Abstract
Human fetal ethanol exposure is strongly associated with ethanol avidity during adolescence. Evidence that intrauterine olfactory experience influences chemosensory-guided postnatal behaviors suggests that an altered response to ethanol odor resulting from fetal exposure may contribute to later abuse risk. Using behavioral and neurophysiological methods, the authors tested whether ethanol exposure via the dam’s diet resulted in an altered responsiveness to ethanol odor in infant and adult rats. Compared with controls, (a) fetal exposure tuned the neurophysiologic response of the olfactory epithelium to ethanol odor at some expense to its responsiveness to other odorants in infantile rats—this effect was absent in adults; (b) the neural effect in infantile rats was paralleled by an altered behavioral response to ethanol odor that was specific to this odorant—this effect was also absent in adults; and (c) a significant component of the infantile behavioral effect was attributable to ethanol’s effect on the olfactory neural modality. These data provide evidence for an important relationship between prenatal ethanol experience and postnatal behavioral responsiveness to the drug that is modulated or determined by olfactory function.
Keywords: fetal ethanol exposure, optical recordings, whole-body plethysmography, ethanol odor preference, olfactory plasticity
The chemical senses are among the earliest systems to develop (Gottlieb, 1971; Schaal & Orgeur, 1992; Smotherman & Robinson, 1990), and in the chemosensory world of the uterus, they may have a unique salience. This statement is of potential significance to the field of ethanol research given that clinical and epidemiological studies provide strong data for a predictive relationship between prenatal ethanol exposure and the risk for ethanol abuse in adolescent and young adults (Alati et al., 2006; Baer, Bar, Bookstein, Sampson, & Streissguth, 1998; Streissguth, 1998; Yates, Cadoret, Troughton, Steward, & Giunta, 1998).
It is well documented that olfactory function is fundamental to regulating a variety of biological processes, such as reproduction, food intake, and different social behaviors. Even communication between conspecific and heterospecific animals relies heavily on the reception and processing of odors produced by body glands and even feces and urine. Given this profound level of functional importance, not surprisingly, experience-induced plasticity in response to odorants is a means by which the olfactory system can be tuned to emphasize the transduction of stimuli that are deemed relevant within the animal’s environment (Hudson, 1993, 1999). A variety of data indicate that the olfactory system is plastic in response to the odorant environment and have been gathered using different experimental manipulations, such as selective odorant exposure in neonates (Coopersmith & Leon, 1984; Johnson, Woo, Duong, Nguyen, & Leon, 1995; McCollum, Woo, & Leon, 1997; McLean & Harley, 2004; Sullivan & Leon, 1986; Sullivan, McGaugh, & Leon, 1991; Sullivan, Wilson, & Leon, 1989; Woo, Coopersmith, & Leon, 1987; Woo, Oshita, & Leon, 1996) and adults (Rochefort, Gheusi, Vincent, & Lledo, 2002; Salcedo, Zhang, Kronberg, & Restrepo, 2005; Youngentob & Kent, 1995). Furthermore, several lines of evidence demonstrate that postnatal behaviors controlled by chemosensory stimuli can be influenced by intrauterine experiences (Pedersen & Blass, 1982; Schaal, Marlier, & Soussignan, 2000; Stickrod, Kimble, & Smotherman, 1982) that can be retained into adulthood, modulating intake and preference patterns (Bond & DiGiusto, 1976; Chotro, Cordoba, & Molina (1991); Chotro & Molina, 1990; Smotherman, 1982b; Smotherman & Robinson, 1985, 1988, 1990). Preferences for odors experienced in utero, as a function of a mother’s diet, have also been shown to result in stimulus-specific enhancement in the responsivity of the olfactory epithelium (Hudson & Distel, 1998; Semke, Distel, & Hudson, 1995). Consequently, viewing ethanol as an odorant, these observations suggest that potentially salient in utero ethanol cues should result in a tuned olfactory system response both behaviorally and neurophysiologically. This hypothesis has received support through animal and human studies indicating that ethanol-related olfactory memories are expressed in neonates and infants as a function of brief experiences with the drug during late gestation (see reviews: Molina, Spear, Spear, Mennella, & Lewis, 2007; N. E. Spear & Molina, 2005).
Accordingly, the present study tested the hypothesis that gestational ethanol exposure results in an altered odorant-induced behavioral responsiveness to ethanol odor that is mediated by an altered neurophysiologic response of the olfactory epithelium in the early postnatal rat and that these alterations persist into adulthood. Furthermore, we examined whether prenatal exposure affects the generalized responsiveness of the olfactory system to other non-fetal-exposure odorants in these age groups.
Method
Overall Design
We evaluated the neurophysiologic and/or stimulus-induced reflexive sniffing responses of Postnatal Day 15 (P15) and P90 Long-Evans rats (both male and female), using our standard optical recording techniques (e.g., Kent, Mozell, Youngentob, & Yurco, 2003; Youngentob, Kent, Sheehe, Schwob, & Tzoumaka, 1995) and whole-body plethysmography (Youngentob, 2005). The P15 time point was chosen because it represents a middle ground for a number of postnatal ages that display a memory of prenatal ethanol experience (e.g., Abate, Pepino, Dominguez, Spear, & Molina, 2000; Abate, Spear, & Molina, 2001; Chotro, Kraebel, McKinzie, Molina, & Spear, 1996; Chotro & Molina, 1990, 1992; Dominguez, Chotro, & Molina, 1993). P90 was chosen because it is beyond the age of developmental changes seen during adolescence (P28–P42: L. P. Spear, 2000; L. P. Spear & Brake, 1983) and well beyond the postadolescent gray zone (L. P. Spear, 2000).
In this study, each of the P15 and P90 experimental groups consisted of 20 (10 male and 10 female) fetal-ethanol-exposed rats (ET), 20 (10 male and 10 female) pair-fed rats (PF), and 20 (10 male and 10 female) ad-libitum chow-fed rats (CH). Each rat was behaviorally evaluated for its stimulus-induced reflexive sniffing response to ethanol odor and neurophysiologically evaluated for its olfactory epithelial response to ethanol and four additional odorants: heptanal, carvone, ethylacetoacetate, and propyl acetate (see below—Optical Recording of Odorant-Induced Mucosal Activity Patterns—for odorant selection rationale). Consequently, these rats permitted an evaluation of the reflexive sniffing response to ethanol odor as well as an evaluation of the specificity of the neural response to the fetal exposure odorant, ethanol, and of a generalized untoward consequence of prenatal ethanol exposure on the response of the olfactory epithelium.
In addition to the above, 60 additional P15 and 60 additional P90 rats (maternal treatment groups as outlined above) were only behaviorally evaluated for their response to ethylacetoacetate. These rats were included to test for the specificity of the reflexive behavioral response to ethanol odor, if any, since only one behavioral assessment could be made in the previous set of rats, whereas all odorants could be administered for neurophysiologic assessment.
Experimental Treatment of Pregnant Dams
On Gestational Day 5 (G5), pregnant Long-Evans female rats (Harlan-Sprague Dawley, Indianapolis, IN) were weighed and divided into blocks of three weight-matched dams. Female rats within a block were randomly assigned to the ET, PF, or CH maternal treatment group. The ET dams were fed an ad-libitum liquid diet (L10251; Research Diets, New Brunswick, NJ) that provided 35% of daily calories from ethanol (6.7% vol/vol) during G11–G20 (Miller, 1992). Rats were weaned onto the diet from G6 to G10 with diets containing an increasing percentage of ethanol (2.2% vol/vol ethanol on G6–G8 and 4.5% vol/vol ethanol on G9–G10). Peak blood ethanol concentration levels were approximately 150 mg/dl on the evening (i.e., 3 hr after lights out in the vivarium) of G17 (Miller, 1992, and sentinel rats [pregnant dams] assayed in this study). This approach to ethanol administration permitted us to achieve a relatively consistent level of ethanol exposure designed to model moderate-to-high ethanol intake (e.g., Driscoll, Streissguth, & Riley, 1990; Vavrousek-Jakuba, Baker, & Shoemaker, 1991). More importantly, it permitted us to do so during an important time period of olfactory system development, that is, during the time the neural response of olfactory sensory neurons is first detectable in rat fetuses around Embryonic Day 14 (E14; i.e., they begin to transduce sensory information; Gesteland, Yancey, & Farbman, 1982) and just before most of the developing axons of these neurons have reached the developing olfactory bulb, which is beginning as an out-pocketing of the rostral end of the cerebral vesicles around this same time (E14–E15; Farbman, 1991).
As noted, there were two dams within a block that served as controls: the first, a weight-matched rat that was pair-fed relative to its respective ET dam within the block and the second, a weight-matched free-choice rat (CH) that had continuous access to normal lab chow and water. Pair-fed dams (PF), received an isocaloric, isonutritive liquid diet (L100252; Research Diets, New Brunswick, NJ) that was matched to the volume consumed by their respective ET-fed dams on the previous day.
Test Subjects
Within 24 hr of birth, all litters were culled to 10 pups and surrogate-fostered to nonexperimental dams that were fed standard lab chow and water during gestation. To avoid artificially inflating the probability of Type I error by treating littermates as independent observations, only one male and one female per litter were assigned to each specific testing condition (Holson & Pearce, 1992). Consequently, in this study, two randomly selected rats of each sex from any given litter within a triad of ET, PF, and CH dams (i.e., an experimental block for analytic purposes) were utilized, and these rats, in turn, were further randomly allocated to either the P15 or P90 time points. A total of 10 blocks of three dams (ET, PF, and CH) were utilized for the combined behavioral and neurophysiologic experiments, and an additional 10 blocks of dams were utilized for the behavioral control experiment testing the non-fetal-exposure odorant ethylacetoacetate.
Monitoring of Stimulus-Induced Reflexive Sniffing
We applied a procedure for the automated rapid evaluation of olfactory function that is based on a rat’s reflexive sniffing response to odorant stimulation. The theoretical basis and technical details of the method have been previously described (Youngentob, 2001, 2005). Briefly, a continuous-flow RM-80 Respiration Frequency and Volume Monitoring System (Columbus Instruments, Columbus, OH) monitored changes in respiration in response to the presentation of odorant stimuli. The RM-80 measured changes in respiration by detecting minute changes created by the unrestrained rat’s breathing inside a chamber. The system was used with a chamber through which a constant flow of air could pass. As such, odorant stimuli were presented and exhausted from the testing chamber with the same level of stimulus control that is standard in our rigorous psychophysical testing paradigms (e.g., Youngentob, Johnson, Leon, Sheehe, & Kent, 2006; Youngentob, Margolis, & Youngentob, 2001; Youngentob, Markert, Mozell, & Hornung, 1990; Youngentob, Schwob, Sheehe, & Youngentob, 1997). The testing chamber consisted of a Plexiglass cylinder with a conical input and output designed to permit rapid onset and cleanout of each stimulus. The testing chambers for the P15 and P90 rats differed in their internal volumes of 300 cc and 1.3 L, respectively. Furthermore, each testing chamber was paired with a unique reference chamber of equivalent size.
In keeping with previously established methods for the monitoring of stimulus-induced sniffing (Youngentob, 2005), a computer controlled the contingencies required for behavioral testing, odorant stimulus generation, and data collection. Stimuli were generated using a standard flow-dilution olfactometer (e.g., Youngentob & Margolis, 1999; Youngentob et al., 1997) and computer-driven electronic mass flow controllers (Teledyne Co., Hampton, VA). The output flow through an odorant stimulus valve, a prestimulus valve, and both a dummy and a blank valve was a constant 400 cc/min, while a carrier stream was maintained at 3,600 cc/min at all times. During an interstimulus period, flow through the dummy valve was normally open to a common line and carrier airstream, whereas the flow through the other valves was normally closed. Each stimulus presentation was preceded by a prestimulus period during which the dummy valve was closed with the simultaneous activation of the prestimulus valve. For the presentation of a stimulus, the prestimulus valve was closed (and the dummy valve remained closed) simultaneously with the activation of the appropriate stimulus valve (either air or odorant). As a consequence of this valve activation scheme, the total flow delivered to the testing chamber was maintained at a constant 2.0 L/min (4.0 L were split to provide equal airflow to both the subject and reference chambers).
For the combined behavioral/neurophysiologic component of the study (either P15 or P90 age groups), each experimental session consisted of monitoring the stimulus-induced sniffing response to ethanol odor of both a male and a female ET rat, as well as sex- and age-matched controls (PF and CH). The order of testing was randomized. Following a habituation period in the apparatus (40 air-only trials), air and ethanol odor stimuli were presented using a 6 s fixed intertrial interval schedule. At the end of the intertrial interval, a 6 s baseline (prestimulus period) was monitored, and the stimulus presented for 6 s. Stimuli were randomly presented in five blocks of 20 trials (10 air and 10 odorant). Using an ascending series of five odorant concentrations (3.125 × 10−3, 6.25 × 10−3, 1.25 × 10−2, 2.5 × 10−2, and 5 × 10−2 of vapor saturation at 20 °C, respectively), each concentration was presented for one block of trials. For each rat and each stimulus presentation, the sniffing patterns were computer analyzed and the numerical values for 14 respiratory response measures determined: sniff frequency; number of inspiratory and expiratory sniffs; duration, volume, average flow rate, and peak flow rate of an inspiratory and an expiratory sniff; total inspiratory and expiratory volume; and total apneic duration (Youngentob, 2005). At the completion of testing, these same rats were killed, and their mucosal response to odorant stimulation was assessed.
The procedures for the rats receiving only behavioral testing for the odorant ethylacetoacetate were identical to the above with the exception that no neurophysiology was performed at the completion of behavioral testing. Note that the odorant ethylacetoacetate was chosen on the basis of preliminary studies that demonstrated a marked decrease in mucosal responsivity to this odorant as a consequence of prenatal ethanol exposure (Youngentob, Kent, Mooney, Spear, & Molina, 2004). Consequently, behavioral assessment of this odorant provided a stark contrast to our hypothesized effects of fetal ethanol exposure on the response to ethanol odor. The ascending series of five odorant concentrations was 7.81 × 10−4, 1.56 × 10−3, 3.125 × 10−3, 6.25 × 10−3, and 1.25 × 10−2 of vapor saturation at 10 °C, respectively.
Optical Recording of Odorant-Induced Mucosal Activity Patterns
For the combined behavioral and neurophysiologic studies, we mapped the odorant-induced spatial activity patterns across the olfactory mucosa. Using optical recording techniques and a voltage-sensitive dye, we simultaneously monitored 14,400 contiguous sites in a 120 × 120 matrix. Prior studies have shown that this technique provides a fine-grained matrix for analyzing the mucosal response to odorant stimulation (e.g., Kent et al., 2003; Kent, Youngentob, & Sheehe, 1995; Youngentob et al., 1995; Youngentob, Kent, & Margolis, 2003). The preparation of the ex vivo mucosa for recording of the responses, the details of stimulus delivery, and the protocol for recording the mucosal response are identical to our published procedures. Briefly, with the optical magnifications used in this study, a 3.5 mm × 3.5 mm (P15 rats) or a 6.0 mm × 6.0 mm (P90 rats) recording area of the mucosa lining the medial face of the turbinates was imaged onto a 120 × 120 pixel array of a Dalsa 12-bit digital CCD camera (Dalsa, Waterloo, Ontario, Canada). The difference in magnification for the two age groups was required to achieve equivalent anatomical recording areas within the array. Rats were anesthetized with Isoflurane and decapitated, and the extraneous tissue was dissected away, retaining only the nasal cavity and the region from the external naris to the cribriform plate. The right nasal cavity was then split, exposing the medial surface of the turbinates. The turbinates were soaked in the voltage-sensitive dye di-4-ANEPPS according to established protocol. Prior to recording, each tissue was placed in a Delrin chamber and covered with a clear plastic plate. Each tissue was aligned in the CCD camera array relative to a standard preparation outline. The Delrin chamber had both an input and an output. The input port to the chamber was connected to a T connector through which air flowed at 600 cc/min perpendicular to the chamber. A 250-cc/min vacuum applied to the output port drew air through the chamber and across the excised mucosa. During stimulation, computer controlled valves switched the flow through the T connector from deodorized to odorized air for 1 s.
In addition to ethanol odor, the set of odorants for study included carvone, heptanal, propyl acetate, and ethylacetoacetate. Prior work has demonstrated that each of these latter four stimuli produce a distinct mucosal response in the rat (Kent et al., 2003; Youngentob et al., 1995), that these responses can be altered by adult experience (Youngentob & Kent, 1995, and unpublished observations), and that the odorant-specific spatial activity patterns of these stimuli predict perceptual differences among the odorants (Kent et al., 1995, 2003). A single concentration was delivered for each odorant such that the responses fell within the linear portion of the optically recorded odorant concentration response curve (e.g., Kent et al., 2003; Youngentob et al., 1995). The concentrations of the odorants were as follows: ethylacetoacetate—45%, carvone—35%, heptanal—6%, ethanol—33%, and propyl acetate—1.25% of vapor saturation at 21 °C. In addition, a sixth odorant, 1.25% amyl acetate, served as a standard stimulus for correction of tissue responsiveness over time.
Each experimental session consisted of recording the mucosal responses from the turbinate surfaces of the behaviorally tested ET, PF, and CH rats. The order of rat neurophysiologic testing followed that of the behavioral testing. For each tissue, a recording session consisted of the randomized presentation of the five odorants (one replication of each odorant). In addition, the odorant standard, amyl acetate, was presented at the beginning and end of a tissue recording session to adjust for gradual changes in mucosal responsiveness that occurred over time. All raw responses were corrected for baseline shifts due to photo bleaching of the dye during the experiment, and the responses were adjusted in accordance with the levels of background fluorescence.
For each stimulus presentation and each of the 14,400 contiguous pixels of the camera’s array, we characterized the neurophysiologic response by determining two measures of the magnitude of the response (i.e., the average response and peak response heights) and three temporal measures (latency; i.e., start, peak, and end times).
Results
Behavioral Analysis
The overall focus of our behavioral analysis was directed toward examining whether in utero ethanol exposure as a consequence of the dam’s diet altered the stimulus-induced reflexive sniffing response of both the infantile and adult rat to ethanol odor. In this respect, it must be emphasized that our previous work has demonstrated that although examination of the individual characteristics of sniffing can be quantitatively useful in determining the physiologic values that rats generate while sampling odors, knowledge about any single characteristic is insufficient to describe the complex pattern of response to odorant stimulation (Youngentob, 2005; Youngentob, Mozell, Sheehe, & Hornung, 1987). Accordingly, to accomplish our evaluation of stimulus-induced reflexive sniffing in the P15 and P90 rats: first, we applied principal components analysis (PCA) to our complex patterns of sniffing behavior to reduce the high dimensionality (i.e., 14) of the respective P15 or P90 data sets to a lesser number of orthogonal (uncorrelated) dimensions (Morrison, 1967); second, using least squares multiple regression, we then evaluated the resultant factors of the PCA analysis to test which, if any, met our predictive error criterion for variable selection (F ≥ 2.0; Mozell, Sheehe, Swieck, Kurtz, & Hornung, 1984). As outlined below, the results from this component of the analysis provided a single univariate measure of the rats’ sniffing behavior for each stimulus presented. These data, in turn, were used to evaluate the consequences of maternal treatment on the reflexive sniffing patterns to a concentration series of ethanol odor.
P15 rats
Only the first factor of the PCA met our predictive error criterion for variable selection. As such, the values from the first factor at each concentration of odorant were used to define an individual rat’s stimulus-induced reflexive response to stimulus presentation.
Figure 1 illustrates the concentration response curve of the ET, PF, and CH rats to ethanol odor, showing the mean and standard error of the reflexive sniffing index for the P15 age group as a function of stimulus concentration. Qualitatively, the behavioral results suggest there was a clear shift in the reflexive response to ethanol odor in the ET rats, relative to the PF and CH treatment groups, at each of the five different odorant concentrations tested. Furthermore, there appeared to be no difference between the responses of the PF and CH rats. In interpreting this figure, it must again be emphasized that our analytic approach was directed toward identifying complex patterns in our data set and expressing them in such a way as to highlight differences, if any, as a function of maternal treatment. Thus, in viewing Figure 1, it is the magnitude of the difference, rather than the direction of the difference, in mean index values at each concentration between the treatment groups that is critical to this determination. Randomized-blocks analysis of variance (ANOVA) demonstrated a highly significant overall effect of maternal treatment on the reflexive sniffing response to ethanol odor in the P15 rats, F(2, 285) = 12.76, p < .0001. Furthermore, there was both an average overall sex difference, F(1, 285) = 4.55, p = .033, and a Sex × Maternal Treatment interaction, F(1, 285) = 3.60, p = .028.
Figure 1.
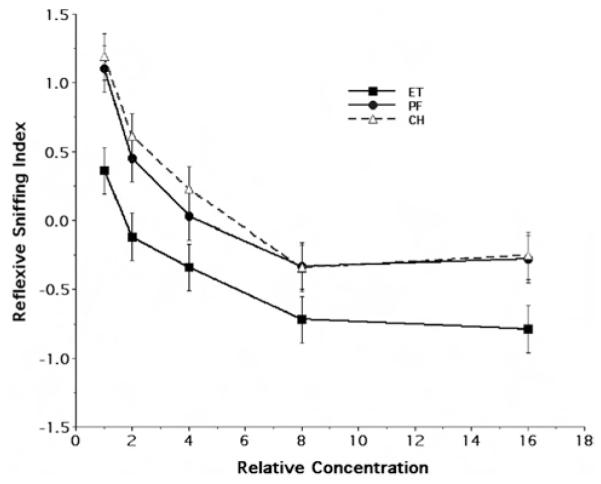
Behavioral response to ethanol odor at Postnatal Day 15. The figure illustrates the concentration response curves for ethanol odor, showing the mean (± SE) reflexive sniffing index values as a function of relative odorant stimulus concentration and maternal treatment group (Steps 1, 2, 4, 8, and 16 refer to odorant concentrations of 3.125 × 10−3, 6.25 × 10−3, 1.25 × 10−2, 2.5 × 10−2, and 5 × 10−2 of vapor saturation at 20 °C, respectively). ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
To further explore the foregoing results, we first examined the lack of an apparent difference in responsivity between the PF and CH rats. Post hoc analysis of treatment means using Tukey’s multiple comparisons criterion suggested there was no evidence of a differential effect of maternal treatment on the reflexive sniffing response between the PF and CH rats (mean difference = 0.090, p = .763). By contrast, the ET rats were significantly different from both the PF (mean difference = 0.514, p < .0003) and CH (mean difference = 0.604, p < .0001) groups. Finally, as illustrated in Figure 2, the observed Sex × Maternal Treatment interaction appeared to be the result of a prominent average difference in reflexive responsiveness of the male PF rats, relative to the female PF rats, to ethanol odor. Taken together, these results suggest there was a unique impact of fetal ethanol exposure on the responsiveness to ethanol odor in the early postnatal rat.
Figure 2.
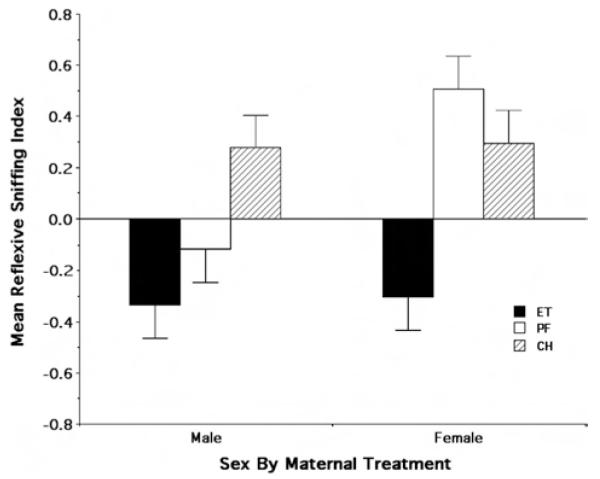
Reflexive sniffing index values averaged across ethanol odorant concentrations as a function of sex and maternal treatment. The values represent the mean (± SE) reflexive sniffing index values. ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
Owing to its potential teratogenicity, fetal exposure to ethanol is known to result in any number of developmental sequelae depending on the timing and dose of the exposure. Therefore, to test the behavioral specificity of the above differential effect on the response to ethanol odor, a separate series of P15 rats were examined for their reflexive sniffing response to the odorant ethylacetoacetate. It should be emphasized that none of the three maternal treatment groups received any form of prior exposure (fetal or otherwise) to this odorant. Figure 3 illustrates the mean and standard error of the reflexive sniffing index of the P15 ET, PF, and CH treatment groups as a function of stimulus concentration. As can be seen in this figure, although there was a clear differential response in each treatment group to the various odorant concentrations presented, by comparison to Figure 1, there was no qualitative evidence of a differential effect of prior fetal treatment. There appears to be substantial overlap in the response of the three treatment groups at each of the five concentrations of odorant tested, with the concentration response curve for the ethanoltreated rats fundamentally located between those for the PF and CH rats.
Figure 3.
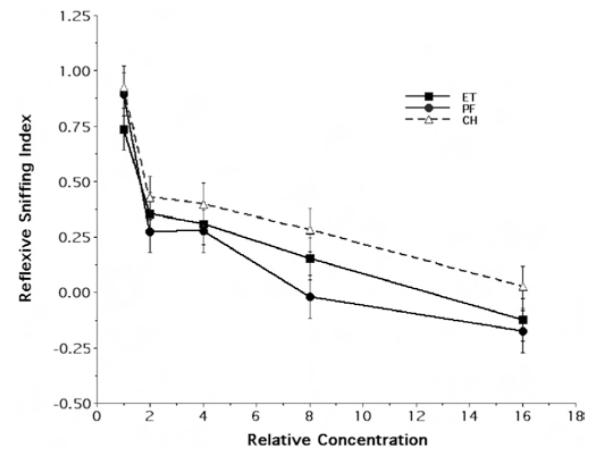
Behavioral response to ethylacetoacetate odor at Postnatal Day 15. The figure illustrates the concentration response curves for ethylacetoacetate odor, showing the mean (± SE) reflexive sniffing index values as a function of relative odorant stimulus concentration (Steps 1, 2, 4, 8, and 16 refer to odorant concentrations of 7.81 × 10−4, 1.56 × 10−3, 3.125 × 10−3, 6.25 × 10−3, and 1.25 × 10−2 of vapor saturation at 10 °C, respectively). ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
Randomized-blocks ANOVA confirmed this subjective impression. The main factors under consideration (maternal treatment and sex), as well as the interaction between these factors, exerted no significant effects upon the reflexive response to the nonexposure odorant ethylacetoacetate.
P90 rats
As was the case for the P15 rats, only the first factor of the PCA for the P90 data set met our predictive error criterion for variable selection. Therefore, in keeping with the above, the first factor values were again used to define our univariate measure of response behavior, or reflexive sniffing index, for each stimulus presentation.
Figure 4 illustrates the concentration response curves for the ET, PF, and CH rats, respectively, to ethanol odor, showing the mean and standard error of the reflexive sniffing index of the P90 age group as a function of stimulus concentration. As expected, the rats in each of the three maternal treatment groups demonstrated a concentration-dependent shift in their reflexive response with increasing odorant concentration. Nonetheless, the curves of the ET and PF groups are completely overlapping, with the added suggestion that the CH rats overlap with both these groups as well. Randomized-blocks ANOVA provided no strong evidence for an overall effect of treatment on the reflexive sniffing response to ethanol odor, F(2, 285) = 2.80, p = .062. Furthermore, there was no overall sex difference, F(1, 285) = 1.87, p = .173, and no strong evidence of a Sex × Maternal Treatment interaction, F(1, 285) = 2.79, p = .063.
Figure 4.
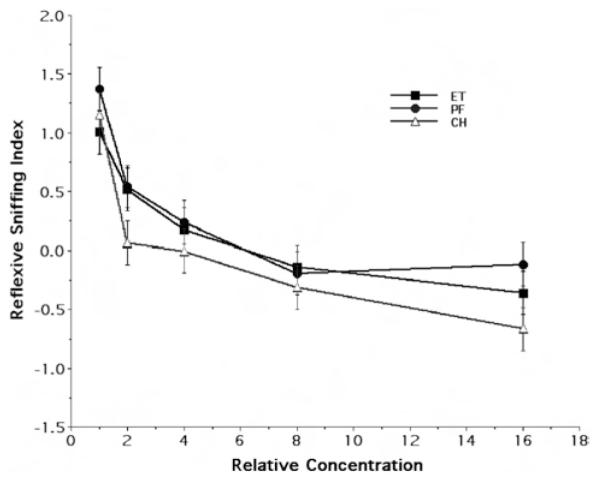
Behavioral response to ethanol odor at Postnatal Day 90. The figure illustrates the concentration response curves for ethanol odor, showing the mean (± SE) reflexive sniffing index values as a function of relative odorant stimulus concentration and maternal treatment group (Steps 1, 2, 4, 8, and 16 refer to odorant concentrations of 3.125 × 10−3, 6.25 × 10−3, 1.25 × 10−2, 2.5 × 10−2, and 5 × 10−2 of vapor saturation at 20 °C, respectively). ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
In keeping with our procedures for the P15 time point, we also examined an additional set of adult rats for their reflexive sniffing response to the odorant ethylacetoacetate. Again, it should be noted that none of the maternal treatment groups received any prior exposure to this odorant. Figure 5 illustrates the mean and standard error of the reflexive sniffing index of the P90 ET, PF, and CH rats in response to the different concentrations of odorant. As can be unambiguously seen in this figure, there is no qualitative evidence of a differential effect of prior maternal fetal treatment. Indeed, the curves for the three fetal exposure groups are demonstrably overlapping. This observation was further emphasized quantitatively. No significant main effects of the factors under consideration or of the interaction between them were observed when inferentially analyzing responsiveness to ethylacetoacetate.
Figure 5.
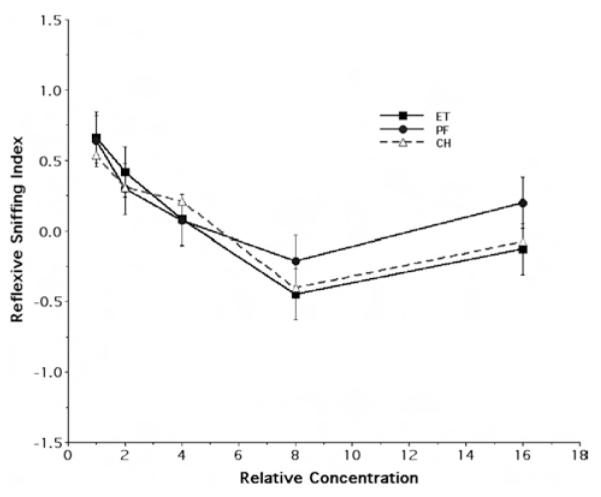
Behavioral response to ethylacetoacetate odor at Postnatal Day 90. The figure illustrates the concentration response curves for ethylacetoacetate odor, showing the mean (± SE) reflexive sniffing index values as a function of relative odorant stimulus concentration (Steps 1, 2, 4, 8, and 16 refer to odorant concentrations of 7.81 × 10−4, 1.56 × 10−3, 3.125 × 10−3, 6.25 × 10−3, and 1.25 × 10−2 of vapor saturation at 10 °C, respectively). ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
Neurophysiologic Analysis
As noted earlier, at the completion of the behavioral testing, each ET rat and an age-matched PF and CH rat were sacrificed and their mucosal activity patterns recorded. The focus of our method was twofold: (a) to explore the specificity of an altered responsiveness, if any, to the in utero exposure odorant, as well as the overall responsivity of the epithelium to the four non-fetal-exposure test odorants; and (b) to examine whether fetal ethanol exposure altered the distinctiveness of each test odorant’s characteristic area of maximal sensitivity on the mucosa.
P15 rats
The neural response of the olfactory epithelium has both temporal and response magnitude components. As such, as described in the Method section, we characterized the response to odorant stimulation in terms of two measures of response magnitude and three temporal measures of the response. Following the same logic as outlined for the behavioral data, our approach focused on encapsulating the five measures (i.e., dimensions) of the mucosal response into a lesser number of orthogonal (uncorrelated) dimensions. Thus, we performed a PCA of the standardized measures of the neural responses. Using the same acceptance criterion as was applied to the behavioral results, only the first factor of the PCA met our predictive error criterion for variable selection (F ≥ 2.0; Mozell et al., 1984). Consequently, the values of the first factor for each odorant were used to define a single measure of the rats’ neural response for each stimulus presented. These data, in turn, were used to evaluate the consequences of maternal treatment.
Figure 6 provides the qualitative impression of a differential effect of prenatal exposure on the responsivity of the epithelium to different odorants. For each of the four non-fetal-exposure odorants, the neural response index of the P15 ET rats was altered in the same direction relative to the PF and CH controls. By contrast, inspection of Figure 6 suggests a similarity in the responsivity to ethanol odor across maternal treatment groups. In interpreting Figure 6, note again that it is the magnitude of the difference between treatment groups in mean neural index values for each odorant that is critical to evaluating the effect of fetal experience. As expected from previous work on the response of the rat olfactory mucosa, randomized-blocks ANOVA demonstrated there was a highly significant effect of odorant on the relative response, F(2, 273) = 45.01, p < .0001. Moreover, there was strong evidence for an overall differential effect of maternal treatment, F(2, 273) = 7.18, p < .0001, with no evidence of an overall main effect of sex or any first-order interactions. Further post hoc analysis of treatment means using Tukey’s multiple comparisons criterion suggested there was no evidence of a differential effect of treatment on the neural responsivity index between the PF and CH rats (mean difference = 0.008, p = .996), whereas, by contrast, the ET rats were significantly different from both the PF (mean difference = 0.354, p = .0033) and CH (mean difference = 0.363, p = .0026) groups.
Figure 6.
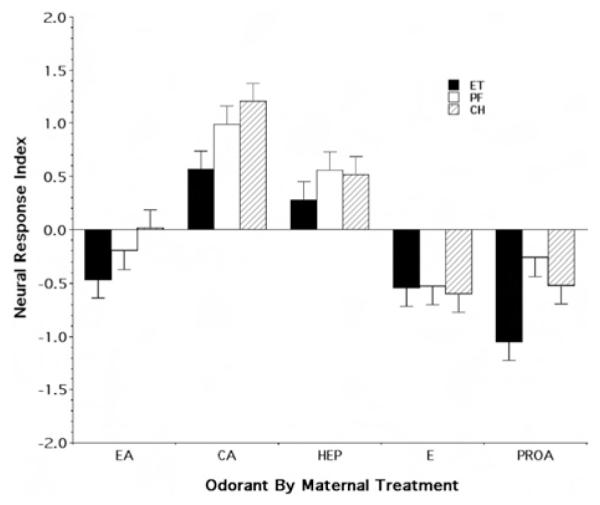
Relative neural response as a function of odorant and maternal treatment group for the Postnatal Day 15 group. The figure illustrates the mean (± SE) index values for the neural response index. EA = ethylacetoacetate; CA = carvone; HEP = heptanal; E = ethanol; PROA = propyl acetate; ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
As indicated above, although the results of our formal analysis found no evidence for an average overall Odorant × Treatment interaction, nonetheless, inspection of Figure 6 is suggestive of a specific interactive effect. To evaluate this observation, an exploratory analysis was performed to test for the specific interaction between the effect of maternal treatment on the response to ethanol odor and the average effect on the response to the nonexposure odorants. That is, we tested the hypothesis that there was no differential in the effects of maternal treatment on the response to ethanol odor relative to these effects on the response to the other odorants. The result of this analysis is consistent with our descriptive observation (see Figure 6) of a preservation of ethanol responsivity in the ET group against a background of untoward (see exploratory analyses below) effects on the response to the remaining odorants (mean ET vs. control difference: ethanol odor = 0.28, nonexposure odorants =−1.872), t(184) = 1.983, nominal p = .043.
To provide broader interpretability to the above results (e.g., explore the directionality of the observed effects) based on the neural response index values, we also performed individual exploratory ANOVAs on the component variables that make up the index. Of the five descriptors of neural response, peak magnitude, average response magnitude, latency of the response initiation, and latency of the response termination, all significantly varied as a consequence of prenatal treatment, F(2, 273) = 7.44, nominal p < .0001; F(2, 273) = 7.85, nominal p < .0001; F(2, 273) = 4.83, nominal p = .009; and F(2, 273) = 3.27, nominal p = .037, respectively. Moreover, the odorant by maternal treatment response profiles of each parameter of the neural response demonstrated the same relative relationships (data not shown) as the neural response index illustrated in Figure 6. By contrast, the time to reach the peak magnitude of the response did not vary significantly as a function of prenatal treatment. Even so, the five-descriptor model did a slightly better job in capturing the variance due to maternal treatment effects than a four-descriptor model that did not incorporate the time to the peak response, F(2, 273) = 7.18 vs. F(2, 273) = 6.99, both nominal ps < 0.001. In short, the foregoing results suggest there was a unique impact of fetal ethanol exposure on the neural responsiveness to both ethanol odor and the four non-fetal-exposure odorants in the early postnatal rats.
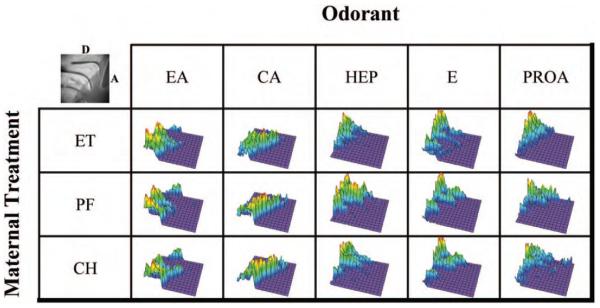
It is well established that different odorants produce different spatial patterns of neural activity across the olfactory epithelium (e.g., Kauer & Moulton, 1974; MacKay-Sim & Kesteven, 1994; MacKay-Sim & Shaman; 1984; Youngentob et al., 1995) and that these differential spatial patterns predict psychophysically determined odorant quality perceptual relationships (Kent et al., 1995, 2003). To evaluate the consequence of prenatal ethanol exposure on the spatially distinct odorant-induced mucosal activity patterns (i.e., “hot spot”; Youngentob et al., 1995) in the P15 rats, the regions of differential responsivity relative to the standard stimulus, amyl acetate, were highlighted by subtracting the equilibrated peak response (equilibration removes differences in overall response magnitude between arrays so that magnitude differences are not confused with pattern differences) for each of the five odorants from the equilibrated response to amyl acetate on a pixel-by-pixel basis (Kent et al., 1995; Loo, Youngentob, Kent, & Schwob, 1996; Youngentob & Kent, 1995; Youngentob et al., 1995). However, only those pixels that demonstrated relatively greater activity were considered (those that showed decreased sensitivity relative to amyl acetate were set to zero). These data were then transformed into enhanced color scale surface plots such that the magnitude of the difference of increased relative responsivity for each pixel of the 120 × 120 optical recording array was represented by the height on the z-axis. As can be seen in Figure 7, each odorant gave a qualitatively different pattern of response, or hot spot, that distinguished it from every other odorant. In this respect, although there were negligible variations across rats in the shape of increased activity for each of the odorants tested, the unique geographic region was, for all rats tested, in a qualitatively similar area of the mucosa (data not shown). As such, the characteristic area of increased activity for each odorant appears similar across the three maternal treatment conditions. This suggests that both the geographic component of the differential spatial response patterns (a hypothesized component of peripheral odorant quality coding; e.g., Kauer & Moulton, 1974; Kent et al., 1995, 2003; MacKay-Sim & Kesteven, 1994; MacKay-Sim & Shaman; 1984; Youngentob et al., 1995) and the primary receptor distribution patterns (Iwema, Fang, Kurtz, Youngentob, & Schwob, 2004; Youngentob et al., 1995) were not altered as a function of maternal treatment.
Figure 7.
Composite color scale enhanced surface plots for the turbinate mucosa for each maternal treatment group in response to ethylacetoacetate (EA), carvone (CA), heptanal (HEP), ethanol (E), and propyl acetate (PROA). The height of the z-axis corresponds to a change in response for a given odorant at a particular pixel compared with the standard, amyl acetate, for that pixel after the entire array of responses have been equilibrated to a value of 100%. Black-and-white panel (upper left corner) illustrates the turbinate mucosa and the orientation of the response panels. A = anterior; D = Dorsal; ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
The panels in Figure 7 also illustrate the typical nonuniformities in responsivity (or differential spatial response) within the regions of relatively greater activity for an individual odorant. Nonetheless, the data also give the impression that the ET, PF, and CH rats did not differ with respect to the degree of relative differential responsivity (i.e., distinctiveness) for each odorant. To assess this observation, the distinctiveness of an odorant’s differential mucosal peak response for each rat was quantitatively expressed as the average percentage difference (APD; Kent & Mozell, 1992; Loo et al., 1996). For each of the rats, the APD for a given odorant was calculated by subtracting, pixel-by-pixel, the equilibrated individual odorant pattern of interest from the equilibrated response pattern of the odorant standard, amyl acetate (as above); calculating the sum of the value of the differences across the pixels greater than zero; dividing by the number of contributing pixels; and multiplying by 100. This APD expression, therefore, quantified, pixel-by-pixel, the APD between the odorant of interest and the standard in a particular rat. As expected, randomized-blocks ANOVA showed a highly significant effect of odorant on the APD, F(4, 273) = 17.96, p < .0001. There was no significant effect of prenatal treatment on the distinctiveness of the odorants’ unique spatial activity pattern. In addition, sex and Sex × Treatment or Odorant × Treatment interactions exerted no significant effects. Thus, it appears that although maternal treatment had an effect on the responsivity of the olfactory epithelium in the P15 rat to odorant stimulation, there was no concomitant effect on the characteristic patterns of spatial activity for these same odorants.
P90 rats
The neurophysiologic data acquired from the P90 rats were subjected to the same analyses as those described for the P15 rats. As with the behavioral effects, our interest here was in examining whether the early neural effects on the offspring of female rats exposed to ethanol during gestation were consolidated with age.
PCA of the standardized measures of the neural responses yielded two factors, neither of which met our predictive error criterion for variable selection with respect to maternal treatment effects. Thus, to continue with our nominal evaluation of the P90 rats, the values for the first factor (which had the larger F of the two factors) were used to define our measure of the mucosal response to each odorant presented. Not surprisingly, given the results of the PCA, Figure 8 unambiguously demonstrates that the differential effect of prenatal exposure observed in the P15 rats ameliorated with time. For each of the five odorants tested, the relative responsivity of the P90 ET rats appears similar to both the PF and CH controls. Indeed, randomized-blocks ANOVA indicated no significant effects of maternal treatment or sex, or any first-order interactions.
Figure 8.
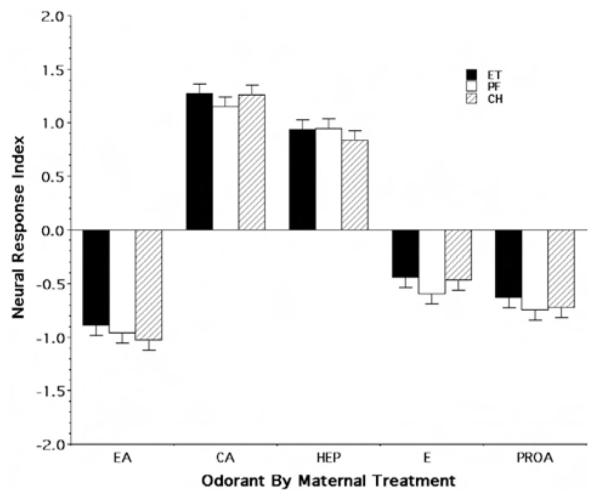
Relative neural response as a function of odorant and maternal treatment group for the Postnatal Day 90 group. The figure illustrates the mean (± SE) index values for the neural response index. EA = ethylacetoacetate; CA = carvone; HEP = heptanal; E = ethanol; PROA = propyl acetate; ET = fetal-ethanol-exposed rats; PF = pair-fed rats; CH = chow-fed rats.
Similar to the results for the P15 rats, we could find no evidence of an alteration in the spatially distinct odorant-induced mucosal activity patterns in the P90 age group. The characteristic area of increased activity for each odorant was similar across the three maternal treatment conditions, and the distinctiveness of an odorant’s differential mucosal response, as expressed by the APD measure, was similar across maternal treatments. Randomized-blocks ANOVA showed an expected significant effect of odorant on the APD, F(4, 272) = 64.21, p < .0001. No other significant main effects or interactions were encountered when analyzing the dependent variable under consideration.
The Mucosal Effect on Reflexive Sniffing Behavior as a Result of Maternal Treatment
The overarching hypothesis of this study is that prenatal ethanol exposure, as a consequence of the dam’s diet, results in an altered behavioral responsiveness to ethanol odor that is mediated, either in whole or in part, by an altered neurophysiologic response of the olfactory epithelium. Thus, as outlined below, our analytic approach was directed toward estimating and testing the significance of the component of behavioral effect we observed in the P15 rats that was attributable to the effect of fetal ethanol exposure on the peripheral olfactory neural modality. Recall that, for each rat, we obtained five behavioral response values to ethanol odor (i.e., from the behavioral PCA, a univariate measure of reflexive response behavior for each concentration of odorant presented) and five neural response values (i.e., from the neural PCA, a single neural responsivity index for each of the five odorants tested). To construct a behavioral composite index for each rat (i.e., one value that incorporates the rat’s behavioral response across all concentrations tested), as well as a corresponding composite mucosal response index for the same rat (i.e., one value that incorporates the rat’s mucosal response across all odorants tested), the following procedures were used: (a) With maternal treatment as the dependent variable, we performed a multiple regression analysis using each rat’s five odorant-induced reflexive index response measures (i.e., one for each concentration of the odorant ethanol) as the regressors in the analysis. The composite behavioral index was defined as the linear compound of the constant plus the five coefficients applied to their respective five reflexive sniffing index measures. (b) Similarly, with maternal treatment as the dependent variable, we performed a multiple regression analysis using each rat’s olfactory neural response index measure to each of the five odorants as the regressors. The mucosal response index for each rat, in turn, was the linear compound of the constant plus the five coefficients applied to the respective five neural response measures. To estimate the coefficient of the mucosal response index so as to predict changes in the composite behavioral response, we performed an analysis of covariance (ANCOVA) to test H0: β= 0, where β is the coefficient of X in the regression of Y (i.e., the average of the male and female composite index behavioral responses for a particular maternal treatment within a block) on X (i.e., the average of the male and female composite neural response indexes for a particular maternal treatment within a block) responses in the following model: Yhj =μ+βXhj + blocks + εhj, where blocks is treated as a categorical variable. The results demonstrated a significant regression, F(1, 28) = 4.719, p = .038, where the estimate of β= .379. With regard to this analysis, it should be emphasized that it is not sufficient just to test the significance of the slope (as was done in the ANCOVA) to evaluate the relationship between the behavioral and neural effects. Rather, the magnitude of the effect of prenatal ethanol exposure on the composite neural response index also has to be estimated. Thus, in this analysis, the differential neural effect between two treatment groups (i.e., μX1–μX2) times the slope (i.e., β) will estimate the effect of prenatal exposure on behavior by way of the olfactory neural modality. To test H0: (μX1–μX2)β= 0, we compared the average composite neural response index of the ET rats (M ± SE = 0.545 ± 0.047) with the mean of the average composite neural responses of the PF and CH controls since an exploratory ANOVA of treatment effects on the composite neural index indicated there was no difference between the PF and CH rats (M ± SE = 0.721 ± 0.047 and 0.728 ± 0.047, respectively). Of major importance for the present study, the results of this analysis demonstrated that a significant component of the observed composite behavioral effect was attributable to ethanol’s effect on the olfactory neural modality, t(18) =−3.21, two-tailed p = .005.
By contrast to the P15 rats and not surprisingly based on the P90 neurophysiological results, we found no relationship between the P90 composite behavioral and neural effects based on maternal treatment, F(1, 28) = 0.067, p = .798, where the estimate of β= .133. As such, we did not further evaluate the mucosal effects on the reflexive sniffing behavior.
Discussion
A substantial literature has developed around the question of what the fetus learns behaviorally about odor contamination of the prenatal environment. These studies have demonstrated the general capacity of the fetus both to process salient chemosensory cues present in the fetal environment and to, perhaps, retain this information over a significant time span (Schaal et al., 2000; Smotherman, 1982a, 1982b; Smotherman & Robinson, 1985, 1987, 1988, 1990; Stickrod et al., 1982). The response to fetal ethanol exposure follows suit with these other chemosensory stimuli, although the results have differed by time, exposure method, and age of evaluation. It is clear, however, that fetal experience with ethanol can modulate infantile responsiveness to certain attributes of the drug (between P8 and P15). The fetus can both acquire information about ethanol’s orosensory cues and display a memory of the prenatal experience with the drug in terms of (a) changes in orienting response to ethanol odor (measured by an autonomic response: Chotro & Molina, 1992; Chotro et al., 1996), (b) enhanced consumption of an ethanol solution (Chotro & Arias, 2003; Chotro & Molina, 1990; Dominguez et al., 1993; Dominguez, Lopez, & Molina, 1998; Molina, Chotro, & Dominguez, 1995), (c) enhanced ethanol odor preference (Chotro et al., 1996; Chotro & Molina, 1990), and (d) ethanol’s ability to act as an unconditioned stimulus that mediates associative learning processes (Abate et al., 2000, 2001). By contrast to these early postnatal observations, adult acceptance and responsiveness to the chemosensory attributes of the drug are less clear. Few studies have tested the effect of prenatal ethanol on later intake. By contrast to the infantile outcomes, adult studies have been quite varied in both implementation and outcome, with some indicating increased adult intake (Bond & DiGiusto, 1976; Phillips & Stainbrook, 1976), others suggesting no enhancement (Abel & York, 1979; McGivern, Clancy, Mousa, Couri, & Noble, 1984), and even others suggesting limited increases (Randall, Hughs, Williams, & Anton, 1983; Reyes, Garcia, & Jones, 1985). Moreover, the consequence of fetal exposure on adult responsiveness to ethanol odor per se has not been subject to published investigation.
Given the above, how might prenatal exposure to ethanol increase its later acceptance? In considering this question, the field of fetal ethanol learning has generally considered the status of olfactory system development in terms of the preparedness of the animal to process stimuli. However, there has been a critical lack of data examining the relationship between olfactory system function and responsiveness to ethanol odor as a consequence of early drug experiences. The results of the present study significantly contribute to an understanding of two theoretical perspectives that have been suggested for later ethanol acceptance, namely, that the simple fetal experience with a food source enhances its liking and, thus, its later ingestion and that during exposure, the fetus acquires an association between ethanol’s orosensory cues (odor/taste) and the drug’s reinforcing properties (N. E. Spear & Molina, 2005). As outlined below, these may not be mutually exclusive processes. With fetal experience comes the potential for stimulus-induced neural plasticity, a fundamental mechanism for which the olfactory system (and odor is an important component of flavor perception) is well organized (e.g., Coopersmith & Leon, 1986; Hudson & Distel, 1998; McLean & Harley, 2004; Salcedo et al., 2005; Semke et al., 1995; Sullivan & Leon, 1986; Sullivan et al., 1989, 1991; Wilson, Guthrie, & Leon, 1990; Woo et al., 1987).
In the present set of experiments, we demonstrated that prenatal ethanol exposure resulted in an alteration in olfactory system function that was manifested both behaviorally and neurophysiologically in the early postnatal rat and that these effects declined by adulthood. This general result is in keeping with the infantile literature (Chotro & Arias, 2003; Chotro & Molina, 1990; Dominguez et al., 1998; Molina et al., 1995) and at least one adult study using a similar exposure paradigm and tests early in development as well as later (Randall et al., 1983). Specifically, compared with isocaloric PF and CH controls, (a) experimental rats exposed to ethanol throughout gestation via the dam’s diet showed a tuned neurophysiologic response of the olfactory epithelium to ethanol odor at some expense to the general responsivity of the system to other odorants; (b) the neural tuning was evident in the early postnatal rat, but this effect was absent by adulthood; (c) the observed neural effect in the infantile rat was paralleled by an altered odorant-induced reflexive sniffing response to ethanol odor; (d) this behavioral effect was both specific to ethanol odor and also declined by adulthood; and (e) a significant component of the observed infantile behavioral effect was attributable to ethanol’s effect on the olfactory neural modality. Taken together, the data suggest that fetal experience-induced olfactory plasticity, in response to ethanol, is a means by which olfactory system function becomes tuned (both behaviorally and neurophysiologically) to emphasize the transduction of ethanol’s chemosensory attributes. Consequently, these effects could either underlie or work in concert with the reinforcing properties of ethanol (Cunningham, 1998; Cunningham, Clemans, & Fidler, 2002; Molina, Ponce, Truxell, & Spear, 2006) to establish the previously described enhanced behavioral responsiveness to ethanol odor as a result of fetal experience. From a clinical perspective, an enhanced preference for ethanol odor may be an important contributor to the risk for an enhanced postnatal avidity for the drug.
In considering the foregoing interpretation, two issues require consideration. The first is the behavioral valence of the observed shift in odorant responsivity we observed, and the second is our definition of neural tuning. It should be emphasized that the plethysmograph data, in and of itself, did not ascribe a valence to the observed shifts in behavioral responsivity that was specific to ethanol odor in the P15 rat. Nonetheless, several lines of evidence give strong support to the interpretation that our behavioral observations (and the neural effects) represent an enhanced olfactory system responsiveness to ethanol odor: (a) Prenatal ethanol exposure results in a preference for its odor (Abate et al., 2000, 2001; Chotro & Molina, 1990; Chotro & Spear, 1997), and (b) using an identical fetal exposure paradigm as in the current study, Youngentob, Molina, Spear, and Youngentob (in press) recently demonstrated an enhanced ethanol acceptance in P15 rats that was absent in adult littermates: an acceptance pattern that parallels both the present behavioral and neurophysiological results. Regarding the question of neural tuning, we have utilized a rather broad definition, namely, any process that results in a concentration of the neural filter’s attention. In this study, we demonstrated that fetal ethanol exposure resulted in a stabilized neural response to ethanol odor that was accompanied by an apparent loss of responsivity to nonexposure odorants. That our definition can be applied to such a result has literature support. Other forms of chronic odor experience have been shown to behaviorally enhance the sensitivity and preference for the exposure odorant (Laing & Panhuber, 1978, 1980), and these effects occurred under the condition of stabilized neural responsiveness to the exposure odorant against a landscape of untoward neural effects. Thus, our data suggest that the responsive neurons to ethanol odor were stabilized in the face of ethanol’s expected toxic effects during development (Barron & Riley, 1992; Sulik & Johnston, 1983). That active neurons are stabilized and survive while inactive ones are compromised is a well-studied observation in development (Ghosh, Carnahan, & Greenberg, 1994; Katz & Shatz, 1996; LeVay, Wiesel, & Hubel, 1980; Zhao & Reed, 2001).
Finally, it is important to consider how the peripheral neural events we observed could manifest themselves in altered odorguided behavior and ultimately in ethanol acceptance patterns. As suggested above, the peripheral neural outcome we observed at P15 is likely a purely activity-dependent phenomenon. However, given the receptotopic projection of the epithelium onto the bulb (Mombaerts et al., 1996; Shepherd, 1991; Vassar et al., 1994), relative changes in glomerular size would be expected to reflect the alterations in the responsivity of the mucosa. That is, we would expect a stabilization of the relevant ethanol-related glomeruli in the face of negative effects to surrounding glomerular regions. This later scenario is consistent with the chronic odor experience noted above. The available data also suggest that the olfactory bulb should be viewed from an information-processing perspective and that the bulb can encode that an odor has gained associative significance (Wilson & Sullivan, 1994). There are centrifugal influences on bulbar function that can modulate the bulb’s responsiveness to particular odor cues on the basis of the previous experience of the rat, its physical or emotional state, and its stage of development. For example, in infant and adult rats, centrifugal inputs signaling reward through monoaminergic and noradrenergic activity to the bulb are necessary to modulate bulbar responses to biologically significant and learned odors (Gervais, Holley, & Keverne, 1988; Gervais & Pager, 1983; Sullivan et al., 1989; Wilson & Leon, 1988a, 1988b). Thus, the paired activation of ascending ethanol-responsive neurons and descending pharmocologic influences of ethanol on bulbar circuits may serve to encode that ethanol odor has gained associative significance via fetal exposure, thereby enhancing odor-guided ethanol behavior, flavor perception, and intake. That ethanol’s effect on the neural modality predicted the behavioral response to ethanol odor and that these effects paralleled ethanol intake patterns under identical fetal exposure conditions suggest these processes are intimately related.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism-National Institutes of Health Grant AA014871 to Steven L. Youngentob.
Contributor Information
Steven L. Youngentob, SUNY Upstate Medical University and SUNY Developmental Exposure Alcohol Research Center
Paul F. Kent, SUNY Upstate Medical University and SUNY Developmental Exposure Alcohol Research Center
Paul R. Sheehe, SUNY Upstate Medical University and SUNY Developmental Exposure Alcohol Research Center
Norman E. Spear, Binghamton University and SUNY Developmental Exposure Alcohol Research Center
Juan C. Molina, SUNY Upstate Medical University, Binghamton University, and SUNY Developmental Exposure Alcohol Research Center
Lisa M. Youngentob, SUNY Upstate Medical University and SUNY Developmental Exposure Alcohol Research Center
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcoholism: Clinical and Experimental Research. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcoholism: Clinical and Experimental Research. 2001;25:989–998. [PubMed] [Google Scholar]
- Abel EL, York JL. Absence of effect of prenatal ethanol on adult emotionality and ethanol consumption in rats. Journal of Studies on Alcohol. 1979;40:547–553. doi: 10.15288/jsa.1979.40.547. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamum A, Williams GM, O’Callagham M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Archive of General Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Baer JS, Bar HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. Journal of Studies on Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurotoxicology and Teratology. 1992;14:291–297. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Bond NW, DiGiusto EL. Effects of prenatal alcohol consumption on open-field behaviour and alcohol preference in rats. Psychopharmacology (Berlin) 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: A conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Cordoba NE, Molina JC. Acute prenatal experience with alcohol in the amniotic fluid: Interactions with aversive and appetitive alcohol orosensory learning in the rat pup. Developmental Psychobiology. 1991;24:431–451. doi: 10.1002/dev.420240605. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear N. Prenatal and postnatal ethanol exposure influences preweanling rats’ behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–385. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during Gestational Day 21: Postnatal changes in alcohol responsiveness in rats. Developmental Psychobiology. 1990;23:535–547. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Bradycardiac responses elicited by alcohol odor in rat neonates: Influence of in utero experience with ethanol. Psychopharmacology (Berlin) 1992;106:491–496. doi: 10.1007/BF02244820. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Spear NE. Repeated exposure to moderate doses of alcohol in the rat fetus: Evidence of sensitization to toxic and chemosensory aspects of alcohol. Alcoholism: Clinical and Experimental Research. 1997;21:360–367. [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984 Aug 24;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Research. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Drug conditioning and seeking behavior. In: O’Donohue W, editor. Learning and behavior therapy. Allyn & Bacon; Boston: 1998. pp. 518–540. [Google Scholar]
- Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacology Biochemistry and Behavior. 2002;72:659–668. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Chotro MG, Molina JC. Alcohol in the amniotic fluid prior to cesarean delivery: Effects of subsequent exposure to the drug’s odor upon alcohol responsiveness. Behavioral and Neural Biology. 1993;60:129–138. doi: 10.1016/0163-1047(93)90229-b. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: Comparability of effects in human and animal models. Neurotoxicology and Teratology. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Developmental neurobiology of the olfactory system. In: Getchell TV, Doty R, Bartoshuk L, Snow J, editors. Smell and taste in health and disease. Raven Press; New York: 1991. pp. 19–33. [Google Scholar]
- Gervais R, Holley A, Keverne B. The importance of central noradrenergic influences on the olfactory bulb in the processing of learned olfactory cues. Chemical Senses. 1988;13:1–12. [Google Scholar]
- Gervais R, Pager J. Olfactory bulb excitability selectively modified in behaving rats after local 6-hydroxydopamine treatment. Behavioral Brain Research. 1983;9:165–179. doi: 10.1016/0166-4328(83)90126-2. [DOI] [PubMed] [Google Scholar]
- Gesteland RC, Yancey RA, Farbman AI. Development of olfactory receptor neuron selectivity in the rat fetus. Neuroscience. 1982;7:3127–3136. doi: 10.1016/0306-4522(82)90235-4. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994 Mar 18;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The biopsychology of development. Academic Press; New York: 1971. pp. 67–128. [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparious species. Neurotoxicology and Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hudson R. Olfactory imprinting. Current Opinion in Neurobiology. 1993;7:548–552. doi: 10.1016/0959-4388(93)90054-3. [DOI] [PubMed] [Google Scholar]
- Hudson R. From molecule to mind: The role of experience in shaping olfactory function. Journal of Comparative Physiology. 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. In: Murphy C, editor. Induced peripheral sensitivity in the developing vertebrate olfactory system; Annals of the New York Academy of Sciences: Vol. 855. Olfaction and Taste XII—An international symposium; New York: New York Academy of Sciences. 1998; pp. 109–115. [DOI] [PubMed] [Google Scholar]
- Iwema CL, Fang H, Kurtz DB, Youngentob SL, Schwob JE. Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium. Journal of Neuroscience. 2004;24:356–369. doi: 10.1523/JNEUROSCI.1219-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Research. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996 Nov 15;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kauer JS, Moulton DG. Response of olfactory bulb neurones to odour stimulation of small nasal areas in the salamander. Journal of Physiology. 1974;243:717–737. doi: 10.1113/jphysiol.1974.sp010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent PF, Mozell MM. The recording of odorant-induced mucosal activity patterns with a voltage-sensitive dye. Journal of Neurophysiology. 1992;68:1804–1819. doi: 10.1152/jn.1992.68.5.1804. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Youngentob SL, Yurco P. Mucosal activity patterns as a basis for olfactory discrimination: Comparing behavior and optical recordings. Brain Research. 2003;981:1–11. doi: 10.1016/s0006-8993(03)02512-5. [DOI] [PubMed] [Google Scholar]
- Kent PF, Youngentob SL, Sheehe PR. Odorant-specific spatial patterns in mucosal activity predict perceptual differences among odorants. Journal of Neurophysiology. 1995;74:1777–1781. doi: 10.1152/jn.1995.74.4.1777. [DOI] [PubMed] [Google Scholar]
- Laing DG, Panhuber H. Neural and behavioral changes in rats following continuous exposure to an odor. Journal of Comparative Physiology. 1978;124:259–265. [Google Scholar]
- Laing DG, Panhuber H. Olfactory sensitivity of rats reared in an odorous or deodorized environment. Physiology & Behavior. 1980;25:555–558. doi: 10.1016/0031-9384(80)90121-3. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. Journal of Comparative Neurology. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: Neurogenesis, response to damage and odorant-induced activity. International Journal of Developmental Neuroscience. 1996;14:881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- MacKay-Sim A, Kesteven S. Topographic patterns of responsiveness to odorants in the rat olfactory epithelium. Journal of Neurophysiology. 1994;71:150–160. doi: 10.1152/jn.1994.71.1.150. [DOI] [PubMed] [Google Scholar]
- MacKay-Sim A, Shaman P. Topographic coding of odorant quality is maintained at different concentrations in the salamander ol-factory epithelium. Brain Research. 1984;297:207–217. doi: 10.1016/0006-8993(84)90562-6. [DOI] [PubMed] [Google Scholar]
- McCollum JF, Woo CC, Leon M. Granule and mitral cell densities are unchanged following early olfactory preference training. Developmental Brain Research. 1997;99:118–120. doi: 10.1016/s0165-3806(96)00201-5. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Clancy AN, Mousa S, Couri D, Noble EP. Prenatal alcohol exposure alters enkephalin levels, without affecting ethanol preference. Life Science. 1984;34:585–589. doi: 10.1016/0024-3205(84)90492-2. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW. Olfactory learning in the rat pup: A model that may permit visualization of a mammalian memory trace. NeuroReport. 2004;15:1691–1697. doi: 10.1097/01.wnr.0000134988.51310.c3. [DOI] [PubMed] [Google Scholar]
- Miller MW. Development of the central nervous system: Effects of alcohol and opiates. Wiley-Liss; New York: 1992. [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning resulting from alcohol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychological perspective. Erlbaum; Hillsdale, NJ: 1995. pp. 419–438. [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: Ethanol reinforcement during the third postnatal week. Alcoholism: Clinical and Experimental Research. 2006;30:1509–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. Alcohol and development: Beyond fetal alcohol syndrome. Developmental Psychobiology. 2007;49:227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, et al. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morrison DF. Multivariate statistical methods. McGraw-Hill; New York: 1967. [Google Scholar]
- Mozell MM, Sheehe PR, Swieck SW, Kurtz DB, Hornung DE. A parametric study of the stimulation variables affecting the magnitude of the olfactory nerve response. Journal of General Physiology. 1984;83:233–267. doi: 10.1085/jgp.83.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Developmental Psychobiology. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Phillips DS, Stainbrook GL. Effects of early alcohol exposure upon adult learning abilities and taste preferences. Physiological Psychology. 1976;4:473–475. [Google Scholar]
- Randall CL, Hughs SS, Williams CK, Anton RF. Effect of prenatal alcohol on consumption of alcohol and alcohol-induced sleep time in mice. Pharmacology Biochemistry and Behavior. 1983;18:325–329. doi: 10.1016/0091-3057(83)90194-6. [DOI] [PubMed] [Google Scholar]
- Reyes E, Garcia KD, Jones BC. Effects of the maternal consumption of alcohol on alcohol selection in rats. Alcohol. 1985;2:323–326. doi: 10.1016/0741-8329(85)90068-0. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. Journal of Neuroscience. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chemical Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Human fetuses learn odors from their pregnant mother’s diet. Chemical Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orgeur P. Olfaction in utero: Can the rodent model be generalized? Quarterly Journal of Experimental Psychology: Journal of Comparative and Physiological Psychology. 1992;44(B):245–278. doi: 10.1080/02724999208250615. [DOI] [PubMed] [Google Scholar]
- Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity associated with fetal learning of food odors in the rabbit. Naturwissenschaften. 1995;82:148–149. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Computational structure of the olfactory system. In: Davis JL, Eichenbaum H, editors. Olfaction: A model system for computational neuroscience. MIT Press; Cambridge, MA: 1991. pp. 3–42. [Google Scholar]
- Smotherman WP. In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behavioral and Neural Biology. 1982a;36:61–68. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Odor aversion learning by the rat fetus. Physiology & Behavior. 1982b;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: Behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behavioral Neuroscience. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Psychobiology of fetal experience in the rat. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Academic Press; Orlando, FL: 1987. pp. 39–60. [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behavioral Neuroscience. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Rat fetuses respond to chemical stimuli in gas phase. Physiology & Behavior. 1990;47:863–868. doi: 10.1016/0031-9384(90)90010-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcoholism: Clinical and Experimental Research. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiology & Behavior. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Streissguth A. Teratogenic and genetic influences on adolescent and adult alcohol use and abuse; Symposium presented at the annual meeting of the Research Society on Alcoholism; Hilton Head, SC. 1998.Jun, [Google Scholar]
- Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice: Craniofacial features of the fetal alcohol syndrome. American Journal of Anatomy. 1983;166:257–259. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Research. 1986;392:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh JL, Leon M. Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Developmental Brain Research. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. Journal of Neuroscience. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vavrousek-Jakuba EM, Baker RA, Shoemaker WJ. Effect of ethanol on maternal and offspring characteristics: Comparison of the formulations fed during gestation. Alcoholism: Clinical and Experimental Research. 1991;15:129–135. doi: 10.1111/j.1530-0277.1991.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Guthrie KM, Leon M. Modification of olfactory bulb synaptic inhibition by early unilateral olfactory deprivation. Neuroscience Letters. 1990;116:250–256. doi: 10.1016/0304-3940(90)90082-k. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Research. 1988a;470:69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. Journal of Neurophysiology. 1988b;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: Early olfactory learning. Behavioral and Neural Biology. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. Journal of Comparative Neurology. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Woo CC, Oshita MH, Leon M. A learned odor decreases the number of Fos-immunopositive granule cells in the olfactory bulb of young rats. Brain Research. 1996;716:149–156. doi: 10.1016/0006-8993(96)00037-6. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Steward M, Giunta TA. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol and drug dependence. Alcoholism: Clinical and Experimental Research. 1998;22:914–920. [PubMed] [Google Scholar]
- Youngentob SL. Developing a strategy for the rapid identification of genetically altered mice: An olfactory system perspective. Lab Animal. 2001;30:32–37. [PubMed] [Google Scholar]
- Youngentob SL. A method for the rapid automated assessment of olfactory function. Chemical Senses. 2005;30:219–229. doi: 10.1093/chemse/bji017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perception from multidimensional scaling of olfactory bulb glomerular activity patterns. Behavioral Neuroscience. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Research. 1995;670:82–88. doi: 10.1016/0006-8993(94)01275-m. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. Journal of Neurophysiology. 2003;90:3864–3873. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Mooney SM, Spear NE, Molina JC. In utero ethanol experience and olfactory plasticity. Alcoholism: Clinical and Experimental Research. 2004;28:94A. [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Schwob JE, Tzoumaka E. Mucosal inherent activity patterns in the rat: Evidence from voltage-sensitive dyes. Journal of Neurophysiology. 1995;73:387–398. doi: 10.1152/jn.1995.73.1.387. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL. OMP gene deletion causes an elevation in behavioral threshold sensitivity. NeuroReport. 1999;10:15–19. doi: 10.1097/00001756-199901180-00003. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL, Youngentob LM. OMP gene deletion results in an alteration in odorant quality perception. Behavioral Neuroscience. 2001;115:626–631. doi: 10.1037//0735-7044.115.3.626. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Markert LM, Mozell MM, Hornung DE. A method for establishing a five odorant identification confusion matrix task in rats. Physiology & Behavior. 1990;47:1053–1059. doi: 10.1016/0031-9384(90)90352-5. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behavioral Neuroscience. doi: 10.1037/0735-7044.121.6.1306. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiology & Behavior. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiology & Behavior. 1997;62:1241–1252. doi: 10.1016/s0031-9384(97)00301-6. [DOI] [PubMed] [Google Scholar]
- Zhao H, Reed RR. X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system. Cell. 2001;104:651–660. doi: 10.1016/s0092-8674(01)00262-8. [DOI] [PubMed] [Google Scholar]