Abstract
Monocytes are frequently described as bone marrow-derived precursors of macrophages. Although many studies support this view, we now appreciate that monocytes neither develop exclusively in the bone marrow nor give rise to all macrophages and dendritic cells. In addition to differentiating to specific leukocyte populations, monocytes, as monocytes, are functionally and ontogenically heterogeneous. In this review we will focus on the development and activity of monocytes and their subsets in mice (Ly-6Chigh/low) and humans (CD14+/dim/− CD16+/−) in the context of atherosclerosis and its complications.
Keywords: monocyte subsets, monocyte heterogeneity, Ly-6C, CD14, CD16, macrophage, dendritic cells, atherosclerosis, myocardial infarction, cardiovascular disease, extramedullary hematopoeisis, inflammation
Introduction
Monocytes, macrophages and dendritic cells are essential to the development of atherosclerosis [1]. According to a current paradigm, monocytes infiltrate atherosclerotic lesions and develop into macrophages and dendritic cells. Macrophages are the most numerous cells in atheromata, accumulate lipids, secrete multiple inflammatory cytokines and growth factors, and participate at all stages of atherogenesis. Depletion of monocytes and their progeny in an experimental mouse model decreases the development of atherosclerosis [2], but wholesale ablation of monocytes and macrophages is not a viable therapeutic option because of these cells’ essential role in immunity.
Leukocytosis and monocytosis have been associated with cardiovascular diseases in numerous epidemiological studies, prompting speculation on the functional role of these cells. Many studies have documented heterogeneity among human monocytes, but it was the discovery and characterization of monocyte subsets in the mouse that enabled investigation into the relevance of monocyte heterogeneity in cardiovascular disease models [3]. In this review we will focus on recent work that has enriched our understanding of monocyte and macrophage biology in atherosclerosis and its complications. We will consider monocyte development and function. We will discuss how insights from experimental studies can be used to test the clinical relevance of monocyte heterogeneity in cardiovascular disease. Finally, we will assess human monocyte heterogeneity in the context of clinical and epidemiological studies.
Development of monocyte subsets
Monocytes are a major population of circulating white blood cells. Morphologically, monocytes are larger than lymphocytes and, unlike granulocytes, contain a characteristic horseshoe or kidney-like nucleus. Functionally, they are phagocytic and can develop into macrophages and dendritic cells in vitro and in vivo. In humans and mice at least two monocyte subsets have been described. In humans they are usually classified according to CD14 and CD16 expression levels (see extended discussion below). In mice, monocytes are defined by their expression of Ly-6C, which is an epitope of Gr-1. Ly-6Chigh (Gr-1+) monocytes are CCR2high CX3CR1low CD62L+, whereas Ly-6Clow (Gr-1−) monocytes are CCR2low CX3CR1high CD62L− [3]. Originally, Ly-6Chigh monocytes were called “inflammatory” because they preferentially accumulate in the peritoneum in response to an inflammatory stimulus, and Ly-6Clow monocytes were called “resident” because of their apparent indiscriminate accumulation in tissue in the steady state. Subsequently, investigators have called Ly-6Clow monocytes “patrolling” because of their ability to crawl along the endothelium and “reparative” because of their role in mediating healing after injury. Others have proposed to call Ly-6Chigh monocytes, in analogy to human monocytes, “classical” and Ly-6Clow monocytes “non-classical”, a nomenclature that, although convenient, offers no functional insight.
Nomenclature aside, an important early question was how subsets relate to each other. It has been known for a long time that monocytes arise from the common myeloid progenitor (CMP), a self-renewing bone marrow precursor that, in addition to monocytes, also gives rise to neutrophils, megakaryocytes, basophils and mast cells. A downstream progenitor of the CMP restricted toward the monocyte lineage, the macrophage and dendritic cell progenitor (MDP) was identified in 2006 [4], and, at the molecular level, transcription factors such as PU.1, KLF4, IRF-8 and MafB have proven to be particularly important for monocyte development [5]. The question remains, however, whether the MDP is a common progenitor for both subsets and whether Ly-6Chigh monocytes convert to Ly-6Clow monocytes. The first study in favor of conversion utilized monocyte-depleting clodronate-loaded liposomes, showing that repopulation of the monocyte pool following depletion occurs in two phases: Ly-6Chigh monocytes appear after 1 day while Ly-6Clow monocytes appear only after 4 days [6]. Direct fate-mapping experiments supported this view. Labeled Ly-6Chigh monocytes adoptively transferred and tracked in the circulation gave rise to Ly-6Clow monocytes, presumably in the bone marrow [7, 8]. Both studies are the foundation of the idea that Ly-6Clow monocytes arise from Ly-6Chigh monocytes.
Conversion alone, however, may not fully explain the relationship between the two subsets. The biphasic re-emergence of monocyte subsets after clodronate-mediated depletion, while intriguing, can also reflect differences in life-span and cell development; Ly-6Clow monocytes are the more long-lived cells. The in vivo adoptive transfer studies are a strong case in favor of conversion, but the number of cells retrieved in those experiments was low, and thus it is difficult to gauge the relative importance of conversion in generating Ly-6Clow monocytes. In vitro, the rapid differentiation of monocytes to macrophages makes it impossible to study conversion. Two insights in gene-knockout animals, however, may help to resolve the issue. First, it has been documented that Nur77−/− mice lack mature Ly-6Clow monocytes but contain a Ly-6Chigh monocyte population [• 9]. Does Nur77 convert Ly-6Chigh monocytes or is it guiding Ly-6Clow monocyte production upstream in ontogeny, perhaps independently of Ly-6Chigh monocytes? Second, Kruppel-Like Factor 4 (KLF4) deficient bone marrow chimeras lack Ly-6Chigh monocytes in blood and spleen but still contain low numbers of Ly-6Clow monocytes [10]. Is this evidence that Ly-6Clow monocytes can develop from a committed progenitor without a Ly-6Chigh intermediate? These mice represent new tools to elucidate the mechanisms that link the development of Ly-6Chigh and Ly-6Clow monocytes.
A comprehensive map of the developmental relationships between monocyte subsets requires a location. Since the 1960s it has been known that monocytes arise in the bone marrow, circulate in the blood, and develop to macrophages in peripheral tissue. The bone marrow contains specialized hematopoietic niches whose composition and function are the subject of multiple studies [11, 12]. Aside from the bone marrow and blood, monocyte subsets can also be found in the spleen as part of a reservoir that can be mobilized in response to distant inflammatory stimuli such as myocardial infarction [13]. The reservoir, which relies on angiotensin II signaling, appears to be important in post-injury healing [14].
In the context of continued or chronic inflammation the spleen becomes monocytopoietic, complementing the bone marrow’s capacity to produce inflammatory cells. In apolipoprotein E deficient (ApoE−/−) and LDL receptor deficient mice hematopoietic stem cells progressively seed the splenic red pulp to produce Ly-6Chigh monocytes locally in response to GM-CSF and IL-3 [• 15]. In myocardial infarction and stroke the continuous demand for monocytes likewise transforms the spleen into a monocytopoietic organ [16]. The phenomenon occurs in a tumor model, thus broadening the scope of extramedullary monocytopoiesis in inflammatory diseases [17]. Although these insights add a new dimension to monocyte heterogeneity – the site of origin – the extent to which different hematopoietic environments influence monocyte function remains to be elucidated.
Function of monocyte subsets in cardiovascular disease
Our understanding of how monocyte subsets participate in cardiovascular disease is largely based on mouse models of atherosclerosis and myocardial infarction (Figure 1). In the steady state, the proportion of circulating Ly-6Chigh and Ly-6Clow monocytes is nearly equivalent. In murine models of atherosclerosis, hypercholesterolemia induces monocytosis in the bone marrow, blood and spleen [18, 19]. Of the two subsets, Ly-6Chigh monocytes increase in number preferentially. Recent work has shown that hypercholesterolemia induces proliferation of hematopoietic stem cell progenitors (HSPC) involving the cholesterol efflux pathway and its central molecular components ApoE and ATP-binding cassette transporters ABCA1 and ABCG1. HSPC secrete ApoE which binds to proteoglycans on the cell surface and mediates cellular cholesterol efflux via ABCA1 and ABCG1 to HDL. ApoE deficiency impairs cholesterol efflux, increases membrane cholesterol content as well as the surface expression of the IL-3/GM-CSF receptor [• 20, 21]. IL-3 and GM-CSF stimulate proliferation and survival of HSPC in the bone marrow and the spleen and thus contribute to monocytosis and neutrophilia [• 15]. Although cholesterol sensing pathways have been known to play important roles in atherosclerosis [22], these recent mechanistic studies have forged a previously unknown link with monocytes.
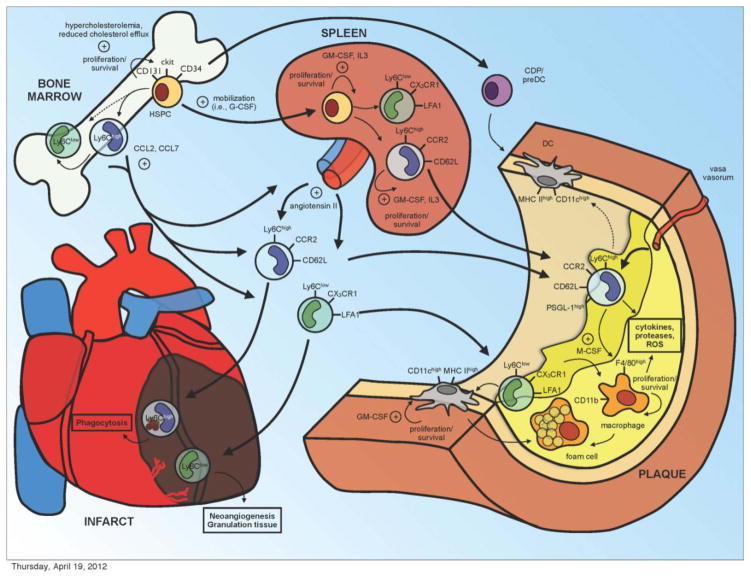
Figure 1.
Monocyte subsets decisively engage in cardiovascular disease. Hypercholesterolemia and impaired cholesterol efflux promote hematopoietic stem progenitor cell (HSPC) proliferation and predominant Ly-6Chigh monocytosis. While monocyte bone marrow egress largely depends on CCR2 signaling, an interplay of multiple growth factors, chemokines and proteases mediates mobilization of progenitor cells. During murine atherosclerosis progenitor cells seed the spleen to locally supplement monocytopoiesis. Ly-6Chigh monocytes from bone marrow and spleen preferentially enter atherosclerotic lesions and develop into macrophages and foam cells. Both monocytes and macrophages are sources of inflammatory cytokines, proteases and reactive oxygen species (ROS). Aortic dendritic cells (DC) derive either from monocytes or common dendritic cell progenitors (CDP) and develop into foam cells. In myocardial infarction Ly-6Chigh and Ly-6Clow monocytes of medullary and extramedullary origins accumulate in the ischemic myocardium sequentially for phagocytosis, debris removal, granulation tissue formation, and neoangiogenesis. Thick arrows depict spatial relationships, thin arrows developmental relationships, and dashed arrows uncertain relationships.
Mice deficient in apolipoprotein E develop large and complex lesions when fed a diet high in fat and cholesterol. Ly-6Chigh monocytes accumulate in the growing atheromata preferentially via CCR2 and CX3CR1 [18, 19, 23]. Invading Ly-6Chigh monocytes of medullary and extramedullary origins are sources of IL-1β, reactive oxygen species, and proteases and may directly contribute to the lipid load in atherosclerotic lesions by ingesting lipids prior to accumulating in lesions [• 15]. Ly6Clow monocytes, which also ingest lipids [24], infiltrate atherosclerotic lesions less frequently via CCR5, but may still contribute to atherosclerosis in other important ways. Nurr77−/− ApoE−/− mice and LDLR−/− mice reconstituted with Nur77−/− bone marrow have fewer Ly-6Clow monocytes. The mice develop large lipid-laden lesions with more macrophages that express higher levels of TNFα, CD36 and SR-A [25]. The observations suggest that Ly-6Clow monocytes are atheroprotective. However, Nur77 deficiency also heightens inflammatory cytokine production by Ly-6Chigh monocytes and macrophages [25, 26], rendering it difficult to judge the relative importance of Ly-6Clow monocytes in atherosclerosis.
In a mouse model of myocardial infarction two monocyte phases can be distinguished. Ly-6Chigh monocytes infiltrate the healing myocardium on day 1 and dominate the first inflammatory phase. This subset secretes proteases and pro-inflammatory TNFα, and removes debris through phagocytosis. The second phase begins on day 4 and is dominated by the preferential accumulation of Ly-6Clow monocytes which secrete VEGF and promote myofibroblast accumulation, angiogenesis, and granulation tissue formation [• 27]. The biphasic monocyte recruitment pattern is orchestrated by increased MCP-1/CCL2 expression in infarcts in the first phase, which attracts Ly-6Chigh CCR2high monocytes, and increased Fractalkine/CX3CL1 expression in the second phase, which favors Ly6Clow CX3CR1high monocytes. A balanced and coordinated subset recruitment is likely important because atherosclerotic ApoE−/− mice with Ly-6Chigh monocytosis have impaired healing of the infarcted myocardium [28].
The functional characterization of monocyte subsets in cardiovascular disease models has raised interesting therapeutic possibilities with regard to monocyte recruitment. Most of the interest has focused on targeting accumulation of Ly-6Chigh monocytes, which are typically regarded as the central inflammatory culprits. Monocyte accumulation occurs through a series of distinct steps. First, chemokines attract monocytes via chemokine receptors. Silencing the chemokine receptor CCR2, which is highly expressed on Ly-6Chigh monocytes, via nanoparticle-mediated transfer of short interfering RNA decreases Ly-6Chigh monocytes and their progenies in peripheral tissues and attenuates inflammation associated with atherosclerosis and myocardial infarction [29]. Second, selectins and their ligands mediate leukocyte capture and rolling, a crucial step to be blocked by specific antibodies. The P-selectin glycoprotein ligand-1 (PSGL-1) on leukocytes interacts with P- and E-selectins on activated endothelium and platelets [30, 31]. Ly-6Chigh monocytes express PSGL-1 at higher levels compared to Ly-6Clow monocytes and thus accumulate in the growing lesion preferentially [32]. L-selectin, which is also selectively overexpressed in Ly-6Chigh monocytes, mediates secondary capture at sites of atherosclerosis [33]. The third stage involves activation of integrins which bind to their cognate receptors on the endothelium to mediate firm arrest. Therapeutically, interference with VLA-4/VCAM-1, LFA-1/ICAM-1 and Mac-1/CD40L interaction reduces monocyte adhesion and macrophage lesion content [34– • 38]. Beyond lipid lowering, statins and other anti-inflammatory drugs are thought to be atheroprotective in part because they interfere with endothelial activation and monocyte recruitment [39, 40]. Many of the therapeutic approaches mentioned above remain to be tested in humans.
Differentiation beyond the monocyte
Monocytes are precursors of macrophages and dendritic cells, the key phagocytic and antigen presenting cells in lymphoid and non-lymphoid tissues. Macrophages and dendritic cells are phenotypically and morphologically heterogeneous, a fact that has fueled considerable debate about how to define and categorize them [41]. Regardless of name, everyone agrees that these populations participate in many important biological processes. From the perspective of monocyte biology, one of the most exciting questions is whether subsets are restricted to give rise to macrophages/dendritic cells with defined functions, or whether the subset differences disappear once monocytes accumulate in tissue.
Functionally distinct macrophages can be derived from monocytes in vitro. Classical macrophage activation, which involves culture of bone marrow cells with M-CSF, LPS or IFN-γ yields M1 macrophages which are TNFα, IL-1β, IL-6, IL-12 and iNOS-secreting, and thus “inflammatory”. Alternative activation involves culture with IL-4, IL-10 or IL-13 and generates M2 macrophages which are defined by their high expression of “anti-inflammatory” arginase 1, IL-10, CD206 and Fizz. These definitions have dominated thinking about macrophage heterogeneity and, although convenient for in vitro studies and elegant in their simplicity, should be used with caution in the in vivo setting. In the steady state, most organs contain their own particular macrophages, many of which do not derive from the bone marrow or from monocytes [42–45]. The spleen, for example, contains red pulp macrophages, metallophilic macrophages, classical dendritic cells, and undifferentiated monocytes, among others [13, 46]. Precisely how monocyte subsets contribute to macrophage and dendritic cell populations in the steady state is still unknown.
In disease, macrophage and dendritic cell heterogeneity magnifies. Uptake of oxidatively modified lipids via scavenger receptors and toll-like receptors drives inflammatory cytokine expression and M1 polarization of macrophages [47, 48], while engulfment of cholesterol crystals activates the NLRP3 inflammasome and leads to the over-expression of the M1 cytokine IL-1β. Cholesterol metabolites and fatty acids serve as ligands for nuclear liver-X-receptor (LXR) and peroxisome-proliferator-activated receptors (PPARs). Activation of these receptors favors alternative macrophage polarization, inhibiting inflammatory gene expression and mediating cholesterol efflux [22, 49]. Platelet chemokine CXCL4 induces differentiation into M4 type macrophages that are weak in phagocytosis and oxLDL uptake [50], whereas Mox macrophages arise when macrophages are stimulated with oxidized phospholipids [51]. Whether these are separate populations or simply states that any lesional macrophage can adopt is perhaps secondary to the fact that the atheroma is a bustling microenvironment of functionally heterogeneous activity [52].
The relationship between circulating monocyte subsets and lesional macrophages is still poorly understood. Ly-6Chigh monocytes give rise to lesional CD11b+ F4/80high macrophages [• 15, 18], but whether they preferentially give rise to a subset of macrophages, and whether macrophages differentiate from one phenotype into another locally, is yet to be clarified. It has been proposed that Ly-6Clow monocytes, which express low levels of CD11c, preferentially give rise to CD11c+ dendritic cells in atherosclerotic lesions [19]. However, low yield of adoptively transferred cells in aortas has compromised sophisticated fate mapping studies. Not all macrophages in aortic lesions derive from a circulating progenitor, however. Recently, it was shown that adventitial Sca-1+ progenitors in C57Bl/6 and ApoE−/− aortas give rise to macrophages locally [53]. Given the rare frequency of these progenitors in aortic tissue their functional in vivo relevance remains to be determined.
In addition to macrophages, the atherosclerotic lesion contains subsets of dendritic cells (DCs). DCs in the steady state aorta and in the growing lesion express high levels of MHCII and CD11c. They stimulate T cells but, unlike macrophages, are poor phagocytes [54, 55]. Aortic CD11c+ DCs may be divided into two major subsets: CD11b+ CD103− F4/80+ DCs and CD11b− F4/80− CD103+ DCs. CD11b+ CD103− DCs are believed to be monocyte derived while CD103+ DCs resemble classical DC and derive from common DC progenitors (CDP) without a monocyte intermediate. Flt3 depletion in LDLR−/− mice selectively ablates CD103+ DC and worsens atherogenesis [55]. Likewise, depletion of plasmacytoid DC, although only sparsely detected in atherosclerotic lesions, increases lesion size [56]. These studies suggest that monocyte-derived macrophages and DC are atherogenic whereas classical DCs are atheroprotective. The complex interplay between these various subsets in shaping disease is yet to be described.
Relationship between mouse and human monocyte subsets
Monocytes are heterogeneous in humans [57]. Human monocytes have been classified according to different expression of surface receptors CD14 and CD16. So-called classical CD14high CD16− monocytes constitute ~70–80% of monocytes in the peripheral blood and can be distinguished from smaller monocytes coexpressing high levels of CD16 and lower levels of CD14 [58]. CD16+ monocytes produce high levels of TNFα in response to LPS stimulation and rise in number during infection. For this reason they were first named “inflammatory” [59, 60]. However, it is the classical CD14high CD16− monocyte subset, and not the “inflammatory” CD16+ subset, that resembles mouse “inflammatory” Ly-6Chigh monocytes: The CD16− subset expresses high levels of CCR2, CD62L, CD64 and low levels of CX3CR1 [3, 57]. This had lead to some confusion as to whether and how human and murine monocytes correspond.
Recent studies have taken advantage of gene array analysis and hierarchical clustering to compare monocyte subsets in the two species. In one study, the investigators gated, or grouped, human monocyte subsets according to their CD16 expression. The dominant “classical” subset was sorted as a CD14+ CD16− cell population whereas the less numerous “non-classical” subset was sorted as a CD16+ cell population that contained a broad range of CD14 expression (CD14+/low/−). The authors concluded that, in general, CD16− monocytes resemble murine Ly-6Chigh monocytes and CD16+ monocytes are similar to murine Ly-6Clow monocytes [61]. Several differences between the species were noted, however, including converse expression of several scavenger receptors.
Another study gated human monocytes into three populations: A numerically dominant CD16− subset, an intermediate CD14+ CD16+ subset, and a CD14low/dim CD16+ subset [62]. By performing principal component analysis and by comparing the three subsets to each other and to murine subsets, the authors concluded that CD14high CD16− and CD14+ CD16+ monocytes cluster with the murine Ly-6Chigh/Gr-1+ monocytes whereas CD14dim CD16+ monocytes cluster with Ly-6Clow monocytes. The study challenges the importance of using CD16 as a discrimination marker and argues for using CD14 as the essential marker for defining 2 monocyte subsets. Even so, the authors supplement their phenotypic characterization with functional assays of all 3 subsets. In response to LPS CD14+ CD16+ monocytes secrete TNFα, IL1β and IL-6 while CD14high CD16− cells preferentially produce CCL2, IL-10, IL-8, reactive oxygen species and myeloperoxidase and high levels of IL-6. CD14dim CD16+ monocytes, which express low levels of CCR2 but highest levels of CX3CR1, and which also exhibit patrolling behavior, resemble murine Ly-6Clow counterparts. Stimulation of these CD14dim cells with TLR7 and 8 agonists selectively upregulated inflammatory cytokine expression [62]. Other groups have reported that CD14dim monocytes can produce TNFα in response to LPS [63, 64]. These observations argue against describing any particular subset as “inflammatory” since every cell is “inflammatory” in its own particular way and is specialized in how it responds to different stimuli. If one were to compare the three transcriptionally distinct human monocyte subsets [64, 65] to the two murine subsets based on phenotype and behavior, both CD16− CD14high and CD16+ CD14+ monocytes resemble Ly-6Chigh monocytes while CD14dim monocytes correspond to Ly-6Clow monocytes (Table).
Table.
Monocyte subset profiles in mice and men
| Mouse | Human | ||||
|---|---|---|---|---|---|
| Subset | Profile | Subset | Profile | ||
| Ly-6Chigh/Gr-1+ | CCR2high, CCR5low, CX3CR1low, CD62L+, PSGL-1high, CD11alow, CD11b+, CD11c−, F480int, CD36−, CD64+, MHCII− | strong phagocytosis, preferential homing to inflamed tissue | Classical CD14high CD16− | CCR2high, CCR5low, CX3CR1low, CD62L+, PSGL-1high, CD11ahigh, CD11b+, CD11C−, CD36high, CD64high, CD86low, HLA-DRlow/− | strong phagocytosis; unclear migratory behavior in vivo |
| intermediate CD14+ CD16+ | CCR2+, CCR5+, CX3CR1+, CD62Llow/−, CD11alow, CD11b+, CD11clow, CD36+ CD64int, CD86+, HLA-DRhigh, Tie2+ | strong phagocytosis; unclear migratory behavior in vivo | |||
| Ly-6Clow/Gr-1− | CCR2low, CCR5low, CX3CR1high, CD62L−, PSGL-1low, CD11ahigh, CD11b+, CD11clow, F480int, CD36+, CD64+, MHCIIlow | strong phagocytosis, patrolling behavior in vivo | non-classical CD14dim CD16+ | CCR2low, CCR5−, CX3CR1high, CD62L−, PSGL-1low, CD11alow, CD11blow, CD11clow, CD36low, CD64−, CD86+, HLA-DR+ | poor phagocytosis; patrolling behavior in vivo |
Monocyte subsets and human cardiovascular disease
Many cohort and case-control studies have documented an association of leukocytosis with cardiovascular diseases. Elevated leukocyte counts were identified as an independent risk factor for developing disease and as a negative prognostic indicator in patients with coronary heart disease, myocardial infarction, peripheral artery disease and stroke [66–69]. Neutrophils show the strongest and most consistent correlation with disease occurrence and outcome [70, 71]. Monocytosis was found to predict cardiovascular events in some studies [70, 72–74] but not in others [75, 76], and lymphocyte counts seem to be inversely correlated with coronary heart disease and its complications [77]. While differences in study design may account for some of the discrepancies regarding the correlation of monocyte counts and adverse cardiac events in epidemiological studies, the use of flow cytometric analysis to distinguish monocyte subsets has allowed for more sophisticated and nuanced risk stratification.
Many studies investigated the relationship between cardiovascular risk factors and monocyte subsets. A weak but positive correlation of CD14dim CD16+ monocytes with total plasma cholesterol and triglyceride levels was first described in 1999 in hypercholesterolemic patients with a positive stress ECG indicative of coronary heart disease [78]. Numbers of CD16+ monocytes but not overall monocyte counts positively correlate with Body-Mass-Index, insulin resistance/diabetes and intima-media-thickness. Weight loss after gastric bypass surgery in severely obese patients is associated with a significant reduction of CD16+ monocytes [79, 80]. Likewise, exercise training reduced numbers of CD16+ monocytes in a physically inactive study population [81]. CD14+ CD16+ monocytes but not total monocyte counts predict cardiovascular events in patients with chronic kidney disease and end stage renal disease on dialysis, a patient population at increased risk for atherosclerotic complications [79, 82].
In patients with symptomatic coronary artery disease compared to healthy controls the percentage of CD16+ monocytes was found to be increased according to a first small case-control study in 2004, even after adjustment for common risk factors [83]. This finding was confirmed in another small study where numbers and proportion of monocyte subsets were measured according to their differential capacity for magnetic nanoparticle uptake [84]. Assessment of plaque vulnerability by optical coherence tomography and computed tomography in patients with both stable and unstable angina pectoris revealed an increase in CD16+ monocytes with more vulnerable plaques. In fact, monocyte subset proportions did not differ significantly between healthy controls and patients with non-vulnerable plaques. Statin treatment decreased the percentage of CD16+ monocytes [85, 86]. CD16+ monocytosis after stent placement in patients with myocardial infarction also positively correlated with late in-stent restenosis [87]. Interestingly however, among a study population with stable coronary artery disease, those patients with more than 5 cardiovascular risk factors and especially with a positive family history for coronary artery disease presented relatively higher percentages of CD14high CD16− monocytes [88]. A prospective cohort study recently reported that an increased number and percentage of classical CD14high CD16− monocytes predicted future cardiovascular events independently of other risk factors in a general population [x• 89].
A number of studies have explored how monocyte subsets change during acute cardiovascular events. In stroke patients, CD14+ CD16+ monocytes increased preferentially within the first week, but the proportion of CD14high CD16− monocytes on admission positively correlated with infarct size and predicted worse clinical outcome and mortality [90]. After acute myocardial infarction (MI) and revascularization total blood monocyte counts tend to rise temporarily, peaking between day 2 and 3 after intervention. However, CD14high CD16− CCR2+ monocytes peak within the first 3–4 days whereas CD16+ CX3CR1+ monocytes peak thereafter, a pattern reminiscent of the biphasic response observed in mice [• 27]. However, no data are yet available on subsets in heart tissue. Patients with unstable angina undergoing intervention do not show a shift in monocyte subsets suggesting that the observed effects are MI related. The peak value of CD16− monocytes shortly after MI negatively correlates with myocardial salvage and gain of left ventricular function at follow up [91]. With worsening congestive heart failure the percentage of intermediate CD14+ CD16+ monocytes has been reported to increase preferentially [92]. More recently, a small study in MI patients compared CD14+ CD16+ and CD14dim CD16+ monocytes. The most pronounced increase was reported for intermediate CD14+ CD16+ monocytes within the first 3 days post MI and intervention. CD14high CD16− monocytes also increased during the first 3 days, as reported earlier, while CD14dim CD16+ monocyte levels did not change over time. At 30 days after MI, monocyte subset distribution and numbers equaled those of patients with stable coronary artery disease [93]. Altogether, most studies report an association of CD14+ CD16+ monocytes with active cardiovascular disease, whereas some suggest that CD14high CD16− monocytosis is a negative prognostic indicator. Altogether, these important epidemiologic studies, which are necessarily descriptive, underscore the need of studying monocyte subset biology in mouse models. The observations that CD14+ CD16+ and CD14high CD16− monocytes are closely related to mouse Ly-6Chigh monocytes, and given that mouse models position Ly-6Chigh monocytes as central culprits of cardiovascular inflammation, lend further credibility to the potential translation of mouse studies to human disease.
Concluding remarks
Cardiovascular diseases are characterized by a highly diversified and heterogeneous response of the myeloid leukocyte population. How cell subsets shape pathogenesis is the current focus of many laboratories, and the therapeutic goal is to neutralize functionally distinct subsets that promote disease while augmenting or sparing those subsets that are protective. Achieving this will rely on mechanistically-oriented, high quality in vivo animal studies that can show how lesion growth, plaque rupture, and infarct healing can be controlled. Translating insights from the mouse to the human will no doubt continue to be a challenge, but it will be the combination of basic insights and increasingly sophisticated clinical studies that will likely harness the significance of monocyte and macrophage heterogeneity in cardiovascular disease.
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 2.Stoneman V, Braganza D, Figg N, et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100:884–893. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 4.Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 5.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 6.Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 7.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 9•.Hanna RN, Carlin LM, Hubbeling HG, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. In this study nuclear receptor Nur77 is being identified as a crucial transcription factor for normal Ly6Clow but not Ly6Chigh monocyte development in the bone marrow. These mice may provide useful tools to study subset conversion versus distinct lineage derivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder JK, Georgantas RWr, Hildreth RL, et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 12.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 13.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Robbins CS, Chudnovskiy A, Rauch PJ, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. This study describes how the spleen becomes a site of extramedullary monocytopoiesis during atherosclerosis directly contributing to lesion formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortez-Retamozo V, Etzrodt M, Newton A, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Murphy AJ, Akhtari M, Tolani S, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. This study identifies impairment of cholesterol efflux in bone marrow stem cells as a mechanism for hypercholesterolemia associated monocytosis in atherosclerotic mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 23.Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Gower RM, Wang H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna RN, Shaked I, Hubbeling HG, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamers AA, Vos M, Rassam F, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 27•.Nahrendorf M, Swirski FK, Aikawa E, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. This is the first study that identifies distinct roles for the two monocyte subsets during infarct healing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panizzi P, Swirski FK, Figueiredo JL, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 31.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 32.An G, Wang H, Tang R, et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson EE, Xie X, Werr J, et al. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–218. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kling D, Fingerle J, Harlan JM, et al. Mononuclear leukocytes invade rabbit arterial intima during thickening formation via CD18-and VLA-4-dependent mechanisms and stimulate smooth muscle migration. Circ Res. 1995;77:1121–1128. doi: 10.1161/01.res.77.6.1121. [DOI] [PubMed] [Google Scholar]
- 35.Nie Q, Fan J, Haraoka S, et al. Inhibition of mononuclear cell recruitment in aortic intima by treatment with anti-ICAM-1 and anti-LFA-1 monoclonal antibodies in hypercholesterolemic rats: implications of the ICAM-1 and LFA-1 pathway in atherogenesis. Lab Invest. 1997;77:469–482. [PubMed] [Google Scholar]
- 36.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 37.Chuang KP, Huang YF, Hsu YL, et al. Ligation of lymphocyte function-associated antigen-1 on monocytes decreases very late antigen-4-mediated adhesion through a reactive oxygen species-dependent pathway. Blood. 2004;104:4046–4053. doi: 10.1182/blood-2004-05-1822. [DOI] [PubMed] [Google Scholar]
- 38•.Wolf D, Hohmann JD, Wiedemann A, et al. Binding of CD40L to Mac-1’s I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis--but does not affect immunity and thrombosis in mice. Circ Res. 2011;109:1269–1279. doi: 10.1161/CIRCRESAHA.111.247684. This work is a good example of novel therapeutic strategies in atherosclerosis. Selective blockade of CD40L interaction with Mac-1 but not with other binding partners attenuates atherogenesis while avoiding harmful side effects caused by indiscriminate inhibition of CD40L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Q, Liao JK. Pleiotropic effects of statins- Basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilgendorf I, Eisele S, Remer I, et al. The oral spleen tyrosine kinase inhibitor fostamatinib attenuates inflammation and atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1991–1999. doi: 10.1161/ATVBAHA.111.230847. [DOI] [PubMed] [Google Scholar]
- 41.Geissmann F, Gordon S, Hume DA, et al. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 44•.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. A comprehensive basic review on monocyte, macrophage and dendritic cell biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 47.Miller YI, Viriyakosol S, Worrall DS, et al. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 48.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 50.Gleissner CA, Shaked I, Little KM, et al. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Psaltis PJ, Harbuzariu A, Delacroix S, et al. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu SN, Chen M, Jongstra-Bilen J, et al. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi JH, Cheong C, Dandamudi DB, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Daissormont IT, Christ A, Temmerman L, et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 58.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 59.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 61.Ingersoll MA, Spanbroek R, Lottaz C, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–9. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, et al. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 64.Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 65.Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 66.Brown DW, Giles WH, Croft JB. White blood cell count: an independent predictor of coronary heart disease mortality among a national cohort. J Clin Epidemiol. 2001;54:316–322. doi: 10.1016/s0895-4356(00)00296-1. [DOI] [PubMed] [Google Scholar]
- 67.Madjid M, Awan I, Willerson JT, et al. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44:1945–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 68.Ohira T, Shahar E, Chambless LE, et al. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 69.Giugliano G, Brevetti G, Lanero S, et al. Leukocyte count in peripheral arterial disease: A simple, reliable, inexpensive approach to cardiovascular risk prediction. Atherosclerosis. 2010;210:288–293. doi: 10.1016/j.atherosclerosis.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Grau AJ, Boddy AW, Dukovic DA, et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke. 2004;35:1147–1152. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 71.Adamsson Eryd S, Smith JG, Melander O, et al. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler Thromb Vasc Biol. 2012;32:533–539. doi: 10.1161/ATVBAHA.111.240416. [DOI] [PubMed] [Google Scholar]
- 72.Nasir K, Guallar E, Navas-Acien A, et al. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 73.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 74.Dragu R, Huri S, Zuckerman R, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. 2008;196:405–412. doi: 10.1016/j.atherosclerosis.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 75.Gurm HS, Bhatt DL, Lincoff AM, et al. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89:1200–1204. doi: 10.1136/heart.89.10.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rana JS, Boekholdt SM, Ridker PM, et al. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk Prospective Population Study. J Intern Med. 2007;262:678–689. doi: 10.1111/j.1365-2796.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 77.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler Thromb Vasc Biol. 2005;25:658–670. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 78.Rothe G, Herr AS, Stohr J, et al. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis. 1999;144:251–261. doi: 10.1016/s0021-9150(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 79.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 80.Poitou C, Dalmas E, Renovato M, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 81.Timmerman KL, Flynn MG, Coen PM, et al. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- 82.Heine GH, Ulrich C, Seibert E, et al. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 83.Schlitt A, Heine GH, Blankenberg S, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 84.Wildgruber M, Lee H, Chudnovskiy A, et al. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One. 2009;4:e5663. doi: 10.1371/journal.pone.0005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imanishi T, Ikejima H, Tsujioka H, et al. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis. 2010;212:628–635. doi: 10.1016/j.atherosclerosis.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 86.Kashiwagi M, Imanishi T, Tsujioka H, et al. Association of monocyte subsets with vulnerability characteristics of coronary plaques as assessed by 64-slice multidetector computed tomography in patients with stable angina pectoris. Atherosclerosis. 2010;212:171–176. doi: 10.1016/j.atherosclerosis.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Imanishi T, Ikejima H, et al. Association between circulating monocyte subsets and in-stent restenosis after coronary stent implantation in patients with ST-elevation myocardial infarction. Circ J. 2010;74:2585–2591. doi: 10.1253/circj.cj-10-0544. [DOI] [PubMed] [Google Scholar]
- 88.Hristov M, Leyendecker T, Schuhmann C, et al. Circulating monocyte subsets and cardiovascular risk factors in coronary artery disease. Thromb Haemost. 2010;104:412–414. doi: 10.1160/TH10-01-0069. [DOI] [PubMed] [Google Scholar]
- 89•.Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5:122–131. doi: 10.1161/CIRCGENETICS.111.960385. This is the first large cohort study identifying increased numbers of classical CD14high CD16− monocytes as an independent risk factor for ischemic cardiovascular events in a general population. [DOI] [PubMed] [Google Scholar]
- 90.Urra X, Villamor N, Amaro S, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 91.Tsujioka H, Imanishi T, Ikejima H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Barisione C, Garibaldi S, Ghigliotti G, et al. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. 2010;28:115–124. doi: 10.3233/DMA-2010-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tapp LD, Shantsila E, Wrigley BJ, et al. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. J Thromb Haemost. 2011 doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]