Abstract
Background
Adenosine, an endogenous purine nucleoside, is involved in several physiological functions. We have previously shown that A2BAR plays a pro-inflammatory role during colitis.
Aims
Our goals were to determine if A2BAR expression was necessary on immune cells/non-immune cells during colitis and if A2BAR was a suitable target for treating intestinal inflammation.
Methods
Wild-type (WT) and A2BAR knockout (KO) mice were utilized in bone marrow transplants to explore the importance of immune/non-immune A2BAR expression during the development of colitis. Additionally, a T-cell transfer model of colitis was induced in Rag1 KO or A2BAR/RAG1 double KO (DKO) recipients. Finally, A2BAR small interfering RNA nanoparticles (NPs) were administered to DSS-treated mice.
Results
WT mice receiving WT or KO bone marrow developed severe colitis after DSS treatment, whereas colitis was significantly attenuated in KO mice receiving WT or KO bone marrow. Colitis induced in Rag1 KO animals was attenuated in A2BAR/RAG1 DKO recipients. Animals receiving NPs exhibited attenuated parameters of colitis severity compared to mice receiving control NPs.
Conclusions
Our results suggest that A2BAR on non-immune cells plays an important role for the induction of colitis and targeting A2BAR expression during colitis may be useful for alleviating symptoms of intestinal inflammation.
Keywords: A2B receptor, inflammation, nanoparticle therapy
1. Introduction
Adenosine is an endogenous purine nucleoside with a variety of physiological functions and modulates many aspects of the inflammatory/immune response.1,2 The effects of adenosine are mediated via four G-protein coupled receptors, A1, A2A, A2B, and A3, following adenosine release from cells or synthesis of adenosine in the extracellular space during inflammation, ischemia, hypoxia or trauma.3–5 Adenosine receptors mediate pro- or anti-inflammatory responses, depending on the cell or tissue type exposed to adenosine.6,7 In terms of intestinal inflammation, the effects of adenosine vary depending on which adenosine receptor is stimulated. Several studies have shown that A2AAR plays an anti-inflammatory role in intestinal inflammation, and the most potent immunosuppressive effects of adenosine are generally attributed to expression of A2AAR on immune cells.8–10 Similar to A2AAR, A1AR signaling was also demonstrated to attenuate intestinal inflammation.11
Whereas A2AAR and A1AR are thought to play anti-inflammatory roles during the development of colitis, we have previously shown that A2BAR exacerbates colitis and mediates proinflammatory events in the colon.12,13 A2BAR is expressed by both immune cells and the intestinal epithelial cells.5,14 Immune cells express multiple adenosine receptors;15 however, A2BAR is the primary adenosine receptor expressed in colonic epithelial cells.16,17 Previous studies demonstrated that colonic A2BAR plays a role in regulating chloride secretion, a mechanism responsible for the transport of isotonic fluid into the intestinal lumen, leading inflammation-associated diarrhea.17,18 Colonic epithelial A2BAR also mediates IL-6 secretion into the lumen, which may activate neutrophils.19 IL-6 secretion induced by A2BAR signaling was shown to increase intracellular calcium levels of neutrophils, indicative of neutrophil degranulation.19
We have previously shown that A2BAR mRNA and protein expression are upregulated in inflammatory bowel disease (IBD) patients.20. Additionally, recent results from our laboratory demonstrated that deletion of A2BAR or blocking A2BAR signaling attenuated several parameters of intestinal inflammation in various murine models of colitis.12,13 Although these findings suggest that A2BAR plays a pro-inflammatory role in intestinal inflammation, another study has shown that A2BAR plays an anti-inflammatory role during colitis. 21 Therefore, the precise mechanism by which this receptor acts during intestinal inflammation remains somewhat controversial. The goal of our current study was to determine whether A2BAR expression on immune and/or non-immune cells was required for colitis. We utilized A2BAR knockout (KO) mice in bone marrow transplant studies, and T-cell-mediated model of colitis, to address possible functional differences of A2BAR expressed on immune cells or locally in the colon. Finally, we investigated the potential therapeutic value of reducing A2BAR expression in the colon by targeting A2BAR siRNA-loaded nanoparticles (NPs) to the gastrointestinal tract.
2. Methods
2.1 Experimental animals
The Animal Care Committee of Emory University approved all procedures performed on animals. The generation of A2BAR−/− mice and their characterization has been described previously.22 Overall, mice lacking A2BAR exhibited a normal phenotype. A2BAR−/− mice were backcrossed to the C57BL/6 background as determined by the PCR-based strain detection method MAX (Charles River Labs, MA). B6 Rag1−/− were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in our facility for > 6 generations. A2BAR/Rag-1 double knockout (DKO) mice were generated by breeding A2BAR−/− and Rag-1−/− mice and verified by genotyping.
2.2 Bone marrow transplantation
Bone marrow transplants were performed as previously described.23 Briefly, the femur and tibia were removed and the bone marrow cells were collected by flushing the bone cavity with basal marrow medium (Iscove’s medium; Cambrex; East Rutherford, NJ). After washing with PBS, bone marrow cells were resuspended in basal marrow medium. Approximately 5 × 106 cells in 50 μL were transplanted retro-orbitally. Four treatment groups with 6 animals per group were used (WT→WT), (WT→KO), (KO→WT) and (KO→KO). Mice were given neomycin at 2 mg/mL for the first week after bone marrow transplants. Four weeks after transplant, colitis was induced using 3% dextran sodium sulfate (DSS) in the drinking water (w/v) and mice were assessed daily for rectal bleeding, weight loss, and diarrhea. Engraftment was verified at the time of sacrifice by genotyping bone marrow cells.
2.3 DSS colitis
Colitis was induced by oral administration of 3% DSS (MP Biomedicals Inc, Aurora, OH) in water ad libitum for 6 days. Age-matched WT and KO receiving normal water served as controls. Upon sacrifice of the mice, samples of the proximal colon, mid-colon, and distal colon were immediately frozen in liquid nitrogen or fixed in buffered 10% formalin. Clinical and histological scores were obtained as described previously.13,24
2.3 T-cell transfer colitis
Naive CD4+CD25−CD45RBhi T cells were isolated from B6 and were FACS sorted, as previously described.25 Sex-matched B6 Rag-1−/− or A2BAR/Rag-1 DKO recipients were injected with 4 × 105 cells via the tail vein, and the development of intestinal inflammation was monitored. Mice were sacrificed when symptoms of clinical disease (significant weight loss or diarrhea) developed in control groups, 8 weeks after the T-cell transfer. Samples of proximal colon, mid-colon, and distal colon were immediately frozen in liquid nitrogen or fixed in buffered 10% formalin. Histology scores were calculated as previously described. 26
2.4 Colonic myeloperoxidase (MPO) activity
Neutrophil infiltration into colon was determined by measuring MPO activity, as described previously.27 One unit of MPO activity was defined as the amount of MPO that degraded 1 μmol of peroxidase per minute.
2.5 Quantification of cytokines in the colon
Cytokine mRNA levels were measured by real-time PCR using previously designed primers.28 Total RNA was extracted from colonic tissue of mice using TRIzol reagent (Invitrogen, Grand Island, NY). A reverse transcription reaction was performed with 2 μg of each sample and oligo-dT primer, using the SuperScript First Strand synthesis system for RT–PCR (Invitrogen). iQ SYBR Green Supermix (Biorad, Hercules, CA) was used for real-time PCR reactions using the real-time RealPlex 4S sequence detection system (Eppendorf, Hauppauge, NY). Expression levels of 36B4 were used as an internal control. The ΔΔCT was calculated as follows: ΔΔCT = (Ct, target − Ct, 36B4)experimental group−(Ct, target − Ct, 36B4)control, with the final values derived from 2−ΔΔCT.
Protein levels of cytokines were measured in supernatant collected from colonic organ culture. Each colon was cut into 1-cm pieces and washed in HBSS with penicillin-streptomycin and cultured in serum-free RPMI-1640 (Mediatech, Manassas, VA), supplemented with penicillin-streptomycin. Cultures were incubated at 37°C in 5% CO2 for 24 hours. After 24 hours, supernatants were centrifuged and supernatant cytokine levels were measured using a MULTI-SPOT plate from Meso Scale Discovery (Gaithersburg, MD).
2.6 Preparation of A2BAR siRNA/PEI NPs covered with PVA
NPs were synthesized via double emulsion/solvent evaporation, as described previously.29,30 Briefly, an internal phase (see method below) containing A2BAR siRNA (Sigma-Aldrich) was mixed with 20 g/L of PLA in dichloromethane to generate a water-in oil (W/O) emulsion after 2 min of vortexing (Maxi Mix II, Thermodyne, Dubuque, Iowa, USA) and 1 min of sonication (Pmax ¼ 400 W) (Digital Sonifier 450, Branson, Danbury, CT, USA). The first emulsion was mixed into a second water phase containing 0.3 g/L of PVA to generate a water/oil/water emulsion (W/O/W). The W/O/W emulsion was dropped in a dispersing phase of 0.1 g/L PVA, and stirred at 45°C under a vacuum to remove dichloromethane. NPs were centrifuged at 9953g and freeze-dried overnight at −50°C under 0.1 mbar pressure.
2.7 Preparation of the internal phase containing A2BAR siRNA
The internal phase had an N/P ratio of the number of negative charges of A2BAR siRNA (P as the phosphorous charge) and positive charges of polyethyleneimine (PEI) (N as the ammonium charge). We used an N/P ratio of 30 for PEI. A mixture of siRNA/PEI: 29 μL A2BAR siRNA (5 μM) or scrambled siRNA was combined with 18 μL PEI (5 mM), and incubated for 10 min at room temperature. After 10 minutes, a polyplex was formed and 750 μL bovine serum albumin (BSA, 50 g/L) was added to generate the first emulsion with dichloromethane.
2.8 Treating DSS-induced colitis with A2BAR siRNA-loaded NPs
C57BL/6 mice were gavaged once daily starting on the first day of DSS treatment through 8 days of DSS treatment with A2BAR siRNA-loaded NPs. The negative control group was gavaged daily with water and hydrogel, while the positive controls for this study were mice treated with DSS and gavaged with hydrogel alone or DSS-treated mice gavaged daily with hydrogel and empty (or scrambled siRNA) NPs.
2.9 Statistical analysis
The data are presented as means ± SEM. Statistical analysis was assessed using Student’s t-test where p < 0.05 was considered significant. In experiments where multiple groups were compared, ANOVA analysis was also used to assess statistical significance.
3. Results
3.1 DSS-induced colitis is attenuated in the absence of mucosal-derived A2BAR
We performed bone marrow transplants to explore the role of both colonic and immune cell-derived A2BAR during the development of colitis. All mice recovered uneventfully from bone marrow transplants. Four weeks after transplant, mice were given drinking water containing 3% (w/v) DSS. Mice were weighed daily and compared with controls in terms of weight loss, stool consistency, and presence of occult stool blood. KO animals receiving bone marrow from WT or KO mice lost less body weight than WT mice receiving WT or KO bone marrow (Figure 1A and 1B). All WT mice that received WT bone marrow developed signs of colitis 5 or 6 days after introduction of DSS-containing drinking water. Colitis was attenuated in KO mice receiving either WT or KO bone marrow. Interestingly, WT mice that received bone marrow from KO animals developed a form of colitis that was not significantly different from that of WT animals receiving WT bone marrow (Figure 1B). These data suggest that A2BAR expression on the colonic epithelium is important for the development of DSS-induced colitis.
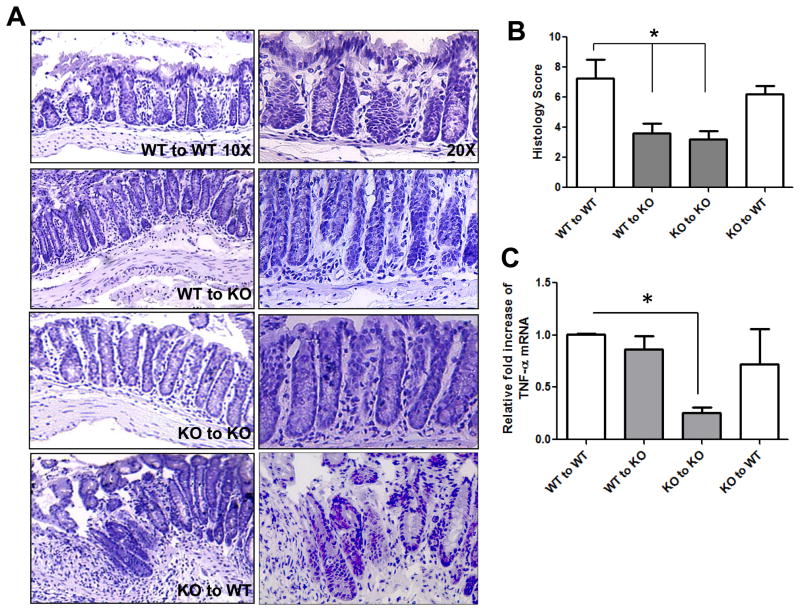
Figure 1. DSS-induced colitis is attenuated in the absence of mucosal-derived A2BAR.
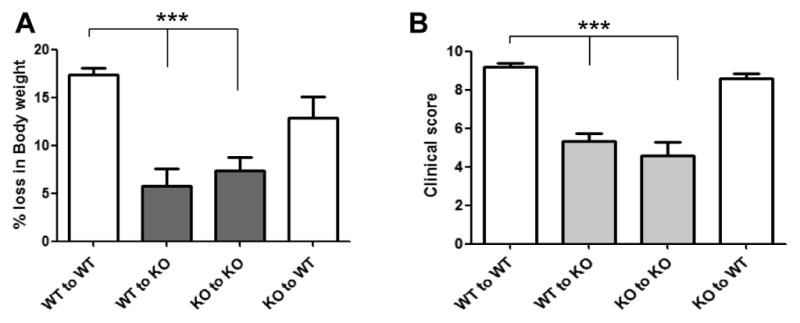
5 × 106 bone marrow cells were transplanted retro-orbitally. Four transplant groups were used; (WT→WT), (WT→KO), (KO→WT) and (KO→KO). Four weeks after transplantation, colitis was induced using 3% DSS in the drinking water (w/v) and mice were assessed daily for weight loss (A), rectal bleeding and diarrhea to calculate the clinical score. Clinical scores were calculated based on the following criteria: Weight loss (1–5% = 1, 5–10% = 2, 10–20% = 3, >20% = 4), stool character (normal = 0, soft with well-formed pellets = 1, soft without pellets = 3, diarrhea = 4) and fecal blood (no blood = 0, positive occult test = 2, gross bleeding = 4). The individual criteria scores were added to give a total clinical score ranging from 0 to 12 (B). Clinical scores and percent weight lost were averaged from 5–6 mice per group and error bars represent the standard deviation. Statistical significance was assessed using Students t-test, where ***, p < .001.
The extent of neutrophil infiltration, assessed by evaluation of MPO levels and histological scoring, was measured to complement the clinical data. WT mice receiving WT bone marrow exhibited obvious signs of colonic inflammation and tissue damage, in contrast to KO mice that received either WT or KO bone marrow (Figure 2A and B). The WT recipients of WT bone marrow had extensive crypt damage, showed epithelial ulceration and formation of crypt abscesses, and had a higher level of infiltration of inflammatory cells into the mucosa, compared to KO recipients of either WT or KO bone marrow (Figure 2A). Finally, we explored whether the level of the pro-inflammatory cytokine TNF-α veried among the different groups because TNF-α is known to be an important effector in both IBD and the DSS colitis model. We found that KO mice receiving KO bone marrow produced less TNF-α-encoding mRNA after DSS treatment than did WT mice receiving WT bone marrow (Figure 2C). In contrast to the clinical and histological data, KO mice receiving WT bone marrow expressed TNF-α at a level similar to that of WT mice receiving either WT or KO bone marrow (Figure 2C). These data suggest that WT bone marrow-derived cells may be able to alter the TNF-α cytokine profile after DSS treatment, but the increase in TNF-α is not enough to exacerbate colitis in KO mice to the severity levels demonstrated in WT mice. Overall, the data suggest that A2BAR expression by non-immune cells most likely mediates the pathology observed after DSS treatment.
Figure 2. Parameters of DSS-induced colitis are attenuated in the absence of mucosal- A2BAR.
H&E staining was performed on colonic sections from bone marrow transplant recipients and visualized at 10X and 20X (A). Histological scores were blindly calculated and averaged for 5–6 mice/group, where the error bars represent standard deviation. The histological scores were based on the following criteria: Inflammatory cells in the laminia propria (rare inflammatory cells = 0, increased number of granulocytes = 1, confluence of inflammatory cells = 2, transmural extension of infiltrate = 3), crypt damage (intact crypt = 0, loss of 1/3 of the basal crypt = 1, loss of 2/3 of the basal crypt = 2, entire crypt loss = 3, change of epithelial surface with erosion = 4, confluent erosion = 5) and ulcer evaluation (no ulcer = 0, 1–2 foci of ulcers = 1, 3–4 foci = 2, extensive ulceration = 3). The total score of these criteria gave a histological score ranging from 0 to 11 (B). TNF-α mRNA levels were measured from isolated colonic RNA using real-time PCR. 36B4 was used as an internal control to account for variances between samples (C). Statistical significance was assessed using Students t-test, where *, p < .05 and the error bars represent the calculated standard deviations.
3.2 A2BAR exacerbates T-cell-mediated intestinal inflammation
Next, we determined the role of mucosal A2BAR in the development of intestinal inflammation using a well-established T-cell-dependent chronic model of colitis. Naïve CD4+CD45RBhigh T cells isolated from C57BL/6 mice were adoptively transferred into sex-matched cohorts of syngeneic RAG1-deficient (RAG1 KO) or A2BAR/RAG1-deficient (DKO) animals, and development of intestinal inflammation was monitored. RAG1 KO and A2BAR/RAG1 DKO mice lost the same percentage of body weight throughout the study (Figure 3A). However, we found that the severe colitis induced by naïve CD4+CD45RBhigh T-cell transfer into RAG1 KO recipients was significantly attenuated in A2BAR/RAG1 DKO mice.
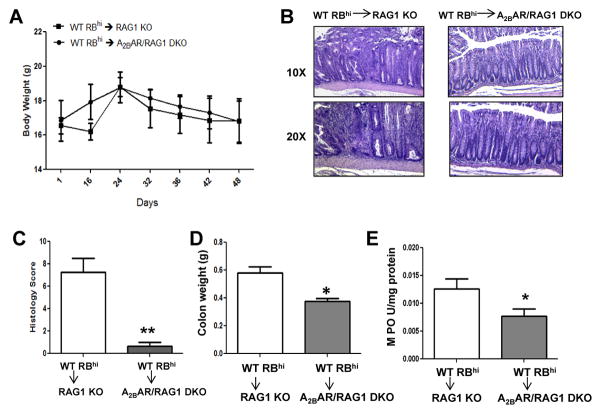
Figure 3. A2BAR exacerbates T-cell mediated intestinal inflammation.
Naive CD4+CD25−CD45RBhi T cells were isolated from WT mice by FACS. Rag1 KO or A2BAR/RAG1 DKO recipients were injected with 4 × 105 cells via the tail vein and the development of intestinal inflammation was monitored. Mice were sacrificed when symptoms of clinical disease (significant weight loss or diarrhea) developed in control groups, around 7–8 weeks after T-cell transfer for both experimental groups. Mice were weighed at the indicated days after T-cell transfer (A). Colonic sections were analyzed by H&E staining (B) and blindly scored for four criteria: degree of epithelial hyperplasia and goblet cell depletion; leukocyte infiltration into the lamina propria; the area of tissue affected; and indicators of inflammation including crypt abscesses, submucosal inflammation and ulcers. The scores of each of these criteria were added to determine the histology score (on a scale of 0–12) (C). Upon sacrifice, the colons were removed and weighed (D) and MPO levels were measured to determine the level of neutrophil infiltration (E). 5–6 mice were used for each experimental group and the error bars represent standard deviation. Statistical significance was assessed using Students t-test, where *, p < .05.
Histological assessment of colonic sections showed that RAG1 KO animals had extensive crypt damage, exhibited epithelial ulceration and formation of crypt abscesses, and had an increased level of mucosal infiltration by inflammatory cells, compared to A2BAR/RAG1 DKO mice (Figure 3B). Thus, the histological scores of RAG1 KO colonic sections were significantly higher than scores from A2BAR/RAG1 DKO sections (Figure 3C), suggesting that absence of endogenous A2BAR, specifically in the colonic epithelium, was sufficient to significantly attenuate colitis in the presence of A2BAR-expressing T cells. In contrast to the observed exacerbation of pathology in RAG1 KO mice, the colon weight was decreased in A2BAR/RAG1 DKO animals (Figure 3D). However, MPO levels rose in RAG1 KO animals, suggesting that more neutrophils were infiltrating the colons of RAG1 KO mice compared to A2BAR/RAG1 DKO mice (Figure 3E). Overall, the data suggest that A2BAR expression on lymphocytes, specifically CD4+ T-cells, may not be required for the development of severe intestinal inflammation in the CD45RBhi transfer model of colitis.
3.3 T-cell-mediated pro-inflammatory cytokine production is attenuated in the absence of A2BAR
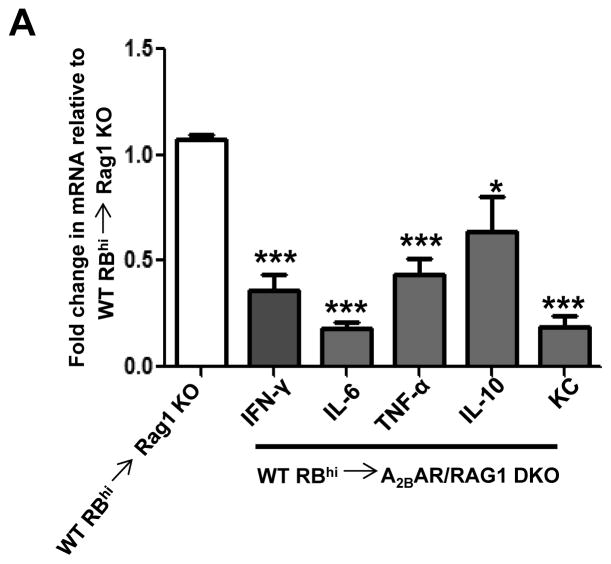
The T-cell transfer colitis model is characterized by an increase in the local Th1 response.31 Therefore, we compared the levels of several proinflammatory cytokines in colon tissue from RAG1 KO and A2BAR/RAG1 DKO recipients. As predicted, colonic tissue isolated from control RAG1 KO recipients had significantly elevated levels of IFN-γ, IL-6, TNF-α and KC. The levels of these cytokines were significantly decreased in A2BAR/RAG1 DKO recipients (Figure 4). IL-10-encoding mRNA was also reduced in A2BAR/RAG1 DKO mice compared to RAG1 KO mice. Together, these data suggest that A2BAR is an important component of the inflammatory response characteristic of the T-cell transfer model of colitis.
Figure 4. T-cell mediated pro-inflammatory cytokine production is attenuated in the absence of A2BAR.
Real-time PCR was utilized to determine the mRNA levels of the listed cytokines in the colons of RAG1 KO and A2BAR/RAG1 DKO mice 7–8 weeks after T-cell transfer. RAG1 KO cytokine levels were normalized to 1.0 and the A2BAR/RAG1 DKO cytokine levels represent the fold-change compared to RAG1 KO cytokine levels. 36B4 was used as an internal control to account for variances in cDNA used in each real-time PCR reaction. Statistical significance was assessed using Students t-test, where ***, p < .001 and *, p < .05 (IL-10 only) when compared to RAG1 KO cytokine levels, which were normalized to 1.0. 6 mice were used for each experimental group and error bars represent standard deviation.
3.4 A2BAR siRNA-loaded nanoparticle therapy attenuates development of DSS-induced colitis
Our bone marrow transplant data suggest that the presence of colonic A2BAR was sufficient to induce colitis. Therefore, we tested the hypothesis that decreased A2BAR levels in the colon would attenuate the development of colitis induced by DSS treatment. Previously, our laboratory prepared nanoparticles (NPs) loaded into a biomaterial formed of alginate and chitosan that can be used to target various NP-loaded substrates to the colon.29 Earlier, it was demonstrated that the biomaterial collapsed in intestinal solution at a pH of 5–6; which is the pH of the colon under both inflammatory and non-inflammatory conditions. We synthesized NPs containing A2BAR siRNA (Figure 5A) to determine if targeting the expression of A2BAR in the colon might be valuable to attenuate symptoms of colitis. WT mice were given NPs alone or NPs loaded with A2BAR siRNA, by daily gavage, during DSS treatment.
Figure 5. A2BAR siRNA-loaded nanoparticle therapy attenuates development of DSS-induced colitis.
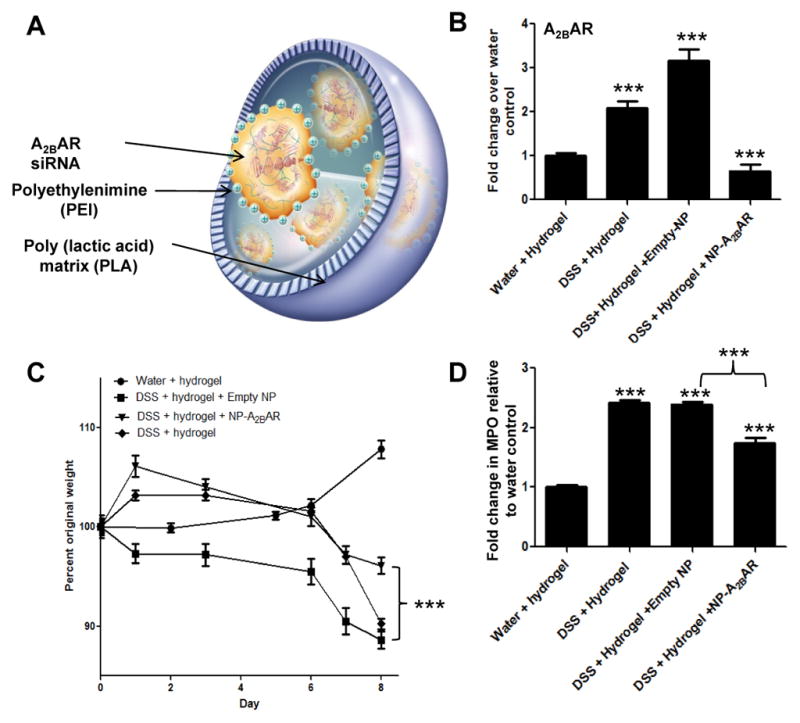
A schematic representation of a A2BAR siRNA-loaded NP, adapted from Laroui et. al 37 (A). After 8 days of DSS treatment, mice were sacrificed and their colons were collected for RNA isolation. A2BAR mRNA levels were determined by real-time PCR using 36B4 as an internal control (B). The different treatment groups were weighed daily and the percent of their original weight was calculated (C). After 8 days of DSS treatment, mice were sacrificed and their colons were collected for MPO analysis. MPO calculations are depicted as fold-increase over water and hydrogel negative control MPO levels. All the experimental groups had significantly increased MPO levels compared to the negative control group receiving water and hydrogel gavages (D). Statistical significance was assessed using Students t-test, where ***, p < .001. 6 mice were used for each experimental group and error bars represent standard deviation.
First, we found that the level of mRNA encoding A2BAR was significantly decreased in the colons of mice treated with A2BAR siRNA-loaded NPs, compared to controls (Figure 5B). Next, we determined that the levels of A2BAR mRNA were significantly elevated both in DSS-treated test mice and in DSS-treated animals gavaged with empty NPs, compared to either control mice or animals treated with A2BAR siRNA-loaded NPs. We found that mice treated with A2BAR siRNA-loaded NPs lost significantly less weight than the DSS-treated animals receiving empty NPs (Figure 5C). Finally, we showed that MPO levels were drastically decreased in mice receiving A2BAR siRNA-loaded NPs, suggesting neutrophil infiltration was decreased upon downregulation of A2BAR expression (Figure 5D).
3.5 Production of DSS-induced proinflammatory cytokines is attenuated upon treatment with A2BAR siRNA-loaded NPs
In addition to decreased MPO levels and weight loss, mice treated with A2BAR siRNA-loaded NPs exhibited reductions in the concentrations of colonic mRNAs encoding proinflammatory cytokines and chemokines, including IL-6; IL-1β and the murine homolog of the human chemokine IL-8; KC. Proinflammatory cytokine protein levels were also decreased in the colons of animals receiving A2BAR siRNA compared to controls. In agreement with the mRNA levels, the levels of IL-6, IL-1β, and KC were all significantly lower in mice A2BAR siRNA-loaded NPs than in controls. Additionally, TNF-α production was downregulated when A2BAR expression was decreased during DSS treatment. Interestingly, we found that IL-10 levels also fell upon treatment with A2BAR siRNA-loaded NPs, compared to the concentrations seen in mice receiving empty NPs, during DSS treatment. This finding supports previous data that demonstrates signaling through A2BAR on various cell types leads to increased production of IL-10.32,33
4. Discussion
A2BAR is increasingly recognized as a mediator of inflammation in chronic diseases including asthma, cancer, and IBD.5,13,34,35 In the present study, we investigated the roles played by immune cells and colonic A2BAR during the progression of colitis using an acute and chronic model of experimental murine colitis. Bone marrow transplant experiments revealed that A2BAR expression on non-bone marrow-derived cells was important in the development of several clinical features of DSS-induced colitis, including weight loss and exacerbation of clinical and histological scores. These data suggest that local expression of A2BAR in the colonic epithelium may be sufficient to induce colitis in the acute DSS model. When KO mice received WT bone marrow, they showed continued protection against clinical and histological signs of colitis, suggesting that A2BAR expression by immune cells may not be as important in the development of intestinal inflammation upon DSS treatment. In agreement with the bone marrow transplant data, we found that the transfer of naïve CD4+ T cells induced severe colitis in RAG1 KO mice, whereas colitis was significantly attenuated in RAG1 KO animals crossed with A2BAR KO mice, suggesting that in the absence of A2BAR in the epithelium, an attenuated form of colitis is observed. These data are important, as the T-cell transfer model represents a chronic colitis model facilitated by T-cells which may model human IBD more accurately than acute models of colitis, due to the important role played of T-cells in chronic intestinal inflammation in human IBD.36
Several studies have suggested that A2BAR signaling may mediate either pro- or anti-inflammatory responses depending upon the cell and/or tissue type exposed to adenosine. For example, reports have indicated that A2BAR plays a protective role in endothelial barrier function and vascular injury models.12,32,34 However, several studies have provided strong evidence that A2BAR mediates pro-inflammatory responses in diseases including asthma and chronic pulmonary disease.34 Additionally, at the cellular level, A2BAR signaling mediates inflammatory effector functions in mast cells, epithelial cells, smooth muscle cells, fibroblasts, and neutrophils.5 Work in our laboratory has consistently shown that A2BAR plays a pro-inflammatory role in intestinal inflammation using several experimental models of colitisincluding DSS, 2,4,6-trinitrobenzene sulfonic acid (TNBS) and Salmonella typhimurium 12,13; however a separate group determined that DSS-induced colitis was exacerbated in the absence of A2BAR expression or upon inhibition of A2BAR signaling.21 Another study showed that the extent of gastrointestinal ischemia/reperfusion injury was more severe in the absence of A2BAR3, supporting the hypothesis that the receptor plays a protective role in hypoxic environments. Currently, no obvious reason for the disparity between our results and those of Frick et al.21 is apparent; the differences may be attributable to variation in the genetic backgrounds of the experimental mice, the presence of different intestinal microbial populations (attributable, perhaps, to variations in institutional animal facilities), or differences between the methods used to induce colitis.
Our data consistently demonstrated that in the absence of A2BAR, several parameters of colitis pathology were attenuated; therefore, we sought to target A2BAR expression specifically in the gastrointestinal tract. Because A2BAR has been shown to play both anti- and pro-inflammatory roles depending upon the cell and tissue type, future therapies should specifically target A2BAR at localized sites of inflammation. Previous studies from our laboratory have optimized the formulation of NPs so that they can be administered via the oral route and breakdown at a pH that is similar to that of the colon.29,37 Our preliminary data suggest that A2BAR, which was previously shown to be upregulated in Crohn’s patients20, may be a plausible target for treating symptoms of IBD by attenuating the effects of inflammatory mediators. Our method of delivering A2BAR siRNA provides a targeted localized delivery of a drug via the oral route that significantly reduced A2BAR mRNA expression during DSS treatment. Our results suggest that targeting A2BAR expression at a local level during colitis may be beneficial for ameliorating symptoms of intestinal inflammation; however, due to differences in clinical parameters and pathology in acute murine models of colitis versus human disease, future experiments will be required to determine the effect of targeting A2BAR in chronic models of colitis as well as human IBD.
Figure 6. Production of DSS-induced proinflammatory cytokines is attenuated upon treatment with A2BAR siRNA-loaded NPs.
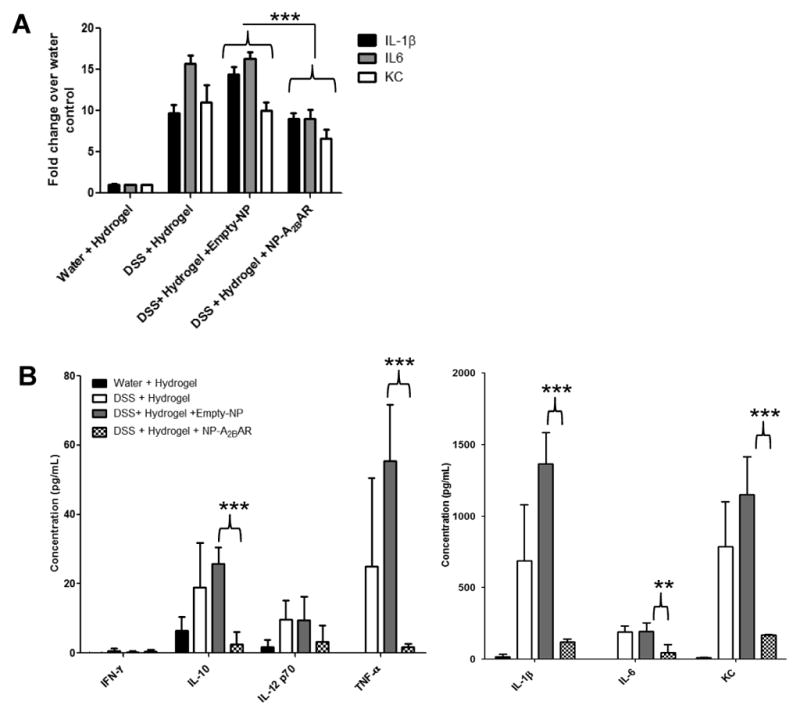
After 8 days of DSS treatment, mice were sacrificed and their colons were collected for isolation of RNA. Proinflammatory cytokine and chemokine mRNA levels were determined by real-time PCR. 36B4 was used as an internal control (A). Protein levels of proinflammatory cytokines were measured in supernatant collected from colonic organ cultures after 24 hours of incubation at 37°C. Supernatants were centrifuged and the cytokine and chemokine levels were measured using a MULTI-SPOT plate (B). 6 mice were used for each experimental group and error bars represent standard deviation. Statistical significance was assessed using Students t-test, where **, p <.01 and ***, p < .001.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants, DK06411
We dedicate this article to the memory of Dr. Shanthi V. Sitaraman, a brilliant scientist, dedicated physician, passionate humanitarian and dearest friend.
Footnotes
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fredholm BB, Irenius E, Kull B, et al. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–8. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB, APIJ, Jacobson KA, et al. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–96. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madara JL, Patapoff TW, Gillece-Castro B, et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–5. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasko G, Csoka B, Nemeth ZH, et al. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30:263–70. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolachala VL, Bajaj R, Chalasani M, et al. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–10. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 7.Feoktistov I, Biaggioni I. Role of adenosine A(2B) receptors in inflammation. Adv Pharmacol. 2011;61:115–44. doi: 10.1016/B978-0-12-385526-8.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naganuma M, Wiznerowicz EB, Lappas CM, et al. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 9.Odashima M, Bamias G, Rivera-Nieves J, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008;83:447–55. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozacmak VH, Sayan H. Pretreatment with adenosine and adenosine A1 receptor agonist protects against intestinal ischemia-reperfusion injury in rat. World J Gastroenterol. 2007;13:538–47. doi: 10.3748/wjg.v13.i4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolachala V, Ruble B, Vijay-Kumar M, et al. Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol. 2008;155:127–37. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolachala VL, Vijay-Kumar M, Dalmasso G, et al. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–70. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novitskiy SV, Ryzhov S, Zaynagetdinov R, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–31. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–12. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–36. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strohmeier GR, Reppert SM, Lencer WI, et al. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–94. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 18.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–72. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Sitaraman SV, Merlin D, Wang L, et al. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–9. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolachala V, Asamoah V, Wang L, et al. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–57. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frick JS, MacManus CF, Scully M, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–64. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Zhang Y, Nguyen HG, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–23. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalmasso G, Nguyen HT, Ingersoll SA, et al. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology. 2011;141:1334–45. doi: 10.1053/j.gastro.2011.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 25.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487–91. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 26.Aranda R, Sydora BC, McAllister PL, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–73. [PubMed] [Google Scholar]
- 27.Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Dalmasso G, Charrier-Hisamuddin L, Thu Nguyen HT, et al. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–78. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laroui H, Dalmasso G, Nguyen HT, et al. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843–53. e1–2. doi: 10.1053/j.gastro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Laroui H, Theiss AL, Yan Y, et al. Functional TNFalpha gene silencing mediated by polyethyleneimine/TNFalpha siRNA nanocomplexes in inflamed colon. Biomaterials. 2011;32:1218–28. doi: 10.1016/j.biomaterials.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth ZH, Lutz CS, Csoka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–70. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson JM, Ross WG, Agbai ON, et al. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009;182:4616–23. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaynagetdinov R, Ryzhov S, Goldstein AE, et al. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol. 2010;42:564–71. doi: 10.1165/rcmb.2008-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryzhov S, Novitskiy SV, Zaynagetdinov R, et al. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–95. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–46. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laroui H, Wilson DS, Dalmasso G, et al. Nanomedicine in GI. Am J Physiol Gastrointest Liver Physiol. 2011;300:G371–83. doi: 10.1152/ajpgi.00466.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]