Abstract
Background
Socioeconomic disparities in treatment and outcomes of non-small cell lung cancer (NSCLC) are well established. To explore whether these differences are secondary to individual or institutional characteristics, we examined treatment selection and outcome in a diverse population treated at a single medical center.
Patient and Methods
We performed a retrospective analysis of consecutive patients diagnosed with NSCLC stages I-III from 2000-2005 at the University of Texas Southwestern Medical Center. Treatment selection was dichotomized as “standard” (surgery for stage I-II; surgery and/or radiation therapy for stage III) or “other.” Associations between patient characteristics (including socioeconomic status) and treatment selection were examined using logistic regression; associations between characteristics and overall survival were examined using Cox regression models and Kaplan-Meier survival analysis.
Results
A total of 450 patients were included. Twenty-eight percent of patients had private insurance, 43% had Medicare, and 29% had an indigent care plan. The likelihood of receiving “standard” therapy was significantly associated with insurance type [indigent plan versus private insurance OR 0.13 (95% CI 0.04-0.43) for stage I-II; OR 0.38 (95% CI 0.14-1.00) for stage III]. For patients with stage I-II NSCLC, survival was associated with age, gender, insurance type (indigent plan versus private insurance HR 1.98; 95% CI 1.16-3.37), stage, and treatment selection. In stage III NSCLC, survival was associated with treatment selection.
Conclusion
Within a single academic medical center, socioeconomically disadvantaged patients with stage I-III NSCLC are less likely to receive “standard” therapy. Socioeconomically disadvantaged patients with stage I-II NSCLC have inferior survival independent of therapy.
Keywords: Surgery, radiation therapy, chemotherapy, underserved, minorities, socioeconomic disparities
Disparities characterize all aspects of health care, from prevention, risk factors and presentation,(1-4) to treatment(5-8) and outcomes.(5, 9-10) Lung cancer provides an excellent opportunity to explore such patterns. Lung cancer affects both genders, all races, and all socioeconomic classes. There are a variety of treatment options. Due to the lethality of lung cancer, patient survival often reflects the behavior of the underlying disease.
The approach to early stage and locally advanced non-small cell lung cancer (NSCLC) is complex. Management requires adequate medical fitness; extensive, often invasive staging procedures; and patient adherence to complex treatment regimens. For localized disease, it is well established that surgical resection offers superior outcomes to conventional radiation therapy or medical treatments. Nevertheless, several studies have demonstrated that black patients are significantly less likely to receive surgical intervention in stage I and II NSCLC than are white patients.(5-6, 11) Similar results have been seen when comparing Hispanic to white patients with stage I disease,(7) and when analyzing individuals by socioeconomic status.(12) In some instances, these disparate treatment patterns appear to account for differing outcomes.(5, 7) Treatment and outcome discrepancies for locally advanced, stage III NSCLC have been less well characterized.
To what extent cancer treatment and outcome disparities reflect individual or institutional factors remains unclear. In lung cancer, a number of studies have suggested that individual behaviors may impact treatment selection. For instance, black patients have been shown to have more reservations about and to be less likely to proceed with surgical resection.(6, 13) Other series have demonstrated that system-level factors are the predominant predictors of treatment and outcomes. As an example, patients who receive care at a hospital which serves a large minority population have been shown to be less likely to undergo surgical resection.(14) It has also been shown that racial differences in survival disappear when patients receive treatment at a National Cancer Institute (NCI)-designated cancer center or have universal access to care in a military health care system.(15-16) However, it is not clear to what extent these patient populations are socioeconomically diverse, a metric thought to be more representative than race of health and health-related qualities.(17-18) Moreover, it is possible that these clinical settings represent self-selected groups that may not fully reflect characteristics of the broader population.
To investigate the role individual factors—in particular socioeconomic status—play in lung cancer treatment and outcome disparities, we studied patients with stage I-III NSCLC at a single medical center providing care to a socioeconomically diverse population. The University of Texas Southwestern Medical Center (UT Southwestern) comprises a large safety-net hospital system, tertiary care referral hospitals, and a freestanding NCI-designated cancer center. A single medical faculty provides care to lung cancer patients at all institutions, and cases are reviewed at a single multidisciplinary thoracic tumor board. We focused on early stage and locally advanced NSCLC because of greater variance in treatment options and outcomes than seen in metastatic disease.
Patients and methods
Sources of Data
This study was approved by the UT Southwestern Institutional Review Board. We acquired data from the American College of Surgeons-approved UT Southwestern and Parkland Health and Hospital System Tumor Registries. The UT Southwestern Tumor Registry collects data from University Hospital and the Harold C. Simmons Cancer Center. Parkland Health and Hospital System, which includes a 968-bed inpatient facility and associated community clinics, is the principal safety-net health care system for Dallas County. Dallas County has a population of 2.45 million, of which 40% are Hispanic, 21% black, and 34% non-Hispanic white.(19) Additional information was obtained through review of electronic and paper medical records.
We included consecutive patients diagnosed with stage I to III NSCLC between January 1, 2000 and December 31, 2005. We focused on stage I-III NSCLC because of greater variation in treatment and outcome compared to stage IV disease. We excluded cases with malignant effusions (“wet IIIB”; stage IVA in the American Joint Committee on Cancer [AJCC] 7th Edition(20)) because treatment (chemotherapy-based) and survival are distinct from other stage III cases and more closely resemble stage IV disease. We selected the 2000-2005 time period because the tumor registries first collected adequate data for this study in the year 2000; the 2005 cut-off allowed for sufficient follow-up for outcome analysis.
Measures
For each patient, we obtained age at diagnosis, sex, race/ethnicity, tumor histology and stage (based on the AJCC 6th Edition), insurance type, treatment selection, and date of death. In the tumor registry, race/ethnicity information is obtained by patient self-report, which hospital or clinic staff enter into the demographic section of the electronic medical record. In instances where this information is not recorded, tumor registry abstractors search clinic notes and other documentation. Staging was based on surgical staging when available and on clinical staging in other instances. For our analyses, we grouped stages I and II together because of the small number of stage II patients and the largely similar treatment paradigms for both stages. Race was dichotomized into non-Hispanic white and other. Insurance type was categorized as private, Medicare, and indigent (which included individuals with Medicaid and individuals with a Dallas County public insurance plan). The county plan provides access to all diagnostic and treatment modalities at PHHS for patients lacking other forms of health coverage. To optimize socioeconomic characterization, we designated patients with both Medicare and Medicaid coverage as Medicaid. We used insurance type rather than census-derived income data as a marker of socioeconomic status because (1) it is patient-specific (in contrast to zip code-derived household income data), and (2) income does not include wealth, which may be more representative of socioeconomic status.(21)
Treatment selection was initially divided into 4 categories: (1) surgery, (2) radiation therapy, (3) chemotherapy alone (including molecular targeted agents), and (4) no treatment. We assigned treatment according to the most aggressive component of a patient’s therapy. For example, a patient who underwent resection was placed in the surgery category, independent of whether radiation or chemotherapy was also administered. Definitive thoracic radiation, with or without chemotherapy, was categorized in the radiation category. In this series, radiation therapy exclusively included conventional fractionated radiation, as opposed to stereotactic radiation, because stereotactic radiation was not employed at our institution during the study period. Palliative radiation therapy alone was not included in the radiation therapy category. For each stage grouping, we dichotomized treatment selection as “standard” or “other” according to widely accepted practice guidelines.(22) For stage I-II disease, “standard” treatment included surgery (with or without other treatment modalities), with all other treatment categories considered “other.” We elected to group radiation therapy, chemotherapy, and no treatment together due to their substantially inferior outcomes compared to surgery for stage I-II disease. For stage III disease, we considered both definitive radiation (with or without chemotherapy) and surgery (with or without other treatment modalities) as “standard” treatment, with “other” including chemotherapy alone or no treatment. Because the 6th edition of the American Joint Commission of Cancer (AJCC) was employed during the time frame of this study, patients with malignant effusions (for whom conventional treatment is chemotherapy alone) were classified as stage IIIB. Through direct review of medical records of all stage IIIB patients in our cohort, we identified patients with malignant effusions and excluded them from the analysis. Overall survival was calculated from date of diagnosis to date of death.
Statistical Analysis
For baseline demographic and clinical characteristics, we used means and standard deviations for continuous variables and percentages for categorical variables. We used univariate and multivariate logistic regression to compare the likelihood of receiving “standard” treatment. All variables included in univariate analyses were retained in the multivariate model. In these analyses, the odds ratio indicated the likelihood of having received “standard” treatment. We used univariate and multivariate Cox regression models to analyze the association between baseline characteristics, treatment selection, and overall survival. As before, all variables included in univariate analyses were retained in the multivariate model. Kaplan-Meier survival curves were drawn and log-rank tests were used to test survival difference among groups. Potential multicolliearity problems associated with multivariate analysis were evaluated using formal detection-tolerance tests. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina) in Microsoft Windows.
Results
Patient Characteristics
We identified 476 consecutive patients diagnosed with stage I to III NSCLC between January 1, 2000 and December 31, 2005. Of these, 26 were malignant effusion (“wet” IIIB; stage IVA in AJCC 7th edition) and were excluded from the analysis. The study cohort therefore included 450 total patients. Baseline patient characteristics are shown in Table 1. Mean age was 64 years. Overall, 63% of the study population was non-Hispanic white, 29% black, 4% Hispanic, and 4% other. Specific stage breakdown was as follows: 43% stage I, 12% stage II, 45% stage III. Specific insurance breakdown was as follows: 28% private insurance, 43% Medicare, 5% Medicaid, 24% county health plan (two-thirds of whom had no insurance coverage prior to cancer presentation). Among the principal insurance categories, there were significant differences with regards to age, gender, race, stage, and histology (see Table 1). Compared to individuals with private insurance, those with an indigent plan were more likely to be male and non-white, have more advanced disease, and have non-adenocarcinoma histology.
Table 1. Baseline characteristics of the entire study sample and according to insurance type.
| Characteristic | Total | Private Insurance |
Medicare | Indigent | Overall P value |
|---|---|---|---|---|---|
| Number of Patients | 450 | 124 | 195 | 131 | <0.001 |
| Age, years, mean ± SD | 63.9 ±10.9 | 58.9 ±8.3 | 72.0 ±7.1 | 57.7 ±10.4 | |
| Gender, no. (%) | |||||
| Male | 234 (52) | 53 (43) | 107(55) | 74 (56) | 0.05 |
| Female | 216(48) | 71 (57) | 88 (45) | 57 (44) | |
| Race, no. (%) | |||||
| White, non-Hispanic | 284 (63) | 82 (66) | 147(75) | 54 (41) | <0.001 |
| Other | 166(37) | 42 (34) | 48 (25) | 77 (59) | |
| Stage, no. (%) | |||||
| Stage I | 194(43) | 52 (42) | 107(55) | 35 (27) | <0.001 |
| Stage II | 53(12) | 23(18) | 16(8) | 14(11) | |
| Stage III | 203 (45) | 49 (40) | 72 (37) | 82 (63) | |
| Histology, no. (%) | |||||
| Adenocarcinoma | 218(48) | 72 (58) | 79 (40) | 46 (35) | <0.001 |
| Squamous Cell | 158(35) | 33 (27) | 95 (49) | 51 (39) | |
| Other * | 74(17) | 19(16) | 21 (11) | 34 (26) |
Includes patients with large cell, adenosquamous or unspecified
Treatment selection
Table 2a shows the type of treatment received according to patient and disease characteristics for stage I-II cases. In univariate analysis, race and type of insurance were significantly associated with treatment selection. Eighty-eight percent of non-Hispanic white patients received “standard” treatment (ie, surgery) compared to 76% of other patients (P=0.01). Sixty-five percent of indigent patients underwent surgery, compared with 87% of patients with Medicare and 93% of patients with private insurance (P<0.001). Additionally, patients with stage I disease were more likely to undergo surgery than were patients with stage II disease. When controlling for age, gender, race, insurance status, stage, and histology in multivariate analysis, only insurance type (P=0.001) and stage (P=0.03) remained significantly associated with treatment selection. In this model, indigent patients were substantially less likely to undergo surgery than were those with private insurance (OR 0.13; 95% CI 0.04-0.43).
Table 2a. Likelihood of receiving “standard” treatment (surgery) for stage I-II NSCLC.
| Univariate OR (95% CI) |
Univariate P-Value |
Multivariate OR (95% CI) |
Multivariate P-Value |
|
|---|---|---|---|---|
| Age | ||||
| < 65 | Reference | 0.78 | Reference | 0.08 |
| ≥ 65 | 0.91 (.045-1.83) | 0.34 (0.11-1.12) | ||
| Gender | ||||
| Male | Reference | 0.59 | Reference | 0.83 |
| Female | 1.21 (0.61-2.42) | 1.08 (0.51-2.30) | ||
| Race | ||||
| White, non-Hispanic | Reference | 0.01 | Reference | 0.17 |
| Other | 0.41(0.20-0.82) | 0.56 (0.24-1.29) | ||
| Insurance | ||||
| Private | Reference | <0.001 | Reference | 0.001 |
| Medicare | 0.48 (0.17-1.36) | 0.76 (0.20-2.86) | ||
| Indigent | 0.13 (0.05-0.40) | 0.13 (0.04-0.43) | ||
| Stage | ||||
| Stage I | Reference | 0.01 | Reference | 0.03 |
| Stage II | 0.39 (0.19-0.83) | 0.40 (0.17-0.92) | ||
| Histology | ||||
| Adenocarcinoma | Reference | 0.09 | Reference | 0.28 |
| Squamous Cell | 0.44 (0.21-0.94) | 0.51 (0.23-1.17) | ||
| Other * | 0.51 (0.18-1.44) | 0.78 (0.25-2.47) |
Includes patients with large cell, adenosquamous or unspecified
Table 2b demonstrates the type of treatment received based on patient and disease characteristics for stage III disease. In univariate analysis, gender was significantly associated with and age, gender and insurance status had non-significant trends toward association with treatment selection. Specifically, among patients under age 65, 80% underwent “standard” treatment (surgery and/or thoracic radiation), compared to 68% of patients age 65 and older. Sixty-eight percent of men received “standard” treatment, compared to 83% of women (P=0.02). Seventy-nine percent of non-Hispanic white received “standard” treatment, compared to 70% of other patients. Eighty-six percent of patients with private insurance received “standard” treatment, compared to 74% of patients with Medicare, and 70% of indigent patients (P=0.04 for indigent plan versus private insurance). In a multivariate model including all variables, treatment selection was associated with age and had a trend toward overall association with gender and insurance type. Specifically comparing those patients with an indigent health plan to those with private insurance, the former remained significantly less likely to receive “standard” treatment (OR 0.38, 95% CI 0.14-1.00; P=0.05). We evaluated for a race by insurance interaction in the analyses of treatment selection. Because these interactions were not statistically significant (P=0.30 for stage I-II; P=0.30 for stage III), the interaction terms were not included in the final analysis..
Table 2b. Likelihood of receiving “standard” treatment (surgery or definitive thoracic radiation) for stage III NSCLC.
| Univariate OR (95% CI) |
Univariate P-Value |
Multivariate OR (95% CI) |
Multivariate P-Value |
|
|---|---|---|---|---|
| Age, no. (%) | ||||
| < 65 | Reference | 0.06 | Reference | 0.05 |
| ≥ 65 | 0.55 (0.29-1.04) | 0.39 (0.16-0.98) | ||
| Gender, no. (%) | ||||
| Male | Reference | 0.02 | Reference | 0.07 |
| Female | 2.28 (1.15-4.40) | 1.95 (0.95-4.00) | ||
| Race, no. (%) | ||||
| White, non-Hispanic | Reference | 0.11 | Reference | 0.23 |
| Other | 0.60 (0.32-1.13) | 0.67 (0.33-1.31) | ||
| Insurance, no. (%) | ||||
| Private | Reference | 0.12 | Reference | 0.09 |
| Medicare | 0.46 (0.18-1.21) | 0.85 (0.27-2.65) | ||
| Indigent | 0.38 (0.15-0.96) | 0.38 (0.14-1.00) | ||
| Histology, no. (%) | ||||
| Adenocarcinoma | Reference | 0.93 | Reference | 0.79 |
| Squamous Cell | 0.88 (0.43-1.79) | 1.32 (0.60-2.89) | ||
| Other * | 0.91 (0.39-2.14) | 1.17 (0.46-2.95) |
Includes patients with large cell, adenosquamous or unspecified
Survival Analysis
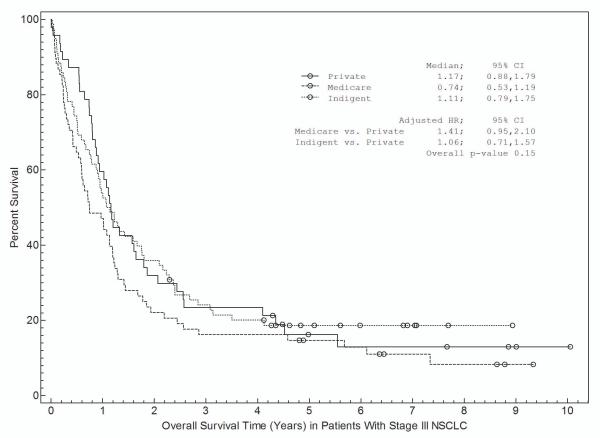
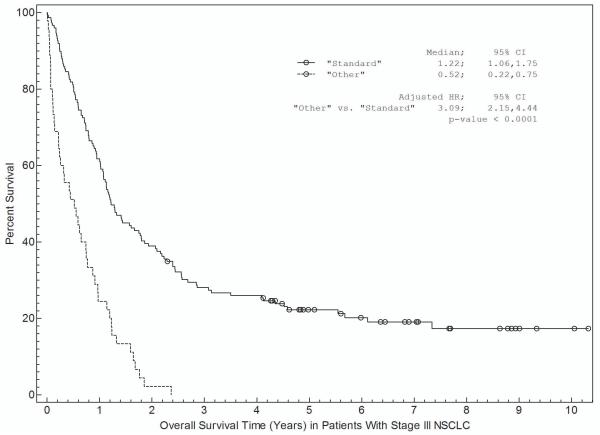
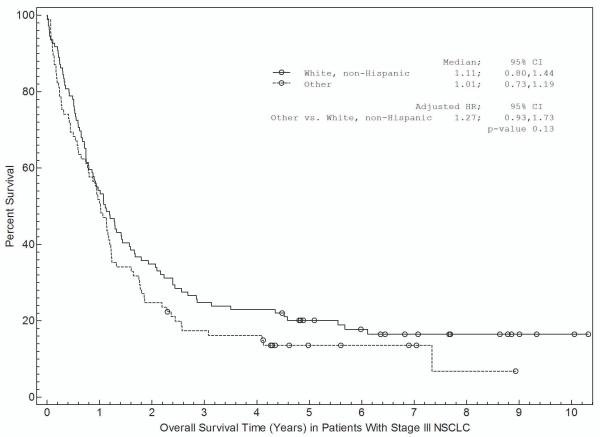
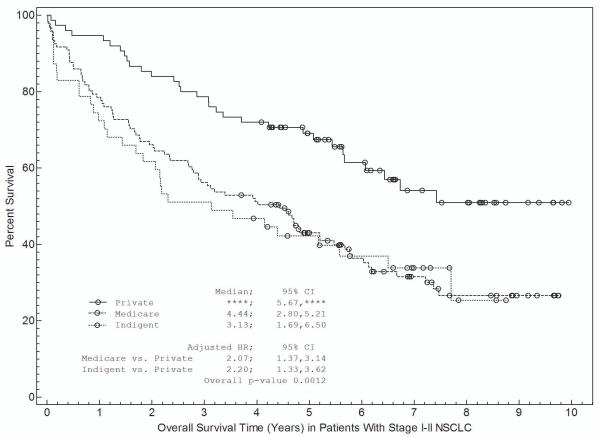
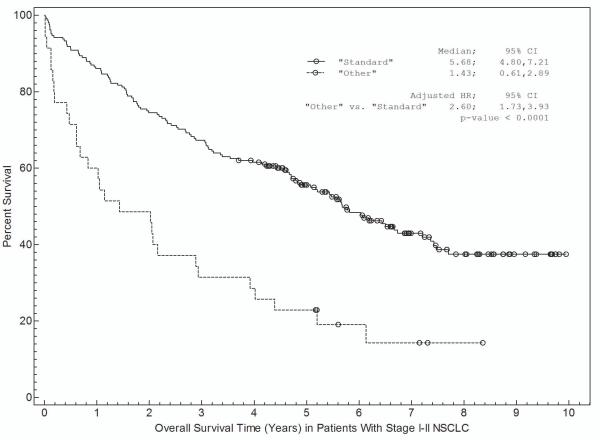
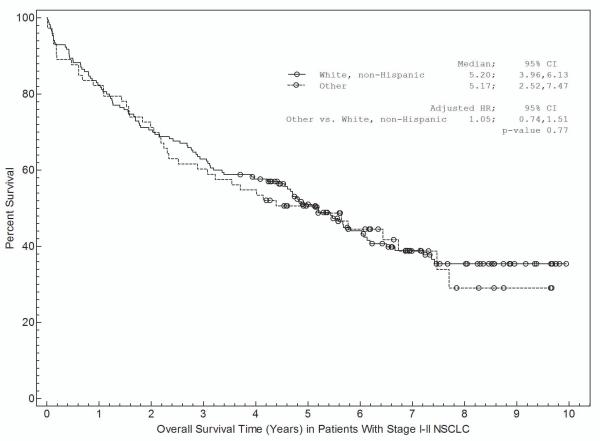
Associations between patient and disease characteristics, treatment selection, and survival are shown in Tables 3a and 3b and in Figures 1 and 2. For stage I-II NSCLC, overall survival was associated with age, gender, insurance, treatment selection, and specific disease stage (Table 3a). Figure 1 shows survival curves grouped by race (Fig 1a), insurance type (Fig 1b), and treatment selection (Fig 1c). Both non-Hispanic white and other individuals had a median survival time of 5.2 years (Fig 1a). Patients with private insurance had a median survival time of greater than 7.4 years, compared to 4.4 years for patients with Medicare and 3.3 years for indigent patients (Fig 1b). Patients who received “standard” treatment (surgery) had a median survival of 5.7 years versus 1.7 years for patients who did not (Fig 1c). In multivariate analysis (Table 3a), age, gender, insurance status, and treatment selection remained significantly associated with outcome.
Table 3a. Association between patient characteristics and survival in Stage I & II NSCLC.
| Univariate HR (95% CI) |
Univariate P-Value |
Multivariate HR (95% CI) |
Multivariate P-Value |
|
|---|---|---|---|---|
| Age | ||||
| < 65 | Reference | <0.001 | Reference | 0.004 |
| ≥ 65 | 1.86 (1.31-2.63) | 2.22 (1.29-3.82) | ||
| Gender | ||||
| Male | Reference | <0.001 | Reference | <0.001 |
| Female | 0.57 (0.41-0.79) | 0.53 (0.38-0.74) | ||
| Race | ||||
| White, non-Hispanic | Reference | 0.77 | Reference | 0.87 |
| Other | 1.05 (0.74-1.51) | 0.97 (0.65-1.44) | ||
| Insurance | ||||
| Private | Reference | 0.001 | Reference | 0.04 |
| Medicare | 2.07 (1.37-3.14) | 1.27 (0.72-2.22) | ||
| Indigent | 2.20 (1.33-3.62) | 1.98 (1.16-3.37) | ||
| Stage | ||||
| Stage I | Reference | 0.06 | Reference | 0.04 |
| Stage II | 1.44 (0.98-2.11) | 1.52 (1.02-2.29) | ||
| Histology | ||||
| Adenocarcinoma | Reference | 0.06 | Reference | 0.06 |
| Squamous Cell | 1.27 (0.88-1.84) | 1.09 (0.75-1.58) | ||
| Other * | 1.74 (1.07-2.83) | 1.83 (1.12-2.99) | ||
| Treatment # | ||||
| “ Standard ” | Reference | <0.001 | Reference | <0.001 |
| Other | 2.60 (1.73-3.93) | 2.13 (1.37-3.31) |
HR>1: shorter survival
Includes patients with large cell, adenosquamous or unspecified
For Treatment, Standard=Surgery. Other =No Surgery
Table 3b. Association between patient characteristics and survival in Stage III NSCLC.
| Univariate HR (95% CI) |
Univariate P-Value |
Multivariate HR (95% CI) |
Multivariate P-Value |
|
|---|---|---|---|---|
| Age | ||||
| < 65 | Reference | 0.02 | Reference | 0.38 |
| ≥ 65 | 1.42 (1.04-1.93) | 1.21 (0.79-1.84) | ||
| Gender | ||||
| Male | Reference | 0.01 | Reference | 0.13 |
| Female | 0.68 (0.50-0.93) | 0.78 (0.56-1.08) | ||
| Race | ||||
| White, non-Hispanic | Reference | 0.13 | Reference | 0.46 |
| Other | 1.27 (0.93-1.73) | 1.14 (0.81-1.59) | ||
| Insurance | ||||
| Private | Reference | 0.15 | Reference | 0.78 |
| Medicare | 1.41 (0.95-2.10) | 1.08 (0.66-1.78) | ||
| Indigent | 1.06 (0.71-1.57) | 0.92 (0.61-1.39) | ||
| Histology | ||||
| Adenocarcinoma | Reference | 0.13 | Reference | 0.26 |
| Squamous Cell | 1.07 (0.76-1.50) | 0.94 (0.65-1.35) | ||
| Other * | 0.69 (0.45-1.06) | 0.70 (0.45-1.08) | ||
| Treatment # | ||||
| “Standard” | Reference | <0.001 | Reference | <0.001 |
| Other | 3.09 (2.15-4.44) | 2.81 (1.91-4.14) |
Includes patients with large cell, adenosquamous or unspecified
For treatment, standard=surgery or definitive thoracic radiation. Other =no surgery or definitive thoracic radiation
Figures 1.
(a-c)-Kaplan-Meier Curves for Stage I and II NSCLC.
a. Association between race and survival.
b. Association between insurance type and survival.
c. Association between treatment selection and survival. “Standard” therapy includes surgery with or without other treatment modalities such as radiation or chemotherapy.
Figures 2.
(a-c)-Kaplan-Meier Curves for Stage III NSCLC.
a. Association between race and survival.
b. Association between insurance type and survival.
c. Association between treatment selection and survival. “Standard” therapy includes surgery- and/or radiation-based treatment with or without other treatment modalities such as chemotherapy.
For stage III NSCLC, overall survival was significantly associated with age, gender, and treatment selection in univariate analysis (Table 3b). Non-Hispanic white patients had a median survival of 1.1 years compared with 1.0 years for other patients (Fig 2a). Patients with private insurance had a median survival of 1.2 years, patients with Medicare had a median survival of 0.7 years, and indigent patients had a median survival of 1.1 years (Figure 2b). Patients who received “standard” treatment (surgery- and/or radiation-based therapy) had a median survival of 1.2 years versus 0.5 years for patients who did not receive surgery or radiation (Figure 2c). In multivariate analysis (Table 4b), only treatment selection remained significantly associated with overall survival. We evaluated for a race by insurance interaction in the survival analyses. Because these interactions were not statistically significant (P=0.44 for stage I-II; P=0.06 for stage III), the interaction terms were not included in the final analysis.
In all multivariate models, we confirmed the non-existence of multicolinearity by tolerances. All tolerances were greater than 0.85 (with multicolinearity generally considered to exist if tolerances are less than 0.1-0.2).
Discussion
In spite of numerous prior studies demonstrating disparities in lung cancer outcomes, several key issues remain unclear. To what extent do survival differences reflect individual or institutional or system factors? To what degree are they related to variance in treatment? Does stage distribution among populations underlie these disparities? We evaluated these and other factors through a cohort study of consecutive patients with stage I-III NSCLC treated at a large, urban North American academic medical center with a diverse patient population. At UT Southwestern, a single medical faculty treats patients in a large safety net hospital system and in a tertiary care NCI-designated cancer center. Health care providers apply a single set of institutional clinical practice guidelines to all patients and present cases at a single multidisciplinary tumor board. Despite the homogeneity of medical care, the present study demonstrates that socioeconomic status was a principal determinant of whether or not a patient received stage-specific “standard” treatment. Specifically, indigent patients were less likely than those with private insurance to undergo surgery for stage I-II NSCLC, and less likely to undergo surgery- or radiation-based therapy for stage III NSCLC. Even when controlling for treatment selection, socioeconomic status remained associated with overall survival in stage I-II disease.
Socioeconomic status was significantly associated with a number of baseline patient and disease characteristics that may impact treatment selection and clinical outcomes. Compared to patients with private insurance, indigent patients were less likely to be female or non-Hispanic white. They presented with more advanced stage disease and were less likely to have adenocarcinoma histology. These findings are consistent with a national study, in which patients without insurance were twice as likely to present with advanced stage disease when compared to individuals with private insurance.(4) Furthermore, inferior outcomes in male patients and non-adenocarcinoma histology are well established.(23-26) The lower rate of adenocarcinoma histology in our indigent population may indicate that this group had a more extensive smoking history, which in turn could potentially influence candidacy for and tolerance of surgery for stage I-II disease. Greater tobacco use could also independently affect overall survival through co-morbidities such as chronic obstructive pulmonary disease and cardiovascular disease, as well as through the development of other tobacco-related malignancies. It has been suggested that, compared to white patients, black patients are less likely to quit smoking after a diagnosis of lung cancer.(27) Despite these potential explanations, when we controlled for all other variables in multivariate models, socioeconomic status remained independently associated with both treatment selection and overall survival in stage I-II disease. Specifically, indigent patients were less likely to undergo surgery than were individuals with private insurance (OR for undergoing surgery 0.13; 95% CI, 0.04 to 0.43), and their risk of death was twice that of individuals with private insurance (HR 1.98; 95% CI, 1.16 to 3.37).
These findings echo those of large, population-based studies for a variety of malignancies. In the 1970s, a retrospective chart review of over 1,400 patients with NSCLC Stage I-III in the New England area found that patients with private insurance were more likely to undergo surgery for lung cancer than others even when controlling for disease stage.(28) Coburn et al found that 96% of patients with private insurance received surgical treatment for breast cancer, compared with only 85% of women without insurance.(29) Because these studies reported data from state registries or from multiple medical centers, it is difficult to discern whether these findings result from system or from individual behaviors. Our current study suggests that individual patient factors contribute to both treatment selection and clinical outcomes, findings that contrast those of prior single-institution analyses.(15-16) However, these results are consistent with studies of first- and second-line chemotherapy administration for advanced NSCLC performed at our institution.(30-31)
Historically, there have been fewer investigations into treatment and outcome disparities for locally advanced NSCLC. Hardy and colleagues found that black patients were significantly less likely than white patients to receive surgery in stage III NSCLC, but found no differences in the receipt of radiation.(32) In multivariate analysis, they also found that patients in the lowest income quartile were less likely to receive radiation in stage III-IV disease. In our study, older patients and indigent patients were less likely to receive “standard” (surgery- and/or radiation-based) treatment. While non-white patients were also less likely to receive “standard” therapy, this trend did not reach statistical significance (P=0.23). In contrast to stage I-II NSCLC, only treatment selection remained associated with overall survival for stage III NSCLC in multivariate analysis. It is possible that underlying patient and disease characteristics may have less influence on clinical outcomes in this setting due to a clinical course that is generally shorter and less variable.
This study suggests that treatment and outcome disparities persist in an environment designed to provide uniform care across populations. However, the underlying reasons for these findings remain unclear. It is possible that under-represented populations elect to receive less aggressive care. For instance, some studies have shown that black patients are less likely to proceed with surgery for lung cancer, even when it is recommended.(6, 33) However, other studies have demonstrated that minorities seek more aggressive care, such as in the acute care setting.(34-35) Physician bias could be another explanation for these findings. One previous study reported that black patients were less likely to be recommended surgery for NSCLC than white patients.(36) However, when limited to a single center—as is our current study—these findings were not reproducible.(37) Potentially limiting individual physician biases, early stage and locally advanced NSCLC cases undergo multidisciplinary tumor board review at our institution, thereby incorporating consensus treatment recommendations. Perhaps, following definitive therapy, differences in clinical and radiographic monitoring schedules and adherence among populations could contribute to survival disparities. Alternatively, greater rates of surgery in the private insurance group might result in upstaging of a greater proportion of these patients and, consequently, relative improvement in their stage-specific survival compared to clinically staged patients. Another reasonable explanation is that socioeconomically disadvantaged patients may be less fit for specific treatments. As mentioned previously, the greater proportion of squamous tumors in our indigent population may indicate greater prevalence and intensity of tobacco use, which in turn could predispose to other medical conditions and limit lung cancer therapeutic options. Unfortunately, a detailed analysis of patient fitness lies beyond the scope of this report. Smoking history, performance status, and medical comorbidities are not routinely captured in tumor registries. Nor are they consistently documented in the primary medical record. We are currently planning an evaluation of these variables. Identification of the causes of treatment and survival disparities—and measures to address them—will be essential to improving the care and outcome of socioeconomically disadvantaged patients with lung cancer.
Principal limitations of this study include its retrospective nature, relatively small size, and single-institution setting. However, it was precisely the single-center setting that permitted a focus on individual, rather than system, disparities. While our center provides access to a diverse patient cohort, certain characteristics may not be fully representative of the larger lung cancer population. Notably, the median age at diagnosis in this study was 64 years, approximately 6 years younger than the national average. We have reported similar age distributions in studies of advanced NSCLC at our institution.(30-31) This trend may be driven by patients with an indigent health plan, who had a median age of 58 years. Whether this reflects the greater tobacco use in medically underserved populations(38-39) and hence earlier tumor initiation and development, is unclear. In the current study, the younger age distribution clearly impacts insurance designation (with Medicare generally available to individuals age 65 years and older), our surrogate marker of socioeconomic status. Additionally, our treatment categorization did not fully capture the nature of a patient’s therapy, such as whether adjuvant chemotherapy was administered for early stage disease (relatively unlikely given the study time period), or whether definitive thoracic radiation was administered alone or with chemotherapy for locally advanced disease. Compared to our primary treatment categorizations (any surgery, any radiation, or neither), though, one would expect these further distinctions to have less impact on outcomes. Key strengths of this study include diversity of the patient population, a uniform approach to NSCLC treatment in the study setting, completeness of data and follow-up, and inclusion of several potentially confounding factors in the multivariable models.
Conclusion
This study demonstrates that socioeconomic disparities in treatment and outcome characterize early stage and locally advanced NSCLC, even within a single medical center with a uniform approach to the care of patients with this disease. Our marker of socioeconomic status— insurance type—may be more representative of these disparities than is race or ethnicity. In early stage disease, socioeconomic status is associated with overall survival, even when controlling for therapeutic modality. In the future, studies and interventions focusing on individual patient factors may be beneficial to address these differences.
Clinical Practice Points
Racial and socioeconomic disparities are well described in the treatment and outcome of lung cancer. However, it is not clear to what extent these differences reflect individual or institutional factors. While it has been shown that such disparities are eliminated when patients receive treatment at a National Cancer Institute (NCI)-designated cancer center or have universal access to care in a military health care system, these settings may represent self-selected groups not generalizable to the wider population. We therefore studied treatment selection and survival among patients with stage I-III non-small cell lung cancer (NSCLC) treated at a medical institution in which a single medical faculty provides care to a racially and socioeconomically diverse population through both an NCI-designated cancer center and a public safety-net hospital system. Despite a uniform approach to lung cancer therapy at the institution, socioeconomically disadvantaged individuals were less likely to receive stage- and evidence-based “standard” therapy (ie, surgery-based treatment for stages I-II; surgery- or radiation-based treatment for stage III). Even when controlling for treatment selection, socioeconomic status was associated with overall survival for early-stage disease. Our findings suggest that treatment and outcome disparities persist at the individual patient level, even when care is provided in a uniform fashion and controlling for disease stage. Therefore, it may be appropriate to focus efforts to overcome these disparities beyond issues of access to care.
Acknowledgements
The authors thank Joan Cox and Alejandra Madrigales for providing data from the Parkland Health and Hospital System and UT Southwestern Tumor Registries.
Biostatistical support provided by the Biostatistics Shared Resource at the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, Texas, USA, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-01.
Funding:
American Cancer Society and Simmons Cancer Center Grant ACS-IRG-02-196, National Institutes of Health CTSA Grant KL2RR024983 (North and Central Texas Clinical and Translational Science Initiative), and a National Cancer Institute Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to D.E.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author note:
J.T.Y. is now at MD Anderson Cancer Center, Houston, Texas, USA.
References
- 1.American Cancer Society . Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 2.Chu SY, Barker LE, Smith PJ. Racial/ethnic disparities in preschool immunizations: United States, 1996-2001. Am J Public Health. 2004;94:973–7. doi: 10.2105/ajph.94.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–88. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 5.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 6.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–76. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisnivesky JP, McGinn T, Henschke C, Hebert P, Iannuzzi MC, Halm EA. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med. 2005;171:1158–63. doi: 10.1164/rccm.200411-1475OC. [DOI] [PubMed] [Google Scholar]
- 8.van Ryn M, Burgess D, Malat J, Griffin J. Physicians’ perceptions of patients’ social and behavioral characteristics and race disparities in treatment recommendations for men with coronary artery disease. Am J Public Health. 2006;96:351–7. doi: 10.2105/AJPH.2004.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joynt K, Orav E, Jha A. Thirty-Day Readmission Rates for Medicare Beneficiaries by Race and Site of Care. JAMA. 2011;305:675–81. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 11.Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–81. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88:1681–4. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis ML, Christie JD, Silvestri GA, Kaiser L, Santiago S, Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: results of a multicenter survey. Ann Intern Med. 2003;139:558–63. doi: 10.7326/0003-4819-139-7-200310070-00007. [DOI] [PubMed] [Google Scholar]
- 14.Lathan CS, Neville BA, Earle CC. Racial composition of hospitals: effects on surgery for early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:4347–52. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onega T, Duell EJ, Shi X, Demidenko E, Goodman DC. Race versus place of service in mortality among medicare beneficiaries with cancer. Cancer. 2010;116:2698–706. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs SL, Schroeder SA. Class - the ignored determinant of the nation’s health. N Engl J Med. 2004;351:1137–42. doi: 10.1056/NEJMsb040329. [DOI] [PubMed] [Google Scholar]
- 18.Winker MA. Measuring race and ethnicity: why and how? JAMA. 2004;292:1612–4. doi: 10.1001/jama.292.13.1612. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau State and County QuickFacts. 2009 [cited 2011 Feb 17]; Available from: http://quickfacts.census.gov/qfd/index.html.
- 20.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 21.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 22.2011 National Comprehensive Cancer Network I The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Non-Small Cell Lung Cancer (Version 3.2011) 2011 [cited 2011 Feb 17]; Available from: http://www.nccn.org/index.asp.
- 23.Chang JW, Asamura H, Kawachi R, Watanabe S. Gender difference in survival of resected non-small cell lung cancer: histology-related phenomenon? J Thorac Cardiovasc Surg. 2009;137:807–12. doi: 10.1016/j.jtcvs.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Hsu LH, Chu NM, Liu CC, et al. Sex-associated differences in non-small cell lung cancer in the new era: is gender an independent prognostic factor? Lung Cancer. 2009;66:262–7. doi: 10.1016/j.lungcan.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Ouellette D, Desbiens G, Emond C, Beauchamp G. Lung cancer in women compared with men: stage, treatment, and survival. Ann Thorac Surg. 1998;66:1140–3. doi: 10.1016/s0003-4975(98)00557-8. discussion 3-4. [DOI] [PubMed] [Google Scholar]
- 26.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Annals of Oncology. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 27.Park ER, Japuntich SJ, Traeger L, Cannon S, Pajolek H. Disparities between blacks and whites in tobacco and lung cancer treatment. Oncologist. 2011;16:1428–34. doi: 10.1634/theoncologist.2011-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg ER, Chute CG, Stukel T, et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 1988;318:612–7. doi: 10.1056/NEJM198803103181006. [DOI] [PubMed] [Google Scholar]
- 29.Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008;14:128–34. doi: 10.1111/j.1524-4741.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 30.Rasco DW, Yan J, Xie Y, Dowell JE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5:1529–35. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerber DE, Rasco DW, Le P, Yan J, Dowell JE, Xie Y. Predictors and impact of second-line chemotherapy for advanced non-small cell lung cancer in the United States: real-world considerations for maintenance therapy. J Thorac Oncol. 2011;6:365–71. doi: 10.1097/JTO.0b013e3181fff142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115:2199–211. doi: 10.1002/cncr.24248. [DOI] [PubMed] [Google Scholar]
- 33.Farjah F, Wood DE, Yanez ND, 3rd, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–8. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepardson LB, Gordon HS, Ibrahim SA, Harper DL, Rosenthal GE. Racial variation in the use of do-not-resuscitate orders. Journal of General Internal Medicine. 1999;14:15–20. doi: 10.1046/j.1525-1497.1999.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenger NS, Pearson ML, Desmond KA, et al. Epidemiology of Do-Not-Resuscitate Orders - Disparity by Age, Diagnosis, Gender, Race, and Functional Impairment. Archives of Internal Medicine. 1995;155:2056–62. [PubMed] [Google Scholar]
- 36.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–8. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 37.McCann J, Artinian V, Duhaime L, Lewis JA, Kvale PA, DiGiovine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–6. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 38.Laaksonen M, Rahkonen O, Karvonen S, Lahelma E. Socioeconomic status and smoking: analysing inequalities with multiple indicators. Eur J Public Health. 2005;15:262–9. doi: 10.1093/eurpub/cki115. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization Tobacco and poverty: a vicious circle. 2004 Available from: www.who.int/tobacco.