Abstract
Myleodysplastic syndromes (MDS) are premalignant diseases characterized by cytopenias, myeloid dysplasia, immune dysregulation with association to autoimmunity, and variable risk for acute myeloid leukemia (AML) transformation. Studies of Forkhead-box P3 (FoxP3)+ regulatory T-cells (Tregs) indicate that the number and/or activation state may influence cancer progression in these patients. Focusing on patients with a lower-risk for leukemia transformation, 18 (34.6%) of 52 patients studied displayed an altered Treg compartment compared to age-matched controls. Delineation of unique Treg subsets revealed that an increase in the absolute number of CD4(+)FoxP3(+)CD25(+)CD127(low)CD45RA(−)CD27(−) Tregs (effector memory Tregs; TregEM) was significantly associated with anemia (p=0.046), reduced hemoglobin (p=0.038), and blast counts ≥5% (p=0.006). In healthy donors, this TregEM population constitutes only 2% of all Tregs (1–6 Treg cells/μl) in peripheral blood, but when isolated, exhibit greater suppressive activity in vitro. With a median follow-up of 3.1 years (range-2.7 to 4.9) from sample acquisition, increased numbers of TregEM cells proved to have independent prognostic importance in survival estimates suggesting that enumeration of this Treg subset may be a more reliable indicator of immunological escape than FoxP3+ T-cells as a whole. Based on multivariate analyses, TregEM impacted survival independently from myeloblast characteristics, cytopenias, karyotype and comorbidities. Based on these findings, TregEM cell expansion may be synonymous with human Treg activation and indicate microenvironmental changes conducive to transformation in MDS.
Introduction
Myelodysplastic syndromes (MDS) refer to a group of pathophysiologically diverse premalignant hematopoietic diseases characterized by inflammation-associated cytopenias, myeloid dysplasia, autoimmunity and variable risk acute myeloid leukemia (AML) progression(1). Several prognostic models have been developed to gauge the risk of AML transformation and overall survival, and thus play a large role in disease management. The International Prognostic Scoring System (IPSS)(2, 3) represents the most widely used model segregating patients into low, intermediate-1 (int-1), intermediate-2 (int-2), and high-risk based on the number of cytopenias, bone marrow blast percentage, and karotype. The IPSS was validated in newly diagnosed and untreated patients(1), but newer prognostic risk models, such as the MD Anderson Scoring System (MDAS), incorporate a broader range of factors that refine prognostic precision, and may better reflect changes that occur during disease progression(4–6). While both systems accurately assess risk and disease outcome, neither system accurately predicts response to FDA-approved drugs, making treatment decisions difficult and nonstandardized.
Overall, approximately 30–40% of lower IPSS-risk patients experience hematopoietic improvement with T-cell depleting therapy with either anti-thymocyte globulin (ATG) or cyclosporine(7–10). Auto-reactivity against abnormally expressed self-antigens in the bone marrow is now widely suspected to play a role in MDS pathogenesis(7–11). However, not all patients respond to such treatment, and clinical benefit seems limited to those with less advanced disease(7–10). This suggests that the role of the T-cell compartment may change over time as MDS progresses from the early autoimmune stages(9) into more advanced stages, where it is likely that classic immune-suppressive mechanisms prevail. Unfortunately, none of the clinical parameters encompassed by prognostic scoring systems like the IPSS or MDAS reflect T-cell reactivity or suppressive state.
Regulatory T-cells (Tregs) have become the quintessential suppressive population within the T-cell compartment, and have been extensively studied for their role in tumor-induced immune suppression(12). Like most cancers, increased numbers of Tregs were found in MDS patients, but were restricted to those with higher risk as defined by IPSS, likely reflecting the immune suppressive state of more advanced disease(13). It is now known that self-antigen-induced T-cell receptor (TCR) signaling is required for Treg suppressive activity(14), and that this activation results in memory populations similar to conventional T-cells(15). We hypothesize that shifts in naive or memory phenotype within the Treg compartment may provide an earlier indicator of active immune suppression, which may result in better prognostic value compared to total Treg numbers alone.
Using phenotypic markers commonly employed to define conventional T-cell memory pools, we demonstrate different suppressive capacities among distinct Treg subpopulations. In a cohort 52 MDS patients, an increase in a Treg subset with more suppressive capacity (i.e., effector memory Tregs, TregEM) was independently associated with increased bone marrow myeloblasts and inferior OS. The prognostication of the MDAS, which reclassified a large number of IPSS-defined lower risk patients, was also improved by the inclusion of TregEM in the analysis. These findings suggest that expansion of a specific Treg subset, rather than expansion of Tregs as a whole, identifies a subset of higher risk patients. Inclusion of Treg memory phenotype analysis into prognostic models may indicate the initiation of an immunosuppressive microenvironment with importance to disease pathobiology and treatment.
Materials and Methods
Patients and Healthy Controls
Fifty-two previously untreated patients diagnosed with MDS at the University of California Los Angeles (UCLA), Penn State University Cancer Institute, Cleveland Clinic Cancer Institute, or the Malignant Hematology Clinic at the H. Lee Moffitt Cancer and Research Institute were studied retrospectively based on data collected by the Bone Marrow Failure Rare Disease Clinical Research Network (BMF-RDCRN). All diagnoses were confirmed at enrollment by experienced hematopathologist through centralized standards implemented at participating institutions and patients were classified according to World Health Organization (WHO) criteria. Metaphase cytogenetic testing was obtained using standard banding techniques. Patients were categorized into lower-risk (low/int-1) or higher-risk (int-2/high) based on IPSS(2, 3) and MD Anderson Assessment Score (MDAS)(4–6). Following consent, 40 ml of peripheral blood was obtained from each patient in heparin tubes. Blood samples from 41 healthy subjects who donated to the Southwest Florida Blood Services, and consented therein, were used as controls. This protocol was approved by the Institutional Review Boards at participating institutions in accordance with the Declaration of Helsinki and all human participants gave written informed consent.
Isolation of Peripheral Blood Mononuclear Cells
Peripheral Blood Mononuclear Cells (PBMCs) were isolated from blood samples using Ficoll-Hypaque (Amersham Pharma Biotech, Piscataway, NJ, USA) gradient centrifugation as per the manufacture’s recommendations. Following collection of PBMCs, cell pellets were resuspended, washed thoroughly with PBS, and then frozen at −80°C in fetal bovine serum (FBS) containing 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) in a polycarbonate container insulated with isopropanol before storage in liquid nitrogen. Cells were then thawed drop-wise in culture medium (RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.02 M HEPES buffer) and then rinsed in phosphate-buffered saline prior to immunoflourescent staining.
Immunoflourescent staining Tregs and Treg Subsets
Nonspecific staining was first blocked for 30 minutes at 4°C with 300 μl of 2% FBS in PBS per 1.0×106 cells. The cells were then labeled with fixable Live/Dead Yellow after thorough PBS washes (Invitrogen, Carlsbad, CA) so that nonviable cells could be excluded during flow cytometric acquisition. Cell surface staining was accomplished by 30 minute incubation at 4°C with 1 μl per 1.0×106 cells of the following Abs: Pacific Blue-CD3, PE-Cy7-CD25, PE-CD127, and APC-CD27 (BD Pharmingen, San Diego, CA); PerCP-Cy5.5-CD45RA (eBioscience, San Diego, CA). For experiments comparing CD127 and FoxP3 expression, the intracellular detection of FoxP3 required fixation and permeabilization with Cytofix/Cytoperm solution (BD Biosciences, San Diego, CA) followed by a 30 minute incubation with APC-FoxP3 (BD Pharmingen, San Diego, CA) antibody at 4°C. Analysis of Treg populations was performed on a LSRII cytometer (BD Biosciences, San Diego, CA) harboring a custom configuration for the H. Lee Moffitt Cancer Center and Research Institute. Using the gating schema outlined in Figure 1 (Figure 1A), viable cells were selected using cells gated on fixable Live/Dead Yellow and then CD4+FoxP3+CD25brightCD127dim cells were discriminated into naïve (TregN) or into central memory (TregCM), effector memory (TregEM), or terminal memory Tregs (Treg™) based on CD27 and CD45RA expression (Figure 1A). TregN cells expressed both CD27 and CD45RA, TregCM cells were CD27+CD45RA−, TregEM cells were double-negative for CD27 and CD45RA, and Treg™ cells were considered CD27−CD45RA+. Intracellular FoxP3 staining and surface analysis confirmed that CD4+CD25brightCD127dim cells accurately defined Tregs based on FoxP3 expression. To visualize these cells, CD4+FoxP3+ Tregs were gated and shown in blue, and CD4+FoxP3− effector cells are shown in orange. FoxP3+ cells are largely contained within the CD25bright and CD127dim region (Figure 1A). For functional studies, fixable Live/Dead Yellow could not be used due to amine reactive nature of this dye. Instead viability staining was performed using 7-Aminoactinomycin D (7-AAD) (Biosciences, San Diego, CA) prior to functional assays and populations were sorted based on phenotypic markers on specific subpopulations as shown in supplemental Figure 1. Analysis of cytometry data was achieved using FlowJo software version 7.6.1 (Tree Star Inc., Ashland, OR).
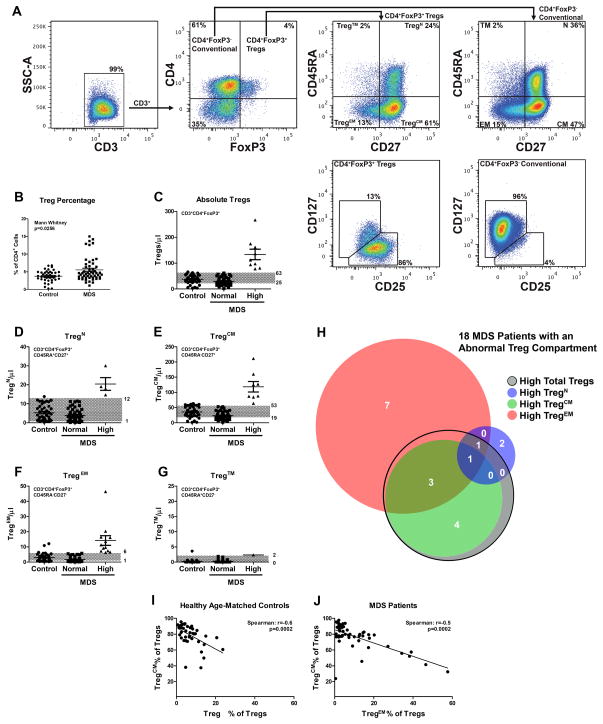
Figure 1. Treg expansion in distinct subsets of lower-risk MDS patients.
(A) Flow cytometry gating schema for defining Treg cells and Treg subsets. Cells were first gated on CD3 expression, and then on CD4 and FoxP3 expression. The resulting Treg (CD4+FoxP3+) or conventional T-cell (CD4+FoxP3−) populations were then analyzed for CD45RA and CD27 expression. Naïve Tregs (TregN) were considered CD45RA+CD27+, central memory Tregs (TregCM) were considered CD45RA−CD27+, and effector memory Tregs (TregEM) were considered CD45RA−CD27−. No significant population of terminal memory Tregs (Treg™) were observed (CD45RA+CD27−). CD4+FoxP3+ Tregs or conventional CD4+ T-cells were then analyzed for CD127 and CD25 expression. (B) Tregs were reported as a percentage of the CD4+ compartment in MDS patients and age-matched controls. (C–G) The normal range of Tregs in peripheral blood was established in age-matched healthy donors. To establish this cutpoint for “normal” versus “high” expression, the mean ± 1 standard deviation (grey areas) was determined based on the ALC. Graphs indicate the data from Control, and MDS patients with Normal and High levels of total Tregs, or of each Treg subtype calculated. (H) A Venn diagram demonstrating the amount of overlap of the 18 MDS patients with high total Treg or high Treg subtype numbers. (I–J) The frequency of TregCM cells negatively correlates with the frequency of TregEM cells within the Treg compartment in age-matched controls (I) and MDS patients (J).
T-cell Suppression Assay
Tregs with distinctive naive and memory phenotypes were isolated following immunoflourescent staining by flow assisted cell sorting (FACS) using a FACS ARIA cell sorter (BD Biosciences, San Diego, CA). Following isolation, increasing ratios of each Treg population was mixed with 1.0×105 conventional CD4+CD25− (responder) T-cells labeled with 0.5 μM Carboxyfluorescein succinimidyl ester (CFSE) according to the manufacturer’s recommendations (Invitrogen, Carlsbad, CA). Each mixture was then placed in a round bottom 96-well plate coated with 5 μM anti-CD3 antibody (eBioscience, San Diego, CA) and 2 μM anti-CD28 antibody (eBioscience, San Diego, CA) was then added to each well for co-stimulation. Proliferation of responding T-cells was assessed by CFSE dilution after 5 days using flow cytometry on a LSRII cytometer (BD Biosciences, San Diego, CA) harboring a custom configuration for the H. Lee Moffitt Cancer Center and Research Institute. The amount of proliferation in each assay was quantified using the proliferation algorithm available with FlowJo analysis software.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software version 5.03 (GraphPad Software, La Jolla, CA) or IBM SPSS Statistics software (version 19). Descriptive statistics such as the mean, median, and standard deviation were determined for continuous variables including the total number and percentage of Tregs, Treg phenotypes, and age. Comparisons between cases and controls were made using Mann-Whitney analysis. Correlation tests were performed using the Spearman analysis. The D’Agostino & Pearson omnibus normality statistic was used to assess normality of the data. The statistical methods used included simple linear regression and analysis of covariance. Kaplan Meier estimates were used for OS rates and log-rank method was used to compare between groups. Univariate and multivariate survival analyses were performed using the Cox Proportional Hazards model. Associations of MDS characteristics and Treg subgroups were determined using Fisher’s exact test. All analyses were performed at the 95% confidence interval and a p-value of less than 0.05 was considered statistically significant.
To compare subsets of patients with high total Tregs or high Treg subsets, dichotomous cut points were used based on the normal ranges established in age-matched controls. In peripheral blood, the normal range (mean ± 1 s.d) was defined for the absolute number of total Tregs (28–77 Tregs/μl) and each of the Treg subsets: TregN cells (1–9 TregN/μl), TregCM cells (19–53 TregCM/μl), TregMEM cells (0–6 TregEM/μl), and Treg™ cells (0–1 Treg™/μl). MDS patients with absolute total Treg or Treg subset numbers above the range of healthy age-matched controls were considered to have “high” levels, while patients within or below this range were considered to have “normal” levels. Because CBC data was not available for each individual healthy control subject, an estimated white blood cell count (WBC) of 7 k/μl, was used to determine the normal range for the absolute number of Tregs in the peripheral blood of controls.
Results
Clinical Characteristics of MDS Patients
Fifty-two consecutive MDS patients enrolled into the Bone Marrow Failure (BMF) Rare Disease Clinical Research Network (RDCRN) were examined for novel aspects of Treg biology with prognostic significance. Median age was 68 years (range 42–82) at the time of sample acquisition. Forty-one control subjects were included with a median age of 65 years (range 45–83 years). There was no statistical difference in age or gender between MDS patients and controls (p=0.097 for age and p=0.530 for gender). Two MDS patients (4%) were classified as isolated deletion 5q- (del (5q)), 12 (24%) as refractory anemia (RA) or refractory anemia with ringed sideroblasts (RARS), 8 (16%) as RA with excessive blasts (RAEB), 18 (35%) as refractory cytopenia with multilineage dysplasia (RCMD) or as RCMD with ringed sideroblasts (RCMD-RS), and 7 (13%) patients were classified as MDS unclassified (MDS-U). Five patients (10%) had the Myelodysplastic/Myeloproliferative neoplasm (MDS/MPN) chronic myelomonocytic leukemia (CMML)(1). In this study, 45 out of 52 (85%) patients were classified as lower-risk (low/int-1) based on IPSS, as shown in Table 1 and the majority of patients studied (69%) retained a lower-risk classification using the MDAS. Subsets of patients displayed thrombocytopenia (35%), neutropenia (56%), and/or anemia (56%), with cytopenias defined by IPSS standards. Thirty-five (67.3%) had a normal karotype, while 17 (32.7%) had an abnormal karyotype.
Table 1.
Characteristics of MDS Patients
| MDS (N=52) | ||
|---|---|---|
| Characteristic | Number | % |
| Age (years)* | 68 ±10 | |
| Sex | ||
| Male | 26 | 50 |
| Female | 26 | 50 |
| WHO** | ||
| 5q- | 2 | 4 |
| RA/RARS | 12 | 24 |
| RAEB | 8 | 16 |
| MDS-U | 7 | 13 |
| RCMD/RCMD-RS | 18 | 35 |
| CMML | 5 | 10 |
| IPSS† | ||
| High (Int-2/High) | 7 | 13 |
| Low (Low/Int-1) | 45 | 87 |
| MDAS†† | ||
| High (Int-2/High) | 16 | 31 |
| Low (Low/Int-1) | 36 | 69 |
| Neutropenia (<1 × 109/L) | ||
| Yes | 29 | 40 |
| No | 23 | 60 |
| Thrombocytopenia (<100 ×109/L) | ||
| Yes | 18 | 35 |
| No | 34 | 65 |
| Anemia (Hb<9 g/dL) | ||
| Yes | 29 | 56 |
| No | 23 | 44 |
| Karyotype‡ | ||
| Normal | 33 | 63 |
| Abnormal: Favorable | 22 | 44 |
| Abnormal: Unfavorable | 17 | 33 |
Age is not statistically different from the control group.
World Health Organization (WHO) includes refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with multilineage dysplasia (RCMD), refractory anemia with multilineage dysplasia with ringed sideroblasts (RCMD-RS), refractory anemia with excessive blasts (RAEB), chronic myelomonocytic leukemia (CMML) and MDS-unclassified (MDS-U)(1).
International Prognostic Scoring System (IPSS) low risk (IPSS score low or intermediate-1) or high risk (IPSS score intermediate-2 or high).
MD Anderson Scoring System (MDAS) classification lower-risk (low or intermediate-1) or higher-risk (intermediate-2 or high)(2, 3).
Karotype was performed by standard cytogenetics and was available for all 52 patients. Favorable karyotype includes del(5q), -Y, del(20q) and an unfavorable karyotype includes chromosome 7 abnormalities, or complex (≥ 3 abnormalities) based on IPSS criteria(1).
Treg Subset Expansion in a group of MDS Patients
Studies suggest that T-cell receptor (TCR) activation is required for suppressive activity from Tregs(14) raising the possibility that Tregs, like conventional T-cells cells, may change their phenotype when induced to expand. The percentage of Tregs in phenotypically distinct subsets (Figure 1A) was determined by flow cytometry and the absolute lymphocyte count (ALC) was used to estimate the total number in peripheral blood. Tregs were phenotypically discriminated into naive (TregN), central memory (TregCM), effector memory (TregEM), and terminal memory Tregs (Treg™) based on CD27 and CD45RA expression, as described in the Methods Section. Using simultaneous overlays of conventional T-cells (shown in orange) and FoxP3+ Tregs (shown in blue), all four phenotypic subtypes were evident within the conventional CD4+ T-cell population(16–19) (Figure 1A). The vast majority of Tregs in patients and controls had a central memory phenotype and a significantly higher percentage of Tregs within the CD4+ T-cell compartment was observed in some MDS cases compared to controls (p=0.026) (Figure 1B). Examining the absolute number of total Tregs and/or Treg subsets estimated from the ALC, 18 of the 52 (34.6%) patients had changes within in the Treg compartment compared to normal ranges established in healthy controls. This included abnormally high absolute numbers of Tregs and/or an increase in the absolute number of TregEM or TregCM subsets, as shown in Figures 1C–1F. Treg™ were low or undetectable in both cases and controls (Figure 1G). To display the profile of the 18 patients with an altered Treg compartment, a Venn diagram of individual MDS patients is shown in Figure 1H. Tregs primarily express high-affinity TCRs against self-antigens as a result of positive selection in the thymus(20–22). We correlated the frequency of TregEM and TregCM by linear regression, and a strong negative correlation is shown in Figure 1I and Figure 1J in both healthy donors and MDS patients (p<0.001) suggesting that recent antigen activation may favor central-to-effector memory transition, similar to conventional T-cells(23).
The TregEM Phenotype is More Suppressive than the TregCM Phenotype
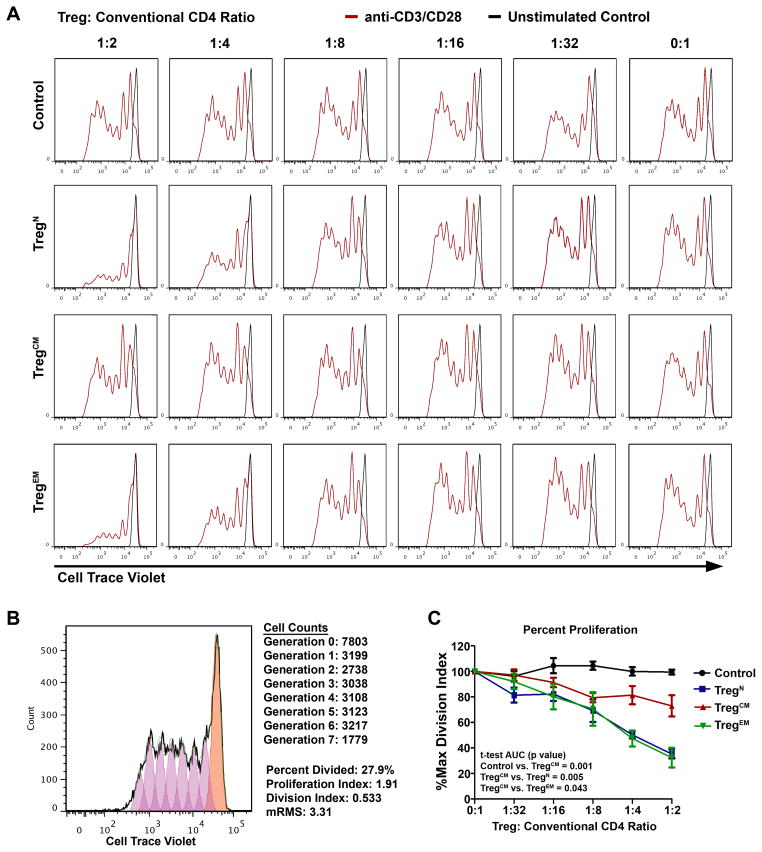
Because cohorts of MDS patients displayed Treg compartmental skewing, we determined the suppressive capacity of each Treg subset. To study the functional differences, sorted cells were cultured at increasing ratios with conventional CFSE-labeled responder T-cells stimulated with plate-bound anti-CD3/soluble anti-CD28 antibodies and CFSE dilution was assessed after five days using flow cytometry (Figure 2) in Tregs isolated from source leukocyte-enriched (“buffy coat”) blood from healthy donors. TregN and TregEM cells represent on average 13% and 2% of the entire Treg compartment, respectively and both cell populations suppress at a 1:8 ratio. TregCM cells, however, represent >80% of all Tregs, but suppress at a 1:2 ratio showing that these individual sorted populations display different suppressive activity in vitro.
Figure 2. Treg subset suppressive function in vitro.
TregN, TregCM, TregEM, or control CD4+CD25− T-cells were isolated via FACS and plated at increasing ratios with Cell Trace Violet labeled responder CD4+CD25− T-cells over plate-bound anti-CD3 antibody (5 μg/ml), and with soluble anti-CD28 (2 μg/ml). Cells were allowed to proliferate for five days and then Cell Trace Violet dilution was then assessed by flow cytometry (red histograms). Unstimulated responder cells are overlaid as a reference for no cellular division (black histograms). A representative inhibition assay from a healthy donor is shown (A). The amount of proliferation in each assay was quantified using the proliferation algorithm available with FlowJo analysis software, as exemplified (B). Briefly, this analysis fits proliferating cells into estimated generations (non-dividing cells: orange histogram, generation 0; dividing cells: purple histograms, generations >0). The events in each resulting generation are enumerated, and the percentage of original dividing cells from generation 0 is calculated, along with the proliferation index (extent of division). The product of these two parameters is the division index. (C) The division index of each assay was then normalized to each Treg subset baseline (each 0:1 ratio), and expressed as a percentage. Comparisons were made using t-test of the area under the curve (AUC) for each subset (n=5).
High TregEM Numbers Are Associated with Higher-Risk MDS Characteristics and Increased Blast Percentage
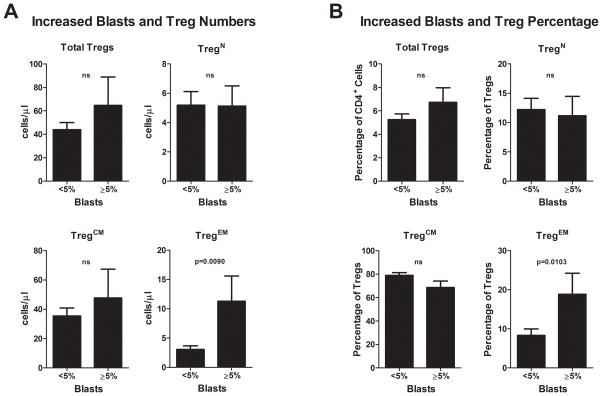
To test the clinical relevance of Tregs in MDS, we investigated the association of Tregs to clinical outcomes and classification. Clinical characteristics were compared among MDS cases stratified into high and normal Treg groups based on the mean + 1s.d. of healthy donors, as shown in Table 2 and Figure 1. TregN (n=2) and Treg™ (n=0) were not included in this analysis because of low sample size. Characteristics of patients with high Tregs (n=9) and high TregCM (n=8)cells were similar (Table 2). Higher numbers of total Tregs and high TregEM (n=12) were correlated with a higher-risk MDAS score (p=0.023) (Table 2). Additionally, the TregEM high group was distinguished by several poor prognostic features including a history of anemia (p=0.046), lower hemoglobin (Hg) (p=0.038 less than 10 g/dL), and increased percentage of bone marrow myeloblasts (≥5% p=0.006) suggesting that the presence of these cells correlates with worse prognosis (Table 2). Patients were stratified into two groups on the basis of bone marrow myeloblast percentage: <5% (n=10) and ≥5% (n=42) and the absolute number of total Tregs, or of TregCM, TregN, and TregEM subsets was then compared among patients in these two groups. Higher TregEM number (Figure 2A) and percentage (Figure 2B) was uniquely found to be associated with myeloblast accumulation demonstrating that the expansion of TregEM cells correlates with negative prognostic features of MDS.
Table 2.
Associations with Disease Characteristics
| MDS Patients (N=52) | High Tregs (>77/μl; N=9) | High TregCM (>53/μl; N=8) | High TregEM (>6/μl; N=12) | |||
|---|---|---|---|---|---|---|
| Characteristic (N) | n/N (%) | p-value | n/N (%) | p-value | n/N (%) | p-value |
| WHO# | ||||||
| 5q- (2) | 0/2 (0) | ns | 0/2 (0) | ns | 0/2 (0) | ns |
| RA/RARS (12) | 4/12 (33) | ns | 4/12 (33) | ns | 2/12 (17) | ns |
| RAEB-1/2 (8) | 1/8 (13) | ns | 1/8 (13) | ns | 3/8 (38) | ns |
| RCMD/RCMD-RS (18) | 1/18 (6) | ns | 0/18 (0) | ns | 5/18 (28) | ns |
| CMML1/2 (5) | 3/5 (60) | 0.031 | 3/5 (60) | 0.022 | 2/5 (40) | ns |
| MDS-U (7) | 0/7 (0) | ns | 0/7 (0) | ns | 0/7 (0) | ns |
| IPSS† | ||||||
| Low (Low/Int-1) (45) | 7/45 (16) | - | 6/45 (13) | - | 10/45 (22) | - |
| High (Int-2/High) (7) | 2/7 (29) | ns | 2/7 (29) | ns | 2/7 (29) | ns |
| MDAS†† | ||||||
| Low (Low/Int-1) (35) | 3/35 (9) | - | 3/35 (9) | - | 5/35 (14) | - |
| High (Int-2/High) (17) | 6/17 (35) | 0.023 | 5/17 (29) | ns | 7/17 (41) | 0.042 |
| Age (years) | ||||||
| <65 (17) | 2/17 (12) | - | 2/17 (12) | - | 2/17 (12) | - |
| ≥65 (35) | 7/35 (20) | ns | 6/35 (17) | ns | 10/35 (29) | ns |
| History of Cytopenias | ||||||
| Anemia (Hb<9 g/dL) No (23) | 5/23 (22) | - | 4/23 (17) | - | 2/23 (9) | - |
| Yes (29) | 4/29 (14) | ns | 4/29 (14) | ns | 10/29 (34) | 0.046 |
| Neutropenia (<1 × 109/L) | ||||||
| No (31) | 6/31 (19) | - | 5/31 (16) | - | 9/31 (29) | - |
| Yes (21) | 3/21 (14) | ns | 3/21 (14) | ns | 3/21 (14) | ns |
| Thrombocytopenia (<100×109/L) | ||||||
| No (31) | 6/31 (19) | - | 5/31 (16) | - | 9/31 (29) | - |
| Yes (21) | 3/21 (14) | ns | 3/21 (14) | ns | 3/21 (14) | ns |
| No. of Cytopenias<2 (33) | 7/33 (21) | - | 6/33 (18) | - | 8/33 (24) | - |
| No. of Cytopenias ≥2 (19) | 2/19 (11) | ns | 2/19 (11) | ns | 4/19 (21) | ns |
| Complete Blood Count (CBC) | ||||||
| Platelets ≥50* (×109/L) (40) | 3/40* (8) | - | 3/40* (8) | - | 10/40* (25) | - |
| Platelets<50*(×109/L) (12) | 4/12* (33) | ns | 3/12* (25) | ns | 2/12* (17) | ns |
| Hg≥10**(g/dL) (33) | 5/33** (15) | - | 4/33** (12) | - | 4/33** (12) | - |
| Hg<10**(g/dL) (19) | 4/19** (21) | ns | 3/19** (16) | ns | 8/19** (42) | 0.038 |
| Bone Marrow Blasts | ||||||
| Myeloblast<5% (42) | 5/42 (12) | - | 6/42 (14) | - | 6/42 (14) | - |
| Myeloblast≥5% (10) | 4/10 (40) | ns | 2/10 (20) | ns | 6/10 (60) | 0.006 |
| Prior Transfusion | ||||||
| No (28) | 4/28 (14) | - | 4/28 (14) | - | 6/28 (21) | - |
| Yes (24) | 5/24 (21) | ns | 4/24 (17) | ns | 6/24 (25) | ns |
| Karyotype | ||||||
| Normal (35) | 6/35 (17) | ns | 6/35 (17) | ns | 8/35 (23) | ns |
| Abnormal‡: Unfavorable (9) | 3/9 (33) | ns | 2/9 (67) | ns | 2/9 (22) | ns |
| Favorable (8) | 0/8 (0) | ns | 0/8 (0) | ns | 2/8 (25) | ns |
Platelet counts were unavailable for four patients. Two of these patients had high total Tregs, and two had high TregCM numbers.
Hemoglobin levels were unavailable for two patients. One of these had high total Tregs, and one had high TregCM numbers.
World Health Organization (WHO) includes refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with multilineage dysplasia (RCMD), refractory anemia with multilineage dysplasia with ringed sideroblasts (RCMD-RS), refractory anemia with excessive blasts (RAEB), chronic myelomonocytic leukemia (CMML) and MDS-unclassified (MDS-U)(1).
International Prognostic Scoring System (IPSS) low risk (IPSS score low or intermediate-1) or high risk (IPSS score intermediate-2 or high).
MD Anderson Scoring System (MDAS) classification lower-risk (low or intermediate-1) or higher-risk (intermediate-2 or high)(2, 3).
Karotype was performed by standard cytogenetics and was available for all 52 patients. Favorable karyotype includes del(5q), -Y, del(20q) and an unfavorable karyotype includes chromosome 7 abnormalities, or complex (≥3 abnormalities) based on IPSS criteria(1).
MDS Patients with Elevated TregEM Cells Have Reduced Overall Survival
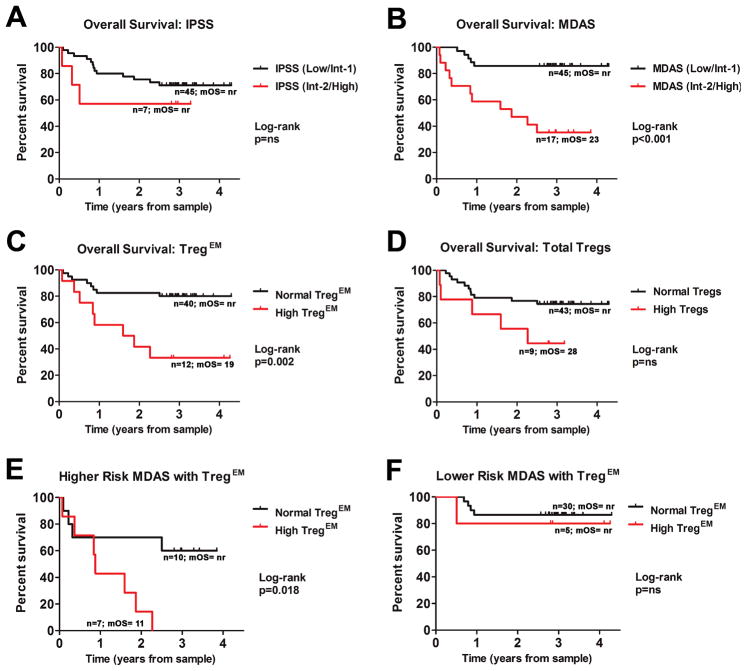
The impact of total Tregs and Treg subsets on OS was then examined. A total of 16 patients (31%) in this cohort had died at the time of retrospective analysis and the median survival of the 52 patients in total was not reached. The median duration of follow-up was 3.1 years (range 2.7 to 4.9) from sample acquisition. In this cohort, there was a trend, but no statistical difference detected in OS by univariate Cox-regression analysis or log-rank test based on IPSS risk (HR 2.0, 95%CI 0.6–7.0, p=0.287) (Figure 4A, Table 3) possibly related to sample size and due to the primary focus on IPSS lower-risk patients in this study. The MDAS model revealed subgroups with different OS (HR 6.3, 95%CI 2.2–18.1, p<0.001) (Figure 4B) and confirmed the ability of this system to refine survival estimates in IPSS lower-risk MDS patients. Cox-regression survival analyses were then performed to determine variables that impacted OS in this cohort of patients (Table 3) and platelet count less than 50k/μl (p=0.008), white blood cell count (WBC) greater than 20×109/L (p=0.007) hemoglobin less than 10g/dL (p=0.018), and blast count 5% (p=0.005) (Table 3) were negatively correlated with OS. Survival data was then examined in two groups of patients segregated by high and normal Treg subsets. By univariate Cox-regression survival analyses and log rank, patients with high TregEM cells had significantly worse OS compared to MDS cases with normal numbers of TregEM cells (HR 4.3, 95%CI 1.6–11.6, p=0.004) (Figure 4C, and Table 3). While the difference based on total Tregs was not significant (Figure 4D, and Table 3), a negative trend was observed (HR 2.6, 95%CI 0.9–7.6, ns). As shown in Table 4, we show that TregEM expansion represents an independent prognostic factor in MDS in multivariate analyses including total Tregs, TregN, and TregCM numbers (TregEM: HR 3.8, 95% CI 1.3–11.1, p=0.017), higher-risk IPSS (TregEM: HR 4.9, 95% CI 1.8–13.6, p=0.002), higher-risk MDAS (TregEM: HR 2.9, 95% CI 1.0–8.1, p=0.047), platelet counts<50×109/L (TregEM: HR 4.6, 95% CI 1.6–13.1, p=0.004), Hg<10g/dL (TregEM: HR 3.2, 95% CI 1.2–9.0, p=0.025), WBC>20×109/L (TregEM: HR 3.4, 95% CI 1.2–9.7, p=0.022), and increased blasts ≥5% (TregEM: HR 3.2, 95% CI 1.1–9.2, p=0.029) when adjusting for each factor individually, or when adjusting for all four factors (TregEM: HR 3.7, 95% CI 1.1–12.2, p=0.036). The presence of high TregEM cells improved upon the MDAS system and was able to independently refine risk estimates of patients with higher MDAS-risk (int-2/high) classification (Figure 4E and Table 4), but did not impact OS estimates in lower MDAS-risk patients (Figure 4F and Table 4). These data indicate that high TregEM numbers an independent prognostic factor, but that it may improve upon already established risk models.
Figure 4. Reduced overall survival (OS) in MDS patients with high TregEM numbers.
OS data of 52 MDS patients was analyzed using the Log-rank method and visualized using Kaplan-Meier plots as stratified by IPSS (A), MDAS (B), by TregEM numbers (C) and by total Treg numbers (D). OS was also analyzed between patients stratified by normal or high TregEM cell numbers in regard to higher-risk (E) and lower-risk (F) MDAS. ns = not significant. mOS= median overall survival (months). nr= not reached.
Table 3.
Kaplan-Meier and Univariate Cox-regression Analyses for Overall Survival
| Univariate Cox-regression (N=52) | ||||
|---|---|---|---|---|
|
| ||||
| Variables | n (%) | HR | 95%CI | P |
| Treg & Treg Phenotypes | ||||
|
| ||||
| Normal Total Tregs | 43 (83) | |||
| High Total Tregs | 9 (17) | 2.6 | 0.9–7.6 | ns |
|
| ||||
| Normal TregN | 48 (91) | |||
| High TregN | 4 (8) | 1.7 | 0.4–7.5 | ns |
|
| ||||
| Normal TregCM | 44 (85) | |||
| High TregCM | 8 (15) | 2.2 | 0.7–6.9 | ns |
|
| ||||
| Normal TregEM | 40 (77) | |||
| High TregEM | 12 (23) | 4.3 | 1.6–11.6 | 0.004 |
|
| ||||
| Risk Scoring Systems | ||||
|
| ||||
| IPSS (Low/Int-1)† | 45 (87) | |||
| IPSS (Int-2/High) | 7 (13) | 2.0 | 0.6–7.0 | ns |
|
| ||||
| MDAS (Low/Int-1)†† | 35 (67) | |||
| MDAS(Int-2/High) | 17 (33) | 6.3 | 2.2–18.1 | 0.001 |
|
| ||||
| Established Risk Factors | ||||
|
| ||||
| Age < 65 | 17 (33) | |||
| Age ≥ 65 | 35 (67) | 4.0 | 0.9–17.7 | ns |
|
| ||||
| No Transfusion | 28 (54) | |||
| Prior Transfusion | 24 (46) | 2.3 | 0.8–6.3 | ns |
|
| ||||
| Platelets ≥ 50×109/L# | 36 (75) | |||
| Platelets < 50×109/L# | 12 (25) | 4.0 | 1.4–11.1 | 0.008 |
|
| ||||
| Hg ≤10g/dL# | 31 (62) | |||
| Hg <10g/dL# | 19 (38) | 3.4 | 1.2–9.3 | 0.018 |
|
| ||||
| ECOG<2 | 51 (98) | |||
| ECOG ≥2* | 1 (2) | - | - | - |
|
| ||||
| WBC < 20×109/L† | 48 (96) | |||
| WBC ≥20×109/L† | 2 (4) | 8.4 | 1.8–39.2 | 0.007 |
|
| ||||
| No. of Cytopenias <2 | 33 (63) | |||
| No. of Cytopenias ≥2 | 19 (37) | 1.2 | 0.4–3.2 | ns |
|
| ||||
| Favorable/Normal Karyotype‡ | 43 (84) | |||
| Unfavorable Karyotype‡ | 9 (16) | 2.6 | 0.9–7.7 | ns |
|
| ||||
| Myeloblasts<5%## | 42 (81) | |||
| Myeloblasts ≥5% | 10 (19) | 4.2 | 1.5–11.2 | 0.005 |
Univariate analysis was not performed due to an insufficient number of patients meeting the criteria for this risk factor.
Data was not available for all 52 patients for this risk factor.
Bone marrow myeloblast count.
International Prognostic Scoring System (IPSS) low risk (IPSS score low or intermediate-1) or high risk (IPSS score intermediate-2 or high).
MD Anderson Scoring System (MDAS) classification lower-risk (low or intermediate-1) or higher-risk (intermediate-2 or high)(2, 3).
Karotype was performed by standard cytogenetics and was available for all 52 patients. Favorable karyotype includes del(5q), -Y, del(20q) and an unfavorable karyotype includes chromosome 7 abnormalities, or complex (≥3 abnormalities) based on IPSS criteria(1)
Table 4.
Multivariate Cox-regression Analyses for Overall Survival
| Multivariate Cox-regression (N=52)
| ||||
|---|---|---|---|---|
| Variables | HR | 95% CI | P | n(%) |
| High Total Tregs | 1.9 | 0.1–36.7 | ns | 9 (17) |
| High TregN | 0.8 | 0.1–6.9 | ns | 4 (8) |
| High TregCM | 0.8 | <0.1–13.0 | ns | 8 (15) |
| High TregEM | 3.8 | 1.3–11.1 | 0.017 | 12 (23) |
|
| ||||
| (N=52) | ||||
| High Treg EM | 4.9 | 1.8–13.6 | 0.002 | 12 (23) |
| IPSS (Int-2/High)† | 2.8 | 0.7–10.1 | ns | 7 (13) |
|
| ||||
| (N=48)* | ||||
| High TregEM | 2.9 | 1.0–8.1 | 0.047 | 12 (23) |
| MDAS (Int-2/High)†† | 4.9 | 1.6–14.8 | 0.005 | 17 (33) |
|
| ||||
| (N=50)* | ||||
| High TregEM | 4.6 | 1.6–9.0 | 0.004 | 11 (23) |
| Platelets< 100×109/L | 4.9 | 1.7–13.8 | 0.003 | 12 (25) |
|
| ||||
| (N=50)* | ||||
| High TregEM | 3.2 | 1.2–9.0 | 0.025 | 12 (24) |
| Hg<10g/dL | 2.6 | 0.9–7.5 | ns | 19 (38) |
|
| ||||
| (N=52) | ||||
| High TregEM | 3.4 | 1.2–9.7 | 0.022 | 12 (24) |
| WBC ≥20 × 109/L | 4.0 | 0.8–20.3 | ns | 2 (4) |
|
| ||||
| (N=48)* | ||||
| High TregEM | 3.2 | 1.1–9.2 | 0.029 | 12 (23) |
| Myeloblasts ≥5% | 2.9 | 1.1–8.4 | 0.045 | 10 (19) |
|
| ||||
| High TregEM | 3.7 | 1.1–12.2 | 0.036 | 11 (23) |
| Platelets<50k/μl | 6.5 | 2.0–21.1 | 0.002 | 12 (25) |
| Hg<10g/dL | 1.2 | 0.3–4.1 | ns | 18 (38) |
| WBC ≥20 ×109/L | 8.9 | 1.4–54.4 | 0.019 | 2 (42) |
| Myeloblasts ≥5% | 4.2 | 1.2–14.4 | 0.023 | 9 (19) |
Data for at least one variable was not available for all 52 patients.
International Prognostic Scoring System (IPSS) low risk (IPSS score low or intermediate-1) or high risk (IPSS score intermediate-2 or high).
MD Anderson Scoring System (MDAS) classification lower-risk (low or intermediate-1) or higher-risk (intermediate-2 or high)(2, 3).
Discussion
A role for Tregs in tumor immune evasion is well defined in animal models and in established solid tumors, but there is limited information about the factors or conditions that contribute to their accumulation in premalignant human diseases. Events that precede diagnosis such as inflammation, constant assault by autoreactive tumor-associated antigens, and establishment of the suppressive microenvironment may contribute to Treg expansion. We hypothesized that studies in a well-defined premalignant neoplasm, such as MDS, would identify the prognostic importance of Treg phenotypic changes that may indicate unique factors that contribute to their expansion in humans. MDS is associated with a heterogeneous clinical presentation and variable rates of leukemia transformation allowing concrete investigations into mechanisms governing disease progression and a role for Tregs in MDS evolution is well established(24). Our investigations demonstrated for the first time that expansion of a phenotypically unique suppressive Treg subpopulation (TregEM cells) is associated with malignant progression. The phenotypic markers expressed by Tregs in MDS suggest that they may be recently activated in a similar manner to conventional effector memory T-cells(16–19) since they lose CD27 expression. TregEM cells were capable of identifying a higher risk subpopulation of patients indicating that an accumulation of these cells may indicate the initiation of an immunosuppressve microenvironment that impacts treatment. In this cohort of patients, the presence of TregEM cells was an independent discriminating factor important for disease prognostication, inferior OS and myeloblast accumulation suggesting that this is an important phenotype within the Treg compartment.
During carcinogenesis, developing neoplasms elicit cytotoxic responses from conventional T-cells through the presentation of immunogenic autoantigens(25–27). Arising in the thymus, naturally occurring Tregs (nTregs) activate in response to self-antigen presentation in the context of MHC-class II(20–22), and activated Tregs control autoreactive effector T-cells that escape central tolerance and become responsive to autoantigens(28, 29). Overexpressed autoantigen presentation, well defined in the bone marrow of IPSS lower-risk MDS patients, may also activate Tregs, leading to their expansion and the escape of the developing neoplasm from immunosurveillance and ultimately to leukemia progression. In addition to naturally occurring Tregs (nTreg), conventional T-cells can be induced to express FoxP3 in the periphery after activation in polarizing conditions (inducible Tregs, iTregs)(30). Following development, all Tregs persist in secondary lymph tissue and in the periphery(31–34) where their numbers are tightly maintained. Significant alteration in the balance of the nTregs or iTreg compartments has pathologic consequences. Accumulation of nTregs is demonstrable in patients with solid tumors(31, 35–42) and in some hematologic malignancies(43), and is associated with anti-tumor immune suppression in animal models(12, 44). The emergence of TregEM cells may originate from either naturally-occurring or inducible populations, but in either case this phenotypic change may reflect cellular activation as their presence in MDS patients is associated with myeloblast accumulation. A mechanistic explanation for the observed differences in the suppressive capacity of the individual Treg subsets would be beneficial, and experiments are currently ongoing to understand these principles.
Current factors used in prognostic MDS models reflect progressive changes inherent to the dysplastic myeloid clone including bone marrow morphology, cytogenetics, mutations, transfusion dependency, the number of cytopenias, as well as age and other comorbidities. The newer models, such as MDAS, has successfully refined prognostic precision by adding age and ECOG performance status, and weighted contribution of blasts and blood cell counts(4–6). None of these factors, however, reflect progressive changes in immune parameters that may impact the disease. An increase in TregEM likely reflects active immune suppression and may fundamentally represent the earliest biomarker to indicate conversion to an immunosuppressive microenvironment.
Several FDA-approved drugs for MDS display variable response rates with preferential activity in select disease subsets including erythroid stimulating agents, hypomethylating agents “azanucleosides” 5-azacytidine and decitabine(45), immunomodulatory drug lenalidomide(46, 47), and immunosuppressive (IST) therapy such as anti-thymocyte globulin (ATG) and cyclosporine(8, 10, 48). Patients included in this study received no prior disease-modifying therapy other than growth factors such as G-CSF for cytopenias. Current prognostic models are incapable of discriminating response to therapies so Treg phenotyping may be a useful tool to segregate MDS patients who are responsive to various drug classes. Therefore, inclusion of Treg status into the current prognostic and treatment models may improve prognostication and better inform therapeutic decisions in MDS. Our study sheds light into unique aspects of T-cell-mediated pathophysiology as it relates to human immunity in a premalignant model of disease and implicates specific TregEM expansion in disease progression in MDS.
Supplementary Material
Figure 3. Association with increased blast percentage is unique to MDS patients with elevated TregEM cells.
The absolute numbers (A) or percentages (B) of total Tregs, TregN, TregCM, and TregEM were compared between patients with normal blast percentage (<5%) and patients with increased blast percentage (≥5%). ns = not significant.
Acknowledgments
Funding for this project was provided by NIH R01 grant CA129952 and a grant from Genzyme Corporation. The authors thank the H. Lee Moffitt Cancer Center Flow Cytometry Core Facility for advice and assistance with acquisition and analyses of flow cytometry data. Statistical analysis was performed in conjunction with the Biostatistics Program at H. Lee Moffitt Cancer Center. Samples were obtained from the Bone Marrow Failure Rare Diseases Clinical Research Network (BMF-RDCRN). The authors thank reviewer from Genzyme Corporation for their comments.
Footnotes
Authorship Contributions
A.W.M performed statistical analyses, Treg functional experiments, collected and analyzed survival data, and wrote the manuscript, C.S. planned the original flow panel and acquired data on Tregs, R.S.K, J.P.M, M.A.S., R.L.P, T.P.L, and A.F.L. are members of the Bone Marrow Failure Rare Diseases Clinical Research Network (BMF-RDCRN) consented patients, obtained samples, collected clinical data. PKE-B is senior author, reviewed all aspects of the study design, data analyses, and wrote the manuscript. All authors reviewed the manuscript and approved publication.
Conflicts of Interest
The authors have no conflicts of interest to declare. The work was supported by a grant from Genzyme Corporation.
References
- 1.Swerdlow S C. International Agency for Research on, and O. World Health. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Germing U, Strupp C, Kuendgen A, Isa S, Knipp S, Hildebrandt B, Giagounidis A, Aul C, Gattermann N, Haas R. Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Haematologica. 2006;91:1596–1604. [PubMed] [Google Scholar]
- 3.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L, Maffioli M, Bernasconi P, Lazzarino M, Cazzola M. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, Freireich EJ, Garcia-Manero G. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao JM, McMillan A, Greenberg PL. International MDS risk analysis workshop (IMRAW)/IPSS reanalyzed: impact of cytopenias on clinical outcomes in myelodysplastic syndromes. American journal of hematology. 2008;83:765–770. doi: 10.1002/ajh.21249. [DOI] [PubMed] [Google Scholar]
- 6.Komrokji RS, Corrales-Yepez M, Ali NA, Kharfan-Dabaja M, Padron E, Fields T, Lancet JE, List AF. Validation of the MD Anderson Prognostic Risk Model for patients with myelodysplastic syndrome. Cancer. 2012 doi: 10.1002/cncr.26567. [DOI] [PubMed] [Google Scholar]
- 7.Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol. 1998;102:1314–1322. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 8.Molldrem JJ, Leifer E, Bahceci E, Saunthararajah Y, Rivera M, Dunbar C, Liu J, Nakamura R, Young NS, Barrett AJ. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Annals of internal medicine. 2002;137:156–163. doi: 10.7326/0003-4819-137-3-200208060-00007. [DOI] [PubMed] [Google Scholar]
- 9.Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, Keyvanafar K, Lu J, Basu A, Barrett AJ, Young NS. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloand EM, Olnes MJ, Shenoy A, Weinstein B, Boss C, Loeliger K, Wu CO, More K, Barrett AJ, Scheinberg P, Young NS. Alemtuzumab treatment of intermediate-1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28:5166–5173. doi: 10.1200/JCO.2010.29.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen D, Culligan D, Jowitt S, Kelsey S, Mufti G, Oscier D, Parker J. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 12.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer research. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 13.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, Lombardi G, Wlodarski MW, Maciejewski JP, Farzaneh F, Mufti GJ. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–850. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 14.Picca CC, Larkin J, 3rd, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunological reviews. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 15.Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 16.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 17.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. The Journal of experimental medicine. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (New York, NY. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. The Journal of experimental medicine. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 22.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. The Journal of experimental medicine. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 24.Bouchliou I, Miltiades P, Nakou E, Spanoudakis E, Goutzouvelidis A, Vakalopoulou S, Garypidou V, Kotoula V, Bourikas G, Tsatalas C, Kotsianidis I. Th17 and Foxp3(+) T regulatory cell dynamics and distribution in myelodysplastic syndromes. Clin Immunol. 2011;139:350–359. doi: 10.1016/j.clim.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein G. Tumor antigens. Annu Rev Microbiol. 1966;20:223–252. doi: 10.1146/annurev.mi.20.100166.001255. [DOI] [PubMed] [Google Scholar]
- 27.Old LJ, Boyse EA. Immunology of Experimental Tumors. Annu Rev Med. 1964;15:167–186. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 29.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 30.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature reviews. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 31.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 33.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. European journal of immunology. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 34.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer research. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 35.Kawaida H, Kono K, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Ooi A, Fujii H. Distribution of CD4+CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 37.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer research. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 38.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 39.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 40.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 41.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–2670. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 42.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer research. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 43.Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. The Journal of clinical investigation. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 46.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, Schmidt M, Zeldis J, Knight R. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 47.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 48.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.