Abstract
Cognitive impairments are now widely acknowledged as an important aspect of major depressive disorder (MDD), and it has been proposed that executive function (EF) may be particularly impaired in patients with MDD. However, the existence and nature of EF impairments associated with depression remain strongly debated. While many studies have found significant deficits associated with MDD on neuropsychological measures of EF, others have not, potentially due to low statistical power, task impurity, and diverse patient samples, and there have been no recent, comprehensive, meta-analyses investigating EF in patients with MDD. The current meta-analysis uses random effects models to synthesize 113 previous research studies that compared participants with MDD to healthy control participants on at least one neuropsychological measure of EF. Results of the meta-analysis demonstrate that MDD is reliably associated with impaired performance on neuropsychological measures of EF, with effect sizes ranging from d = 0.32–0.97. While patients with MDD also have slower processing speed, motor slowing alone cannot account for these results. In addition, some evidence suggests that deficits on neuropsychological measures of EF are greater in patients with more severe current depression symptoms, and those taking psychotropic medications, while evidence for effects of age was weaker. The results are consistent with the theory that MDD is associated with broad impairment in multiple aspects of EF. Implications for treatment of MDD and theories of EF are discussed. Future research is needed to establish the specificity and causal link between MDD and EF impairments.
Keywords: executive function, major depressive disorder, meta-analysis
Major depressive disorder (MDD) is one of the most common mental illnesses (with an estimated lifetime prevalence of 16.6%), and is associated with significant impairments in social, occupational, and educational functioning (Kessler, Berglund, et al., 2005). Cognitive impairments are now widely acknowledged as an important aspect of MDD. Indeed, the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria for MDD include “diminished ability to think or concentrate, or indecisiveness” (American Psychiatric Association, 2000). Like this criterion, many theories have posited non-specific impairments in cognitive function associated with MDD, for example low motivation leading to difficulty with effortful tasks (e.g., Weingartner, Cohen, Murphy, Martello, & Gerdt, 1981), depleted cognitive resources in general (e.g., Mathews & MacLeod, 1994), difficulty initiating efficient cognitive strategies (e.g., Hertel & Gerstle, 2003), or slowed processing speed (e.g., Den Hartog, Derix, van Bemmel, Kremer, & Jolles, 2003; Nebes et al., 2000).
More recently, it has been proposed that executive function (EF) may be particularly impaired in individuals with MDD, and that problems in other domains, such as memory, attention, and problem-solving, may arise because these abilities rely heavily on aspects of EF and prefrontal function (Levin, Heller, Mohanty, Herrington, & Miller, 2007; Nitschke, Heller, Imig, McDonald, & Miller, 2004). While EF has been defined in different ways, these definitions all share the idea that EFs are higher-level cognitive processes, which control and regulate lower-level processes (e.g., perception, motor responses) to effortfully guide behavior towards a goal, especially in non-routine situations (e.g., Alvarez & Emory, 2006; Banich, 2009). Thus, EFs are distinct from more automatic cognitive processes that have been over-learned by repetition (e.g., motor, reading, and language skills, semantic memory, object recognition; Shallice & Burgess, 1996). EFs allow us to respond flexibly to the environment: to break out of habits, make decisions and evaluate risks, plan for the future, prioritize and sequence actions, and cope with novel situations, among many other things. In other words, EFs are essential for successfully navigating nearly all of our daily activities. Impairments in EF thus have serious consequences, which may be as important to quality of life and functional outcomes as affective symptoms.
EF appears to be especially vulnerable to disruption, with evidence for EF impairments associated with disorders including schizophrenia (e.g., Fioravanti, Carlone, Vitale, Cinti, & Clare, 2005), attention-deficit/hyperactivity disorder (e.g., Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), and obsessive-compulsive disorder (e.g., Olley, Malhi, & Sachdev, 2007), among others. Thus, it has been proposed that EF deficits may be transdiagnostic intermediate phenotypes or risk factors for emotional, behavioral, and psychotic disorders (Nolen-Hoeksema & Watkins, 2011). However, the existence and nature of EF impairments associated with MDD remain strongly debated, with some arguing that patients with MDD have no appreciable impairments in cognition (e.g., Grant, Thase, & Sweeney, 2001) and others that they have pronounced neuropsychological impairments (e.g., Porter, Gallagher, Thompson, & Young, 2003). Addressing this debate will be critical not only for understanding cognitive symptoms associated with MDD, but also for clarifying the degree to which EF deficits span disorders.
Executive and Prefrontal Function
Many specific components of EF have been proposed as scientists build a broad concept of EF, including creating, maintaining, and switching between task goals; sequencing behavior; inhibiting habitual behaviors (prepotent responses) and distracting information; decision-making; and selecting among competing options, among many others (e.g., Aron, 2008; Badre & Wagner, 2007; Banich, 2009; Miyake, et al., 2000; Thompson-Schill, Bedny, & Goldberg, 2005). Across models of EF, it is nearly universally recognized that while there is a unitary aspect of EF, a number of these components are also behaviorally, genetically, and neurally dissociable (e.g., Baddeley, 1996; Collette et al., 2005, Friedman et al., 2008; Miyake et al., 2000).
One influential model of EF, the three-component model (Friedman, et al., 2008; Miyake, et al., 2000) describes three key aspects of EF: (1) updating (adding relevant and removing no longer relevant information from working memory), (2) shifting between tasks or mental sets, and (3) inhibiting prepotent responses, as well as a common EF component tapped by all EF tasks (and which may subsume inhibition, Friedman et al., 2008). Multiple studies have found that while aspects of EF are moderately correlated (i.e., share a common EF component), they are separable (i.e., have unique components; e.g., Fisk & Sharp, 2004; Friedman et al., 2006; Hedden & Yoon, 2006; Huizinga, Dolan, & van der Molen, 2006; Miyake et al., 2000; Willcutt et al., 2001). Importantly, EF components are also differentially associated with aspects of psychopathology and cognition. For example, poor common EF and inhibition predict attention, conduct, and substance use problems in adolescents (Friedman et al., 2007; Young et al., 2009), while only updating predicts IQ (Friedman et al., 2006). Given these dissociations, and the unique cognitive processes, genetic influences, and neural substrates supporting different aspects of EF, it is important to consider both specific EF domains and what is common across them.
Components of EF
While updating, shifting, and inhibition are important aspects of EF, this model in no way posits that these are the only components. Indeed, several other EF domains have been well defined in the literature, including verbal and visuospatial working memory, planning, and verbal fluency. In each domain, studies using latent variable and correlational approaches have provided support for the existence of correlated but separable EF components, and confirmed that tasks posited to tap each aspect of EF are related to one another (Table 1).
Table 1.
Commonly Used Neuropsychological Measures of Executive Function (EF) and their Construct Validity
| EF Component | Task | Description | Construct Validitya | Prefrontal Involvement | Other Cognitive Demandsb |
|---|---|---|---|---|---|
| Shifting | Wisconsin Card Sorting Test (WCST) | Learn from feedback to sort cards by one dimension (e.g., color), and then switch to a different dimension (e.g., shape) when given negative feedback on the first dimension (repeats with multiple sorting rules). | Loads on a shifting latent variable (Miyake et al., 2000) | VLPFC, medial PFC, ACC (meta-analysis, Buchsbaum, Greer, Chang, & Berman, 2005) | Learning from feedback, visual processing, numerical processing, categorization, rule induction, working memory for current rule. |
| Trail Making Test Part B | Alternately connect letters and numbers in sequence (A-1-B-2 etc.) Often compared to Trail Making A (connect letters or numbers only, does not require shifting). |
Correlates with switch-cost on task shifting tasks Arbuthnott & Frank, 2000), and WCST (Sanchez-Cubillo et al., 2009) | VLPFC, DLPFC, medial PFC, ACC (TMT-B > TMT-A; e.g., Jacobson, Blanchard, Connolly, Cannon, & Garavan, 2011; Moll, de Oliveira-Souza, Moll, Bramati, & Andreiuolo, 2002; Zakzanis, Mraz, & Graham, 2005). | Visual search, motor speed, sequencing, working memory for task rule. | |
| Intradimensional/Extradimensional Shift | Learn from feedback to select a stimulus based on one dimension, switch to the previously non-rewarded stimulus (intradimensional shift), then to a different stimulus dimension (extradimensional shift). | Designed as analog to WCST, correlates with WCST (Jazbec et al., 2007). | VLPFC, DLPFC (e.g., Hampshire & Owen, 2005). | Reversal learning, feedback learning, visual processing, categorization, rule induction, working memory for current rule. | |
| Inhibition | Color-Word Stroop | Identify the color ink a color word is printed in. Trials are incongruent (e.g., “red” written in blue ink) and congruent (e.g., “red” written in red ink) or neutral (non-color word). | Loads on inhibition latent variables (e.g., Friedman et al., 2008; Miyake et al., 2000) | VLPFC, DLPFC, medial PFC, ACC (meta-analysis; Nee at al., 2007). | Visual processing, language production, working memory for task rule. |
| Hayling | Read sentences where the final word is omitted but highly predictable. First complete sentences correctly (Part A), then with an unrelated word (part B). | Loads on an inhibition latent variable with the Stroop task (de Fries, Dixon, & Strauss, 2009) | VLPFC, DLPFC (e.g., Allen et al., 2008; Collette et al., 2001, Nathaniel-James & Frith, 2002). | Reading, language production, working memory for task rules, selection among options. | |
| Updating | n-Back | Indicate if the stimulus (usually letter) matches the stimulus n (e.g., 3) items back. | Loads on updating latent variable (Friedman et al., 2008) | VLPFC, DLPFC, anterior PFC, ACC (meta-analyses; McMillan, Laird, Witt & Meyerand, 2007; Owen, McMillan, Laird, & Bullmore, 2005). | Visual processing, sequencing |
| Visuospatial Working Memory | Corsi Block Tapping/Spatial Span | Tap irregularly arranged blocks/squares in the same order as experimenter (Corsi blocks) or computer (Spatial Span). | Loads on visuospatial working memory latent variable (e.g., Fournier-Vicente et al., 2008; Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001) | VLPFC, DLPFC (e.g., Owen, Evans, & Petrides, 1996; Toepper et al., 2010). | Visual and motor processing, sequencing. |
| Delayed-Match-to-Sample | View a complex shape (the sample), then indicate after a delay if a probe matches the sample. | Loads on latent variable with other visual memory tasks (Robbins et al., 1994). | VLPFC, DLPFC, ACC (e.g., Kaiser et al., 2010; Schon, Quiroz, Hasselmo, & Stern, 2009). | Visual processing. | |
| Self-Ordered Pointing | Search an array of boxes for hidden tokens. Token is only in each location once. | Loads on latent variable with spatial span (Robbins et al., 1998), correlates with spatial span (Ross, Hanouskova, Giarla, Calhoun, & Tucker, 2007). | VLPFC, DLPFC, medial PFC, ACC (e.g., Owen, et al., 1996; Provost, Petrides, & Monchi, 2010 | Visual search, strategy formation, reversal learning. | |
| Verbal Working Memory | Forward Digit Span | Repeat sequence of numbers in the same order presented. | Loads on latent variables with other verbal working memory tasks (e.g., Kane et al., 2004; Mertens, Gagnon, Coulombe, & Messier, 2006; Roberts, Stankov, Pallier, & Dolph, 1997). | VLPFC, DLPFC, medial PFC/ACC (e.g., Gerton et al., 2004; Owen, Lee, & Williams, 2000) | Auditory or visual processing, language production, sequencing. |
| Backward Digit Span | Repeat sequence of numbers in the reverse order presented. | Loads on latent variables with other verbal working memory tasks (e.g., Fournier-Vicente et al., 2008; Mertens et al., 2006; Waters & Caplan, 2003) | VLPFC, DLPFC, medial PFC, ACC (e.g., Gerton et al., 2004; Owen et al., 2000) | Auditory or visual processing, language production, sequencing. | |
| Planning | Tower of London/Stockings of Cambridge | Move rings on pegs from a starting position to a target position in as few moves as possible, following a set of rules. | Correlates with Tower of Hanoi planning task (Welsh & Huizinga, 2001; Welsh, Satterlee-Cartmell, & Stine, 1999) | VLPFC, DLPFC, anterior PFC (e.g., Kaller, Rahm, Spreer, Weiller, & Unterrainer, 2011; Wagner, Koch, Reichenbach, Sauer, & Schlösser, 2006) | Visual processing, sequencing, working memory for task rules. |
| Verbal Fluency | Phonemic Verbal Fluency | Say as many items starting with a certain letter (usually F, A, S) as possible in 1 (or 3) min. | Loads on verbal fluency latent variable (Unsworth, Spillers, & Brewer, 2011) | VLPFC, DLPFC, ACC (meta-analysis; Costafreda et al., 2009). | Vocabulary, language production, memory retrieval, strategy formation |
| Semantic Verbal Fluency | Say as many words from a semantic category (e.g., animals) as possible in 1 (or 3) min. | Loads on verbal fluency latent variable (Unsworth et al., 2011) | VLPFC, DLPFC, ACC (e.g., Hirshorn & Thompson-Schill, 2006; Whitney et al., 2009 | Semantic knowledge, semantic memory retrieval, language production, strategy formation |
Note. PFC = prefrontal cortex; VLPFC = ventrolateral prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex.
Examples of evidence that each task is related to other tasks within the relevant EF component.
Examples of some other cognitive demands imposed by each task (there may be other demands not listed).
Updating is defined as monitoring and coding incoming information for task-relevance, and replacing no longer relevant information with newer, more relevant information (Miyake et al., 2000). The most common updating task in the MDD literature is the n-back task, in which participants indicate if the stimulus (usually a letter or number) matches the stimulus n (e.g., 3) items back. The dependent measures are reaction time and accuracy.
Shifting is defined as switching between task sets or response rules (Miyake et al., 2000). The most common shifting tasks in the MDD literature are the Wisconsin Card Sorting Test, the Trail Making Test part B, and the Intradimensional/Extradimensional Shift task. In the Wisconsin Card Sorting Test (Berg, 1948; Strauss, Sherman, & Spreen, 2006), participants sort cards by one dimension (e.g., color), and then switch to a different dimension (e.g., shape) when given negative feedback. This process repeats with multiple sorting rules. The measures of shifting are perseverative errors (sorting by the old rule) and category sets achieved (number of successful switches). The Trail Making Test part B (Partington & Leiter, 1949; Strauss et al., 2006) requires alternately connecting letters and numbers (A-1-B-2 etc.). It is often compared to the Trail Making Test part A, which does not require switching (connecting letters or numbers only). In the standard version of the task, the tester points out errors immediately, and the participant must correct them before going on. Thus, the dependent measure, total time, reflects a combination of slow and error-prone performance. The Intradimensional/Extradimensional Shift task (Robbins et al., 1998) requires learning from feedback to select a stimulus based on one dimension, switching to the previously non-rewarded stimulus (intradimensional shift), and then switching to a different stimulus dimension (extradimensional shift). Dependent variables include the number of trials needed to switch and number of switches achieved.
Inhibition is defined as suppressing or avoiding a prepotent (automatic) response in order to make a less automatic but task-relevant response (Miyake et al., 2000). The most common inhibition task in the MDD literature is the color-word Stroop task (Strauss et al., 2006; Stroop, 1935), in which participants name the color of the ink that color words are printed in (e.g., the word blue printed in red ink), overriding the automatic response of reading the word. This incongruent condition is compared to a neutral condition in which participants name ink colors in the absence of conflicting color word information. Dependent measures include time to complete the incongruent condition, the difference in the time required to complete the incongruent and neutral condition (interference), and accuracy on the incongruent condition.
Working memory is defined as actively maintaining (i.e., ‘holding on line’) or manipulating information across a short delay, and can be divided into verbal (e.g., words, letters, and numbers) and visuospatial (e.g., shapes, patterns, and spatial locations) components (e.g., Baddeley, 1992, 1996; Repovs & Baddeley, 2006). The most common verbal working memory tasks in the MDD literature are forward and backward digit span, in which participants hear a sequence of numbers and repeat it in forward or reverse order. The dependent measure is the participant's span, which is the longest sequence successfully repeated. The most common visuospatial tasks are spatial span, delayed-match-to-sample, and self-ordered pointing. In the spatial span task (also known as the Corsi block tapping or block span task; e.g., Strauss, et al., 2006) participants watch a pattern of taps on irregularly arranged blocks/squares and repeat it in the same order (forward span) or reverse order (backward span). The dependent measure is the participant's span (longest correct sequence). In the delayed-match-to-sample task participants maintain a complex shape in working memory across a delay (4-12 seconds) and then indicate if a probe stimulus matches it. Dependent measures are reaction time and accuracy. In the self-ordered pointing task (Owen, Downes, Sahakian, Polkey, & Robbins, 1990; also known as the spatial working memory task in the CANTAB; Robbins et al., 1998), participants search an array of boxes/images for hidden tokens. The primary dependent measure is between-search errors, when the participant returns to a previously searched location.
Planning is defined as identifying and organizing a sequence of steps to achieve a goal (e.g., Lezak, Howieson, & Loring, 2004). Planning tasks involve multiple cognitive demands (e.g., Goel & Grafman, 1995) and so may not represent a single EF ability. However, they are frequently used in clinical studies, perhaps because this complexity may be seen as a benefit for relating laboratory task performance to complex real-world tasks. The most common planning task in the MDD literature is the Tower of London, in which participants move beads across pegs from a starting position to target position in as few moves as possible (Shallice, 1982). Dependent measures include the number of moves needed to reach the target position and the number of problems solved in the minimum number of moves.
Verbal fluency is defined as the ability to generate words in a limited period of time, from semantic categories (semantic verbal fluency; e.g., animals) or starting with certain letters (phonemic verbal fluency; e.g., Troyer, Moscovitch, & Winocur, 1997). The dependent measure reported is the number of words generated. Like planning, verbal fluency tasks likely tap multiple cognitive processes (e.g., Rende, Ramsberger, & Miyake, 2002). However, they form a distinct component separable from other EF components (Fisk & Sharp, 2004), depend on prefrontal function (e.g., Alvarez & Emory, 2006), and are widely used in the clinical literature.
What is common across EF measures?
Both theoretical perspectives and empirical evidence suggest that along with these specific components of EF, there is also a common mechanism across EFs (e.g., Duncan & Owen, 2000; Engle, Tuholski, Laughlin, & Conway, 1999; Friedman et al., 2008; Miyake et al., 2000), which is separable from perceptual speed and fluid intelligence (Friedman et al., 2008). This common mechanism is hypothesized to be the ability to maintain goal and context information in working memory (Miyake et al., 2000). This view is compatible with accounts of EF that view the central role of the frontal lobes to be active maintenance of goals, plans, and other task-relevant information in working memory (e.g., Engle et al., 1999; Hazy, Frank, & O'Reilly, 2007). Thus, the ability to keep task-relevant information active in working memory may be essential for all aspects of EF (Miyake et al., 2000).
Prefrontal cortex and EF
‘Frontal lobe tasks’ and EF are often used synonymously in the literature, and indeed EF relies heavily on prefrontal cortex (PFC), although EF tasks also recruit broader neural networks, including posterior cortical and subcortical areas, and connectivity between these regions. Neuroimaging research in healthy individuals demonstrates that all of the neuropsychological measures of EF included in the current meta-analysis activate PFC (Table 1). Neuroimaging methods (fMRI and PET) provide powerful, non-invasive measures of brain function during EF tasks, by measuring hemodynamic correlates of neural activity. These methods can provide important insight into the mechanisms underlying EF deficits in MDD, by making contact with the wider cognitive neuroscience literature.
Multiple theories have been proposed for the organization of PFC and the role of different PFC regions in EF (e.g., Badre, 2008; Banich, 2009; Christoff & Gabrieli, 2000; Duncan & Owen, 2000; Petrides, 2005; Stuss & Alexander, 2007). A full discussion of these theories is beyond the scope of the current paper, but the PFC neuroanatomy relevant to understanding neuroimaging findings in patients with MDD is briefly described here. Although many neural areas have been implicated in EF, across multiple theories and empirical studies, three main subdivisions of PFC emerge as key for EF: dorsolateral PFC (DLPFC), ventrolateral PFC (VLPFC) and anterior cingulate cortex (ACC). For many different EF tasks, there is joint recruitment of these regions (e.g., Duncan & Owen, 2000). Meta-analyses of neuroimaging studies have found reliable activation of DLPFC, VLPFC, and dorsal ACC for inhibition (Nee, Wager, & Jonides, 2007), shifting (Wager, Jonides, & Reading, 2004), working memory (Wager & Smith, 2003), and verbal fluency (Costafreda, David & Brammer, 2009), while a qualitative review concluded that these regions were also active for planning (Collette et al., 2006).
However, different EF components have also been found to recruit some unique neural substrates (e.g., Collette et al., 2005; Sylvester et al., 2003), with updating and inhibition associated with more anterior prefrontal areas than shifting. Within the domain of working memory, meta-analytic evidence suggests that verbal working memory more consistently activates left PFC, while visuospatial working memory more consistently activates right PFC (Wager & Smith, 2003). Additionally, manipulating items held in working memory is associated with VLPFC activation, while updating the contents of working memory is associated with DLPFC activation (Wager & Smith, 2003). Thus, as for behavioral performance, there are both shared and unique neural substrates for different components of EF.
Is EF Impaired in Patients with MDD?
MDD is associated with structural and functional abnormalities in PFC, including DLPFC, VLPFC and ACC (for reviews see Levin et al., 2007; Rogers et al., 2004), and a meta-analysis found that patients with depressive disorders had decreased DLPFC and ACC activation during a resting state (Fitzgerald, Laird, Maller, & Daskalakis, 2008). This prefrontal hypoactivity may be related to reduced levels of the main excitatory neurotransmitter, glutamate, associated with MDD (for a review, see Yüksel & Ongur, 2010). As discussed in the previous section, the PFC regions that are hypoactive in MDD are implicated in multiple aspects of EF. Thus, it has been posited that impaired PFC function in MDD may lead to broad impairment in EF (e.g., Davidson, Pizzagalli, Nitschke, & Putnam, 2002). Specifically, decreased PFC function may lead to decreased goal setting and ability to override established behaviors, and subsequent decreases in the formation of organizational strategies for action in patients with MDD (avolition; Nitschke & Mackiewicz, 2005). This theory is compatible with the view that common EF, conceptualized as maintaining task-relevant information in working memory, may be impaired in patients with MDD, leading to deficits across all aspects of EF.
However, evidence for EF impairments associated with MDD is mixed. While many studies have reported significant deficits on many neuropsychological measures of EF, others have reported no significant differences between patients with MDD and healthy control participants. Authors have consequently reached a wide range of conclusions about the association between MDD and EF, from no appreciable impairments in cognitive functioning (e.g., Grant et al. , 2001) to pronounced neuropsychological impairment (e.g., Porter et al., 2003). Several recent reviews have reported partial support for impairments across multiple aspects of EF, including shifting, inhibition, working memory, planning, and verbal fluency (DeBattista, 2005; Hammar & Ardal, 2009; Ottowitz, Dougherty, & Savage, 2002; Rogers et al., 2004).
Previous meta-analyses also present mixed conclusions. While there have been no recent, comprehensive meta-analyses investigating neuropsychological measures of EF in MDD, previous meta-analyses found mixed results, based on a small number of studies. One reported significant impairments for patients with MDD on verbal fluency (semantic verbal fluency, d = 0.97, k = 2; phonemic verbal fluency, d = 0.61, k = 7), and inhibition (Stroop, d = 0.69, k = 2), but not shifting (Trail Making Test part B, d = 0.77, k = 5; Wisconsin Card Sorting Test, d = 0.32, k = 3), or verbal working memory (backward digit span, d = 0.32, k = 10; Zakzanis, Leach, & Kaplan, 1998). Another found reliable deficits on verbal fluency (phonemic verbal fluency, d = 0.55, k = 3) and ‘mental flexibility and control’ (Trail Making Test part B and Stroop, d = 1.31, k = 3), but not working memory (digit span and spatial span, d = 0.18, k = 3; Veiel, 1997). Two additional meta-analyses examined EF in mood disorders more broadly, but did not provide separate analyses for patients with MDD. One found broad impairments in verbal working memory (forward digit span, d = 0.37, k = 9; backward digit span, d = 0.39, k = 8), verbal fluency (phonemic verbal fluency, d = 0.64, k = 14; semantic verbal fluency, d = 1.07, k = 4), shifting (Trailing Making Test part B, d = 0.66, k = 5), and inhibition (Stroop, d = 1.00, k = 5; Christensen, Griffiths, Mackinnon, & Jacomb, 1997). A second meta-analysis primarily investigated verbal fluency and found significant effects for both phonemic (d = 0.30, k = 53) and semantic (d = 0.44, k = 15) fluency, with a larger effect for semantic fluency (Henry & Crawford, 2005). Within studies reporting verbal fluency, other tasks were also analyzed, with small but significant effects for shifting (Wisconsin Card Sorting Test, d = 0.27, k = 14) and inhibition (Stroop, d = 0.17, k = 11). Thus, while there has been extensive investigation of EF in patients with MDD, divergent results and a lack of recent, comprehensive meta-analyses limit the conclusions that can be drawn.
One source of mixed findings in the literature may be methodological factors, including low statistical power, the use of only one or a few neuropsychological tasks to assess EF, and failure to control for non-EF impairments. First, many studies have fewer than 30 MDD participants, which provides adequate power for detecting only large effect sizes, not more realistically moderate effects (e.g., Cohen, 1992). Most of these studies report at least one null result, which is often interpreted as showing a lack of impairment, when lack of power is a viable alternative explanation. Meta-analysis is well suited to address low power, because even results that were non-significant in the original study can contribute to a significant effect across studies.
Second, many studies use only a small number of neuropsychological measures of EF. This approach is problematic because using single tasks creates a task impurity problem, which is particularly pervasive in studies of EF (Burgess, 1997; Phillips, 1997). This is because EFs necessarily operate on other cognitive processes (e.g., shifting occurs between tasks such as identifying colors and shapes, which require non-EF abilities like visual processing; Miyake et al., 2000). Thus, a low score on a single EF task does not necessarily mean impaired EF– it could instead be due to impairment on other aspects of the task. This problem can be addressed by investigating performance on tasks that have the target component (e.g., updating) in common, but have very different non-EF aspects (e.g., the nature of the information to be updated); thus, while each task suffers from the task impurity problem, what they have in common is only the EF component of interest (Miyake et al., 2000). Ideally, more latent variable studies are needed which extract only the variance shared across multiple measures of each EF component. However, in practice such studies may be difficult to conduct with clinical populations, as it may be unfeasible to recruit a sufficiently large sample for latent-variable analysis, and patients may be unwilling or unable to complete long testing sessions. Meta-analysis provides an alternative, if imperfect, solution. Although multiple tasks may not be used by each study, multiple tasks are used across studies. Thus, effect sizes can be compared across tasks that share a target EF component but differ in other task demands.
Finally, many studies fail to control for non-EF aspects of cognition. Using multiple measures designed to tap specific aspects of EF is a critical step in establishing the specificity of EF impairments in MDD. However, it may not fully accomplish this, since some non-EF abilities, such as processing speed, may be shared across EF measures. Thus, this problem must be addressed by including additional control conditions within EF tasks and/or separate control tasks. Control conditions within tasks share the non-EF demands of the task, differing only in the key EF demand. Since it is difficult to include control conditions for tasks tapping certain EF components, it may be necessary to include separate control tasks as well. For example, psychomotor speed tasks, which are thought not to rely on EF, may be appropriate control tasks.
Are EF Deficits Moderated by Clinical and Demographic Factors?
An additional source of variance leading to mixed results across studies may be the diversity of MDD patient samples, including variability in current depression symptom severity, psychotropic medication use, age, and comorbidity.
Depression symptom severity
There is some evidence that EF impairment is greater in patients with more severe depressive symptoms (McClintock, Husain, Greer, & Cullum, 2010; McDermott & Ebmeier, 2009). A recent meta-analysis found a significant correlation between depression severity and performance on neuropsychological measures of EF (McDermott & Ebmeier, 2009). However, this meta-analysis only included the small number of studies (k = 10) that conducted correlation analyses between depression severity and task performance, rather than investigating associations with depression severity across studies. In addition, it did not examine specific aspects of EF separately, and included studies of patients with minor depression. Thus, it is uncertain whether these findings apply to MDD specifically, and whether depression symptom severity predicts performance on all aspects of EF equally. Moreover, some studies have failed to find a relationship between depression symptom severity and EF impairments (e.g., Harvey et al., 2004; Porter et al., 2007). Likewise, while some studies have found improvements in one aspect of EF, verbal fluency, as depression symptoms improved (Beblo, Baumann, Bogerts, Wallesch, & Herman, 1999; Reppermund, Ising, Lucae, & Zihl, 2009; Trichard et al., 1995), studies of other aspects of EF have found relatively stable impairments (Biringer et al., 2005; Trichard et al., 1995). Thus, it remains unclear whether impairments in some or all aspects of EF are sensitive to the current level of depression symptomatology or represent stable traits independent of current depression severity.
Medication
Cognitive deficits in depression are not merely an artifact of drug side effects, as a number of studies have found significant EF impairment in medication-free participants (Hinkelmann et al., 2009; Merriam, Thase, Haas, Keshavan, & Sweeney, 1999; Porter, Gallagher, Thompson, & Young, 2003; Tavares et al., 2007) and medication-naïve adolescents (Cataldo, Nobile, Lorusso, Battaglia, & Molteni, 2005; Matthews, Coghill, & Rhodes, 2008). However, some evidence suggests that long-term or repeated use of some antidepressant medications may impair cognitive function (McClintock et al., 2010). Tricyclic and tetracyclic antidepressants may produce larger impairments than selective serotonin re-uptake inhibitors (SSRIs) or monoamine oxidase inhibitors (Porter et al., 2007), although there is some evidence for negative cognitive effects of SSRIs with anticholinergic or antihistamine actions (Lane & O'Hanlon, 1999).
Age
Age-related declines in brain function are most pronounced for PFC (e.g., Fuster, 1989; Woodruff-Pak, 1997), and performance on neuropsychological measures of EF declines with age (e.g., Bryan & Luszcz, 2000; Cepeda, Kramer, & de Sather, 2001; Salthouse, Atkinson, & Berish, 2003; Troyer et al., 1997). Thus, it seems possible that depression and age may have superadditive effects on EF, leading to more pronounced EF deficits in depressed older adults. Indeed, some researchers have argued, based on reviews of the literature, that MDD is more reliably related to cognitive deficits in elderly patients than in young adults (Elliott, 1998; Porter et al., 2007). However, this possibility has not been systematically tested, with few studies directly comparing age groups. Two studies that did compare younger and older adults with MDD found greater impairment in older MDD patients on some neuropsychological measures of EF but not others (Lockwood, Alexopoulos, & van Gorp, 2002; Nakano et al., 2008). Thus, it remains unclear whether age and depression are merely independently associated with poor EF, or whether they interact to produce larger EF deficits in depressed older adults.
Comorbidity
Nearly 60% of patients with MDD also meet criteria for at least one anxiety disorder (Kessler, Chiu, Demler, & Walters, 2005). These high rates of comorbidity pose special problems for research (and research reviews). Although findings are mixed, evidence suggests that trait anxiety and anxiety disorders are associated with impairments on neuropsychological measures of EF (e.g., Castaneda, Tuulio-Henriksson, & Marttunen, 2008; Eysenck & Derakshan, 2011; Olley et al., 2007; Snyder et al., 2010). Thus, it is possible that some deficits attributed to MDD could actually be due to a comorbid disorder (e.g., an anxiety disorder), or co-occurring disorders may contribute additively or interactively to EF impairments. For example, some studies found that only depressed patients with comorbid anxiety had impaired EF (Basso, et al., 2007; Lyche, Jonassen, Stiles, Ulleberg, & Landrø, 2011). In other cases, comorbid anxiety may mask the effect of depression (e.g., Engels, et al., 2010; Keller, et al., 2000). Many studies fail to assess or control for comorbidity, and few studies have directly investigated the effects of comorbid anxiety and depression. However, some studies have excluded participants with comorbid Axis I disorders (including anxiety disorders), while others have not. Thus, while it is not possible to fully address the issue of comorbidity given the current literature, a meta-analytic approach may provide some insight. For example, if only studies that include participants with comorbid Axis I disorders show deficits on neuropsychological measures of EF, it would suggest that comorbid disorders, rather than depression per se, are associated with EF deficits in patients with MDD.
Can EF Impairments be Explained by Deficits in Psychomotor Speed?
Psychomotor retardation, which is related to the cognitive concept of processing speed, is an important symptom of MDD (although it is not required for a diagnosis of MDD, and psychomotor agitation may also occur). Manifestations of psychomotor retardation may include slowed movements, reduced speaking rate, and delayed motor initiation (Caligiuri & Ellwanger, 2000). Some have therefore proposed that impairments on neuropsychological measures of EF associated with MDD might actually be due to slowed processing speed.
The motor slowing hypothesis posits that depression causes motor slowing, independent of higher cognitive processes (which may also be impaired; e.g., Sabbe, Hulstijn, van Hoof, Tuynman-Qua, & Zitman, 1999; van Hoof, Jogems-Kosterman, Sabbe, Zitman, & Hulstijn, 1998). Supporting this theory, studies have found significant motor slowing for MDD patients (who were not pre-selected on the basis of psychomotor retardation) on tasks with minimal higher-level cognitive demands, such as pointing at a target (Caligiuri & Ellwanger, 2000) and drawing simple lines (Pier, Hulstijn, & Sabbe, 2004; Sabbe et al., 1999). This hypothesis implies that motor slowing in MDD may account for deficits on speeded tasks or reaction time measures, while impairment of higher-level cognitive processes might affect both timed and untimed tasks.
A broader theory, the cognitive speed hypothesis, has also been proposed. However, in its current form it is not empirically falsifiable, and thus is only briefly discussed here. The cognitive speed hypothesis posits that the rate of processing limits performance on higher-level operations because if processing steps are carried out too slowly the products of earlier operations may be lost or no longer relevant by the time later operations occur (Nebes et al., 2000). This theory therefore posits that the effect of processing speed is not restricted to timed or speeded tasks, and could result in overall decrements in task performance. Thus, it is not clear what would constitute definitive evidence against the cognitive speed hypothesis. Since cognitive slowing is posited to affect even untimed and unspeeded tasks, impairments on self-paced accuracy measures of EF would not be considered evidence against this hypothesis. Nor would greater impairment on EF tasks than processing speed tasks, since it is always possible to argue that more complex tasks may require more processing steps, and are thus more affected by cognitive slowing. Thus, while the motor speed hypothesis can be empirically evaluated (including by meta-analyses), evaluation of the cognitive speed hypothesis must await more complete specification of the theory in a way that makes it empirically falsifiable.
Objectives of the Current Meta-Analysis
The current meta-analysis synthesizes research findings to addresses three central questions: (1) whether neuropsychological measures of EF are reliably impaired in patients with MDD compared to healthy control participants, (2) whether apparent EF impairments can be explained by deficits in psychomotor speed, and (3) whether deficits on neuropsychological measures of EF associated with MDD are modulated by clinical and demographic factors. To address the first question, the meta-analysis tests whether there is reliable cumulative evidence across studies for impairments on neuropsychological measures of EF in patients with MDD compared to healthy control participants, despite mixed results from individual studies. Furthermore, the current meta-analysis evaluates whether deficits on measures designed to tap particular aspects of EF are consistent across tasks, providing evidence that impairments are not task-specific. To address the second question, the meta-analysis compares effect sizes for psychomotor speed tasks with those for neuropsychological measures of EF, and evaluates impairment on self-paced, accuracy measures of EF, which provide evidence of higher-level deficits not caused by motor slowing. To address the third question, the meta-analysis includes the following moderator variables: current depression symptom severity and remission status, medication (percentage of patients receiving psychotropic medications at the time of testing), patient age, and whether patients with comorbid Axis I disorders were excluded from the study.
The current meta-analysis is limited to patients with MDD in order to reduce heterogeneity across patient samples. In comparing patients with MDD to healthy control participants, the objective is not to identify deficits specific to MDD, but rather to clarify the pattern of impairments on neuropsychological measures of EF associated with MDD. This is the first step towards understanding a clinically significant problem and provides a foundation for future work to identify which aspects of EF impairment may be specifically associated with MDD and which may represent transdiagnostic features of psychopathology.
Methods
Inclusion and Exclusion Criteria
Studies were required to include a patient group with a diagnosis of MDD or major depressive episode, and a healthy control group with no diagnosed psychopathology. Patients could be currently experiencing an episode of depression or in remission at the time of testing. Studies were excluded if they reported only mixed diagnostic groups (e.g., all mood disorders), or if depression was secondary to organic brain damage (e.g., traumatic brain injury, Alzheimer's disease) or a medical condition (e.g., heart failure). Studies were included if they tested MDD and control groups on at least one neuropsychological measure of EF and reported sufficient information to calculate effect sizes. Only tasks with emotionally neutral materials were included in the meta-analysis. This was done, first, to avoid confounding altered emotional processing with EF impairments, and second, because a recent meta-analysis examined the relation between depression and cognitive control over emotional materials (Peckham, McHugh, & Otto, 2010), and thus the current meta-analysis does not duplicate this effort.
Search Strategies
The author, who has a background in EF research, conducted the search and screening process. Searches were conducted in PubMed and ISI Web of Science through July 2011 using the keywords depression or depressive paired with executive function, working memory, inhibition, shifting, switching, planning, verbal fluency, cognitive, or neuropsychological for studies published in English at any time prior to the search date. An initial screen was conducted by examining titles to eliminate studies that clearly did not meet the inclusion criteria. Next, the abstracts of all remaining articles were examined, and if an article appeared likely to meet the inclusion criteria the full text was obtained. In addition, the reference lists of included articles, and articles citing included articles, were screened for any studies missed in the database search process. This process initially identified 145 studies for inclusion. Of these, 32 were excluded because they did not report sufficient information to calculate effect sizes (k = 17), did not include a healthy control group (k = 5), reported only mixed diagnostic groups (k = 7), or reported the same data as another study included in the meta-analysis (e.g., re-analyses; k = 8). Thus, a total of 113 studies were included in the meta-analysis (see Appendix A). Only peer-reviewed, published studies were included, as they are likely to be of higher quality. Trim and fill analyses (Duval & Tweedie, 2000; implemented in the Comprehensive Meta-Analysis software package) and funnel plots were examined for evidence of publication bias (Appendix B, Figures S1-S8 and Table S1).
Coding Procedures
The tasks in the included studies determined the aspects of EF covered in the meta-analysis. Neuropsychological measures of EF were coded as tapping one of the following EF components, as detailed below: inhibition, shifting, updating, verbal and visuospatial working memory, planning, and verbal fluency. This list is not meant to be exhaustive of all EF abilities, but rather of the range of neuropsychological measures of EF included in the MDD literature. The author coded all studies. In addition, for 25% of studies, a second coder with a background in cognitive psychology coded the EF component tapped by each EF task. Intercoder agreement was high (96%)1; thus, the author's coding was used in all analyses. All other aspects of coding were objective, as they were supplied directly by the included manuscripts. For each EF component, all tasks tapping that component (listed below) were combined in composite score analyses. In addition, measures with five or more studies were also analyzed separately.
In addition, two types of non-EF comparison measures were also coded. First, two neuropsychological measures of EF reported by many studies, the Trail Making Test and the Stroop task, have baseline conditions that control for many non-EF aspects of the task (see Shifting and Inhibition sections). Second, some studies report measures of processing speed or vocabulary (see the Processing speed and vocabulary section).
Inhibition
The most reported inhibition task in the studies included in the meta-analysis is the color-word Stroop task (k = 40), with studies reporting one or more of the following dependent variables: time to complete the incongruent condition (k = 19), interference (k = 20), and accuracy (k = 10). Each of these measures was analyzed individually, and also included in a composite inhibition score averaging across all inhibition tasks in each sample. In addition, time to complete the neutral condition was included as a non-EF comparison measure (k = 14). The Hayling task (see Table 1 for description, k = 5) was analyzed individually and also included in the composite inhibition score. In addition, seven studies reported other inhibition tasks. The dependent measures are reaction time and errors. The go/no-go (k = 4) and stop-signal (k = 1) tasks both require making a response to some stimuli and withholding a response to others. The Simon task is a Stroop-like task where participants ignore the location of a stimulus (k = 1), while the flanker task requires ignoring incongruent surrounding stimuli to make a judgment about the central target (k = 1). These tasks were included in the composite inhibition score.
Shifting
The three most reported shifting tasks in studies in the meta-analysis were the Wisconsin Card Sorting Test (WCST, k = 25), the Trail Making Test part B (TMT-B, k = 35), and the Intradimensional/Extradimensional Shift task (ID/ED Shift, k = 15). In addition, the Trail Making Test part A (TMT-A) was included as a non-EF comparison measure (k = 32). These tasks were each analyzed individually and also included in a composite shifting score averaging across all shifting effect sizes for each study. For the Wisconsin Card Sorting Test, the dependent measure was perseverative errors (when reported), or the number of stages achieved (when perseverative errors were not reported). The primary measure reported for both parts of the Trail Making Test is total time to complete the task; a few studies used a version without error correction and reported perseverative errors (e.g., A-B instead of A-1) or number correct. For the Intradimensional/Extradimensional Shift task, the dependent measures reported vary, and include intradimensional shift, extradimensional shift, and total errors; trials to criterion; and number of stages achieved. Since reporting across studies was not consistent enough to examine each measure independently, a composite score was calculated for each study.
In addition, five studies reported other shifting tasks: two variants on the Wisconsin Card Sorting Test (Cogtest Set Shifting, k = 1; BADS Rule Shift Cards, k = 1), a cued task-switching task (color-shape, k = 2), and a ‘rule-shift’ task in which participants switch which color stimuli receive a yes response (k = 1). These tasks were also included in the composite shifting score.
Updating
The n-back task was the most reported updating task (k = 7). It was analyzed individually, and also included in a composite updating score averaging across all updating tasks in each sample. In addition, three studies reported other updating tasks: the letter-memory task, in which participants continuously repeat the last four numbers in a sequence (k = 1); a task in which participants say the number n back on each trial (k = 1); and a recent-probes task in which participants update their representation of the stimuli on each trial to avoid false alarms to previously presented items (k = 1). These tasks were included in the composite updating score.
Verbal working memory
The most reported verbal working memory tasks were digit span forward (k = 27) and backward (k = 23), which were analyzed individually, and also included in a composite verbal WM score averaging across all verbal working memory measures reported for each sample. Forward digit span was also included in a verbal working memory maintenance composite score (tasks requiring maintenance of information in the order presented), and backward digit span was also included in a verbal working memory manipulation composite score (tasks requiring re-arrangement of information in working memory).
In addition, ten studies reported other verbal working memory tasks. The following tasks were included in the verbal working memory composite and the maintenance composite scores: California Verbal Learning Test trial one immediate recall (k = 2, repeat a list of words), Sternberg and letter maintenance tasks (k = 2, recall three numbers or letters after a delay), and reading span (k = 1, recall the last word of sentences in order). The following tasks were included in the verbal working memory composite and manipulation composite scores: digit or letter sequencing tasks (k = 2, repeat a list of numbers, reordering from lowest to highest, or letter, reordering in alphabetical order) and a letter-number sequencing task (k = 3, repeat a list of numbers and letters, reordering to say letters first, then numbers).
Visuospatial working memory
The most reported visuospatial working memory tasks were spatial span forward (k = 18) and backward (k = 9), delayed-match-to-sample (DMTS, k = 11), and self-ordered pointing (k = 12). These tasks were each analyzed individually and also included in a composite visuospatial working memory score averaging across all visuospatial working memory measures for each study. In addition, two studies reporting a delayed spatial match-to-sample task were included in the composite visuospatial working memory score.
Planning
The planning tasks reported by the studies included in the meta-analysis were the Tower of London (TOL) and closely related Stockings of Cambridge (SOC) task from the CANTAB (Robbins et al., 1998; k = 17). Dependent measures reported varied by study, and included number of moves to solve problems, number of moves in excess of the minimum number of moves required, and number of problems solved in the minimum number of moves (perfect solutions). These measures were included in a composite planning score.
Verbal fluency
Semantic (k = 24) and phonemic (k = 37) verbal fluency tasks were analyzed individually and also included in a composite verbal fluency score.
Processing speed and vocabulary
Processing speed and vocabulary measures were analyzed as non-EF comparison measures. The most reported processing speed task was the digit-symbol substitution task (k = 23). The dependent measure is the number of items correctly completed within the time limit. This task was analyzed individually, as it may impose some EF demands (see Discussion). In addition, 23 studies reported one or more psychomotor speed measure, including simple RT (k = 12), choice RT (k = 9), finger-tapping (tap fingers as quickly as possible, k = 5), and grooved pegboard (put pegs into holes in a board as quickly as possible, k = 1). These tasks were included in a psychomotor speed composite score.
The most reported vocabulary test was the National Adult Reading Test (k = 17, including versions in languages other than English), in which participants read a list of irregular words aloud (e.g., quadruped). The dependent measure is the number of pronunciation mistakes, which is converted to a verbal IQ estimate. Other measures included the WAIS-R vocabulary subtest (k = 9), Stanford-Binet vocabulary subtest (k = 1), Binois-Pichot vocabulary subtest (k = 2), Ammons Quick Test (k = 1), British picture vocabulary test (k = 1), Mill Hill vocabulary test (k = 1), and German vocabulary test (k = 1), which all yield standardized scores or verbal IQ estimates. These tests were included in a single vocabulary analysis.
Moderator Analyses
Information was coded on current depression symptom severity, age, psychotropic medication use, and comorbidity exclusions.2 When a moderator variable was not reported by a study, that study was not included in the applicable moderator analysis, but was included in all other analyses for which it reported data.
Current depression symptom severity
The mean score of the MDD group on at least one of the following standardized rating scales of depression severity was reported by 92% of studies. The Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) is a clinician-administered scale based on 17 items (some versions include more items, but they are not included in the score). Reliability (internal, interrater, and retest) and validity (convergent, discriminant, and predictive) are generally good, although concerns have been raised about its congruence with the DSM-IV MDD criteria (Bagby, Ryder, Schuller, & Marshall, 2004). The Montgomery-Asberg Depression Rating Scale (MADRS) is also a clinician-administered scale, based on 10 items, with high interrater reliability and a significant correlation with the Hamilton Depression Rating Scale (Montgomery & Asberg, 1979). The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and Beck Depression Inventory II (BDI-II; Beck, Steer, & Brown, 1996) are self-report scales based on 21 items. The BDI has been shown to have high internal validity and adequate retest reliability, as well as good convergent, discriminant and predictive validity (Beck, Steer, & Garbin, 1988). The BDI-II has similarly high internal reliability and good convergent validity (Beck Steer, Ball, & Ranieri, 1996; Osman et al., 1997). In addition, a few studies report the Geriatric Depression Scale (GDS, 15 items, self-report; Brink et al., 1982), Hospital Anxiety and Depression Scale depression subscale (HADS, 7 items, self-report; Zigmond & Snaith, 1983), or Kessler Psychological Distress Scale (K10, 10 items, self-report; Kessler et al., 2002).
While they differ from each other in some ways, these rating scales have been shown to correlate highly with one another (e.g., Carmody et al., 2006; Uher et al., 2008). Thus, they were combined into a single severity moderator variable. Since the rating scales have different numbers of items and severity level categories, they were re-coded into a common metric to make moderator analysis possible (Table 2). The lowest severity level on each scale was coded as 0 (remission), and the following levels as 1 (mild), 2 (moderate), and 3 (severe/very severe). The HAM-D and MADRS use a fourth, very severe level not used by the other scales; thus, ratings of severe or very severe on these measures were both coded as 3. In rare cases where there was disagreement on severity level between measures reported for a sample (k = 3), the average of the rating was used (e.g., a sample rated severe on one questionnaire and moderate on another would be coded 2.5). To directly test whether neuropsychological measures of EF remain impaired in remission, dichotomous analyses comparing current depressive episode vs. remission were also conducted for those measures with at least five samples in remission.
Table 2.
Recoding of Measures of Current Depression Symptom Severity into a Common Metric
| Severity Measure | Cut-Point Source | Scoring | Recoding |
|---|---|---|---|
| HAM-D | Kearns et al., 1982 | 0-7= normal | 0 |
| 8-13 = mild | 1 | ||
| 14-18= moderate | 2 | ||
| 19-22= severe | 3 | ||
| ≥ 23= very severe | 3 | ||
| MADRS | Kearns et al., 1982 | 0-7 = recovered | 0 |
| 8-15= mild | 1 | ||
| 16-25= moderate | 2 | ||
| 26-30= severe | 3 | ||
| 31≥ = very severe | 3 | ||
| BDI | Beck, 1978 | 0-9= normal | 0 |
| 10-16= mild | 1 | ||
| 17-29= moderate | 2 | ||
| ≥ 30= severe | 3 | ||
| BDI-II | Beck et al., 1996 | 0-13 = minimal | 0 |
| 14-19= mild | 1 | ||
| 20-28= moderate | 2 | ||
| ≥ 29= severe | 3 | ||
| K10 | Andrews & Slade, 2001 | 10-19= well | 0 |
| 20-24= mild | 1 | ||
| 25-29= moderate | 2 | ||
| 30≥= severe | 3 | ||
| GDS | Yesavage et al., 1983 | 0-9= normal | 0 |
| 10-19= mild | 1 | ||
| ≥20 = severe | 3 | ||
| HADS | Zigmond & Snaith, 1983 | 0-7=normal | 0 |
| 8-10=mild | 1 | ||
| 11-14=moderate | 2 | ||
| 15-21=severe | 3 |
Note. The HAM-D and MADRS use a fourth, very severe level not used by the other scales; thus, ratings of severe or very severe on were both coded as 3. HAM-D = Hamilton Depression Rating Scale; MADRS = Montgomery-Asberg Depression Rating Scale; BDI = Beck Depression Inventory; BDI-II = Beck Depression Inventory II; K10 = Kessler Psychological Distress Scale; GDS = Geriatric Depression Scale; HADS = Hospital Anxiety and Depression Scale.
Age
The mean age of the MDD group was included as a continuous variable in meta-regression analyses.3 Age was reported by all studies, and nearly all studies (92%) used age-matched MDD and control groups.
Medication
The percentage of the MDD group currently taking psychotropic medications was coded for each sample. Medication usage was reported by 88% of studies. Many studies only reported the total number of medicated patients; thus, a more detailed analysis of the types or duration of medication could not be conducted.
Comorbidity
The inclusion or exclusion of patients with comorbid DSM Axis I disorders was coded as a categorical variable. Studies that did not provide information about comorbidity were assumed to have not excluded such patients. Thus, all studies were coded as either excluding or not excluding patients with comorbid Axis I disorders. Few studies reported more detailed information on comorbidity, such as number and type of other disorders or measures of subclinical anxiety, so these factors could not be considered in the moderator analyses (in the studies which did list specific comorbid disorders, all were anxiety disorders).
Statistical Methods
For each study, effect sizes comparing the performance of MDD and control groups on each measure were calculated as Cohen's d (m1 - m2 / SDpooled, where m = group mean and SDpooled is the pooled standard deviation of the two groups). The number of studies for 22 of 30 measures was sufficient to achieve adequate power (> 80%) for detecting a small effect size (d = 0.3) even with large heterogeneity, with the remaining 8 analyses having power between 40–80% (Borenstein, Hedges, Higgins, & Rothstein, 2009). The sign of d was set such that a positive value always indicated poorer performance for the MDD group relative to the control group (e.g., lower accuracy, higher error rates, or longer RTs). Hedges’ small sample bias correction was applied to each effect size (dadj = d(1- (3/4N) - 9, where N = number of participants in the patient and control samples combined; Hedges, 1980). Effect sizes were weighted by sample size using inverse variance weights (w = (2(n1 + n2)n1n2) / (2(n1 + n2)2 + n1n2d2), where n1 and n2 are the number of participants in the patient and control groups respectively). Finally, outliers with effect sizes > d = 2.0, or +/- 3 SD from the mean effect size in each analysis, were excluded. Effect sizes were only excluded from the analysis in which they were statistical outliers.
Only one effect size from each MDD and control group comparison was included in each analysis to avoid statistical dependence. When five or more studies reported a measure, individual tasks and dependent measures were analyzed separately. In addition, all effect sizes were included in composite scores, which were calculated by averaging effect sizes within a construct (e.g., multiple inhibition measures; see Methods and Results). Nineteen studies reported two different MDD samples compared to the same control sample. In these cases, to avoid introducing statistical dependence, the MDD samples were first combined into a single sample using weighted means and standard deviations. (However, comparisons between the groups are reviewed in the Discussion section). Three studies reported both younger and older adult MDD samples, compared to their own age-matched control samples. In these cases, both sample comparisons were included since there is no statistical dependence. In addition, when patients were tested more than once (e.g., at different points in treatment), only the first test was analyzed, as practice effects may diminish the EF demands of tasks.
Random effects meta-analytic models were used for all analyses, as there are likely to be many sources of variability between study samples beyond sampling error, violating the assumptions of fixed-effects models (Raudenbush, 2009). Importantly, random effects models allow inferences to be drawn about the population as a whole, rather than only the samples tested. Mean effect size analyses were conducted using the SPSS meta-analysis macro developed by David B. Wilson (Wilson, 2006). For each analysis, weighted mean effect sizes with 95% confidence intervals were calculated. The null hypothesis that the mean effect size is zero was tested with the z statistic at the alpha=.05 significance level. The random effects variance component v represents the estimate of the amount of variance due to random effects. Heterogeneity in effect sizes was tested with the Qt statistic (Hedges & Olkin, 1985). Qt quantifies the degree to which the studies contributing to each weighted mean effect size can be considered homogeneous. If Qt is significant, it suggests that there are substantive differences between the studies in that analysis. In some cases, these differences may be partly accounted for by moderators, while in other cases they may result from other sources of variability between studies. The I2 statistic ((Q - (k - 1)) / Q) represents the percentage of total variability in the set of effect sizes due to true heterogeneity.
Moderator analyses were conducted using the SPSS meta-analysis macros developed by Wilhelm Hofmann (Hofmann, 2009), using mixed effects models with method of moments estimation. Current depression symptom severity (0 - 3), patient age, and medication status (% receiving psychotropic medications) were included as continuous variables in separate and combined meta-regression analyses. Comorbidity (patients with other Axis I disorders excluded or not), and remission status (in remission vs. current depressive episode) were included as categorical variables in meta-ANOVA analyses, whenever there were at least five studies in the smaller category. Moderator analyses were only conducted for measures with 20 or more effect sizes, as analyses with fewer studies have inadequate power and may produce unstable estimates (Marín-Martínez & Sánchez-Meca, 1998; Sánchez-Meca & Marín-Martínez, 1998).
Results
In total, the 113 studies in the meta-analysis included 7,707 participants: 3,936 patients and 3,771 healthy control participants. MDD and control groups were similar in age and gender composition. MDD groups included 2,404 females (61%), 1,395 males (35%) and 137 participants for whom gender was not reported (4%). Control groups included 2,246 females (60%), 1,428 males (38%) and 97 participants for whom gender was not reported (2%). The mean age of patients was 46 years, and of controls 45 years. In those samples reporting medication use, 41% of patients were taking psychotropic medications at the time of testing (medication information was not reported by 12% of studies). At the time of testing, depression symptom severity of the MDD group was severe in 52 studies, moderate in 25 studies, mild in 10 studies, and in remission in 19 studies (9 studies did not report symptom severity). Demographics for the subjects in each analysis are provided in Appendix B, Table S2. Age, medication use, and current depression symptom severity were not correlated with one another (ps > .10). Thirty-eight studies matched MDD and control participants on both IQ and education, and 23 studies excluded participants with comorbid Axis I disorders.
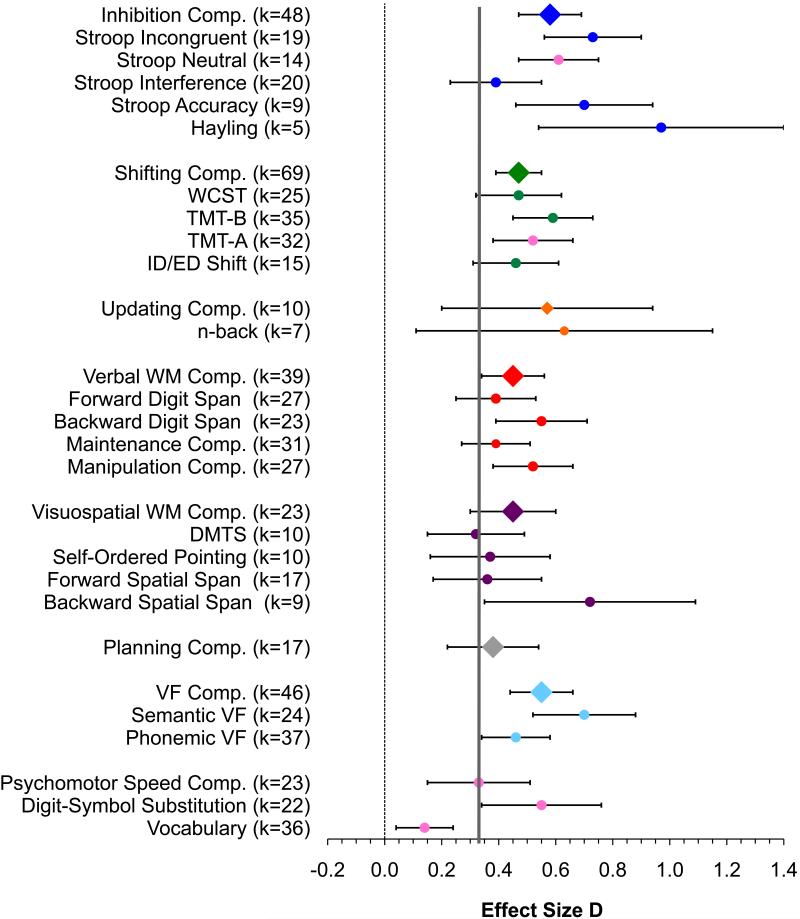
Outlier screening resulted in the exclusion of a total of 13 effect sizes, with one outlier each excluded in 10 analyses, and two outliers excluded in two analysis. Outlier effect sizes and mean effect sizes with outliers included are listed in Table 3 notes. Weighted mean effect sizes for all analyses comparing patients with MDD to healthy control participants are presented in Table 3 and plotted in Figure 1. Meta-multiple regression analyses for continuous moderators (current symptom severity, medication, and age) are presented in Table 4 (simple regression analyses are available in Appendix B, Table S3). Meta-ANOVA analyses of categorical moderators (remission status and comorbidity) are presented in Table 5 (categorical age analyses are available in Appendix B, Table S4). Analyses for IQ and education matched samples only (Table S5) and medication-free samples only (Table S6) are available in Appendix B, and complete data sets are available from the author upon request.
Table 3.
Weighted Mean Effect Size Analyses
| Measure | N | k | d | 95% Confidence Interval | SE | Z | p | Homogeneity Test | Sensitivity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | v | Q | df | p | I2 | Trim & Fill Adjusted d | IQ & Education Matched d | |||||||
| Inhibition: | |||||||||||||||
| Stroop Incongruenta | 1494 | 19 | 0.73 | 0.56 | 0.90 | 0.09 | 8.34 | <.001 | 0.08 | 42.76 | 18 | .001 | 58.87 | 0.58 | 0.88 |
| Stroop Neutral (comparison condition) | 1078 | 14 | 0.61 | 0.47 | 0.74 | 0.07 | 8.84 | <.001 | 0.01 | 14.54 | 13 | .3 | 17.47 | 0.49 | 0.68 |
| Stroop Interference | 1482 | 20 | 0.39 | 0.23 | 0.54 | 0.08 | 4.81 | <.001 | 0.06 | 39.22 | 19 | .004 | 51.56 | 0.30 | 0.47 |
| Stroop Accuracyb | 628 | 9 | 0.70 | 0.46 | 0.93 | 0.12 | 5.82 | <.001 | 0.06 | 14.60 | 8 | .067 | 45.21 | 0.50 | - |
| Hayling | 247 | 5 | 0.97 | 0.54 | 1.40 | 0.22 | 4.45 | <.001 | 0.13 | 9.23 | 4 | .056 | 56.66 | 0.97 | - |
| Inhibition Comp.c | 3274 | 48 | 0.58 | 0.47 | 0.69 | 0.06 | 9.95 | <.001 | 0.09 | 109.67 | 47 | <.001 | 57.14 | 0.42 | 0.62 |
| Shifting: | |||||||||||||||
| WCSTd | 1943 | 25 | 0.47 | 0.32 | 0.61 | 0.07 | 6.31 | <.001 | 0.07 | 52.66 | 24 | .001 | 54.42 | 0.35 | 0.43 |
| TMT-B | 2825 | 35 | 0.59 | 0.45 | 0.73 | 0.07 | 8.08 | <.001 | 0.12 | 101.66 | 34 | <.001 | 66.56 | 0.43 | 0.35 |
| TMT-A (comparison condition)e | 2794 | 32 | 0.52 | 0.38 | 0.66 | 0.07 | 7.42 | <.001 | 0.09 | 85.09 | 31 | <.001 | 63.57 | 0.35 | 0.37 |
| ID/ED Shiftf | 899 | 15 | 0.46 | 0.31 | 0.61 | 0.18 | 5.88 | <.001 | 0.02 | 17.01 | 14 | .3 | 17.70 | 0.35 | 0.41 |
| Shifting Comp.g | 5154 | 69 | 0.47 | 0.39 | 0.55 | 0.04 | 10.93 | <.001 | 0.06 | 133.83 | 68 | <.001 | 49.19 | 0.36 | 0.40 |
| Updating: | |||||||||||||||
| n-Back | 324 | 7 | 0.63 | 0.11 | 1.15 | 0.26 | 2.37 | .018 | 0.38 | 27.02 | 6 | .001 | 77.79 | 0.63 | - |
| Updating Comp. | 528 | 10 | 0.57 | 0.20 | 0.94 | 0.19 | 3.00 | .003 | 0.26 | 35.22 | 9 | .001 | 74.45 | 0.57 | - |
| Verbal Working Memory: | |||||||||||||||
| Forward Digit Span | 1820 | 27 | 0.39 | 0.25 | 0.53 | 0.07 | 5.56 | <.001 | 0.06 | 47.55 | 26 | .006 | 45.32 | 0.39 | 0.42 |
| Backward Digit Span | 1700 | 23 | 0.55 | 0.39 | 0.71 | 0.08 | 6.86 | <.001 | 0.08 | 49.30 | 22 | .001 | 55.38 | 0.55 | 0.46 |
| Maintenance Comp. | 2093 | 31 | 0.39 | 0.27 | 0.51 | 0.06 | 6.32 | <.001 | 0.04 | 49.66 | 30 | .013 | 39.59 | 0.23 | 0.40 |
| Manipulation Comp. | 2051 | 27 | 0.52 | 0.38 | 0.66 | 0.07 | 7.42 | <.001 | 0.07 | 55.86 | 26 | .001 | 53.46 | 0.52 | 0.43 |
| Verbal WM Comp. | 2840 | 39 | 0.45 | 0.34 | 0.56 | 0.05 | 8.38 | <.001 | 0.04 | 65.59 | 38 | .004 | 42.06 | 0.45 | 0.40 |
| Visuospatial Working Memory: | |||||||||||||||
| DMTSh | 624 | 10 | 0.32 | 0.15 | 0.48 | 0.08 | 3.76 | <.001 | 0.00 | 9.18 | 9 | .4 | 1.96 | 0.23 | 0.40 |
| Self-Ordered Pointingi | 722 | 10 | 0.37 | 0.16 | 0.58 | 0.11 | 3.42 | .001 | 0.05 | 16.54 | 9 | .056 | 54.59 | 0.33 | 0.33^ |
| Forward Spatial Spanj | 1037 | 17 | 0.36 | 0.17 | 0.55 | 0.10 | 3.70 | <.001 | 0.08 | 33.41 | 16 | .007 | 52.11 | 0.36 | 0.21^ |
| Backward Spatial | 598 | 9 | 0.72 | 0.35 | 1.09 | 0.19 | 3.85 | <.001 | 0.23 | 31.57 | 8 | <.001 | 74.66 | 0.63 | 0.75 |
| Span | |||||||||||||||
| Visuospatial WM | 1493 | 23 | 0.45 | 0.30 | 0.59 | 0.07 | 6.07 | <.001 | 0.05 | 38.01 | 22 | .018 | 42.12 | 0.29 | 0.47 |
| Compk | |||||||||||||||
| Planning: | |||||||||||||||
| Planning Comp. | 1037 | 17 | 0.38 | 0.22 | 0.53 | 0.08 | 4.70 | <.001 | 0.03 | 23.40 | 16 | .1 | 31.62 | 0.26 | 0.29 |
| Verbal Fluency: | |||||||||||||||
| Semantic VF | 1642 | 24 | 0.70 | 0.52 | 0.88 | 0.09 | 7.56 | <.001 | 0.13 | 66.94 | 23 | <.001 | 65.64 | 0.53 | 0.75 |
| Phonemic VF | 2850 | 37 | 0.46 | 0.34 | 0.57 | 0.06 | 7.72 | <.001 | 0.06 | 73.12 | 36 | <.001 | 50.77 | 0.33 | 0.44 |
| VF Comp. | 3466 | 46 | 0.55 | 0.44 | 0.66 | 0.06 | 9.74 | <.001 | 0.07 | 100.43 | 45 | <.001 | 55.19 | 0.42 | 0.51 |
| Comparison Measures: | |||||||||||||||
| Psychomotor Speed | 1757 | 23 | 0.33 | 0.15 | 0.50 | 0.09 | 3.68 | <.001 | 0.11 | 63.24 | 22 | <.001 | 65.21 | 0.20 | 0.30^ |
| Comp. | |||||||||||||||
| Digit-Symbol | 1904 | 22 | 0.55 | 0.34 | 0.75 | 0.10 | 5.25 | <.001 | 0.17 | 88.76 | 21 | <.001 | 75.21 | 0.55 | 0.30 |
| Substitutionl | |||||||||||||||
| Vocabulary | 2175 | 36 | 0.14 | 0.04 | 0.25 | 0.05 | 2.68 | .007 | 0.03 | 47.81 | 35 | .073 | 26.79 | 0.08^ | n/a |
Note. Comp. = composite score; WCST= Wisconsin Card Sorting Test; TMT-B = Trail Making Test part B; TMT-A = Trail Making Test part A; ID/ED = Intradimensional/Extradimensional; WM = working memory; DMTS = delayed-match-to-sample VF= verbal fluency; d = weighted mean effect size; K = number of studies; N = number of participants; v = random effects variance component; Q = heterogeneity; I2 = percentage of total variability in the set of effect sizes due to true heterogeneity; – = Too few IQ and education matched samples to conduct analysis (k<5).
1 outlier excluded (d = 3.57): with this outlier included, the weighted mean effect size was d = 0.83.
1 outlier excluded (d = 2.52): with this outlier included, the weighted mean effect size was d = 0.75.
1 outlier excluded (d = 2.14): with this outlier included, the weighted mean effect size was d = 0.61.
1 outlier excluded (d = 2.66): with this outlier included, the weighted mean effect size was d = 0.57.
1 outlier excluded (d = 1.96): with this outlier included, the weighted mean effect size was d = 0.55.
1 outlier excluded (d = 2.96): with this outlier included, the weighted mean effect size was d = 0.60.
2 outliers excluded (d = 2.66, d = 2.96): with these outliers included, the weighted mean effect size was d = 0.54.
1 outlier excluded (d = 2.56): with this outlier included, the weighted mean effect size was d = 0.54.
2 outliers excluded (d = 4.90, d = 2.55): with these outliers included, the weighted mean effect size was d = 0.83.
1 outlier excluded (d = 2.12): with this outlier included, the weighted mean effect size was d = 0.45.
1 outlier excluded (d = 3.20): with this outlier included, the weighted mean effect size was d = 0.56.
1 outlier excluded (d = 2.10): with this outlier included, the weighted mean effect size was d = 0.61.
Non-significant (p > .05).
Figure 1.
Weighted mean effect sizes for all analyses. Error bars are 95% confidence intervals. Compared to healthy control participants, patients with MDD are significantly impaired on all tasks. EF composite measures are indicated with diamond symbols, and individual measures within each EF component by circle symbols in the same color. Pink circles indicate non-EF comparison measures. The solid vertical line indicates the psychomotor speed composite score effect size: measures for which the lower error b ar (95% confidence interval) does not pass the grey line have significantly larger effect sizes than the psychomotor speed effect size. Comp. = composite score; WCST = Wisconsin Card Sorting Test; TMT-B = Trail Making Test part B; TMT-A = Trail Making Test part A; ID/ED = Intradimensional/Extradimensional; WM = working memory; DMTS = delayed-match-to-sample; VF = verbal fluency.
Table 4.
Simultaneous Moderator Regression Analyses
| DV | IV | Beta | 95% Confidence Interval |
B | SE | p | K | N | QB(df) | QW(df) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||||||
| Inhibition: | |||||||||||
| Stroop Interference | Severity | 0.20 | -0.47 | 0.86 | 0.057 | 0.098 | .6 | 17 | 1482 | 0.70 (3) | 12.2 (13) |
| Age | 0.12 | -0.58 | 0.82 | 0.003 | 0.008 | .7 | |||||
| Medication | 0.18 | -0.44 | 0.81 | 0.001 | 0.002 | .6 | |||||
| Inhibition Comp. | Severity | 0.32 | 0.01 | 0.64 | 0.100 | 0.050 | .045* | 43 | 3260 | 6.11 (3) | 38.69 (39) |
| Age | 0.08 | -0.22 | 0.37 | 0.002 | 0.004 | .6 | |||||
| Medication | 0.32 | 0.01 | 0.64 | 0.003 | 0.002 | .046* | |||||
| Shifting: | |||||||||||
| WCST | Severity | 0.40 | -0.03 | 0.83 | 0.156 | 0.086 | .069# | 23 | 1943 | 3.36 (3) | 20.91 (19) |
| Age | -0.01 | -0.41 | 0.39 | 0.000 | 0.005 | .9 | |||||
| Medication | 0.18 | -0.25 | 0.62 | 0.002 | 0.002 | .4 | |||||
| TMT-B | Severity | 0.27 | -0.10 | 0.63 | 0.100 | 0.069 | .2 | 27 | 2787 | 8.69 (3) | 23.46 (23) |
| Age | 0.33 | -0.02 | 0.68 | 0.010 | 0.005 | .066# | |||||
| TMT-A (Comparison Condition) | Medication | 0.36 | 0.00 | 0.72 | 0.004 | 0.002 | .048* | ||||
| Severity | 0.09 | -0.36 | 0.54 | 0.030 | 0.076 | .7 | 22 | 2756 | 1.69 (3) | 18.80 (18) | |
| Age | 0.02 | -0.42 | 0.46 | 0.001 | 0.007 | .9 | |||||
| Medication | 0.29 | -0.15 | 0.74 | 0.003 | 0.002 | .2 | |||||
| Shifting Comp. | Severity | 0.29 | 0.04 | 0.55 | 0.089 | 0.040 | .025* | 55 | 5116 | 6.71 (3) | 54.44 (51) |
| Age | 0.11 | -0.14 | 0.36 | 0.003 | 0.003 | .4 | |||||
| Medication | 0.18 | -0.08 | 0.43 | 0.001 | 0.001 | .2 | |||||
| Verbal Working Memory: | |||||||||||
| Forward Digit Span | Severity | -0.01 | -0.43 | 0.41 | -0.003 | 0.063 | .9 | 22 | 1782 | 5.34 (3) | 17.33 (18) |
| Age | -0.06 | -0.48 | 0.36 | -0.002 | 0.006 | .8 | |||||
| Medication | 0.49 | 0.07 | 0.91 | 0.004 | 0.002 | .022* | |||||
| Backward Digit Span | Severity | 0.43 | -0.00 | 0.87 | 0.155 | 0.079 | .051# | 17 | 1662 | 7.63 (3) | 14.42 (13) |
| Age | -0.29 | -0.76 | 0.17 | -0.011 | 0.009 | .2 | |||||
| Medication | 0.40 | -0.06 | 0.86 | 0.004 | 0.003 | .086# | |||||
| Maintenance Comp. | Severity | 0.14 | -0.23 | 0.51 | 0.038 | 0.052 | .5 | 26 | 2055 | 7.85 (3) | 21.37 (22) |
| Age | 0.14 | -0.23 | 0.51 | 0.003 | 0.004 | .5 | |||||
| Medication | 0.53 | 0.16 | 0.90 | 0.004 | 0.001 | .005* | |||||
| Manipulation Comp. | Severity | 0.43 | 0.04 | 0.82 | 0.136 | 0.063 | .031* | 20 | 2080 | 9.04 (3) | 17.27 (16) |
| Age | -0.11 | -0.50 | 0.28 | -0.003 | 0.005 | .6 | |||||
| Medication | 0.29 | -0.10 | 0.68 | 0.003 | 0.002 | .139 | |||||
| Verbal WM Comp.a | Severity | 0.32 | 0.00 | 0.63 | 0.080 | 0.041 | .049* | 30 | 2840 | 17.05 (4) | 24.93 (25) |
| Age | 0.02 | -0.29 | 0.34 | 0.000 | 0.003 | .9 | |||||
| Medication | 0.55 | 0.21 | 0.89 | 0.004 | 0.001 | .001* | |||||
| Task | 0.10 | -0.23 | 0.43 | 0.043 | 0.074 | .6 | |||||
| Visuospatial Working Memory: | |||||||||||
| Visuospatial WM Comp. | Severity | 0.30 | -0.15 | 0.75 | 0.095 | 0.073 | .2 | 17 | 1455 | 7.64 (3) | 12.41 (13) |
| Age | -0.03 | -0.49 | 0.43 | -0.001 | 0.006 | .9 | |||||
| Medication | 0.50 | 0.04 | 0.96 | 0.005 | 0.002 | .035* | |||||
| Verbal Fluency: | |||||||||||
| Semantic VF | Severity | 0.72 | 0.32 | 1.12 | 0.408 | 0.116 | <.001* | 17 | 1643 | 13.97 (3) | 13.95 (15) |
| Age | 0.00 | -0.40 | 0.39 | 0.000 | 0.007 | .9 | |||||
| Medication | 0.22 | -0.16 | 0.60 | 0.003 | 0.003 | .3 | |||||
| Phonemic VF | Severity | 0.66 | 0.28 | 1.03 | 0.194 | 0.057 | .001* | 29 | 2850 | 11.85 (3) | 24.79 (25) |
| Age | 0.16 | -0.19 | 0.50 | 0.003 | 0.004 | .4 | |||||
| Medication | 0.32 | -0.04 | 0.67 | 0.002 | 0.001 | .085# | |||||
| VF Comp.b | Severity | 0.58 | 0.24 | 0.91 | 0.203 | 0.060 | .001* | 34 | 3466 | 15.61 (3) | 29.73(29) |
| Age | 0.07 | -0.23 | 0.37 | 0.002 | 0.004 | .7 | |||||
| Medication | 0.28 | -0.05 | 0.62 | 0.003 | 0.002 | .099# | |||||
| Task | 0.13 | -0.19 | 0.44 | 0.073 | 0.094 | .4 | |||||
| Comparison Measures: | |||||||||||
| Psychomotor Speed Comp. | Severity | 0.48 | 0.03 | 0.92 | 0.209 | 0.101 | .038* | 18 | 1743 | 7.21 (3) | 13.61 (14) |
| Age | 0.44 | -0.02 | 0.90 | 0.013 | 0.007 | .060# | |||||
| Medication | 0.17 | -0.28 | 0.62 | 0.002 | 0.003 | .5 | |||||
| Digit-Symbol Substitution | Severity | 0.32 | -0.17 | 0.80 | 0.171 | 0.134 | .2 | 18 | 1944 | 9.84 (3) | 14.65 (14) |
| Age | 0.28 | -0.20 | 0.75 | 0.011 | 0.010 | .3 | |||||
| Medication | 0.40 | -0.01 | 0.82 | 0.006 | 0.003 | .056# | |||||
| Vocabulary | Severity | 0.45 | 0.13 | 0.76 | 0.098 | 0.035 | .006* | 30 | 2175 | 19.18 (3) | 23.25 (26) |
| Age | 0.55 | 0.22 | 0.88 | 0.013 | 0.004 | .001* | |||||
| Medication | -0.20 | -0.54 | 0.15 | -0.001 | 0.001 | .3 | |||||
Note. Comp. = composite score; WCST= Wisconsin Card Sorting Test; TMT-B = Trail Making Test part B; TMT-A = Trail Making Test part A; WM = working memory; VF = verbal fluency; IV = independent variable; DV = dependent variable; d = weighted mean effect size; K = number of studies; N = number of participants; Qw = residual heterogeneity; QB= heterogeneity accounted for by moderators.
Analysis controlling for task (maintenance, manipulation or both), because of significant differences between verbal working memory maintenance and manipulation effect sizes.
Analysis controlling for task (semantic, phonemic or both), because of significant differences between semantic and phonemic effect sizes.
Significant (p<.05).
Marginal (p<.10).
Table 5.
Moderator ANOVA Analyses
| DV | IV | Group | d | 95% Confidence Interval |
SE | K | N | Q w (df) | Between Groups Homogeneity Test |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Q B (df) | p B | ||||||||
| Inhibition: | |||||||||||
| Stroop Interference | Comorbidity | Other Axis I Excluded | 0.38 | 0.08 | 0.68 | 0.16 | 6 | 1482 | 16.54 (18) | 0.00 (1) | .9 |
| Other Axis I Included | 0.39 | 0.46 | 0.72 | 0.07 | 14 | ||||||
| Inhibition Comp. | Comorbidity | Other Axis I Excluded | 0.55 | 0.32 | 0.79 | 0.12 | 12 | 3260 | 43.46 (46) | 0.08 (1) | .8 |
| Other Axis I Included | 0.59 | 0.46 | 0.72 | 0.07 | 36 | ||||||
| Remission Status | Currently Depressed | 0.60 | 0.48 | 0.73 | 0.07 | 37 | 3102 | 43.87 (45) | 0.02 (1) | .9 | |
| Remission | 0.58 | 0.35 | 0.81 | 0.12 | 11 | ||||||
| Shifting: | |||||||||||
| WCST | Comorbidity | Other Axis I Excluded | 0.35 | 0.02 | 0.68 | 0.17 | 5 | 1943 | 26.33 (23) | 0.63 (1) | .4 |
| Other Axis I Included | 0.50 | 0.33 | 0.66 | 0.08 | 20 | ||||||
| TMT-B | Remission Status | Currently Depressed | 0.63 | 0.47 | 0.80 | 0.09 | 28 | 2499 | 33.07 (31) | 0.43 (1) | .5 |
| Remission | 0.50 | 0.19 | 0.87 | 0.19 | 5 | ||||||
| TMT-A (comparison condition) | Remission Status | Currently Depressed | 0.58 | 0.41 | 0.75 | 0.08 | 26 | 2508 | 30.70 (29) | 0.00 (1) | .9 |
| Remission | 0.59 | 0.22 | 0.95 | 0.19 | 5 | ||||||
| Shifting Comp. | Comorbidity | Other Axis I Excluded | 0.36 | 0.32 | 0.79 | 0.11 | 11 | 5116 | 70.62 (67) | 1.23 (1) | .3 |
| Other Axis I Included | 0.49 | 0.40 | 0.57 | 0.05 | 58 | ||||||
| Remission Status | Currently Depressed | 0.52 | 0.42 | 0.62 | 0.05 | 37 | 4600 | 64.63 (61) | 3.66 (1) | .056# | |
| Remission | 0.30 | 0.09 | 0.50 | 0.11 | 11 | ||||||
| Verbal Working Memory: | |||||||||||
| Forward Digit Span | Remission Status | Currently Depressed | 0.40 | 0.24 | 0.57 | 0.08 | 21 | 1737 | 24.01 (24) | 0.02 (1) | .9 |
| Remission | 0.38 | 0.04 | 0.71 | 0.17 | 5 | ||||||
| Maintenance Comp. | Remission Status | Currently Depressed | 0.41 | 0.27 | 0.55 | 0.07 | 24 | 2010 | 28.10 (28) | 0.11 (1) | .7 |
| Remission | 0.36 | 0.07 | 0.63 | 0.14 | 6 | ||||||
| Manipulation Comp. | Comorbidity | Other Axis I Excluded | 0.54 | 0.24 | 0.84 | 0.15 | 6 | 2051 | 28.17 (25) | 0.02 (1) | .9 |
| Other Axis I Included | 0.52 | 0.36 | 0.68 | 0.08 | 21 | ||||||
| Verbal WM. Comp. | Comorbidity | Other Axis I Excluded | 0.48 | 0.21 | 0.75 | 0.14 | 6 | 2840 | 36.35 (37) | 0.05 (1) | .8 |
| Other Axis I Included | 0.45 | 0.33 | 0.56 | 0.06 | 33 | ||||||
| Remission Status | Currently Depressed | 0.48 | 0.35 | 0.60 | 0.06 | 29 | 2600 | 33.55 (34) | 1.94 (1) | .2 | |
| Remission | 0.28 | 0.03 | 0.53 | 0.13 | 7 | ||||||
| Verbal Fluency: | |||||||||||
| Phonemic VF | Comorbidity | Other Axis I Excluded | 0.27 | 0.02 | 0.53 | 0.13 | 8 | 2850 | 35.93 (35) | 2.45 (1) | .117 |
| Other Axis I Included | 0.50 | 0.37 | 0.63 | 0.06 | 29 | ||||||
| Remission Status | Currently Depressed | 0.51 | 0.39 | 0.62 | 0.06 | 29 | 2555 | 32.65 (32) | 5.68 (1) | .017* | |
| Remission | 0.15 | -0.12 | 0.42 | 0.14 | 5 | ||||||
| VF Comp. | Comorbidity | Other Axis I Excluded | 0.44 | 0.21 | 0.67 | 0.12 | 11 | 3466 | 46.04 (44) | 1.11 (1) | .3 |
| Other Axis I Included | 0.58 | 0.46 | 0.70 | 0.06 | 35 | ||||||
| Remission Status | Currently Depressed | 0.59 | 0.48 | 0.71 | 0.06 | 37 | 3171 | 43.58 (41) | 5.35 (1) | .021* | |
| Remission | 0.25 | -0.03 | 0.52 | 0.14 | 6 | ||||||
| Comparison Measures: | |||||||||||
| Vocabulary | Comorbidity | Other Axis I Excluded | 0.07 | -0.17 | 0.30 | 0.12 | 6 | 2175 | 33.01 (34) | 0.49 (1) | .5 |
| Other Axis I Included | 0.16 | 0.04 | 0.28 | 0.06 | 30 | ||||||
| Remission Status | Currently Depressed | 0.23 | 0.11 | 0.35 | 0.06 | 22 | 2057 | 31.06 (31) | 8.04 (1) | .005* | |
| Remission | -0.07 | -0.24 | 0.10 | 0.09 | 11 | ||||||
Note. Comp. = composite score; WCST= Wisconsin Card Sorting Test; TMT-B = Trail Making Test part B; TMT-A = Trail Making Test part A; WM = working memory; VF = verbal fluency; IV = independent variable; DV = dependent variable; d = weighted mean effect size; K = number of studies; N = number of participants; Qw = within-group (residual) heterogeneity; QB= between-group (moderator) heterogeneity.
Significant (p<.05).
Marginal (p<.10).
Inhibition
Weighted mean effect size analyses
Patients with MDD were impaired on the inhibition composite score (d = 0.58, k = 48) as well as on the Stroop and Hayling tasks. In the Stroop task, patients with MDD were significantly slower than healthy control participants to name the ink color of incongruent color words (d = 0.73, k = 19); however, patients were also slower to name colors in the neutral condition (d = 0.61, k = 14), with an upper confidence limit (d = 0.74) just above the incongruent effect size. Thus, it may be more informative to focus on Stroop interference. Patients had a larger interference effect (d = 0.39, k = 20): that is, the difference between incongruent and neutral condition response times was larger for patients than for healthy controls. In addition, patients were significantly less accurate in the incongruent condition (d = 0.70, k = 9). On the Hayling task, patients were significantly slower and less accurate in responding with a word that did not complete each sentence (d = 0.97, k = 5).
Moderator analysis
Moderator analyses were only conducted for Stroop interference and inhibition composite scores, since other measures did not have adequate power (k < 20). For inhibition composite scores, effect sizes increased significantly with greater symptom severity. However, in the categorical analysis, there was no effect of remission status (remission vs. current depressive episode). Effect sizes increased significantly with the percentage of patients receiving psychotropic medications. There were no effects of age or comorbidity. For Stroop interference scores, there were no moderator effects.
Shifting
Weighted mean effect size analyses
Patients with MDD were impaired on the shifting composite score (d = 0.47, k = 69) as well as on all individual shifting tasks. In the Wisconsin Card Sorting Test, patients shifted rules more poorly than healthy control participants (d = 0.47, k = 25). For the Intradimensional/Extradimensional Shift task, patients had significantly lower scores than healthy controls (d = 0.46, k = 15). In addition, patients took significantly longer to complete the Trail Making Task part B (d = 0.59, k = 35). However, they were also significantly slower on the Trail Making Task part A comparison measure (d = 0.52, k = 32), with an upper confidence limit (d = 0.66) above the effect size for the Trail Making Task part B. Therefore, it cannot be concluded that shifting performance in Trail Making Task part B is impaired to a greater extent than sequencing and processing speed in Trail Making Task part A.
Moderator analysis
Moderator analyses revealed larger impairments on some measures with increasing severity and medication use. For the shifting composite score, effect sizes increased with symptom severity. In the categorical analysis, there was a marginal effect of remission status, with larger effect sizes for currently depressed samples than those in remission; however, those in remission remained significantly impaired. There were no effects of age, medication, or comorbidity. For the Wisconsin Card Sorting Test, effect sizes increased marginally with symptom severity. There were no effects of age, medication, or comorbidity. For the Trail Making Test part B, effect sizes increased marginally with average patient age and significantly with the percentage of patients on psychotropic medications. There were no effects of symptom severity or remission status. For the Trail Making Test part A comparison measure, there were no moderator effects. Moderator analyses were not conducted for the ID/ED Shift task, because it did not have adequate power (k < 20).
Updating
Weighted mean effect size analysis
Patients with MDD performed significantly worse on updating composite scores (d = 0.57, k = 10) and the n-back task (d = 0.63, k = 7) than healthy control participants.
Moderator analysis
Moderator analyses were not conducted for updating, since the measures did not have adequate power (k < 20).
Verbal working memory
Weighted mean effect size analyses
Patients with MDD were impaired on verbal working memory composite scores (d = 0.45, k = 39), as well as on all verbal working memory measures. Patients had significantly shorter forward (d = 0.39, k = 27) and backward (d = 0.55, k = 23) digit spans than healthy control participants, and significantly impaired performance on both the verbal working memory maintenance (d = 0.39, k = 31) and manipulation (d = 0.53, k = 28) composite scores. Effect sizes were significantly smaller for forward digit span and working memory maintenance compared to backward digit span and working memory manipulation.
Moderator analysis
Moderator analyses revealed larger impairments on some measures with increasing severity and medication use. For overall verbal working memory composite scores, effect sizes increased with the percentage of patients receiving psychotropic medication. There were no effects of current symptom severity, remission status, comorbidity, or age. For forward digit span and verbal working memory maintenance composite scores, effect sizes increased with the percentage of patients receiving psychotropic medication. There were no effects of current symptom severity, remission status, or age. Effects of comorbidity could not be analyzed because there were too few samples that excluded participants with comorbid Axis I disorders (k < 5). For verbal working memory manipulation composite scores, effect sizes increased significantly with greater symptom severity. There were no effects of medication, comorbidity, or age. Remission status could not be analyzed because there were too few samples in remission (k < 5). For backward digit span, effect sizes increased marginally with greater symptom severity and percentage of patients receiving psychotropic medication. There was no effect of age. Remission status and comorbidity could not be analyzed because there were too few samples in each group (k < 5).
Visuospatial Working Memory
Weighted mean effect size analyses
Patients with MDD were impaired on the visuospatial WM composite score (d = 0.45, k = 23) and all individual visuospatial working memory tasks: delayed-match-to-sample (d = 0.32, k = 10), self-ordered pointing (d = 0.37, k = 10), spatial span forward (d = 0.36, k = 17) and spatial span backward (d = 0.72, k = 9).
Moderator analysis
Moderator analyses were only conducted for visuospatial working memory composite scores, because individual measures did not have adequate power (k < 20). Effect sizes increased with the percentage of patients receiving psychotropic medication. There were no effects of current symptom severity or age. Remission status and comorbidity could not be analyzed because there were too few samples in each group (k < 5).
Planning
Weighted mean effect size analysis
Patients with MDD had significantly lower composite planning scores than healthy control participants (d = 0.38, k=17).
Moderator analysis
Moderator analyses were not conducted for planning, since it did not have adequate power (k < 20).
Verbal fluency
Weighted mean effect size analyses
Patients with MDD were impaired on the composite verbal fluency score (d = 0.55, k = 46), as well as on semantic (d = 0.70, k = 24) and phonemic (d = 0.46, k = 37) verbal fluency individually. Patients produced significantly fewer words than healthy control participants in both the semantic and phonemic verbal fluency tasks, with a significantly larger effect size for semantic fluency.
Moderator analyses
For verbal fluency composite scores, since there was an effect size difference between semantic and phonemic verbal fluency, task (semantic, phonemic, or average of both) was included as a covariate in the regression analysis. For semantic, phonemic, and composite verbal fluency scores, effect sizes increased with symptom severity. For phonemic and composite verbal fluency scores, there were also effects of remission status, with smaller and non-significant effects for samples in remission. Remission status could not be analyzed for semantic verbal fluency because there were too few samples in remission (k < 5). For phonemic and composite verbal fluency scores, effect sizes increased marginally with the percentage of patients receiving psychotropic medication. There was no effect of medication for semantic verbal fluency. There were no effects of age for any verbal fluency analysis. For phonemic and composite verbal fluency scores, there were no significant effects of comorbidity; comorbidity could not be analyzed for semantic verbal fluency, because too few samples excluded participants with comorbid Axis I disorders (k < 5).
Processing Speed and Vocabulary
Weighted mean effect size analyses
Patients with MDD were significantly slower than healthy controls on both the psychomotor speed composite score (d = 0.33, k = 23) and digit-symbol substitution measures (d = 0.55, k = 22). Patients also had lower scores on measures of vocabulary, but with a very small effect size (d = 0.14, k = 36).
Moderator analysis
For vocabulary, effect sizes increased with symptom severity and mean patient age. There was also an effect of remission status, with smaller and non-significant effect sizes for samples in remission. There were no effects of medication or comorbidity. For psychomotor speed composite scores, effect sizes increased with symptom severity and marginally with mean patient age. There was no effect of medication. Remission status and comorbidity could not be analyzed because there were too few samples in each group (k < 5). For digit-symbol substitution, effect sizes increased marginally with the percentage of patients receiving psychotropic medication. There were no effects of symptom severity or age. Remission status and comorbidity could not be analyzed because there were too few samples in each group (k < 5).
Heterogeneity Analyses
There was significant heterogeneity among effect sizes for all measures except Stroop accuracy, Hayling, self-ordered pointing, and vocabulary, which all had marginal heterogeneity, and the Stroop neutral condition and planning, which were homogenous.
Sensitivity Analyses
Publication Bias
Trim and fill analyses are reported in Table S1, and adjusted weighted mean effect sizes in Table 3. Funnel plots (Figures S1-S8) for most analyses appeared generally symmetrical, and trim and fill analyses were highly robust. In all cases, adjusted effect sizes for neuropsychological measures of EF remained significant. Only one non-EF comparison measure, vocabulary, became marginal. Thus, publication bias cannot account for the finding that MDD is associated with impairments on the neuropsychological measures of EF in the meta-analysis.
IQ and Education Matching
Analyses restricted to IQ and education matched samples are reported in Table S5, and effect sizes for IQ and education matched samples only in Table 3. All analyses with IQ and education matched samples only remained significant, except for spatial span forward and self-ordered pointing, which became marginal, and the psychomotor speed composite score, which became non-significant. However, this should be interpreted with caution given the small number of IQ and education matched studies in the analyses that did not remain significant (k < 10).
Discussion
MDD is Associated with Broad Impairments on Neuropsychological Measures of EF
Overall, the meta-analysis revealed significantly impaired performance for MDD patients, compared to healthy control participants, on all neuropsychological measures of EF, with similar effect sizes across EF domains. Specifically, the composite scores for each domain ranged from d = 0.45–0.58, and did not statistically differ from one another, with the exception of inhibition composite scores (d = 0.58), which fell just above the upper confidence limits for shifting (d = 0.47, upper limit 0.55) and verbal working memory (d = 0.45, upper limit 0.56) composite scores. Thus, there is some limited evidence that inhibition tasks may demonstrate larger impairments in patients with MDD than tasks tapping some other EF domains. However, the fairly narrow range of effect sizes across EF domains is most consistent with broad impairments affecting performance on all neuropsychological measures of EF in patients with MDD. The following sections discuss which cognitive processes may best account for these findings, drawing on a consideration of the demands of each task, as well as relevant neuroimaging findings in the literature.
Inhibition
Patients with MDD were significantly impaired on all inhibition measures. However, patients were also significantly slower to name colors in the neutral condition of the Stroop task, suggesting that poor Stroop performance may be partly due to other demands of the task, such as processing speed or working memory for the task goal, even in the absence of interference. Deficits on the Stroop task likely cannot be entirely attributed to overall slowing however, as patients also had significantly lower Stroop accuracy and higher Stroop interference costs, reflecting differentially slower performance on the incongruent than neutral condition. Thus, slower Stroop performance appears to reflect a combination of deficits specific to the incongruent condition and deficits in working memory and/or non-EF processes that affect both incongruent and congruent conditions. However, many studies calculated interference as a simple RT difference between the incongruent and neutral conditions, rather than the ratio of RTs in these conditions. This approach is problematic: since difference scores scale with overall RT, when participants are slower overall the difference between conditions is also greater. Thus, based on the current evidence, the possibility cannot be fully ruled out that greater Stroop interference scores in patients with MDD are due to slow processing speed alone. To resolve this issue, further research is needed using ratio measures of Stroop interference that control for overall differences in RT.
Comparing the results of the current and previous meta-analyses, the effect size for the Stroop incongruent condition (d = 0.73) is consistent with the effect sizes for Stroop reported by two previous meta-analyses (Christensen et al., 1997; d = 1.00, k = 5, not limited to MDD; Zakzanis et al., 1998; d = 0.69, k = 2), and larger than that reported by a third meta-analysis (Henry & Crawford, 2005; d = 0.17, k = 11). Since these meta-analyses had fewer studies than the current meta-analysis, their effect size estimates are likely to be less stable and representative of the population. Other inhibition tasks were not analyzed in previous meta-analyses.
A further question is which EF processes account for increased Stroop interference effects in MDD. Neuroimaging and theoretical work suggest that selecting and biasing attention towards task-relevant representations may be the specific EF processes contributing most strongly to increased Stroop interference in MDD. The cascade-of-control model (Banich, 2009; Banich et al., 2000; Milham & Banich, 2005) identifies four aspects of EF critical for inhibiting prepotent responses, which depend on distinct neural substrates within PFC: (1) biasing responses towards task-relevant processes (the relevant task or mental set), (2) biasing attention towards task-relevant representations (the relevant stimulus or response required), (3) selecting the information that should guide responding, and (4) evaluating the response. Thus, impaired performance could result from deficits in any one, or a combination, of these processes.
However, some suggestive evidence is provided by neuroimaging studies of the Stroop task. One study found that compared to healthy control participants, patients with MDD had larger behavioral Stroop interference costs and significantly reduced dorsal ACC and left DLPFC activation in the Stroop interference contrast (Holmes & Pizzagalli, 2007). However, another study, which found no behavioral differences between patients with MDD and healthy control participants, reported greater activation for patients in left DLPFC in the Stroop interference contrast (Wagner et al., 2006). While seemingly contradictory, the performance differences across studies suggest that patients with MDD may have larger Stroop interference effects when they fail to adequately recruit left DLPFC and require greater left DLPFC activation than healthy control participants to achieve the same level of performance. In the cascade-of-control model, an area of mid-DLPFC consistent with the DLPFC area identified in these studies is posited to be critical for selecting the most task-relevant representation and biasing attention towards that representation. Thus, this aspect of the Stroop task may be particularly affected in patients with MDD, although further behavioral and fMRI evidence will be needed to confirm this possibility.
Shifting
Patients with MDD were significantly impaired on all measures tapping shifting between task-sets or response rules. However, since both the Wisconsin Card Sorting Test and Intradimensional/Extradimensional Shift task require learning from negative feedback as well as shifting, deficits on both of these tasks could result from either difficulty with shifting itself, or with inducing the new task rules based on feedback. Providing some evidence on this question, MDD was also associated with slowed performance on the Trail Making Test part B, which requires switching between connecting numbers and letters, but does not require learning from negative feedback. However, patients with MDD were also significantly slower on the Trail Making Test part A, which does not require switching, and the effect size for the Trail Making Test part B was numerically but not significantly larger than for the Trail Making Test part A. Thus, slowed performance on the Trail Making Test may primarily reflect deficits in sequencing, processing speed, or working memory for the task goals, rather than shifting processes per se. To determine whether patients with MDD have specific impairments in shifting, more research is needed using tasks that more specifically assess shifting, such as cued task-switching paradigms.
Comparing the results of the current meta-analysis to previous meta-analyses, the effect sizes for the Wisconsin Card Sorting Test (d = 0.47) and Trail Making Test part B (d = 0.59) are consistent with the effect sizes previously reported for these tasks (Christensen et al., 1997; Trail Making Test part B, d = 0.66, k = 5, not limited to MDD; Henry & Crawford, 2005; Wisconsin Card Sorting Test, d = 0.27, k = 14, not limited to MDD; Zakzanis et al., 1998; Trail Making Test part B, d = 0.77, k = 5; Wisconsin Card Sorting Test, d = 0.32, k = 3). Other shifting tasks were not analyzed in previous meta-analyses.
Working memory: updating, verbal working memory, and visuospatial working memory
Patients with MDD were impaired on all tasks requiring updating, manipulating, or maintaining the contents of working memory. The effect sizes for working memory manipulation and updating tasks were significantly larger than for working memory maintenance tasks (above the upper confidence limits for maintenance composite scores and forward digit span). Thus, while MDD was associated with impaired performance even for simple maintenance of verbal information in working memory, there appear to be additional deficits in processes required to add, remove, and re-order information in working memory. Despite the variation in mean effect sizes across the visuospatial working memory tasks, these differences were generally not significant due to the relatively small number of studies reporting each task. Thus, further research is needed to determine which aspects of visuospatial working memory are most impaired in patients with MDD.
Comparing the results of the current meta-analysis to previous meta-analyses, effect sizes for digit and spatial span tasks (d = 0.36–0.72) were somewhat larger than those reported by previous meta-analyses, especially in the case of backward digit span (d = 0.55 in the current meta-analysis; Veiel, 1997; digit span and spatial span, d = 0.18, k = 3; Zakzanis et al., 1998; backward digit span, d = 0.32, k = 10). Since these meta-analyses had fewer studies than the current meta-analysis, their effect size estimates are likely to be less stable and representative of the population. Other working memory tasks were not analyzed in previous meta-analyses.
Neuroimaging studies have found widespread differences in brain activity for patients with MDD versus healthy control participants performing working memory tasks. In the n-back task, two studies found no behavioral effect of MDD, but widespread increases in activation, including VLPFC, DLPFC and ACC (Fitzgerald et al., 2008; Harvey et al., 2005). Similarly, patients with MDD had more DLPFC activation in a delayed-match-to-sample task than healthy control participants, and were also slower and less accurate (Walter, Wolf, Spitzer, & Vasic, 2007). However, one study found no overall differences between groups, although there was a greater increase in VLPFC activation for patients with MDD as load increased in the n-back task (Walsh et al., 2007). A study using a multi-voxel pattern classification technique was able to classify participants into MDD and control groups with 65% sensitivity and 70% specificity based on brain activation during the n-back task (Marquand, Mourão-Miranda, Brammer, Cleare, & Fu, 2008). Brain regions with the highest contribution to this classification included VLPFC, DLPFC, and several posterior cortical areas. Thus, there is evidence for hyper-activation of VLPFC and DLPFC, and perhaps ACC, in patients with MDD performing working memory tasks, consistent with inefficient recruitment of working memory networks. Further evidence for disruptions to working memory networks is provided by a study showing that patients with MDD performing a verbal working memory manipulation task had reduced functional connectivity in PFC-parietal (inferior parietal, superior PFC, and orbitofrontal) and PFC-ACC (ACC, VLPFC, and superior PFC) networks (Vasic, Walter, Sambataro, & Wolf, 2009).
Multifaceted EF tasks: planning and verbal fluency
The Tower of London and its variants require multiple aspects of EF collectively referred to as planning, including formulating goals, selecting moves, sequencing and maintaining moves in working memory, and monitoring progress towards the goal. Thus, planning tasks might be expected to be particularly vulnerable to broad EF deficits, since they include multiple EF demands. However, the effect size for the composite score was the smallest of all neuropsychological EF measures in the meta-analysis. This may be due to methodological factors: some frequently reported dependent measures, such as number of perfect solutions, may lack sensitivity. More studies are needed which report potentially more sensitive measures, such as error rates and reaction times. Two studies using neuroimaging methods, which may be more sensitive than the frequently reported behavioral measures, have found differences in PFC activation between MDD and control groups, but in opposite directions: One study found decreased activation in DLPFC and several posterior cortical regions in patients with MDD (Elliott et al., 1997), while one found increased activation in VLPFC, DLPFC, and several posterior cortical areas (Fitzgerald et al., 2008). Thus, while there is some evidence for altered PFC function in MDD patients during planning tasks, the nature of these changes is unclear.
Verbal fluency tasks are also complex, requiring a variety of EF and non-EF cognitive processes. Maximal performance on verbal fluency tasks requires clustering (production of words within semantic or phonemic subcategories) and switching (shifting between subcategories). To accomplish this, participants must retrieve words from semantic memory, detect the need to switch (e.g., when retrieval of more items from a subcategory fails) and select what to switch to (one of many possible other subcategories and items). In addition, participants must keep track of the words already produced, and monitor their production to avoid repeating words. Patients with MDD were significantly impaired on all verbal fluency measures, and the effect size was significantly larger for semantic than phonemic verbal fluency, consistent with earlier findings (Henry & Crawford, 2005). Why might semantic fluency be more impaired than phonemic fluency? One possibility is that semantic fluency may place heavier demands on switching, and particularly on selecting what to switch to, since category cues are likely to lead to the activation of many category members, which then compete for production (Snyder & Munakata, 2010). The current meta-analysis demonstrates impairments on shifting tasks in MDD that might lead to switching deficits in verbal fluency tasks. Supporting this theory, patients with MDD had reduced activation during verbal fluency in left VLPFC (Okada, Okamoto, Morinobu, Yamawaki, & Yokota, 2003), the same area activated by switching during verbal fluency in healthy participants (Hirshorn & Thompson-Schill, 2006). To test the possibility that switching during verbal fluency is particularly sensitive to MDD, further research is needed using the more sensitive and informative measures of cluster size and switch rate, rather than the total number of words generated.
Comparing the current meta-analysis to previous meta-analyses, the effect sizes for phonemic verbal fluency (d = 0.46) and semantic verbal fluency (d = 0.70) are somewhat larger than those reported by one previous meta-analysis (Henry & Crawford, 2005; phonemic verbal fluency, d = 0.30, k = 53; semantic fluency, d = 0.44, k = 15; not limited to MDD) and somewhat smaller than those reported by two other meta-analyses (Veiel, 1997; phonemic verbal fluency, d = 0.55, k = 3; Zakzanis, et al., 1998; semantic verbal fluency, d = 0.97, k = 2; phonemic verbal fluency, d = 0.61, k = 7). However, the current meta-analysis concurs with the previous meta-analyses in finding larger impairments on semantic verbal fluency than phonemic verbal fluency.
Effect sizes are highly variable across studies
While mean effect sizes reveal broad impairment on neuropsychological measures of EF for patients with MDD, there is also high variability in effect sizes across studies. Specifically, there was significant heterogeneity among effect sizes (as indexed by Qt) in 22 of 30 analyses (73%). There may be multiple sources of this variability. First, some variability is likely due to differences in methodology across studies. In composite score analyses, tasks are likely to vary in sensitivity (e.g., standard neuropsychological tests are less sensitive to subtle impairments than those designed to assess individual differences in the normal range). Even in analyses of single tasks, task versions may vary in sensitivity (for example, the standard neuropsychological version of the Stroop task, with separate blocks of neutral and incongruent stimuli, is easier than versions in which trial types are intermixed). In addition, studies often report different dependent measures for the same task (e.g., number of errors versus number of perfect solutions for the Tower of London), which also vary in sensitivity. Second, some variability is due to differences in the clinical and demographic characteristics of the patient groups included in each study.
Clinical and Demographic Factors Moderate Some Deficits on Neuropsychological Measures of EF
The ability to examine the role of potential moderators was somewhat limited by insufficient numbers of studies for some tasks and domains, and limited reporting of moderator information in the primary research literature. However, the current meta-analysis did provide some evidence that the magnitude of deficits on neuropsychological measures of EF associated with MDD may be vary depending on current depression symptom severity and psychotropic medication use, while evidence for effects of age was weaker, and there was no evidence for effects of excluding comorbid Axis I disorders (Table 6 and discussed in detail below). In addition, analyses including only samples in which MDD and control groups were matched on education level and IQ suggest that deficits on neuropsychological measures of EF associated with MDD are unlikely to be explained by failure to match groups on these important factors. Finally, while differences between MDD subtypes could not be evaluated in the meta-analysis due to inadequate reporting of subtype information, individual studies provide some evidence that the magnitude of impairment on neuropsychological measures of EF may differ between disorder subtypes (Gomez et al., 2006; Michopoulos et al., 2006, 2008; Murata et al., 2001; Rapp et al., 2005; Salloway et al., 1996; Schatzberg et al., 2000).
Table 6.
Summary of Moderator Findings
| Construct | Measure | Severity |
Medication | Age | Comorbidity | |
|---|---|---|---|---|---|---|
| Symptom Severity | Remission Status | |||||
| Inhibition | Inhibition Comp. | √ | X | √ | X | X |
| Stroop Interference | X | - | X | X | X | |
| Shifting | Shifting Comp. | √ | # | X | X | X |
| WCST | # | - | X | X | X | |
| TMT-B | X | X | √ | # | X | |
| Verbal WM | Verbal WM Comp. | X | X | √ | X | X |
| Forward Digit Span | X | X | √ | X | - | |
| Backward Digit Span | # | - | # | X | - | |
| Maintenance Comp. | X | X | √ | X | - | |
| Manipulation Comp. | √ | - | X | X | X | |
| Visuospatial WM | Visuospatial WM Comp. | X | - | √ | X | - |
| Verbal | VF Comp. | √ | √ | # | X | X |
| Fluency | Phonemic VF | √ | √ | # | X | X |
| Semantic VF | √ | - | X | X | - | |
| Comparison | TMT-A | X | X | X | X | X |
| Measures | Psychomotor Speed Comp. | √ | - | X | # | - |
| Digit-Symbol Substitution | X | - | # | X | - | |
| Vocabulary | √ | √ | X | √ | X | |
Note. Comp. = composite score; WCST= Wisconsin Card Sorting Test; TMT-B = Trail Making Test part B; TMT-A = Trail Making Test part A; WM = working memory; VF = verbal fluency; – = Analysis cannot be run (> 5 samples/category).
√= significant effect. # = marg. effect. X= no significant effect.
Current depression symptom severity
Consistent with some previous findings that EF deficits are greater in patients with more severe depressive symptoms (McClintock et al., 2010; McDermott & Ebmeier, 2009), depression symptom severity predicted greater impairment on inhibition composite scores, shifting composite scores, Wisconsin Card Sorting Test, verbal working memory manipulation composite scores, backward digit span, and verbal fluency (composite scores, semantic verbal fluency, and phonemic verbal fluency), controlling for age and medication. Thus, while EF appears to be impaired across multiple psychiatric disorders, performance on some neuropsychological measures of EF is sensitive to the specific symptomatology of depression. However, effects of current symptom severity were not significant for Stroop interference, Trail Making Test part B, verbal working memory overall and maintenance composite scores, or forward digit span (although there were trends in the same direction for all measures except forward digit span). There may be methodological reasons for these mixed findings. First, power to detect symptom severity effects may vary among analyses due to differing distributions of symptom severity levels. Second, it was necessary to recode symptom severity ratings into a common scale with only four levels for the meta-analysis, which may have reduced sensitivity overall. Finally, many samples were quite heterogeneous in terms of symptom severity, so the mean symptom severity rating for the group might not adequately describe the symptom severity level for all patients. To address these issues, more studies are needed which correlate task performance on multiple EF tasks with symptom severity on an individual participant level.
While impairments associated with MDD on many neuropsychological measures of EF are sensitive to current symptom severity, they mainly persist even in remission, although a few measures may be unimpaired during remission. For inhibition composite scores, Trail Making Test part B (and the part A comparison measure), verbal working memory overall and maintenance composite scores, and forward digit span, there were no significant differences between samples with current depression and those in remission. This suggests that at least some EF impairments associated with MDD are present even when patients are not currently depressed. For shifting composite scores, the effect size was marginally larger for samples with current depression, but remained significantly above zero for samples in remission. For verbal fluency composite scores, phonemic verbal fluency, and the vocabulary non-EF comparison measure, effect sizes were significantly larger for samples with current depression, and were not significantly above zero for samples in remission. In the case of measures that show smaller effect sizes in samples with depression in remission, further longitudinal research is needed to determine whether EF improves with recovery from depression, or conversely whether pre-existing EF abilities predict recovery from depression.
Medication
Consistent with previous evidence that antidepressant medications can adversely affect cognitive function (McClintock et al., 2010), the percentage of patients receiving psychotropic medications predicted greater impairment on the inhibition composite score, Trail Making Task part B, verbal working memory measures (overall composite scores, maintenance composite scores, forward and backward digit span), visuospatial working memory composite scores, verbal fluency measures (composite scores and phonemic verbal fluency), and one non-EF comparison measure (digit-symbol substitution), controlling for current symptom severity and age. Importantly, although medication use was associated with larger deficits on these measures, there were significant deficits even in medication-free MDD samples (Table S6)4. There were no effects of medication on Stroop interference, shifting composite scores, Wisconsin Card Sorting Test, or verbal working memory manipulation composite scores (although there were trends in the same direction for all measures).
The strongest association between medication use and task performance was on the verbal working memory composite score. Thus, it is possible that the negative associations between medication and performance on neuropsychological measures of EF may be driven by the working memory demands of each task. However, in the absence of studies using random assignment to medication conditions, correlations between medication use and cognitive function can be difficult to interpret. Apparent negative effects of medication on neuropsychological function could instead reflect other characteristics of patients taking antidepressants. Medication effects in the meta-analysis remained after controlling for current symptom severity. However, other clinical characteristics, such as treatment duration and degree of treatment resistance, which are not captured in symptom severity ratings, may both influence the likelihood that patients receive medication and affect EF. Alternately, medication use may reflect past levels of symptom severity (since medication may have reduced symptom severity in the interim), which may also correspond with poorer EF. Furthermore, an apparent lack of medication effects could arise from a combination of negative and positive effects on cognition, which effectively cancel each other out (Rogers et al., 2004). Thus, it is important to interpret apparent effects, or lack of effects, of medication on EF cautiously.
Age
Mean patient age predicted greater impairment on the Trail Making Test part B and two non-EF comparison measures (psychomotor speed composite scores and vocabulary), controlling for current symptom severity and medication. However, there were no effects of age on any other measures. Thus, while some have argued that EF deficits are more robust in depressed older adults (Elliott, 1998; Porter et al., 2003), the current meta-analysis found little support for this theory. Instead, aging and depression may have additive (rather than superadditive) effects on EF. That is, while older adults may have poorer EF overall, age-related declines may be equal in MDD and healthy control participants, such that difference between groups remains constant with age. However, it is possible that older adults with MDD still face greater functional impairment than younger adults with MDD, due to the combined effect of age and depression related cognitive impairments, and more research is needed to clarify this question. In addition, many samples were quite heterogeneous in terms of age, so the mean age for the group might not adequately describe the age of all patients. To address these issues, more studies are needed which correlate task performance with age on an individual participant level.
Comorbidity
There were no significant differences in effect sizes between studies that excluded patients with comorbid Axis I disorders and those that did not exclude such participants. However, the ability to detect effects of comorbidity was limited in two ways by the methodology of the studies in the meta-analysis. First, the effects of comorbidity could not be investigated for many measures, because too few studies excluded patients with comorbid disorders. Second, most studies report little information about the number and type of comorbid disorders present in their MDD samples. If this information was reported, more sensitive continuous moderator analyses could be conducted, rather than treating comorbidity as a categorical variable. Furthermore, even when patients with comorbid disorders are excluded, MDD patients are still likely to have higher levels of trait anxiety, which may also be related to EF (e.g., Bunce, Handley, & Gaines, 2008; Eysenck & Derakshan, 2011; Gershuny & Sher, 1995; Snyder et al., 2010). Future research would thus benefit from including measures of trait anxiety. Thus, while the meta-analysis provided no evidence that comorbid disorders moderate the association between MDD and neuropsychological measures of EF, more research is needed to differentiate the effects of depression, anxiety, and other aspects of psychopathology.
Education and IQ matching
For the most part, impairments on neuropsychological measures of EF associated with MDD could not be attributed to differences in education or IQ between patient and control groups. Even using the stringent standard of including only studies that reported both education and IQ, and where MDD and control groups were matched on both measures, effect sizes remained significant for all but two analyses of neuropsychological measures of EF (self-ordered pointing and forward spatial span, which became marginally significant). In addition, the non-EF comparison measure psychomotor speed became non-significant. In all analyses where effect sizes were no longer significant there were few studies (k<10), mainly because many studies did not report IQ measures. Thus, these effects should be interpreted with caution. In addition, analysis of a measure of crystallized IQ, vocabulary, revealed only a very small difference between patients with MDD and healthy control participants, which became non-significant in the trim and fill analysis. While future research should certainly assess and report education and IQ to ensure that they do not confound the association between MDD and EF deficits, the current meta-analysis suggests that failure to match on education or IQ cannot explain deficits on most neuropsychological measures of EF. In addition, these results partly address the possibility that deficits on neuropsychological measures of EF could be due to general factors that affect all taxing tasks, such as poor motivation, fatigue, or poor concentration. Specifically, most IQ tests would be considered quite taxing, so the finding that MDD is associated with impairments on neuropsychological measures of EF in IQ matched samples suggests that deficits beyond such general factors may be present.
MDD subtypes
As few studies reported MDD subtype information, potential differences between subtypes could not be formally analyzed. As described below, a few individual studies have directly compared different MDD subtypes, but more research is needed.
Because psychomotor disturbance is a prominent symptom of melancholic depression, some have proposed that cognitive function may be more impaired in melancholic than non-melancholic MDD (Austin et al., 1999), but evidence for larger EF impairments associated with melancholic MDD is inconclusive. Two studies found that patients with melancholic MDD performed significantly more poorly than patients with non-melancholic MDD on a measure of shifting (Intradimensional/Extradimensional Shift task), but not on a measure of planning (Stockings of Cambridge; Michopoulos et al., 2006, 2008). Similarly, another study found poorer performance for patients with melancholic MDD on measures of shifting (Trail Making Test part B and Wisconsin Card Sorting Test), and verbal working memory (backward digit span) compared to healthy control participants, while those with non-melancholic MDD were only impaired on the Wisconsin Card Sorting Test; however, the patient groups did not significantly differ from each other (Austin et al., 1999). On the other hand, a third study found that patients with non-melancholic MDD were impaired on the Stroop task compared to healthy controls, while patients with melancholic MDD were not, although the difference between MDD groups was not significant (Markela-Lerenc, Kaiser, Fiedler, Weisbrod, & Mundt, 2006).
Some have suggested that MDD with psychotic features may share features with schizophrenia, and hence might be associated with more severe cognitive impairment (Schatzberg et al., 2000). One study found greater impairment for patients with psychotic features on multiple neuropsychological measures of EF, including measures of verbal working memory (digit span backward, letter-number sequencing), shifting (Trail Making Test part B), and inhibition (Stroop), but not on forward digit span or verbal fluency (phonemic and semantic; Gomez et al., 2006). Another study found significantly worse performance for patients with psychotic MDD compared to those without psychotic features on measures of shifting (Trail Making Test part B) and inhibition (Stroop; Schatzberg et al., 2000). However, one study found that patients with severe MDD with and without psychotic features were equally impaired on the Wisconsin Card Sorting Test (Ilonen et al., 2000), and one study found that patients with non-psychotic MDD were significantly slower than healthy control participants on the n-back task, while those with psychotic MDD were not (Garrett et al., 2011). Thus, while there is some evidence that MDD with psychotic features may be associated with greater deficits on neuropsychological measures of EF, more research is needed to determine whether this difference is reliable across studies and tasks.
Finally, studies comparing older adults with late-onset versus earlier-onset recurrent MDD have reported mixed results. Two studies found greater impairment for late-onset MDD patients on shifting (Trail Making Test part B; Murata et al., 2001; Rapp et al., 2005), while one did not (Salloway et al., 1996). Two studies found no difference in verbal fluency performance between early and late onset groups (Brodaty et al., 2001; Rapp et al., 2005), while one found poorer performance in the late-onset group (Salloway et al., 1996). A meta-analysis of six studies comparing patients with late and early onset depressive disorders found that those with late onset depression had significantly poorer performance (Herrmann, Goodwin, & Ebmeier, 2007). However, two of the included studies were not limited to patients with MDD. More research is needed comparing patients with early versus late-onset, and recurrent versus first-episode, MDD.
Relation Between EF and Psychomotor Speed Impairments associated with MDD
Are the deficits on neuropsychological measures of EF associated with MDD due to specific EF impairments, or might they be better explained by reduced processing speed? The current meta-analysis demonstrates that MDD is associated with slowed performance on tasks designed to assess processing speed. However, two pieces of evidence suggest that motor slowing cannot fully account for impaired performance on neuropsychological measures of EF. First, impairments on neuropsychological measures of EF are larger than on motor speed tasks. The psychomotor speed composite score effect size (d = 0.33) was numerically smaller than all neuropsychological measures of EF except delayed-match-to-sample (a simple visuospatial maintenance task), and was below the lower 95% confidence limit of 13 measures: (1) inhibition composite scores, Stroop incongruent, Stroop accuracy, and Hayling, (2) shifting composite scores and the Trail Making Test part B, (3) verbal working memory overall composite scores, manipulation composite scores, and backward digit span, (4) backward spatial span, and (5) all verbal fluency measures (composite scores, semantic, and phonemic). Moreover, most of the effects that where not significantly larger than the psychomotor speed composite effect were either accuracy measures (Wisconsin Card Sorting Test, Intradimensional/Extradimensional Shift task, forward digit span, forward spatial span, self-ordered pointing, planning composite score), or difference scores designed to control for processing speed (Stroop interference). Thus, it is unlikely that motor slowing accounts for deficits on these tasks.
Second, the motor slowing hypothesis posits that processing speed deficits should lead to longer reaction times and impaired performance on speeded tasks, but not performance decrements on self-paced accuracy measures. Contrary to this hypothesis, there were robust impairments for patients with MDD on accuracy measures from self-paced neuropsychological measures of EF, including the Wisconsin Card Sorting Test, Intradimensional/Extradimensional Shift task, planning tasks, and self-ordered pointing task. Motor slowing could not account for impairments on these tasks, since they do not require or assess fast motor responses. Thus, while there is significant motor slowing in MDD patients that may contribute to poor performance on timed tasks, motor slowing cannot account for the presence or extent of deficits on all neuropsychological measures of EF. Moreover, these findings suggest that poor performance by patients with MDD on reaction time based neuropsychological measures of EF are unlikely to be due to a speed-accuracy trade-off (e.g., a more ‘cautious’ response style in patients with MDD), since patients with MDD are impaired on both speed and accuracy measures.
Compared to the small effect for simple psychomotor speed tasks, there were larger effects for the more complex comparison tasks. However, while these tasks have been considered measures of processing speed, they may in fact impose working memory and other EF demands as well. Indeed, it has long been known that the digit-symbol substitution task makes demands on multiple cognitive abilities, including working memory and sustained attention (e.g., Lezak, 1983; van Hoof et al., 1998). Likewise, the Trail Making Test part A is likely to impose some working memory demands, as participants must remember the task goal of connecting the items in sequence, as well as remembering the next item in the sequence. The working memory demands of naming colors in the Stroop neutral condition would seem to be more minimal. However, since only a few colors are generally used, it is possible that repeatedly naming the same colors causes response competition in the same way as repeatedly naming semantically related pictures (Schnur, Brecher, Rossi, & Schwartz, 2004; Schnur, Schwartz, Brecher, & Hodgson, 2006). Thus, these tasks may not be well suited to differentiating the effects of MDD on processing speed and EF. In sum, while patients with MDD are impaired on processing speed tasks, deficits on simple psychomotor speed tasks cannot fully explain impairments on neuropsychological measures of EF, and deficits on more complex processing speed tasks may be partly due to their unintended EF demands.
Is subclinical dysphoria associated with deficits on neuropsychological measures of EF?
The clinical phenotype of MDD may be the end point of underlying dysfunction in neural networks and cognitive and emotional control processes (e.g., Clark, Chamberlain, & Sahakian, 2009). From this perspective, deficits associated with MDD might also occur, perhaps in milder form, in those with subclinical dysphoria. While there have been relatively few studies, there is some evidence that dysphoria in non-clinical samples is associated with impairments on neuropsychological measures of EF. Depressive symptoms are associated with poorer performance on a composite EF score (Trail Making Test part B, phonemic verbal fluency, and clock drawing; Ganguli, Snitz, Vander Bilt, & Chang, 2009), the Wisconsin Card Sorting Test (Channon, 1996), the Stroop and Simon tasks (Holmes & Pizzagalli, 2007), and verbal fluency (but not Stroop; Baune, Suslow, Engelien, Arolt, & Berger, 2006). However, one study found no association between depression symptoms and a composite EF score (updating, task switching, and Stroop tasks; Bunce et al., 2008). In addition, neural function during neuropsychological measures of EF may be altered in participants with dysphoria. Studies with the Stroop task have found that dysphoria is associated with altered patterns of prefrontal activity (increased left DLPFC, left VLPFC, and ACC, and decreased right DLPFC activation; Killgore, Gruber, & Yurgelun-Todd, 2007); decreased amplitude in the ERP pre-stimulus slow wave, considered a measure of proactive cognitive control (West, Choi, & Travers, 2010); and larger P300 ERP amplitudes, interpreted as inefficient neural processing (Krompinger & Simons, 2011). Thus, there is preliminary but mixed evidence that dysphoria in non-clinical samples is associated with impairments on neuropsychological measures of EF, and differences in associated brain activity. However, it is important to note that participants in these studies were not formally assessed for MDD, so it is possible that some were clinically depressed. More research is needed with a wider variety of tasks, and in samples screened for clinical disorders, to determine whether the widespread deficits associated with MDD extend to subclinical dysphoria.
Clinical Implications
EF impairments associated with MDD may have important implications for treatment. Impaired cognitive functioning affects family and social life, as well as school and work performance (Hammar & Ardal, 2009). Therefore, full assessment of EF may be useful in developing therapeutic goals tailored to each patient. For example, a patient who has trouble shifting may benefit from help planning strategies to transition more easily between daily tasks. In addition, EF may be related to the ability to regulate emotion and responses to negative information: lateral PFC provides top-down control over responses to emotional material in the amygdala and associated limbic regions (for a recent review, see Heatherton & Wagner, 2011). Patients with MDD are impaired at inhibiting attention to negative emotional stimuli (see Peckham et al., 2010 for a meta-analysis), and may have difficulty preventing negative information from entering and remaining in working memory, leading to rumination (Joormann, 2010). Thus, addressing deficits in EF, including working memory, may lead to new approaches to improve emotion regulation and reduce rumination. Simply being aware of how EF deficits can affect daily life function may also be helpful for patients with MDD. For example, while patients with MDD in remission are often expected to function at a pre-morbid level, this may not be a realistic expectation if EF continues to be impaired (Hammar & Ardal, 2009). Unrealistic expectations may lead to frustration and feelings of worthlessness, which could contribute to the risk of relapse (Hammar & Ardal, 2009). Cognitive training and rehabilitation during remission could thus help improve quality of life and prevent relapse.
EF may also be important for predicting treatment response. While the reason is not known, depressed patients with EF deficits appear to have a slow or poor response to antidepressant treatment (DeBattista, 2005; Dunkin et al., 2000). In addition, the role of cognitive processes, including EFs, in treatment compliance is often overlooked. For example, the efficacy of cognitive behavioral therapy, a widely used treatment for MDD, is believed to partly depend on patients’ use of EF (Mohlman & Gorman, 2005). Patients are asked to do thought restructuring exercises, formulate and implement behavioral plans, and monitor their own cognition and behavior, all of which involve EF. If clinicians are aware of how poor EF may be interfering with treatment compliance, they may be able to work around these deficits and increase the likelihood that patients understand and implement intervention strategies.
Implications for Theories of Executive and Prefrontal Function
Better understanding the nature of EF deficits associated with disorders such as MDD may also have implications for refining theories of EF and prefrontal function. The results of the current meta-analysis are consistent with the theory that patients with MDD may have impairments in the unitary component of EF (i.e., common EF; Friedman et al., 2008; Miyake et al., 2000). Although other explanations are also possible (e.g., multiple specific aspects of EF could be independently impaired in MDD), impairment in common EF is the most parsimonious interpretation. Indeed, a recent latent-variable analysis likewise concluded that depressive symptoms were associated with poor common EF (Sabella, Miyake, Friedman, Young, & Hewitt, in preparation). Together with the current meta-analysis, this suggests that further investigating the possibility of impaired common EF in patients with MDD is a fruitful area for future research. If this theory is born out, understanding what is altered neurobiologically in patients with MDD may lead to a better understanding of the processes and neural mechanisms underlying common EF. For example, evidence for reduced PFC excitatory neurotransmission in patients with MDD (e.g., Yüksel & Ongur, 2010) could suggest a reduced ability to actively maintain representations (e.g., of task goals, rules, and information) in working memory. This would support one theory of the nature of common EF, which has thus far been speculative (Miyake et al., 2000). Active maintenance of representations in PFC has been posited as a critical mechanism involved in multiple aspects of EF (e.g., Chatham et al., in press). Clinical research using genetic, neuroimaging, and behavioral methods could be profitably combined with basic cognitive neuroscience research to develop more complete models of how active maintenance and other aspects of prefrontal function are neurally implemented and how they support EF.
Conclusions and Future Directions
In sum, MDD is associated with significant impairments on all neuropsychological measures of EF. In addition, some evidence suggests that these deficits are greater in patients with more severe depression, and those taking psychotropic medications. While patients with MDD also have slower processing speed, motor slowing alone cannot account for these results. Further research is needed with cognitively demanding non-EF measures to rule out the possibility that general problems with concentration, alertness, or motivation could account for impairments on neuropsychological measures of EF. However, samples matched on measures of IQ, which are generally cognitively demanding, still show robust impairments on neuropsychological measures of EF, suggesting that the results are not due to such non-specific problems. Rather, these findings are consistent with the view that these deficits can be accounted for by impairment of the general EF component shared by all EF tasks, posited to be maintenance of task goals in working memory (Sabella et al., in preparation). Future research is needed to refine understanding of how such broad deficits arise. One possibility is that impaired function in brain networks involved in EF, including PFC, may lead to broad impairment in EF (e.g., Davidson et al., 2002). Indeed, neuroimaging evidence suggests that areas of the PFC are inefficiently recruited during neuropsychological measures of EF in patients with MDD.
Patterns of EF impairment across disorders
Future research is needed to clarify which EF deficits may be specific to MDD, and which may be shared across disorders. EF impairments have been identified in many psychiatric disorders (e.g., Fioravanti et al., 2005; Olley et al., 2007; Willcutt et al., 2005), but it remains unclear whether the profile of deficits across EF components differs among disorders. Differences in brain structure and function across disorders (e.g., Konarski et al., 2008; Mana, Paillere Martinot, & Martinot, 2010) suggest that different patterns of cognitive function might be expected, and some studies have reported differences on neuropsychological measures of EF between patients with MDD and those with other disorders (e.g., Egeland, Sundet, & Rund, 2003; Tavares et al., 2007). For example, some studies have reported that only patients with MDD were impaired on measures of planning (while euthymic bipolar patients were not; Maalouf et al., 2010), visuospatial working memory and shifting (while bipolar patients were not; Tavares et al., 2007), and verbal working memory manipulation (while schizophrenic patients were not; Fossati, Amar, Rauoux, Ergis, & Allilaire, 1999). In addition, the current meta-analysis and a previous meta-analysis (McDermott & Ebmeier, 2009) both found that impairments on neuropsychological measures of EF correlated with depression severity, suggesting that EF is sensitive to the specific symptomatology of depression. On the other hand, compared to patients with MDD, several studies have reported greater impairment on some neuropsychological measures of EF for patients with schizophrenia (Stroop; Egeland et al., 2003; phonemic verbal fluency; Fossati et al., 1999) and bipolar disorder (spatial working memory; Sweeney, Kmiec, & Kupfer, 2000). While these studies suggest that there may be some differences in the pattern or magnitude of EF deficits across diagnostic groups, other studies have found similar deficits on neuropsychological measures of EF associated with multiple disorders (e.g., MDD, bipolar, and schizophrenia/schizoaffective disorders; Harris, Reilly, Thase, Kashavan, & Sweeney, 2009; Hill et al., 2009; Neu, Kiesslinger, Schlattmann, & Reischies, 2001; MDD and obsessive-compulsive disorder; Meiran, Diamond, Toder, & Namets, 2011).
It is likely that there are both differences in EF among disorders (e.g., due to unique genetic and environmental influences) and shared aspects of EF impairment across disorders. Specifically, PFC hypoactivity that leads to impaired EF may be a transdiagnostic risk factor for psychopathology (Nolen-Hoeksema & Watkins, 2011). For example, an impaired ability to update working memory based on current context and goals may allow irrelevant negative material to enter and remain in working memory (e.g., Joormann, 2010; Joormann & Gotlib, 2008). This may lead to rumination and difficulty breaking away from habitual thought patterns to engage in effective reappraisal and problem solving (Joormann, 2010). Individuals who engage in rumination rather than adaptive reappraisal and problem-solving strategies are at risk for multiple forms of psychopathology (see Aldao, Nolen-Hoeksema, & Schwizer, 2010 for a meta-analysis). This general vulnerability may combine with unique genetic, neurobiological, and environmental factors to produce divergent trajectories, determining the specific clinical and neuropsychological profiles associated with each disorder (Nolen-Hoeksema & Watkins, 2011). Future studies that systematically investigate relations between EF impairments, other risk factors, and psychopathology will be needed to test this possibility.
Causal links between EF and MDD
While EF impairments as a transdiagnostic risk factor for psychopathology is one possible causal link between EF deficits and MDD, it is not the only one. There are (at least) four non-mutually-exclusive possibilities. First, EF impairments could be caused by factors such as neurobiological differences, neurovascular changes, stress exposure, or trait anxiety, which also confer risk for MDD in particular or psychopathology more broadly. Some of these risk factors are likely environmental. For example, rodent models demonstrate the PFC neurons are highly sensitive to stress exposure, suggesting that stress-induced alterations in PFC function may be the main neural insult underlying EF deficits in many neuropsychiatric disorders (Holmes & Wellman, 2009). However, other risk factors are genetic, as MDD is approximately 30-40% heritable (e.g., Sullivan, Neale, & Kendler, 2000), and a number of genes have been identified which confer risk for MDD, including glutamate receptor genes (e.g., Tsunoka et al., 2009). Some of these genes could also contribute to PFC dysfunction, and thus EF impairments. Importantly, genetic and environmental risk factors may interact: for example, early life stress interacts with the effects of genes in the serotonin, hypothalamic-pituitary-adrenal axis, and neurotrophin systems in predicting depressive and anxiety disorders (Nugent, Tyrka, Carpenter, & Price, 2011).
Second, depression could cause neurobiological changes that in turn cause EF impairment. Recurrent MDD is associated with more functional impairments than single-episode MDD, suggesting that there may be cumulative effects of depression (McClintock et al., 2010). For example, glutamate levels in PFC are correlated with the duration of recurrent MDD (Portella et al., 2011). In addition, increased production of inflammatory cytokines (which may in turn be triggered by stress) in MDD could lead to brain damage via neurodegeneration and reduced neurogenesis (Catena-Dell'Osso, Bellantuono, Consoli, Rotella, & Marazziti, 2011).
Third, current depression may directly impair EF, either because of changes in brain function or because symptoms like rumination occupy cognitive resources. For example, experimentally induced rumination impaired Stroop performance in dysphoric participants, suggesting that task-irrelevant, distracting thoughts may play a role in EF deficits (Philippot & Brutoux, 2008). Lastly, EF impairments could contribute to relapse or maintenance of depressive episodes, perhaps by contributing to feelings of frustration, helplessness and low self-worth (Hammar & Ardal, 2009). Understanding which of these models best accounts for EF deficits in patients with MDD will be critical for developing strategies for prevention and remediation. In particular, there is a need for longitudinal studies that provide evidence about the directional links between EF and depression at neuropsychological, neurobiological and behavioral levels. However, regardless of the causal link, understanding impairments in EF is a key aspect of understanding the challenges patients with MDD face as they navigate their daily lives.
Supplementary Material
Acknowledgments
Preparation of this manuscript was supported by a fellowship from the National Institutes of Health (F31-MH087073). I thank Yuko Munakata, Mark Whisman, Marie Banich, Wendy Heller, Akira Miyake, Roselinde Henderson, and members of the Cognitive Development Center for valuable discussions.
Appendix A
Studies Included in the Meta-Analysis
- Airaksinen E, Larsson M, Lundberg I, Forsell Y. Cognitive functions in depressive disorders: Evidence from a population-based study. Psychological Medicine. 2004;34:83–91. doi: 10.1017/s0033291703008559. doi:10.1017/S0033291703008559. [DOI] [PubMed] [Google Scholar]
- Austin M, Mitchell P, Wilhelm K, Parker G, Hickie I, Brodaty H, Hadzi-Pavlovic D. Cognitive function in depression: A distinct pattern of frontal impairment in melancholia? Psychological Medicine. 1999;29:73–85. doi: 10.1017/s0033291798007788. doi:10.1017/S0033291798007788. [DOI] [PubMed] [Google Scholar]
- Austin MP, Ross M, Murray C, O'Carroll RE. Cognitive function in major depression. Journal of Affective Disorders. 1992;25:21–30. doi: 10.1016/0165-0327(92)90089-o. doi:10.1016/0165-0327(92)90089-O. [DOI] [PubMed] [Google Scholar]
- Ayotte BJ, Potter GG, Williams HT, Steffens DC, Bosworth HB. The moderating role of personality factors in the relationship between depression and neuropsychological function among older adults. International Journal of Geriatric Psychiatry. 2009;24:1010–1019. doi: 10.1002/gps.2213. doi:10.1002/gps.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Baba H, Noguchi I, Arai R, Suzuki T, Mimura M, Arai H. Executive dysfunction in remitted late-life depression: Juntendo University Mood Disorder Projects (JUMP). The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22:70–74. doi: 10.1176/jnp.2010.22.1.70. doi:10.1176/appi.neuropsych.22.1.70. [DOI] [PubMed] [Google Scholar]
- Baldwin R, Jeffries S, Jackson A, Sutcliffe C, Thacker N, Scott M, Burns A. Treatment response in late-onset depression: Relationship to neuropsychological, neuroradiological and vascular risk factors. Psychological Medicine. 2004;34:125–136. doi: 10.1017/s0033291703008870. doi:10.1017/S0033291703008870. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biological Psychiatry. 2003;53:376–384. doi: 10.1016/s0006-3223(02)01674-8. doi:10.1016/S0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- Baudic S, Tzortzis C, Barba GD, Traykov L. Executive deficits in elderly patients with major unipolar depression. Journal of Geriatric Psychiatry and Neurology. 2004;17:195–201. doi: 10.1177/0891988704269823. doi:10.1177/0891988704269823. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26:591–603. doi: 10.1017/s0033291700035662. doi:10.1017/S0033291700035662. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. Journal Of Nervous And Mental Disease. 2010;198:513–515. doi: 10.1097/NMD.0b013e3181e4c5ba. doi:10.1097/NMD.0b013e3181e4c5ba. [DOI] [PubMed] [Google Scholar]
- Boone KB, Lesser IM, Miller BL, Wohl M. Cognitive functioning in older depressed outpatients: Relationship of presence and severity of depression to neuropsychological test scores. Neuropsychology. 1995;9:390–398. doi:10.1037/0894-4105.9.3.390. [Google Scholar]
- Borkowska A, Drozdz W. The Wisconsin Card Sorting Test and the n-back test in mild cognitive impairment and elderly depression. World Journal of Biological Psychiatry. 2009;10:870–876. doi: 10.1080/15622970701557985. doi:10.1080/15622970701557985. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. doi:10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Canuto A, Giannakopoulos P, Moy G, Rubio MM, Ebbing K, Meiler-Mititelu C, Weber K. Neurocognitive deficits and personality traits among euthymic patients with mood disorders in late life. Journal of Neurological Sciences. 2010;299:24–29. doi: 10.1016/j.jns.2010.08.045. doi:10.1016/j.jns.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Castaneda AE, Suvisaari J, Marttunen M, Perälä J, Saarni SI, Aalto-Setälä T, Tuulio-Henriksson A. Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. Journal of Affective Disorders. 2008;110:36–45. doi: 10.1016/j.jad.2007.12.239. doi:10.1016/j.jad.2007.12.239. [DOI] [PubMed] [Google Scholar]
- Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M. Impulsivity in depressed children and adolescents: A comparison between behavioral and neuropsychological data. Psychiatry Research. 2005;136:123–133. doi: 10.1016/j.psychres.2004.12.012. doi:10.1016/j.psychres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Channon S, Baker J, Robertson M. Working memory in clinical depression: An experimental study. Psychological Medicine. 1993;23:87–91. doi: 10.1017/s0033291700038873. doi:10.1017/S0033291700038873. [DOI] [PubMed] [Google Scholar]
- Channon S, Green PS. Executive function in depression: The role of performance strategies in aiding depressed and non-depressed participants. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66:162–171. doi: 10.1136/jnnp.66.2.162. doi:10.1136/jnnp.66.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant EL, Adam S, Gillain B, Seron X, Bruyer R, Seghers A. Effects of sertaline on depressive symptoms and attentional and executive functions in major depression. Depression and Anxiety. 2005;21:78–89. doi: 10.1002/da.20060. doi:10.1002/da.20060. [DOI] [PubMed] [Google Scholar]
- Degl'Innocenti A, Agren H, Bäckman L. Executive deficits in major depression. Acta Psychiatrica Scandinavica. 1998;97:182–188. doi: 10.1111/j.1600-0447.1998.tb09985.x. doi:10.1111/j.1600-0447.1998.tb09985.x. [DOI] [PubMed] [Google Scholar]
- Delaloye C, Baudois S, de Bilbao F, Dubois Remund C, Hofer F, Lamon M, Giannakopoulos P. Cognitive impairment in late-onset depression. Limited to a decrement in information processing resources? European Neurology. 2008;60:149–154. doi: 10.1159/000144086. doi:10.1159/000144086. [DOI] [PubMed] [Google Scholar]
- Den Hartog H, Derix M, van Bemmel A, Kremer B, Jolles J. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: Testing the effort and cognitive speed hypotheses. Psychological Medicine. 2003;33:1443–1451. doi: 10.1017/s003329170300833x. doi:10.1017/S003329170300833X. [DOI] [PubMed] [Google Scholar]
- Egeland J, Rund BR, Sundet K, Landrø NI, Asbjørnsen A, Lund A, Hugdahl K. Attention profile in schizophrenia compared with depression: Differential effects of processing speed, selective attention and vigilance. Acta Psychiatrica Scandinavica. 2003;108:276–284. doi: 10.1034/j.1600-0447.2003.00146.x. doi:10.1034/j.1600-0447.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- Egeland J, Sundet K, Rund BR, Asbjørnsen A, Hugdahl K, Landrø NI, Stordal KI. Sensitivity and specificity of memory dysfunction in schizophrenia: A comparison with major depression. Journal of Clinical and Experimental Neuropsychology. 2003;25:79–93. doi: 10.1076/jcen.25.1.79.13630. doi:10.1076/jcen.25.1.79.13630. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Hellemann G, Pham D, Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. International Journal of Geriatric Psychiatry. 2009;24:459–468. doi: 10.1002/gps.2137. doi:10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology. 2006;21:669–676. doi: 10.1016/j.acn.2006.05.011. doi:10.1016/j.acn.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: A proof of principle study. Psychological Medicine. 2007;37:1229–1238. doi: 10.1017/S0033291707001110. doi:10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker S, Rogers R, O'leary D, Paykel E, Frith C, Sahakian B. Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychological Medicine. 1997;27:931–942. doi: 10.1017/s0033291797005187. doi:10.1017/S0033291797005187. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychological Medicine. 1996;26:975–989. doi: 10.1017/s0033291700035303. doi:10.1017/S0033291700035303. [DOI] [PubMed] [Google Scholar]
- Exner C, Lange C, Irle E. Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. Journal of Affective Disorders. 2009;119:156–162. doi: 10.1016/j.jad.2009.03.015. doi:10.1016/j.jad.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Favre T, Hughes C, Emslie G, Stavinoha P, Kennard B, Thamas C. Executive functioning in children and adolescents with major depressive disorder. Child Neuropsychology. 2008;15:85–98. doi: 10.1080/09297040802577311. doi:10.1080/09297040802577311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, Egan GF. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Human Brain Mapping. 2008;29:490–501. doi: 10.1002/hbm.20414. doi:10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis A, Allilaire J. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. doi:10.1016/S0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Fossati P, Ergis AM, Allilaire JF. Problem-solving abilities in unipolar depression: Comparison on the modified version of the Wisconsin and the California sorting tests. Psychiatry Research. 2001;104:145–156. doi: 10.1016/s0165-1781(01)00307-9. doi:10.1016/S0165-1781(01)00307-9. [DOI] [PubMed] [Google Scholar]
- Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. American Journal of Psychiatry. 2011;168:173–182. doi: 10.1176/appi.ajp.2010.09121718. doi:10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, Le Gall D. Cognitive inhibition and working memory in unipolar depression. Journal of Affective Disorders. 2009;116:100–105. doi: 10.1016/j.jad.2008.10.028. doi:10.1016/j.jad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, Schatzberg AF. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biological Psychiatry. 2006;60:472–478. doi: 10.1016/j.biopsych.2005.11.010. doi:10.1016/j.biopsych.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Posener JA, Keller J, Debattista C, Solvason B, Schatzberg AF. Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology. 2009;34:1012–1018. doi: 10.1016/j.psyneuen.2009.01.017. doi:10.1016/j.psyneuen.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biological Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. doi:10.1016/S0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber O, Zilles D, Kennel J, Gruber E, Falkai P. A systematic experimental neuropsychologial investigation of the functional integrity of working memory circuits in major depression. European Archives of Psychiatry and Clinical Neuroscience. 2010;261:179–184. doi: 10.1007/s00406-010-0165-3. doi:10.1007/s00406-010-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:217–225. doi: 10.1176/jnp.2006.18.2.217. doi:10.1176/appi.neuropsych.18.2.217. [DOI] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50:307–316. doi: 10.1111/j.1469-7610.2008.01972.x. doi:10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Hammar A, Sørensen L, Ardal G, Oedegaard KJ, Kroken R, Roness A, Lund A. Enduring cognitive dysfunction in unipolar major depression: A test-retest study using the Stroop paradigm. Scandinavian Journal of Psychology. 2009;51:304–308. doi: 10.1111/j.1467-9450.2009.00765.x. doi:10.1111/j.1467-9450.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Hammar A, Strand M, Ardal G, Schmid M, Lund A, Elliott R. Testing the cognitive effort hypothesis of cognitive impairment in major depression. Nordic Journal of Psychiatry. 2010:1–7. doi: 10.3109/08039488.2010.494311. online. doi:10.3109/08039488.2010.494311. [DOI] [PubMed] [Google Scholar]
- Harvey P-O, Fossati P, Pochon J-B, Levy R, Lebastard G, Lehéricy S, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. doi:10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P. Executive functions and updating of the contents of working memory in unipolar depression. Journal of Psychiatric Research. 2004;38:567–576. doi: 10.1016/j.jpsychires.2004.03.003. doi:10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Northoff G, Boeker H, Boesiger P, Grimm S. Emotional processing and executive functions in major depressive disorder: Dorsal prefrontal activity correlates with performance in the intra-extra dimensional set shift. Acta Neuropsychiatrica. 2010;22:269–279. doi: 10.1111/j.1601-5215.2010.00494.x. doi:10.1111/j.1601-5215.2010.00494.x. [DOI] [PubMed] [Google Scholar]
- Herrera-Guzman I, Herrera-Abarca JE, Gudayol-Ferre E, Herrera-Guzman D, Gomez-Carbajal L, Pena-Olivera M, Joan G-O. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Research. 2010;177:323–329. doi: 10.1016/j.psychres.2010.03.006. doi:10.1016/j.psychres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Ehlis AC, Fallgatter AJ. Bilaterally reduced frontal activation during verbal fluency task in depressed patients as measured by near-infrared spectroscopy. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16:170–175. doi: 10.1176/jnp.16.2.170. doi:10.1176/appi.neuropsych.16.2.170. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Harris MSH, Rosen C, Marvin RW, DeLeon O, Sweeney JA. A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophrenia Research. 2009;113:167–175. doi: 10.1016/j.schres.2009.04.020. doi:10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: Association with salivary cortisol. Biological Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. doi:10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. doi:10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueng T-T, Lee IH, Guog Y-J, Chen KC, Chen SS, Chuang SP, Yang YK. Is a patient-administered depression rating scale valid for detecting cognitive deficits in patients with major depressive disorders? Psychiatry and Clinical Neurosciences. 2011;65:70–76. doi: 10.1111/j.1440-1819.2010.02166.x. doi:10.1111/j.1440-1819.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Ilonen T, Taiminen T, Karlsson H, Lauerma H, Tuimala P, Leinonen K, Salokangas R. Impaired Wisconsin Card Sorting Test performance in first-episode severe depression. Nordic Journal of Psychiatry. 2000;54:275–280. doi: 10.1053/comp.2000.9017. doi:10.1053/comp.2000.9017. [DOI] [PubMed] [Google Scholar]
- Kaneda Y. Verbal working memory impairment in patients with current episode of unipolar major depressive disorder and in remission. Clinical Neuropharmacology. 2009;32:346–347. doi: 10.1097/WNF.0b013e3181b130a0. doi:10.1097/WNF.0b013e3181b130a0. [DOI] [PubMed] [Google Scholar]
- Katz R, De Sanctis P, Mahoney JR, Sehatpour P, Murphy CF, Gomez-Ramirez M, Foxe JJ. Cognitive control in late-life depression: Response inhibition deficits and dysfunction in the anterior cingulate cortex. The American Journal of Geriatric Psychiatry. 2010;18:1017–1025. doi: 10.1097/JGP.0b013e3181d695f2. doi:10.1097/JGP.0b013e3181d695f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymak SU, Demir B, Senturk S, Tatar I, Aldur MM, Ulug B. Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:217–223. doi: 10.1007/s00406-009-0045-x. doi:10.1007/s00406-009-0045-x. [DOI] [PubMed] [Google Scholar]
- Kertzman S, Reznik I, Hornik-Lurie T, Weizman A, Kotler M, Amital D. Stroop performance in major depression: selective attention impairment or psychomotor slowness? Journal of Affective Disorders. 2010;122(1-2):167–173. doi: 10.1016/j.jad.2009.08.009. doi:10.1016/j.jad.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Korhonen V, Laukkanen E, Antikainen R, Peiponen S, Lehtonen J, Viinamaki H. Effects of major depression on cognitive performance among treatment-seeking adolescents. Nordic Journal of Psychiatry. 2002;56:187–193. doi: 10.1080/080394802317607165. doi:10.1080/080394802317607165. [DOI] [PubMed] [Google Scholar]
- Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:995–1005. doi: 10.1111/j.1469-7610.2004.00400.x. doi:10.1111/j.1469-7610.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Lampe IK, Sitskoorn MM, Heeren TJ. Effects of recurrent major depressive disorder on behavior and cognitive function in female depressed patients. Psychiatry Research. 2004;125:73–79. doi: 10.1016/j.psychres.2003.12.004. doi:10.1016/j.psychres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Landrø NI, Stiles TC, Sletvold H. Neuropsychological function in nonpsychotic unipolar major depression. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2001;14:233–240. [PubMed] [Google Scholar]
- Lemelin S, Baruch P, Vincent A, Everett J, Vincent P. Distractibility and processing resource deficit in major depression. Evidence for two deficient attentional processing models. Journal Of Nervous And Mental Disease. 1997;185:542–548. doi: 10.1097/00005053-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Levens SM, Muhtadie L, Gotlib IH. Rumination and Impaired Resource Allocation in Depression. Journal of Abnormal Psychology. 2009;118:757–766. doi: 10.1037/a0017206. doi:10.1037/a0017206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. The American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. doi:10.1097/00005053-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landro NI. Attentional functions in major depressive disorders with and without comorbid anxiety. Archieves of Clinical Neuropsychology. 2011;26:38–47. doi: 10.1093/arclin/acq095. doi:10.1093/arclin/acq095. [DOI] [PubMed] [Google Scholar]
- Maalouf FT, Klein C, Clark L, Sahakian BJ, Labarbara EJ, Versace A, Phillips ML. Impaired sustained attention and executive dysfunction: Bipolar disorder versus depression-specific markers of affective disorders. Neuropsychologia. 2010;48:1862–1868. doi: 10.1016/j.neuropsychologia.2010.02.015. doi:10.1016/j.neuropsychologia.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahurin RK, Velligan DI, Hazleton B, Mark Davis J, Eckert S, Miller AL. Trail making test errors and executive function in schizophrenia and depression. Clinical Neuropsychology. 2006;20:271–288. doi: 10.1080/13854040590947498. doi:10.1080/13854040590947498. [DOI] [PubMed] [Google Scholar]
- Markela-Lerenc J, Kaiser S, Fiedler P, Weisbrod M, Mundt C. Stroop performance in depressive patients: A preliminary report. Journal of Affective Disorders. 2006;94:261–267. doi: 10.1016/j.jad.2006.04.011. doi:10.1016/j.jad.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Matthews K, Coghill D, Rhodes S. Neuropsychological functioning in depressed adolescent girls. Journal of Affective Disorders. 2008;111:113–118. doi: 10.1016/j.jad.2008.02.003. doi:10.1016/j.jad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. The American Journal of Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Michopoulos I, Zervas IM, Pantelis C, Tsaltas E, Papakosta V-M, Boufidou F, Lykouras L. Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:217–225. doi: 10.1007/s00406-007-0781-8. doi:10.1007/s00406-007-0781-8. [DOI] [PubMed] [Google Scholar]
- Michopoulos I, Zervas IM, Papakosta VM, Tsaltas E, Papageorgiou C, Manessi T, Soldatos CR. Set shifting deficits in melancholic vs. non-melancholic depression: Preliminary findings. European Psychiatry. 2006;21:361–363. doi: 10.1016/j.eurpsy.2006.03.008. doi:10.1016/j.eurpsy.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, Arai H. Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders. 2008;111:46–51. doi: 10.1016/j.jad.2008.01.027. doi:10.1016/j.jad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, Reynolds CF. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30:679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, Reynolds CF. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: A randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. doi:10.1016/S0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Neu P, Bajbouj M, Schilling A, Godemann F, Berman RM, Schlattmann P. Cognitive function over the treatment course of depression in middle-aged patients: Correlation with brain MRI signal hyperintensities. Journal of Psychiatric Research. 2005;39:129–135. doi: 10.1016/j.jpsychires.2004.06.004. doi:10.1016/j.jpsychires.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Neu P, Kiesslinger U, Schlattmann P, Reischies FM. Time-related cognitive deficiency in four different types of depression. Psychiatry Research. 2001;103:237–247. doi: 10.1016/s0165-1781(01)00286-4. doi:10.1016/S0165-1781(01)00286-4. [DOI] [PubMed] [Google Scholar]
- O'Brien JT, Lloyd A, Mckeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. The American Journal of Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. doi:10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003;47(1):21–26. doi: 10.1159/000068871. doi:10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. Journal of Affective Disorders. 2005;89(1-3):125–135. doi: 10.1016/j.jad.2005.09.006. doi:10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JRC, Segrati A, Akkal D, Hassel S, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:602–611. doi: 10.1016/j.jaac.2011.03.018. doi:10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG. Cognitive impairment in the euthymic phase of chronic unipolar depression. Journal Of Nervous And Mental Disease. 1997;185:748–754. doi: 10.1097/00005053-199712000-00005. doi:10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Pelosi L, Slade T, Blumhardt LD, Sharma VK. Working memory dysfunction in major depression: an event-related potential study. Clinical Neurophysiology. 2000;111:1531–1543. doi: 10.1016/s1388-2457(00)00354-0. doi:10.1016/S1388-2457(00)00354-0. [DOI] [PubMed] [Google Scholar]
- Porter R, Gallagher P, Thompson J, Young A. Neurocognitive impairment in drug-free patients with major depressive disorder. The British Journal of Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. doi:10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Portella MJ, Marcos T, Rami L, Navarro V, Gast C, Salamero M. Residual cognitive impairments in late-life depression after a 12-month period follow-up. International Journal of Geriatric Psychiatry. 2003;18:571–576. doi: 10.1002/gps.895. doi:10.1002/gps.895. [DOI] [PubMed] [Google Scholar]
- Preiss M, Kramska L, Dockalova E, Holubova M, Kucerova H. Attentional networks in euthymic patients with unipolar depression. European Psychiatry. 2010;25:69–74. doi: 10.1016/j.eurpsy.2009.08.007. doi:10.1016/j.eurpsy.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Preiss M, Kucerova H, Lukavsky J, Stepankova H, Sos P, Kawaciukova R. Cognitive deficits in the euthymic phase of unipolar depression. Psychiatry Research. 2009;169:235–239. doi: 10.1016/j.psychres.2008.06.042. doi:10.1016/j.psychres.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Pu S, Matsumura S, Yamada T, Ikezawa S, Mitani H, Adachi A, Nakagome K. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: Multi-channel near-infrared spectroscapy study. Psychiatry and Clinical Neurosciences. 2008;62:728–737. doi: 10.1111/j.1440-1819.2008.01882.x. doi:10.1111/j.1440-1819.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- Pu S, Yamada T, Yokoyama K, Matsumura H, Kobayashi H, Sasaki N, Adachi A. A multi-channel near-infrared spectroscopy study of prefrontal cortex activation during working memory task in major depressive disorder. Neuroscience Research. 2011;70:91–97. doi: 10.1016/j.neures.2011.01.001. doi:10.1016/j.neures.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological function in young patients with unipolar major depression. Psychological Medicine. 1997;27:1277–1285. doi: 10.1017/s0033291797005448. doi:10.1017/S0033291797005448. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. The American Journal of Psychiatry. 2005;162:691–698. doi: 10.1176/appi.ajp.162.4.691. doi:10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian Journal of Psychology. 2002;43:239–251. doi: 10.1111/1467-9450.00292. doi:10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Reischies F, Neu P. Comorbidity of mild cognitive disorder and depression: A neuropsychological analysis. European Archives of Psychiatry and Clinical Neuroscience. 2000;250:186–193. doi: 10.1007/s004060070023. doi:10.1007/s004060070023. [DOI] [PubMed] [Google Scholar]
- Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: Further evidence for the final common pathway disorder hypothesis. Psychological Medicine. 2009;39:603–614. doi: 10.1017/S003329170800411X. doi:10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Beschoner P, Hermle L, Ebert D, Falkenstein M. Electrophysiological evidence for reduced inhibitory control in depressed patients in partial remission: A go/nogo study. International Journal of Psychophysiology. 2008;68:209–218. doi: 10.1016/j.ijpsycho.2008.01.010. doi:10.1016/j.ijpsycho.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. The American Journal of Psychiatry. 2000;157:1095–1100. doi: 10.1176/appi.ajp.157.7.1095. doi:10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- Siegle G, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. doi:10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Muir WJ, Blackwood DH. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disorders. 2006;8:40–46. doi: 10.1111/j.1399-5618.2006.00275.x. doi:10.1111/j.1399-5618.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Stoddart S, Craddock N, Jones L. Differentiation of executive and attention impairments in affective illness. Psychological Medicine. 2007;37:1613–1623. doi: 10.1017/S0033291707000712. doi:10.1017/S0033291707000712. [DOI] [PubMed] [Google Scholar]
- Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjørnsen A, Landrø NI, Lund A. Impairment across executive functions in recurrent major depression. Nordic Journal of Psychiatry. 2004;58:41–47. doi: 10.1080/08039480310000789. doi:10.1080/08039480310000789. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders. 2001;12:265–280. doi: 10.1159/000051269. doi:10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. doi:10.1016/S0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Taconnat L, Baudouin A, Fay S, Raz N, Bouazzaoui B, El-Hage W, Ergis A-M. Episodic memory and organizational strategy in free recall in unipolar depression: the role of cognitive support and executive functions. Journal of Clinical and Experimental Neuropsychology. 2010;32:719–727. doi: 10.1080/13803390903512645. doi:10.1080/13803390903512645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami H, Okamoto Y, Yamashita H, Okada G, Yamawaki S. Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. American Journal of Geriatric Psychiatry. 2007;15:594–603. doi: 10.1097/01.JGP.0b013e31802ea919. doi:10.1097/01.JGP.0b013e31802ea919. [DOI] [PubMed] [Google Scholar]
- Tavares JVT, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biological Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. doi:10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Tsaltas E, Kalogerakou S, Papakosta V-M, Kontis D, Theochari E, Koutroumpi M, Oulis P. Contrasting patterns of deficits in visuospatial memory and executive function in patients with major depression with and without ECT referral. Psychological Medicine. 2010;41:983–996. doi: 10.1017/S0033291710001443. doi:10.1017/S0033291710001443. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: An event related potentials study. Biological Psychology. 2009;81:169–176. doi: 10.1016/j.biopsycho.2009.03.009. doi:10.1016/j.biopsycho.2009.03.009. [DOI] [PubMed] [Google Scholar]
- van der Meere J, Borger NA, Pirila S, Sallee F. Interference control in children with first episode major depression: A brief report. Child Neuropsychology. 2011;17:96–104. doi: 10.1080/09297049.2010.533165. doi:10.1080/09297049.2010.533165. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H-J, Schlösser RGM. Cortical inefficiency in patients with unipolar depression: An event-related fMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. doi:10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: An event-related, parametric, performance-controlled fMRI study. Journal of Affective Disorders. 2007;101:175–185. doi: 10.1016/j.jad.2006.11.017. doi:10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Neumeister A. Evidence for continuing neuropsychological impairments in depression. Journal of Affective Disorders. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. doi:10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Westheide J, Wagner M, Quednow BB, Hoppe C, Cooper-Mahkorn D, Strater B, Kuhn K-U. Neuropsychological performance in partly remitted unipolar depressive patients: Focus on executive functioning. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:389–395. doi: 10.1007/s00406-007-0740-4. doi:10.1007/s00406-007-0740-4. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhu W, Zhang Z, Bai F, Yu H, Shi Y, Liu Z. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: An optimized voxel-based morphometry study. Biological Psychiatry. 2008;64:541–544. doi: 10.1016/j.biopsych.2008.04.032. doi:10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
Footnotes
The coders agreed on the EF component assignment of all tasks included in the meta-analysis. The second coder identified two additional tasks as potential working memory tasks, which the author excluded because they placed clear demands on other aspects of cognition (serial addition task), or did not clearly require working memory (spatial recognition task).
In addition, gender (MDD group % female) was considered as a moderator, but was not significant in any analysis. The composition of MDD samples in terms of subtypes of depression and duration of depressive episodes were also noted, but were provided by too few studies to be included as moderators. However, studies that directly compared different subtypes of depression are reviewed in the Discussion section.
Since potential differences between older and younger adults may be of interest, age was also coded dichotomously in meta-ANOVA analyses to contrast samples with a mean age of 60 or older (k = 24) to those with a mean age > 60. Results were similar to continuous analyses of age (see Supplementary Material Table S4).
Analyses of medication-free MDD samples were conducted for all measures with at least five medication-free samples. All effect sizes for neuropsychological measures of EF remained significant except for forward spatial span, which became marginal. In addition, psychomotor speed composite scores became non-significant and vocabulary marginally significant. There were too few medication-free samples in remission to analyze these samples separately. However, three studies did examine medication-free patients in remission, and found significant impairments on neuropsychological measures of EF (Vanderhasselt & De Raedt, 2009; Weiland-Fiedler et al., 2004; Yuan et al., 2008). Thus, there is evidence for impairments on neuropsychological measures of EF in medication-free patients, even those in remission.
References
References marked with an asterisk indicate studies included in the meta-analysis.
- Aldao A, Nolen-Hoeksema S, Schwizer S. Specificity of cognitive emotion regulation strategies: A transdiagnostic examination. Behaviour Research and Therapy. 2010;48:974–983. doi: 10.1016/j.brat.2010.06.002. doi:10.1016/j.brat.2010.06.0 02. [DOI] [PubMed] [Google Scholar]
- Allen P, Mechelli A, Stephan KE, Day F, Dalton J, Williams S, McGuire PK. Fronto-temporal interactions during overt verbal initiation and suppression. Journal of Cognitive Neuroscience. 2008;20:1656–1669. doi: 10.1162/jocn.2008.20107. doi:10.1162/jocn.2008.20107. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. doi:10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andrews G, Slade T. Interpreting scores on the Kessler Psychological Distress Scale (K10). Australian and New Zealand Journal of Public Health. 2001;25:494–497. doi: 10.1111/j.1467-842x.2001.tb00310.x. doi:10.1111/j.1467-842X.2001.tb00310.x. [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J. Trail Making Test, part B as a measure of executive control: Validation using a set-shifting paradigm. Journal of Clinical and Experimental Neuropsychology. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. doi:10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Aron AR. Progress in executive-function research. Current Directions in Psychological Science. 2008;17:124–129. doi:10.1111/j.1467-8721.2008.00561.x. [Google Scholar]
- *Austin M, Mitchell P, Wilhelm K, Parker G, Hickie I, Brodaty H, Hadzi-Pavlovic D. Cognitive function in depression: A distinct pattern of frontal impairment in melancholia? Psychological Medicine. 1999;29:73–85. doi: 10.1017/s0033291798007788. doi:10.1017/S0033291798007788. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. doi:10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Exploring the central executive. The Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1996;49A:5–28. doi:10.1080/713755608. [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. doi:10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. doi:10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: Has the gold standard become a lead weight? The American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. doi:10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive Function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. doi:10.1111/j.1467-8721.2009.01615.x. [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: Evidence from fMRI. Cognitive Brain Research. 2000;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. doi:10.1016/S0926-6410(00)00015-X. [DOI] [PubMed] [Google Scholar]
- Basso MR, Lowery N, Ghormley C, Combs D, Purdie R, Neel J, Bornstein R. Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cognitive Neuropsychiatry. 2007;12:437–456. doi: 10.1080/13546800701446517. doi:10.1080/13546800701446517. [DOI] [PubMed] [Google Scholar]
- Baune BT, Suslow T, Engelien A, Arolt V, Berger K. The association between depressive mood and cognitive performance in an elderly general population: The MEMO Study. Dementia and Geriatric Cognitive Disorders. 2006;22:142–149. doi: 10.1159/000093745. doi:10.1159/000093745. [DOI] [PubMed] [Google Scholar]
- Beblo T, Baumann B, Bogerts B, Wallesch CW, Herman M. Neuropsychological correlates of major depression: A short-term follow-up. Cognitive Neuropsychiatry. 1999;4:333–341. doi:10.1080/135468099395864. [Google Scholar]
- Beck AT. Depression inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. doi:10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. doi:10.1016/0272-7358(88)90050-5. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archieves of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. doi:10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. doi:10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, Lund A. Executive function improvement upon remission of recurrent unipolar depression. European Archieves of Psychiatry and Clinical Neuroscience. 2005;255:373–380. doi: 10.1007/s00406-005-0577-7. doi:10.1007/s00406-005-0577-7. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Wiley; New York: 2009. [Google Scholar]
- Brink TL, Yesavage JA, Lum O, Heersema P, Abey MB, Rose TL. Screening tests for geriatric depression. Clinical Gerontologist. 1982;1:37–44. doi:10.1300/J018v01n01_06. [Google Scholar]
- Brodaty H, Luscombe G, Parker G, Wilhelm K, Hickie I, Austin M-P, Mitchell P. Early and late onset depression in old age: Different aetiologies, same phenomenology. Journal of Affective Disorders. 2001;66:225–236. doi: 10.1016/s0165-0327(00)00317-7. doi:10.1016/S0165-0327(00)00317-7. [DOI] [PubMed] [Google Scholar]
- Bryan J, Luszcz MA. Measurement of executive function: Considerations for detecting adult age differences. Journal of Clinical and Experimental Neuropsychology. 2000;22:40–55. doi: 10.1076/1380-3395(200002)22:1;1-8;FT040. doi:10.1076/1380-3395(200002)22:1;1-8;FT040. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta□analysis of neuroimaging studies of the Wisconsin Card□Sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. doi:10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Handley R, Gaines SO. Depression, anxiety, and within-person variability in adults aged 18 to 85 years. Psychology and Aging. 2008;23:848–858. doi: 10.1037/a0013678. doi:10.1037/a0013678. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Theory and methodology in executive function research. In: Rabbitt P, editor. Methodology of frontal and executive function. Psychology Press; Hove, UK: 1997. pp. 81–116. [Google Scholar]
- Caligiuri MP, Ellwanger J. Motor and cognitive aspects of motor retardation in depression. Journal of Affective Disorders. 2000;57:83–93. doi: 10.1016/s0165-0327(99)00068-3. doi:10.1016/S0165-0327(99)00068-3. [DOI] [PubMed] [Google Scholar]
- Carmody T, Rush A, Bernstein I, Warden D, Brannan S, Burnham D, Trivedi M. The Montgomery Äsberg and the Hamilton ratings of depression: A comparison of measures. European Neuropsychopharmacology. 2006;16:601–611. doi: 10.1016/j.euroneuro.2006.04.008. doi:10.1016/j.euroneuro.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda A, Tuulio-Henriksson A, Marttunen M. A review of cognitive impairments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. doi:10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- *Cataldo MG, Nobile M, Lorusso ML, Battaglia M, Molteni M. Impulsivity in depressed children and adolescents: A comparison between behavioral and neuropsychological data. Psychiatry Research. 2005;136:123–133. doi: 10.1016/j.psychres.2004.12.012. doi:10.1016/j.psychres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Catena-Dell'Osso M, Bellantuono C, Consoli G, Rotella F, Marazziti D. Inflammatory and neurodegenerative pathways in depression: A new avenue for antidepressant development? Current Medical Chemistry. 2011;18:245–255. doi: 10.2174/092986711794088353. doi:10.2174/092986711794088353. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, de Sather JCMG. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–730. doi:10.1037//0012-1649.37.5.715. [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: The Wisconsin Card Sorting Test. Journal of Affective Disorders. 1996;39:107–114. doi: 10.1016/0165-0327(96)00027-4. doi:10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Herd SA, Brant AM, Hazy TE, Miyake A, O'Reilly M, Friedman NP. From an executive function network to executive control: A computational model of the n-back task. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_00047. in press. doi:10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Griffiths K, Mackinnon A, Jacomb P. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. Journal of the International Neuropsychological Society. 1997;3:631–651. Retrieved from http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=49233. [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. Retreived from http://www.christofflab.ca/pdfs/Christoff_2000_Psychobiology.pdf. [Google Scholar]
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: Implications for treatment. Annual Review of Neuroscience. 2009;32:57–74. doi: 10.1146/annurev.neuro.31.060407.125618. doi:10.1146/annurev.neuro.31.060407.125618. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. doi:10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. doi:10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Delfiore G, Degueldre C, Luxen A, Salmon E. The functional anatomy of inhibition processes investigated with the Hayling task. NeuroImage. 2001;14:258–267. doi: 10.1006/nimg.2001.0846. doi:10.1006/nimg.2001.0846. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive function. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. doi:10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, David AS, Brammer MJ. A parametric approach to voxel-based meta-analysis. Neuroimage. 2009;46:115–122. doi: 10.1016/j.neuroimage.2009.01.031. doi:10.1016/j.neuroimage.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. doi:10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- DeBattista C. Executive dysfunction in major depressive disorder. Expert Review of Neurotherapeutics. 2005;5:79–83. doi: 10.1586/14737175.5.1.79. doi:10.1586/14737175.5.1.79. [DOI] [PubMed] [Google Scholar]
- de Fries CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitive elite to cognitively impaired. Neuropsychology. 2009;23:778–791. doi: 10.1037/a0016743. doi:10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Den Hartog H, Derix M, van Bemmel A, Kremer B, Jolles J. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: Testing the effort and cognitive speed hypotheses. Psychological Medicine. 2003;33:1443–1451. doi: 10.1017/s003329170300833x. doi:10.1017/S003329170300833X. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. doi:10.1016/S0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter A, Cook I, Kasl-Godley J, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of Affective Disorders. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. doi:10.1016/S0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association. 2000;95:89–98. doi:10.2307/2669529. [Google Scholar]
- Egeland J, Sundet K, Rund BR. Sensitivity and specificity of memory dysfunction in schizophrenia: A comparison with major depression. Journal of Clinical and Experimental Neuropsychology. 2003;25:79–93. doi: 10.1076/jcen.25.1.79.13630. doi:10.1076/jcen.25.1.79.13630. [DOI] [PubMed] [Google Scholar]
- Elliott R. The neuropsychological profile in unipolar depression. Trends in Cognive Science. 1998;2:447–454. doi: 10.1016/s1364-6613(98)01235-2. doi:10.1016/S1364-6613(98)01235-2. [DOI] [PubMed] [Google Scholar]
- *Elliott R, Baker S, Rogers R, O'leary D, Paykel E, Frith C, Sahakian B. Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychological Medicine. 1997;27:931–942. doi: 10.1017/s0033291797005187. doi:10.1017/S0033291797005187. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, Miller GA. Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective & Behavioral Neuroscience. 2010;10:141–156. doi: 10.3758/CABN.10.1.141. doi:10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. doi:10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Eysenck M, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. doi:10.1016/j.paid.2010.08.019. [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficts in adults with a diagnosis of schizophrenia. Neuropsychology Review. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. doi:10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Sharp CA. Age-related impairments in executive functioning: Updating, inhibition, shifting, and acess. Journal of Clinical and Experimental Neuropsychology. 2004;26:874–890. doi: 10.1080/13803390490510680. doi:10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. doi:10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, Egan GF. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Human Brain Mapping. 2008;29:490–501. doi: 10.1002/hbm.20414. doi:10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fossati P, Amar G, Raoux N, Ergis A, Allilaire J. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. doi:10.1016/S0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Fournier-Vincente S, Larigauderie P, Gaonach D. More dissociations and interactions within central executive functioning: A comprehensive latent-variable analysis. Acta Psychologica. 2008;129:32–48. doi: 10.1016/j.actpsy.2008.04.004. doi:10.1016/j.actpsy.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. doi:10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries J, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. doi:10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. doi:10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. The prefrontal cortex: Anatomy, physiology and neuropsychology of the frontal lobe. Raven; New York: 1989. [Google Scholar]
- Ganguli M, Snitz B, Vander Bilt J, Chang C-CH. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. International Journal of Geriatric Psychiatry. 2009;24:1277–1284. doi: 10.1002/gps.2257. doi:10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activity during a working memory task in psychotic major depression. American Journal of Psychiatry. 2011;168:173–182. doi: 10.1176/appi.ajp.2010.09121718. doi:10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- Gershuny BS, Sher KJ. Compulsive checking and anxiety in a nonclinical sample: Differences in cognition, behavior, personality, and affect. Journal of Psychopathology and Behavioral Assessment. 1995;17:19–38. doi:10.1007/BF02229201. [Google Scholar]
- Gerton BK, Brown TT, Meyer-Lindenberg A, Kohn P, Holt JL, Olsen RK, Berman KF. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia. 2004;42:1781–1787. doi: 10.1016/j.neuropsychologia.2004.04.023. doi:10.1016/j.neuropsychologia.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33:623–642. doi: 10.1016/0028-3932(95)90866-p. doi:10.1016/0028-3932(95)90866-P. [DOI] [PubMed] [Google Scholar]
- *Gomez R, Fleming S, Keller J, Flores B, Kenna H, DeBattista C, Schatzberg A. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biological Psychiatry. 2006;60:472–478. doi: 10.1016/j.biopsych.2005.11.010. doi:10.1016/j.biopsych.2005.11.010. [DOI] [PubMed] [Google Scholar]
- *Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. doi:10.1016/S0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. doi:10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar Å, Ardal G. Cognitive functioning in major depression: A summary. Frontiers in Human Neuroscience. 2009;3:1–7. doi: 10.3389/neuro.09.026.2009. doi:10.3389/neuro.09.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2005;16:1679–1689. doi: 10.1093/cercor/bhj116. doi:10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Harris MSH, Reilly JL, Thase ME, Keshavan MS, Sweeney JA. Response suppression deficits in treatment-naïve first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic majaor depression. Psychiatry Research. 2009;170:150–156. doi: 10.1016/j.psychres.2008.10.031. doi:10.1016/j.psychres.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Harvey PO, Fossati P, Pochon J-B, Levy R, Lebastard G, Lehéricy S, Dubois B. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. doi:10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- *Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P. Executive functions and updating of the contents of working memory in unipolar depression. Journal of Psychiatric Research. 2004;38:567–576. doi: 10.1016/j.jpsychires.2004.03.003. doi:10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O'Reilly RC. Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. doi:10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. doi:10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Yoon C. Individual differences in executive processing predict suseptibility to interference in verbal working memory. Neuropsychology. 2006;20:511–528. doi: 10.1037/0894-4105.20.5.511. doi:10.1037/0894-4105.20.5.511. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Unbiased estimation of effect size. Evaluation in Education: An International Review Series. 1980;4:25–27. doi:10.1016/0191-765X(80)90005-1. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; Orlando, FL: 1985. [Google Scholar]
- Henry J, Crawford JR. A meta-analytic review of verbal fluency deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:78–101. doi: 10.1080/138033990513654. doi:10.1080/138033990513654. [DOI] [PubMed] [Google Scholar]
- Herrmann LL, Goodwin GM, Ebmeier KP. The cognitive neuropsychology of depression in the elderly. Psychological Medicine. 2007;37:1693–1702. doi: 10.1017/S0033291707001134. doi:10.1017/S0033291707001134. [DOI] [PubMed] [Google Scholar]
- Hertel PT, Gerstle M. Depressive deficits in forgetting. Psychological Science. 2003;14:573–578. doi: 10.1046/j.0956-7976.2003.psci_1467.x. doi:10.1046/j.0956-7976.2003.psci_1467.x. [DOI] [PubMed] [Google Scholar]
- *Hill SK, Reilly JL, Harris MSH, Rosen C, Marvin RW, DeLeon O, Sweeney JA. A comparison of neuropsychological dysfunction in first-episode psychotic patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophrenia Research. 2009;113:167–175. doi: 10.1016/j.schres.2009.04.020. doi:10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hinkelmann K, Moritz S, Botzenhardt J, Riedesel K, Wiedemann K, Kellner M, Otte C. Cognitive impairment in major depression: Association with salivary cortisol. Biological Psychiatry. 2009;66:879–885. doi: 10.1016/j.biopsych.2009.06.023. doi:10.1016/j.biopsych.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. doi:10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hofmann W. Extension of SPSS for Windows meta-analysis macros (D. Wilson, 2001) 2009 Retrieved from http://wilhelmhofmann.info/wordpress/?page_id=120.
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: Relationship to subclinical measures of depression. Emotion. 2007;7:68–76. doi: 10.1037/1528-3542.7.1.68. doi:10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. doi:10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related changes in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. doi:10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- *Ilonen T, Taiminen T, Karlsson H, Lauerma H, Tuimala P, Leinonen K, Salokangas R. Impaired Wisconsin Card Sorting Test performance in first-episode severe depression. Nordic Journal of Psychiatry. 2000;54:275–280. doi: 10.1053/comp.2000.9017. doi:10.1080/080394800448156. [DOI] [PubMed] [Google Scholar]
- Jacobson SC, Blanchard M, Connolly CC, Cannon M, Garavan H. An fMRI investigation of a novel analogue to the Trail-Making Test. Brain and Cognition. 2011;77:60–70. doi: 10.1016/j.bandc.2011.06.001. doi:10.1016/j.bandc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set shifting performance in schizophrenia: Impact of distractors. Schizophrenia Research. 2007;89:339–349. doi: 10.1016/j.schres.2006.08.014. doi:10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Joormann J. Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19:161–166. doi:10.1177/0963721410370293. [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. doi:10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Kopka M-L, Rentrop M, Walther S, Kronmüller K, Olbrich R, Stippich C. Maintenance of real objects and their verbal designations in working memory. Neuroscience Letters. 2010;469:65–69. doi: 10.1016/j.neulet.2009.11.045. doi:10.1016/j.neulet.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM. Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cerebral Cortex. 2011;21:307–317. doi: 10.1093/cercor/bhq096. doi:10.1093/cercor/bhq096. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. Journal of Experimental Psychology: General. 2004;133:189–217. doi: 10.1037/0096-3445.133.2.189. doi:10.1037/0096-3445.133.2.189. [DOI] [PubMed] [Google Scholar]
- Kearns NP, Cruickshank CA, McGuigan KJ, Rily SA, Shaw SP, Snaith RP. A comparison of depression rating scales. The British Journal of Psychiatry. 1982;141:45–49. doi: 10.1192/bjp.141.1.45. doi:10.1192/bjp.141.1.45. [DOI] [PubMed] [Google Scholar]
- Keller J, Nitschke J, Bhargava T, Deldin P, Gergen J, Miller G, Heller W. Neuropsychological differentiation of depression and anxiety. Journal of Abnormal Psychology. 2000;109:3–10. doi:10.1037//0021-843X.109.1.3. [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Zaslavsky AM. Short screening scales to monitor population prevalence and trends in non-specific psychological distress. Psychological Medicine. 2002;32:959–976. doi: 10.1017/s0033291702006074. doi:10.1017\S0033291702006074. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archieves of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. doi:10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu W, Demler O, Walters E. Prevalence, severity, and comorbidity of 12-Month DSM-IV disorders in the National Comorbidity Survey Replication. Archieves of General Psychiatry. 2005;62:617–628. doi: 10.1001/archpsyc.62.6.617. doi:10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neuroscience Letters. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. doi:10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Biplar Disorders. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. doi:10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Simons RF. Cognitive inefficiency in depressive undergraduates: Stroop processing and ERPs. Biological Psychology. 2011;86:239–246. doi: 10.1016/j.biopsycho.2010.12.004. doi:10.1016/j.biopsycho.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Lane RM, O'Hanlon JF. Cognitive and psychomotor effects of antidepressants with emphasis on selective serotonin reuptake inhibitors and the depressed elderly patient. German Journal of Psychiatry. 1999;1:1–42. Retrieved from http://www.gjpsy.unigoettingen.de/gjp-article-lane.htm. [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. doi:10.1007/s10608-007-9128-z. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 2nd ed. Oxford University Press; New York: 1983. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessement. 4th ed. Oxford University Press; New York: 2004. [Google Scholar]
- *Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. The American Journal of Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. doi:10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- *Lyche P, Jonassen R, Stiles TC, Ulleberg P, Landrø NI. Attentional functions in major depressive disorder with and without comorbid anxiety. Archieves of Clinical Neuropsychology. 2011;26:38–47. doi: 10.1093/arclin/acq095. doi:10.1093/arclin/acq095. [DOI] [PubMed] [Google Scholar]
- *Maalouf FT, Klein C, Clark L, Sahakian BJ, Labarbara EJ, Versace A, Phillips ML. Impaired sustained attention and executive dysfunction: Bipolar disorder versus depression-specific markers of affective disorders. Neuropsychologia. 2010;48:1862–1868. doi: 10.1016/j.neuropsychologia.2010.02.015. doi:10.1016/j.neuropsychologia.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mana S, Paillere Martinot M-L, Martinot J-L. Brain imaging findings in children and adolescents with mental disorders: A cross-sectional review. European Pyschiatry. 2010;25:345–354. doi: 10.1016/j.eurpsy.2010.04.010. doi:10.1016/j.eurpsy.2010.04.010. [DOI] [PubMed] [Google Scholar]
- *Markela-Lerenc J, Kaiser S, Fiedler P, Weisbrod M, Mundt C. Stroop performance in depressive patients: A preliminary report. Journal of Affective Disorders. 2006;94:261–267. doi: 10.1016/j.jad.2006.04.011. doi:10.1016/j.jad.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Marín-Martínez F, Sánchez-Meca J. Testing for dichotomous moderators in meta-analysis. The Journal of Experimental Education. 1998;67:69–81. doi:10.1080/00220979809598345. [Google Scholar]
- Marquand AF, Mourão-Miranda J, Brammer MJ, Cleare AJ, Fu CHY. Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. Neuroreport. 2008;19:1507–1511. doi: 10.1097/WNR.0b013e328310425e. doi:10.1097/WNR.0b013e328310425e. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. doi:10.1146/annurev.psych.45.1.25. [DOI] [PubMed] [Google Scholar]
- *Matthews K, Coghill D, Rhodes S. Neuropsychological functioning in depressed adolescent girls. Journal of Affective Disorders. 2008;111:113–118. doi: 10.1016/j.jad.2008.02.003. doi:10.1016/j.jad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. doi:10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. doi:10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- McMillan KM, Laird AR, Witt ST, Meyerand ME. Self-paced working memory: Validation of verbal variations of the n-back paradigm. Brain Research. 2007;1139:133–142. doi: 10.1016/j.brainres.2006.12.058. doi:10.1016/j.brainres.2006.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiran N, Diamond GM, Toder D, Nemets B. Cognitive rigidity in unipolar depression and obsessive-compulsive disorder: Examination of task switching, Stroop, working memory updating and post-conflict adaptation. Psychiatry Research. 2011;185:149–156. doi: 10.1016/j.psychres.2010.04.044. doi:10.1016/j.psychres.2010.04.044. [DOI] [PubMed] [Google Scholar]
- *Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. The American Journal of Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. Retrieved from http://ajp.psychiatryonline.org/article.aspx?volume=156&page=780. [DOI] [PubMed] [Google Scholar]
- Mertens V, Gagnon M, Coulombe D, Messier C. Exploratory factor analysis of neuropsychological tests and their relationship to the Brown-Peterson task. Archives of Clinical Neuropsychology. 2006;21:733–739. doi: 10.1016/j.acn.2006.08.005. doi:10.1016/j.acn.2006.08.005. [DOI] [PubMed] [Google Scholar]
- *Michopoulos I, Zervas IM, Pantelis C, Tsaltas E, Papakosta V-M, Boufidou F, Lykouras L. Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. European Archieves of Psychiatry and Clinical Neuroscience. 2008;258:217–225. doi: 10.1007/s00406-007-0781-8. doi:10.1007/s00406-007-0781-8. [DOI] [PubMed] [Google Scholar]
- *Michopoulos I, Zervas IM, Papakosta VM, Tsaltas E, Papageorgiou C, Manessi T, Soldatos CR. Set shifting deficits in melancholic vs. non-melancholic depression: Preliminary findings. European Psychiatry. 2006;21:361–363. doi: 10.1016/j.eurpsy.2006.03.008. doi:10.1016/j.eurpsy.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. doi:10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. Journal of Experimental Psychology: General. 2001;130:621–640. doi: 10.1037//0096-3445.130.4.621. doi:10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: A pilot study with anxious older adults. Behaviour Research and Therapy. 2005;43:447–465. doi: 10.1016/j.brat.2004.03.007. doi:10.1016/j.brat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting: An fMRI study of the trail making test. Arquivos de Neuro-Psiquiatria. 2002;60:900–905. doi: 10.1590/s0004-282x2002000600002. doi:10.1590/S0004-282X2002000600002. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. doi:10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murata T, Kimura H, Omori M, Kado H, Kosaka H, Iidaka T, Wada Y. MRI white matter hyperintensities, 1H-MR spectroscopy and cognitive function in geriatric depression: A comparison of early- and late-onset cases. International Journal of Geriatric Psychiatry. 2001;16:1129–1135. doi: 10.1002/gps.501. doi:10.1002/gps.501. [DOI] [PubMed] [Google Scholar]
- *Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, Arai H. Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders. 2008;111:46–51. doi: 10.1016/j.jad.2008.01.027. doi:10.1016/j.jad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Nathaniel-James DA, Frith CD. The role of the dorsolateral prefrontal cortex: Evidence from the effects of contextual constraint in a sentence completion task. NeuroImage. 2002;16:1094–1102. doi: 10.1006/nimg.2002.1167. doi:10.1006/nimg.2002.1167. [DOI] [PubMed] [Google Scholar]
- *Nebes R, Butters M, Mulsant B, Pollock B, Zmuda M, Houck P, Reynolds C. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychological Medicine. 2000;30:679–691. doi: 10.1017/s0033291799001968. doi:10.1017/S0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. doi:10.3758/CABN.7.1.1. [DOI] [PubMed] [Google Scholar]
- *Neu P, Kiesslinger U, Schlattmann P, Reischies FM. Time-related cognitive deficiency in four different types of depression. Psychiatry Research. 2001;103:237–247. doi: 10.1016/s0165-1781(01)00286-4. doi:10.1016/S0165-1781(01)00286-4. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2004;25:1–22. doi:10.1023/A:1026485530405. [Google Scholar]
- Nitschke JB, Mackiewicz KL. Frontal and anterior cingulate contributions to volition in depression. International Review of Neurobiology. 2005;67:73–94. doi: 10.1016/S0074-7742(05)67003-1. doi:10.1016/S0074-7742(05)67003-1. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. doi:10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: Early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. doi:10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003;47:21–26. doi: 10.1159/000068871. doi:10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- Olley A, Malhi G, Sachdev P. Memory and executive functioning in obsessive–compulsive disorder: A selective review. Journal of Affective Disorders. 2007;104:15–23. doi: 10.1016/j.jad.2007.02.023. doi:10.1016/j.jad.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Osman A, Downs WR, Barrios FX, Kopper BA, Gutierrez PM, Chiros C. Factor structure and psychometric characteristics of the Beck Depression Inventory-II. Journal of Psychopathology and Behavioral Assessment. 1997;19:359–376. doi:10.1007/BF02229026. [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: Implications for application of the medical disease model to psychiatric disorders. Harvard Reviews of Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. doi:10.1093/hrp/10.2.86. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal-lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. doi:10.1016/0028-3932(90)90137-D. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: A positron emission tomography study. Cerebral Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. doi:10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- Owen AM, Lee ACH, Williams EJ. Dissociating aspects of verbal working memory within the human frontal lobe: Further evidence for a“ process-specific” model of lateral frontal organization. Psychobiology. 2000;28:146–155. Retrieved from http://cambridgebrainsciences.com/assets/default/files/pdf/Owen2000.pdf. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. doi:10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington JE, Leiter RG. Partington pathways test. Psychological Services Center; Washington, DC: 1949. [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. doi:10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophial Transactions of the Royal Society of London Series B: Biologial Sciences. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. doi:10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot P, Brutoux F. Induced rumination dampens executive processes in dysphoric young adults. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:219–227. doi: 10.1016/j.jbtep.2007.07.001. doi:10.1016/j.jbtep.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Phillips L. Do “frontal tests” measure executive function? Issues of assessment and evidence from fluency tests. In: Rabbitt P, editor. Methodology of frontal and executive function. Psychology Press; Hove, UK: 1997. pp. 191–213. [Google Scholar]
- Pier MPBI, Hulstijn W, Sabbe BGC. Differential patterns of psychomotor functioning in unmedicated melancholic and nonmelancholic depressed patients. Journal of Psychiatric Research. 2004;38:425–435. doi: 10.1016/j.jpsychires.2003.11.008. doi:10.1016/j.jpsychires.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Portella MJ, de Diego-Adelino J, Gomez-Anson B, Morgan-Ferrando R, Vives Y, Puigdemont D, Perez V. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: A comparison among first episode, remitted recurrent and chronic patients. Journal of Psychiatric Research. 2011;45:227–234. doi: 10.1016/j.jpsychires.2010.08.010. doi:10.1016/j.jpsychires.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Bourke C, Gallagher P. Neuropsychological impairment in major depression: Its nature, origin and clinical significance. Australian and New Zealand Journal of Psychiatry. 2007;41:115–128. doi: 10.1080/00048670601109881. doi:10.1080/00048670601109881. [DOI] [PubMed] [Google Scholar]
- *Porter RJ, Gallagher P, Thompson J, Young A. Neurocognitive impairment in drug-free patients with major depressive disorder. The British Journal of Psychiatry. 2003;182:214–220. doi: 10.1192/bjp.182.3.214. doi:10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Provost J-S, Petrides M, Monchi O. Dissociating the role of the caudate nucleus and dorsolateral prefrontal cortex in the monitoring of events within human working memory. European Journal of Neuroscience. 2010;32:873–880. doi: 10.1111/j.1460-9568.2010.07333.x. doi:10.1111/j.1460-9568.2010.07333.x. [DOI] [PubMed] [Google Scholar]
- *Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. The American Journal of Psychiatry. 2005;162:691–698. doi: 10.1176/appi.ajp.162.4.691. doi:10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW. Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. Russel Sage Foundation; New York: 2009. [Google Scholar]
- Rende B, Ramsberger G, Miyake A. Commonalities and differences in the working memory components underlying letter and category fluency tasks: A dual-task investigation. Neuropsychology. 2002;16:309–321. doi: 10.1037//0894-4105.16.3.309. doi:10.1037//0894-4105.16.3.309. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. doi:10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- *Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: Further evidence for the final common pathway disorder hypothesis. Psychological Medicine. 2009;39:603–614. doi: 10.1017/S003329170800411X. doi:10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive function and cognitive aging. Journal of the International Neuropsychological Soceity. 1998;4:474–490. doi: 10.1017/s1355617798455073. doi:10.1017/S1355617798455073. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbit P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia and Geriatric Cognitive Disorders. 1994;5:266–281. doi: 10.1159/000106735. doi:10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Roberts RD, Stankov L, Pallier G, Dolph B. Charting the cognitive sphere: Tactilel-kinesthetic performance within the structure of intelligence. Intelligence. 1997;25:111–148. doi:10.1016/S0160-2896(97)90048-9. [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Kato N. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. doi:10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ross TP, Hanouskova E, Giarla K, Calhoun E, Tucker M. The reliability and validity of the self-ordered pointing task. Archieves of Clinical Neuropsychology. 2007;22:449–458. doi: 10.1016/j.acn.2007.01.023. doi:10.1016/j.acn.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Sabella SA, Miyake A, Friedman NP, Young SE, Hewitt JK. Depression symptoms are associated with lower general executive control. in preparation.
- Sabbe B, Hulstijn W, van Hoof J, Tuynman-Qua HG, Zitman F. Retardation in depression: Assessment by means of simple motor tasks. Journal of Affective Disorders. 1999;55:39–44. doi: 10.1016/s0165-0327(98)00087-1. doi:10.1016/S0165-0327(98)00087-1. [DOI] [PubMed] [Google Scholar]
- Salloway S, Malloy R, Kohn E, Gillard E, Duffy J, Rogg J, Westlake R. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. Retrieved from http://www.neurology.org/content/46/6/1567.short. [DOI] [PubMed] [Google Scholar]
- Salthouse T, Atkinson T, Berish D. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. doi:10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Parianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society. 2009;15:438–450. doi: 10.1017/S1355617709090626. doi:10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J, Marín-Martínez F. Testing continuous moderators in meta-analysis: A comparison of procedures. British Journal of Mathematical and Statistical Psychology. 1998;51:311–326. doi:10.1111/j.2044-8317.1998.tb00683.x. [Google Scholar]
- *Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. The American Journal of Psychiatry. 2000;157:1095–1100. doi: 10.1176/appi.ajp.157.7.1095. doi:10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- Schnur TS, Brecher A, Rossi N, Schwartz MF. Errors of lexical selection during high and low semantic competition. Brain and Language. 2004;91:7–8. doi:10.1016/j.bandl.2004.06.007. [Google Scholar]
- Schnur TS, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language. 2006;54:199–227. doi:10.1016/j.jml.2005.10.002. [Google Scholar]
- Schon K, Quiroz YT, Hasselmo ME, Stern CE. Greater working memory load results in greater medial temporal activity at retrieval. Cerebral Cortex. 2009;19:2561–2571. doi: 10.1093/cercor/bhp006. doi:10.1093/cercor/bhp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. doi:10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess DF. The domain of suppervisor processes and temporal organization of behavior. Philosophical Transactions of the Royal Society of London: Part B, Biological Science. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. doi:10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Snyder HR, Hutchison N, Nyhus E, Curran T, Banich MT, O'Reilly RC, Munakata Y. Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16483–16488. doi: 10.1073/pnas.1002291107. doi:10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Munakata Y. Becoming self-directed: Abstract representations support endogenous flexibility in children. Cognition. 2010;116:155–167. doi: 10.1016/j.cognition.2010.04.007. doi:10.1016/j.cognition.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; Oxford: 2006. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. doi:10.1037/h0054651. [Google Scholar]
- Stuss D, Alexander M. Is there a dysexecutive syndrome? Philisopical Transactions of the Royal Society of London Series B: Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. doi:10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. The American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. doi:10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- *Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. doi:10.1016/S0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Sylvester C-YC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. doi:10.1016/S0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- *Tavares JVT, Clark L, Cannon DM, Erickson K, Drevets WC, Sahakian BJ. Distinct profiles of neurocognitive function in unmedicated unipolar depression and bipolar II depression. Biological Psychiatry. 2007;62:917–924. doi: 10.1016/j.biopsych.2007.05.034. doi:10.1016/j.biopsych.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg R. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. doi:10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Toepper M, Markowitsch HJ, Gebhardt H, Beblo T, Thomas C, Gallhofer B, Sammer G. Hippocampal involvement in working memory encoding of changing locations: An fMRI study. Brain Research. 2010;1354:91–99. doi: 10.1016/j.brainres.2010.07.065. doi:10.1016/j.brainres.2010.07.065. [DOI] [PubMed] [Google Scholar]
- Trichard C, Martinot JL, Alagille M, Masure MC, Hardy P, Ginestet D, Féline A. Time course of prefrontal lobe dysfunction in severely depressed in-patients: A longitudinal neuropsychological study. Psychological Medicine. 1995;25:79–85. doi: 10.1017/s0033291700028105. doi:10.1017/S0033291700028105. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. doi:10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Tsunoka T, Kishi T, Masashi I, Tsuyoshi K, Yamanouchi Y, Konoshita Y, Iwata N. Association analysis of Group II metabotropic glutamate receptor genes (GRM2 and GRM3) with mood disorders and fluvoxamine response in a Japanese population. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;35:875–879. doi: 10.1016/j.pnpbp.2009.04.007. doi:10.1016/j.pnpbp.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Aitchison K. Measuring depression: Comparison and integration of three scales in the GENDEP study. Psychological Medicine. 2008;38:289–300. doi: 10.1017/S0033291707001730. doi:10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers GJ, Brewer GA. Variation in verbal fluency: A latent variable analysis of clustering, switching, and overall performance. Quarterly Journal of Experimental Psychology. 2011;64:447–466. doi: 10.1080/17470218.2010.505292. doi:10.1080/17470218.2010.505292. [DOI] [PubMed] [Google Scholar]
- *Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: An event related potentials study. Biological Psychology. 2009;81:169–176. doi: 10.1016/j.biopsycho.2009.03.009. doi:10.1016/j.biopsycho.2009.03.009. [DOI] [PubMed] [Google Scholar]
- van Hoof JJ, Jogems-Kosterman BJ, Sabbe BG, Zitman FG, Hulstijn W. Differentiation of cognitive and motor slowing in the Digit Symbol Test (DST): Differences between depression and schizophrenia. Journal of Psychiatric Research. 1998;32:99–103. doi: 10.1016/S0022-3956(98)00057-0. doi:10.1016/S0022-3956(98)00057-0. [DOI] [PubMed] [Google Scholar]
- Vasic N, Walter H, Sambataro F, Wolf RC. Aberrant functional connectivity of dorsolateral prefrontal and cingulate networks in patients with major depression during working memory processing. Psychological Medicine. 2009;39:977–987. doi: 10.1017/S0033291708004443. doi:10.1017/S0033291708004443. [DOI] [PubMed] [Google Scholar]
- Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology. 1997;19:587–603. doi: 10.1080/01688639708403745. doi:10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. doi:10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. doi:10.3758/CABN.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlösser RGM. The special involvement of the rostrolateral prefrontal cortex in planning abilities: An event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. doi:10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- *Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H-J, Schlösser RGM. Cortical inefficiency in patients with unipolar depression: An event-related fMRI study with the Stroop task. Biological Psychiatry. 2006;59:958–965. doi: 10.1016/j.biopsych.2005.10.025. doi:10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Walsh ND, Williams SCR, Brammer MJ, Bullmore ET, Kim J, Suckling J, Fu CHY. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biological Psychiatry. 2007;62:1236–1243. doi: 10.1016/j.biopsych.2006.12.022. doi:10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- *Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: An event-related, parametric, performance-controlled fMRI study. Journal of Affective Disorders. 2007;101:175–185. doi: 10.1016/j.jad.2006.11.017. doi:10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Waters GS, Caplan D. The reliability and stability of verbal working memory measures. Behavior Research Methods, Instruments, & Computers. 2003;35:550–564. doi: 10.3758/bf03195534. doi:10.3758/BF03195534. [DOI] [PubMed] [Google Scholar]
- *Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Neumeister A. Evidence for continuing neuropsychological impairments in depression. Journal of Affective Disorders. 2004;82:253–258. doi: 10.1016/j.jad.2003.10.009. doi:10.1016/j.jad.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Cohen RM, Murphy DL, Martello J, Gerdt C. Cognitive processes in depression. Archives of General Psychiatry. 1981;38:42–47. doi: 10.1001/archpsyc.1981.01780260044004. doi:10.1001/archpsyc.1981.01780260044004. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Huizinga M. The development and preliminary validation of the Tower of Hanoi-Revised. Assessment. 2001;8:167–176. doi: 10.1177/107319110100800205. doi:10.1177/107319110100800205. [DOI] [PubMed] [Google Scholar]
- Welsh MC, Satterlee-Cartmell T, Stine M. Towers of Hanoi and London: Contribution of working memory and inhibition performance. Brain and Cognition. 1999;41:231–242. doi: 10.1006/brcg.1999.1123. doi:10.1006/brcg.1999.1123. [DOI] [PubMed] [Google Scholar]
- West R, Choi P, Travers S. The influence of negative affect on the neural correlates of cognitive control. International Journal of Psychophysiology. 2010;76:107–117. doi: 10.1016/j.ijpsycho.2010.03.002. doi:10.1016/j.ijpsycho.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Whitney C, Weis S, Krings T, Huber W, Grossman M, Kircher T. Task-dependent modulations of prefrontal and hippocampal activity during intrinsic word production. Journal of Cognitive Neuroscience. 2009;21:697–712. doi: 10.1162/jocn.2009.21056. doi:10.1162/jocn.2009.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E, Doyle A, Nigg J, Faraone S, Pennington B. Validity of the executive function theory of Attenion-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi:10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt E, Pennington BF, Boada R, Ogline J, Tunick RA, Chhabildas NA, Olson RK. A comparision of cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. doi:10.1037/0021-843X.110.1.157. [DOI] [PubMed] [Google Scholar]
- Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata. 2006 Retrieved from http://mason.gmu.edu/~dwilsonb/ma.html.
- Woodruff-Pak D. The neuropsychology of aging. Blackwell; Oxford: 1997. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Abey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. doi:10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. doi:10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Yuan Y, Zhu W, Zhang Z, Bai F, Yu H, Shi Y, Liu Z. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: An optimized voxel-based morphometry study. Biological Psychiatry. 2008;64:541–544. doi: 10.1016/j.biopsych.2008.04.032. doi:10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Ongur D. Magnetic resonance spectroscapy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. doi:10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry. Neuropsychology, and Behavioral Neurology. 1998;11:111–119. Retrieved from http://ovidsp.tx.ovid.com/sp-3.5.1a/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&AN=00010291-199807000-00001&NEWS=N&CSC=Y&CHANNEL=PubMed. [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the trail making test. Neuropsychologia. 2005;43:1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. doi:10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. doi:10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.