Abstract
Though osteonecrosis of the jaw (ONJ) is temporally-associated with the use of nitrogen-containing bisphosphonates (N-BPs), a cause/effect relationship has not yet been established. We hypothesize that ONJ is a two-stage process in which: a) risk factors initiate pathologic processes in the oral cavity that lead to a supranormal rate of hard tissue necrosis, and b) powerful anti-resorptives reduce the rate of removal of necrotic bone sufficiently to allow its net accumulation in the jaw. To test this hypothesis, we used the rice rat model of periodontitis. At age 28 days, rats (n=15/group) were placed on a high sucrose and casein diet to exacerbate the development of periodontitis. Animals were injected SC biweekly with vehicle or alendronate (ALN, 15μg/kg), or IV once monthly with vehicle, a low dose (LD), or a high dose (HD) of zoledronic acid (ZOL) and sacrificed after 6, 12, 18, and 24 wks. Mandibles and maxillae were analyzed to determine the effects on the: a) progression of periodontitis, b) integrity of alveolar bone, c) status of bone resorption and formation, d) vascularity, and e) osteocyte viability. We found that only HD-ZOL induced ONJ-like lesions in mandibles of rice rats after 18 and 24 wks of treatment. These lesions were characterized by areas of exposed necrotic alveolar bone, osteolysis, a honey comb-like appearance of the alveolar bone, presence of bacterial colonies, and periodontal tissue destruction. In addition, inhibition of bone formation, a paradoxical abolition of the antiresorptive effect of only HD-ZOL, increased osteocyte necrosis/apoptosis, and decreased blood vessel number were found after 18 and/or 24 wks. Our study suggests that only HD-ZOL exacerbates the inflammatory response and periodontal tissue damage in rice rats, inducing bone lesions that resemble ONJ.
Keywords: ONJ, Zoledronic acid, alendronate, periodontal disease, rice rats
Introduction
Osteonecrosis of the jaw (ONJ) has been temporally associated with the use of nitrogen (N)-containing bisphosphonates (N-BPs)(1-5) and more recently, with the use of receptor activator of nuclear factor kappa B (RANK) ligand (L) antibodies.(6-9) However, no cause/effect relationship has yet been established. N-BPs are powerful antiresorptive agents which bind to bone surfaces. They are ingested by osteoclasts, after which they decrease the activity and survival of these bone resorbing cells.(10,11) RANKL antibodies inhibit the formation, function and survival of osteoclasts by specifically binding to RANKL, preventing its binding to the RANK receptor and hence, inhibiting RANKL/RANK signaling.(12-14) Because of the earlier introduction and wider use of N-BPs compared to RANKL antibodies, more research has been conducted on N-BP-associated ONJ than RANKL-associated ONJ. N-BP-associated ONJ has been defined as an area of exposed bone in the maxillofacial region that does not heal within 8 weeks after identification by a health care provider, in a patient who is receiving or had been exposed to an N-BP and had not had radiation therapy to the craniofacial region.(1,15,16) ONJ was reported to occur in 6-10% of N-BP-treated cancer patients.(1,4,5,17) It is observed most frequently in patients with multiple myeloma or breast cancer treated intravenously with high doses of N-BPs, particularly zoledronic acid (ZOL) or pamidronate,(3,18,19) for inhibition of bone metastases. ONJ has also been reported to occur in postmenopausal women undergoing treatment for osteoporosis with the N-BPs alendronate (ALN), ibandronate, and risedronate.(4,5,19,20) Although ONJ incidence in N-BP-treated osteoporotic patients is much less than in cancer patients, more total ONJ cases may be identified in osteoporotic patients because many more women are treated with N-BPs for osteoporosis than for cancer.
Periodontitis has been identified as one of the most important risk factors for the development of ONJ.(1,4) The rice rat has been shown to be extraordinarily susceptible to the initiation and progression of a spontaneous form of periodontitis that does not require mechanical manipulation.(21-25) Periodontal lesions develop more rapidly when rats are fed a diet high in sucrose and casein (H-SC).(22-24,26-28) Importantly, periodontitis in rice rats appears somewhat similar to that observed in humans in its development, location and appearance.(22,23,27,29)
We have recently reexamined the rice rat model for periodontitis and characterized the periodontal lesions.(25) We confirmed that rice rats, particularly when consuming a pelleted H-SC diet, represent a good small animal model for the accelerated horizontal and vertical alveolar bone loss (ABL) (moderate periodontitis) observed in humans with early or late onset periodontitis. Based on these findings, we considered that rice rats would represent a useful tool to study ONJ. We made use of this animal model of periodontitis and hypothesized that ONJ in this model is a two-stage process in which: a) risk factors, such as periodontitis, initiate pathologic processes in the oral cavity that lead to a supranormal rate of hard tissue necrosis, and b) a reduced level of bone resorption (e.g. as produced by powerful anti-resorptives) decreases the rate of removal of necrotic bone sufficiently to allow its net accumulation in the jaw.
We aimed to establish the effects of osteoporosis doses of ZOL and ALN, and oncologic doses of ZOL on: a) the progression of periodontitis, b) the integrity of alveolar bone, c) the status of bone resorption and formation, d) angiogenesis, and e) osteocyte viability. This information allowed us to determine whether N-BPs can induce ONJ-like lesions in rice rats with periodontitis.
Materials and methods
Animals and experimental design
Study 1: Effects of N-BPs on the jaws of rice rats with inducible periodontitis after 6, 12 and 18 wks of treatment
Seventeen pairs of marsh rice rats (Oryzomys palustris) were provided by Dr. Kent Edmonds, Department of Biology, Indiana University Southeast, New Albany, IN. Rice rats were maintained in quarantine for 12 wks before being transferred to a conventional room. Rice rats were paired using a monogamous continuous-breeding system until the complete number of experimental animals was generated. After a lactation period of 25-28 days, litters were weaned, separated by gender, and randomized to nine different experimental groups, composed of 15-19 (9-11 males and 5-9 females) rats in each group.
Experimental rats were fed a pelleted H-SC diet (TestDiet, Purina Feed, Richmond, IN) for periods of 6, 12 and 18 wks to induce periodontitis as previously reported.(23,25,27,30) The composition of the H-SC diet was based on the formula of the powdered Harvard high sucrose 700 diet and the ration 100 diet previously used.(21-24,26,27) Simultaneously, groups of rice rats were injected either with vehicle, ALN, or a low (LD) or high dose (HD) of ZOL. The high dose of ZOL (HD-ZOL) is referred to as an “oncologic dose” throughout the text. Vehicle or ALN (15 μg/kg) were injected SC twice weekly for 6, 12 or 18 weeks. The ALN dose was based on a clinically relevant dosage for osteoporosis that was used previously. (31,32) Vehicle, LD (8 μg/kg) or HD (80 μg/kg) of ZOL were intravenously (IV) injected in the tail vein once monthly for the same periods of time. The LD and HD of ZOL were recommended by Dr. J. Gasser (Novartis Pharma; Basel, Switzerland; personal communication) as the equivalent doses for osteoporosis and oncology treatments, respectively. In order to minimize the use of rats, vehicle control groups for ALN and ZOL were combined, in which half of the rats were injected SC with the vehicle used to dissolve ALN and the other half was injected IV with the vehicle used for ZOL dilution. Alendronate was obtained from Merck & Co. (Rahway, NJ) and dissolved in a vehicle of sterile PBS. ZOL was obtained from Novartis Pharma AG (Basel, Switzerland) and dissolved in sterile saline.
Study 2: Effects of HD of ZOL in rice rats after 24 wks of treatment
The definition of N-BP-associated ONJ states that a non-healed area of exposed bone must persist for at least a period of 8 weeks after identification,(15,16) or for 6 to 8 weeks according to Bilezikian.(1) Based on results from study 1, we conducted a second study to determine whether ONJ-like lesions could also be present in rats treated with HD-ZOL for 24 wks. For this purpose, 25-28 day-old rice rats (10 males and 5 females/group) were fed the pelleted H-SC diet for periods of 24 wks and simultaneously injected IV with vehicle or HD-ZOL monthly until the end of the study. The presence of ONJ-like lesions after 24 wks of treatment would suggest persistence of lesions for more than 6 wks.
All experimental rats were group-housed (2-5 animals per cage) in static filter top cages (area: 143 in2), with pine shavings bedding and continuous access to food and water. The housing room was maintained at 55-70 °F, with an average humidity between 30-70%, and under a 14:10-h light:dark cycle. The Animal Care Services resource at the University of Florida is an AAALAC-accredited animal care and use program. The animal protocol was approved by the University of Florida Institutional Animal Care and Use Committee (IACUC). All animal care and experimental procedures were in accordance with federal policies and guidelines of the University of Florida IACUC. Adequate measures were taken to minimize pain and discomfort in the rats.
Necropsy and tissue collection
Rats were injected SC with declomycin and calcein (Sigma) at a dose of 15 mg/kg on the 7th and 2nd days prior to sacrifice, respectively, to label sites of bone formation. Rice rats were then euthanized by CO2 inhalation followed by thoracotomy. Maxillae and mandibles were stripped of musculature, leaving the periosteum intact. All right maxillae were processed for analyses of horizontal ABL. Left maxillae of males were placed in 10% buffered formalin for analyses of histology and vertical ABL. Left mandibles of males were placed in 70% ethanol to assess static and dynamic histomorphometric parameters and vertical ABL. Left mandibles of females were placed in 4% paraformaldehyde for histology, histochemical, immunohistochemical, and vertical ABL analyses. Right mandibles of females were placed in 70% ethanol for microCT analyses. Right mandibles of male rats were frozen at -80°C and processed to histochemically determine lactate dehydrogenase (LDH) activity in osteocytes as a marker of cell viability.
Determination of horizontal alveolar bone loss (ABL)
Right maxillae were processed as previously described.(25) Digital images of both palatal and buccal root surfaces of all molar teeth were captured using a stereo dissecting microscope (SteReo Discovery V8; Carl Zeiss MicroImaging, Inc., Thornwood, NY), and the surface perimeter of the CEJ and the alveolar bone crest (ABC) was calculated.(25)
Vertical alveolar bone loss (ABL)
Maxillae of male and female rice rats were processed as previously described.(25,33,34) Fifty consecutive 10 μm sections were discarded to eventually obtain two consecutive sections more adjacent to the buccal surface (buccal surface sections). Vertical alveolar bone height (ABH) was determined by measuring the distance from a line between the CEJs of adjacent teeth to the ABC of the interproximal alveolar bone between the first and second molars (M1-M2), and second and third molars (M2-M3). These measurements were obtained in the palatal surface and buccal surface sections using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA) at a magnification of 100×.(25) Sections of mandibles from males that were stained enbloc with basic fuchsin and embedded in methyl methacrylate were used to assess vertical alveolar bone height at the lingual surface (see bone histomorphometry below).
Histomorphometry
Region of interest (ROI)
Histomorphometric evaluation for bone parameters, necrotic alveolar bone, and histochemical and immunohistochemical analyses were performed in the ROI located at the mandibular interproximal space between molar (M)-1 and M2, the site for the highest prevalence and severity of periodontal lesions in rice rats.(23,27,29) The ROI was similar to that previously reported(27,30) except for minor modifications(25) (See supplement Figure 1 for details).
Evaluation of bone parameters
Left mandibles from male rats (n=9-11/group) were processed by enbloc basic fuchsin staining and methyl methacrylate embedding to assess bone static and dynamic histomorphometric parameters within the ROI as previously described.(25,35-37) Sections obtained from each rat that were adequately aligned and contained alveolar bone processes from the lingual surface were used for the quantitative analysis. Variables assessed in the ROI included the % of mineralized alveolar bone present within the ROI (alveolar bone volume) and fluorochrome-based indices of bone formation, including mineralizing surface (% cancellous bone perimeter bearing double fluorochrome labels plus one half of singly labeled surfaces), mineral apposition rate, and bone formation rate. Bone formation rate (surface referent, BFR/BS) was calculated by multiplying mineralizing surface by mineral apposition rate.(38) The terminology used, when applicable, was based on recommendations by the Histomorphometry Nomenclature Committee of the American Society of Bone and Mineral Research.(39)
Osteoclast analyses
Left mandibles from female rats (n= 5-9/group) were decalcified, embedded in paraffin, sectioned at 5 μm and stained for tartrate-resistant acid phosphatase (TRAP; Sigma).(40) Bone resorption parameters, including eroded surface, osteoclast surface and osteoclast number, were measured on alveolar bone surfaces within the ROI using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA).
Assessment of necrotic alveolar bone
Necrotic alveolar bone within the ROI was defined using 3 complementary approaches: 1) quantification of bone areas containing non-viable osteocytes using sections histochemically stained for lactate dehydrogenase (LDH) enzyme reaction, 2) evaluation of osteocyte apoptosis by TUNEL staining, and 3) measurement of bone areas devoid of basic fuchsin stain.
LDH reactivity
Right mandibles from male rice rats (n=9-11/group) were used to calculate the amount of necrotic bone present in the alveolar bone of the ROI. Necrotic bone was histologically defined as those mineralized bone areas lacking viable osteocytes. Osteocyte viability was measured using in situ histochemical analysis of lactate dehydrogenase (LDH) activity as previously described.(41,42) Briefly, three randomized 7 μm undecalcified cryosections per animal were obtained with a Leica Cryostat (Leica, Inc.) equipped with a CryoJane Frozen Sectioning Kit (Instrumedics Inc.).(43) Cryosections were incubated for 3 hours at 37°C in 40 % Polypep solution containing nicotinamide adenine dinucleotide (NAD) disodium salt, lactic acid and nitroblue tetrazolium (NBT). Following incubation, sections were rinsed in warm water to remove the reaction mixture prior to fixation in 4% paraformaldehyde, and then mounted in Farrant's mounting medium. The total number of LDH positive and negative osteocyte lacunae was determined within the alveolar bone area of the ROI. Results were expressed as the percentage of LDH-negative osteocyte lacunae over the total number of osteocyte lacunae (with/without osteocytes present) within the ROI using the Osteomeasure System (Osteometrics, Inc.).
TUNEL assay
Paraffin embedded, 5 μm longitudinal sections of mandibles from female rats (n=5/group) were deparaffinized and rehydrated through a graded series of ethanol and water. Slides were then placed in 0.1M citrate buffer (pH 6.0) and permeabilized by exposure to one minute of microwave irradiation (350W). A known positive sample and two negative control slides were included in the runs. Staining was performed using a commercially available apoptosis kit (In situ cell death Detection Kit, Roche, Applied Science) following the manufacturer's instructions. TUNEL reaction mixture was incubated on the slides for 1 hour at 37°C, with negative control slides receiving labeling mixture devoid of TdT enzyme. After 3 washes in 1× PBS, slides were coverslipped using VectaShield with DAPI (Vector Labs, Burlingame, CA). Detection was observed as a fluorescent green signal contributed by Fluorescein Isothiocyanate (FITC).
Basic fuchsin stain using the en bloc staining method
The same bulk stained mandible sections from male rats (n=9-11) were used for histomorphometry were used to determine necrotic bone. Necrotic bone was defined as those regions of the bone matrix devoid of basic fuchsin stain and was assessed in the ROI at a magnification of 200×. Necrotic bone was expressed as a percentage of the total bone area within the ROI using the Osteomeasure System (Osteometrics, Inc.). This method has been used to identify areas of necrotic bone in the mandibles of dogs treated with N-BPs.(35-37)
Immunohistochemical evaluation of angiogenesis/vascularity
Left mandibles from female rats (n=5/group) were fixed in 4% paraformaldehyde and decalcified in 5% formic acid. Five μm decalcified paraffin-embedded sections were cut in the mesio-distal plane and processed for the morphometric evaluation of angiogenesis/vascularity by immunocytochemistry. Briefly, sections were deparaffinized and rehydrated through a graded series of ethanol and water. Slides were then stained with mouse anti-CD31 (DADO cotomation, Carpinteria, CA). Slides were blocked for endogenous peroxidase activity and then unmasked in Target Retrieval Solution (DaKoCytomation, Carpinteria, CA). Antibody was applied at 1:50 overnight at 4C prior to identification with an anti-mouse detection kit using DAB (Elite Kit, Vector Labs, Burlingame, CA). An isotype and concentration matched negative control section was included. The specificity of immunostaining was demonstrated by the absence of signal in sections incubated with isotype and concentration-matched control mouse IgGs (Vector Laboratories) instead of the primary antibody. Slides were counterstained with hematoxylin and examined by light microscopy. Blood vessel number, expressed as total number per mm2 of the ROI area minus bone area, was quantified in a blinded manner at a magnification of 200 ×.
Microscopic Analyses
Five μm decalcified paraffin-embedded sections of maxillae from males (n=9-11/group) and mandibles from females (n=5-9/group) rats were cut in a mesio-distal plane, stained with H&E, and examined in a blind-coded fashion. As described for the vertical ABL assessment, sectioning was performed from the palatal towards the buccal surface. Blocks were oriented so as to visualize the mesial and distal roots of all molars as an indicator of proper alignment. Ten consecutive sections obtained from each rat that were adequately aligned and contained alveolar bone processes from the palatal or lingual surface were obtained to perform microscopic analyses. An inflammation scoring system was used, ranging from 0-5, where 0 corresponded to no histological lesions, 1 to slight, 2 to mild, 3 to moderate, 4 to severe periodontitis, and 5 to ONJ-like lesions (see Table 1 for details).
Table 1.
Scoring system used to characterize periodontal lesions observed in maxillae and mandibles of rice rats.
| Score | degree | lesions |
|---|---|---|
| 0 | absence | None |
| 1 | slight | Gingivitis: slight hyperplasia of GE, intraepithelial inflammatory cell infiltration. Accumulation of bacterial plaque. No substantial changes in the LP, PDL or ABC. |
| 2 | mild | Gingival hyperplasia, inflammatory cell infiltration of the GE and LP, accumulation of bacterial plaque. No substantial changes in the PDL or ABC. |
| 3 | moderate | Erosion/ulceration and hyperplasia of the GE and accumulation of bacterial plaque. Moderate inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and ABC resorption. |
| 4 | severe | Erosion/ulceration of GE and hyperplasia of the GE. Severe inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and ABC resorption. |
| 5 | ONJ-like | Extensive ulceration of GE. Exposed necrotic bone and/or osteolysis. Severe destruction of the periodontium accompanied with inflammatory cell infiltration of the LP and a pseudoepitheliomatous-like hyperplasia of GE. Bacterial colonies adhered to alveolar bone or deep in the LP. |
GE: gingival epithelium, LP: lamina propria, PDL: periodontal ligament and ABC: alveolar bone crest.
MicroCT Assessment
MicroCT assessment of mandibular alveolar bone was performed in right mandibles of females (n=5/group) as previously described.(44) Vertical ABH was measured in the mandibular interproximal space M1-M2 in the customary fashion (distance from a line connecting the CEJs of adjacent teeth to the ABC) in up to ten 8μm slices that subtended the buccal-to-lingual dimension. Furthermore, a 3D reconstruction of the microCT scan of the whole rat mandible was performed to display horizontal alveolar bone on the buccal and lingual aspects of the first and second molars.(45) In addition, bone mineral density (BMD) and bone volume (BV/TV) of alveolar bone were also assessed. All microCT measurements were performed in a blinded and randomized fashion.
Peripheral quantitative computed tomography (pQCT)
For the pQCT analysis, left femurs from male (n=9-11/group) and female (n=5-9/group) rice rats were scanned using a Stratec XCT Research M instrument (Norland Medical Systems; Fort Atkinson, WI) with software version 5.40. Scans were performed at a distance of 5 mm proximal to the distal end of the femur. This site is at the level of the secondary spongiosa of the distal femoral metaphysis. Volumetric content, bone density and bone area were determined for total bone (trabecular and cortical bone) as previously described.(46)
Statistical analysis
Data are expressed as mean ± SD for each group. For Study 1, data were evaluated with ANOVA followed by the Holm-Sidak test for multiple comparisons. When ANOVA assumptions regarding normality of data were not met, the non-parametric Kruskal-Wallis test was used. For Study 2, differences in variables between rice rats treated with vehicle or HD-ZOL for 24 wks were evaluated using the unpaired Student's t-test. Regardless of the test employed, P values less than 0.05 were considered to be statistically significant.
Results
Study 1: Effects of N-BPs on the jaws of rice rats with inducible periodontitis after 6, 12 and 18 wks of treatment
Rice rats fed the H-SC diet and simultaneously treated with vehicle, ALN or ZOL (LD or HD) consistently grew and increased body weight from weaning (4 wks of age) to the end of the study (22 wks of age) (supplement Figure 2). Male rice rats became progressively larger in size and heavier than females after 7 wks of age. However, there were no intra-gender differences in BW between N-BP-treated groups compared to age-matched, vehicle-treated groups.
Gross examination of the jaws revealed no lesions of any type in rice rats treated for 6 wks in any of the treatment groups. In contrast, serious gross lesions were observed more frequently in mandibles from rice rats treated with oncologic doses of ZOL for 18 wks (36% in male and 40% in female rats) compared to age-matched controls (0 % in male and 11% in female rats) (data not shown). These serious lesions appeared to resemble ONJ-like lesions described in humans. They were usually bilateral and located on the lingual surface of the mandible, particularly at the interproximal area between molars 1 and 2. ONJ-like lesions were grossly characterized by erosion or ulceration of the gingiva, exposed alveolar bone, molar furcation, and loss of alveolar bone support for the nearby teeth (Figure 1). Consistent with the gross findings, histologic examination revealed minimal lesions in maxillae and mandibles of rice rats treated for six wks in any of the groups. Except for one male rat treated with LD-ZOL and another with HD-ZOL for 18 wks, which developed histologic inflammatory lesions with a score of 5, there were no significant differences in the degree of periodontal lesions in maxillae of rats treated with N-BPs compared to age-matched, vehicle-treated controls (Figure 2C).
Figure 1. ONJ-like lesions are frequently observed in mandibles from rice rats treated with a high dose (HD; oncologic doses) of zoledronic acid (ZOL) for 18 wks.
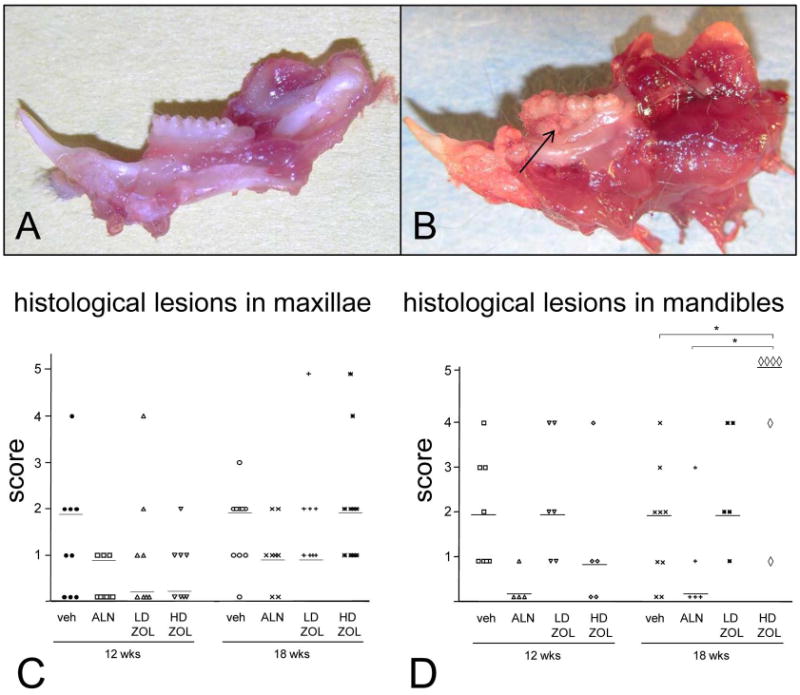
Mandibles from male rice rats treated with vehicle (A) or HD-ZOL (B) for 18 wks. ONJ-like lesions are grossly characterized by erosion or ulceration of the gingiva (arrow), exposed alveolar bone, molar furcation, and loss of tooth support. The degree of histological lesions in maxillae (C) and mandibles (D) from rice rats treated with vehicle, alendronate (ALN), and low (LD; 8 μg/kg) or HD (80 μg/kg)-ZOL for 12 and 18 wks are shown Graphs did not include the 6 wk time-point since there were no differences among treatment groups. Maxillary and mandibular histologic lesions were assessed using an inflammation scoring system ranging from 0-5. Details regarding this scoring system are described in Table 1. Each symbol represents an individual rat. Horizontal lines represent the median for each experimental group, which are composed of 9-11 male and 5-9 female rice rats. An asterisk above brackets indicates a significant difference between compared groups. (P < 0.05).
Figure 2. ONJ-like lesions develop in mandibles of rice rats that received a high dose (HD, oncologic doses) of zoledronic acid (ZOL) for 18 wks.
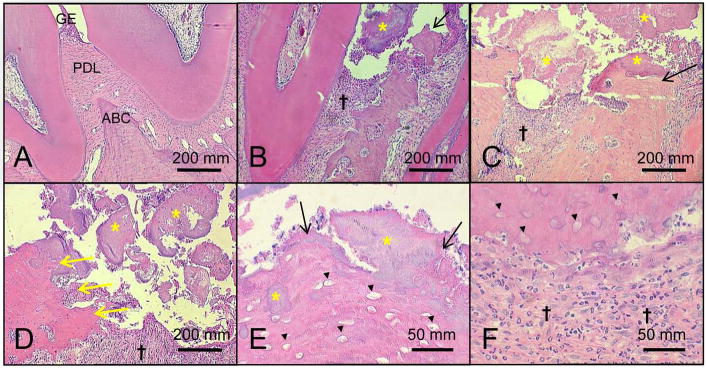
Representative histologic photos of mandibles from female rice rats treated with vehicle (A) and HD-ZOL for 18 wks (B-F). Photos were taken at the interproximal space between the first and second molars. Note the normal histologic features of the gingival epithelium (GE), periodontal ligament (PDL) and the alveolar bone crest (ABC) in a mandible from a rice rat treated with vehicle for 18 wks (A). In contrast, ulceration of the gingival epithelium accompanied by exposed necrotic alveolar bone (black arrows) (B, C and E), osteolysis (yellow arrows) (D), moderate to severe inflammatory cell infiltration (†) (B-D and F), and massive accumulation of bacterial plaque (yellow asterisk) (B-E) are frequently observed in mandibles of rice rats treated with HD-ZOL. Note bacterial plaque on the surface of the ulcerated epithelium, attached to the ABC surface, or embedded in the necrotic bone (yellow asterisk) (B-E). The osteolysis in the HD-ZOL rats (yellow arrows) was sometimes so severe that all the alveolar bone and part of the body of the mandible disappeared (yellow arrows) (D). Necrotic alveolar bone was histologically characterized by extensive areas of tissue with empty osteocyte lacunae (black arrowheads) (E and F). Neutrophils are the predominant inflammatory cells present in the infiltrates (F). H&E. Bars = 200 μm (A-D), and 50 μm (G and H), respectively.
In contrast, a significant increase in the degree of periodontal lesions with a score of 5 was observed in mandibles of female rice rats treated with HD-ZOL for 18 wks compared to vehicle controls (Figure 2D). Periodontal lesions with a score of 5 were defined as ONJ-like lesions and were characterized by extensive ulceration of the gingival epithelium (GE), exposed necrotic bone and/or osteolysis, severe destruction of the periodontium accompanied by inflammatory cell infiltration of the lamina propria (LP), pseudoepitheliomatous-like hyperplasia of the GE, and the presence of bacterial colonies adhered to the surface, within the necrotic alveolar bone, or deep in the LP (Figure 2).
As expected, we observed a potent antiresorptive effect of ALN and ZOL (LD and HD) at the distal femoral metaphyses in rice rats treated for 6, 12 and 18 wks compared to age-matched, vehicle-treated rats (supplement Figure 3). This effect was inferred by the significant increase in BMC and BMD detected by pQCT at this appendicular location. Remarkably, the effects of ALN and ZOL were different in the jaw bones than in the long bones. In maxillae, ALN and HD-ZOL prevented horizontal ABL only after 18 wks of treatment (Figure 3). In mandibles, HD-ZOL significantly increased BMD at 6 and 12 wks but not at 18 wks (Figure 4A). Furthermore, HD-ZOL significantly increased bone volume and reduced vertical ABL at the lingual and buccal surfaces at 12 wks, but not at 18 wks (Figure 4B-D). Consistent with this, the histometric analysis showed that HD-ZOL ameliorated maxillary and mandibular vertical ABL in male rice rats at 12 wks but not at 18 wks (data not shown). Importantly, severe mandibular ABL and bone osteolysis were apparent in mandibles of rice rats treated with HD-ZOL for 18 wks by microCT analysis (Figure 4 E,F). Furthermore, increased porosity and a honeycomb-like appearance were frequently observed in the ONJ-like lesions in the alveolar bone of rats treated with HD-ZOL for 18 wks (Figure 4 G,H).
Figure 3. Alendronate (ALN) and a high dose of zoledronic acid (ZOL) prevent maxillary alveolar bone loss (ABL) observed with the high sucrose and casein diet after 18 wks of treatment.
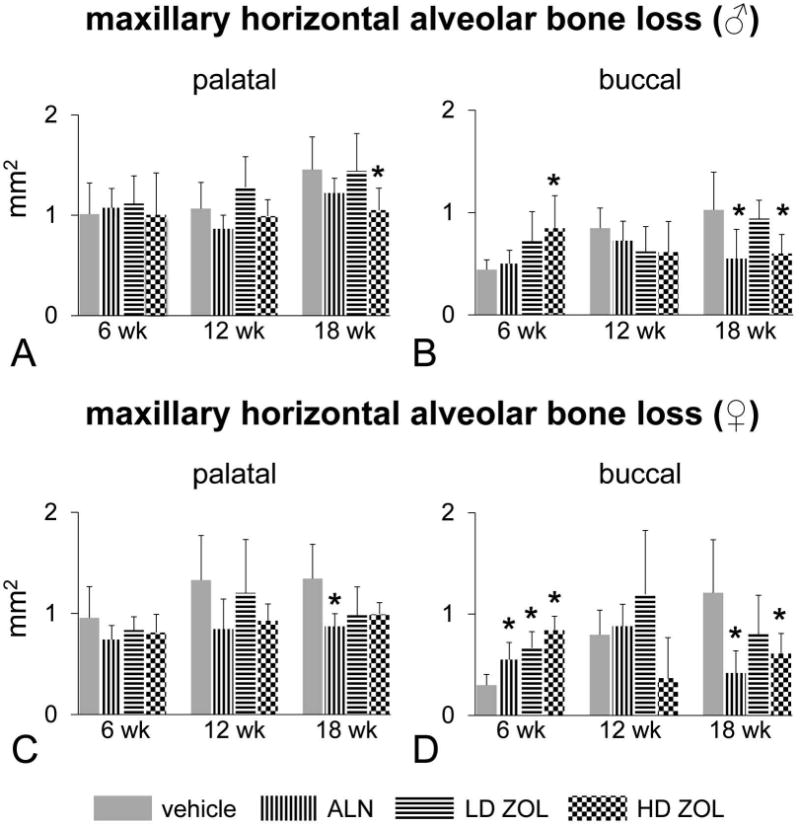
ALN (15μg/kg) was given SC biweekly. ZOL was administered either at a low dose (LD; 8 μg/kg) or high dose (HD; 80 μg/kg) IV once monthly. Maxillary horizontal ABL at the palatal surface of males (A), buccal surface of males (B), palatal surface of females (C), and buccal surface of females (D). An asterisk denotes a significant difference from its respective time-matched vehicle-treated group. ANOVA. (P < 0.05).
Figure 4. High dose (HD) of zoledronic acid (ZOL) prevents mandibular alveolar bone loss at 12 wks but this effect is lost and accompanied by an altered bone structure after 18 wks of treatment.
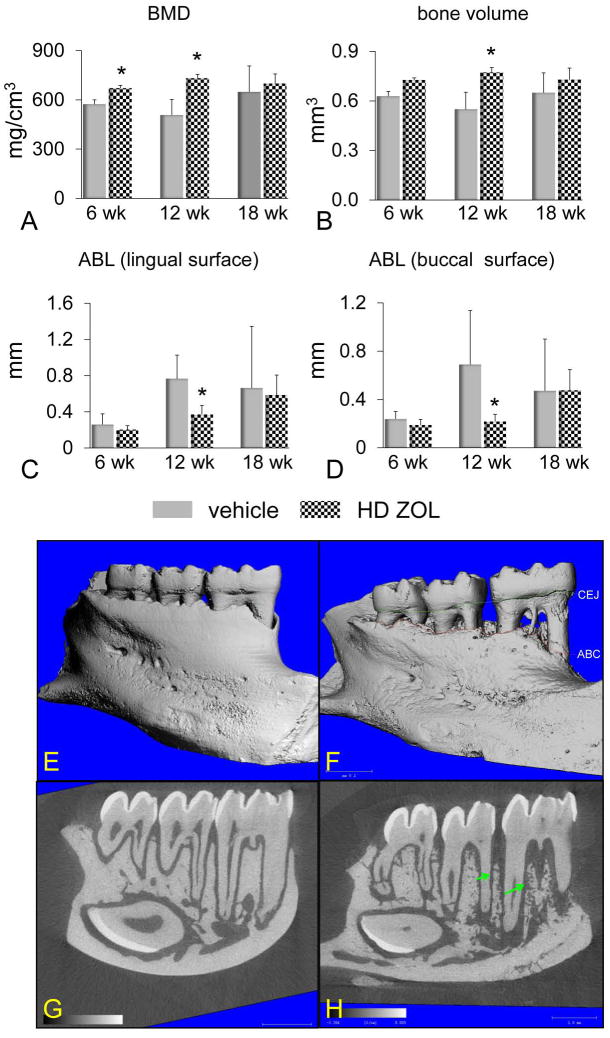
A microCT analysis was performed on mandibles of female rice rats treated with vehicle or HD-ZOL (80 μg/kg) IV once monthly. BMD (A), bone volume (B), vertical alveolar bone loss (ABL) at the lingual surface (C) and vertical ABL at the buccal surface (D). All measurements were performed within the ROI described in supplemental Figure 1. An asterisk denotes a significant difference from its respective time-matched vehicle-treated group. ANOVA. (P < 0.05). Comparative microCT photos taken at the lingual surface of mandibles from female rice rats treated with vehicle (E,G) or HD-ZOL (F,H) for 18 wks. Note the severe mandibular ABL and bone destruction [increased area between the cementoenamel junction (CEJ) (green line) and the alveolar bone crest (ABC) (red line)] depicted in a reconstructive microCT image of a rat treated with HD-ZOL for 18 wks (F) compared to a lesser degree of ABL in a vehicle-treated rat (E). Also note the increased porosity and the honeycomb-like appearance (green arrows) of alveolar bone in the mandible of a rice rat treated with HD-ZOL (H) compared to the mandible of a vehicle-treated rat (G).
The histomorphometric analysis performed in mandibles showed that both LD-ZOL and HD-ZOL dramatically reduced bone formation at 6, 12 and 18 wks, whereas ALN only decreased it after 18 wks of treatment (Figure 5). Osteoclast number was reduced by ALN and ZOL at 6 wks, but not at later time points (Figure 5D). In addition, no significant differences were observed in blood vessel number between N-BP-treated rice rats compared to vehicle-treated control rats. However, a trend towards a reduction in blood vessel number was observed between HD-ZOL and vehicle-treated groups at 12 and 18 wks (Figure 5E).
Figure 5. Effects of alendronate (ALN) and zoledronic acid (ZOL) on mandibular alveolar bone formation, osteoclast number and vascularity after 6-18 wks of treatments in rice rats.
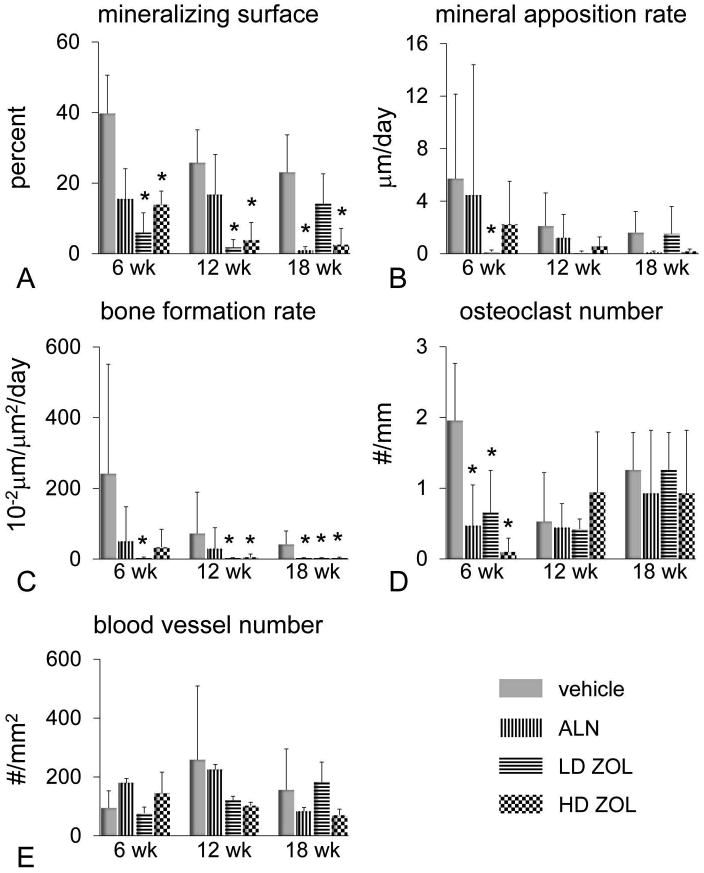
A histomorphometric analysis was performed in the mandibles of male rats treated with vehicle, ALN, or a low dose (LD) (8 μg/kg) or high dose (HD) of ZOL (80 μg/kg) for 6, 12 and 18 wks. Mineralizing surface (A), mineral apposition rate (B), bone formation rate (C), osteoclast number (D) and blood vessel number (E). All measurements were performed within the ROI described in supplemental Figure 1. An asterisk denotes a significant difference from its respective time-matched vehicle-treated group. (P < 0.05).
Histologic examination revealed that ONJ-like lesions contained areas of exposed alveolar bone with empty osteocyte lacunae and/or osteolytic lesions. We observed a significant decrease in LDH reactivity in osteocyte lacunae in the mandibular alveolar bone of male rice rats treated with HD-ZOL for 18 wks (Figure 6A, D,E). Furthermore, we observed an increased percentage of osteocyte apoptosis in the mandibular alveolar bone of female rice rats treated with HD-ZOL for 18 wks (Figure 6B, H-K). Consistent with the decreased LDH negative osteocyte lacunae observed in males, we found extensive areas of DAPI negative osteocyte lacunae in the alveolar bone of female rice rats treated under the same regimen (Figure 6J). In addition, the percentage of areas devoid of basic fuchsin was significantly higher in mandibular alveolar bone of rats treated with HD-ZOL for 18 wks compared to age-matched, vehicle-treated controls (Figure 6F, G).
Figure 6. Oncologic dose of zoledronic acid (ZOL) increases the percentage of necrotic and apoptotic osteocytes, and the area of alveolar bone devoid of basic fuchsin stain in mandibles of rice rats treated for 18 wks.
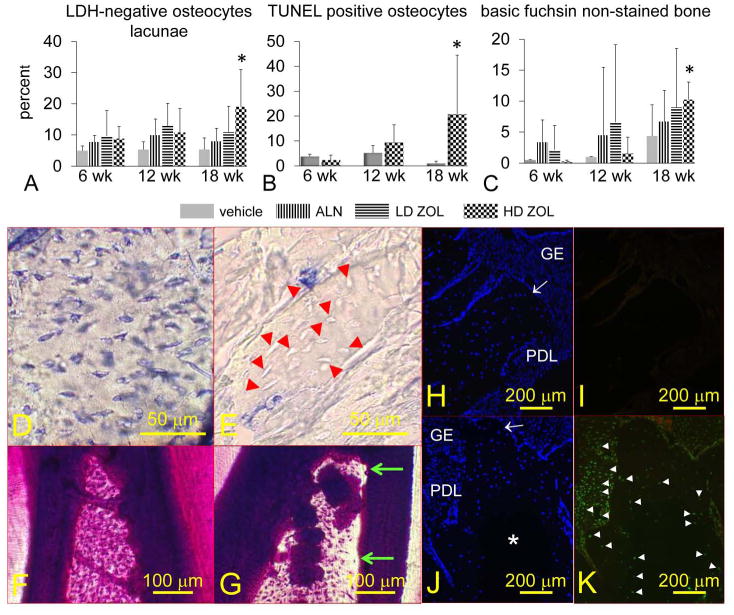
The percentage of osteocytes that do not show lactate dehydrogenase (LDH) expression (necrotic osteocytes) (A), TUNEL positive osteocytes (apoptotic) (B), and areas devoid of basic fuchsin stain (C) are shown. Rice rats were treated with vehicle, alendronate (ALN), and low (8 μg/kg) or high doses (80 μg/kg) of ZOL for 6, 12 and 18 wks. An asterisk denotes a significant difference from its respective time-matched vehicle-treated group (P < 0.05). Representative photos of mandibular alveolar bone histochemically stained for LDH in a rat treated with vehicle (D) or HD-ZOL (E) for 18 wks. Osteocytes that are blue-stained express LDH (viable). Osteocytes that are dead are unreactive (unstained). Most of the osteocytes present at the interdental alveolar bone in the mandibles of rice rats treated with vehicle are viable (D). In contrast, rats treated with HD-ZOL for 18 wks show extensive areas of alveolar bone containing empty osteocyte lacunae or lacunae with unstained osteocytes (E) (red arrowheads). En bloc basic fuchsin stained mandibles at the interdental alveolar bone of a rat treated with vehicle (F) or HD-ZOL for 18 wks (G). Note areas devoid of basic fuchsin stain at the coronal region of the interdental alveolar bone in the rat treated with ZOL (green arrows). Photos depicting DAPI (H, J) and TUNEL (I, K) staining in mandibles from vehicle and HD-ZOL treated rats for 18 wks, respectively. Gingival epithelium (GE), periodontal ligament (PDL), alveolar bone crest (white arrow). DAPI positive cells are stained blue (H, J). Note a big area in the center of the alveolar bone that is devoid of DAPI reaction in a rat treated with HD-ZOL (J) compared to a more uniform DAPI reaction in a vehicle-treated rat (H). Note the TUNEL-positive reaction (white arrowheads) in osteocytes adjacent to the DAPI negative area, and in many cells of the periodontal ligament area in a rat treated with ZOL for 18 wks (K). In contrast no TUNEL-positive reaction is observed in the mandible of the vehicle-treated rat (I). Bars = 50 μm (D, E), 100 μm (F, G), and 200 μm (H-K), respectively.
Study 2: Effects of HD of ZOL in rice rats after 24 wks of treatment
HD-ZOL administered for 24 wks induced mandibular gross lesions that resemble ONJ lesions (Figure 7A,B) (33% in male and 67% in female rats) compared to age-matched controls (0 % in male and 0% in female rats) (data not shown). These lesions were grossly characterized by extensive ulceration of the gingiva, exposed alveolar bone, molar furcation, and tooth loss. We found that several vehicle-treated rats developed moderate periodontitis after 24 wks, (Figure 7C). Importantly, only rice rats treated with HD-ZOL developed ONJ-like lesions (Figure 7G). These lesions were similar or with an even more destructive pattern compared to those observed after 18 wks of treatment. Furthermore, bacterial biofilms morphologically resembling Actinomyces sp. were found attached to or within the necrotic alveolar bone, and also on the surface of the ulcerated gingival epithelium (Figure 7 C-F). As observed in rats treated with HD-ZOL for 18 wks, this potent N-BP reduced mineralizing surface and BFR/BS, but not osteoclast number (Figure 7 H-K). Finally, blood vessel number was significantly decreased in rice rats treated with HD-ZOL compared to vehicle controls (Figure 7L).
Figure 7.
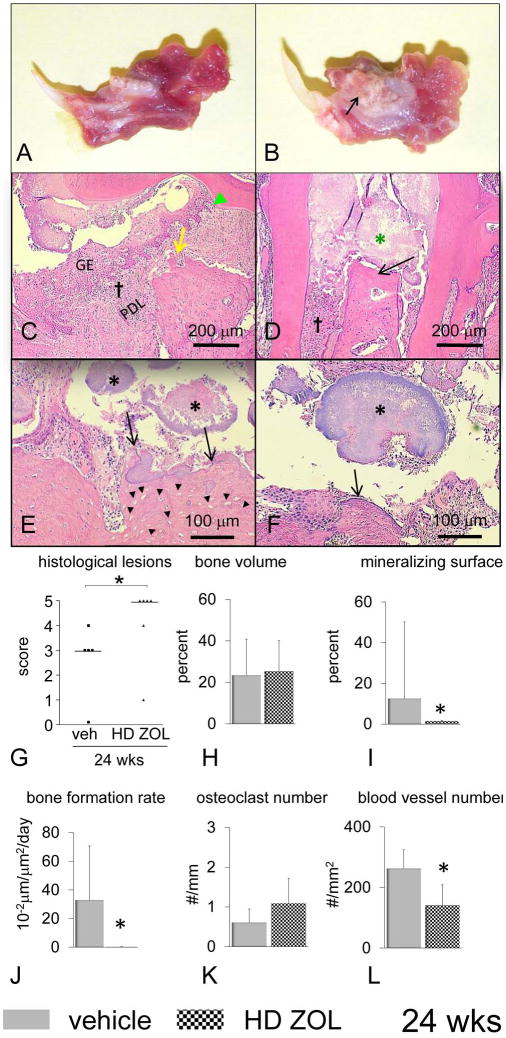
ONJ-like lesions, decreased bone formation and reduced vascularity are observed in rice rats that received an oncologic dose (high dose) of zoledronic acid (ZOL) for six more weeks (Total 24 wks). Mandibles from male rice rats treated with vehicle (A) or a high dose (HD) (80 μg/kg) of ZOL (B) for 24 wks. ONJ-like lesions are grossly characterized by extensive ulceration of the gingiva (arrow), exposed alveolar bone, molar furcation, and loss of tooth support. Histological images of mandibles (except D from maxilla) from female rice rats treated with vehicle (C) and HD-ZOL for 24 wks (D-F). Photos were taken at the interproximal space between the first and second molars. Several vehicle-treated rats developed severe periodontitis after 24 wks of treatment (C). Note the substantial hyperplasia of the gingival epithelium (GE), inflammatory cell infiltration of the lamina propria (†, disruption of the periodontal ligament (PDL), apical migration of the junctional epithelium (green ▴), and alveolar bone crest resorption (yellow arrow). Only rats treated with HD-ZOL develop ONJ-like lesions. These lesions are characterized by the presence of exposed necrotic alveolar bone (black arrows) (D-F), severe inflammatory cell infiltrate (†), and bacterial biofilm morphologically resembling Actinomyces sp. (black asterisks). Note the substantial disruption of the periodontium, ulceration of the gingival epithelium, massive accumulation of bacterial plaque (green asterisk), exposed necrotic alveolar bone (black arrow) in the maxilla of a rat treated with HD-ZOL for 24 wks (D). Actinomyces-like colonies (*) are found attached to necrotic alveolar bone (D-F), or on the surface of ulcerated periodontium adjacent to soft tissue or bone (E-F). A classic sulphur granule of Actinomyces-like organism is observed in the interproximal space (*). Necrotic alveolar bone was characterized histologically by extensive areas with empty osteocyte lacunae (black arrowheads) (E). H&E. Bars = 200 μm (C-D), 100 μm (E and F), respectively. Degree of histological lesions in mandibles (G) of rice rats treated with vehicle and HD-ZOL. Histologic lesions were assessed using an inflammation scoring system ranging from 0-5. Details regarding this scoring system are described in Table 1. Each symbol represents an individual rat. Horizontal lines represent the median for each experimental group. Experimental groups are composed of 5-6 female rice rats. An asterisk above brackets indicates a significant difference between compared groups (P < 0.05). A histomorphometric analysis was performed in mandibles of male rats treated with vehicle and HD-ZOL for 24 wks. Bone volume (H), mineralizing surface (I), bone formation rate (J), osteoclast number (K) and blood vessel number (L). All measurements were performed within the ROI described in supplemental Figure 1. An asterisk denotes a significant difference from its respective time-matched vehicle-treated group (P < 0.05).
Discussion
Thirty years ago, Gotcher and Jee(30) used the rice rat model of periodontitis to determine whether dichloromethylene diphosphonate (Cl2MDP), a non-nitrogen containing BP, could retard or prevent the bone loss associated with periodontal disease. They observed that Cl2MDP indeed prevented alveolar bone loss. However, and perhaps relevant to the pathophysiology of ONJ, they found an abnormal amount, location, and morphologic pattern of the alveolar bone in mandibles of rats that were treated for 18 wks. The authors(30) referred to these lesions as “areas of alveolar bone protruded and exposed in the oral cavity with portions that were devoid of bone cells and devitalized”. This description attracted our attention since it has similarities to the ONJ lesions described in N-BP-treated patients. Nevertheless, the study(30) did not provide conclusive evidence to support the existence of ONJ, leaving open the possibility to infer that a different bone disorder occurred. Inspired by these observations, we reexamined the periodontitis model in rice rats, previously described,(22-24,27,29) with methodologies now commonly used in periodontal research, including morphometry, histomorphometry, immunocytochemistry, histochemistry, and microCT techniques.(25,47) We next performed the present study to determine the effects of two N-BPs now approved for the treatment of osteoporosis and, in the latter case, for metastatic cancer, ALN and ZOL, on: the progression of periodontitis, the integrity of alveolar bone, bone resorption and formation, vascularity, and osteocyte viability.
To our knowledge, very few studies(30,48) have considered periodontal disease as a comorbid factor in the development of an animal model for ONJ. Taking this risk factor into account improves the potential relevance of an animal model of ONJ, since clinically and radiographically evident periodontitis has been identified as one of the most important risk factors for the development of ONJ in humans.(1,4,49)
Our study shows that about 40% of the rice rats (males and females) treated with oncologic doses of ZOL for 18 wks developed gross mandibular lesions that appear to resemble those observed in ONJ patients. In contrast, only 20% of these animals developed ONJ-like lesions in maxillae. This observation represents an interesting epizootiologic aspect of the model, since it is well established that the incidence of ONJ in humans is two-fold greater in the mandible than in the maxilla.(1,5,17) Similar incidence of ONJ-like lesions, including osteonecrosis (47%) and exposed bone (21%), were found and elegantly illustrated by Aghaloo et al(48,49) in maxillae of ZOL-treated rats with ligature-induced periodontitis. Our study also shows that none of the rats treated with ALN developed ONJ-like lesions. Our findings are in agreement with epidemiological data in humans showing a high incidence (6-10%) of ONJ lesions in N-BP-treated cancer patients(3,18,19) and a very low incidence (∼1/10,000) in postmenopausal women undergoing treatment for osteoporosis.(4,5,19,20) This study did not investigate the effects of N-BPs on rice rats that were fed a standard diet instead of the H-SC diet. Data obtained from such an experiment, in which rice rats would have little or no periodontitis, could contribute to determining whether predisposing factors, in this particular case, moderate/severe periodontitis, are sine qua non conditions for the development of ONJ. This study also did not investigate the effect of introducing N-BPs into rice rats with periodontitis that had been established by 18wks administration of the H-SC diet. Data obtained from such an experiment, would more closely mimic the situation in adult humans with established periodontitis who begin to take N-BPs as oncologic or osteoporosis therapy.
Remarkably, we observed that rice rats that had mandibular histologic lesions with an inflammatory score of 5 (ONJ-like lesions), were those that displayed evident serious gross lesions including erosion or ulceration of the gingiva, exposed alveolar bone, molar furcation, and loss of alveolar bone supporting the teeth. This correspondence suggests that in our model, gross examination of mandibles can be used similar to a clinical examination, to study the progression of ONJ or to test different treatment modalities for ONJ in vivo before euthanizing the animals for ex vivo tissue analysis.
We further observed that ONJ-like lesions were not only present in rats that received HD-ZOL for 18 wks but also in those treated for 24 wks. This is important, because it suggests that ONJ-like lesions in this model were not transient, since they likely persisted for at least 6 wks after their identification at 18 wks of treatment. Although this time period does not fully comply with the most recent definitions of N-BP-associated ONJ,(15,16) it could represent a comparable persistence for a rodent animal model.
The definition of ONJ only specifies “an area of exposed bone in the maxillofacial region.” However, numerous clinical reports in humans have described additional bone lesions present in ONJ patients, including osteolysis, osteosclerosis, presence of woven bone, honeycomb-like appearance of affected areas of jaw bones, and pathologic fractures.(4,50-57) Importantly, we observed some of these lesions in our model. Rice rats treated with oncologic doses of ZOL for 18-24 wks displayed, concomitantly with or without exposed bone, extensive areas of osteolysis, and alveolar bone areas with a honey comb-like appearance as evidenced by microCT. The histologic and histomorphometric analyses of these honey comb-like areas showed a correspondence to alveolar bone areas that were replaced by woven bone, periodontal ligament and/or fibrous tissue, and were sometimes mineralized. Taken together, these data suggest that our model can reproduce not only the presence of exposed necrotic bone in the oral cavity, but also other pathological bone lesions frequently observed in patients with ONJ.
The clinical staging system updated on the 2009 AAOMS expanded abbreviation guidelines (16) has served to more accurately categorize patients with ONJ. We attempted to use this system for ONJ-like lesions found in rice rats. We observed that HD-ZOL administered for 18 wks induced the development of ONJ-like lesions that could be comparable to a stage 1/2, since the rats did not lose weight (as an indicator of well-being and lack of pain or distress), but had periodontal lesions frequently accompanied by infection. On the other hand, ONJ-like lesions present in rice rats treated with HD-ZOL for 24 wks could be comparable to those in stage 2/3, because exposed necrotic bone was frequently accompanied by infection, osteolytic lesions, and the loss of a significant amount of body weight (data not shown). The development of a staging system for the rice rat model would be highly desirable in order to advance the development of early diagnostic protocols and treatments, particularly in the early stages of the disease (phase 0), when no clinical evidence of necrotic bone but nonspecific clinical findings and symptoms appear to be present in humans.
In contrast to the consistent and potent antiresorptive effect of ALN and ZOL observed at the distal femoral metaphyses, different responses were seen in jaw bones. Responses were associated with the degree of histological lesions found at these locations. When periodontal lesions were absent or present to a low degree, as found in maxillae at 18 wks and in mandibles at 12 wks, HD-ZOL exerted a potent antiresorptive effect. In contrast, the antiresorptive effect of HD-ZOL was lost when a high degree of damage, destruction and commensal bacterial (Actinomyces-like) growth were present. It is well established that oral bacterial infection, particularly with Actinomyces sp., is a common finding in patients with ONJ.(52,58-62) When this takes place, microorganisms can stimulate bone resorption by inducing cytokines and/or RANKL.(63-65) Our findings are in agreement with what Reid and Cornish(52) defined as the “ONJ paradox”, where aggressive local osteolysis could be present in patients treated with antiresorptives as a result of infection-induced bone resorption.
We also observed that rice rats treated with oncologic doses of ZOL for 18 wks have areas of exposed alveolar bone that contain empty osteocyte lacunae and/or osteolytic lesions. In line with these findings, we also observed decreased reactivity to LDH in osteocyte lacunae in males and an increased percentage of osteocyte apoptosis in female rice rats at this time point. Taken together, these data suggest that indeed, HD-ZOL is associated with increased levels of apoptosis/necrosis in alveolar bone osteocytes of rats with induced periodontitis. There are some differences in the assessments of these tests. The assessment of LDH negative osteocyte lacunae considers not only the presence of unreactive osteocytes (necrotic), but also empty osteocyte lacunae. Therefore, the presence of cellular remnants and absence of osteocytes in lacunae are also expressions of necrotic cells. In contrast, the percentage of TUNEL positive osteocytes, accounted only for those cells that have fragmented DNA, a characteristic of both apoptotic as well as necrotic cells, from the total of DAPI positive osteocytes in the ROI. Therefore, empty osteocyte lacunae were excluded from this calculation. The presence of empty osteocyte lacunae is a characteristic of the alveolar bone described in ONJ patients.(19,59,62) Taken together, these findings suggest that the LDH assay would be a better indicator of the index of osteocyte necrosis in the alveolar bone compared to TUNEL staining, which underestimates the real number of necrotic/apoptotic osteocytes. Whether apoptosis/necrosis of osteocytes results from side effects of the inflammatory response, bacterial endotoxins and/or end-products, a direct toxic effect of HD-ZOL, or other mechanisms, remains to be defined.
Finally, we observed a tendency toward reduced blood vessel number in the periodontium of rice rats treated with HD-ZOL, but only after 24 wks of treatment. We have previously described an antiangiogenic effect of another N-BP, ALN, in a tooth extraction rat model.(32) However, in the latter study, the effect appeared early and was transient.
Zoledronic acid has been reported to inhibit oral keratinocytes proliferation,(66) induce oral epithelial cell apoptosis,(67) and exert a potent antiangiogenic effect.(68-70) It is well established that the integrity of the gingival epithelium is a physical barrier for the oral microbiota to invade, colonize and induce inflammation and disease. Taken together, our data suggest that high doses of ZOL could enhance or promote the persistence of periodontitis and tissue destruction by inhibiting re-epithelization of damaged gingival epithelium, allowing commensal bacterial growth and periodontal infection. Bacterial infection can then stimulate production of pro inflammatory cytokines and increase the expression of RANKL by different cell types in the periodontal tissue, resulting in more tissue destruction, decreased angiogenesis, and the paradoxical local elimination of the antiresorptive effect of this potent N-BP.
Most ONJ cases reported in the literature of the past five years have been associated with the use of oncologic doses of N-BP's(1-5). The more recent observation of ONJ in N-BP-naïve patients receiving RANKL antibodies(6-9) suggests that it may be the physiologic effect of strongly inhibiting bone resorption by any means, rather than through the specific use of an N-BP, that drives much of ONJ's pathophysiology. In this report, we show that rice rats with moderate/severe periodontitis develop ONJ-like lesions when co-administered oncologic doses of ZOL. A similar experiment in which RANKL antibodies or osteoprotogerin were administered to rice rats with moderate/severe periodontitis would test this hypothesis.
In summary, our data show that rice rats treated with oncologic doses of ZOL appear to be a promising animal model for studies of the pathogenesis and treatment of ONJ. Our study also showed that osteoporosis doses of ALN and ZOL produced no such lesions in a similar timeframe. Our study suggests that HD-ZOL exacerbates the inflammatory response and periodontal tissue damage in rice rats, inducing lesions that resemble ONJ. Our data show that HD-ZOL inhibits bone resorption in the jaw of rice rats when the integrity of the periodontium is preserved. However, the antiresorptive effect is lost simultaneously with extensive ulceration of gingival epithelium, exposed necrotic bone and/or osteolysis, severe inflammatory cell infiltration of the lamina propria, and presence of bacterial biofilm adhered to alveolar bone or deep in the lamina propria.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research (NIH/NIDCR) NIH grant R03DE018924-01A1
This research was supported by NIH grant R03DE018924-01A1 from the National Institute of Dental and Craniofacial Research (NIDCR). We thank M Rivera, X. Xia, Katherine Neuville and A. Lepper for technical support. We thank Dr. Kent Edmonds, Department of Biology, Indiana University Southeast, New Albany, IN for providing the original rice rat breeders.
Footnotes
Author's roles: Study design: JIA, DBK, TJW. Study conduct: JIA, JEP, AW, MJ. Data collection: JIA, JEP, MPA. Data analysis: JIA, MPA. Data interpretation: JIA, DBK, TJW, MPA, MJ, LK. Drafting manuscript: JIA. Revising manuscript content: JIA, DBK, TJW, MPA, MJ, LK. Approving final version of manuscript: JIA, MPA, DBK, JEP, AW, MJ, LK, and TJW. JIA takes responsibility for the integrity of the data analysis.
Supplemental data: Three supplement figures with figure legends are included.
Conflict of interest: All authors except for DBK have no conflict of interest.
DBK: Consultant to Xradia, Amgen, Lexicon, and Bayer, and own Merck stock and options.
Contributor Information
J. I. Aguirre, Email: aguirrej@ufl.edu.
M. P. Akhter, Email: mohammedakhter@creighton.edu.
D. B. Kimmel, Email: kimmeldb@comcast.net.
J. E. Pingel, Email: jpingel@ufl.edu.
A. Williams, Email: williamsaly@ufl.edu.
M. Jorgensen, Email: marda@ufl.edu.
L. Kesavalu, Email: kesavalu@dental.ufl.edu.
T. J. Wronski, Email: wronskit@ufl.edu.
Reference List
- 1.Bilezikian JP. Osteonecrosis of the jaw--do bisphosphonates pose a risk? N Engl J Med. 2006;355:2278–81. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- 2.Hellstein JW, Marek CL. Bis-phossy jaw, phossy jaw, and the 21st century: bisphosphonate-associated complications of the jaws. J Oral Maxillofac Surg. 2004;62:1563–5. doi: 10.1016/j.joms.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–7. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–75. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 6.Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB. RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Support Care Cancer. 2011;19:2035–40. doi: 10.1007/s00520-010-1061-0. [DOI] [PubMed] [Google Scholar]
- 7.Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 9.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von MR, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 10.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 11.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–6. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipton A, Goessl C. Clinical development of anti-RANKL therapies for treatment and prevention of bone metastasis. Bone. 2011;48:96–9. doi: 10.1016/j.bone.2010.10.161. [DOI] [PubMed] [Google Scholar]
- 13.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–92. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 14.McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821–31. doi: 10.1056/NEJMoa044459. [DOI] [PubMed] [Google Scholar]
- 15.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 16.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw - 2009 update. Aust Endod J. 2009;35:119–30. doi: 10.1111/j.1747-4477.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7:508–14. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 18.Migliorati CA, Schubert MM, Peterson DE, Seneda LM. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 19.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Yarom N, Yahalom R, Shoshani Y, Hamed W, Regev E, Elad S. Osteonecrosis of the jaw induced by orally administered bisphosphonates: incidence, clinical features, predisposing factors and treatment outcome. Osteoporos Int. 2007;18:1363–70. doi: 10.1007/s00198-007-0384-2. [DOI] [PubMed] [Google Scholar]
- 21.Gupta O, Shaw J. The relation of a chelating agent to smooth-surface lesions in the white rat. J Nutr. 1956;60:311–22. doi: 10.1093/jn/60.3.311. [DOI] [PubMed] [Google Scholar]
- 22.Gupta O, Shaw J. Periodontal disease in the rice rat. II. Methods for the evaluation of the extent of periodontal disease. Oral Surg Oral Med Oral Pathol. 1956;9:727–35. doi: 10.1016/0030-4220(56)90249-3. [DOI] [PubMed] [Google Scholar]
- 23.Gupta O, Shaw J. Periodontal disease in the rice rat. I. Anatomic and histopathologic findings. Oral Surg Oral Med Oral Pathol. 1956;9:592–603. doi: 10.1016/0030-4220(56)90319-x. [DOI] [PubMed] [Google Scholar]
- 24.Ryder MI. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat. I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. J Periodontal Res. 1980;15:502–15. doi: 10.1111/j.1600-0765.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 25.Aguirre JI, Akhter M, Kimmel D, Pingel J, Xia X, Williams A, Jorgensen M, Edmonds K, Lee J, Reinhard M, Battles A, Kesavalu L, Wronski TJ. Enhanced alveolar bone loss in a model of non-invasive periodontitis in rice rats. Oral Dis. 2011 doi: 10.1111/j.1601-0825.2011.01893.x. http://dx.doi/10.1111/j.1601-0825.2011.01893.x. [DOI] [PMC free article] [PubMed]
- 26.Auskaps A, Gupta O, Shaw J. Periodontal disease in the rice rat. III. Survey of dietary influences. J Nutr. 1957;63:325–43. doi: 10.1093/jn/63.3.325. [DOI] [PubMed] [Google Scholar]
- 27.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J Periodontal Res. 1981;16:275–91. doi: 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 28.Hattler AB, Snyder DE, Listgarten MA, Kemp W. The lack of pulpal pathosis in rice rats with the periodontal syndrome. Oral Surg Oral Med Oral Pathol. 1977;44:939–48. doi: 10.1016/0030-4220(77)90038-x. [DOI] [PubMed] [Google Scholar]
- 29.Leonard EP. Periodontitis. Animal model: periodontitis in the rice rat (Oryzomys palustris) Am J Pathol. 1979;96:643–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res. 1981;16:441–55. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs RK, Shea M, Durski SL, Winters-Stone KM, Widrick J, Snow CM. Individual and combined effects of exercise and alendronate on bone mass and strength in ovariectomized rats. Bone. 2007;41:290–6. doi: 10.1016/j.bone.2007.04.179. [DOI] [PubMed] [Google Scholar]
- 32.Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16:674–85. doi: 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- 33.Verma RK, Rajapakse S, Meka A, Hamrick C, Pola S, Bhattacharyya I, Nair M, Wallet SM, Aukhil I, Kesavalu L. Porphyromonas gingivalis and Treponema denticola Mixed Microbial Infection in a Rat Model of Periodontal Disease. Interdiscip Perspect Infect Dis. 2010;2010:605125–31. doi: 10.1155/2010/605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verma RK, Bhattacharyya I, Sevilla A, Lieberman I, Pola S, Nair M, Wallet SM, Aukhil I, Kesavalu L. Virulence of major periodontal pathogens and lack of humoral immune protection in a rat model of periodontal disease. Oral Dis. 2010;16:686–95. doi: 10.1111/j.1601-0825.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 35.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–65. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 36.Allen MR. Animal models of osteonecrosis of the jaw. J Musculoskelet Neuronal Interact. 2007;7:358–60. [PubMed] [Google Scholar]
- 37.Burr DB, Hooser M. Alterations to the en bloc basic fuchsin staining protocol for the demonstration of microdamage produced in vivo. Bone. 1995;17:431–3. doi: 10.1016/s8756-3282(95)00241-3. [DOI] [PubMed] [Google Scholar]
- 38.Frost HM. Bone Histomorphometry: Analysis of trabecular bone dynamics. In: Recker RR, editor. Bone Histomorphometry: Techniques and Interpretation. 1st. CRC Press; Boca Raton, FL: 1983. pp. 109–32. [Google Scholar]
- 39.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 40.Bainbridge B, Verma RK, Eastman C, Yehia B, Rivera M, Moffatt C, Bhattacharyya I, Lamont RJ, Kesavalu L. Role of Porphyromonas gingivalis phosphoserine phosphatase enzyme SerB in inflammation, immune response, and induction of alveolar bone resorption in rats. Infect Immun. 2010;78:4560–9. doi: 10.1128/IAI.00703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble BS, Stevens HY. Techniques for the study of apoptosis in bone. Methods Mol Med. 2003;80:225–36. doi: 10.1385/1-59259-366-6:225. [DOI] [PubMed] [Google Scholar]
- 42.Mann V, Huber C, Kogianni G, Jones D, Noble B. The influence of mechanical stimulation on osteocyte apoptosis and bone viability in human trabecular bone. J Musculoskelet Neuronal Interact. 2006;6:408–17. [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang X, Kalajzic Z, Maye P, Braut A, Bellizzi J, Mina M, Rowe DW. Histological analysis of GFP expression in murine bone. J Histochem Cytochem. 2005;53:593–602. doi: 10.1369/jhc.4A6401.2005. [DOI] [PubMed] [Google Scholar]
- 44.Park C, Abramson Z, Taba MJ, Jin Q, Kreider J, Goldstein S, Giannobile WV. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78:273–81. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu XM, Wiswall AT, Rutledge JE, Akhter MP, Cullen DM, Reinhardt RA, Wang D. Osteotropic beta-cyclodextrin for local bone regeneration. Biomaterials. 2008;29:1686–92. doi: 10.1016/j.biomaterials.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ke HZ, Qi H, Chidsey-Frink KL, Crawford DT, Thompson DD. Lasofoxifene (CP-336,156) protects against the age-related changes in bone mass, bone strength, and total serum cholesterol in intact aged male rats. J Bone Miner Res. 2001;16:765–73. doi: 10.1359/jbmr.2001.16.4.765. [DOI] [PubMed] [Google Scholar]
- 47.Aguirre JI, Altman MK, Franz SE, Bassit ACF, Wronski TJ. Alendronate transiently impairs removal of alveolar bone and healing of the root socket after tooth extraction in rats. J Bone Miner Res. 2008;23:S76. [Google Scholar]
- 48.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 2011;26:1871–82. doi: 10.1002/jbmr.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Favia G, Pilolli GP, Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009;45:406–13. doi: 10.1016/j.bone.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Hutchinson M, O'Ryan F, Chavez V, Lathon PV, Sanchez G, Hatcher DC, Indresano AT, Lo JC. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg. 2010;68:2232–40. doi: 10.1016/j.joms.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Reid IR, Cornish J. Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.181. [DOI] [PubMed] [Google Scholar]
- 53.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38–46. doi: 10.1111/j.1749-6632.2010.05768.x. [DOI] [PubMed] [Google Scholar]
- 54.Treister N, Sheehy N, Bae EH, Friedland B, Lerman M, Woo S. Dental panoramic radiographic evaluation in bisphosphonate-associated osteonecrosis of the jaws. Oral Dis. 2009;15:88–92. doi: 10.1111/j.1601-0825.2008.01494.x. [DOI] [PubMed] [Google Scholar]
- 55.Migliorati CA, Epstein JB, Abt E, Berenson JR. Osteonecrosis of the jaw and bisphosphonates in cancer: a narrative review. Nat Rev Endocrinol. 2011;7:34–42. doi: 10.1038/nrendo.2010.195. [DOI] [PubMed] [Google Scholar]
- 56.Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, Tregnaghi A, Pietrogrande F, Procopio O, Saia G, Ferretti M, Bedogni G, Chiarini L, Ferronato G, Ninfo V, Lo RL, Lo ML, Nocini PF. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:358–64. doi: 10.1016/j.tripleo.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 57.Wutzl A, Eisenmenger G, Hoffmann M, Czerny C, Moser D, Pietschmann P, Ewers R, Baumann A. Osteonecrosis of the jaws and bisphosphonate treatment in cancer patients. Wien Klin Wochenschr. 2006;118:473–8. doi: 10.1007/s00508-006-0644-8. [DOI] [PubMed] [Google Scholar]
- 58.Maahs MP, Azambuja AA, Campos MM, Salum FG, Cherubini K. Association between bisphosphonates and jaw osteonecrosis: a study in Wistar rats. Head Neck. 2011;33:199–207. doi: 10.1002/hed.21422. [DOI] [PubMed] [Google Scholar]
- 59.Pautke C, Otto S, Reu S, Kolk A, Ehrenfeld M, Sturzenbaum S, Wolff KD. Bisphosphonate related osteonecrosis of the jaw--manifestation in a microvascular iliac bone flap. Oral Oncol. 2011;47:425–9. doi: 10.1016/j.oraloncology.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Reid IR. Osteonecrosis of the jaw: who gets it, and why? Bone. 2009;44:4–10. doi: 10.1016/j.bone.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Hellstein JW, Marek CL. Bisphosphonate osteochemonecrosis (bis-phossy jaw): is this phossy jaw of the 21st century? J Oral Maxillofac Surg. 2005;63:682–9. doi: 10.1016/j.joms.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Hansen T, Kunkel M, Weber A, James KC. Osteonecrosis of the jaws in patients treated with bisphosphonates - histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 2006;35:155–60. doi: 10.1111/j.1600-0714.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 63.Belibasakis GN, Meier A, Guggenheim B, Bostanci N. Oral biofilm challenge regulates the RANKL-OPG system in periodontal ligament and dental pulp cells. Microb Pathog. 2011;50:6–11. doi: 10.1016/j.micpath.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Belibasakis GN, Bostanci N, Hashim A, Johansson A, Aduse-Opoku J, Curtis MA, Hughes FJ. Regulation of RANKL and OPG gene expression in human gingival fibroblasts and periodontal ligament cells by Porphyromonas gingivalis: a putative role of the Arg-gingipains. Microb Pathog. 2007;43:46–53. doi: 10.1016/j.micpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–80. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohnuki H, Izumi K, Terada M, Saito T, Kato H, Suzuki A, Kawano Y, Nozawa-Inoue K, Takagi R, Maeda T. Zoledronic acid induces S-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch Oral Biol. 2011 doi: 10.1016/j.archoralbio.2011.11.015. http://dx.doi/10.1016/j.archoralbio.2011.11.015. [DOI] [PubMed]
- 67.Scheper MA, Badros A, Chaisuparat R, Cullen KJ, Meiller TF. Effect of zoledronic acid on oral fibroblasts and epithelial cells: a potential mechanism of bisphosphonate-associated osteonecrosis. Br J Haematol. 2009;144:667–76. doi: 10.1111/j.1365-2141.2008.07504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clezardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–44. [PubMed] [Google Scholar]
- 69.Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–7. [PubMed] [Google Scholar]
- 70.Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010;28:165–75. doi: 10.1007/s00774-009-0128-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


