issues. LabMedicine. 2009;40:368–373. [Google Scholar]
- 26.The Action to Control Cardiovascular Risk in Diabetes Study Group Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]