Abstract
Adaptor molecules are essential in organizing signaling molecules and in coordinating and compartmentalizing their activity. 3BP2 is a cytoplasmic adaptor protein mainly expressed by haematopoietic cells that has been shown to act as a positive regulator in T, B and NK cell signal transduction. 3BP2 is an important regulator of cytotoxic granule release in NK cells. Mast cells similarly degranulate following antigen-dependent aggregation of the high affinity receptor for IgE (FcεRI) on the cell surface. Activation of these cells induces the release of preformed inflammatory mediators and the de novo synthesis and secretion of cytokines and chemokines. Thus, mast cells participate in both innate and acquired responses. We observed that 3BP2 is expressed in human mast cells from diverse origins. Moreover 3BP2 co-immunoprecipitates with essential mast cell signaling mediators such as Lyn, Syk, and PLCγ, thus a role for this adaptor in mast cell function was postulated. In the present work, we used shRNA lentiviral targeting approach to silence 3BP2 expression in human mast cells. Our findings point to a requirement for 3BP2 in optimal immediate and late mast cells responses such as degranulation and IL-8 or GM-CSF secretion. 3BP2 was determined to be necessary for optimal phosphorylation of Syk, LAT and PLCγ1, critical signals for calcium release from intracellular stores. Taken together, our results show that by participating in FcεRI- mediated signal transduction 3BP2 is an important regulator of human mast cell activation. Thus, 3BP2 could be a potential therapeutic target for IgE-dependent mast cell-mediated inflammatory disease.
INTRODUCTION
Mast cells are key effector cells in IgE-dependent hypersensitivity, as well as in allergic and inflammatory disorders. Ligation of the high affinity receptor for IgE (FcεRI), constitutively expressed on mast cells, promotes cell activation and immediate release and production of proinflammatory mediators (1, 2). FcεRI-mediated mast cell activation can be dramatically enhanced by concurrent activation of the tyrosine kinase type III KIT (CD117), that plays a role in cell survival, proliferation and differentiation (3, 4). The signals generated as a consequence of antigen interaction with specific IgE bound to FcεRI is a highly ordered process where adaptor molecules are essential in organizing, coordinating and compartmentalizing signaling molecules in order to direct specific cellular responses. Adaptors display several motifs and domains that coordinate protein-protein and protein-lipid interactions and can be transmembrane or cytoplasmic (5). SH3-binding protein 2 (3BP2) is a cytoplasmic adaptor originally identified as a protein interacting with the SH3 domain of the protein tyrosine kinase (PTK) Abl (6). The 3BP2 encoding gene is located on human chromosome 4 (4p16.3 region). Human 3BP2 is a 561-aa protein containing an N-terminal pleckstrin homology (PH) domain, an SH3-binding proline-rich regions, and a C-terminal SH2 domain. The proline-rich region (PR1, PR2 and PR3) are known to associate to Src family PTKs (Fyn, Lyn and Lck) (7, 8). The SH2 domain of 3BP2 interacts with Syk family PTKs, Syk and ZAP-70 (7). 3BP2 is known to regulate bone homeostasis through osteoclast activation and osteoblast differentiation and function (9). Mutations in the proline rich region of 3BP2 are responsible for the cherubism autosomal dominant inherited disorder characterized by excessive bone degradation of the upper and lower jaws which results in facial swelling (10). It has been recently shown that these mutations uncouple 3BP2 from the poly (ADP-ribose) polymerase Tankyrase reducing ADP ribosylation and subsequent destruction of 3BP2 via ubiquitylation. Tankyrase Ankyrin repeats bind 3BP2 RxxPDG hexapeptide, and disruption of such interaction stabilizes 3BP2 protein (11). Consequently, elevated 3BP2 protein levels result in increased Src, Syk and Vav activity and osteoclasts hyperactivation which leads to cherubism (12). 3BP2 is preferentially expressed in hematopoietic tissues where it contributes to the regulation of immune responses (13). This molecule regulates transcriptional activities via calcineurin- and Ras-dependent pathways in T lymphocytes (7). 3BP2 mediated signals are regulated on T cells via SHP-1 tyrosine phosphatase which acts as a negatively regulated by dephosphorylation of the adaptor (14). A positive regulatory role for 3BP2 in B cell receptor (BCR) function has been described (15) wherein 3BP2-deficient mice show impaired optimal B cell activation and thymus-independent humoral responses (16, 17). Regarding 3BP2 phosphorylation, in B cells 3BP2 gets tyrosine phosphorylated in a Syk dependent manner after BCR stimulation (18). 3BP2 also plays an important function in NK cells, where it regulates cell-mediated cytotoxicity via its PH, SH2, and proline-rich regions (19). Moreover, phosphorylation of 3BP2Tyr 183, which mediates the interaction with Vav-1 and PLC-γ, is critical for the ability of 3BP2 to positively regulate NK cell-mediated killing (19). In NK cells, 3BP2 was described to directly associate with the SLAM family member, CD244 (2B4), and to regulate CD244-mediated cytotoxicity through a SAP -and PKC-dependent mechanism, that does not affect IFNγ secretion (20, 21). Although it has been described that the overexpression of 3BP2 SH2 domain impairs FcεRI dependent degranulation in rat basophilic leukaemia cell line RBL-2H3 (22), evidence for a role for endogenous 3BP2 in human mast cells (huMCs) function has remained elusive. Furthermore, as overexpression of dominant negative mutant constructs can cause off-target effects by interfering with other SH2-domain-containing proteins and/or may have incomplete effects on their specific targets, how 3BP2 regulates mast cell function in general is not clear. Thus, in this work we investigated the role of endogenous 3BP2 on FcεRI signaling and function in huMCs using a shRNA knock down approach. Our data indicates that 3BP2 is important for Syk phosphorylation and, consequently, for short and long term events in mast cells activation through FcεRI. Altogether our data show an important role for this adaptor in mast cell function through one of the major mast cell receptors.
MATERIALS AND METHODS
Cells and antibodies
The LAD2 human mast cell line, kindly provided by Drs. A Kirshenbaum and D.D. Metcalfe (NIH, Bethesda, MD, USA), was grown in StemPro-34 Serum-Free Medium (Invitrogen Life Technologies, Carlsbad, CA, USA), supplemented with StemPro-34 Nutrient and with L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), and 100 ng/ml recombinant stem cell factor (SCF) (Amgen, CA, USA). The LUVA human mast cell line, kindly provided by Dr. J. Steinke (University of Virginia) was grown as indicated above without SCF. Primary HuMCs derived from CD34-positive peripheral blood cells were obtained from healthy donors following informed consent on a protocol (98-I-0027; PI: Dr. A. Kirshenbaum) approved by the NIAID IRB and differentiated in vitro for 8 weeks in the presence of 100 ng/ml IL-6 and 100 ng/ml SCF as described (23). Mouse anti-3BP2 (clone 470) and rabbit anti-PLCγ antibodies were purchased from Santa Cruz (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA). Monoclonal anti-Syk was from Biolegend (San Diego, CA, USA). Anti-phosphotyrosine monoclonal and anti-total ERK antibodies were from Zymed Laboratories (Invitrogen). Anti-LAT, anti-pLAT Y191, anti-pSyk Y352, anti-pPLCγ 1Y783, anti-pP38 Thr180/Tyr182, anti-p-ERK Thr202/Tyr204 and anti p-JNK Thr183/Tyr185 were purchased from Cell Signaling (Cell Signaling Technology, Inc. Danvers, MA, USA). Biotinylated human IgE was from Abbiotec (San Diego, CA, USA).
ShRNA silencing by lentiviral infection
Lentiviral particles to silence the 3BP2 gene expression were generated using Mission ® shRNA technology according to manufacturer’s instructions (Sigma, St. Louis, MO, USA). The 3BP2 specific shRNA sequence selected was: 5´CCG GGC GAA GTG GAA AGG TTG TTC ACT CGA GTG AAC AAC CTT TCC ACT TCG CTT TTT G 3´. Non-target shRNA was used as a control. Virus preparations were done by transfection of 293LTV (Cell Biolabs, San Diego, CA, USA) cells and harvesting of supernatant 3 days post transfection as described (24). Briefly, supernatants were concentrated 35X by centrifugation and used to infect huMCs. 5×106 of 6 week huMCs per shRNA preparation were infected. Selection was started 1 week later with 0, 2 µg/ml Puromycin (Sigma) and the extent of 3BP2 knock down was determined by western blot 1 week after starting selection. All experiments in huMCs were performed at weeks 8 and 9 of cell culture.
RNA extraction and retrotranscription
Total RNA was extracted with RNAeasy Mini Kit Qiagen (Qiagen, Hilden, Germany) from 2×106 non-transduced, non-target control and 3BP2 knockdown mast cells sensitized with biotinylated IgE and challenged with streptavidin (SA) (Sigma). cDNA was generated from mRNA High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Real Time PCR
Real-time PCR for IL-8 was performed using TaqMan Gene Expression Assay (Applied Biosystems) on an ABI-Prism 7300 Sequence Detector (Applied Biosystems). 18S RNA amplification control was used for cycle normalization. Data were analyzed with 7500 SDS Software (Applied Biosystems). All PCR reactions were set up in triplicates.
Degranulation assays
Human mast cells were sensitized overnight with 100 ng/ml biotinylated IgE, in StemPro-34 media with SCF and IL-6. After washing, cells were stimulated with 100 ng/ml streptavidin (SA) at 37 °C for 30 min in Tyrode´s buffer (10 mM Hepes, 137 mM NaCl, 2.7 mM, KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 1.3 mM MgSO4, 5.6 mM glucose and 0.025% BSA) for 30 min at 37 °C. IgE-dependent mast cell degranulation was monitored by β-hexosaminidase release as described (25). The resulting β-hexosaminidase activity was expressed as the percentage of total cell content (samples lysed with triton X-100). % β-hexosaminidase release = (sample release minus spontaneous release/ maximum release minus spontaneous release) X 100.
Calcium mobilization
Calcium mobilization in CD34+-derived huMCs and LAD2 cells was followed by fluorimetric analysis of free calcium with Fluo 4 AM fluorescent dye (Molecular Probes ®, Invitrogen). 0.2×106 cells/ point were loaded with 5µM Fluo 4 AM for 30 min at 4°C in the dark, washed twice with Tyrode´s buffer and resuspended. Fluorimetric measurements were done in a Modulus™ II Microplate Multimode Reader, Turner Biosystems (Promega, CA, USA) according to the manufacturer’s instructions.
Cell activation, immunoprecipitation and immunoblotting
Cells were sensitized overnight with biotinylated-IgE (100 ng/ml) in culture media. The following day, the cells were stimulated with 100 ng/ml of SA in the Tyrode’s buffer for the indicated times. Whole cell lysate preparations were obtained as described elsewhere (26). For immunoprecipitation experiments, cells were washed twice with ice-cold PBS and solubilized in lysis buffer (1% Triton X-100, 50 mM Tris, pH 7.4, 150 mM NaCl, 20mM octyl-β-glucoside, 100 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium pyrophosphate and protease inhibitor cocktail (Roche Molecular Biochemical, IN, USA)). Cell lysates were pre-cleared by centrifugation. Pre-cleared lysates were first incubated with the indicated primary antibodies and, subsequently with protein A-sepharose beads (Amersham Pharmacia Biotech, Sweden). After rotation for 3 h at 4°C, the beads were washed three times with lysis buffer and immunoprecipitated proteins were eluted by heat treatment at 100°C for 5 min with 3 fold 4X concentrated sampling buffer. The immunoprecipitates and total cell lysates were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene difluoride membranes PVDF (Millipore, Bedford, MA, USA). Blots were probed with the indicated antibodies. In all blots, proteins were visualized by enhanced chemiluminescence (Santa Cruz Biotechnology, Inc).
Immunofluorescence microscopy
Unstimulated and FcεRI-activated LAD2 cells at various times (1 × 106 cells of each), or unstimulated non-target shRNA and 3BP2 shRNA infected LAD2 cells (Supplementary Figure 1) were fixed with 4% paraformaldehyde in PBS and permeabilized with 0, 05% Saponin in PBS. After washing with PBS and incubated for 1 h with blocking buffer (0,2% non fat dry milk, 2%FCS,1%BSA, 20% rabbit serum, 0,01% NaN3 and 0,01% Triton). Cells were labeled in suspension with anti-human 3BP2 or mouse IgG1 isotype control (1 µg/ml) at 4°C for 1 h. After washing with ice-cold PBS, cells were incubated with 1 µg/ml anti-mouse Cy3 (Jackson ImmunoResearch Laboratories) at 4°C (control) for 30 min. For supplementary figure 2 anti-pSyk (Cell Signalling) was incubated for 1h at 4°C and developed with Alexa488 conjugated anti-rabbit secondary (Molecular Probes). Cells were then washed twice with ice-cold PBS, immobilized on polylysine-treated coverslips at 4°C for 30 min. Samples were washed and mounted in Fluoromount-G (Southern Biotechnology Associates). Fluorescence images were obtained using an Axio Observer.Z1 microscope (Zeiss) or a confocal microscope (TCS NT; Leica) for Supplementary Figure 2. Digital images were processed using Adobe Phostoshop 8.0 (Adobe Systems Inc, San Jose CA) programs.
GM-CSF and IL-8 production
IgE/antigen-dependent GM-CSF and IL-8 production and release was measured by ELISA with the DuoSet® ELISA Development System as described by the manufacturer (R&D Systems, Inc. MN, USA). Briefly, after overnight sensitization with biotinylated IgE (100ng/ml), cells were incubated with SA (1 µg/ml) for 6 h. Supernatants were collected and analyzed by ELISA.
Statistical data analysis
All results are expressed as mean ± standard deviation of the mean (SD). Student’s t-test was used to determine significant differences (p value) between two experimental groups after determination of normal distribution of the sample and variance analysis.
RESULTS
3BP2 is expressed and relocalized towards membrane upon FcεRI activation
In order to elucidate the role of the 3BP2 adaptor in huMCs we first determined its expression in primary huMCs as well as in LAD2, LUVA and tissue-derived lung mast cells by western blot (Fig. 1A). We also examined its cellular distribution in resting and FcεRI dependent-activated human mast cells by fluorescent microscopy (Fig.1 B). 3BP2 shows a cytoplasmic staining pattern in human mast cells in resting conditions. Interestingly, the protein distribution in the cytoplasm is not diffuse but shows a punctate expression pattern. Similar punctuated pattern was observed in non-target shRNA infected cells, whereas no 3BP2 staining was observed in 3BP2 knock down cells (Supplementary Fig. 1). Moreover, 3BP2 relocalizes mainly at the plasma membrane after early FcεRI activation, where it co-localizes with phosphorylated Syk kinase (Supplementary Figure 2). Ten minutes following activation the 3BP2 distribution resembles the basal situation. This data suggests a rapid and transient translocation of the adaptor to the plasma membrane induced by FcεRI aggregation.
Figure 1. 3BP2 is expressed in human mast cells.
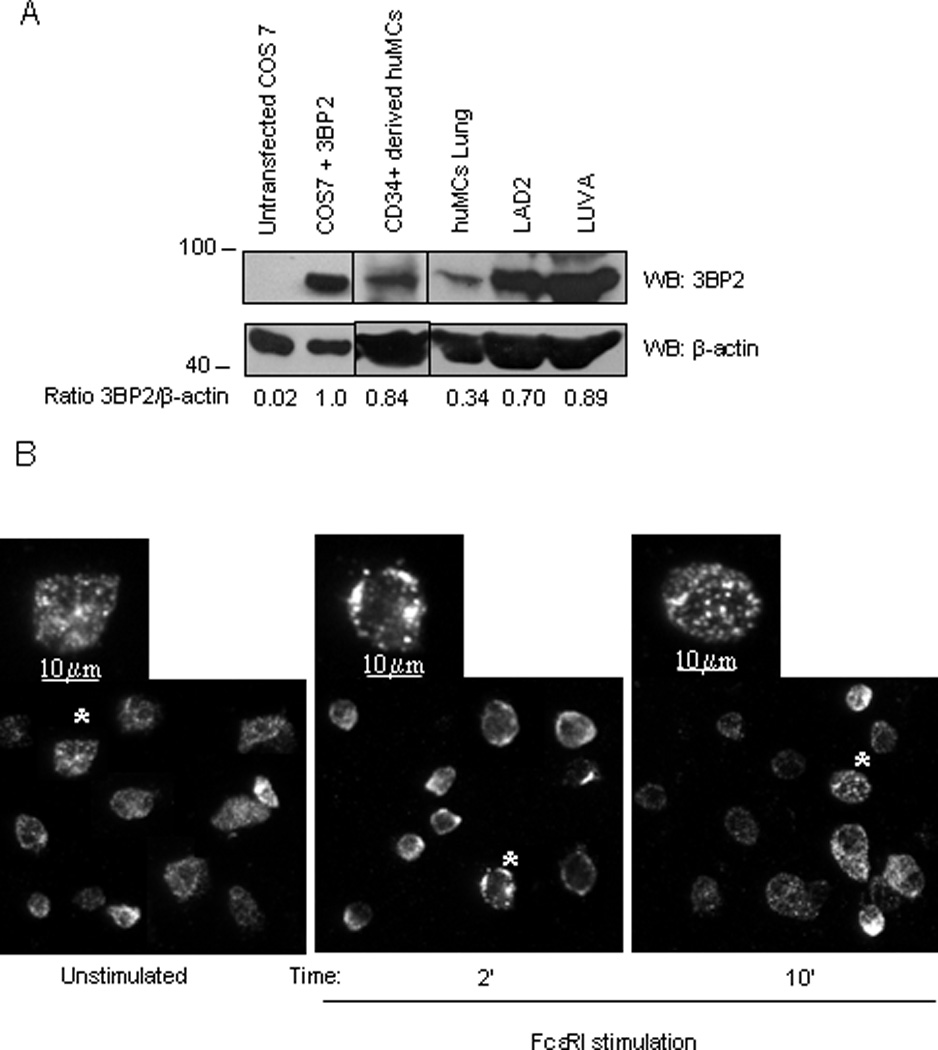
Anti-3BP2 western blot analysis was performed on non-transfected and 3BP2- transfected COS 7, LAD2 and CD34+ peripheral blood-derived huMCs whole cell lysates, LUVA and lung mast cells. Membrane was reprobed with anti-β-actin, to calculate 3BP2/total protein ration and to check loading levels per lane. (A). IgE-bound LAD2 cells were activated with streptavidin (SA) at 2 and 10 min. Non-stimulated and activated cells were fixed, permeabilized and stained with anti-3BP2 and isotype control antibodies (data not shown) and further stained with anti-mouse-Cy3 as indicated in Materials and Methods section (B). Bars equal to 10µm
3BP2 recruits essential signaling mediators in human mast cells
We next analyzed 3BP2 phosphorylation and signalosome assembly after FcεRI signaling. Biotinylated IgE-sensitized LAD2 cells were stimulated with streptavidin (SA) for various times and 3BP2 was immunoprecipitated. As shown in Figure 2, 3BP2 is phosphorylated as early as 5” after receptor ligation, and co-immunoprecipitates with several phosphoproteins. The essential FcεRI signalling molecules Lyn, Syk, and PLCγ were identified as 3BP2 binding partners in huMCs. After the interaction of cell-bound IgE with cognate Ag, Lyn kinase phosphorylates the β- and γ-chain ITAMs of proximal receptors (27). These phosphorylated ITAMs become docking sites for Syk kinase (28), which propagates downstream signals by phosphorylation of several mediators including LAT and PLCγ (29). In our experiments, 3BP2 consistently co-immunoprecipitated with a 35–40kD phosphoprotein with apparent characteristics of LAT, however, we failed to identify this interaction by immunoblots with the anti-LAT available antibodies. Nevertheless, others have reported that LAT associates with the SH2-domain of 3BP2 (22). Given that these findings suggested that 3BP2 could play a central role in the FcεRI-anchored signalosome, we investigated the consequences of 3BP2 depletion to FcεRI signaling. We first analyzed the efficiency of lentiviral-encoded shRNA sequences for their ability to silence 3BP2, as compared to non-transduced cells or control /non target (NT) shRNA transduced cells. As shown in Fig 3A, we identified shRNA sequences that could effectively suppress 3BP2 expression in human mast cell lines and CD34+ derived huMCs. We evaluated Lyn (Fig. 3B) as well as FcεRI expression (Fig. 3C) in NT and 3BP2-silenced LAD2 and CD34+ derived huMCs cells. Our results show that both FcεRI and Lyn expression were unaffected by 3BP2 knock down.
Figure 2. 3BP2 coprecipitates with Lyn, Syk and PLCγ in human mast cells.
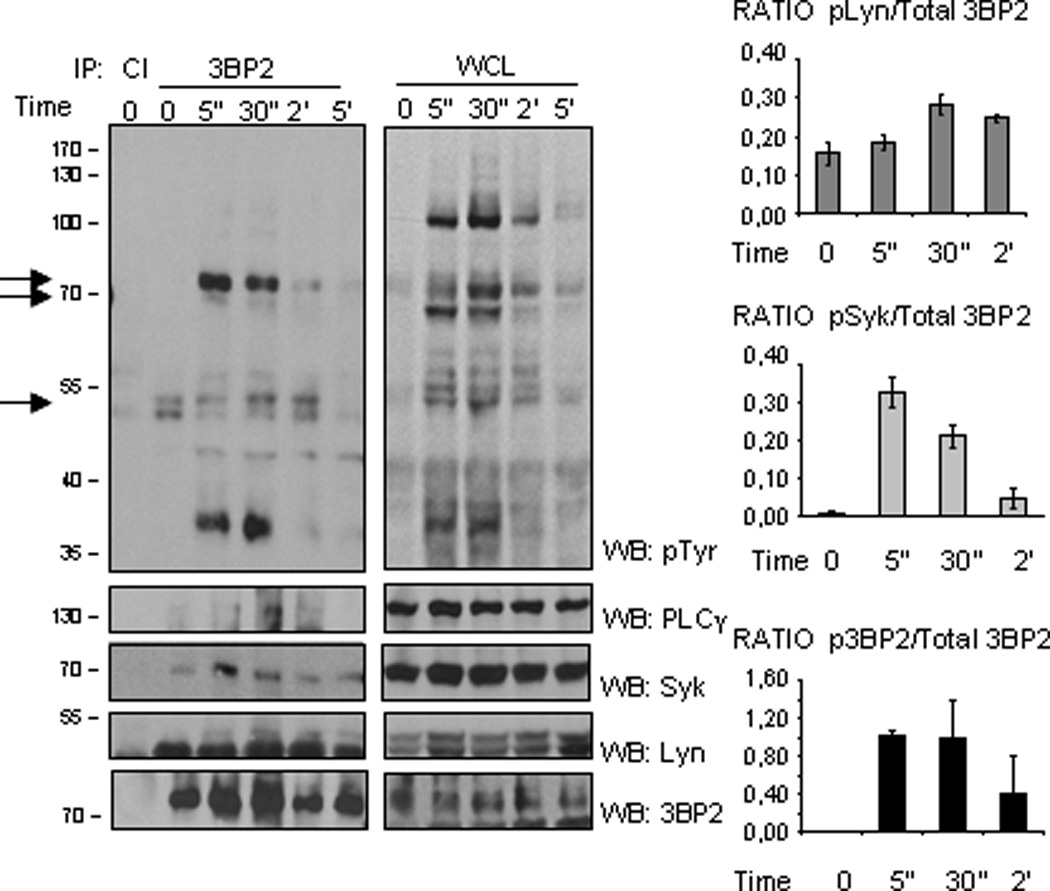
Biotinylated IgE-bound LAD2 cells (25 million cells per point) were activated with streptavidin (SA) at the indicated times and lysed as discussed in Materials and Methods. The lysates were immunoprecipitated using control Ig (CI) and 3BP2 antibodies. Western blot was performed with the following antibodies: anti-phosphotyrosine, anti-PLCγ, anti-Lyn and anti-Syk. The immunoprecipitates and WCL (one million and a half) were loaded in the same gel. WCL stands for whole cell lysate. The experiment has been performed various times and the graphics comparing phosphorylation of Lyn, Syk and 3BP2 from 3BP2 immunoprecipitation versus total 3BP2 immunoprecipitated are the mean of three independent experiments
Figure 3. Selective knock down of 3BP2 in human mast cells.
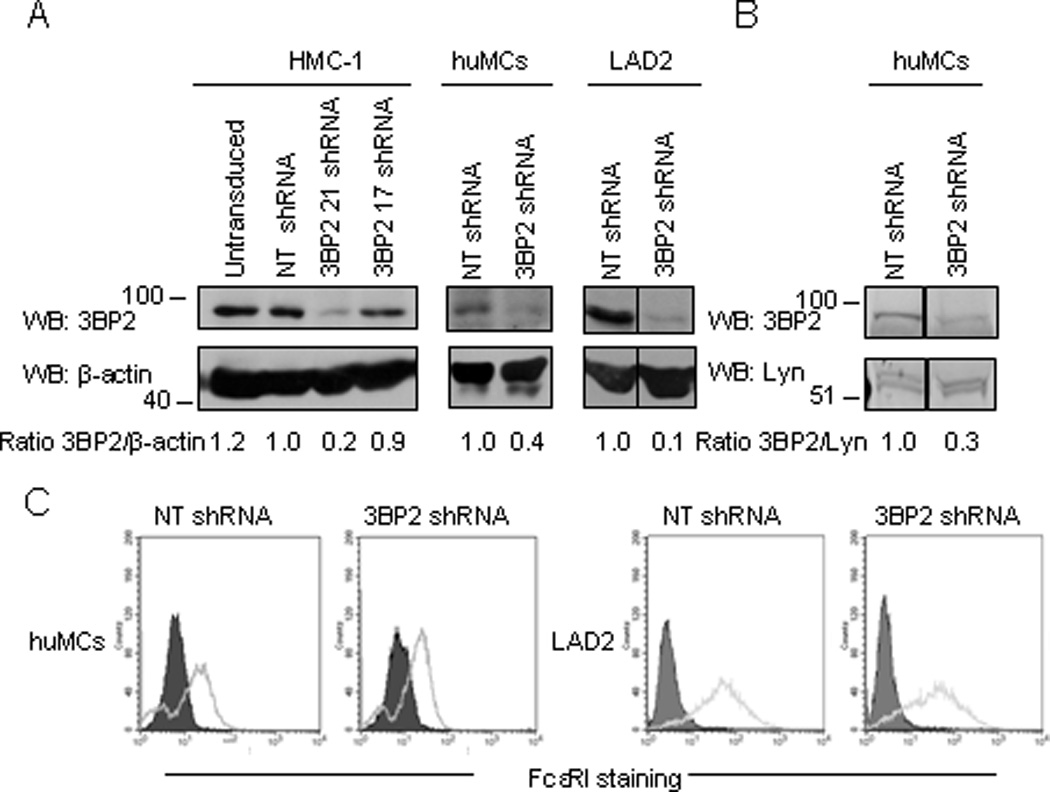
3BP2 expression was analyzed in HMC-1 cells by western blot following treatment of the cells with control (NT shRNA) and 2 different 3BP2-targetting shRNAs. The sequence corresponding to shRNA clone 21 was chosen for further assays in CD34+ peripheral blood-derived huMCs and LAD2 cells (A). Quantitation of the band intensity was performed by densitometry. Lyn (B) and FcεRI expression (C) were similar in 3BP2 shRNA and control- transduced huMCs and LAD2 cells. FcεRI expression was determined by FACS using PE-conjugated α-FcεRI. The isotype control is grey filled. Data are representative of 3 experiments.
3BP2 is required for FcεRI-dependent Syk phosphorylation
3BP2 was first identified as a Syk binding partner in a two hybrid screening (7). This interaction involves the 3BP2 SH2 domain and Syk, suggesting that the 3BP2 SH2 domain associates with autophosphorylated Tyr residues in the Syk linker or kinase domain (7). In our work, we found that 3BP2 interacts with Syk in human mast cells after FcεRI activation (Figure 2). Syk has an essential role in early and late mast cell responses (30), binding the phosphorylated FcεRIγ chain ITAMs after receptor aggregation resulting in a conformational change that increases its enzymatic activity (29) which leads to downstream propagation of signals. To evaluate whether 3BP2 participates in the regulation of Syk activation we examined the kinase phosphorylation in 3BP2-silenced versus NT shRNA transduced huMCs following FcεRI stimulation. We found reduced Syk phosphorylation in Tyr352 when 3BP2 expression is reduced both in LAD2 and huMCs cells while totals levels of the kinase remain unaffected (Fig.4 A and B). Reduction in Syk phosphorylation in 3BP2 knock down cells occurs as early as 5” after FcεRI stimulation (Supplementary Figure 3).
Figure 4. Silencing of 3BP2 reduces FcεRI-induced Syk Y352 phosphorylation.
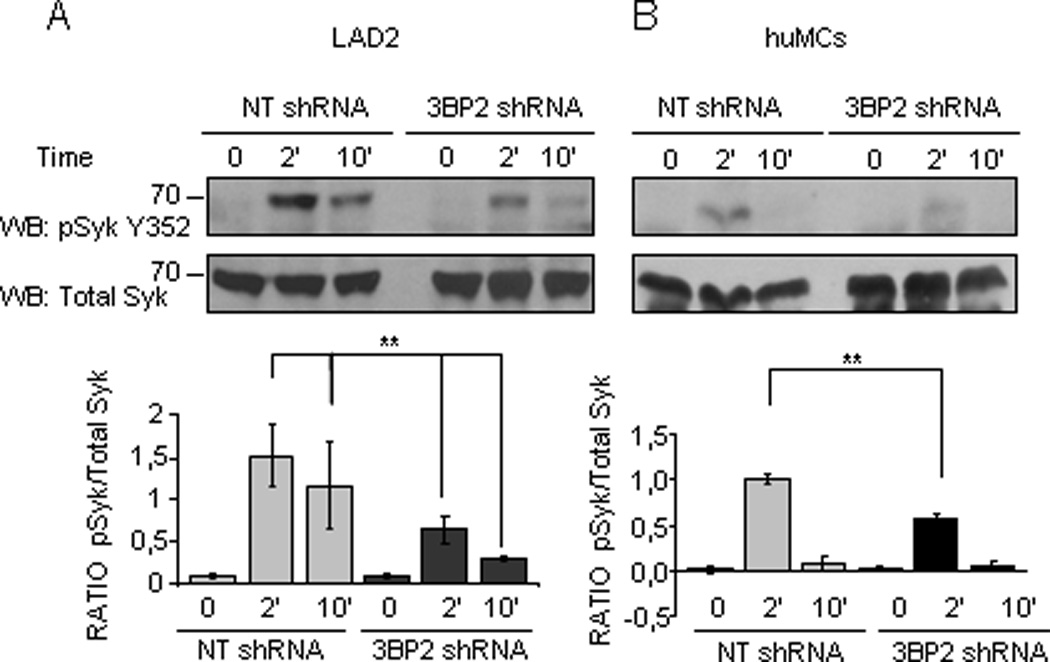
Specific antibodies recognizing phosphorylated Syk Y352 and total Syk were used to immunoprobe for these proteins in huMC lysates of 3BP2 shRNA- and NT shRNA- transduced cells following biotinylated IgE crosslinking with 100 ng/ml SA at indicated times. Western blots correspond to LAD2 cells (A) and CD34+ peripheral blood-derived huMCs (B). The band intensity quantitation was performed by densitometry. Densitometric analysis and phosphorylated/total ratio for each molecule from 3 independent experiments is represented in the bar charts on the bottom (C). Statistical significance (**p< 0.001,) is relative to NT shRNA.
3BP2 is required for FcεRI-dependent LAT and PLCγ phosphorylation and calcium signaling
Activated Syk phosphorylates LAT which acts as a scaffold for a multimolecular signaling complex that includes PLCγ among other molecules (31). As 3BP2 expression modulates Syk phosphorylation, we expected that LAT and PLCγ phosphorylation could also be negatively affected after 3BP2 silencing. Thus, we examined phosphorylation of these molecules in 3BP2-silenced versus NT shRNA-transduced huMCs following FcεRI stimulation and we found a decrease in LAT and PLCγ1 phosphorylation when 3BP2 expression was reduced (Fig.5 A). PLCγ activation and phosphorylation facilitated by LAT, are key steps in Ca2+ release from intracellular stores (29), (32). Hence, we measured calcium fluxes following FcεRI aggregation in 3BP2-silenced huMCs and LAD2 cells and compared these responses to those obtained in the NT shRNA-transduced cells. Our analysis showed that the initial calcium rise was similar in both control and 3BP2-silenced huMCs, but calcium mobilization in the 3BP2-silenced cells was not equally sustained with time (Fig.5 B). Conversely, the calcium mobilization was equal in both control and 3BP2-silenced cells after ionomycin treatment (Fig. 5C). Importantly, the maintenance of high calcium concentration is mediated through the influx of calcium from the extracellular environment and is a requirement for optimal MC degranulation (33). Thus, our findings indicate that 3BP2 is an important regulator of calcium influx after FcεRI stimulation.
Figure 5. FcεRI-mediated phosphorylation of PLCγ and LAT and resulting calcium signal are reduced in 3BP2 shRNA- silenced cells.
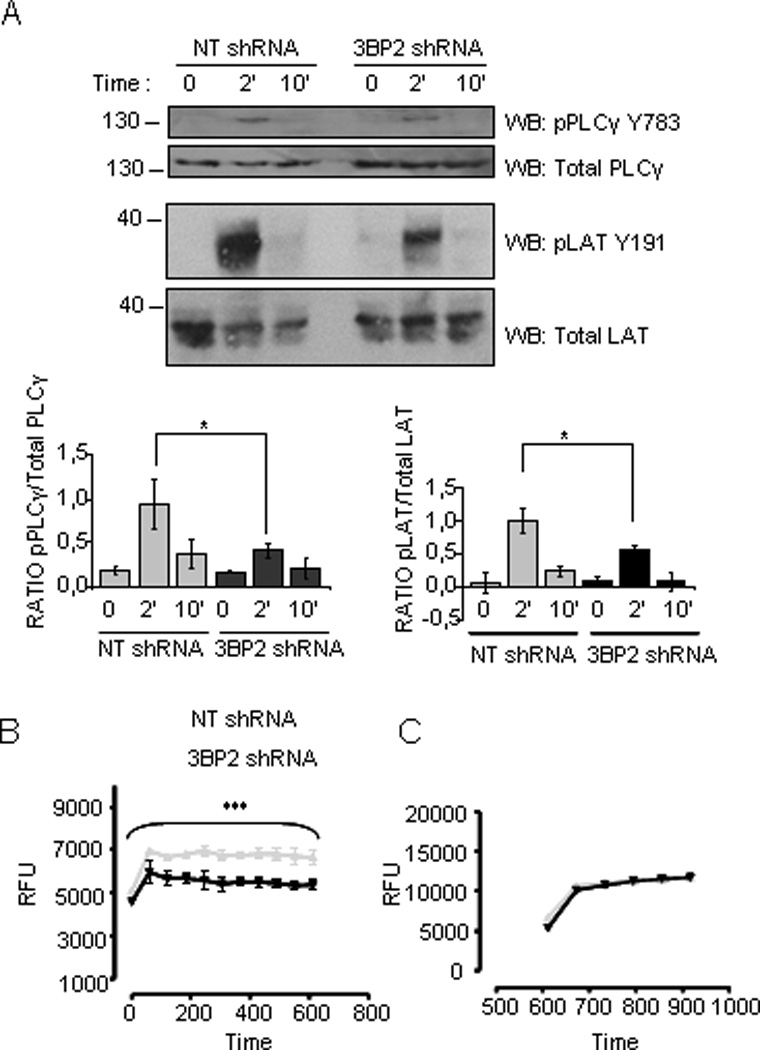
Specific anti-phosphorylated LATY191 and PLCγ Y783 antibodies were used to blot membranes with 3BP2 knockdown versus control shRNA huMCs cell lysates upon biotinylated IgE crosslinking with 100ng/ml SA for various times as indicated. Densitometric analysis and phosphorylated/total ratio for each molecule from 3 independent experiments is represented in the bar charts next to the blot (A). Calcium fluxes following FcεRI stimulation were measured in biotinylated IgE sensitized 3BP2 shRNA silenced and NT shRNA transduced LAD2 (B) cells loaded with Fluo4. Cells were stimulated with 100 ng/ml streptavidin (SA) and analyzed by fluorimetric measure of free calcium concentrations. Results are expressed as mean +/− SD of triplicates and it is representive of 6 independent experiments. 3BP2 shRNA and NT shRNA transduced LAD2 cells responses to ionomycin, respectively are shown in (C). Statistical significance (*p< 0.005, ***p< 0.0001) is relative to NT shRNA. RFU means relative fluorescence units.
3BP2 silencing affects ERK1/2 and p38 phosphorylation
Once phosphorylated LAT recruits a number of signalling intermediaries that include Gab2, guanosine triphosphate exchangers, SOS that further activate small GTPases such as Ras resulting in the stimulation of the MAP kinase pathways. Previously, we have reported that overexpression of 3BP2 increases ERK1/2 phosphorylation, without affecting p38 phosphorylation, in NK cells after CD244 triggering, which correlate with enhancement of cytotoxicity (20). Herein, we studied how these pathways were altered after 3BP2 silencing in huMCs and LAD2 cells. Our data shows that FcεRI-mediated ERK1/2 and p38 phosphorylation was decreased following 3BP2 knockdown (Figure 6), supporting the idea of an important role for 3BP2 in signal propagation from the FcεRI.
Figure 6. ERK and p38 MAPK phosphorylation is reduced in 3BP2 silenced huMCs.
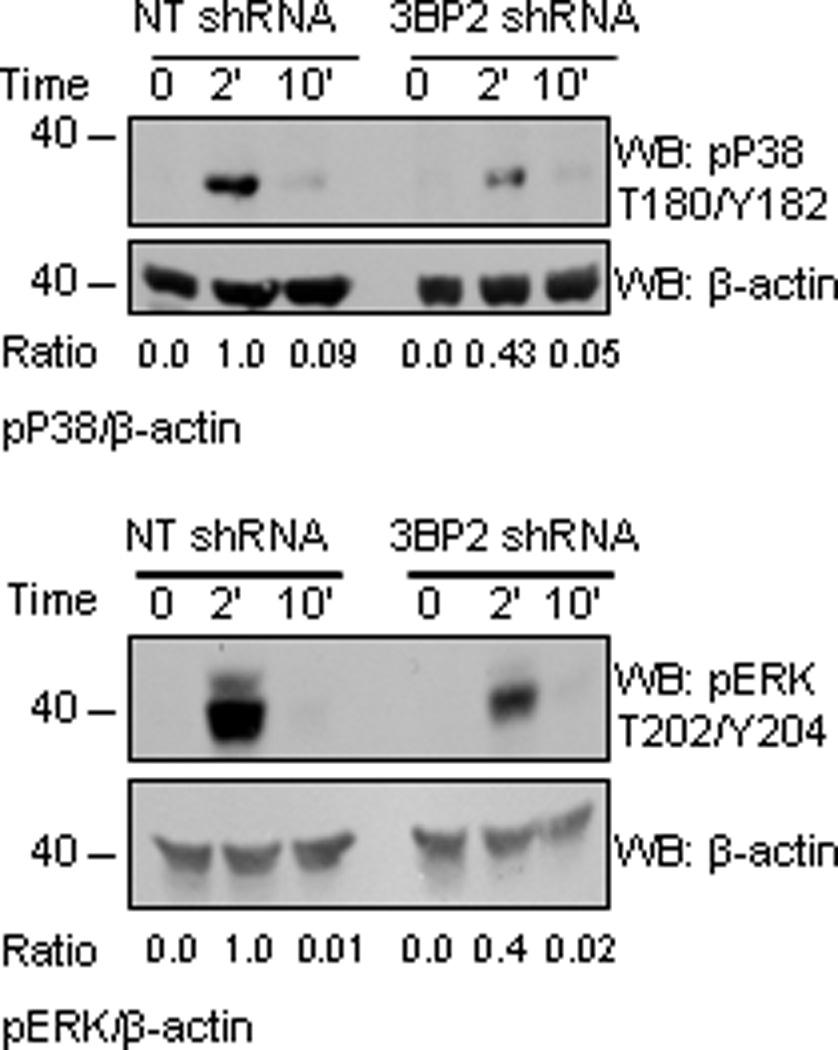
Biotinylated IgE-sensitized 3BP2 shRNA- and NT shRNA-transduced huMCs were stimulated with 100 ng/ml SA for 2 and 10 min. Cells were lysed and total cell lysates were analyzed for ERK1/2 and p38 MAPK phosphorylation. Actin was used as loading control. Band intensity quantitation for each blot was performed by densitometry. The experiment is representative of 3 independent assays.
3BP2 is a positive regulator of MC degranulation and cytokine responses
Given the relationship between calcium mobilization and MC activation, we explored the effect of 3PB2 silencing on huMC degranulation. Thus, we examined β-hexosaminidase release in 3BP2 shRNA- versus NT-infected cells. Reduced levels of 3BP2 expression in huMCs resulted in reduced FcεRI-mediated degranulation compared to that observed in NT transduced cells. When the non immunologic stimulus PMA plus ionomycin (P+I) was added, 3BP2 silencing did not affect granule release indicating that 3BP2 acts specifically downstream of FcεRI and upstream of late events (Figure 7). Late responses after IgE stimulation on MCs include the de novo synthesis and secretion of cytokines and chemokines (34). IL-8 and GM-CSF constitute two of the most abundant of such mediators; hence we evaluated the role of 3BP2 in their production. Biotinylated IgE-sensitized 3BP2-silenced and NT shRNA- transduced huMCs were stimulated with SA for 6h and IL-8 and GM-CSF contents were measured in cell culture supernatants (Fig. 8A and 8B). In addition, in the case of IL-8, 3BP2 was required for optimal production at the transcriptional level, as evidenced by a marked reduction in IL-8 mRNA levels after 3BP2 silencing (Fig. 8C). Collectively, the findings demonstrate that the 3BP2 adaptor molecule is necessary for optimal triggering of early and late FcεRI signaling events.
Figure 7. 3BP2 silencing impairs FcεRI mediated degranulation in huMCs.
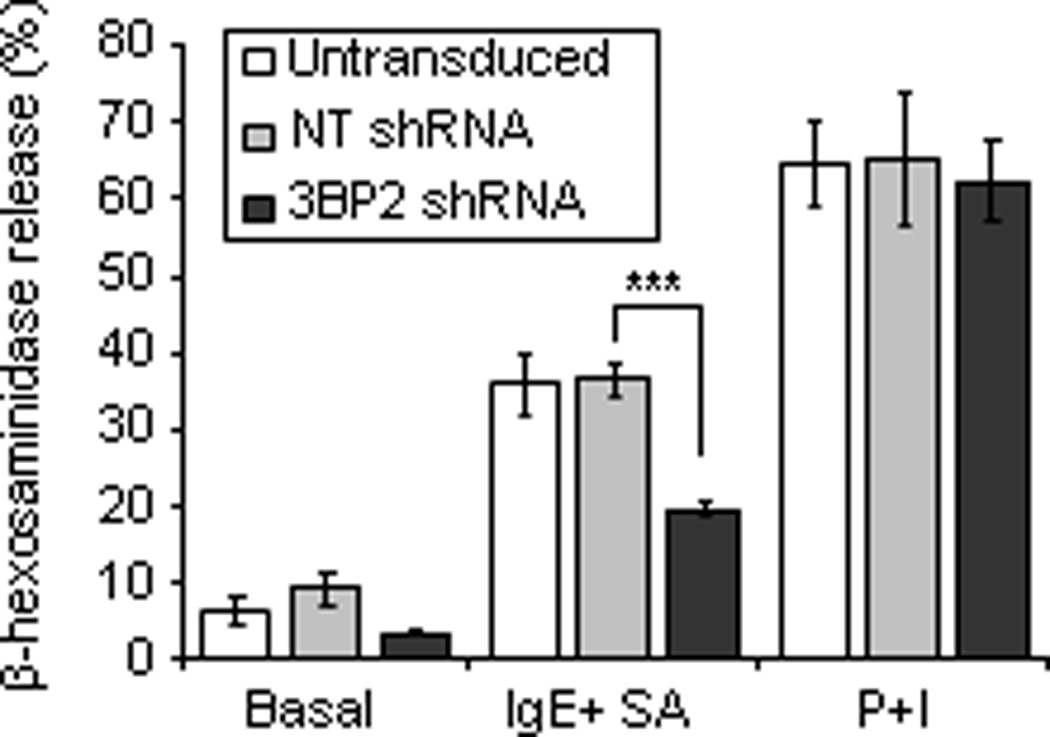
Biotinylated-IgE-sensitized CD34+ peripheral blood-derived huMCs with normal or reduced levels of 3BP2 (shRNA silenced) were stimulated with 100 ng/ml SA for 30 min. β-hexosaminidase release was measured in collected supernatants. Results are expressed as a percent of total β-hexosaminidase content reported as mean +/− SE. Data represent the mean of 3 independent experiments. Statistical significance (***p< 0.0001) is relative to NT shRNA.
Figure 8. 3BP2 is required for normal huMC cytokine production.
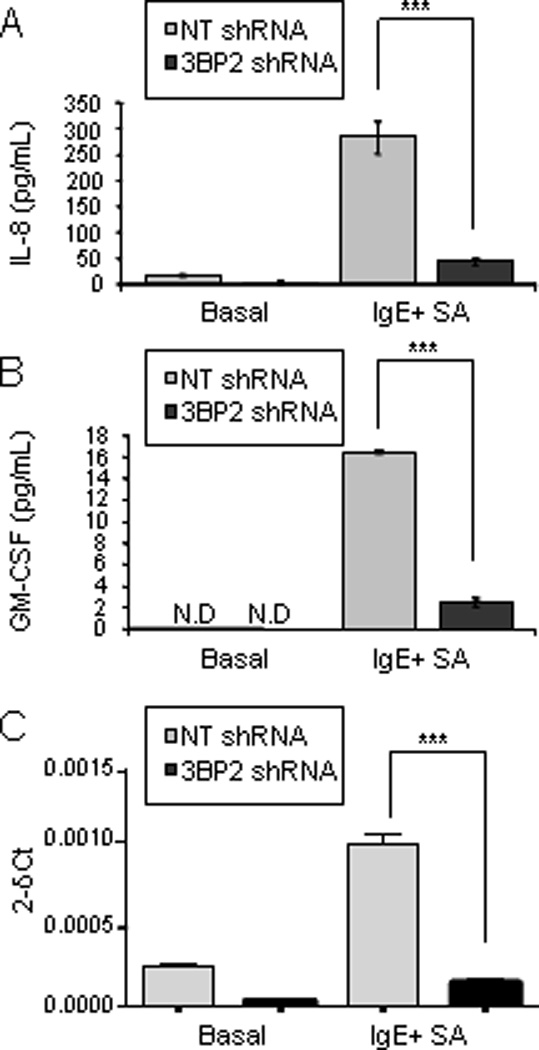
CD34+ peripheral blood-derived huMCs with normal or reduced levels of 3BP2 (NT shRNA and 3BP2 shRNA respectively) were sensitized O/N with biotinylated IgE and stimulated for 6 h with 1 µg/ml streptavidin (SA). IL-8 and GM-CSF production were measured by ELISA (A and B) and IL-8 transcripts relative to a control RNA were measured by real time PCR (C). Results are expressed as the mean +/− SD. Significant (***p<0.0001) difference was found between NT shRNA and 3BP2 shRNA. Data are the mean of triplicates and it is representative of 3 independent experiments from 3 different donors. N.D. means not detectable.
DISCUSSION
Mast cells are involved in both innate and adaptive immunity and they induce the release of preformed and the de novo synthesis of inflammatory mediators after activation. The final outcome will depend on the balance of engaging inhibitory and activating cell surface receptors as well as the positive and negative intracellular molecular events that involve kinases and phosphatases as well as other protein and lipid mediators with adaptor functions. Here we report for first time that 3BP2 is expressed in human mast cells and that it is required for FcεRI signaling.
3BP2 was tyrosine phosphorylated at early times after FcεRI stimulation and consequentially was observe to co-immunoprecipitate with the src kinase Lyn implicating this adaptor in the organization of early events in mast cell signaling. Lyn is constitutively associated with the β subunit of the FcεRI and activated upon antigen-mediated FcεRI aggregation (35). Following antigen stimulation, 3BP2 was recruited to the plasma membrane, presumably by its PH domain. Once in close proximity to the plasma membrane, 3BP2 could interact by its proline rich domain with the Lyn SH3 domain and be a target for phosphorylation by this Src kinase. This view is supported by experiments in COS-7 cells showing that 3BP2 interacts with, and is a substrate for, Lyn. In addition, phosphorylation of Y446 in 3BP2 can create a binding site for the Lyn SH2 thereby increasing Lyn autophosphorylation (8). Our data show that 3BP2 also binds Syk in human mast cells after FcεRI stimulation, Both 3BP2 and phosphorylated Syk are translocated to the membrane where they colocalize following receptor crosslinking. 3BP2 (via its SH2 domain) has been shown to interact with phosphorylated and activated Syk after antigen receptor engagement of B cells (15). Syk, also binds to the tyrosine phosphorylated γ ITAM of the FcεRI trough its SH2 domains (35) and is essential for all known FcεRI-mediated responses (36). 3BP2 also undergoes rapid dephosphorylation within two minutes upon stimulation, which could be mediated by SHP-1 phosphatase which has been described as a 3BP2 substrate in T cells (Yu, 2006). The afore mentioned early phosphorylation events lead to the recruitment of molecules such as LAT, and to the activation of enzymes like phospholipase Cγ, which regulates intracellular calcium release and PKC activation (31, 35). We observed that 3BP2 can bind PLCγ when the adaptor is phosphorylated. Indeed, it has been described that Syk can also induce phosphorylation of Y183 in 3BP2 (18), which then binds PLCγ1 stabilizing the interaction of this enzyme with LAT and contributing to optimal phosphorylation of these molecules in B cells (18). The above interactions of 3BP2 would likely constitute an amplification mechanism that promotes Lyn -Syk - LAT- PLCγ signaling through stabilizing this complex. Reduction in 3BP2 expression did not completely ablate signaling but we observed weaker phosphorylation of Syk Y352 as early as 5” after stimulation, and downstream of this, reduced LAT Y191 and PLCγ1 Y738 phosphorylation. Pharmacological inhibitors of Syk kinase catalytic activity bearing therapeutic potential have been developed (37). However, Syk is widely distributed in different cell types, and inhibiting its catalytic activity could result in unwanted consequences. Inhibition of Syk interactions with its cellular partners, even while maintaining its kinase activity, might well result in the dampening of cellular responses and could be useful as a strategy with therapeutic potential (38). Because 3BP2 is a Syk binding protein it constitutes an interesting candidate for potential intervention in Syk mediated-signals. In addition to participating in early signaling events, 3BP2 also affects other downstream signaling molecules, which would account for the observed reduction in cytokine production following 3BP2 knock down. Our findings show that ERK1 and 2 and p38 MAPKs are less active in 3BP2 silenced cells. Optimal activation of MAPKs requires LAT function as revealed in LAT deficient BMMCs (39, 40). Thus the contribution of 3BP2 in achieving complete phosphorylation of LAT could explain the observed effects in late FcεRI responses, including MAPKs and transcription factor activity. In support of this view, 3BP2 has been reported to contribute to B cell antigen receptor (BCR) (18) dependent activation of transcription factors like NFAT, creating a multiprotein signalosome with the SH2 domain(s) of PLCγ2 and Vav1.
In this work, we show that in mast cells, 3BP2 regulates and is necessary for GM-CSF production following FcεRI stimulation. Transcription of GM-CSF is known to be NFAT-dependent (41). The two murine models in which the 3BP2 gene is deleted have generated some controversy as to whether there is a role for 3BP2 in cell viability and in Syk, PLCγ2 and MAP kinase activation. However, is quite clear that B cell proliferation and BCR signaling are impaired while there is normal T cell development, proliferation, cytokine secretion, and signaling in response to TCR ligation in 3BP2 deficient mice (16), (17). These findings suggest that 3BP2 is critical for BCR, and humoral responses but not for TCR signaling. T cells could still have a homologous molecule which has a redundant function of 3BP2. The presence of a 3BP2 homologue is consistent with the observation of wild type sequence of 3BP2 in 3 out of 12 families with cherubism, a disease where mutation of 3BP2 results in molecule´s gain of function (42).
Cherubism involves a chronic non-infectious inflammation of the bone which occurs in the absence of a detectable adaptive immune response. Cherubism mice exhibit increased myeloid responses to macrophage-colony-stimulating factor (M-CSF) and RANK (receptor activation of NFkB) ligand (RANKL) leading to the activation of osteoclast. As we learn more about the immunological features of this disease it appears that TNF-α secreting myeloid cells are emerging as primary players (9) (43). Although such cells are likely to be important players, mast cell involvement can not be excluded. Recently, it has been demonstrated that cherubism mutations disrupt the interaction between 3BP2 and Tankyrase, a poly (ADP-ribose) polymerase resulting in higher 3BP2 levels due to increased protein stability, leading to hyperactive Src, Syk, and Vav pathways in osteoclasts (12). Structural evidence that deregulation of substrate recognition by Tankyrase is indeed the underlying mechanistic basis of cherubism has been as well provided (11). Conversely, it was published that overexpression of the cherubism 3BP2 mutants suppress Vav and MAP kinase activation attenuating the FcεRI-mediated activation in RBL-2H3 cells, suggesting that point mutations of 3BP2 cause dysfunction of 3BP2 in vivo (44). These data revealed the importance of elucidating the role of the protein in the most appropriate and physiological context.
In conclusion, herein we present evidence for the expression and critical role of 3BP2 in human mast cells activation. This function is related to its essential role as an adaptor molecule for both early and late signal transduction responses required for respectively degranulation and the generation and release of cytokine Increasing our knowledge on how 3BP2 functions will be crucial towards further understanding its role in the regulation of FcεRI signaling, which may also open new avenues of research with therapeutic promise in mast cell derived diseases.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Unitat Microscopia Confocal, Serveis Cientificotècnics, Universitat de Barcelona-IDIBAPS) for technical assistance.
This work was supported by grant SAF2009-07548 from the Plan Nacional Ministerio de Ciencia e Innovación. Spain. E. A-E is supported by a FPI fellowship both from Ministerio de Ciencia e Innovación. D. A-E is supported by a Juan de la Cierva contract. J.S. is supported by a contract Miguel Servet from Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III (CP06/00058). Work conducted in the laboratory of A.M.G. is supported by the Intramural Research Program within the National Institute of Allergy and Infectious Disease of the National Insitutes of Health, USA. Work done in J.R. laboratory is funded by the Intramural Research Program within the National Institute of Arthritis, Musculoskeletaland Skin Disease of the National Institutes of Health, USA.
REFERENCES
- 1.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mekori YA, Oh CK, Metcalfe DD. The role of c-Kit and its ligand, stem cell factor, in mast cell apoptosis. Int Arch Allergy Immunol. 1995;107:136–138. doi: 10.1159/000236955. [DOI] [PubMed] [Google Scholar]
- 4.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cellular and molecular life sciences : CMLS. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Errico D, Lessmann E, Rivera J. Adapters in the organization of mast cell signaling. Immunol Rev. 2009;232:195–217. doi: 10.1111/j.1600-065X.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 7.Deckert M, Tartare-Deckert S, Hernandez J, Rottapel R, Altman A. Adaptor function for the Syk kinases-interacting protein 3BP2 in IL-2 gene activation. Immunity. 1998;9:595–605. doi: 10.1016/s1074-7613(00)80657-3. [DOI] [PubMed] [Google Scholar]
- 8.Maeno K, Sada K, Kyo S, Miah SM, Kawauchi-Kamata K, Qu X, Shi Y, Yamamura H. Adaptor protein 3BP2 is a potential ligand of Src homology 2 and 3 domains of Lyn protein-tyrosine kinase. J Biol Chem. 2003;278:24912–24920. doi: 10.1074/jbc.M301201200. [DOI] [PubMed] [Google Scholar]
- 9.Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E, Olsen BR. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 "cherubism" mice. Cell. 2007;128:71–83. doi: 10.1016/j.cell.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Hatani T, Sada K. Adaptor protein 3BP2 and cherubism. Current medicinal chemistry. 2008;15:549–554. doi: 10.2174/092986708783769795. [DOI] [PubMed] [Google Scholar]
- 11.Guettler S, LaRose J, Petsalaki E, Gish G, Scotter A, Pawson T, Rottapel R, Sicheri F. Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. Cell. 2011;147:1340–1354. doi: 10.1016/j.cell.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Levaot N, Voytyuk O, Dimitriou I, Sircoulomb F, Chandrakumar A, Deckert M, Krzyzanowski PM, Scotter A, Gu S, Janmohamed S, Cong F, Simoncic PD, Ueki Y, La Rose J, Rottapel R. Loss of Tankyrase-mediated destruction of 3BP2 is the underlying pathogenic mechanism of cherubism. Cell. 2011;147:1324–1339. doi: 10.1016/j.cell.2011.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deckert M, Rottapel R. The adapter 3BP2: how it plugs into leukocyte signaling. Adv Exp Med Biol. 2006;584:107–114. doi: 10.1007/0-387-34132-3_8. [DOI] [PubMed] [Google Scholar]
- 14.Yu Z, Maoui M, Zhao ZJ, Li Y, Shen SH. SHP-1 dephosphorylates 3BP2 and potentially downregulates 3BP2-mediated T cell antigen receptor signaling. Febs J. 2006;273:2195–2205. doi: 10.1111/j.1742-4658.2006.05233.x. [DOI] [PubMed] [Google Scholar]
- 15.Foucault I, Le Bras S, Charvet C, Moon C, Altman A, Deckert M. The adaptor protein 3BP2 associates with VAV guanine nucleotide exchange factors to regulate NFAT activation by the B-cell antigen receptor. Blood. 2005;105:1106–1113. doi: 10.1182/blood-2003-08-2965. [DOI] [PubMed] [Google Scholar]
- 16.de la Fuente MA, Kumar L, Lu B, Geha RS. 3BP2 deficiency impairs the response of B cells, but not T cells, to antigen receptor ligation. Mol Cell Biol. 2006;26:5214–5225. doi: 10.1128/MCB.00087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Dimitriou ID, La Rose J, Ilangumaran S, Yeh WC, Doody G, Turner M, Gommerman J, Rottapel R. The 3BP2 adapter protein is required for optimal B-cell activation and thymus-independent type 2 humoral response. Mol Cell Biol. 2007;27:3109–3122. doi: 10.1128/MCB.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla U, Hatani T, Nakashima K, Ogi K, Sada K. Tyrosine phosphorylation of 3BP2 regulates B cell receptor-mediated activation of NFAT. J Biol Chem. 2009;284:33719–33728. doi: 10.1074/jbc.M109.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jevremovic D, Billadeau DD, Schoon RA, Dick CJ, Leibson PJ. Regulation of NK cell-mediated cytotoxicity by the adaptor protein 3BP2. J Immunol. 2001;166:7219–7228. doi: 10.4049/jimmunol.166.12.7219. [DOI] [PubMed] [Google Scholar]
- 20.Saborit-Villarroya I, Del Valle JM, Romero X, Esplugues E, Lauzurica P, Engel P, Martin M. The adaptor protein 3BP2 binds human CD244 and links this receptor to Vav signaling, ERK activation, and NK cell killing. J Immunol. 2005;175:4226–4235. doi: 10.4049/jimmunol.175.7.4226. [DOI] [PubMed] [Google Scholar]
- 21.Saborit-Villarroya I, Martinez-Barriocanal A, Oliver-Vila I, Engel P, Sayos J, Martin M. The adaptor 3BP2 activates CD244-mediated cytotoxicity in PKC- and SAP-dependent mechanisms. Mol Immunol. 2008;45:3446–3453. doi: 10.1016/j.molimm.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Sada K, Miah SM, Maeno K, Kyo S, Qu X, Yamamura H. Regulation of FcepsilonRI-mediated degranulation by an adaptor protein 3BP2 in rat basophilic leukemia RBL-2H3 cells. Blood. 2002;100:2138–2144. doi: 10.1182/blood-2001-12-0340. [DOI] [PubMed] [Google Scholar]
- 23.Radinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Current protocols in immunology / edited by John E. Coligan ... [et al.] Chapter 7:Unit 7. 2010:37. doi: 10.1002/0471142735.im0737s90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Errico D, Oliver-Vila I, Ainsua-Enrich E, Gilfillan AM, Picado C, Sayos J, Martin M. CD84 negatively regulates IgE high-affinity receptor signaling in human mast cells. J Immunol. 2011;187:5577–5586. doi: 10.4049/jimmunol.1101626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in Fc epsilon RI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. Journal of immunological methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- 27.Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci U S A. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouvin MH, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet JP. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 29.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 30.Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 32.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Critical reviews in immunology. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 35.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 37.Riccaboni M, Bianchi I, Petrillo P. Spleen tyrosine kinases: biology, therapeutic targets and drugs. Drug discovery today. 2010;15:517–530. doi: 10.1016/j.drudis.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Villoutreix BO, Laconde G, Lagorce D, Martineau P, Miteva MA, Dariavach P. Tyrosine kinase syk non-enzymatic inhibitors and potential anti-allergic drug-like compounds discovered by virtual and in vitro screening. PloS one. 2011;6:e21117. doi: 10.1371/journal.pone.0021117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh S, Odom S, Gomez G, Sommers CL, Young HA, Rivera J, Samelson LE. The four distal tyrosines are required for LAT-dependent signaling in FcepsilonRI-mediated mast cell activation. J Exp Med. 2003;198:831–843. doi: 10.1084/jem.20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson BV, Bert AG, Ryan GR, Condina A, Cockerill PN. Granulocyte-macrophage colony-stimulating factor enhancer activation requires cooperation between NFAT and AP-1 elements and is associated with extensive nucleosome reorganization. Mol Cell Biol. 2004;24:7914–7930. doi: 10.1128/MCB.24.18.7914-7930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, Ninomiya C, doAmaral C, Peters H, Habal M, Rhee-Morris L, Doss JB, Kreiborg S, Olsen BR, Reichenberger E. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson PJ, El-Shanti HI. Autoinflammatory bone disorders. Current opinion in rheumatology. 2007;19:492–498. doi: 10.1097/BOR.0b013e32825f5492. [DOI] [PubMed] [Google Scholar]
- 44.Miah SM, Hatani T, Qu X, Yamamura H, Sada K. Point mutations of 3BP2 identified in human-inherited disease cherubism result in the loss of function. Genes Cells. 2004;9:993–1004. doi: 10.1111/j.1365-2443.2004.00784.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


