Abstract
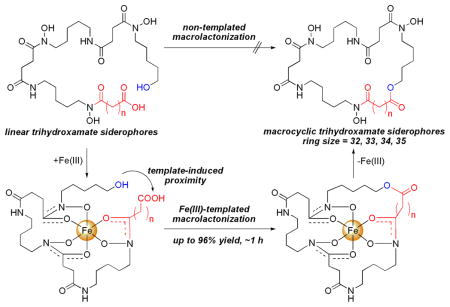
A method was developed to synthesize macrocyclic trihydroxamate siderophores using optimized Yamaguchi macrolactonization conditions. The natural ability of siderophores to bind iron(III) was exploited to template the reactions and allowed for rapid reaction rates, high product conversions, and the formation of large macrolactone rings up to 35 atoms. An X-ray structure of a 33-membered macrolactone siderophore-Fe(III) complex is presented.
Siderophores are low molecular weight, multidentate iron(III) chelators used by microorganisms for assimilation of otherwise insoluble and bio-unavailable iron(III) minerals.1 Siderophore structures are finely tuned to selectively bind iron(III) with high affinity from a mixed metal environment, which makes them ideal candidates for chelation-based treatments of human iron overload diseases such as β-thalassemia.2 The limited choice of drugs and far from ideal treatment regimes for currently approved iron chelation therapies has driven the search for more effective iron(III) chelating agents.3
One approach adapted by microbes to improve a siderophore’s ability to chelate iron(III) is to biosynthesize macrocyclic siderophore scaffolds. A macrocyclic scaffold preorganizes the ligands for metal chelation, increases iron(III) binding constants, and stabilizes the resulting siderophore-iron(III) complexes which gives the producing organism a competitive advantage for sequestering the essential nutrient.4 Herein we report a method to synthesize macrocyclic trihydroxamate siderophores using an iron(III)-templated Yamaguchi macrolactonization reaction.
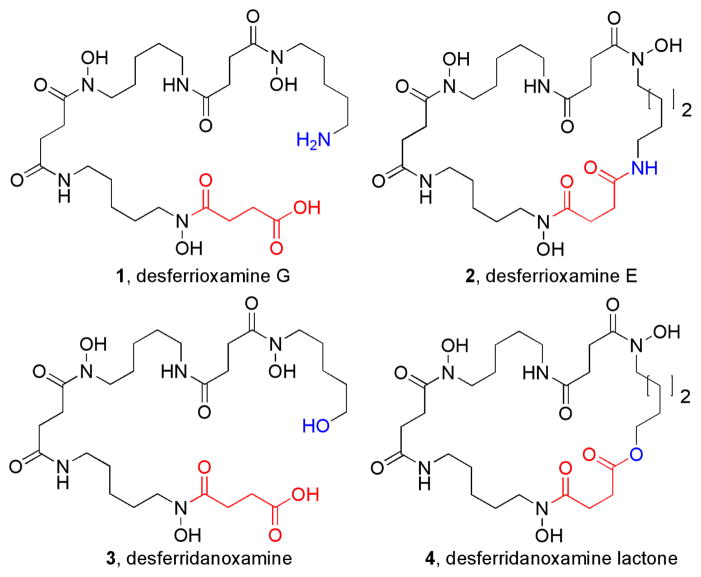
Linear trihydroxamate siderophores from the ferrioxamine family were some of the first siderophores isolated and have been used extensively in the clinic for treating iron-overload diseases in humans.1–2 Linear ferrioxamine siderophores form 1:1 octahedral complexes with iron(III) and have iron(III) binding constants of 1030, while a value of 1032.5 was reported for the macrolactam ferrioxamine siderophore desferrioxamine E, 2.5 Desferrioxamine G, 1, is a direct biosynthetic precursor for desferrioxamine E, 2, in Streptomyces coelicolor and the macrolactonization event is facilitated by the action of DesD in an ATP-dependent fashion via the mixed anhydride with release of PPi.6 The existence of the hydroxy acid siderophore desferridanoxamine, 3, suggests that a macrolactone counterpart, 4, might exist in nature since an increase in iron(III)-binding affinity would give the producing organism a competitive growth advantage to compensate for extra energy spent on biosynthesis of the macrolactone ring.
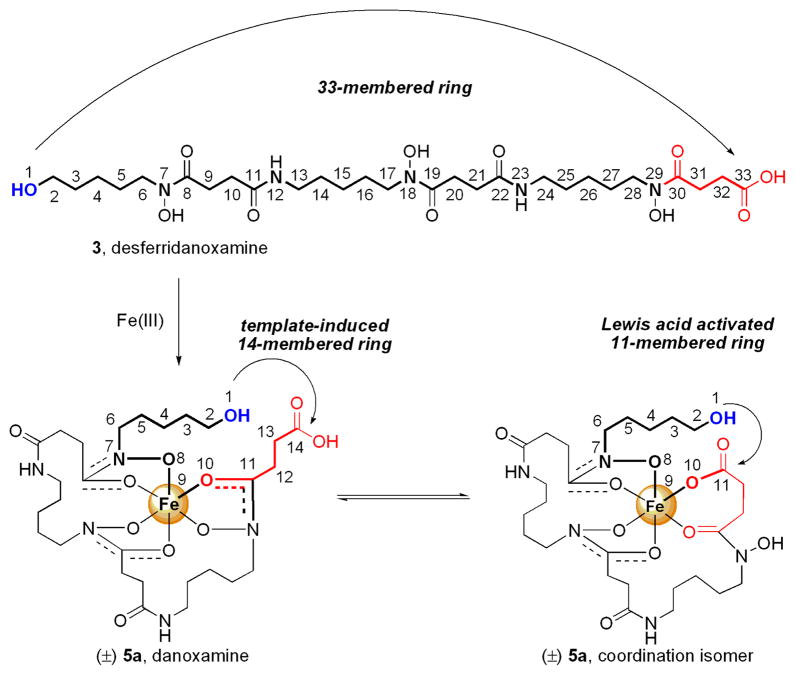
The total synthesis of desferrioxamine E was accomplished by Bergeron and coworkers using a DPPA-mediated macrolactamization of tri-O-benzyl-desferrioxamine G. The macrolactamization took 4 days and gave 54% yield of the benzyl protected desferrioxamine E precursor.7 A similar benzyl protecting group strategy was adopted by our group to synthesize desferridanoxamine, 3.8 However, a macrocyclization approach to desferridanoxamine macrolactone, 4, using a protected hydroxy acid precursor was not possible since the terminal alcohol was also protected as a benzyl ether.9 Thus, a different synthetic approach was envisioned using an iron(III)-templated macrolactonization (Figure 2). Precomplexation of desferridanoxamine, 3, with iron(III) was anticipated to bring the alcohol and carboxylate in close proximity in the resulting siderophore-iron(III) complex, 5a. The template-induced proximity decreases the effective macrolactone ring size to 14, compared to the untemplated 33-membered ring, and was expected to promote a more facile macrolactonization event.
Figure 2.
Design for Fe(III)-templation to bring the hydroxyl and carboxyl groups into closer proximity.
While there are many reagents and methods for performing macrolactonizations of large rings,10 we first tested the iron(III)-templation model by screening the well known Keck11 (DCC, DMAP, DMAP-HCl) and Yamaguchi12 (2,4,6-trichlorobenzoyl chloride, iPr2EtN, DMAP) macrolactonization conditions using danoxamine, 5a, as a substrate. Keck macrolactonization conditions failed to give any detectable macrolactone product according to LC-MS analyses (data not shown) while the initial Yamaguchi conditions screened gave 43% conversion to the macrolactone, 5b (Table 1, Entry 1). When desferridanoxamine, 3, was used as substrate under Keck and Yamaguchi conditions no macrolactone product was detected by LC-MS (data not shown).
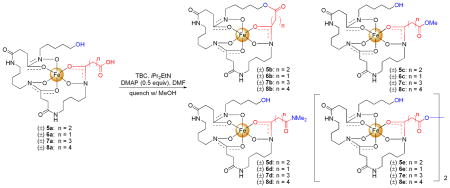
Table 1.
Optimization and scope of the Yamaguchi macrolactonization for trihydroxamate siderophore-iron(III) complexes 5a–8a.
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| entrya | substrate | n | ring size | TBCb (equiv) | iPr2EtN (equiv) | temp (°C) | relative % composition after 72 hc
|
||||
| % acid (5a–8a) | % lactone (5b–8b) | % Me ester (5c–8c) | % NMe2 amide (5d–8d) | % dimer (5e–8e) | |||||||
| 1 | 5a | 2 | 33 | 1.0 | 2.0 | 22 | 16 | 43 | 12 | 24 | 5 |
| 2 | 5a | 2 | 33 | 1.0 | 2.0 | 50 | 26 | 39 | 8 | 20 | 7 |
| 3 | 5a | 2 | 33 | 3.0 | 6.0 | 22 | 0 | 73 | 9 | 10 | 8 |
| 4 | 5a | 2 | 33 | 3.0 | 6.0 | 50 | 0 | 74 | 8 | 7 | 12 |
| 5 | 6a | 1 | 32 | 3.0 | 6.0 | 50–22d | 0 | 77e | 0 | 0 | 23 |
| 6 | 5a | 2 | 33 | 3.0 | 6.0 | 22 | 0 | 47 | 28 | 9 | 16 |
| 7 | 7a | 3 | 34 | 3.0 | 6.0 | 50–22d | 0 | 89e | 0 | 0 | 11 |
| 8 | 8a | 4 | 35 | 3.0 | 6.0 | 50 | 0 | 96f | 0 | 0 | 4 |
Reactions were performed in anhydrous DMF using 5 mg of danoxamine 5a (Entries 1–4) or 1 mg of siderophore-Fe(III) hydroxy acids 5a–8a (Entries 5–8) at a concentration of 0.005 M and 0.5 equivalent of DMAP.
TBC: 2,4,6-trichlorobenzoyl chloride.
Relative percent compositions were determined after 72 h by quenching the reaction mixture with MeOH and integrating peak areas from HPLC chromatograms monitored at 427 nm.
Reaction mixture was heated to 50 °C to solublize the siderophore-Fe(III) complex then cooled to rt for the remaining reaction time.
Percent composition was reached after 1 h and was stable throughout the course of the reaction.
Percent composition was reached after 6 h and was stable throughout the course of the reaction.
Inspired by these initial results the Yamaguchi conditions were optimized using danoxamine, 5a, as a model substrate (Table 1, Entries 1–4). Reactions were monitored by quenching aliquots of the reaction mixture with methanol followed by analysis with LC-MS and analytical HPLC using visible detection at a wavelength of 427 nm (λmax for 1:1 trihydoxamate siderophore:Fe(III) complexes)1a,5 which allowed for accurate calculation of product ratios. The LC-MS studies showed the presence of four siderophore products: macrolactone 5b, methyl ester 5c, dimethyl amide 5d, and dimer 5e. Increasing the reaction temperature to 50 °C enhanced the reaction rate, but did not increase conversion to the desired macrolactone (Table 1, Entry 2). Increasing the amount of Yamaguchi reagent (2,4,6-trichlorobenzoyl chloride) and Hunig’s base noticeably increased conversion to the desired macrolactone 5b and suppressed formation of byproducts 5d and 5e (Table 1, Entry 3). Increasing the reaction temperature in combination with excess Yamaguchi reagent and Hunig’s base enhanced the reaction rate, but did not increase conversion to the macrolactone (Table 1, Entry 4). The presence of methyl ester (formed during the MeOH quench) after 72 hours indicated that the activated ester is long-lived under the reaction conditions.
To evaluate the scope of the optimized Fe(III)-templated Yamaguchi macrolactonization additional siderophore substrates, 6a–8a, with carboxyl termini of variable chain length (n = 1–4; ring sizes = 32–35) were screened using the same HPLC assay described previously. All of the siderophore substrates, 6a–8a, successfully gave the macrolactone (Table 1, Entries 5–8). Surprisingly, the percent conversion to macrolactone followed the trend 8b (96%, n = 4) > 7b (89%, n = 3) > 6b (77%, n = 1) > 5b (47%, n = 2). The reason for this trend is unknown, but we hypothesize that the carboxylic acid terminus could interact with the Lewis acidic Fe(III) center and participate in chelation which might hinder formation of the Yamaguchi activated ester (Figure 2).
To probe for this Lewis acid effect we studied the relative rates of hydrolysis of purified siderophore-Fe(III) complex methyl esters 5c–8c (Table 2). Reaction half-lives were calculated using first order rate plots generated by integrating siderophore methyl ester and carboxylic acid HPLC peak areas at 427 nm. At pH 10, all the methyl ester siderophore-Fe(III) complexes were stable, as indicated by UV/Vis analysis of the reaction mixtures, and gave clean hydrolysis reactions. The half-lives of ester hydrolysis followed the order 6c (1.6 h, n = 1) > 5c (8.1 h, n = 2) > 7c (13.0 h, n = 3) > 8c (19.9 h, n = 4), showing a clear trend that increasing the distance of the ester from the Fe(III) center increased the ester’s half-life.13 Since all the esters have similar steric hindrance, the observed trend is consistent with the Fe(III) coordination center acting as a Lewis acid to polarize the ester carbonyl. This supports the possibility of the terminal carboxylate chelating the siderophore’s Fe(III) metal center, as discussed previously (Figure 2).
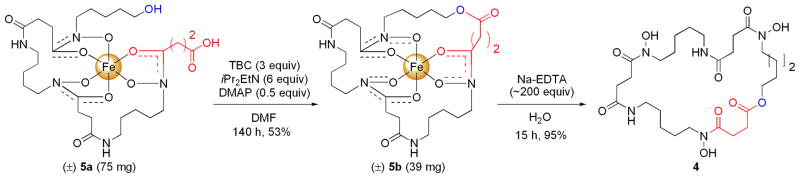
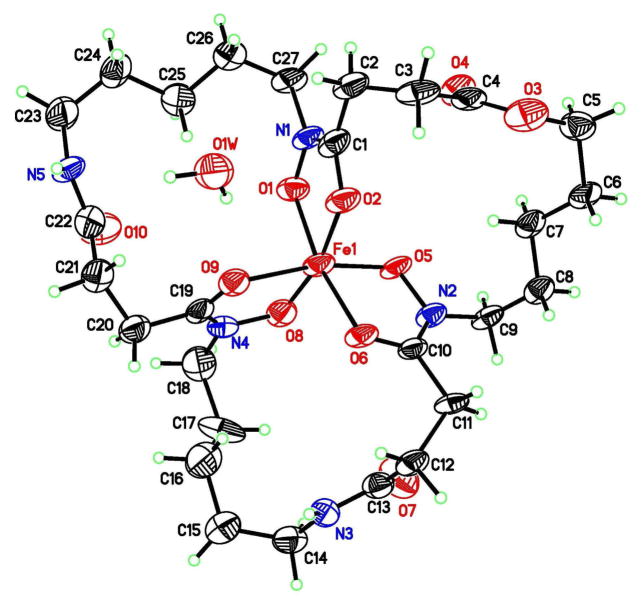
With reliable macrolactonization conditions in hand, the reaction was scaled up using danoxamine, 5a (Scheme 2). The scaled up reaction (75 mg of 5a) gave the macrolactone 5b in 53% yield (39 mg) after purification by Prep-HPLC. A single crystal of macrolactone siderophore-Fe(III) complex 5b was obtained from wet MeOH/Et2O and the crystal structure was determined by X-ray diffraction (Figure 3). This is one of only four ferrioxamine siderophore structures that has been determined.14 Further structural characterization of the macrolactone was obtained by removing the Fe(III) using excess EDTA (Scheme 2) which allowed for NMR analysis of desferridanoxamine macrolactone 4.
Scheme 2.
Scaled up macrolactonization of danoxamine, 5a, and deferration to give desferridanoxamine macrolactone 4.
Figure 3.
X-ray structure of the danoxamine macrolactone-Fe(III) complex, 5b, displayed with 50% probability ellipsoids.
The Fe(III)-templated Yamaguchi macrolactonizations of siderophore-Fe(III) complex hydroxy acids 5a–8a were fast and efficient. The use of an Fe(III) template brought the terminal alcohol and carboxylates of the siderophore substrates in closer proximity which enhanced the reaction rate and percent conversion to the desired macrolactones 5b–8b. Iron also conveniently served as a chromophore (λmax = 427 nm) for monitoring reaction progress and a protecting group for the nucleophilic hydroxamates. The Fe(III) was removed using EDTA providing access to desferrisiderophore macrolactones which are necessary for treating iron-overload diseases.
Other metal-templated macrolactonizations are cited in the literature that use oxophilic ionophores complexed to Na,15a K,15b Sn,15c and Cu.15d To the best of our knowledge, this is the first example using an Fe(III)-template for macrolactonization of a siderophore. We are currently exploring the use of catalytic metals (Fe(III), Ga(III), Al(II), etc.) in the macrolactonization reactions and evaluating the bioactivity and Fe(III)-affinity constants of the macrocyclic siderophores
Supplementary Material
Figure 1.
Structures of linear and macrocyclic trihydroxamate siderophores from the ferrioxamine family.
Acknowledgments
TAW thanks UND Chemistry-Biochemistry-Biology Interface program (NIH T32GM075762) for fellowship support.
Footnotes
Supporting Information Available General experimental details and characterization data (X-ray, NMR, MS, HPLC) for synthesis of siderophores 4 and 5a,b,c–8a,b,c. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Winkelmann G, editor. Handbook of Microbial Iron Chelates. CRC Press; Boca Raton, FL: 1991. [Google Scholar]; (b) Miethke M, Marahiel MA. Microbiol Mol Bio Rev. 2007:71, 413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nick H. Curr Opin Chem Biol. 2007;11:419–423. doi: 10.1016/j.cbpa.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kontoghiorghes GJ, Eracleous E, Economides C, Kolnagou A. Curr Med Chem. 2005;12:2663–2681. doi: 10.2174/092986705774463003. [DOI] [PubMed] [Google Scholar]; (b) Liu J, Obando D, Schipanski LG, Groebler LK, Witting PK, Kalinowski DS, Richardson DR, Codd R. J Med Chem. 2010;53:1370–1382. doi: 10.1021/jm9016703. [DOI] [PubMed] [Google Scholar]
- 4.Hider RC, Kong X. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 5.(a) Evers A, Hancock RD, Martell AE, Motekaitis RJ. Inorg Chem. 1989;28:2189–2195. [Google Scholar]; (b) Schwarzenbach G, Schwarzenbach K. Helv Chim Acta. 1963;46:1390–1400. [Google Scholar]; (c) Anderegg G, L’Eplattenier F, Schwarzenbach G. Helv Chim Acta. 1963;46:1409–1422. [Google Scholar]
- 6.Kadi N, Oves-Costales D, Barona-Gomez F, Challis GL. Nature Chem Biol. 2007;3:652–656. doi: 10.1038/nchembio.2007.23. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron RJ, McManis JS. Tetrahedron. 1990;46:5881–5888. [Google Scholar]
- 8.Roosenberg JM, Jr, Miller MJ. J Org Chem. 2000;65:4833–4838. doi: 10.1021/jo000050m. [DOI] [PubMed] [Google Scholar]
- 9.See Scheme S1 of the Supporting Information.
- 10.(a) Parenty A, Moreau X, Campagne JM. Chem Rev. 2006;106:911–939. doi: 10.1021/cr0301402. [DOI] [PubMed] [Google Scholar]; (b) Schweitzer D, Kane JJ, Strand D, McHenry P, Tenniswood M, Helquist P. Org Lett. 2007;9:4619–4622. doi: 10.1021/ol702129w. [DOI] [PubMed] [Google Scholar]
- 11.(a) Boden EP, Keck GE. J Org Chem. 1985;50:2394–2395. [Google Scholar]; (b) Keck GE, Sanchez C, Wager CA. Tetrahedron Lett. 2000;41:8673–8676. [Google Scholar]
- 12.(a) Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull Chem Soc Jpn. 1979;52:1989–1993. [Google Scholar]; (b) Dhimitruka I, SantaLucia J., Jr Org Lett. 2006;8:47–50. doi: 10.1021/ol0524048. [DOI] [PubMed] [Google Scholar]
- 13.See Supporting Information for exact experimental details, HPLC chromatograms, and first order rate plots.
- 14.(a) van der Helm D, Poling M. J Am Chem Soc. 1976;98:82–86. doi: 10.1021/ja00417a014. [DOI] [PubMed] [Google Scholar]; (b) Bilayet Hossain M, Jalal MAF, van der Helm D. Acta Cryst. 1986;C42:1305–1310. [Google Scholar]; (c) Bilayet Hossain M, Jalal MAF, van der Helm D, Shimizu K, Akiyama M. J Chem Crystallography. 1998;28:53–56. [Google Scholar]; (d) Dhungana S, White PS, Crumbliss AL. J Biol Inorg Chem. 2001;6:810–818. doi: 10.1007/s007750100259. [DOI] [PubMed] [Google Scholar]
- 15.(a) Carrillo R, Martin VS, Martin T. Tetrahedron Lett. 2004;45:5215–5219. [Google Scholar]; (b) Fürstner A. Eur J Org Chem. 2004:943–958. [Google Scholar]; (c) Ramirez RJA, Karamanukyan L, Ortiz S, Gutierrez CG. Tetrahedron Lett. 1997;38:749–752. [Google Scholar]; (d) Palomo C, Oiarbide M, García JM, González A, Pazos R, Odriozola JM, Bañuelos P, Tello M, Linden A. J Org Chem. 2004;69:4126–4134. doi: 10.1021/jo0497499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.