Abstract
Cytochrome c Oxidase (CcO) is the terminal oxidase of the mitochondrial electron transport chain. This bigenomic enzyme in mammals contains 13 subunits, of which, three catalytic subunits are encoded by the mitochondrial genes. The remaining ten subunits with suspected roles in the regulation, and/or, assembly are coded by the nuclear genome. The enzyme contains two heme groups (heme a and a3) and two Cu2+ centers (Cu2+ A and Cu2+ B) as catalytic centers and handles more than 90% of molecular O2 respired by the mammalian cells and tissues. CcO is a highly regulated enzyme which is believed to be the pace setter for mitochondrial oxidative metabolism and ATP synthesis. The structure and function of the enzyme is affected in a wide variety of diseases including cancer, neurodegenerative diseases, myocardial ischemia/reperfusion, bone and skeletal diseases and diabetes. Despite handling a high O2 load the role of CcO in the production of reactive oxygen species still remains a subject of debate. However, a volume of evidence suggests that CcO dysfunction is invariably associated with increased mitochondrial reactive oxygen species production and cellular toxicity. In this article we review literature on mechanisms of multimodal regulation of CcO activity by a wide spectrum of physiological and pathological factors. We also review an array of literature on the direct or indirect roles of CcO in reactive oxygen species production.
Keywords: Cytochrome C Oxidase, mitochondrial dysfunction, oxidative stress, alcohol toxicity, drug effects, myocardial ischemia, regulation of enzyme activity, phosphorylation, Protein kinse A, cAMP, respirasome assembly, mtDNA mutations
Introduction
Under normal physiological conditions, mitochondrial oxidative phosphorylation accounts for more than 90% of the cellular ATP production in most cells and tissues. Mitochondria are also involved in the maintenance of Ca2+ homeostasis, in addition to carrying out critical reaction steps of steroid hormone metabolism, pyrimidine synthesis, elimination of ammonia through urea cycle, and programmed cell death. In addition, they are the major cellular source for reactive oxygen species (ROS) [23,75]. Almost all functions of mitochondria are either directly or indirectly linked to the working of oxidative phosphorylation machinery and energy coupling. Most part of this machinery is in the inner mitochondrial membrane and comprises the four electron transfer chain complexes (complex I, II, III and IV), ATP synthase (complex V) and ubiquinone, and cytochrome c as electron carriers. Complex IV or CcO is the terminal enzyme of the electron transport chain, which catalyses the final step of electron transfer from reduced cytochrome c to oxygen to make H2O. CcO is also one of the three proton pumps along with complexes I and III that generate the proton gradient across the inner mitochondrial membrane, which powers the ATP synthesis. This review focuses on function and the multilayered regulation of cytochrome c oxidase (CcO) and explores the mechanisms by which CcO defects play a role in mitochondrial dysfunction and oxidative stress.
Highly oxidative tissues and cells such as the heart, brain and kidney nephrons produce generally higher levels of ROS compared to less oxidative tissues due to higher rates of electron transfer chain activities and highly polarized transmembrane potential. Mechanisms of ROS production by highly polarized mitochondria with high Δφm have been discussed elegantly in a recent review by Murphy [99]. It should however be recognized that high transmembrane potential is not the only cause of ROS production since mitochondria with defective, dysfunctional electron transport complexes, or imbalance in the levels of different complexes and electron donors also produce higher rates of ROS. The literature on this aspect of mitochondrial biology and oxidative stress is highlighted in this review.
CcO is a bigenomic complex with subunits coded by both mitochondrial and nuclear DNA. A coordinated expression of these subunits and their assembly in the right stoichiometry provides cells different modes of regulation of enzyme content in mitochondria. Of the thirteen subunits of the mammalian CcO, the mitochondrial genome encodes subunits I, II and III, which form the catalytic core of the enzyme [31]. During a catalytic cycle, the copper center CuA associated with subunit II, accepts electrons from reduced cytochrome C and transfers to the heme a in subunit I. From heme a, electrons are transferred to the bimetallic center heme a3/CuB, also in subunit I, to reduce molecular oxygen to water; the function of subunit III is not clearly understood [31]. The nuclear genome codes for the remaining ten subunits of CcO, which are synthesized in cytosol and imported into mitochondria. While some of the nuclear subunits provide structural stability to the complex, in general, they are thought to be involved in the regulation of enzyme activity [31]. The importance of CcO is underlined by the fact that organisms have evolved different levels of regulation of its activity. Under normal physiological conditions, CcO acts as the rate limiting step of respiratory chain and its activity is an indicator of the oxidative capacity of the cells.
Regulation of Cytochrome c Oxidase Function
Subunit Isoforms
Organisms from different phylogenetic order contain CcO with variable number of subunits ranging from 4-13 in different species; 4 in bacteria, 7 in slime-molds, 12 in Yeast and 13 in vertebrates [88]. The three largest and evolutionarily conserved subunits I, II and III carry the heme and Cu+2 redox centers and form the catalytic core of the enzyme. In eukaryotes, these subunits are encoded by the mitochondrial genome and the remaining are coded by nuclear genes. Many of the nuclear subunits exist as multiple isoforms that are constitutively expressed in a tissue specific manner or are induced under certain physiological conditions. In vertebrates, subunits VIa, VIIa and VIII are expressed as L and H isologs [6,87,158]. The L isologs are expressed ubiquitously in all tissues, while the H isologs are expressed exclusively in the skeletal and cardiac muscle [87,130,132]. It has been suggested that subunits VIaH and L isologs may contribute to the tissue specific differences in ADP/ATP binding to Cytochrome oxidase [71]. As explained in a later section, binding of ADP/ATP is an important mechanism for allosteric regulation of CcO activity which directly relates to the bioenergetic status of the cell.
Regulation by subunit switching
In Yeast, COX 5 is expressed as 5a and 5b isologs. Subunit 5a is expressed under aerobic conditions and is replaced by 5b under low oxygen levels [28]. The main difference between the yeast enzymes containing Va and Vb subunits is the rate of electron transfer to the binuclear center and thus the catalytic function [155]. The mammalian homolog of this subunit, designated as IV, is expressed as two isoforms, IVi1 and IVi2 [72]. Unlike the yeast homolog, regulation of mammalian subunit IV appears to be more complex. This stems from the fact that isoform IVi2 is both inducible and differentially expressed in mammalian tissues. Huttemann and coworkers, showed high levels of constitutively expressed IVi2 in lung and trachea, with undetectable levels in other tissues like heart, brain, muscle and liver [72]. Mouse models with CcO IVi2 gene knock out show decreased CcO activity and ATP levels in lung and develop lung pathology that deteriorates with age [73]. Promoter analysis showed presence of a 24bp proximal promoter region called oxygen response element (ORE), which is essential for constitutive expression of IVi2 in human lung cells [74]. Semenza’s lab on the other hand, showed that in both liver and lung, hypoxic environment causes induction of isoform IVi2 that switches subunit composition of CcO from IVi1 to IVi2 [59]. Further, they showed two hypoxia response elements (HRE) in the 5′ flanking region and intron 1 of Cox4I2 gene. Both hypoxia (1% O2) and over expression of Hif 2 in HeLa cells and 293T cells strongly induced the transcription activity of Cox4I2 promoter luciferase reporter constructs showing the presence of a Hif inducible mechanism of IVi2 up regulation [59]. These studies show that altered subunit composition by switching isoforms is an important mechanism by which the catalytic function of CcO complex is regulated.
Tissue distribution
Vijayasarathy et al. reported that CcO activity varies in heart, brain, kidney and liver and in different heart compartments depending on the oxidative capacity and workload of the tissue [150]. Immunoblots and mRNA analysis showed a variation in CcO subunit content in these tissues as well as in different cardiac compartments. Tissues with low energy demand such as liver exhibited lower levels of CcO subunits IV and Vb and CcO activity compared to high oxygen consuming tissues such as the brain and kidney [150]. Interestingly, subunit IVi1 expression in rat testis was similar to heart although CcO enzyme activity is among the lowest in testis [152]. Similarly among the cardiac compartments, left ventricle and right atrium contain nearly twice the amount of Vb and VIa compared to left atrium and right ventricle. Liver CcO exhibited a high Km for cytochrome C and low turnover number (TN) while heart had the highest TN with low Km. Kidney and brain showed intermediate kinetic values [150].
Substrate availability and allosteric regulation
A host of small molecules have been shown to interact with CcO subunits, affecting the kinetic properties of the enzyme.
Both ATP and ADP bind to CcO. Using kinetic analysis, seven ATP and ten ADP binding sites have been determined in bovine CcO [102]. Subunits, IV and VIa have been identified as ATP binding subunits [17,102]. Titration of ATP versus ADP showed that while all of the ATP binding sites can also be occupied by ADP, three of the ADP binding sites are exclusive. This suggested that rather than the concentration of ATP, the ratio of ATP to ADP determines the allosteric status of the enzyme. A higher ADP content increases enzyme activity while higher steady state ATP results in allosteric inhibition [102]. Based on ATP/ADP mediated regulation of CcO activity Kadenbach and co workers proposed a second mechanism of respiratory control termed RC2 to explain the variable relationships between respiration and Δp (proton gradient) [10]. They showed that ATP/ADP mediated allosteric regulation of CcO activity in turn regulates the rate of respiration, independent of Δp [10,76]. More recently, using isolated cow and rat heart mitochondria these investigators showed that high matrix ATP/ADP ratios keep Δφm at low level possibly through allosteric modulation of CcO activity to reduce ROS production [76,117,118].
Although allosteric modulation provides a mechanism to regulate enzyme activity in response to ATP levels, cells can bypass the ATP/ADP dependent allosteric regulation to meet their changing energy demands. At least three mechanisms have been described by which allosteric inhibition of CcO by ATP is reversed. One such mechanism is by assembling CcO with subunit IVi2, an isoform that is not subject to allosteric inhibition by ATP [59,69]. Hypoxia in cortical astrocytes and neurons induces expression of subunit IVi2 [69]. In astrocytes, ATP levels even under hypoxia are sufficient to negatively regulate CcO activity [69]. Subunit IVi2 expression under hypoxia in these cells therefore prevents any further inhibition of CcO by ATP when cells are under bioenergetic crisis. Similarly, in HeLa and 293T cells, hypoxia causes an isoform switch from subunit IV i1 to IV i2 [59]. CcO complexes containing IVi2 have also been shown to be catalytically more efficient with higher turnover number (TN) and lower Km for cytochrome C [69,137]. Similarly in yeast, at low oxygen concentrations substitution of COX5b, the yeast homolog of mammalian IVi2 for COX5a resulted in higher turnover number of cytochrome c oxidase, which is an adaptive advantage under hypoxia [4,155]. The second mechanism of relieving ATP mediated regulation of CcO activity is by binding thyroid hormone 3,5 diiodothyronine (T2). T2 has been shown to directly bind to subunit Va and abrogate ATP mediated allosteric inhibition of CcO [9]. This effect represents a forced activation of CcO activity in response to hormonal stimulation and is in accordance with the effect of T2 on energy metabolism [9]. Phosphorylation and dephosphorylation of specific subunits are yet another mechanism to either abrogate or activate ATP dependent allosteric inhibition and is discussed in detail in the next section.
Apart from ATP/ADP, other small molecules have been shown to interact with Cytochrome oxidase, although their physiological significance has not been as extensively studied. CcO subunit Vb interacts through its amino terminal end with RI , the regulatory subunit of cAMP dependent protein kinase (PKA) [159]. In CHO cells, binding of RI is sensitive to cAMP level and has been shown to regulate CcO activity. In these cells, under normal conditions, binding of RI is required for basal CcO activity. It is speculated that conditions leading to increased cAMP levels might dissociate RI from Vb leading to inhibition of CcO activity [159].
Four different gases, Nitric oxide (NO), Carbon monoxide (CO), Hydrogen Sulfide (H2S) and Hydrogen Cyanide bind to CcO and invariably inhibit the enzyme activity. Their interaction with CcO and its physiological significance have been reviewed in detail by Cooper and Brown [48]. NO has been established as an important second messenger, which is involved in diverse physiological and pathological functions. Although soluble guanylate cyclase is one of its most prominent targets, NO interacts with metal centers of many proteins. Reversible inhibition of CcO by NO was demonstrated nearly two decades ago [25,43,131]. Since then, physiological significance of this interaction and its role in cellular pathologies have been extensively investigated. Since O2 and NO compete for the same binding site in CcO, an important question has been whether endogenously generated NO can reach concentrations that are inhibitory to CcO under physiological oxygen levels. Several lines of experimental evidence indeed suggest that this is the case. In endothelial cells under basal conditions, NOS inhibitor N monomethyl l arginine, dramatically increased oxygen consumption [44]. Similarly NOS inhibitors increased oxygen consumption in whole body and specific tissues of conscious dogs [134,144]. Conversely, treatment with bradykinin and ATP, which increase endogenous NO by Ca2+ dependent NOS, decreased respiration [44]. An interesting physiological role for NO CcO signaling has been put forward by Darley-Usmar’s group. According to their model, inhibition of CcO by NO controls oxygen gradients in tissues by regulating consumption of O2 in actively respiring mitochondria [135,136]. Changes in local oxygen levels, when CcO is inhibited by NO, have been shown by multiple studies [64,89,142]. In addition, actively respiring mitochondria (State 3) have been shown to be more susceptible to NO inhibition than those undergoing state 4 respiration [19,24]. These findings have led to the suggestion that NO increases the availability of oxygen by minimizing its consumption by active CcO, thus preventing hypoxia in deeper regions of tissue [19,24].
CO is generated endogenously by degradation of Heme by Heme Oxygenase. Zuckerbraun et. al, showed that anti inflammatory effects of CO in macrophages is a result of direct inhibition of CcO [165]. However, this study used exogenously added CO and did not provide insight on physiological conditions that generate high CO in cells. In case of both NO and CO, since CcO inhibition is by competing with O2, the effects are likely to be more significant under pathological conditions of low oxygen tension such as hypoxia or when CcO complex is altered leading to lower affinity for oxygen. H2S is produced endogenously by both enzymatic and non enzymatic reactions and has been shown to affect vasodilation, neurotransmission and both proinflammatory and anti inflammatory pathways [48]. Hydrogen sulfide non competively inhibits CcO activity with Ki of 0.2 μM [113]. The resulting decrease in ATP level was thought to activate KATP channels and mediate many of the biological effects of H2S [48]. However, a more recent estimate of H2S concentration in tissue was found to be about 15 nM [60]. This suggests that physiological effects of H2S are likely to be due to its direct action on ion channels, transcription factors and other cellular proteins as reviewed in [85].
Covalent modification
Phosphorylation at eighteen different sites of CcO complex has been reported thus far under physiological and pathological conditions [67]. The kinases involved and the precise physiological role of phosphorylation are still not completely understood. Depending on the residues and subunits involved, the effect of phosphorylation has been shown to both activate and inhibit enzyme activity. T11 of subunit VIa, S34 and S58 of subunit IV, and S4 and T35 of subunit Va have been shown to be phosphorylated under normal physiological conditions [2,68]. Phosphorylation at S58 and its function was reported only recently by Manfredi’s lab [2]. Previous work by their group showed that metabolically generated CO2 increases mitochondrial cAMP by activating a CO2 bicarbonate regulated soluble adenylyl cyclase [3]. This in turn activates cAMP dependent protein kinase (PKA) in the mitochondrial matrix and phosphorylates CcO subunits I and IV [3]. Subunit IV was shown to be phosphorylated at S58, one of the ATP binding sites involved in allosteric regulation of CcO activity [2]. By computational modeling and induced fit, the authors propose that phosphorylation of S58 reduces ATP binding to the complex and abolishes ATP dependent inhibition of CcO activity. The proposed model allows regulation of CcO activity in accordance with the metabolic flux of the cell. The phosphorylation site on subunit I by this signaling and its role in CcO activation is yet to be determined.
Earlier studies showed that, in vitro phosphorylation of CcO in presence of cAMP and PKA resulted in phosphorylation of only subunits II and Vb [18]. Intriguingly, under these conditions phosphorylation resulted in inhibition of CcO activity, possibly by increasing ATP dependent allosteric inhibition [18]. cAMP dependent phosphorylation of CcO, however appears to be more complex than just regulation by metabolic flux. Lee et al. showed that in presence of theophylline, Tyrosine 304 of CcO subunit I is phosphorylated in a cAMP dependent manner [81]. In this case phosphorylation resulted in a decrease of enzyme activity and increased affinity for cytochrome c binding. However, phosphorylation by theophylline action differs in that Tyr 304 of CcO subunit I faces the intermembrane space, and thus, likely to be different from the site phosphorylated by mitochondrial matrix PKA. In addition, effect of cAMP is indirect and probably through activation of a tyrosine kinase [81]. Glucagon, a hormone produced during starvation to conserve energy also promotes subunit I phosphorylation at Y304 and CcO inhibition [81]. Prabu et al, have shown that subunits I, IVi1 and Vb are phosphorylated under hypoxia and ischemia/reperfusion stress in a cAMP and PKA dependent manner [115]. Although the phosphorylation sites on subunits IVi1 and Vb characterized in this study are not canonical PKA sites, results using H89 and specific inhibitor PKI, strongly suggest the role of PKA [115]. The nature of these phosphorylations and their effect on CcO activity will be discussed in a later section. In osteoclasts, phosphorylation of CcO is used to meet the high energy demand of their bone resorption activity. c-Src, a non-receptor tyrosine kinase was shown to phosphorylate subunit II [97]. Although not experimentally determined, phosphorylation site prediction suggests that Tyr 113 is the likely residue involved. Phosphorylation increases CcO activity and is positively correlated with increasing c-Src activity [97]. Apart from phosphorylation, the only other covalent modification of CcO reported is acetylation. Several mitochondrial proteins have been shown to be acetylated, and significance of some of them has been demonstrated only by activity of deacetylases. A recent high resolution mass spectrometry has identified N6 acetylation of subunit Vb at K121 and of subunit IVi1 at K53 and K60 [41]. However, the physiological significance of these modifications still remains to be established.
Biogenesis and organization in supercomplexes
CcO assembly is a concerted process and involves a sequential addition of subunits to form the final complex. More than 30 assembly factors have been identified so far [56,57]. They are involved at different stages of assembly like biosynthesis of heme, import and incorporation of nuclear subunits to the membrane, insertion of copper and regulation of assembly itself [56,57]. Strikingly, a majority of inherited CcO deficiencies are associated with defects in CcO assembly [51,53,111]. CcO assembly starts with a seed subunit, identified as Cox1 in mammals and is pulsated with formation of stable assembly intermediates as additional subunits are incorporated to the growing complex [57]. In the yeast S. cerevesiae, synthesis of Cox1p itself has been shown to be regulated by the availability of other assembly factors shy1p, Mss51 and Cox14p [15,16,112].
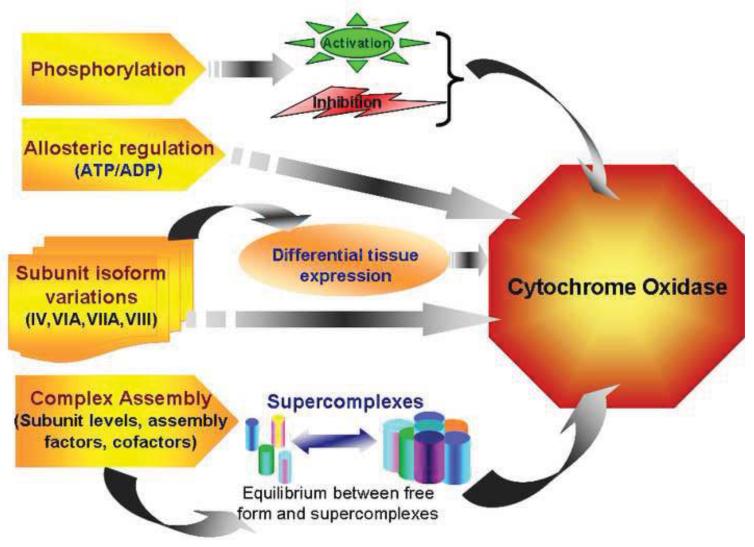
Fully assembled CcO has been shown to exist along with other electron transfer chain complexes, specifically complex I and III in larger assemblies called super complexes [1]. It has been proposed that super complexes increase the efficiency of OXPHOS by substrate channeling and reducing the distances of electron transfer in between centers [1,125,153]. They also prevent leakage of electrons and their premature oxidation to form reactive oxygen radicals. Interestingly, the composition of super complexes is variable resulting in a pool of free OXPHOS complexes and those present as supercomplexes [1,125,153]. The individual complexes are in a dynamic state of association with supercomplexes depending on the metabolic state of the cell. A single functional unit of supercomplex consisting of I III2 IV4 is called respirasome [128]. Experimental evidence suggests that these repirasomes are arranged in rows to form supramolecular structures called respiratory strings. Through its existence in a supercomplex, CcO (complex IV) has been shown to be an essential component for the assembly of functional complex I in fibroblasts [52]. Similarly assembly and stability of CcO itself has been shown to require the presence of cytochrome C and ATP synthase in mitochondria [121]. The multimodal regulation of CcO by allosteric and hormonal activators, subunit phosphorylation and organization in respirosome complexes has been summarized in Figure 1.
Figure 1.
Regulation of Cytochrome C Oxidase. Cells control CcO function by regulating both protein content and enzyme activity. Co ordinated expression of subunits, their assembly into complexes and supercomplexes provide a global control over protein levels of the enzyme. Differential cell and tissue specific expression of subunit isoforms and their relative abundance are used to change enzyme composition and alter kinetic properties of CcO activity in accordance with oxidative capacity of the tissues. Rapid adaptation to cellular bioenergetic requirements is brought about by binding small molecules like ATP/ADP, di-iodo thyronine, NO and by phosphorylation of specific subunits that either increase or decrease CcO activity. The multimodal regulation of CcO activity is summarized in this figure.
Cytochrome Oxidase Dysfunction and Oxidative Stress
As a critical component of the electron transport chain, CcO activity affects all aspects of mitochondrial function. While a robust CcO activity setting up steeper Δφm is expected to promote higher rates of mitochondrial superoxide production, dysfunctional CcO also promotes oxidative stress in many diseases. A diverse array of pathological conditions cause altered cytochrome oxidase function in cells and tissues. In addition to diseases, age related loss of CcO activity has been reported in many studies [108,138]. Except in cases of genetic defects, it is commonly seen that mitochondrial dysfunction is a cumulative effect of failure of more than one complex of the electron transport chain. Some of the common mechanisms of CcO dysfunction include assembly defects, covalent modifications and loss of subunits, disassembly of supercomplex organization and direct inhibition of enzyme activity. The impact of these events include energy crisis due to lower ATP production, lactic acidosis and increased formation of ROS in mitochondria. Although there is a volume of literature on CcO activity in many diseases, detailed investigation on the mechanism of CcO dysfunction and its consequence in the disease process are not completely understood. In this section, we review mechanisms of CcO dysfunction with reference to some of the conditions and assess their impact on mitochondrial function leading to oxidative stress.
Nuclear and mitochondrial mutations which cause defective assembly
Genetic defects resulting in either specific loss of mitochondrial subunits or defects in complex assembly are perhaps the only conditions leading to exclusive loss of CcO activity. They are ideal for understanding the role of CcO in mitochondrial dysfunction. MtDNA mutations that involve large scale rearrangements causing deletion or insertions and point mutations have been reported in all three mitochondrially encoded subunits of CcO [77,91,92]. They are responsible for mild to severe depletion of the subunits resulting in varying levels of loss of CcO activity. At least five different mutations have been identified for CoxI including nonsense mutations, microdeletion and heteroplasmic point mutations [14,27,47,62]. Mutations of CoxII include missense and initiation codon mutations [42,116]. CoxIII mutations include nonsense mutations, frame shift and micro deletions [66,77,143]. However, a majority of the mutations leading to CcO deficiency are those occurring in nuclear encoded assembly factors. The most common form is associated with Leigh disease, with mutations in SurfI, a protein involved in CcO assembly [164]. Other assembly factor mutations are found in LRPPRC and Taco1 [94,156]. Mutations in Copper chaperones Sco1 and Sco2, and factors involved in heme biosynthesis, Cox10 and Cox15 have been shown to cause varied CcO deficiencies with different clinical manifestations [7,45,147,148]. More recently, a single mutation in subunit COX VIb1 has been reported as the first ever structural subunit mutation of CcO [93]. Phenotypically these mutations are responsible for hypertrophic cardiomyopathy, encephalomyopathy, sideroblastic anemia, amyotrophic lateral sclerosis like syndrome, Leigh’s syndrome and MELAS. The severity and type of symptom are dependent on the mutation load and the organ affected. Energy crisis leading to lactic acidosis appears to be an underlying cause of most of the symptoms of genetic CcO deficiency.
Alterations in nuclear subunit levels
The prototypic cytochrome oxidase complex consists of three catalytic core subunits, I, II and III, which are sufficient to carry out the catalytic cycle of the enzyme. The additional subunits coded by nuclear genome found in higher eukaryotes, are used for regulating enzyme activity and to provide structural stability to the complex. Not surprisingly, many pathological conditions involving CcO dysfunction are due to alterations in the expression and regulation of some of these nuclear subunits.
Hypoxia and ischemia cause a time dependent damage to the electron transport chain in mitochondria. While short duration of hypoxia affects activities of complexes I and III [55,83,120], prolonged hypoxia (0.2 to 2% O2 for 8 12h) and global or focal ischemia of hearts have been shown to significantly decrease CcO activity [82,114,115,146,151]. Prabu et. al, showed in RAW 264.7 macrophages, 12h of hypoxia results in more than 50% decrease in CcO activity. Analysis of immunoprecipitated holoenzyme complex showed that under both hypoxia and rabbit heart ischemia/reperfusion the levels of subunits I, IVi1 and Vb were reduced [115]. Interestingly, using phospho specific antibodies, these same subunits were shown to be phosphorylated at serine and threonine residues under hypoxia. Mitochondrial PKA activity was increased under these conditions and inhibition of PKA activity by either H89 (50nM) or specific inhibitor, myristoylated peptide inhibitor (MPI, 40 nM) prevented both phosphorylation and loss of the subunits [115]. Although at higher concentrations, H89 is known to inhibit other kinases such as certain MAP kinases and AMPK, at the concentration of 50 100 nM used in these studies, it is more specific for PKA [100]. By mass spectrometry, the PKA responsive phosphorylations were shown to be at S115 and S116 of subunit I, S40 of subunit Vb and T52 of subunit IVi1 [54]. It is likely that phosphorylation of these subunits under hypoxia is a trigger for their degradation. Notably, mass spectrometric analysis of rabbit heart enzyme did not show any other phosphorylation thought to occur under physiological conditions [54]. It is possible that the phosphorylation dependent regulation of CcO under low oxygen tension is quite different from the regulation under normoxic conditions and involves different kinases, phosophorylation sites and perhaps different phosphatases.
Fukuda et. al., reported the mechanism of degradation of subunit IVi1 under hypoxia [59]. Exposing HeLa cells to 1% O2 or hypoxia of lung and liver of mice induces the expression of Lon protease in a Hypoxia inducible factor (HIF) dependent manner [59]. Lon is a stress responsive mitochondrial protease that degrades oxidatively damaged proteins [20,21,103]. Fukuda et. al showed that under hypoxia, Lon degrades subunit IVi1 of CcO and that under same conditions, there is an upregulation of subunit IVi2, as an adaptive response to hypoxia [59]. Specific, although modest decrease in subunits I and IV of CcO has also been reported in cardiac mitochondria of transgenic rat model of heart failure [124]. Loss of these subunits resulted in about 50% decrease in TMPD/ascorbate stimulated respiration in mitochondria [124]. Similarly, a rat model of cardiac hypertrophy exhibited loss of subunit Vb of CcO [80]. Differential loss of CcO subunits due to ischemia reperfusion has also been reported in rat heart. Prolonged ischemia/reperfusion of rat heart resulted in about 70% decrease in subunit I and more than 50% in subunit Va [162]. However, there was no significant difference in levels of subunit IV and Vb in these hearts. The differences in the subunits involved in all these studies could arise from species specific variation in sequences that might alter their susceptibility to oxidative damage.
Loss of subunit IV and Vb has also been reported in ethanol toxicity [12,36]. Chronic alcohol consumption has long been shown to decrease CcO protein content and activity [8,141]. A consistent observation is that about 50 to 70% decrease in the activity and heme aa3 content occurs under ethanol treatment in rodents [141]. Cunningham and others have demonstrated that ethanol consumption decreases synthesis of the 13 mitochondrially encoded proteins that are components of respiratory complexes I, III, and IV and the ATP synthase [110]. It is proposed that the inhibition of mitochondrial protein synthesis following chronic ethanol exposure is due to defects in mtDNA and a decline in the number of functional mitochondrial ribosomes resulting from chronic ethanol exposure [110]. However, this does not completely explain the observed decreases in nuclear encoded subunits of CcO. Recent work from our lab has shown that in both COS and HepG2 cells, ethanol treatment results in lower levels of subunits IV and Vb of CcO and accompanying decrease in enzyme activity [12]. Loss of CcO activity was exacerbated in cells expressing higher levels of mitochondrial targeted P450 2E1, suggesting its role in CcO dysfunction [12]. Excessive ROS produced during ethanol toxicity has an important role in oxidatively damaging subunits of CcO. Chen et. al., have shown 4 hydroxy nonenal (HNE), a toxic lipid peroxidation product produced when rats are exposed to ethanol, forms aldehyde adducts mainly with subunit IVi1 of CcO [35,36]. In addition to HNE, ethanol treatment generates malondialdehyde (MDA), another lipid peroxidation product. MDA also forms adduct with CcO and in vitro treatment showed greater inhibition of enzyme activity by MDA compared to HNE [34]. The more potent effect of MDA could be due to the fact that it forms adducts with both subunits IVi1 and Va/b of CcO [34]. Proteins modified by HNE and other lipid peroxidation derived aldehydes are actively degraded by proteases and probably explains the loss of subunits IVi1 and Vb in ethanol toxicity.
Loss of supercomplex organization
The supercomplex arrangement of the electron transport chain is a fairly recent observation. Supercomplexes are proposed to increase the efficiency of electron transfer and minimize electron leakage that could generate partially reduced oxygen species. The nature of interaction between complexes and the dynamic equilibrium between free forms of the complex and supercomplex forms are not completely understood. However, in a growing number studies effect of pathological conditions on supercomplex assembly is being reported. CcO is found in two major forms of mitochondrial supercomplexes, I/III2/IV1 4 and III2/IV1 2 [126,129]. Pathologies that result in decreased protein levels of CcO eventually affect stoichiometry and assembly of these supercomplexes. Heart failure in dogs induced by coronary microembolism resulted in the loss of Complex IV containing supercomplexes of the electron transport chain [61,119]. Similarly, in RAW 264.7 macrophages knockdown of either subunit CcO Vb or CcO IV resulted in significant decrease in cytochrome oxidase containing supercomplexes [61]. Liver mitochondria from ethanol treated rat also showed a lower level of supercomplexes concomitant with the loss of CcO protein [12]. CcO has been shown to be necessary for maintaining stability of complex I in the supercomplexes. In mouse fibroblast cell lines reduced expression of subunit IVi1 or nonsense mutation in subunit I, not only resulted in lower CcO content, but also caused significant reduction in complex I [86]. Similarly, mouse fibroblasts from COX10 knockout mice, which have defective CcO assembly, also exhibited reduced complex I levels [52]. However, RAW 264.7 macrophages with more than 90% reduced subunit Vb levels and in mouse cell lines with reduced expression of IV, Va, VIa, VIIa and VIIb, there was no significant reduction in complex I content [58,61]. Similarly, muscle mitochondria from patients with SurfI mutation had defective CcO assembly with no effect on complex I level [127]. This apparent discrepancy can be explained by a recent finding that structural intermediates of CcO, which usually accumulate during CcO assembly defects, are involved in the initial stabilization of complex I during supercomplex formation [98].
Drug induced inhibition of enzyme activity
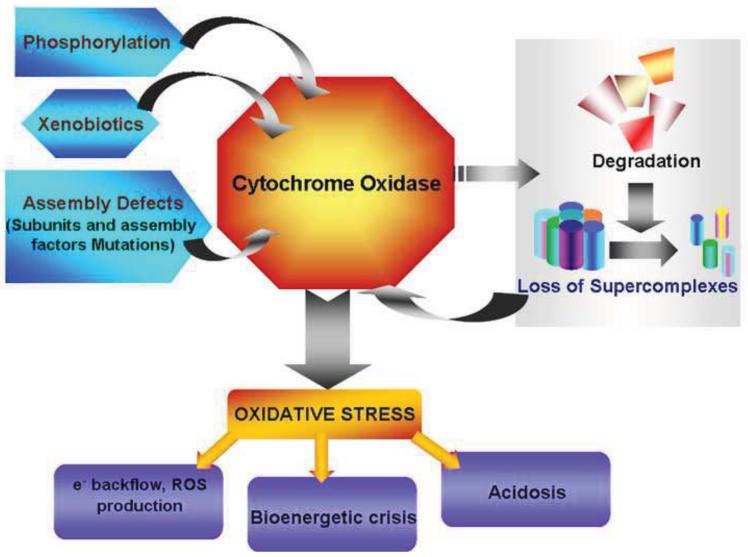
Direct inhibition of CcO activity occurs by inhibitors interfering with the catalytic cycle or by binding at distal allosteric sites. Nitric oxide, which has been shown to have both physiological and pathological effects, inhibits CcO activity by competing with oxygen for the binuclear center. Inhibition by NO is shown to be reversible. However, under oxidative stress, NO reacts with superoxide radical to form peroxynitrite. In vitro experiments show that peroxynitrite interacts irreversibly with CcO subunits inhibiting its activity [133]. Carbon monoxide and Hydrogen sulphide are other gaseous molecules that inhibit CcO activity. Although enzyme systems in the cell are capable for producing CO (Heme oxygenase) and H2S (Cystathionine gamma lyase and Cystathionine –beta synthase), the cellular concentrations are unlikely to affect CcO activity. Poisoning by inhalation of these gases is a more common reason for high cellular concentration and inhibition of CcO activity. Many externally administered drugs and xenobiotics have also been shown to inhibit CcO activity leading to mitochondrial stress. Mechanisms of action of most of these have not been elucidated in great detail. Anthracyclines like daunomycin (DAU) and doxorubicin (DOX), are antitumor drugs that cause cardiomyopathy as a side effect during long term treatment [33,107]. Cell culture and animal studies showed DAU directly inhibits CcO activity in a dose dependent manner. Further, DAU toxicity could be reversed by treatment with hemin, suggesting that DAU may act by binding to heme groups in CcO [107]. Interestingly, DOX treatment resulted in both CcO inhibition and lower levels of CcO subunits. DOX effects were reversed by treatment with MitoQ, a mitochondria targeted antioxidant [33]. Anthracyclines probably act by more than one mechanism to affect CcO expression and activity. CcO activity is significantly inhibited during inflammatory condition like sepsis [84]. Tyr 304 phosphorylation of subunit I induced by the inflammatory cytokine TNF is thought to be the mechanism of inhibition of CcO [122]. The pathophysiological factors that induce mitochondrial dysfunction have been summarized in Figure 2.
Figure 2.
Factors which cause dysfunction of Cytochrome C Oxidase. Multiple factors affect CcO function leading to mitochondrial stress and dysfunction. Genetic mutations of subunits and assembly factors are responsible for significant decrease in fully assembled complexes. Environmental changes such as hypoxia induce phosphorylation which either increase the activity or decrease activity by targeting the subunits for degradation. Many xenobiotics act by directly binding to active site heme and inhibit enzyme activity. Dysfunctional CcO contributes greatly to oxidative stress by increasing ROS production, ATP depletion and lactic acidosis.
Role of dysfunctional CcO in oxidative stress
Energy crisis and acidosis
As one of the three proton pumps that contribute to mitochondrial membrane potential and as the enzyme that completes electron transport chain by transferring electrons to oxygen, CcO dysfunction has a direct effect on cellular ATP level. Knockdown of CcO subunits that cause loss of activity also resulted in lower membrane potential and reduced ATP generation [58,61]. Cells compensate for decreased mitochondrial ATP production through oxidative metabolism by up regulating less efficient glycolytic pathway. However, a consequence of this adaptation is increased acidification due to lactate accumulation. In Leigh’s disease CcO assembly defect leads to more than 80% reduction in enzyme activity [63,65,101]. Fibroblasts generated from patient samples were found to generate only 15 20% of the ATP compared to control cells [65]. A compensatory increase in glycolysis is thought to cause severe lactic acidosis and pyruvate depletion affecting aerobic regions of the patient’s brain [63].
ROS production
Mitochondria are the principal source of cellular reactive oxygen species (ROS). Mechanisms of ROS production by mitochondrial respiratory chain complexes have been reviewed extensively [30]. ROS, particularly superoxide anions are formed invariably as by products of electron transport chain and other redox reactions in mitochondria through one electron reduction of molecular oxygen (O2). Superoxide is then converted to hydrogen peroxide (H2O2) by superoxide dismutases (SODs), present both within the mitochondria and in the cytosol. Depending on their type and rate of production, ROS have both physiological role and pathological effects in the context of mitochondrial as well as whole cell function. Excessive production of ROS and the associated cytotoxic effects are generally called oxidative stress. Peroxidation of membrane lipids, direct oxidation of amino acids and oxidative cleavage of peptide bonds in proteins and DNA damage are some of the hallmarks of oxidative stress and are responsible for many of the disease symptoms. Although several redox reactions take place in mitochondria, only a few of them have been shown to generate detectable oxygen free radicals [5]. While Complexes I and III are the major sites of ROS formation [23,29,30,38,83,145], recent reports show that complex II can readily generate superoxide radicals in the absence of electron acceptors [105,163]. In addition, ketoglutarate dehydrogenase, electron transfer flavoprotein:CoenzymeQ oxidoreductase, mono amine oxidase, and few other mitochondrial enzymes have been shown to produce ROS [30,75,139,140].
One of the major challenges in the field of free radical biology has been to determine which of the respiratory sites generate pathological levels of ROS. It is widely accepted that mitochondria with robust respiration coupled electron transport chain activity and high Δφm produce higher levels of O2.− [75,79,99]. A recent review nicely highlights possible mechanisms by which complex I, II and III contribute to ROS production both as part of forward and reveres electron flow [99].
The contribution of CcO, the terminal enzyme of the electron transport chain to ROS production and oxidative stress remains controversial. It is important to note that, in sequential systems that carry out a series of redox reactions such as the electron transport chain, ROS production from a particular site can be influenced by dysfunction of other complexes or redox centers in the series. Irrespective of the state of other complexes, most cases of CcO deficiency induce mitochondrial ROS production. The direct and indirect roles of CcO in ROS production have been investigated by a number of groups over the years.
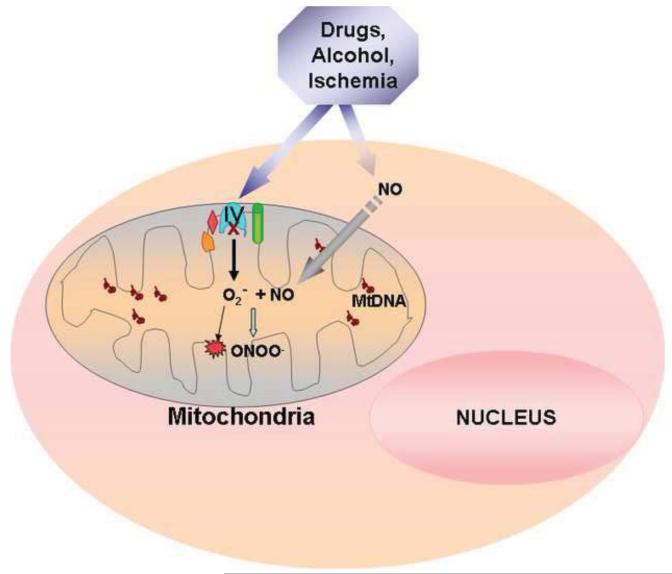
A notable characteristic of hypoxic stress is the massive burst of reactive oxygen species produced in mitochondria. Decreased flux through CcO due to low oxygen availability results in accumulation of reduced intermediates upstream in the ETC. Loss of electrons from reduced intermediates of the electron transport chain is considered one of the mechanisms of ROS formation [49,50]. Contribution of CcO dysfunction to ROS production under ischemia/reperfusion has been shown in an earlier study from our group [115]. In this study, CcO containing vesicles of asolectin and cardiolipin were prepared from sub mitochondrial particles from control and ischemic rabbit hearts. When reduced cytochrome c was added as the electron donor, CcO vesicles prepared from ischemic heart generated nearly 2 fold higher reactive oxygen species compared to control heart [115]. Since global stress conditions such as ischemia and hypoxia affect more than one complex, a more exclusive role of CcO in increasing mitochondrial ROS was shown in a stable CcO knock down cell line. Stable expression of sh-RNA to CcO Vb mRNA produced cells containing only 10% CcO activity compared to control wild type macrophages. In these cells, even under normoxic conditions, isolated mitochondria generated much higher ROS compared to control cells [61]. The specificity and mitochondrial origin of this ROS was verified by electron paramagnetic resonance spectroscopy using mito DEPMPO, a mitochondria targeted spin trap [61]. Similarly, HeLa cells expressing sh-RNA to subunit IV 1 exhibited increased H2O2 level compared to control cells [59]. Another mechanism of CcO dysfunction that leads to higher ROS formation during hypoxia and ischemia is possibly by the loss of cardiolipin [37]. CcO is associated with cardiolipin in the inner membrane and this interaction is essential for maximal CcO activity. ROS produced during hypoxia/ischemia depletes cardiolipin by peroxidation [109]. This decreases cytochrome C level resulting in higher ROS formation by complex I. As mentioned above, paradoxically, a more robust CcO activity has also been shown to increase ROS production [137]. In astrocytes, hypoxic and toxin mediated induction of subunit IV i2 is thought to play a role in elevated mitochondrial peroxide production [69,137]. Knockdown of IV i2 by siRNA in these cells resulted in decreased mitochondrial peroxide formation in addition to decreasing CcO activity [96,137]. This observation is however opposite to an earlier report in which knockdown of subunit IV i2 under hypoxia increased H2O2 production [59]. The difference could be due to the cell types used and the stress condition used to induce subunit IV i2. CcO dysfunction can also contribute to higher ROS formation by the loss of supercomplex structures. Heart failure in dogs induced by coronary microembolism resulted in a loss of Complex IV containing supercomplexes of the electron transport chain [61,119]. Similarly, in both CcO Vb and CcO IV knockdown RAW 264.7 macrophages, there was a significant decrease in cytochrome oxidase containing supercomplexes [61]. As discussed earlier in this review, an important function of these supercomplexes is thought to reduce electron leakage to minimize ROS formation, which seems to be affected under conditions when cytochrome oxidase levels are reduced. Chronic ethanol treatment augments CcO subunit degradation, which is one of the factors leading to high levels of mitochondrial O2.− and H2O2, resulting in oxidative damage. Ethanol treatment also increases NO production in hepatocytes which has been shown to affect mitochondrial functions [13,149,160]. Inhibition of CcO by NO itself has been shown to increase ROS formation from other complexes of the electron transport chain [22]. In addition, NO reacts spontaneously with superoxide anion in the mitochondria to form peroxinitrite, a highly reactive nitrogen radical [70]. Peroxinitrite covalently modifies mitochondrial proteins by nitrosation of tyrosine and cysteine residues and damages Fe S centers causing futher oxidative damage to the mitochondrial compartment [104]. The interaction of CcO and NO pathway to increase mitochondrial ROS production under ethanol and hypoxic stress is summarized in figure 3.
Figure 3.
Mechanism of ethanol, ischemia and drug induced mitochondrial ROS production. Results show that ethanol, myocardial ischemia and drugs such as doxorubicin cause CcO dysfunction and induce ROS production. In the case of ethanol induced toxicity, increased mitochondrial superoxide anion reacts with NO to form the highly reactive peroxynitrite which covalently modifies proteins and Fe S centers.
All of these mechanisms describe indirect contribution of CcO to ROS formation in mitochondria. So far, there is no evidence for CcO acting as a direct source of ROS, although mechanistic studies on the catalysis by CcO have shown formation of reactive oxygen intermediates at the metal centers of the enzyme [95]. Additionally, Electron paramagnetic resonance (EPR) spin trap studies suggest the formation of protein derived radicals on mammalian CcO [39,40,90], though other studies suggest that amino acid radicals may be part of the proton loading and release mechanism [161]. While many redox centers can leak reactive intermediates, the rapid kinetics of CcO ensures full reduction without releasing the reactive species into the medium. The rapid kinetics of electron transfer to oxygen prevents formation of reactive oxygen species and release into medium. Interestingly, however, in cells subjected to hypoxia or loss of some select CcO subunits, there is a marked accumulation of subcomplexes [61]. It would be interesting to see if such partially assembled complexes exhibit kinetics that is different from fully assembled CcO. Using functional models of CcO active site, Collman et al, have shown the importance of correct spatial arrangement of elements of active site [46]. In their model, masking of a phenol, representing Tyr244 of the bovine CcO active site resulted in much greater leakage of partially reduced oxygen species (PROS). Recently Khalimonchuk et al. showed in yeast that CcO subunit I haem a is a pro oxidant intermediate formed during the assembly of cytochrome oxidase [78]. However, evidence for the direct contribution of CcO to ROS production is still lacking.
Role of CcO in the formation of Nitric Oxide has been shown under hypoxic conditions. Castello et al, have shown that isolated yeast CcO produces NO in presence of Nitrite under oxygen limiting conditions [32]. Nitrite reductase activity of CcO has also been shown by earlier studies [26,106,123]. Further, under hypoxia, NO generated by CcO has been shown to form peroxynitrite by reacting with superoxide and modify mitochondrial proteins by nitration. HEK293 cells that do not possess nitric oxide synthase were shown to generate mitochondrial NO under hypoxia, which was prevented by CcO inhibitor, sodium azide [11]. NO generated in these cells was shown to stabilize Hif . Both NO inhibitors and CcO inhibitors prevented stabilization of Hif under hypoxia in these cells proving the role of CcO generated NO in hypoxia signaling [11].
Calcium homeostasis and apoptosis
Mitochondria have an important function in modulating cellular calcium homeostasis. They act as calcium stores by taking up calcium in an energy dependent manner. Calcium enters mitochondria through a uniporter in the inner mitochondrial membrane and is driven by membrane potential. Physiological levels of calcium are required for stimulating mitochondrial metabolism. Any impairment of mitochondrial function leading to loss of membrane potential and decreased ATP levels reduces the activity of the calcium pump and mitochondrial uptake. Given the important role of CcO in ATP synthesis it seems logical to expect aberrant calcium homeostasis in CcO deficient cells. Surprisingly there has been only a single report of abnormal calcium homeostasis in fibroblasts of a CcO deficient patient [154]. Leigh syndrome is caused by mutations in Surf I leading to impaired CcO assembly. The authors report a greatly impaired influx of calcium into mitochondria of patient fibroblasts, even in presence of Thapsigargin that activates mitochondrial store operated calcium entry by blocking ER calcium entry [154]. Mitochondria play an important role in programmed cell death. They are the sites of initiation of the intrinsic pathway of apoptosis. Myocardial infarction (MI) resulting from cardiac ischemia reperfusion is characterized by tissue damage due to apoptosis. Stress by MI leads to specific up regulation of COX III [157]. A recent study has established a connection between COX III over expression and apoptosis. Over expression of COX III in HL 1 myocytes resulted in decreased expression of COX I and lower Cytochrome oxidase activity. Interestingly the cells became susceptible to oxidative stress and had increased cell death due to apoptosis [157].
Summary and conclusions
Cytochrome oxidase is a major regulatory enzyme of the electron transport chain. Its importance is shown by multiple levels of regulation of its activity and content in the mitochondria. Disruption of any of these regulatory cues can result in a pathological condition. Defective cytochrome oxidase activity either directly or indirectly affects all aspects of mitochondrial function and consequently an array of diseases are associated with reduced contents or altered activity of CcO. While it is expected to see a severe impairment of aerobic ATP generation due to CcO deficiency, there is also evidence of CcO affecting calcium homeostasis. Further, there is considerable literature to show the role of defective CcO in increased mitochondrial ROS, though the precise mechanism of its contribution remains unclear.
Highlights.
Mammalian Cytochrome oxidase (CcO) is a bigenomic enzyme with 13 subunits
The enzyme is regulated by subunit phosphorylation and allosteric effectors
CcO function is affected by hypoxia/ischemia, drugs and by alcohol
CcO function is also compromised in many diseases
CcO dysfunction often leads to increased production of reactive oxygen species
Acknowledgements
The research in the author’s laboratory was supported by NIH grants AA 017749 and GM 34883. The authors thank Dr.Manti Guha for illustrations in this manuscript.
Abbreviations
- OXPHOS
Oxidative phosphorylation system
- ROS
Reactive oxygen species
- sh-RNA
Short hairpin RNA
- ER
Endoplasmic reticulum
- cAMP
Cyclic adenosine mono phosphate
- Δψm
Mitochondrial transmembrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [2].Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Allen LA, Zhao XJ, Caughey W, Poyton RO. Isoforms of yeast cytochrome c oxidase subunit V affect the binuclear reaction center and alter the kinetics of interaction with the isoforms of yeast cytochrome c. J Biol Chem. 1995;270:110–118. doi: 10.1074/jbc.270.1.110. [DOI] [PubMed] [Google Scholar]
- [5].Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry. Biokhimii Inverted Question Marka Inverted Question Mark. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- [6].Anthony G, Stroh A, Lottspeich F, Kadenbach B. Different isozymes of cytochrome c oxidase are expressed in bovine smooth muscle and skeletal or heart muscle. FEBS Lett. 1990;277:97–100. doi: 10.1016/0014-5793(90)80817-3. [DOI] [PubMed] [Google Scholar]
- [7].Antonicka H, Mattman A, Carlson CG, Glerum DM, Hoffbuhr KC, Leary SC, Kennaway NG, Shoubridge EA. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2003;72:101–114. doi: 10.1086/345489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arai M, Gordon ER, Lieber CS. Decreased cytochrome oxidase activity in hepatic mitochondria after chronic ethanol consumption and the possible role of decreased cytochrome aa3 content and changes in phospholipids. Biochim. Biophys. Acta. 1984;797:320–327. doi: 10.1016/0304-4165(84)90252-6. [DOI] [PubMed] [Google Scholar]
- [9].Arnold S, Goglia F, Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur. J Biochem. 1998;252:325–330. doi: 10.1046/j.1432-1327.1998.2520325.x. [DOI] [PubMed] [Google Scholar]
- [10].Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- [11].Ball KA, Nelson AW, Foster DG, Poyton RO. Nitric oxide produced by cytochrome c oxidase helps stabilize HIF-1alpha in hypoxic mammalian cells. Biochem. Biophys. Res. Commun. 2012;420:727–732. doi: 10.1016/j.bbrc.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bansal S, Srinivasan S, Anandasadagopan S, Roy CA, Selvaraj V, Kalyanaraman B, Joseph J, Avadhani NG. Additive effects of mitochondria-targeted Cytochrome P4502E1 and alcohol toxicity on cytochrome c oxidase function and the stability of respirosome complexes. J. Biol. Chem. 2012 doi: 10.1074/jbc.M111.314062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS. Ethanol consumption increases nitric oxide production in rats, and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin. Exp. Res. 2002;26:883–889. [PubMed] [Google Scholar]
- [14].Barrientos A, Barros MH, Valnot I, Rotig A, Rustin P, Tzagoloff A. Cytochrome oxidase in health and disease. Gene. 2002;286:53–63. doi: 10.1016/s0378-1119(01)00803-4. [DOI] [PubMed] [Google Scholar]
- [15].Barrientos A, Korr D, Tzagoloff A. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh’s syndrome. EMBO J. 2002;21:43–52. doi: 10.1093/emboj/21.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barrientos A, Zambrano A, Tzagoloff A. Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 2004;23:3472–3482. doi: 10.1038/sj.emboj.7600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Beauvoit B, Rigoulet M. Regulation of cytochrome c oxidase by adenylic nucleotides. Is oxidative phosphorylation feedback regulated by its end-products? IUBMB. Life. 2001;52:143–152. doi: 10.1080/152165401317316545. [DOI] [PubMed] [Google Scholar]
- [18].Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- [19].Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem. J. 1996;315(Pt 1):295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- [21].Bota DA, Van RH, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- [22].Boveris A, Cadenas E. Mitochondrial production of hydrogen peroxide regulation by nitric oxide and the role of ubisemiquinone. IUBMB. Life. 2000;50:245–250. doi: 10.1080/713803732. [DOI] [PubMed] [Google Scholar]
- [23].Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radical Biology and Medicine. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- [24].Brookes PS, Kraus DW, Shiva S, Doeller JE, Barone MC, Patel RP, Lancaster JR, Jr., Darley-Usmar V. Control of Mitochondrial Respiration by NO. Effects of Low Oxygen and Respiratory State. J. Biol. Chem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- [25].Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- [26].Brudvig GW, Stevens TH, Chan SI. Reactions of nitric oxide with cytochrome c oxidase. Biochemistry. 1980;19:5275–5285. doi: 10.1021/bi00564a020. [DOI] [PubMed] [Google Scholar]
- [27].Bruno C, Martinuzzi A, Tang Y, Andreu AL, Pallotti F, Bonilla E, Shanske S, Fu J, Sue CM, Angelini C, DiMauro S, Manfredi G. A stop-codon mutation in the human mtDNA cytochrome c oxidase I gene disrupts the functional structure of complex IV. Am. J. Hum. Genet. 1999;65:611–620. doi: 10.1086/302546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burke PV, Raitt DC, Allen LA, Kellogg EA, Poyton RO. Effects of oxygen concentration on the expression of cytochrome c and cytochrome c oxidase genes in yeast. J Biol Chem. 1997;272:14705–14712. doi: 10.1074/jbc.272.23.14705. [DOI] [PubMed] [Google Scholar]
- [29].Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biology and Medicine. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- [31].Capaldi RA. Structure and function of cytochrome c oxidase. Annu. Rev. Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- [32].Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes 4. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- [33].Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys. J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen J, Petersen DR, Schenker S, Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin. Exp. Res. 2000;24:544–552. [PubMed] [Google Scholar]
- [35].Chen J, Robinson NC, Schenker S, Frosto TA, Henderson GI. Formation of 4-hydroxynonenal adducts with cytochrome c oxidase in rats following short-term ethanol intake. Hepatology. 1999;29:1792–1798. doi: 10.1002/hep.510290611. [DOI] [PubMed] [Google Scholar]
- [36].Chen J, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE) Role of HNE adduct formation with the enzyme subunits. Biochim. Biophys. Acta. 1998;1380:336–344. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- [37].Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radical Biology and Medicine. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- [38].Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. J. Biol. Chem. 2004;279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- [39].Chen YR, Gunther MR, Mason RP. An electron spin resonance spin-trapping investigation of the free radicals formed by the reaction of mitochondrial cytochrome c oxidase with H2O2. J. Biol. Chem. 1999;274:3308–3314. doi: 10.1074/jbc.274.6.3308. [DOI] [PubMed] [Google Scholar]
- [40].Chen YR, Sturgeon BE, Gunther MR, Mason RP. Electron spin resonance investigation of the cyanyl and azidyl radical formation by cytochrome c oxidase. J. Biol. Chem. 1999;274:24611–24616. doi: 10.1074/jbc.274.35.24611. [DOI] [PubMed] [Google Scholar]
- [41].Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- [42].Clark KM, Taylor RW, Johnson MA, Chinnery PF, Chrzanowska-Lightowlers ZM, Andrews RM, Nelson IP, Wood NW, Lamont PJ, Hanna MG, Lightowlers RN, Turnbull DM. An mtDNA mutation in the initiation codon of the cytochrome C oxidase subunit II gene results in lower levels of the protein and a mitochondrial encephalomyopathy. Am. J. Hum. Genet. 1999;64:1330–1339. doi: 10.1086/302361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- [44].Clementi E, Brown GC, Foxwell N, Moncada S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1559–1562. doi: 10.1073/pnas.96.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coenen MJ, van den Heuvel LP, Ugalde C, Ten BM, Nijtmans LG, Trijbels FJ, Beblo S, Maier EM, Muntau AC, Smeitink JA. Cytochrome c oxidase biogenesis in a patient with a mutation in COX10 gene. Ann. Neurol. 2004;56:560–564. doi: 10.1002/ana.20229. [DOI] [PubMed] [Google Scholar]
- [46].Collman JP, Devaraj NK, Decreau RA, Yang Y, Yan YL, Ebina W, Eberspacher TA, Chidsey CE. A cytochrome C oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux 1. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Comi GP, Bordoni A, Salani S, Franceschina L, Sciacco M, Prelle A, Fortunato F, Zeviani M, Napoli L, Bresolin N, Moggio M, Ausenda CD, Taanman JW, Scarlato G. Cytochrome c oxidase subunit I microdeletion in a patient with motor neuron disease. Ann. Neurol. 1998;43:110–116. doi: 10.1002/ana.410430119. [DOI] [PubMed] [Google Scholar]
- [48].Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- [49].Cooper CE, Davies NA. Effects of nitric oxide and peroxynitrite on the cytochrome oxidase K(m) for oxygen: implications for mitochondrial pathology. Biochim. Biophys. Acta. 2000;1459:390–396. doi: 10.1016/s0005-2728(00)00176-6. [DOI] [PubMed] [Google Scholar]
- [50].Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am. J. Physiol. 1993;264:C961–C967. doi: 10.1152/ajpcell.1993.264.4.C961. [DOI] [PubMed] [Google Scholar]
- [51].Diaz F. Cytochrome c oxidase deficiency: patients and animal models. Biochim. Biophys. Acta. 2010;1802:100–110. doi: 10.1016/j.bbadis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- [52].Diaz F, Fukui H, Garcia S, Moraes CT. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol. Cell Biol. 2006;26:4872–4881. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].DiMauro S, Lombes A, Nakase H, Mita S, Fabrizi GM, Tritschler HJ, Bonilla E, Miranda AF, DeVivo DC, Schon EA. Cytochrome c oxidase deficiency. Pediatr. Res. 1990;28:536–541. doi: 10.1203/00006450-199011000-00025. [DOI] [PubMed] [Google Scholar]
- [54].Fang JK, Prabu SK, Sepuri NB, Raza H, Anandatheerthavarada HK, Galati D, Spear J, Avadhani NG. Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Letters. 2007;581:1302–1310. doi: 10.1016/j.febslet.2007.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Flameng W, Andres J, Ferdinande P, Mattheussen M, van BH. Mitochondrial function in myocardial stunning. J. Mol. Cell Cardiol. 1991;23:1–11. doi: 10.1016/0022-2828(91)90034-j. [DOI] [PubMed] [Google Scholar]
- [56].Fontanesi F, Soto IC, Barrientos A. Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB. Life. 2008;60:557–568. doi: 10.1002/iub.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fontanesi F, Soto IC, Horn D, Barrientos A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol Cell Physiol. 2006;291:C1129–C1147. doi: 10.1152/ajpcell.00233.2006. [DOI] [PubMed] [Google Scholar]
- [58].Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem. J. 2010;428:363–374. doi: 10.1042/BJ20091714. [DOI] [PubMed] [Google Scholar]
- [59].Fukuda R, Zhang H, Kim J. w., Shimoda L, Dang CV, Semenza GL. HIF-1 Regulates Cytochrome Oxidase Subunits to Optimize Efficiency of Respiration in Hypoxic Cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- [60].Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol Regul. Integr. Comp Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- [61].Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, Lopez M, Kalyanaraman B, Avadhani NG. Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem. J. 2009;420:439–449. doi: 10.1042/BJ20090214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gattermann N, Retzlaff S, Wang YL, Hofhaus G, Heinisch J, Aul C, Schneider W. Heteroplasmic point mutations of mitochondrial DNA affecting subunit I of cytochrome c oxidase in two patients with acquired idiopathic sideroblastic anemia. Blood. 1997;90:4961–4972. [PubMed] [Google Scholar]
- [63].Glerum M, Robinson BH, Spratt C, Wilson J, Patrick D. Abnormal kinetic behavior of cytochrome oxidase in a case of Leigh disease. Am. J. Hum. Genet. 1987;41:584–593. [PMC free article] [PubMed] [Google Scholar]
- [64].Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- [65].Haginoya K, Miyabayashi S, Iinuma K, Okino E, Maesaka H, Tada K. Cytochrome C oxidase-deficient mitochondria in mitochondrial myopathy. Pediatr. Neurol. 1992;8:13–18. doi: 10.1016/0887-8994(92)90046-2. [DOI] [PubMed] [Google Scholar]
- [66].Hanna MG, Nelson IP, Rahman S, Lane RJ, Land J, Heales S, Cooper MJ, Schapira AH, Morgan-Hughes JA, Wood NW. Cytochrome c oxidase deficiency associated with the first stop-codon point mutation in human mtDNA. Am. J. Hum. Genet. 1998;63:29–36. doi: 10.1086/301910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Helling S, Huttemann M, Ramzan R, Kim SH, Lee I, Muller T, Langenfeld E, Meyer HE, Kadenbach B, Vogt S, Marcus K. Multiple phosphorylations of cytochrome c oxidase and their functions. Proteomics. 2012;12:950–959. doi: 10.1002/pmic.201100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, Kadenbach B. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol. Cell Proteomics. 2008;7:1714–1724. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Horvat S, Beyer C, Arnold S. Effect of hypoxia on the transcription pattern of subunit isoforms and the kinetics of cytochrome c oxidase in cortical astrocytes and cerebellar neurons. J. Neurochem. 2006;99:937–951. doi: 10.1111/j.1471-4159.2006.04134.x. [DOI] [PubMed] [Google Scholar]
- [70].Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic. Res. Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- [71].Huttemann M, Frank V, Kadenbach B. The possible role of isoforms of cytochrome c oxidase subunit VIa in mammalian thermogenesis. Cell Mol. Life Sci. 1999;55:1482–1490. doi: 10.1007/s000180050387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Huttemann M, Kadenbach B, Grossman LI. Mammalian subunit IV isoforms of cytochrome c oxidase 3. Gene. 2001;267:111–123. doi: 10.1016/s0378-1119(01)00385-7. [DOI] [PubMed] [Google Scholar]
- [73].Huttemann M, Lee I, Gao X, Pecina P, Pecinova A, Liu J, Aras S, Sommer N, Sanderson TH, Tost M, Neff F, guilar-Pimentel JA, Becker L, Naton B, Rathkolb B, Rozman J, Favor J, Hans W, Prehn C, Puk O, Schrewe A, Sun M, Hofler H, Adamski J, Bekeredjian R, Graw J, Adler T, Busch DH, Klingenspor M, Klopstock T, Ollert M, Wolf E, Fuchs H, Gailus-Durner V, Hrabe de AM, Weissmann N, Doan JW, Bassett DJ, Grossman LI. Cytochrome c oxidase subunit 4 isoform 2 knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology. FASEB J. 2012 doi: 10.1096/fj.11-203273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huttemann M, Lee I, Liu J, Grossman LI. Transcription of mammalian cytochrome c oxidase subunit IV-2 is controlled by a novel conserved oxygen responsive element. FEBS J. 2007;274:5737–5748. doi: 10.1111/j.1742-4658.2007.06093.x. [DOI] [PubMed] [Google Scholar]
- [75].Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism 1. Int. J Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- [76].Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol Med. 2000;29:211–221. doi: 10.1016/s0891-5849(00)00305-1. [DOI] [PubMed] [Google Scholar]
- [77].Keightley JA, Hoffbuhr KC, Burton MD, Salas VM, Johnston WS, Penn AM, Buist NR, Kennaway NG. A microdeletion in cytochrome c oxidase (COX) subunit III associated with COX deficiency and recurrent myoglobinuria. Nat. Genet. 1996;12:410–416. doi: 10.1038/ng0496-410. [DOI] [PubMed] [Google Scholar]
- [78].Khalimonchuk O, Bird A, Winge DR. Evidence for a pro oxidant intermediate in the assembly of cytochrome oxidase. J. Biol. Chem. 2007;282:17442–17449. doi: 10.1074/jbc.M702379200. [DOI] [PubMed] [Google Scholar]
- [79].Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- [80].Kuo WW, Chu CY, Wu CH, Lin JA, Liu JY, Ying TH, Lee SD, Hsieh YH, Chu CH, Lin DY, Hsu HH, Huang CY. The profile of cardiac cytochrome c oxidase (COX) expression in an accelerated cardiac-hypertrophy model. J. Biomed. Sci. 2005;12:601–610. doi: 10.1007/s11373-005-7373-2. [DOI] [PubMed] [Google Scholar]
- [81].Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Huttemann M. cAMP-dependent Tyrosine Phosphorylation of Subunit I Inhibits Cytochrome c Oxidase Activity. J. Biol. Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- [82].Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- [83].Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic Injury to Mitochondrial Electron Transport in the Aging Heart: Damage to the Iron-Sulfur Protein Subunit of Electron Transport Complex III. Archives of Biochemistry and Biophysics. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- [84].Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome C oxidase in sepsis. Shock. 2004;21:110–114. doi: 10.1097/01.shk.0000108400.56565.ab. [DOI] [PubMed] [Google Scholar]
- [85].Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- [86].Li Y, D’Aurelio M, Deng JH, Park JS, Manfredi G, Hu P, Lu J, Bai Y. An assembled complex IV maintains the stability and activity of complex I in mammalian mitochondria. J. Biol. Chem. 2007;282:17557–17562. doi: 10.1074/jbc.M701056200. [DOI] [PubMed] [Google Scholar]
- [87].Lightowlers R, Ewart G, Aggeler R, Zhang YZ, Calavetta L, Capaldi RA. Isolation and characterization of the cDNAs encoding two isoforms of subunit CIX of bovine cytochrome c oxidase. J. Biol. Chem. 1990;265:2677–2681. [PubMed] [Google Scholar]
- [88].Linder D, Freund R, Kadenbach B. Species specific expression of cytochrome c oxidase isozymes. Comp Biochem. Physiol B Biochem. Mol. Biol. 1995;112:461–469. doi: 10.1016/0305-0491(95)00093-3. [DOI] [PubMed] [Google Scholar]
- [89].Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].MacMillan F, Kannt A, Behr J, Prisner T, Michel H. Direct evidence for a tyrosine radical in the reaction of cytochrome c oxidase with hydrogen peroxide. Biochemistry. 1999;38:9179–9184. doi: 10.1021/bi9911987. [DOI] [PubMed] [Google Scholar]
- [91].Manfredi G, Servidei S, Bonilla E, Shanske S, Schon EA, DiMauro S, Moraes CT. High levels of mitochondrial DNA with an unstable 260-bp duplication in a patient with a mitochondrial myopathy. Neurology. 1995;45:762–768. doi: 10.1212/wnl.45.4.762. [DOI] [PubMed] [Google Scholar]
- [92].Manfredi G, Vu T, Bonilla E, Schon EA, DiMauro S, Arnaudo E, Zhang L, Rowland LP, Hirano M. Association of myopathy with large-scale mitochondrial DNA duplications and deletions: which is pathogenic? Ann. Neurol. 1997;42:180–188. doi: 10.1002/ana.410420208. [DOI] [PubMed] [Google Scholar]
- [93].Massa V, Fernandez-Vizarra E, Alshahwan S, Bakhsh E, Goffrini P, Ferrero I, Mereghetti P, D’Adamo P, Gasparini P, Zeviani M. Severe infantile encephalomyopathy caused by a mutation in COX6B1, a nucleus-encoded subunit of cytochrome c oxidase. Am. J. Hum. Genet. 2008;82:1281–1289. doi: 10.1016/j.ajhg.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Merante F, Petrova-Benedict R, MacKay N, Mitchell G, Lambert M, Morin C, De BM, Laframboise R, Gagne R, Robinson BH. A biochemically distinct form of cytochrome oxidase (COX) deficiency in the Saguenay-Lac-Saint-Jean region of Quebec. Am. J. Hum. Genet. 1993;53:481–487. [PMC free article] [PubMed] [Google Scholar]
- [95].Michel H, Behr J, Harrenga A, Kannt A. Cytochrome c oxidase: structure and spectroscopy 2. Annu. Rev. Biophys. Biomol. Struct. 1998;27:329–356. doi: 10.1146/annurev.biophys.27.1.329. [DOI] [PubMed] [Google Scholar]
- [96].Misiak M, Singh S, Drewlo S, Beyer C, Arnold S. Brain region-specific vulnerability of astrocytes in response to 3-nitropropionic acid is mediated by cytochrome c oxidase isoform expression. Cell Tissue Res. 2010;341:83–93. doi: 10.1007/s00441-010-0995-3. [DOI] [PubMed] [Google Scholar]
- [97].Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, Ugalde C. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–335. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci. Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- [101].Nagai T, Goto Y, Matsuoka T, Sakuta R, Naito E, Kuroda Y, Nonaka I. Leigh encephalopathy: histologic and biochemical analyses of muscle biopsies. Pediatr. Neurol. 1992;8:328–332. doi: 10.1016/0887-8994(92)90084-c. [DOI] [PubMed] [Google Scholar]
- [102].Napiwotzki J, Kadenbach B. Extramitochondrial ATP/ADP-ratios regulate cytochrome c oxidase activity via binding to the cytosolic domain of subunit IV. Biol Chem. 1998;379:335–339. doi: 10.1515/bchm.1998.379.3.335. [DOI] [PubMed] [Google Scholar]
- [103].Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic. Biol. Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissmann N, Braun-Dullaeus RC, Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. American Journal Of Physiology. Lung Cellular And Molecular Physiology. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- [106].Paitian NA, Markossian KA, Nalbandyan RM. The effect of nitrite on cytochrome oxidase. Biochem. Biophys. Res. Commun. 1985;133:1104–1111. doi: 10.1016/0006-291x(85)91250-1. [DOI] [PubMed] [Google Scholar]
- [107].Papadopoulou LC, Tsiftsoglou AS. Mitochondrial cytochrome c oxidase as a target site for daunomycin in K-562 cells and heart tissue. Cancer Res. 1993;53:1072–1078. [PubMed] [Google Scholar]
- [108].Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decrease in the cytochrome c oxidase activity and changes in phospholipids in rat-heart mitochondria. Arch. Gerontol. Geriatr. 1993;16:263–272. doi: 10.1016/0167-4943(93)90037-i. [DOI] [PubMed] [Google Scholar]
- [109].Paradies G, Petrosillo G, Pistolese M, Ruggiero FM. The effect of reactive oxygen species generated from the mitochondrial electron transport chain on the cytochrome c oxidase activity and on the cardiolipin content in bovine heart submitochondrial particles. FEBS Letters. 2000;466:323–326. doi: 10.1016/s0014-5793(00)01082-6. [DOI] [PubMed] [Google Scholar]
- [110].Patel VB, Cunningham CC. Altered hepatic mitochondrial ribosome structure following chronic ethanol consumption. Arch. Biochem. Biophys. 2002;398:41–50. doi: 10.1006/abbi.2001.2701. [DOI] [PubMed] [Google Scholar]
- [111].Pecina P, Houstkova H, Hansikova H, Zeman J, Houstek J. Genetic defects of cytochrome c oxidase assembly. Physiol Res. 2004;53(Suppl 1):S213–S223. [PubMed] [Google Scholar]
- [112].Perez-Martinez X, Broadley SA, Fox TD. Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 2003;22:5951–5961. doi: 10.1093/emboj/cdg566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]
- [114].Piper HM, Sezer O, Schleyer M, Schwartz P, Hutter JF, Spieckermann PG. Development of ischemia-induced damage in defined mitochondrial subpopulations. J. Mol. Cell Cardiol. 1985;17:885–896. doi: 10.1016/s0022-2828(85)80102-4. [DOI] [PubMed] [Google Scholar]
- [115].Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury 2. J Biol Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rahman S, Taanman JW, Cooper JM, Nelson I, Hargreaves I, Meunier B, Hanna MG, Garcia JJ, Capaldi RA, Lake BD, Leonard JV, Schapira AH. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am. J. Hum. Genet. 1999;65:1030–1039. doi: 10.1086/302590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ramzan R, Staniek K, Kadenbach B, Vogt S. Mitochondrial respiration and membrane potential are regulated by the allosteric ATP-inhibition of cytochrome c oxidase. Biochim. Biophys. Acta. 2010;1797:1672–1680. doi: 10.1016/j.bbabio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- [118].Ramzan R, Weber P, Kadenbach B, Vogt S. Individual biochemical behaviour versus biological robustness: spotlight on the regulation of cytochrome C oxidase. Adv. Exp. Med. Biol. 2012;748:265–281. doi: 10.1007/978-1-4614-3573-0_11. [DOI] [PubMed] [Google Scholar]
- [119].Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc. Res. 2008;80:30–39. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am. J. Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- [121].Saddar S, Dienhart MK, Stuart RA. The F1F0-ATP synthase complex influences the assembly state of the cytochrome bc1-cytochrome oxidase supercomplex and its association with the TIM23 machinery. J. Biol. Chem. 2008;283:6677–6686. doi: 10.1074/jbc.M708440200. [DOI] [PubMed] [Google Scholar]
- [122].Samavati L, Lee I, Mathes I, Lottspeich F, Huttemann M. Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J. Biol. Chem. 2008;283:21134–21144. doi: 10.1074/jbc.M801954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sarti P, Giuffre A, Forte E, Mastronicola D, Barone MC, Brunori M. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem. Biophys. Res. Commun. 2000;274:183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- [124].Sayen MR, Gustafsson AB, Sussman MA, Molkentin JD, Gottlieb RA. Calcineurin transgenic mice have mitochondrial dysfunction and elevated superoxide production. Am. J. Physiol Cell Physiol. 2003;284:C562–C570. doi: 10.1152/ajpcell.00336.2002. [DOI] [PubMed] [Google Scholar]
- [125].Schafer E, Seelert H, Reifschneider NH, Krause F, Dencher NA, Vonck J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006;281:15370–15375. doi: 10.1074/jbc.M513525200. [DOI] [PubMed] [Google Scholar]
- [126].Schagger H. Respiratory chain supercomplexes. IUBMB. Life. 2001;52:119–128. doi: 10.1080/15216540152845911. [DOI] [PubMed] [Google Scholar]
- [127].Schagger H, de CR, Bauer MF, Hofmann S, Godinot C, Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J. Biol. Chem. 2004;279:36349–36353. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- [128].Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Schagger H, Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 2001;276:37861–37867. doi: 10.1074/jbc.M106474200. [DOI] [PubMed] [Google Scholar]
- [130].Schlerf A, Droste M, Winter M, Kadenbach B. Characterization of two different genes (cDNA) for cytochrome c oxidase subunit VIa from heart and liver of the rat. EMBO J. 1988;7:2387–2391. doi: 10.1002/j.1460-2075.1988.tb03083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schweizer M, Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem. Biophys. Res. Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- [132].Seelan RS, Grossman LI. Cytochrome c oxidase subunit VIIa isoforms. Characterization and expression of bovine cDNAs. J. Biol. Chem. 1991;266:19752–19757. [PubMed] [Google Scholar]
- [133].Sharpe MA, Cooper CE. Interaction of peroxynitrite with mitochondrial cytochrome oxidase. Catalytic production of nitric oxide and irreversible inhibition of enzyme activity. J. Biol. Chem. 1998;273:30961–30972. doi: 10.1074/jbc.273.47.30961. [DOI] [PubMed] [Google Scholar]
- [134].Shen W, Xu X, Ochoa M, Zhao G, Wolin MS, Hintze TH. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ. Res. 1994;75:1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- [135].Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic. Biol. Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- [136].Shiva S, Darley-Usmar VM. Control of the nitric oxide-cytochrome c oxidase signaling pathway under pathological and physiological conditions. IUBMB. Life. 2003;55:585–590. doi: 10.1080/152165430310001640489. [DOI] [PubMed] [Google Scholar]
- [137].Singh S, Misiak M, Beyer C, Arnold S. Cytochrome c oxidase isoform IV-2 is involved in 3-nitropropionic acid-induced toxicity in striatal astrocytes. Glia. 2009;57:1480–1491. doi: 10.1002/glia.20864. [DOI] [PubMed] [Google Scholar]
- [138].Sohal RS. Aging, cytochrome oxidase activity, and hydrogen peroxide release by mitochondria. Free Radic. Biol. Med. 1993;14:583–588. doi: 10.1016/0891-5849(93)90139-l. [DOI] [PubMed] [Google Scholar]
- [139].St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- [140].Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Thayer WS, Cummings JJ., Jr Effects of chronic alcohol consumption on the steady-state kinetics properties of cytochrome oxidase in rat liver. Biochim. Biophys. Acta. 1990;1016:333–338. doi: 10.1016/0005-2728(90)90165-z. [DOI] [PubMed] [Google Scholar]
- [142].Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. U. S. A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tiranti V, Corona P, Greco M, Taanman JW, Carrara F, Lamantea E, Nijtmans L, Uziel G, Zeviani M. A novel frameshift mutation of the mtDNA COIII gene leads to impaired assembly of cytochrome c oxidase in a patient affected by Leigh-like syndrome. Hum. Mol. Genet. 2000;9:2733–2742. doi: 10.1093/hmg/9.18.2733. [DOI] [PubMed] [Google Scholar]
- [144].Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circ. Res. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- [145].Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- [146].Ueta H, Ogura R, Sugiyama M, Kagiyama A, Shin G. O2-. spin trapping on cardiac submitochondrial particles isolated from ischemic and non-ischemic myocardium. J. Mol. Cell Cardiol. 1990;22:893–899. doi: 10.1016/0022-2828(90)90120-q. [DOI] [PubMed] [Google Scholar]
- [147].Valnot I, Osmond S, Gigarel N, Mehaye B, Amiel J, Cormier-Daire V, Munnich A, Bonnefont JP, Rustin P, Rotig A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am. J. Hum. Genet. 2000;67:1104–1109. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Valnot I, Von Kleist-Retzow JC, Barrientos A, Gorbatyuk M, Taanman JW, Mehaye B, Rustin P, Tzagoloff A, Munnich A, Rotig A. A mutation in the human heme A:farnesyltransferase gene (COX10 ) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- [149].Venkatraman A, Shiva S, Davis AJ, Bailey SM, Brookes PS, Darley-Usmar VM. Chronic alcohol consumption increases the sensitivity of rat liver mitochondrial respiration to inhibition by nitric oxide. Hepatology. 2003;38:141–147. doi: 10.1053/jhep.2003.50293. [DOI] [PubMed] [Google Scholar]
- [150].Vijayasarathy C, Biunno I, Lenka N, Yang M, Basu A, Hall IP, Avadhani NG. Variations in the subunit content and catalytic activity of the cytochrome c oxidase complex from different tissues and different cardiac compartments. Biochim. Biophys. Acta. 1998;1371:71–82. doi: 10.1016/s0005-2736(97)00278-2. [DOI] [PubMed] [Google Scholar]
- [151].Vijayasarathy C, Damle S, Prabu SK, Otto CM, Avadhani NG. Adaptive changes in the expression of nuclear and mitochondrial encoded subunits of cytochrome c oxidase and the catalytic activity during hypoxia 1. Eur. J Biochem. 2003;270:871–879. doi: 10.1046/j.1432-1033.2003.03447.x. [DOI] [PubMed] [Google Scholar]
- [152].Virbasius JV, Scarpulla RC. The rat cytochrome c oxidase subunit IV gene family: tissue-specific and hormonal differences in subunit IV and cytochrome c mRNA expression. Nucleic Acids Res. 1990;18:6581–6586. doi: 10.1093/nar/18.22.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Vonck J, Schafer E. Supramolecular organization of protein complexes in the mitochondrial inner membrane. Biochim. Biophys. Acta. 2009;1793:117–124. doi: 10.1016/j.bbamcr.2008.05.019. [DOI] [PubMed] [Google Scholar]
- [154].Wasniewska M, Karczmarewicz E, Pronicki M, Piekutowska-Abramczuk D, Zablocki K, Popowska E, Pronicka E, Duszynski J. Abnormal calcium homeostasis in fibroblasts from patients with Leigh disease. Biochem. Biophys. Res. Commun. 2001;283:687–693. doi: 10.1006/bbrc.2001.4834. [DOI] [PubMed] [Google Scholar]
- [155].Waterland RA, Basu A, Chance B, Poyton RO. The isoforms of yeast cytochrome c oxidase subunit V alter the in vivo kinetic properties of the holoenzyme. J. Biol. Chem. 1991;266:4180–4186. [PubMed] [Google Scholar]
- [156].Weraarpachai W, Antonicka H, Sasarman F, Seeger J, Schrank B, Kolesar JE, Lochmuller H, Chevrette M, Kaufman BA, Horvath R, Shoubridge EA. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 2009;41:833–837. doi: 10.1038/ng.390. [DOI] [PubMed] [Google Scholar]
- [157].Wu C, Yan L, Depre C, Dhar SK, Shen YT, Sadoshima J, Vatner SF, Vatner DE. Cytochrome c oxidase III as a mechanism for apoptosis in heart failure following myocardial infarction. Am. J. Physiol Cell Physiol. 2009;297:C928–C934. doi: 10.1152/ajpcell.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Yanamura W, Zhang YZ, Takamiya S, Capaldi RA. Tissue-specific differences between heart and liver cytochrome c oxidase. Biochemistry. 1988;27:4909–4914. doi: 10.1021/bi00413a048. [DOI] [PubMed] [Google Scholar]
- [159].Yang WL, Iacono L, Tang WM, Chin KV. Novel function of the regulatory subunit of protein kinase A: regulation of cytochrome c oxidase activity and cytochrome c release. Biochemistry. 1998;37:14175–14180. doi: 10.1021/bi981402a. [DOI] [PubMed] [Google Scholar]
- [160].Yin M, Gabele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001;34:935–942. doi: 10.1053/jhep.2001.28888. [DOI] [PubMed] [Google Scholar]
- [161].Yu MA, Egawa T, Shinzawa-Itoh K, Yoshikawa S, Yeh SR, Rousseau DL, Gerfen GJ. Radical formation in cytochrome c oxidase. Biochim. Biophys. Acta. 2011;1807:1295–1304. doi: 10.1016/j.bbabio.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yu Q, Nguyen T, Ogbi M, Caldwell RW, Johnson JA. Differential loss of cytochrome-c oxidase subunits in ischemia-reperfusion injury: exacerbation of COI subunit loss by PKC-epsilon inhibition. Am. J. Physiol Heart Circ. Physiol. 2008;294:H2637–H2645. doi: 10.1152/ajpheart.91476.2007. [DOI] [PubMed] [Google Scholar]
- [163].Zhang L, Yu L, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. The Journal Of Biological Chemistry. 1998;273:33972–33976. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- [164].Zhu Z, Yao J, Johns T, Fu K, De B,I, Macmillan C, Cuthbert AP, Newbold RF, Wang J, Chevrette M, Brown GK, Brown RM, Shoubridge EA. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 1998;20:337–343. doi: 10.1038/3804. [DOI] [PubMed] [Google Scholar]
- [165].Zuckerbraun BS, Chin BY, Bilban M, d’Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]