Abstract
Background
Alcohol abuse is involved in the pathogenesis of multiple organ disorders; the underlying mechanism is incompletely understood. The ubiquitin editing enzyme A20 is involved in regulating activities in the cell. Suppression of A20 is suggested as one factor in the initiation of inflammation. This study investigates the mechanism by which chronic alcohol consumption modulates the levels of ubiquitin editing enzyme A20 in macrophages and further contributes to induce endothelial barrier dysfunction in the lung.
Methods
Mice were gavage-fed with 40% alcohol daily for 0-3 weeks. Airway macrophages were collected by lung lavage. Expression of ubiquitin editing enzyme A20 in isolated macrophages was assessed at both mRNA and protein levels. The endothelial barrier function of the lung was evaluated by the Evans blue method.
Results
Mice treated with alcohol for 3 weeks showed an increase in cell infiltration in the lung in response to exposure to peptidoglycan; over 80% of the infiltrated cells were macrophages. Furthermore, we observed that A20 level was suppressed in macrophages of mice treated with alcohol; the levels of tumor necrosis factor, interleukin-6 and nuclear factor kappa B in macrophage were increased. In addition, the endothelial barrier function of the lung was compromised, showing excessive infiltration of Evans blue in the lung indicating lung edema. Pretreatment with synthesized A20 inhibited alcohol-induced lung endothelial barrier dysfunction.
Conclusions
We conclude that chronic alcohol ingestion disturbs the endothelial barrier function in the lung by modulating macrophage properties. Increase in A20 in the cell may have potential for the treatment of inflammatory disorders.
Keywords: Alcohol, endothelial barrier function, lung, macrophage, ubiquitin
Introduction
Alcohol abuse is common in all social classes in the world [1]. Chronic consumption or acute excessive consumption of alcohol is harmful for important organs including the lung [2]. Although the absorbed alcohol is mainly metabolized in the liver, the unmetabolized portion enters the circulation and via pulmonary vessels reaches the lung where it affects its homeostasis or causes injury of local tissue [3]. It has been reported that the inhalation of alcohol compromises lung epithelial barrier function and induces lung edema [2]. The mechanism is not fully understood yet.
Ubiquitination is a process that occurs in almost all biochemical activities of the body [4]. Ubiquitin editing enzyme A20 is one of the enzymes involved in the process of ubiquitination [5]. Under physiological conditions, A20 restricts the activities of nuclear factor (NF)κB; the expression of A20 is tightly restricted in the cell. Suppressing A20 may result in over expression of NFκB and result in over-production of proinflammatory cytokines such as tumor necrosis factor (TNF) and induce an inflammatory reaction [6]. However, the factors affecting the A20 expression are unclear.
Macrophage (Mφ) is one of the inflammatory cell types in the body. In general, Mφ functions to eliminate invading pathogens in the body by producing a number of cytokines to destroy the pathogens. However, over-production of these cytokines, such as TNF, may result in tissue damage, e.g. impairment of the epithelial barrier function [7]. Whether alcohol induced-lung epithelial barrier dysfunction is induced by Mφ-derived TNF is, however, as yet unknown.
Since alcoholism affects the epithelial barrier integrity [2], and since Mφ-derived TNF is one of the factors inducing epithelial barrier dysfunction [7], and depression of A20 can strengthen the expression of TNF in Mφ [6], we hypothesized that chronic alcohol consumption induces lung endothelial barrier dysfunction via modulating the A20 and TNF expression in Mφ. The hypothesis was tested in this study with a mouse model, observing step by step the expression of A20 and TNF in lung Mφ and lung endothelial barrier function.
Materials and methods
Reagents
Antibodies against major basic protein, NIMP-R14, 3F12 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Curcumin, Evans blue, ethanol, peptidoglycan (PGN) were purchased from Sigma Aldrich (Shanghai, China). The dehydrogenase assay kit was purchased from Roche (Germany). Flow cytometry reagents were purchased from BD Biosciences (San José, CA, USA). Reagents for real time polymerase chain reaction (RT-PCR) and Western blotting were purchased from Invitrogen (Shanghai, China). Diff-Quik stain was purchased from Baxter Diagnostics (McGaw Park, IL, USA). NE-PER® nuclear and cytoplasmic extraction kit was purchased from Pierce Biotech (Rockford, IL, USA). A20 was synthesized by GL Biotech Ltd. (Shanghai, China).
Animals
Male C57BL/6 mice (body weight 25-30 g) were purchased from the Beijing Experimental Animal Institute. TNF-deficient mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Animals were housed in a pathogen-free environment and given free access to food and water. The animal experimental procedures were approved by the Animal Ethics Committee at Sun Yat-sen University.
Administration of alcohol
Grouped mice (6 mice per group; in the time-point experiments, 3 mice per group) received 40% alcohol (0-3 g/kg) via gavage-feeding daily for 0-3 weeks. Control mice were fed with saline daily at time points that matched the alcohol administration. A group of 6 mice was pretreated with recombinant A20 (100 ng/mouse) 30 min prior to each alcohol administration.
Assessing water in the lung
The lung was excised from each mouse and weighed, dried in an oven at 60°C overnight, and then weighed again. The rate of water in the lung was calculated by the formula: water in the lung (%) = (dry lung/wet lung) × 100.
Evans Blue assay for lung endothelial permeability
Evans blue dye (2% in saline, 4 ml/kg) was injected into the experimental mice (each group consisted of 6 mice) via the tail vein 2 h after the administration of ethanol; 30 min later, mice were killed by cervical dislocation; the chest was opened and the lung was excised and weighed. The lung tissue was homogenized in 2.5 ml phosphate-buffered saline and mixed by vortexing for 2 min after the addition of 2.5 ml of 60% trichloroacetic acid, to precipitate the protein. Samples were cooled and then centrifuged for 30 minutes at 1000 g. The supernatant was measured at 620 nm for absorbance of Evans blue using a microplate reader (Bio-Tek ELx800™, London, UK). Evans blue was expressed as μg/g tissue against a standard curve.
Western blotting
Protein extracts were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Blots were blocked for 1 h at room temperature in phosphate buffered saline (PBS) containing 5% skim milk and 0.05% Tween-20. The membranes were incubated overnight with the primary antibody (1 μg/ml, or an isotype IgG) at 4°C. The target protein was detected using horseradish peroxidase-conjugated second antibody (1:1000). After three washes in tris-buffered saline (TBS) containing 0.05% Tween-20, signals were revealed by enhanced chemiluminescence (ECL) Western blotting and recorded by X ray film.
Lung lavage
Bronchoalveolar lavage (BAL) was performed from each experimental mouse; the lavage fluid (BALF) was obtained by cannulating the trachea with a 20-gauge catheter. The right lung was lavaged with two aliquots (0.7 ml) of PBS without calcium; total returns after lavage were 0.8-1.2 ml/mouse. BALF was centrifuged (600 g, 10 min at 4°C), and cell-free supernatants were stored at -80°C. The cell pellet was diluted in PBS, and the total cell number was counted with a hemocytometer after staining with trypan blue. Differential cell counts were done with cytocentrifuge preparations stained with Diff-Quik stain. Cell populations were determined by counting 300 cells/sample, and a percentage was calculated based on 6 mice per group. Total protein was measured in the cell-free supernatant.
Flow cytometry
Cells were fixed with 1% paraformaldehyde mixed with permeable agents for 30 min on ice. Cells were then stained with fluorescence labeled antibodies against cell markers of alveolar macrophage, eosinophil, neutrophil and lymphocyte (1:100) for 30 min on ice. After washing, cells were analyzed by a FACSarray bioanalyzer (BD Bioscience, San Jose, CA, USA). Data were analyzed by the software FlowJo.
Extraction of nuclear proteins
Nuclear protein was extracted from purified Mφs with a commercial NE-PER® kit. The cells were treated with hypotonic buffer (provided in NE-PER® kit) for 30 min on ice. Detergent (1/100) was added to the homogenates and vortexed for 10 sec and then centrifuged for 30 sec at 14,000 × g at 4°C. The supernatant was removed and the nuclear pellet was resuspended in 50 μl of complete lysis buffer and vortexed for 10 sec. The mixture was incubated on ice for 30 min, vortexed briefly and pelleted at 14,000 × g for 10 min at 4°C. The supernatant (nuclear fraction) was aliquoted, protein content measured and stored at -80°C until use.
Real time RT-PCR
Using real time reverse-transcriptase polymerase chain reaction (qRT-PCR), we performed a quantitative analysis of the mRNA expression of NFκB. The mouse NFκB mRNA sequence was obtained from NCBI (AY521463). The primers were designed with software primer3. Forward: ctgacctgagccttctggac; reverse: gcaggctattgctcatcaca. Total RNA was extracted from the cells using an RNeasy Mini kit (Qiagen, Shanghai, China). cDNA was synthesized using the iScriptTMcDNA Synthesis Kit (Bio-Rad, Mississauga, ON, Canada). The resulting cDNA was subjected to qPCR that was performed with a LightCycler using a SuperScript III Platinum SYBR Green Two-Step qPCR Kit (Invitrogen, Burlington, ON, Canada) (annealing temperature: 60°C, for 30 seconds, 39 cycles). The amplified product was detected by the presence of an SYBR green fluorescent signal. The standard curve was designed with β-actin cDNA. The resulted amplicon was quantified with the standard curve. β-actin: forward, 5'-ggacttcgagcaagagatgg-3'; reverse, 5'-agcactgtgttggcgtacag-3' (456 bp. NCBI, DQ407611).
Statistics
The data were expressed as means ± SD of at least 5 independent experiments. The values were analyzed using the two-tailed unpaired Student's t-test when the data consisted of two groups or by ANOVA when three or more groups were compared. P < 0.05 was set as the statistically significant threshold.
Step by step results
Chronic alcohol consumption increases Mφ infiltration in the airways in response to microbial product exposure
Chronic alcohol consumption increases the risk for lung injury [8,9]; the underlying mechanism is incompletely understood. Mφ is one of the major inflammatory cells in a number of lung diseases. We thus hypothesized that the chronic alcohol consumption may associate with Mφ extravasation in the lung. Using a murine model, we fed mice with 40% alcohol (this concentration was determined in preliminary experiments). As demonstrated by the time-course data, 30 min after the alcohol gavage, the serum alcohol reached its peak value and returned to 0 value 48 h later (Figure 1A). We then fed mice with graded doses of alcohol daily for 3 weeks. Mice were challenged with peptidoglycan (PGN) on the last day and sacrificed 3 h later. After sacrifice, BAL was collected from the mice and processed for cell counting. The results showed that the total cell counts in BAL were significantly increased in mice that received alcohol and alcohol plus PGN in an alcohol dose-dependent manner; the latter had a higher cell number than the former. The total cell counts in mice treated with PGN alone did not show significant differences from the saline control group (Figure 1B). The collected cells were also stained with anti-F4/80 (a cell marker of Mφ) and analyzed by flow cytometry. The frequency of Mφ in BAL was increased in the alcohol group and further increased in mice treated with both alcohol and PGN (Figure 1C). The data indicate that chronic alcohol consumption induces Mφ infiltration in the airways in response to microbial product (such as PGN) exposure.
Figure 1.
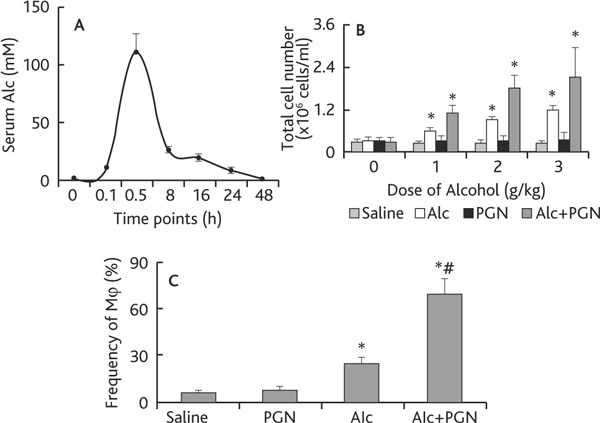
Chronic alcohol consumption facilitates the airway macrophage infiltration. Definition of abbreviations: Alc, alcohol; PGN, peptidoglycan. A: C57BL/6 mice (3 mice/each time-point) were treated with alcohol (Alc) via gavage-feeding at a dose of 3 g/kg. Mice were sacrificed at the indicated time points. The curve indicates the serum levels of alcohol. B-C: Mice (6 mice per group) were treated with saline, or 40% alcohol, or PGN, or alcohol/PGN via gavage-feeding at the indicated doses daily for 3 weeks. On the last day, mice were challenged with PGN via nasal drops. Six hours after PGN challenge, mice were sacrificed; BAL was performed. The total cell number was counted under a light microscope (B); the frequency of Mφ was analyzed by flow cytometry (C). Data are presented as mean ± SD. * p < 0.05, compared with saline group. # p < 0.05, compared with the group treated with alcohol alone.
Chronic alcohol consumption inhibits ubiquitin editing enzyme A20 in lung Mφ
A20 is a ubiquitin editing enzyme that plays a critical role in the maintenance of homeostasis in the body [10] and is involved in the regulation of airway inflammation [11]. Since chronic alcohol consumption increases the Mφ infiltration in the airways as shown in Figure 1, we wondered if the levels of A20 in Mφs were modulated by chronic alcohol consumption. Thus, using the same mouse model in Figure 1, we collected BAL cells; F4/80+ Mφs were purified by magnetic cell sorting (MACS); total proteins were then extracted. The proteins were analyzed by Western blotting. As depicted in Figure 2, the A20 levels were markedly suppressed in airway Mφs from mice treated with chronic alcohol consumption in an alcohol dose-dependent manner (Figure 2). The results indicate that chronic alcohol consumption affects the expression of A20 in Mφs.
Figure 2.
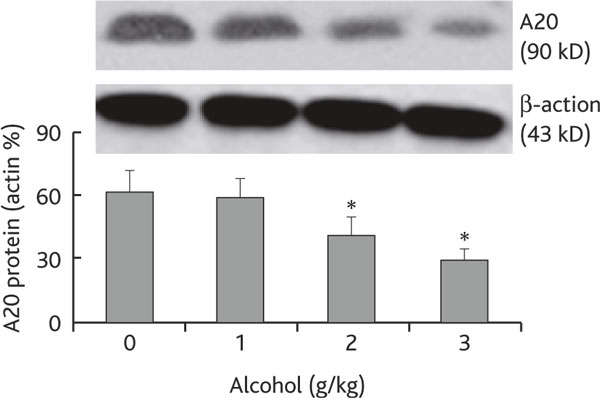
Chronic alcohol consumption compromises the expression of A20 in airway Mφ. Definition of abbreviation: Mφ, macrophage. Cells were collected from BAL of each mouse as described in Figure 1; F4/80+ Mφ were purified from the cells by MACS and processed to determine A20 level by Western blotting. The immune blots indicate A20 level in protein extracts from purified Mφ. Bars present the density analytic results of immune blots that were presented as the percentage of β-actin. Data are expressed as means ± SD. * p < 0.05, compared with group "0". The data represent 3 experiments.
Chronic alcohol consumption increases the levels of NFκB in airway Mφs
A20 is a critical factor in restricting NFκB activities in Mφ [6]. Since chronic alcohol consumption suppressed the levels of A20 in airway Mφs, the levels of NFκB in airway Mφs may be upregulated. To test this hypothesis, we further analyzed the expression of NFκB in airway Mφs. Using the same mouse model in Figure 1, we collected BAL Mφs from the experimental mice. The levels of NFκB in nuclear extracts were analyzed. As shown by qPCR and Western blotting data, the levels of NFκB were significantly higher in Mφ from mice treated with chronic alcohol consumption than in control mice, and were further increased in mice treated with both alcohol and PGN, which was abrogated by pretreatment with exogenous A20 (Figure 3).
Figure 3.
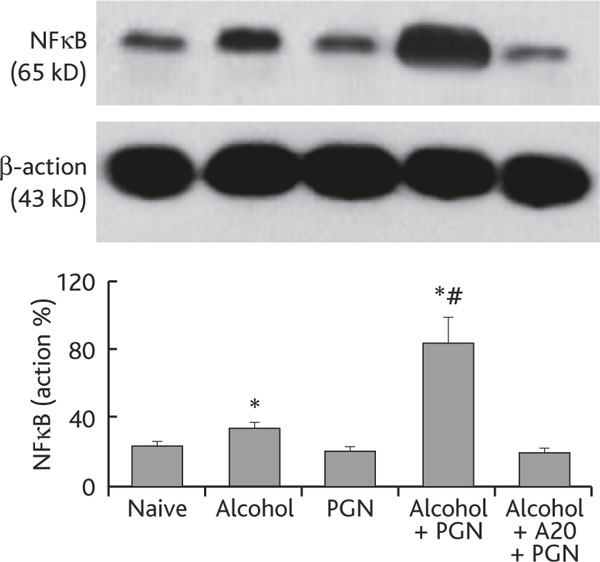
Chronic alcohol consumption increases the NFκB level in airway Mφ. Definition of abbreviations: MΦ, macrophage; NFκB, nuclear factor-κB; PGN, peptidoglycan. Airway Mφs were prepared from each experimental mouse in the same way as described in Figure 2. Total proteins were extracted from purified Mφs and subjected to Western blotting to determine the NFκB level in airway Mφ. The immune blots show levels of NFκB. The bars show densitometrical data that are presented as percentage of β-actin (the internal control). Alcohol+A20+PGN: Mice were treated with synthesized A20 (100 ng/mouse) via i.p. 30 min prior to alcohol gavage. Other treatments were the same as for the group "Alcohol+PGN". Data represent 3 experiments.
Chronic alcohol consumption increases the levels of TNF in BAL
TNF and IL-6 are the major proinflammatory cytokines released by activated Mφs that contribute to various inflammations. Therefore, the levels of TNF and IL-6 were also measured in our experimental system. High levels of TNF and IL-6 were detected in BAL of mice treated with chronic alcohol consumption and exposure to PGN, and this could be inhibited by pretreatment with synthesized A20 (Figure 4).
Figure 4.
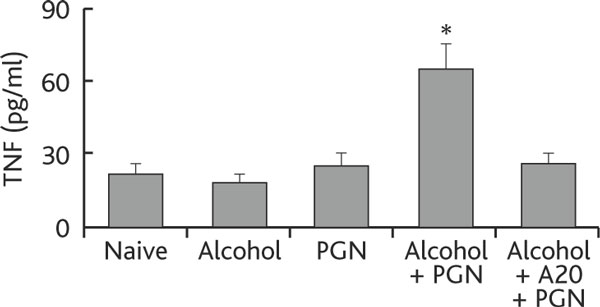
Chronic alcohol consumption increases tnf production by airway Mφs. Definition of abbreviations: BALF, bronchoalveolar lavage fluid; Mφ macrophage; PGN, peptidoglycan; TNF, tumor necrosis factor. Mice (6 mice per group) were treated with alcohol or saline and challenged with PGN as described in Figure 1. BALF and Mφs were prepared as described in the text. Total proteins were extracted from purified Mφs and the levels of TNF determined by ELISA. The annotations are the same as in Figures 1-3.
Chronic alcohol consumption impairs airway endothelial barrier function
Epithelial barrier function plays a critical role in maintaining the homeostasis in the lung. Dysfunction of the epithelial barrier is an important causal factor in a number of lung inflammations such as acute lung injury [7]. Based on the results that chronic alcohol consumption increases TNF expression in airway Mφs, and given that TNF plays a critical role in compromising epithelial barrier integration, we postulated that chronic alcohol consumption may damage the airway endothelial barrier function in the lung. To test the hypothesis, we employed the Evans blue method to evaluate the lung endothelial barrier function in mice. After treatment with chronic alcohol consumption, we treated the mice with PGN as described above; meanwhile, we injected Evans blue into the tail vein. After sacrifice, the amounts of Evans blue in lung tissue were assessed. The results showed that Evans blue amounts in the lung were significantly higher in mice treated with alcohol and PGN than control mice (Figure 5A). The results were further supported by examining the water/lung tissue ratio that demonstrated markedly that more water was extravasated into lung tissue in alcohol/PGN treated mice than in control mice (Figure 5B). On the other hand, a group of TNF-deficient mice was treated with alcohol for three weeks in the same way as described above. However, in contrast to the wild type mice (C57/B6 in mice; data not shown), the TNF-deficient mice did not have endothelial barrier dysfunction. Most strikingly, in mice pretreated with exogenous A20 at a dose of 100 ng/mouse/time 30 min prior to each alcohol administration, the alcohol-induced epithelial barrier dysfunction in the lung was abolished. These results indicate that chronic alcohol consumption can compromise lung endothelial barrier function.
Figure 5.
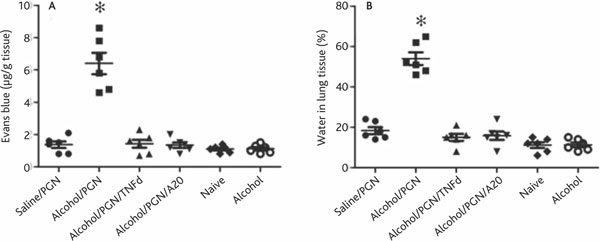
Chronic alcohol consumption impairs airway endothelial barrier function and induces inflammation in the lung. Definition of abbreviations: PGN, peptidoglycan; TNF, tumor necrosis factor. C57/B6 or TNF-deficient mice (6 mice per group) were treated with chronic alcohol consumption or saline pretreatement with or without exogenous PGN. Mice were exposed to PGN on the last day. Mouse lung endothelial permeability was determined with the Evans blue method. The permeability of lung endothelial was also assessed by evaluating the ratio of water in lung tissue. A, the scatter plots show Evans blue contents in the lung. Each dot represents the Evans blue level in lung tissue of one mouse. B, the scatter plots show the amount of water in the lung of mice presenting as percent of lung tissue. Each dot represents the amount of water in lung tissue of one mouse.
Discussion
Chronic alcohol consumption can induce multiple organ injury [12-14]. The mechanism remains to be fully clarified. The present study provides a set of novel data showing that chronic ingestion of alcohol caused hyperpermeability of lung endothelial barrier in response to exposure to PGN that resulted in inflammatory cell (mainly Mφ) infiltration in the lung. NFκB and TNF play a critical role in alcohol induced lung endothelial barrier dysfunction. The ubiquitin editing enzyme A20 is a critical molecule in regulationof NFκB and TNF production in Mφ during chronic alcohol consumption-induced lung endothelial barrier dysfunction.
Mφ infiltration in the alveoli is a common pathogenic phenomenon in airway inflammation, such as in allergic asthma [15]. Mφ is one of the major inflammatory cells in a number of immune disorders; activated Mφ releases an array of proinflammatory cytokines to induce tissue injury. The present data provide a mechanistic explanation for chronic alcohol consumption-induced lung endothelial barrier injury. After chronic ingestion of alcohol, the level of ubiquitin enzyme A20 in Mφ was suppressed. In response to PGN challenge, over-production of NFκB was induced in Mφ. This results in the production of proinflammatory cytokines such as TNF, as observed in the present study, that can be a causal factor for lung injury. On the other hand, a control group was also treated with PGN that did not cause lung endothelial hyperpermeability. This indicates that chronic alcohol consumption injures the lung's endothelial barrier function. The lung then becomes susceptible to microbial stimuli.
In synergy with several regulatory proteins, including Tax1 binding protein 1 (TAX1BP1) and the E3 ubiquitin ligases Itch and ring finger protein 11 (RNF11), A20 restricts NFκB activation [6]. Our data are in line with pioneer studies showing that, after treating mice with chronic alcohol consumption, A20 expression in Mφ was suppressed, which resulted in a significant increase in NFκB activation in Mφ. A20 functions as a ubiquitin-editing enzyme with both deubiquitinating (DUB) and ubiquitin E3 ligase activity toward the adaptor protein and death-domain containing protein kinase, receptor-interacting protein 1 (RIP1) in the TNFR pathway [16]. Suppression of A20 induces an increase in the production of proinflammatory cytokines such as TNF in Mφ. The present data demonstrate that chronic alcohol consumption can suppress the A20 production in Mφ that further contributes to induction of lung injury.
Normal lung endothelial barrier maintains an integrated condition that plays a critical role in the homeostasis of the lung. Cumulative research evidence indicates that various factors can affect the lung endothelial barrier function, including circulating oxidized phospholipids [17], cancer [18], reactive oxygen, nitrogen species [19] and hypoxia [20]. Previous studies have demonstrated that alcoholism is one of the factors involved in impaired barrier function in the airway. Our data link chronic alcoholism to lung endothelial barrier dysfunction by providing evidence that administration of alcohol suppresses the expression of A20 in Mφ, which over-produces proinflammatory cytokine TNF; the latter induces endothelial barrier dysfunction and causes edema in the lung. The pretreatment with exogenous A20 results in suppression of both NFκB activation and TNF production in Mφ and thus suppresses the endothelial hyperpermeability in the lung, implying that A20 may have the potential to be used in interventions related to chronic alcohol consumption-related lung disorders.
Conclusion
In conclusion, the present study demonstrates that chronic alcohol consumption can affect the expression of A20 in lung Mφ that further impairs the lung endothelial barrier function and induces Mφ infiltration and edema in the airway, in which TNF plays a critical role.
Conflict of interest statement
None of the authors has any conflict of interest to declare in relation to the subject matter of this manuscript.
Acknowledgements
This study was supported by a grant (No: XQ-0426) from Guangdong Medical College.
References
- Hingson RW, Zha W, Weitzman ER. Magnitude of and trends in alcohol-related mortality and morbidity among U.S. college students ages 18-24, 1998-2005. J Stud Alcohol Drugs Suppl. 2009;16:12–20. doi: 10.15288/jsads.2009.s16.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkoulias K, Tsitsaras H, Patouchas D, Sampsonas F, Likouras D, Kaparianos A, Spiropoulos K. The alcoholic lung disease: historical background and clinical features. Medicina (Kaunas) 2008;44:651–664. [PubMed] [Google Scholar]
- Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- Kanarek N, London N, Schueler-Furman O, Ben-Neriah Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb Perspect Biol. 2010;2:a000166. doi: 10.1101/cshperspect.a000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, Formisano S, Vito P, Leonardi A. ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in inhibiting NF-kappaB. J Biol Chem. 2006;281:18482–18488. doi: 10.1074/jbc.M601502200. [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu O, Ahmad RC, Tavares R, Wang M, Philpott D, Turer EE, Lee BL, Shiffin N, Advincula R, Malynn BA, Werts C, Ma A. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28:381–390. doi: 10.1016/j.immuni.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achur RN, Freeman WM, Vrana KE. Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J Neuroimmune Pharmacol. 2010;5:83–91. doi: 10.1007/s11481-009-9185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30:383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AA. Immunomodulators in the treatment of asthma. Allergy Asthma Proc. 2009;30:109–119. doi: 10.2500/aap.2009.30.3203. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen SH, Zhang Y, Yu CH, Li SD, Li YM. Is the hypoxia-inducible factor-1 alpha mRNA expression activated by ethanol-induced injury, the mechanism underlying alcoholic liver disease? Hepatobiliary Pancreat Dis Int. 2006;5:560–563. [PubMed] [Google Scholar]
- Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, Becher R. Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPARalpha. Inhal Toxicol. 2010;22:140–150. doi: 10.3109/08958370903019885. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Fu P, Birukov KG. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl Res. 2009;153:166–176. doi: 10.1016/j.trsl.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Jiang WG. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 2009;1788:872–891. doi: 10.1016/j.bbamem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Miserocchi G. Lung interstitial pressure and structure in acute hypoxia. Adv Exp Med Biol. 2007;618:141–157. doi: 10.1007/978-0-387-75434-5_11. [DOI] [PubMed] [Google Scholar]


