Abstract
RNA receptors such as TLR3 and RIG-I/MDA5 play essential roles in innate immunity to RNA viruses. However, how innate immunity to RNAs is controlled at the molecular level is not well understood. We describe here a new regulatory pathway of anti-RNA immunity that comprises PI3K (phosphoinositide kinase-3), its target GTPase Rac, and the newly described immune regulator TIPE2 (TNF-α-induced protein 8 like-2, or TNFAIP8L2). Poly (I:C), a double-stranded RNA receptor ligand, activates Rac via its guanine nucleotide exchange factor Tiam; this leads to the activation of cytokine genes, and paradoxically down-regulation of Tipe2 gene. TIPE2 is a negative regulator of immunity; its deficiency leads to hyper-activation of the PI3K–Rac pathway as exemplified by enhanced AKT, Rac, PAK, and IRF3 activities. As a consequence, TIPE2 knockout myeloid cells are hyper-reactive to Poly (I:C) stimulation, and TIPE2 knockout mice are hypersensitive to Poly (I:C)-induced lethality. These results indicate that TIPE2 controls innate immunity to RNA by targeting the PI3K–Rac pathway. Therefore, manipulating TIPE2 or Rac functions can be effective for controlling RNA viral infections.
Keywords: TNFAIP8, RNA, Innate Immunity, Rac, TLR, PI3K
Introduction
TLR3 and RIG-I (retinoid acid-inducible gene I)/MDA5 (melanoma differentiation-associated gene 5) have been identified as receptors for double stranded RNAs (1–3). RNA ligation of its receptors induces type I IFN and pro-inflammatory cytokines via a MyD88-independent pathway, which involves TIR domain-containing adaptor molecule-1 (TRIF) or interferon-β promoter stimulator (IPS) (2, 4). TRIF relays signals to the kinases TBK1 and IKK, which in turn activate the downstream transcription factors IRF3, NF-κB, and AP1, leading to the production of type I IFN and pro-inflammatory cytokines (4–7). Recent studies indicate that the PI3K–AKT pathway and small GTPases may also be involved in TLR signaling (8–12). Small GTPases are enzymes that hydrolyze guanosine triphosphate (GTP). They are active when bound to GTP and inactive when bound to GDP, and therefore serve as molecular “On-and-Off” switches of signaling pathways that control a wide variety of cellular processes including growth, motility, vesicle trafficking, gene transcription, and death (13, 14). However, whether and how the small GTPases are involved in innate immunity to dsRNAs is not clear.
TIPE2, or tumor necrosis factor-alpha-induced protein 8 (TNFAIP8)-like 2 (TNFAIP8L2), is a newly described immune regulator of the TNFAIP8 family (15, 16). It is preferentially expressed in hematopoietic cells and is significantly down-regulated in patients with infectious or autoimmune disorders (17). The mammalian TNFAIP8 family consists of four members: TNFAIP8 (TIPE), TIPE1, TIPE2, and TIPE3, whose functions are largely unknown. We report here that TIPE2 regulates Poly (I:C)-induced innate immune responses by targeting Rac GTPases in a PI3K-dependent manner.
Materials and Methods
Mice
Wild type C57BL/6 (B6) mice were purchased from the Jackson Laboratory. The Tipe2−/− deficent B6 mice were generated by backcrossing Tipe2−/− 129 mice to B6 mice for 12 generations. Age- and sex-matched wild type and Tipe2-deficient mice were used in all experiments. Mice were housed in the University of Pennsylvania Animal Care Facilities under specific pathogen-free conditions. All animal procedures were pre-approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Reagents
Poly (I:C), CpG, Imiquimod, and Rhodamine-labeled Poly (I: C) were purchased from InvivoGen. Lipopolysaccharide (LPS) and peptidoglycan (PGN) were from Sigma. PI3K inhibitor LY294002 were obtained from Cell signaling. The p38 inhibitor SB203580, ERK kinase I inhibitor PD98059, JNK inhibitor SP600123, and NF-κB inhibitor Bay were purchased from Promega. Rac inhibitor NSC23766 was obtained from Tocris. GM-CSF was from R&D systems. ELISA reagents were purchased from BD Biosciences, which include purified and biotinylated rat anti-mouse IL6 and TNFα. Antibodies used were as follows: Rabbit anti-Rac1/2/3, Rabbit anti-Na/K ATPase, Rabbit anti-phospho-PAK1(Ser144)/PAK2(Ser141), Rabbit anti-PAK1/2/3, Rabbit anti-pAKT, Rabbit anti-total AKT, Rabbit anti-IRF3 (Cell signaling), Rabbit anti-Tiam (Santa Cruz), HRP-conjugated anti-mouse or anti-rabbit Ig (GE healthcare). Quantitative Real-time PCR primers for IL6, TNFα, IL-1β, IFNβ1, and IFNα4 were purchased from Qiagen.
Preparation of bone marrow-derived dendritic cells and macrophages
Bone marrow-derived dendritic cells (BMDCs) were generated as described (18). Briefly, 2×106 BM precursors from B6 and Tipe2-deficent mice were seeded in complete IMDM supplemented with 3.3 ng/ml GM-CSF in 6-well plates. Two milliliters of medium were added to the culture on day 3, and half of the medium was replaced with new medium on days 5, 6, and 7. To generate bone marrow-derived macrophages (BMMs), bone marrow cells were cultured for 7 days in DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, 1% glutamine, and 30% L-929 cell culture supernatant. At the end of culturing, the cells were washed twice with cold DPBS and rested overnight in complete DMEM before assays. BMDCs were 80% CD 11c+ and BMMs were >95% CD11b+ and F4/80+ as determined by flow cytometry.
TLR ligand and inhibitor treatment
TLR2 ligand PGN ((10 ug/ml), TLR3 ligand Poly (I:C) (20 ug/ml), TLR9 ligand CpG (5uM), TLR4 ligand LPS (100 ng/ml), and TLR7 ligands imiquimod (5 ug/ml) were added to BMDC or BMM cultures for various times. For inhibitor assays, all the inhibitors were added to the culture 30 minutes before TLR ligand stimulation.
RNA isolation and real-time quantitative PCR
The total RNA was isolated using Trizol reagent (invitrogen) and purified using RNeasy Mini kits (Qiagen) according to manufacturer’s description. After treatment with RNase-free DNase I (invitrogen), RNA samples were reversely transcribed with oligo(dT) and SuperScript II transcriptase (Invitrogen). Real-time quantitative PCR analysis was performed using specific Quantitect Primers for mouse GAPDH, TIPE2, IL6, IFNβ 1, IFNα4, IL-1β, and TNFα (Qiagen) in an Applied Biosystems 7500 system using Power SYBR Green PCR Master Mix (Applied Biosystems). Relative levels of gene expression were determined using GAPDH as the control.
Enzyme-linked immunosorbent assay (ELISA)
The cell culture supernatants were collected and stored at −80°C. Quantitative ELISA was performed using paired mAbs specific for corresponding cytokines according to the manufacturer’s protocols (BD Biosciences).
Rac pulldown assay
To assess Rac activation, the cell extracts were incubated with PAK-glutathione-S-tranferase fusion protein beads (Cytoskeleton) at 4°C for 60 min. The collected beads were then washed three times and resuspended in SDS protein sample buffer. The bound proteins and total cell lysates were analyzed by SDS-PAGE, and blotted with anti-Rac (Cell Signaling).
Protein extraction, cell subcellular fractionation, and immunoblotting
Whole cell lysate was prepared by lysing cells in a buffer containing 150 mM NaCl, 50 mM HEPEs, pH 7.0, 1mM EDTA, 1% NP-40, 1x complete protease inhibitors cocktail (Roche), and 1x phosphatase inhibitor cocktail (Roche). In certain experiments, cell membrane proteins and cytoplasmic proteins were prepared using a Subcellular Protein Fractionation Kit (Pierce) according to the manufacturer’s protocols. Protein concentration was determined by BCA assay (Pierce). Equal quantities of proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with specific antibodies.
IRF-3 dimerization
Whole cell extracts were prepared in NP40 lysis buffer (50mM Tris-HCl, pH8.0, 1% NP40, 150mM NaCl, 1x protease inhibitor cocktail (Roche), and 1x phosphatase imhibitor cocktail (Roche)), then were subjected to electrophoresis on 8% native acrylamide gels, which were pre-run at 40mA for 30min at 4°C. The electrophoresis buffer were composed of a upper chamber buffer (25mM Tris-HCl, pH8.4, 192mM glycine, and 0.2% sodium doxycholate) and a lower chamber buffer (25mM Tris-HCl, pH8.4, 192mM glycine). Protein for each sample were mixed with 2x loading buffer (125mM Tris-HCl pH6.8, 30% glycerol, and BPB), electrophoresis by native PAGE at 25mA for 90 min at 4°C, then transferred to a nitrocellulose membrane, and blotted with IRF-3 antibodies.
Retrovirus preparation and retrovirus-mediated gene transfer
The murine RAW264.7 macrophages (ATCC) and HEK293T cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 2mM L-glutamine, and 100U/ml penicillin/streptomycin. To prepare retroviruses, packaging cells (293T) were cultured in 10-mm culture dishes and transfected with NGFR MSCV-based retroviral vector containing wt Rac, Rac1 T17N, and Rac1 Q61L along with pVSVG and pCGP sequences using CalPhos mammalian transfection kit (Clontech). The transfection medium was replaced with fresh medium 6 hours post transfection. At 24 and 48 hours post transfection, the culture medium containing recombinant retroviruses was harvested, filtered (with a 0.20 μm filter), and used to infect Raw cells. The infection efficiency was ~70% as determined by flow cytometry.
Poly (I:C) injection
6–8 week old WT and Tipe2−/− mice were intraperitoneally injected with Poly (I:C) (40mg/kg body weight). Mice were observed for sickness for 5 days. The Serum was collected at 24 hours after injection.
Statistical analyses
The differences in mRNA and proteins were analyzed by 2-tailed Student’s t test. The differences in survival rate were analyzed by Mann-Whitney U test.
Results
Inverse relationship between TIPE2 and cytokine gene expressions in myeloid cells following TLR stimulation
Myeloid cells play important roles in innate immunity to pathogens. TIPE2 is highly expressed in resting myeloid cells including dendritic cells and macrophages (15). To explore the roles of TIPE2 in dendritic cell-mediated innate immunity, TIPE2 expression was examined in murine bone marrow-derived dendritic cells (BMDCs) before and after stimulation with different Toll-like receptor (TLR) ligands/agonists. Upon stimulation with lipopolysaccharide (LPS, the TLR4 ligand), Poly (I:C) (the TLR3/MDA5 agonist), CpG (the TLR9 agonist), and peptidoglycan (PGN, the TLR2 ligand), TIPE2 mRNA expression was significantly reduced (Fig. 1a); by contrast, the mRNA (Figure 1b and 1c) and/or protein levels (Figure 1d) of cytokine genes (IFNβ1, IFNα4, IL6, and TNFα) were significantly increased in BMDCs. Similar effects were observed in the murine bone marrow-derived macrophages (Fig. 1e)(16). This inverse correlation between TIPE2 and cytokine levels in the innate immune cells treated with TLR ligands/agonist suggests a role for TIPE2 in regulating innate immune responses.
Figure 1. TLR stimulation markedly diminishes TIPE2 expression while increasing cytokine expression.
(a–b) Bone marrow-derived dendritic cells (BMDCs) were stimulated with or without lipopolysaccharide (100 ng/ml), Poly (I:C) (20 ug/ml), CpG (5 uM), and peptidoglycan (PGN) (10 ug/ml) for 6 hours, and total RNAs were isolated, treated with RNase-free DNase, and reversely transcribed. TIPE2 (a) and IFNβ1 (b) mRNA levels were determined by real-time PCR. Control (ctr), cultures not treated with TLR ligands. (c) BMDCs were stimulated with poly (I:C) (10 ug/ml) for the indicated times. IFNα4, IFNβ1, IL6, and TNFα mRNA levels were determined by real-time PCR. (d) BMDCs were stimulated with poly (I:C) (10 ug/ml) for the indicated times. Cytokine concentrations were determined by ELISA. (e) Bone marrow-derived macrophages (BMDMs) were stimulated with poly (I:C) (10 ug/ml) for the indicated times. Cytokine concentrations were determined by ELISA. Data in this figure are representative of three independent experiments. Error bars represent the standard deviations of the means. *p<0.05, **p<0.01, ***p<0.001.
Tipe2−/− dendritic cells have increased cytokine expression, Rac activation, and IRF3 phosphorylation, and Tipe2−/− mice are hypersensitive to Poly (I:C) lethality
Type I IFNs and inflammatory cytokines such as IL6 produced by activated dendritic cells play crucial roles in anti-microbial immunity. To determine the potential roles of TIPE2 in Poly (I:C)-induced cytokine production, we compared cytokine gene expression in wild type and Tipe2−/− bone marrow-derived dendritic cells. We observed significantly increased IFNβ1 and IL6 expression in Tipe2−/− dendritic cells (Figure 2a). Transcription factor IRF3 mediates IFNβ1 production in innate immune cells. We observed constitutively active IRF3 in Tipe2−/− BMDCs upon poly (I:C) stimulation (Figure 2b).
Figure 2. Tipe2−/− dendritic cells are hyper-reactive to Poly (I:C) stimulation and Tipe2−/− mice are hyper-sensitive to Poly (I:C) lethality.
(a) Wild-type (WT) and Tipe2−/− BMDCs were stimulated with Poly (I:C) (10 ug/ml) for 5 hours. The total RNAs were isolated, treated with RNase-free DNase I, and reversely transcribed. IFNβ1 and IL6 mRNA levels were determined by real-time PCR. (b) Wild-type (WT) and Tipe2−/− BMDCs were treated with poly (I:C) for the indicated times. The total proteins were isolated, separated by native PAGE, and immunoblotted with anti-IRF3 and anti-beta-actin. D, dimer, M, monomer. (c) Increased survival rate of Tipe2−/− mice after poly (I:C) injection. Poly (I:C) (40mg/Kg body weight) was peritoneally injected into wild-type and Tipe2−/− mice (n=4). The mice were monitored for sickness and survival for 5 days. (d) Mice were treated as in Panel c, and sera were collected at the 24th hour. Seral IL6 and TNFα levels were determined by ELISA. (e) TIPE2 deficiency does not affect the uptake of poly (I:C). BMDCs were incubated with Rhodomine-labeled poly (I:C) for the indicated times at 37°C, and washed 3 times with cold PBS. The fluorescence levels of WT (solid lines) and Tipe2−/− (broken lines) BMDCs were determined by flow cytometry. (f) Rac is constitutively active in Tipe2−/− BMDCs. Total cells were lysed and incubated with PAK-glutathione-S-tranferase fusion protein beads. The activated Rac was resolved by SDS-PAGE and detected by anti-Rac blotting (top panel). Total Rac in the cell lysate was detected by immunoblotting with anti-Rac (bottom panel). (g) Increased active TBK1, PAK, and AKT in Tipe2−/− BMDCs. Wild type and Tipe2−/− BMDCs were treated with poly (I:C) for the indicated times. The levels of total (t) and phosphorylated (p) proteins were determined by Western blotting. Results shown are means ± SEM. *p<0.01, **p<0.001. Data presented in this figure are representative of at least three independent experiments.
To establish the in vivo relevance of these findings, we injected poly (I:C) intra-peritoneally into wild type and Tipe2−/− mice. Remarkably, all Tipe2−/− mice were very sick 24 hours after poly (I:C) injection and died after blood withdraw whereas none of wild type littermates did (Figure 2c). The serum IL6 and TNFα levels were dramatically elevated in Tipe2−/− mice (Figure 2d), indicating the important role of TIPE2 in poly (I:C)-mediated inflammatory cytokine expression.
To explore the mechanism of TIPE2 action in Poly (I:C)-induced cytokine expression, we first compared ligand uptake between wild-type and Tipe2−/− bone marrow-derived dendritic cells. The Rhodomine-labeled Poly (I:C) was comparably taken up by BMDCs from the wild type and Tipe2−/− mice (Figure 2e), indicating that increased cytokine expression in Tipe2−/− cells was not due to enhanced uptaking of the TLR agonists. We recently found that endogenous TIPE2 can constitutively bind to the small GTPase Rac in immune cells (Z. Wang and Y. Chen, unpublished data). We compared Rac activation between wild type and Tipe2−/− bone marrow-derived dendritic cells. An elevated constitutive Rac activation was observed in Tipe2−/− BMDCs (Figure 2f), indicating that Rac was activated and involved in Poly (I:C)-mediated cytokine expression. PAK and AKT are downstream effectors of Rac and PI3K, respectively. As shown in Figure 2g, PAK and AKT were also constitutively active in Tipe2−/− bone marrow-derived dendritic cells. Rac1 and PAK1 have been reported to act upstream of TBK1/IKK-ε in the viral activation of IRF3 (19). Increased constitutive activation of TBK1 was also observed in Tipe2−/− cells as compared to the wild type cells (Figure 2g) indicating that TIPE2 regulates TBK1 via the Rac/PAK pathway.
PI3K and Rac inhibition significantly diminishes poly (I:C)-induced cytokine gene expression
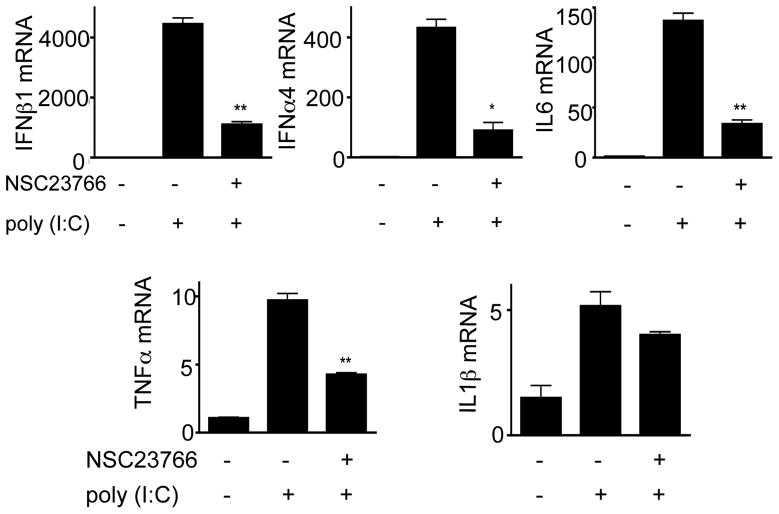
To further dissect the pathways involved in poly (I:C)-mediated cytokine production, we utilized inhibitors of several signaling pathways. As shown in Figure 3a, PI3K inhibitor LY294002 and p38 inhibitor SB203580 significantly diminished the expression of IFNβ1 and IL6, whereas PD98059 (an ERK inhibitor), SP600123 (a JNK inhibitor), and Bay (an NF-κB inhibitor) did not. Next, we examined whether LY294002 could also affect Rac activation. As shown in Figure 3b, LY294002 significantly blocked Rac activation. To directly confirm the role of Rac in IFNβ1 and IL6 production, the Rac inhibitor NSC 23766 was added to cells 20 minutes before Poly (I:C) stimulation. Similar to LY294002 (Figure 3c), NSC23766 effectively inhibited IFNβ1 and IL6 production in a dose-dependent manner (Figure 3d). Similar results were obtained for bone morrow-derived macrophages. NSC23766 significantly inhibited IFNβ1, IL6, IFNα4, and TNFα mRNA expression but not IL-1β expression (Figure 4). The inhibitors tested in Figures 3 and 4 did not significantly affect the apoptotic levels of the cells in the cultures as determined by flow cytometry (data not shown).
Figure 3. Effects of PI3K and Rac inhibition on poly (I:C)-induced responses in BMDCs.
(a) BMDCs were treated with indicated inhibitors for 20 minutes, and then stimulated with or without (ctr, control) poly (I:C) (10ug/ml) for 5 hours. IFNβ1 and IL6 mRNA were determined by real-time PCR. (b) BMDCs were treated with or without LY294002 (10 uM) for 20–30 minutes before stimulated with poly (I:C) for 5 hours. Cells were lysed, and total and activated Rac were detected by Western blot. (c) BMDCs were treated with or without the indicated concentrations of LY294002 or NSC23766 for 20 minutes before stimulated with poly (I:C) for 5 hours. Cytokine expressions were determined by real-time PCR. Ctr, culture not treated. Results are means ± SEM. *p<0.05, **p<0.01, ***p<0.001. Data are representative of at least three independent experiments.
Figure 4. Effects of Rac inhibition on poly (I:C)-induced responses in BMMs.
BMMs were treated with or without Rac inhibitor NSC23766 (100uM) for 20–30 minutes, and then stimulated with or without poly (I:C) (10ug/ml) for 5 hours as indicated. IFNβ1, IFNα4, IL1β, IL6, and TNFα mRNAs were determined by real-time PCR. Results are means ± SEM. *p<0.05, **p<0.01. Data are representative of at least three independent experiments.
To directly examine whether Rac activation is involved in IFNβ1 and IL6 production, we retrovirally transduced Raw macrophages with a dominant negative Rac mutant (Rac17N) or a constitutively active Rac (Rac61L). The expression of Rac17N significantly suppressed Poly (I:C)- induced IFNβ1 and IL6 production whereas that of Rac61L had the opposite effect (Figure 5). These results indicate that Rac activation is directly involved in Poly (I:C)-mediated cytokine expression.
Figure 5. Roles of Rac in poly (I:C)-induced cytokine responses.
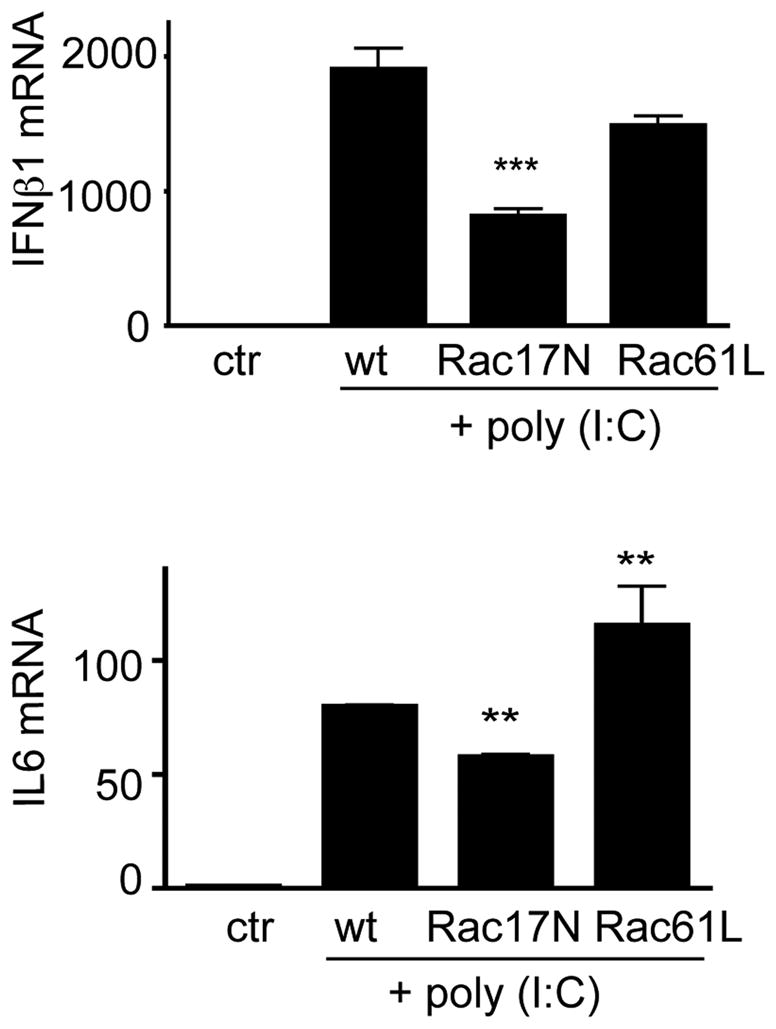
Wild type, a dominant negative form of Rac1, Rac17N, or a constitutively active form of Rac1, Q61L, were retrovirally transduced into Raw cells. The transduced cells were treated with or without poly (I:C) (10ug/ml) for 5 hours. IFNβ1 and IL6 mRNA expression was determined by real-time PCR. Results are means± SEM. **p<0.01, *** p<0.001, as compared to the WT group. Data are representative of at least two independent experiments.
PI3K activates the Rac–PAK pathway independent of Rac GEF Tiam
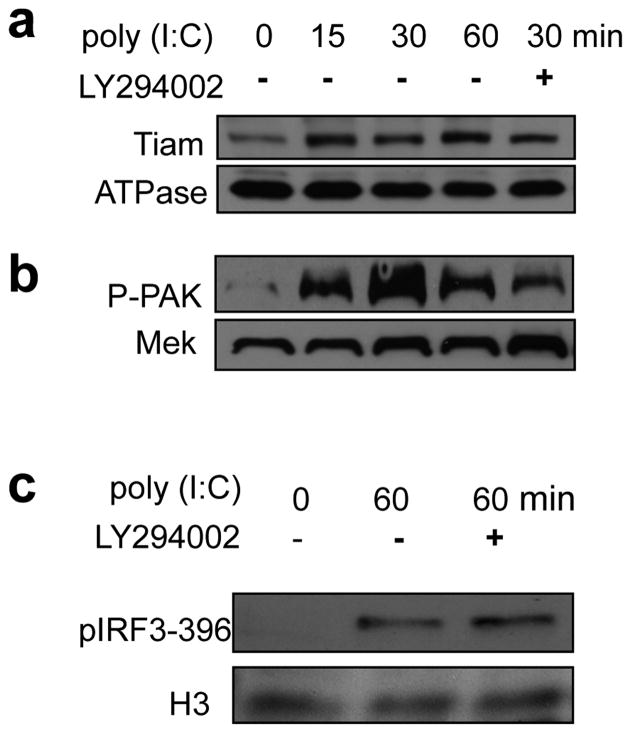
Small GTPases are activated by guanine nucleotide exchange factors (GEFs). NSC23766 effectively blocks the binding of Rac1 to the Rac-specific GEF Tiam, thus inhibiting Rac1 activity. To determine whether Poly (I:C) also affects membrane Tiam levels via PI3K pathway, we checked membrane Tiam and cytosolic PAK levels. As shown in Figure 6a, Tiam membrane recruitment was enhanced by poly (I:C) stimulation but the enhancement was not affected by PI3K inhibitor LY294002. Similarly, the phosphorylation at serine 396 was not affected by inhibition of PI3K (Figure 6c). However, the activation of Rac downstream effector PAK was significantly affected. Thus, the activation of PAK was markedly elevated upon poly (I:C) stimulation, which was significantly blocked by LY294002 (Figure 6b). Taken together, we have found that Poly (I:C) stimulated the recruitment of Rac GEF Tiam to the membrane but in a PI3K-independent manner wheareas Rac effector PAK was activated by Poly (I:C) in a PI3K-dependent manner.
Figure 6. Roles of PI3K in Rac GEF membrane translocation and Rac signaling.
BMDCs were treated with poly (I:C) (10ug/ml) for the indicated times, and PI3K inhibitor LY294002 (5 uM) was added to the indicated cultures for 20 minutes before the poly (I:C) treatment. Membrane (a) and cytosolic proteins (b) were fractionated and analyzed by Western blotting using antibodies specific for the indicated proteins. (c) Wild-type (WT) and Tipe2−/− BMDCs were treated with poly (I:C) for the indicated times. Nuclear proteins were isolated, separated by SDS-PAGE, and immunoblotted with anti-p-IRF3 Ser396 and anti-histone H3 (H3). Data are representative of two independent experiments.
Discussion
Dendritic cells play important roles in innate anti-microbial immune responses and the initiation of adaptive immune responses. The activated dendritic cells trigger large quantities of type I IFN and inflammatory cytokine production (20). Poly (I:C) activates various immune cells through two major dsRNA sensors: TLR3 and RIG-I/MDA5. RIG-I/MDA5 are cytosolic dsRNA sensors detecting cytosolic Poly (I:C) via adaptor protein IPS while TLR3 is located in intracellular endosome recognizing double-strand RNA and triggering type I IFN and inflammatory cykokine production via a MyD88-independent pathway. However, signaling through both receptors converges on the induction of type I IFN. Results reported here indicate that TIPE2 serves as an important regulator of type I IFN and pro-inflammatory cytokine production in Poly (I:C)-stimulated dendritic cells by targeting Rac activation. TIPE2 is constitutively expressed at high levels in immune cells. Exposure to TLRs markedly down-regulates TIPE2 levels, which removes its inhibitory effect on Rac GTPases; the activated Rac GTPases in turn activate their downstream effector targets in a PI3K-dependent manner. Thus, TIPE2 plays an important role in cytokine expression mediated by Poly (I:C) in innate immune cells.
Rac proteins constitute a subgroup of the Rho family of small GTPases, which includes Rac1, Rac2, Rac3, and Rac1b, the splice variant of Rac1 (13, 21). They function as molecular switches that control signaling pathways regulating cytoskeleton organization, gene expression, cell cycle progression, cell motility (22), innate immune responses related to pathogen sensing, intracellular uptake, and destruction (23, 24). They can be activated through interaction with Dbl family guanine nucleotide exchange factors (GEFs), e.g., Tiam, Trio, Dock180, and inactivated by GAPs (GTPase-Activating Proteins), e.g., RacGAP. NSC23766 specifically block the binding of Tiam to Rac1. Tiam contains two PH (pleckstrin homology) domains flanking its DH (Dbl homology) domain. DH domain is responsible for GEF activity while in general, the N-terminal PH domains have been implicated in directing the subcellular localization by binding to phosphatidylinositides and/or membrane-associated protein targets (25). In our study, NSC23766, a small molecule blocking the binding of Tiam to Rac1 (26), leads to downregulation of cytokine expression induced by Poly (I:C), indicating that the binding of Tiam and Rac1 plays crucial roles in the poly (I:C)-mediated cytokine expression in dendritic cells. Although PI3K inhibitor, LY294002 could inhibit Rac activation and block the cytokine production induced by Poly (I:C) as shown in our experiments, it does not block the Tiam membrane translocation, indicating an alternative mechanism for Tiam membrane translocation independent of PI3K. Rac activation could occur at several steps: removal of inhibitory molecules such as the GDI, recruitment or activation of GEFs, and removal or inhibition of GAP; the current available evidence suggests that it depends largely on the activation of the GEF (27). However, the mechanism of Tiam activation remains unclear. Although the N-terminal PH domain of Tiam binds to PIP3, its membrane translocation, as reported by several laboratories, does not require PI3K activity (28, 29). The role of PI3K in Tiam activation is still controversial (30).
The activation of the transcription factor IRF3 is crucial for the production of type I IFNs. The dormant form of IRF3 is present as a monomer in cytosol. Viral infection and treatment with dsRNA induce phosphorylation of IRF3, leading to its dimerization and eventual activation (31). As reported here, the transcription factor IRF3 was constitutively activated in Tipe2−/− cells but the phosphorylation of IRF3 at serine 396 was not affected by poly (I:C), which is consistent with a previous report (9). Although phosphorylation at serine 396 is not affected by PI3K, PI3K is necessary for the phosphorylation of additional residues of IRF3 and its full activation (9). Poly (I:C)-induced Rac-activation and downstream signaling molecule PAK activation is PI3K-dependent. PI3K inhibitor LY294002 removed the majority of type I IFN and cytokine expression while the dominant negative Rac T17N only partially blocked the cytokine expression in poly (I:C)-stimulated DCs, indicating that PI3K is most likely functioning at the receptor-proximal end while Rac signaling is one of the PI3K downstream events.
Thus, upon ligation of TLR3 by Poly (I:C), TIPE2 level decreases, removing the inhibitory effects on the Rac-TBK1 pathway. The activated TBK1 in turn causes the activation of IRF3 and subsequent expression of cytokine genes. These findings raise additional questions. How does TIPE2 regulate PI3K-Rac pathway? Do they interact with each other directly? What are the roles, if any, of other TIPE2-interacting proteins in innate immunity? Answering these questions will broaden our knowledge of innate immune regulation and help develop strategies to manipulate innate immune responses in humans.
Acknowledgments
This work is supported by grants from the National Institutes of Health, USA (AI-077533, AI-050059, and GM-085112).
References
- 1.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 3.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol Res. 2006;34:33–48. doi: 10.1385/IR:34:1:33. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 10.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, Higashi S, Kawaguchi Y, Enjoji M, Takayanagi R, Kaisho T, Yoshikai Y, Fukui Y. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med. 2010;207:721–730. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo CC, Lin WT, Liang CM, Liang SM. Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J Immunol. 2006;176:5943–5949. doi: 10.4049/jimmunol.176.10.5943. [DOI] [PubMed] [Google Scholar]
- 13.Sun D, Xu D, Zhang B. Rac signaling in tumorigenesis and as target for anticancer drug development. Drug Resist Updat. 2006;9:274–287. doi: 10.1016/j.drup.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 14.William CM, Tanabe Y, Jessell TM. Regulation of motor neuron subtype identity by repressor activity of Mnx class homeodomain proteins. Development. 2003;130:1523–1536. doi: 10.1242/dev.00358. [DOI] [PubMed] [Google Scholar]
- 15.Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S, Chen YH. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell. 2012;45:610–618. doi: 10.1016/j.molcel.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Song L, Fan Y, Li X, Li Y, Chen J, Zhu F, Guo C, Shi Y, Zhang L. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol. 2009;133:422–427. doi: 10.1016/j.clim.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Sriram U, Biswas C, Behrens EM, Dinnall JA, Shivers DK, Monestier M, Argon Y, Gallucci S. IL-4 suppresses dendritic cell response to type I interferons. J Immunol. 2007;179:6446–6455. doi: 10.4049/jimmunol.179.10.6446. [DOI] [PubMed] [Google Scholar]
- 19.Ehrhardt C, Kardinal C, Wurzer WJ, Wolff T, von Eichel-Streiber C, Pleschka S, Planz O, Ludwig S. Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 2004;567:230–238. doi: 10.1016/j.febslet.2004.04.069. [DOI] [PubMed] [Google Scholar]
- 20.Gauzzi MC, Del Corno M, Gessani S. Dissecting TLR3 signalling in dendritic cells. Immunobiology. 2010;215:713–723. doi: 10.1016/j.imbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Dyer JO, Demarco RS, Lundquist EA. Distinct roles of Rac GTPases and the UNC-73/Trio and PIX-1 Rac GTP exchange factors in neuroblast protrusion and migration in C. elegans. Small Gtpases. 2010;1:44–61. doi: 10.4161/sgtp.1.1.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 23.Dinauer MC. Regulation of neutrophil function by Rac GTPases. Curr Opin Hematol. 2003;10:8–15. doi: 10.1097/00062752-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Baumeister MA, Martinu L, Rossman KL, Sondek J, Lemmon MA, Chou MM. Loss of phosphatidylinositol 3-phosphate binding by the C-terminal Tiam-1 pleckstrin homology domain prevents in vivo Rac1 activation without affecting membrane targeting. J Biol Chem. 2003;278:11457–11464. doi: 10.1074/jbc.M211901200. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan FG, Elliot CM, Gibbs M, Exton JH. Translocation of the Rac1 guanine nucleotide exchange factor Tiam1 induced by platelet-derived growth factor and lysophosphatidic acid. J Biol Chem. 2000;275:9742–9748. doi: 10.1074/jbc.275.13.9742. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 30.Welch HC, Coadwell WJ, Stephens LR, Hawkins PT. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546:93–97. doi: 10.1016/s0014-5793(03)00454-x. [DOI] [PubMed] [Google Scholar]
- 31.Yoneyama M, Suhara W, Fujita T. Control of IRF-3 activation by phosphorylation. J Interferon Cytokine Res. 2002;22:73–76. doi: 10.1089/107999002753452674. [DOI] [PubMed] [Google Scholar]