Abstract
Dendritic cells (DC) in the gut promote immune tolerance by expressing retinal dehydrogenase (RALDH), an enzyme that promotes retinoic acid (RA), which aids differentiation of inducible Foxp3+ Treg (iTreg) in the intestinal mucosa. How RALDH expression is regulated is unclear. We found that 4-1BB (CD137), a member of the TNFR family, together with CD103, marked mesenteric lymph node DC with the highest level of RALDH activity, and ligation of 4-1BB maintained RALDH expression in these gut DC. Moreover, 4-1BB signals synergized with those through TLR2 or GM-CSFR to promote RALDH activity in undifferentiated DC. Correspondingly, 4-1BB-deficient mice were impaired in their ability to generate iTreg in the GALT when exposed to oral antigen, and 4-1BB-deficient mesenteric lymph node DC displayed weak RALDH activity and were poor at promoting iTreg development. Thus, our data demonstrate a novel activity of 4-1BB in controlling RALDH expression and the regulatory activity of DC.
Introduction
The mucosa of the gut is the largest surface in the body, continuously exposed to exogenous antigens that may be innocuous (food, commensal bacteria) or pathogenic (invasive bacteria, food allergens) (1, 2). As such, lymphocytes distributed throughout the gut-associated lymphoid tissues (GALT) must remain immunologically hypo-responsive to many antigens. Migratory intestinal dendritic cells (DC), marked by CD103 expression, control immune homeostasis in the GALT through promoting high frequencies of Foxp3+ Treg that suppress Th1 and Th17 populations in effector sites such as the lamina propria (LP) (3, 4). This regulatory DC activity is specifically associated with expression of retinal dehydrogenases (RALDHs or ALDHs) that direct retinoic acid (RA) production (5, 6). RA is made by DC in both Peyer’s patches (PP) and mesenteric lymph nodes (MLN) and can direct T cells to have gut-homing potential by inducing α4β7 and CCR9 (7, 8). Furthermore, RA synergizes with TGF-β in promoting the differentiation of Foxp3+ inducible Treg (iTreg) while suppressing Th17 development (9–11). Although the RA-producing GALT DC have been well characterized in terms of activity, the factors that control the capacity of these DC to express RALDH, and therefore exhibit suppressive function, are not clear.
4-1BB (CD137, TNFRSF9) (12) is a receptor in the TNFR superfamily that is mostly known as a cosignaling molecule promoting the activation and expansion of peripheral effector T cells (13, 14). Although the majority of research on 4-1BB has been directed to T cells, it is becoming clear that this molecule can influence other lineages of cells in both positive and negative manners, exemplified by our recent data showing that 4-1BB expressed on bone marrow progenitors limits myelopoiesis and DC development (15). In further pursuing this latter observation, we found unusual constitutive expression of 4-1BB on mesenteric lymph node (MLN) DC that overlapped to a great extent with CD103. We now show that these 4-1BB positive gut DC expressed the highest levels of RALDH. 4-1BB was also induced on undifferentiated DC through TLR2 and GM-CSFR, molecules that have been implicated in driving the development of RALDH+ regulatory DC. Moreover, signaling through 4-1BB promoted optimal RALDH in these DC and imparted regulatory activity in the cells. Correlating with these observations, Foxp3+ iTreg conversion to oral antigen was dampened in the GALT of 4-1BB-deficient mice, and MLN DC from 4-1BB-deficient mice displayed an attenuated ability to promote iTreg differentiation due to reduced expression of RALDH. These data define a new and novel role for 4-1BB in DC biology.
Materials and Methods
Mice
4-1BB-deficient (16) and OT-II mice on a BL/6 background were bred at LIAI. C57BL/6J (CD45.2+) and C57BL/6Pep3b/BoyJ (CD45.1+) mice were purchased from the Jackson Laboratories. All experiments were in compliance with the regulations of the LIAI animal care committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
Cells
DC from spleens and mesenteric lymph nodes (SDC and MDC, respectively) were isolated by MACS with CD11c-microbeads as described previously (15). Naïve OT-II CD4 T cells were further purified by cell sorting by gating cells as CD4+CD25−CD44loCD62Lhi, which results in negligible contamination of Foxp3+ cells as described previously (17). Lymphocytes were isolated from spleens, lymph nodes, and PP by treatment with collagenase D (Roche) followed by mashing through 70 μM cell strainer. Intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were isolated as described (11).
RALDH induction in spleen DC
2 ~ 3 × 105 SDC were cultured with a cytokine, GM-CSF (10~1000 pg/ml), or TLR2 ligands Zymosan (0.25 ~ 25 μg/ml, Sigma-Aldrich) and Pam-3-cys (0.1 ~ 10 μg/ml, Invivogen) for 48 h. Where indicated, rat IgG (KLH/G1-2-2, 25 μg/ml), anti-4-1BB (3H3, 25 μg/ml), and inhibitors of signaling pathways, were added at the start of culture. For blockade of signaling, the following were used; ERK inhibitor U0126 (5 μM); PI3K inhibitor Ly294002 (5 μM); NFκB inhibitor Bay 11–7082 (1 μM, all from Sigma-Aldrich); Wnt/β-catenin inhibitor XAV939 (5 μM, sc-296704, Santa Cruz Biotechnology). The activity of RALDH was determined by ALDEFLUOR staining as described (18).
In vitro lineage differentiation of CD4+ T cells
1 × 105 naïve OT-II T cells and 2 × 104 DC (MDC or SDC) were cultured in the presence of OVA323–339 peptide (0.1 ~ 10 μM) for four days. In some cultures, SDC were pre-activated with Zymosan (2.5 μg/ml) together with IgG or anti-4-1BB (25 μg/ml) for 48h, washed three times with PBS, and then co-cultured with OT-II cells. Where indicated, exogenous cytokines and reagents were added at the start of culture. T helper cell lineages were determined by intracellular staining for IFN-γ (Th1) and Foxp3 (Treg) after restimulating cells with PMA and Ionomycin (Sigma-Aldrich) for 5 h in the presence of Golgi-Plug (BD).
Oral tolerance and in vivo conversion of Treg
To see the conversion of naïve OT-II cells into Foxp3+ Treg in vivo we exploited an oral tolerance model (6). Briefly, wt or 4-1BB−/− mice (CD45.2+) received naïve OT-II cells (1×106, CD45.1+) and then one day later were challenged with OVA (1 %, Sigma-Aldrich) in the drinking water for five days. Water containing OVA was changed approximately every 36 h. The conversion of Treg was analyzed by intracellular staining of Foxp3 from donor (CD45.1+) and recipient (CD45.2+) CD4 T cells.
Statistics
All statistical analyses were done with two-tailed Student t test. *, P > 0.05, **, P > 0.01, and ***, P > 0.001.
Results and Discussion
4-1BB is expressed on CD103+ regulatory MLN DC
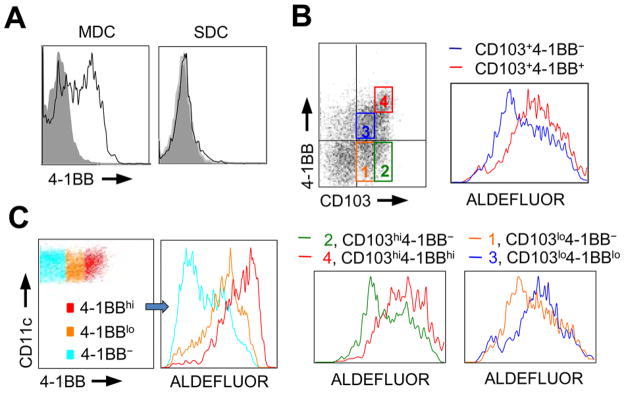
Whereas spleen DC (SDC) do not constitutively express 4-1BB, we found that approximately half of MLN DC (MDC) expressed 4-1BB when analyzed immediately ex-vivo (Figure 1A). Moreover, the 4-1BB+ DC population overlapped with the CD103+ population to a great extent, implying that 4-1BB expression might correlate with RALDH activity and production of RA that has been described to associate with CD103 expression (Figure 1B, top left). To assess this, we used a sensitive flow technique with a fluorescent substrate for aldehyde dehydrogenase (ALDEFLUOR). Fluorescence strongly corresponds with both the level of mRNA for Aldh1a2 (RALDH2) that is expressed in DC, and the ability of DC to promote generation of Foxp3+ iTreg (18, 19).
Figure 1. 4-1BB is expressed on mesenteric lymph node dendritic cells and correlates with RALDH activity.
A, 4-1BB expression on gated CD11c+MHC class IIhi dendritic cells in mesenteric lymph nodes (MDC) and spleen (SDC) isolated from wt mice (8 weeks-old). Shaded histograms represent isotype control. B, MDC stained for CD103 vs. 4-1BB expression (top left). MDC gated into CD103+4-1BB- and CD103+4-1BB+ subsets (top right). MDC gated based on levels of CD103 and 4-1BB (1, orange, CD103lo4-1BB−; 2, green, CD103hi4-1BB−; 3, blue, CD103lo4-1BBlo; 4, red, CD103hi4-1BBhi) (bottom, left and right). RALDH activity was determined by ALDEFLUOR staining (top right, and bottom left and right). C, MDC were stained for 4-1BB, and divided into subsets based on 4-1BB levels (left). RALDH activity was assayed by ALDEFLUOR staining (right) with histograms representing gated subsets: Cyan, 4-1BB−; Orange, 4-1BBlo; Red, 4-1BBhi. Results are representative of three experiments.
Most interestingly, the subset of MDC positive for both CD103 and 4-1BB displayed a higher level of activity of RALDH than CD103+ cells that were negative for 4-1BB (Figure 1B, top right; CD103+4-1BB+ vs. CD103+4-1BB−, 57.1 vs. 38.9 % ALDEFLUOR+, and 19128 vs. 11441 MFI of ALDEFLUOR). To control for RALDH activity, the assay was performed in the presence of diethylaminobenzaldehyde (DEAB), a RALDH inhibitor (not shown). When CD103+ MDC were further subdivided based on the levels of CD103 and 4-1BB, the activity of RALDH was found to be markedly stronger in 4-1BB high or low cells compared to 4-1BB negative cells (Figure 1B, bottom, quadrant gates 1–4; CD103hi4-1BB− vs. CD103hi4-1BBhi, 46.2 vs. 71.9 % ALDEFLUOR+ and 12944 vs. 28097 MFI of ALDEFLUOR; CD103lo4-1BB− vs. CD103lo4-1BBlo, 35.7 vs. 45.1 % ALDEFLUOR+ and 10804 vs. 12222 MFI of ALDEFLUOR). Similarly, gating only on the level of expression of 4-1BB revealed MDC with progressively increasing RALDH activity (Figure 1C; 4-1BB− vs. 4-1BBlo vs. 4-1BBhi, 20.2 vs. 46.8 vs. 62 % ALDEFLUOR+ and 6339 vs. 15265 vs. 20879 MFI of ALDEFLUOR). Thus, 4-1BB is a marker of RALDH activity in migratory GALT DC.
4-1BB signaling maintains and augments RALDH
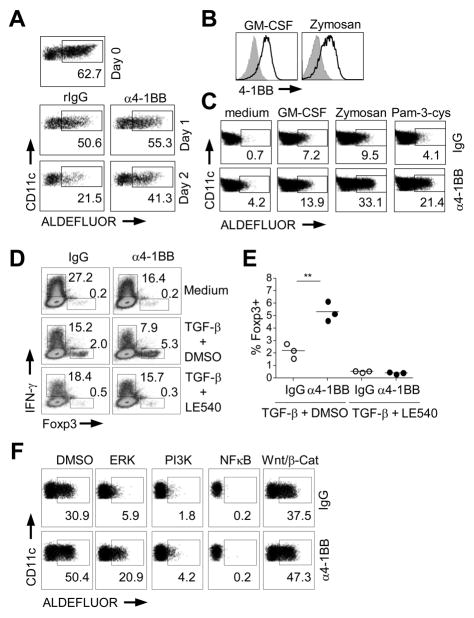
To assess whether 4-1BB signaling might control RALDH expression or activity, MDC were cultured in vitro in the absence of stimulation or were stimulated with an agonist antibody to 4-1BB. Whereas MDC strongly lost RALDH activity over a 48 hr period without further stimulation, triggering 4-1BB allowed a large proportion of the cells to maintain high levels of active RALDH (Fig. 2A).
Figure 2. 4-1BB is a signaling receptor for RALDH induction in DC.
A, MLN DC, isolated by MACS, were cultured for 1 or 2 days in the absence of stimulation with control IgG, or stimulated with an agonist antibody to 4-1BB (25 μg/ml). Activity of RALDH was assayed by ALDEFLUOR staining. Percentages of ALDEFLUOR+ cells are indicated. B–F, MACS- isolated SDC were stimulated with GM-CSF and Zymosan for 48 h. Where indicated, agonist anti-4-1BB (25 μg/ml) or rat IgG was added at the start of culture. B, 4-1BB expression in SDC stimulated with GM-CSF (100 pg/ml) and Zymosan (25 μg/ml). C, Activity of RALDH in SDC was determined by ALDEFLUOR staining when treated with GM-CSF (10 pg/ml), Zymosan (2.5 μg/ml), and Pam-3-cys (1 μg/ml) for 48h. D–E, SDC, pre-treated with Zymosan in the presence of IgG or agonist anti-4-1BB for 48h, were cultured with naïve OT-II CD4 T cells and OVA peptide (0.01 μM) for 4 days. Where indicated, TGF-β (5 ng/ml) and LE540, the pan antagonist of RA receptor, (2 μM) were added. Treg/Th1 development was determined by intracellular staining after restimulation of cells with PMA and ionomycin. Representative flow plot (D) and % Foxp3+ T cells in individual cultures (E) are shown. F, Inhibitors for signaling pathways were added at the start of culture when SDC were stimulated with Zymosan (25 μg/ml). ERK, U0126 (5 μM); PI3K, Ly (5 μM); NFκB (1 μM); Wnt/β-Catenin (5 μM). Results are representative of three experiments.
Regulatory GALT DC are thought to develop or mature in the periphery from RALDH negative precursors in response to GM-CSFR and/or TLR2 signals (18, 20). To potentially mimic this process, and assess whether 4-1BB might participate in such a differentiation event, we cultured SDC that do not constitutively express RALDH or 4-1BB with GM-CSF or Zymosan (a TLR2-ligand). Importantly, 4-1BB was induced when SDC were stimulated through either pathway (Figure 2B). Moreover, when 4-1BB signaling was induced with an agonistic antibody this led to significant up-regulation of RALDH activity compared to that promoted by either GM-CSF or Zymosan alone, although this was more pronounced with Zymosan or another TLR2-ligand Pam-3cys (Figure 2C). The level of RALDH activity induced in SDC with these combinations of stimuli was lower than that observed in MDC isolated ex-vivo, indicating that other factors from the gut environment are also likely to contribute to RALDH expression in GALT DC.
Next, we wanted to confirm that RALDH induced through 4-1BB signaling was functional. SDC, pretreated with Zymosan together with agonist anti-4-1BB, were cultured with naïve OT-II T cells and antigen. Under neutral conditions, naïve Th cells stimulated with Zymosan-treated SDC predominantly differentiated into IFN-γ-producing Th1 cells, while Foxp3 induction and weaker Th1 differentiation was seen in cultures supplemented with exogenous TGF-β (Figure 2D). SDC stimulated through TLR2 and 4-1BB promoted fewer Th1 cells in either culture condition (Figure 2D), and more Foxp3+ T cells in the presence of TGF-β (Figures 2D and 2E). Most importantly, these activities of 4-1BB were dependent on production of RA from the DC as LE540, a pan antagonist of the RA receptor (6, 9), abolished iTreg development and reversed the suppression of Th1 differentiation (Figures 2D and 2E).
4-1BB can trigger several signaling pathways in T cells, notably activation of NF-κB, PI3K/Akt, and ERK1/2 (13, 14). Of potential significance, these intracellular mediators have been reported to be induced by TLR2 and GM-CSFR. We therefore added inhibitors to SDC cultures to determine if these pathways drove RALDH through 4-1BB. Blocking activation of ERK, PI3K, or NF-κB strongly suppressed RALDH activity induced by 4-1BB as well as that induced by TLR2 alone or GM-CSF alone (Figure 2F and not shown). In contrast, manipulation of the Wnt/β-Catenin pathway revealed no significant role in RALDH induction.
Defective Foxp3 iTreg generation to oral antigen in 4-1BB-deficient mice
The expression of RALDH in GALT DC is thought crucial for inducing tolerance against orally encountered antigens by promoting RA production that contributes to the development of Foxp3+ iTreg (5, 6). We then performed oral tolerance experiments in 4-1BB-deficient mice. To assess induction of Foxp3+ Treg, and focus on a potential role of 4-1BB on APC rather than T cells, we adoptively transferred wild-type naïve (Foxp3-CD25−) OVA-specific OT-II T cells (CD45.1+) into 4-1BB-deficient mice and fed the animals OVA in their drinking water. When transferred into wild-type mice, a significant number of T cells strongly upregulated Foxp3 in the GALT after oral antigen treatment (Figures 3A and 3B). In contrast, Treg induction was dramatically attenuated in the MLN, PP, and LPL, when T cells were transferred into 4-1BB−/− mice. Defective Treg generation was specific to the GALT since conversion was moderately increased in the spleen of 4-1BB−/− recipients (Figure 3). This implied that 4-1BB−/− mice possess an environment in the GALT that is less favorable for inducible Treg development, related to attenuated regulatory activity in mucosa-resident APC populations.
Figure 3. Defective iTreg conversion to oral antigen in the GALT of 4-1BB-deficient mice.
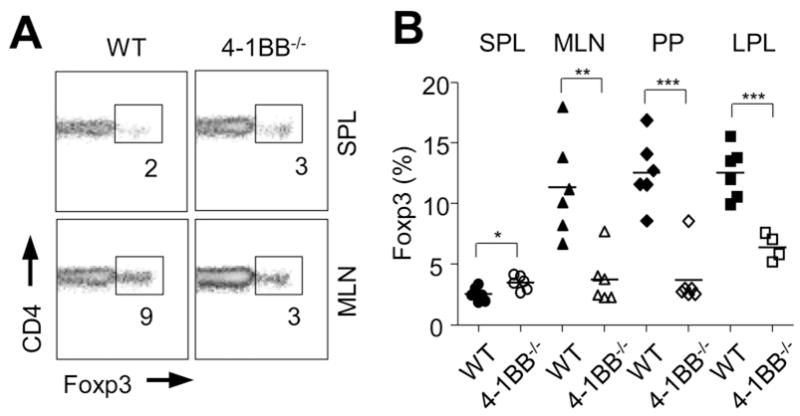
Naïve wt OT-II CD4 T cells (CD45.1+ and CD25-Foxp3−) were adoptively transferred into wt or 4-1BB−/− mice (CD45.2+) that were subsequently given OVA (1%) in the drinking water for five days. Lymphocytes from the indicated organs were analyzed on day 6 for the conversion of naïve OT-II cells into Treg by gating on CD45.1 and staining for intracellular Foxp3. A, concatenate histograms of four individual samples shown in spleen (top) and MLN (bottom). Percentages of Foxp3+ cells in CD4+ populations positive for CD45.1 are shown. B, Percentage of Foxp3+ cells within donor OT-II cells (CD45.1+) in each organ from individual mice. Results are representative of three experiments.
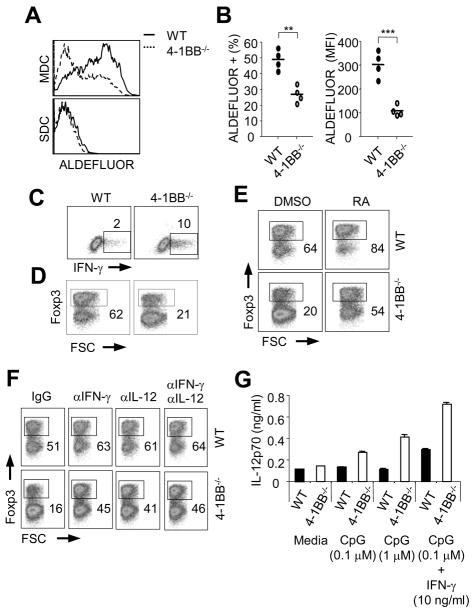
4-1BB-deficient MDC express low levels of RALDH and are poor at inducing Treg
Although DC are believed to be the primary inducer of Treg induced by oral antigens in the GALT, some studies have suggested that macrophages and intestinal epithelial cells may also contribute to the generation and/or the maintenance of these iTreg (21, 22). To then investigate whether the defective Treg induction we observed in 4-1BB-deficient animals was likely related to altered DC activity, we then isolated DC from the mesenteric lymph nodes. This is the main location where naïve CD4 T cells are converted into iTreg with oral antigen (5, 21). Significantly, the percentage of MDC in 4-1BB−/− mice expressing RALDH activity was strongly reduced and the level of activity was much lower than in wt MDC (Figures 4A and 4B). Moreover, the level of active RALDH in 4-1BB−/− MDC was comparable to that observed in 4-1BB negative MDC from wt mice (compare to data in Figure 1). In contrast, SDC from wt or 4-1BB−/− mice were indistinguishable and essentially negative for RALDH (Figure 4A). The reduced activity of RALDH in 4-1BB−/− MDC was paralleled by a lower level of Aldh1a2 (RALDH2) mRNA (Supplemental Fig. 1A) and was not due to decreased numbers of the CD103+ population (Supplemental Fig. 1B).
Figure 4. Defective regulatory function of MLN DC from 4-1BB−/− mice.
A–B, Activity of RALDH in MDC or SDC from wt or 4-1BB−/− mice was determined by ALDEFLUOR staining. A, Representative RALDH activity displayed as histogram of ALDEFLUOR staining in gated CD11c+ class IIhi DC. B, RALDH activity in MDC from individual mice. Left, percent. Right, MFI. C–F, Naïve wt OT-II CD4 T cells (CD25-Foxp3−) were cultured for 4 days with OVA peptide (1 μM) presented on MDC isolated from wt or 4-1BB−/− mice. Cells were cultured without exogenous cytokines (C) or with TGF-β (5 ng/ml, D–F) for four days to determine default Th1 cell generation by intracellular staining of IFN-γ after restimulation of cells with PMA and ionomycin, and Treg differentiation by intracellular Foxp3 staining in CD4+ T cells, respectively. Percent positive indicated. E–F, Where indicated, cells were cultured with retinoic acid (RA, 100 nM) or neutralizing antibodies against IFN-γ and IL-12 (10 μg/ml) added at the start of culture. G, MDC from wt and 4-1BB−/− mice were stimulated as indicated for 24h. IL-12p70 production was measured by ELISA in culture supernatants. Results are representative of two (F–G) and three experiments (A–E), respectively.
MDC from 4-1BB−/− mice did not show any obvious surface phenotypic differences compared to wt MDC, including expression of CD80, CD86, and MHC class II (Supplemental Fig. 1B). 4-1BBL was found on wt MDC at low levels and at higher levels in 4-1BB−/− MDC (Supplemental Fig. 1C), corresponding to our previous results that showed that endogenously expressed 4-1BB antagonizes the expression of 4-1BBL (15). To assess the functional activity of MDC, we cultured naïve wt OT-II CD4 T cells with cells isolated from wt or 4-1BB−/− mice, and determined their relative ability to support Th1 (medium only culture) or Foxp3 iTreg (TGF-β culture) development driven by OVA peptide. MDC from 4-1BB−/− mice induced more Th1 cells than MDC from wt mice (Figure 4C), and most significantly promoted fewer Foxp3+ iTreg (Figure 4D). In contrast, SDC isolated from 4-1BB−/− mice displayed normal activity for the development of Th1 cells and Treg (Supplemental Fig. 2A and 2B).
The defective iTreg-promoting ability of 4-1BB−/− MDC was strongly recovered when RA was added exogenously into culture, suggesting it was largely due to the reduced activity of RALDH (Figure 4E). Treg conversion in the presence of RA was however moderately lower with 4-1BB−/− MDC than with wt MDC, indicating that other factors might also contribute to the aberrant regulatory activity. Neutralization of IFN-γ or IL-12 strongly enhanced the differentiation of iTreg induced by 4-1BB−/− MDC (Figure 4F), but no synergy was observed when both where inhibited, implying that IL-12 was aberrantly expressed in these cultures. In accordance, more IL-12p70 was produced from 4-1BB-deficient MDC when stimulated with CpG DNA (Figure 4G). Interestingly IL-12p70 production was augmented when MDC were co-stimulated with IFN-γ, implying that a feed forward mechanism from IFN-γ produced from responding T cells might also contribute to enhanced IL-12 production. Thus, 4-1BB-deficient MDC display a primary defect in RALDH expression associated with poor Treg-inducing activity, as well as deregulated IL-12 activity.
Collectively, these data suggest that 4-1BB expression on GALT DC is functionally relevant in terms of regulating the expression of active RALDH. This is a novel activity of 4-1BB distinct from its known effects as a costimulatory molecule for T cells.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI42944 and AI85291 to M.C, and NIH grant AI050265 to H.C. This is manuscript #1149 from the La Jolla Institute for Allergy and Immunology.
Abbreviations used
- RALDH
retinal dehydrogenase
- RA
retinoic acid
- GALT
gut-associated lymphoid tissue
- SDC
spleen DC
- MDC
mesenteric lymph node DC
References
- 1.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature reviews Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 2.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nature reviews Immunology. 2010;10:735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 3.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature reviews Immunology. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 10.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. The Journal of experimental medicine. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature immunology. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 12.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunological reviews. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 14.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nature reviews Immunology. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, Croft M. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nature immunology. 2008;9:917–926. doi: 10.1038/ni.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS. Immune responses in 4-1BB (CD137)-deficient mice. Journal of immunology. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 17.Duan W, So T, Mehta AK, Choi H, Croft M. Inducible CD4+LAP+Foxp3-regulatory T cells suppress allergic inflammation. Journal of immunology. 2011;187:6499–6507. doi: 10.4049/jimmunol.1101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song SY, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. International immunology. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 20.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal immunology. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.