Abstract
Background
Prospective studies have consistently found that postmenopausal breast cancer risk increases with circulating estrogens; however, findings from studies of estrogens and mammographic density (MD), an intermediate marker of breast cancer risk, have been inconsistent. We investigated the cross-sectional associations of urinary estrogens, and their 2-, 4-, and 16-hydroxylated metabolites with MD.
Methods
Postmenopausal women without breast cancer (n=194), ages 48-82 years, and reporting no current menopausal hormone therapy use were enrolled at a clinic in Western NY in 2005. Urinary estrogens and estrogen metabolites were measured using mass spectrometry. Percent MD and dense area (cm2) were measured using computer-assisted analyses of digitized films. Linear regression models were used to estimate associations of log-transformed estrogen measures with MD while adjusting for age, body mass index (BMI), parity, and past hormone therapy use.
Results
Urinary concentrations of most individual estrogens and metabolites were not associated with MD; however, across the interdecile range of the ratio of parent estrogens (estrone and estradiol) to their metabolites, MD increased by 6.8 percentage points (p=0.02) and dense area increased by 10.3 cm2 (p=0.03). Across the interdecile ranges of the ratios of 2-, 4-, and 16-hydroxylation pathways to the parent estrogens, MD declined by 6.2 (p=0.03), 6.4 (p=0.04), and 5.7 (p=0.05) percentage points, respectively. All associations remained apparent in models without adjustment for BMI.
Conclusions
In this study of postmenopausal women, less extensive hydroxylation of parent estrogens was associated with higher MD.
Impact
Hydroxylation of estrogens may modulate postmenopausal breast cancer risk through a pathway involving MD.
Keywords: Estrogens, metabolism, mammography, breast neoplasms, risk factors, human, female, middle-aged
INTRODUCTION
Estrogens play important roles in the pathophysiology of breast tumors and are recognized as causal etiologic factors. This central insight has led to many of the available preventive and therapeutic interventions for breast cancer. Numerous laboratory and small observational studies have suggested that estrogen metabolism may also play a role in breast cancer risk and that its study could provide clues about underlying mechanisms of estrogen-mediated carcinogenesis (reviewed in (1)).
Because estrogens can enhance cellular proliferation through receptor-mediated signaling, it is plausible that elevated estrogen levels may be associated with a greater extent of radiodense breast tissues. Mammographic density (MD), a measure of the extent of radiodensity, has consistently been associated with both breast cancer risk (2-4) and many established risk factors (5). That estrogens could increase breast cancer risk through effects on MD is supported by observations that menopausal hormone therapy use, known to increase breast cancer risk, is associated with increased MD (6), while tamoxifen, a selective estrogen-receptor modulator used to prevent breast cancer, often results in decreased MD (7). While prospective studies have consistently found that postmenopausal breast cancer risk increases with circulating estrogens (8), the analogous relation has not consistently been observed for MD (9-19).
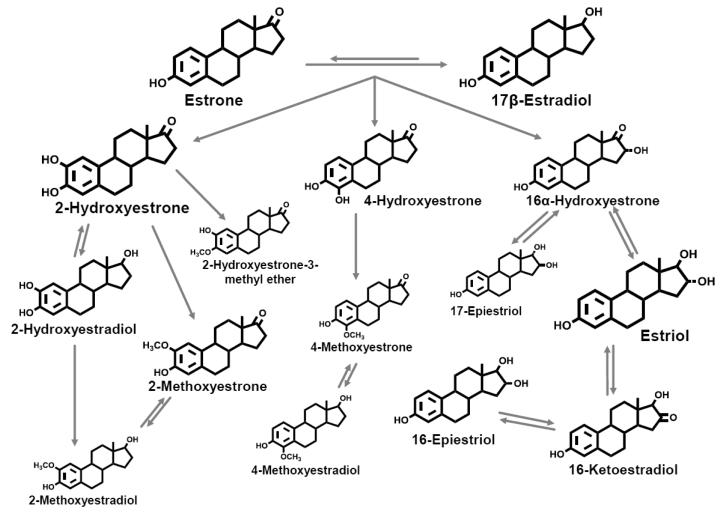
The parent estrogens, estrone and estradiol, can each be hydroxylated at the C2, C4, or C16 positions of the steroid ring to produce an array of metabolites (Figure 1) with different affinities for estrogen receptors (20). Catechol estrogens, characterized by adjacent hydroxyl groups, can be oxidized to form mutagenic semi-quinones (21), while methylation prevents formation of these reactive species (22). Wide inter-individual variation in estrogen metabolism results in diverse exposure profiles (23).
Figure 1. Pathways of estrogen metabolism.
Adapted from Ziegler et al. (43) and reproduced with permission from Environmental Health Perspectives.
The systematic study of estrogen metabolites has become possible with the development of a highly reliable liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for concurrent measurement of 15 estrogens and estrogen metabolites (jointly referred to as EM) in urine or serum (24, 25). A recent prospective study utilizing this assay identified two patterns of estrogen metabolism associated with reduced postmenopausal breast cancer risk even after adjusting for estradiol: greater extent of 2-hydroxylation of parent estrogens and greater methylation of 4-pathway catechol estrogens (26). Riza and colleagues (27) used an immunoassay and found elevated urinary concentrations of 2-hydroxyestrone and a higher ratio of this metabolite to 16α-hydroxyestrone in association with higher risk MD patterns.
We measured urinary EM using LC-MS/MS within a cross-sectional study of postmenopausal women to examine whether estrogens, estrogen metabolites, or patterns of estrogen metabolism are associated with MD.
METHODS
Study Design and Population
We conducted a cross-sectional study of MD and its determinants, with a focus on dietary and hormonal exposures (28). Participants were enrolled when they sought mammograms at a radiology clinic near Buffalo, NY between March-August 2005. Eligible subjects had to be at least 45 years old and postmenopausal at study entry (last menstrual period > 12 months prior or a history of bilateral oophorectomy; for those with a history of hysterectomy and at least one intact ovary, menopause was defined as age > 51 years). Women who reported a history of cancer other than non-melanoma skin cancer, use of menopausal hormone therapy or antibiotics within the previous month, a history of breast augmentation or breast reduction surgery, or an allergy to soy or peanuts (the protocol included a soy challenge to determine equol status) were excluded from the study. Participants completed a questionnaire, underwent anthropometric measures by trained personnel, provided first morning urine specimens before undergoing a soy challenge, and provided access to mammographic films.
Of 330 enrolled women, 24 were later excluded: 10 who received digital, rather than film-screen, mammograms; 9 who were premenopausal, 2 who were taking menopausal hormones, and 3 who were diagnosed with breast cancer. Urine specimens were available for 212 of the 306 eligible participants; others either did not provide a morning urine sample prior to a soy intervention (n=68) or did not give consent for future research (n=26). Of those remaining, we excluded those who had unavailable questionnaires (n=11), anthropometric measures (n=2), mammographic films (n=3), or urinary EM measures (n=2). Thus, 194 participants remain in the present analysis.
Urine Collection
Participants provided first-morning urine samples, collected between 6-8 a.m. at home and delivered to the clinic with an icepack, usually by 10 a.m. Urine samples were filtered and aliquoted into 2.0 mL cryovials and stored at −80 °C. Urinary EM have previously been observed to remain stable under similar conditions of processing and storage (29). Urinary creatinine was measured to adjust for variation in urine volumes (28).
Laboratory Assays
LC-MS/MS was used to measure 15 urinary EM, as shown in Figure 1 (24). Details of the method, including sample preparation and assay conditions, have been published previously (24). We used six stable isotopically labeled standards to account for losses during sample preparation and assays: deuterated 2-hydroxyestradiol, 2-methoxyestradiol and estriol (C/D/N Isotopes, Inc., Pointe-Claire, Quebec, Canada); deuterated 16-epiestriol (Medical Isotopes, Inc., Pelham, NH); and 13C-labeled estrone and estradiol (Cambridge Isotope Laboratories, Andover, MA). Assay reliability was monitored using 10% masked quality control (QC) samples inserted randomly into each batch. Coefficients of variation were <5% for each measured EM.
Measurement of Mammographic Density (MD)
Right and left cranio-caudal films were selected for measurement. Mammographic films were collected and then digitized at 100 pixels / cm with a Kodak Lumisys 85 laser film scanner, which covers an optical density range of 0 - 4.0 absorbance. MD was measured by a single reader (B.T.) using computer-assisted analysis to quantify total breast area (cm2), and dense area (cm2) (30); MD measures for each woman represent means of the measures for left and right breasts. Percent density was calculated as 100*dense area / total area. Comparisons of repeated measures for 10% of the films yielded overall coefficients of variation of 8.5% and 8.8%, for percent density and dense area, respectively, and intra-class correlation coefficients of 0.95 and 0.89.
Estrogen Measurements
MD associations were evaluated for each individual EM and for groups based on metabolic pathways. We also investigated ratios representing measures of individual propensities for site-specific hydroxylation of parent estrogens and for methylation of 2- and 4-pathway catechols. Ratios of competing metabolic pathways, in particular, the ratio of 2- to 16-hydroxylation pathways were investigated because they have been studied in association with breast cancer (31-33) and MD (27).
Statistical Analysis
Neither percent density nor its log or square root transformation were normally distributed; therefore we used it without transformation. In contrast, dense area and total area did not deviate from normality. The main independent variables were urinary concentrations of EM in picomoles per milligram of creatinine. Following log-transformation, distributions of estrogens and estrogen metabolite concentrations did not deviate significantly from normality. We examined the correlation among urinary EM using Pearson’s correlation coefficients. Associations of MD measures with participant characteristics were evaluated by testing for differences across categories using analysis of variance (ANOVA), or t-tests, as appropriate.
Linear regression models were used to evaluate associations of each EM with percent density, dense area, and total area. For regression models, all estrogen metabolism measures were log transformed to a base corresponding to the ratio of the 90th to the 10th percentile observed for that measure. This was done so that the regression coefficients associated with each estrogen, metabolite, group or ratio correspond to the average change in density across its interdecile range; thus, regression coefficients are comparable across measures in spite of differences in scale. Residuals from linear models were assessed by visual inspection and were not found to deviate substantially from normality.
Breast cancer risk factors were evaluated as potential confounders of EM associations with MD using stepwise linear models. Final multivariable-adjusted models included continuous age and BMI, combined parity and age at first birth ( nulliparous, parous < 21 y; parous 22-29 y; parous 30+ y) and past use of combination menopausal hormone therapy (former, never). The proportion of variation in density measures attributable to estrogen metabolism was assessed by adding EM to final adjusted models and observing the change in the model R-square. The impact of adiposity on any estrogen-density associations was assessed by comparing results from regression models with and without BMI.
To evaluate whether associations of EM were modified by factors known to influence estrogens and/or MD, we stratified analyses by age, years since menopause, BMI, parity, and past use of menopausal hormones. We calculated Wald p-values for interaction by including interaction terms in regression models.
Scatterplots with a fitted line were created for associations of estrogens with the multivariable-adjusted percent density. In sensitivity analyses, women with indications other than routine screening for mammography and those reporting histories of surgical menopause were excluded; results were similar and are not shown here.
P-values <0.05 were considered statistically significant. All tests were two-sided. Analyses were conducted using SAS v. 9.1 (SAS Institute, Cary, NC).
RESULTS
In this sample of 194 postmenopausal women, the mean (standard deviation, SD) age was 58 (6) years. Most participants were non-Hispanic and white (98%), and 92% reported seeking a routine screening mammogram. The mean (SD) percent density was 34.3 (17.7); median percent density was 38.8, and the range was 3.0 to 77.0. Mean (SD) dense area was 49.0 (27.2) cm2 and mean (SD) total area was 145.7 (60.5) cm2. Characteristics of the study participants and associations with MD are shown in Table 1. Thirty-four percent of participants were overweight (BMI 25.0-29.9) and 35% were obese (BMI >30.0). Only 15% of participants were nulliparous and 69% had a first full-term birth before 30 years of age. In this study sample 63% of participants reported past use of menopausal hormones, and 33% combination menopausal hormones (estrogen + progesterone).
Table 1.
Mammographic density by participant characteristics (n=194)
| Percent Density | Dense Area (cm2) | Total Area (cm2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| n | %* | Mean | S.D. | P † | Mean | S.D. | P † | Mean | S.D. | P † | |
| Age (y) | 0.06 | 0.99 | 0.27 | ||||||||
| 48- 54 | 55 | 28.4 | 43.1 | 18.8 | 49.7 | 25.1 | 132.2 | 64.0 | |||
| 55- 58 | 45 | 23.2 | 36.7 | 20.3 | 48.1 | 28.1 | 152.3 | 71.4 | |||
| 59- 64 | 56 | 28.9 | 35.2 | 18.7 | 49.5 | 28.0 | 151.6 | 52.8 | |||
| 65+ | 38 | 19.6 | 33.2 | 17.4 | 48.2 | 28.7 | 148.6 | 50.1 | |||
|
| |||||||||||
| Education | 0.45 | 0.47 | 0.01 | ||||||||
| High school | 46 | 23.7 | 35.2 | 18.8 | 53.0 | 29.2 | 168.1 | 68.3 | |||
| Some college / technical school | 52 | 26.8 | 36.1 | 20.6 | 46.4 | 30.6 | 141.8 | 58.7 | |||
| Completed college | 96 | 49.5 | 39.1 | 18.5 | 48.4 | 24.1 | 137.0 | 55.1 | |||
|
| |||||||||||
| Body mass index (kg/m2) | <0.0001 | 0.09 | <0.001 | ||||||||
| ≤ 24.9 (underweight - normal) | 60 | 30.9 | 46.3 | 17.1 | 44.2 | 20.4 | 99.8 | 35.0 | |||
| 25.0- 29.9 (overweight) | 66 | 34.0 | 36.9 | 18.1 | 47.7 | 24.2 | 136.9 | 38.1 | |||
| ≥30.0 (obese) | 68 | 35.1 | 30.1 | 18.8 | 54.4 | 33.8 | 194.7 | 60.4 | |||
|
| |||||||||||
| Age at menarche (y) | 0.78 | 0.53 | 0.04 | ||||||||
| ≤11 | 46 | 24.2 | 36.5 | 21.1 | 52.6 | 31.1 | 164.4 | 70.6 | |||
| 12-13 | 106 | 55.8 | 38.2 | 19.1 | 47.3 | 25.2 | 137.1 | 54.4 | |||
| 14+ | 38 | 20.0 | 35.8 | 17.9 | 47.8 | 28.4 | 147.1 | 62.8 | |||
|
| |||||||||||
| Parity / age at first birth (y) | 0.03 | 0.36 | 0.68 | ||||||||
| Nulliparous | 29 | 15.0 | 42.8 | 21.5 | 54.4 | 30.7 | 143.7 | 60.8 | |||
| Parous, ≤ 21 | 25 | 12.9 | 31.7 | 19.0 | 42.9 | 24.0 | 149.1 | 54.6 | |||
| Parous, 22-29 | 108 | 55.7 | 35.4 | 18.0 | 47.8 | 27.0 | 148.7 | 62.7 | |||
| Parous, 30+ | 32 | 16.5 | 43.8 | 18.9 | 52.7 | 26.6 | 134.5 | 57.9 | |||
|
| |||||||||||
| Indication for mammogram | 0.67 | 0.74 | 0.33 | ||||||||
| Screening | 179 | 92.3 | 37.6 | 18.8 | 49.2 | 27.2 | 144.4 | 60.3 | |||
| Other / missing | 15 | 7.7 | 35.4 | 23.1 | 46.7 | 27.6 | 160.5 | 62.7 | |||
|
| |||||||||||
|
Family history of breast cancer in first
degree female relative |
0.72 | 0.92 | 0.85 | ||||||||
| No | 137 | 76.5 | 37.5 | 18.7 | 49.3 | 27.1 | 145.2 | 60.7 | |||
| Yes | 42 | 23.5 | 36.3 | 19.5 | 48.8 | 28.3 | 147.2 | 57.8 | |||
|
| |||||||||||
| Menopause type | 0.01 | 0.35 | 0.004 | ||||||||
| Natural menopause | 143 | 76.1 | 38.9 | 18.6 | 49.8 | 27.5 | 139.6 | 59.1 | |||
| Surgical menopause | 45 | 23.9 | 30.7 | 19.6 | 45.4 | 27.5 | 169.3 | 61.4 | |||
|
| |||||||||||
| Years since menopause | 0.02 | 0.97 | 0.02 | ||||||||
| 0-5 | 69 | 35.6 | 41.9 | 18.5 | 49.4 | 24.5 | 129.6 | 53.6 | |||
| 6-8 | 35 | 18.0 | 38.8 | 20.4 | 49.3 | 30.0 | 150.9 | 77.8 | |||
| > 8 | 90 | 46.4 | 33.4 | 18.4 | 48.5 | 28.2 | 155.9 | 55.7 | |||
|
| |||||||||||
| Past use of menopausal hormones | 0.79 | 0.08 | 0.14 | ||||||||
| No | 72 | 37.1 | 36.9 | 19.8 | 44.5 | 22.6 | 137.4 | 55.4 | |||
| Yes | 122 | 62.9 | 37.7 | 18.8 | 51.6 | 29.3 | 150.6 | 63.0 | |||
|
| |||||||||||
| Past use of unopposed estrogens | 0.003 | 0.24 | 0.01 | ||||||||
| No | 146 | 75.3 | 39.7 | 18.2 | 50.3 | 26.4 | 139.3 | 59.6 | |||
| Yes | 48 | 24.7 | 30.4 | 20.3 | 44.9 | 29.4 | 165.1 | 59.5 | |||
|
| |||||||||||
| Past use of combination hormones | 0.01 | 0.003 | 0.54 | ||||||||
| No | 130 | 67.0 | 35.0 | 19.7 | 44.9 | 24.5 | 147.6 | 59.9 | |||
| Yes | 64 | 33.0 | 42.3 | 17.1 | 57.2 | 30.4 | 141.8 | 61.8 | |||
|
| |||||||||||
|
Time since menopausal hormone therapy
was last used among past users (y) |
0.32 | 0.58 | 0.59 | ||||||||
| ≤ 3 | 53 | 54.6 | 39.1 | 19.4 | 49.5 | 28.2 | 147.9 | 68.9 | |||
| 4+ | 44 | 45.4 | 35.3 | 17.1 | 52.7 | 29.5 | 155.2 | 60.8 | |||
Percentages exclude participants with missing data
P values result from tests for differences in mammographic density across categories of each covariate. For covariates with 3 or more categories, ANOVA was performed; for covariates with 2 categories, t-tests were performed.
Percent MD declined with increasing age, BMI, and years since menopause, and was higher in women who were nulliparous or had first births after age 30y. Histories of natural vs. surgical menopause, and past vs. never use of combination menopausal hormone therapy were each associated with higher percent density. Among these factors, only past use of combination menopausal hormone therapy was statistically significantly associated with increased dense area; in contrast, BMI, surgical menopause and years since menopause were positively associated with total breast area.
Table 2 shows medians and interdecile ranges for each urinary EM. On average, parent estrogens represented 16% of total urinary estrogens, while 2-, 4-, and 16-hydroxylated metabolites represented, respectively, 32%, 5%, and 43% of total urinary estrogens. Log-transformed urinary concentrations of EM were moderately to highly correlated. The Pearson correlation coefficients for associations of the parent estrogens with total estrogens and metabolites, and with the 2-, 4-, and 16-hydroxylation pathways were 0.77, 0.52, 0.44, and 0.57, respectively.
Table 2.
Median urinary concentrations of estrogens and estrogen metabolites (pmol/mg creatinine) and linear associations between log-transformed urinary estrogens and estrogen metabolites* and percent density, dense area (cm2), and total breast area (cm2).
| Estrogens and measures of estrogen metabolism |
Urinary concentrations of
estrogens and metabolites |
Multivariable-adjusted associations of estrogens and
their metabolites* with mammographic density |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Median | Interdecile Range | Percent density | Dense area (cm2) | Total area (cm2) | ||
| 10th | 90th | Beta (P) † | Beta (P) † | Beta (P) † | ||
| Total Estrogens and Metabolites | 54.3 | 32 | 93.1 | −3.38 (0.25) | 0.67 (0.89) | −5.35 (0.47) |
|
| ||||||
| Parent Estrogens | 8.9 | 4.4 | 18.7 | 1.73 (0.53) | −8.46 (0.09) | −7.90 (0.25) |
| Estrone | 6.3 | 3.1 | 14.2 | 1.63 (0.54) | −7.61 (0.14) | −7.12 (0.29) |
| Estradiol | 2.4 | 1.1 | 5.5 | 1.25 (0.68) | −9.32 (0.07) | −9.01 (0.23) |
|
| ||||||
| 2-Hydroxylation pathway | 17.6 | 8.4 | 31.0 | −5.17 (0.11) | −1.10 (0.82) | −4.39 (0.58) |
| 2-Pathway catechols | 13.3 | 6 | 25.2 | −4.67 (0.16) | −8.41 (0.08) | −3.55 (0.67) |
| 2-Hydroxyestrone | 10.1 | 4.3 | 19.9 | −5.37 (0.11) | −10.09 (0.03) | −3.83 (0.65) |
| 2-Hydroxyestradiol | 3.1 | 1.2 | 6.4 | −0.38 (0.90) | −2.87 (0.57) | −6.75 (0.38) |
| 2-Pathway methylated catechols | 3.9 | 2.1 | 7.7 | −4.99 (0.11) | −7.79 (0.10) | −4.23 (0.59) |
| 2-Methoxyestrone | 2.1 | 1.2 | 4.8 | −7.71 (0.01) | −8.68 (0.09) | 2.90 (0.71) |
| 2-Methoxyestradiol | 1.0 | 0.4 | 2.7 | 0.43 (0.90) | −6.90 (0.16) | −10.54 (0.20) |
| 2-Hydroxyestrone-3-methyl ether | 0.5 | 0.3 | 1.1 | −3.07 (0.31) | −8.11 (0.10) | −10.2 (0.17) |
|
| ||||||
| 4-Hydroxylation pathway EM | 2.7 | 1.4 | 5.5 | −5.50 (0.10) | −8.99 (0.07) | −4.44 (0.60) |
| 4-Pathway catechol: 4-Hydroxyestrone | 2.0 | 0.9 | 4.1 | −4.52 (0.15) | −2.64 (0.59) | −4.29 (0.59) |
| 4-Pathway methylated catechols | 0.7 | 0.4 | 1.4 | −4.32 (0.17) | 0.67 (0.89) | −2.60 (0.74) |
| 4-Methoxyestrone | 0.4 | 0.2 | 0.8 | −6.78 (0.03) | −8.46 (0.09) | 5.62 (0.48) |
| 4-Methoxyestradiol | 0.2 | 0.1 | 0.6 | 1.12 (0.72) | −7.61 (0.14) | −12.68 (0.10) |
|
| ||||||
| 16-Hydroxylation pathway | 23.4 | 13.5 | 44.5 | −3.96 (0.18) | −5.68 (0.21) | −4.35 (0.55) |
| 16α-Hydroxyestrone | 4.3 | 1.9 | 8.6 | −3.08 (0.38) | −4.20 (0.44) | −13.33 (0.13) |
| Estriol | 11.8 | 6.1 | 24 | −3.18 (0.27) | −4.67 (0.30) | −3.49 (0.63) |
| 17-Epiestriol | 0.5 | 0.2 | 1.3 | −1.78 (0.57) | −2.27 (0.64) | −7.69 (0.32) |
| 16-Ketoestradiol | 4.4 | 2.2 | 9.0 | −4.76 (0.10) | −7.53 (0.09) | −2.56 (0.73) |
| 16-Epiestriol | 1.3 | 0.6 | 2.6 | −4.38 (0.18) | −6.00 (0.24) | −3.00 (0.72) |
|
| ||||||
| Ratios | ||||||
| Parent estrogens / estrogen metabolites | 0.2 | 0.11 | 0.38 | 6.80 (0.02) | 10.33 (0.03) | −7.39 (0.32) |
| 2-Hydroxylation pathway / parent estrogens | 1.98 | 0.91 | 3.6 | −6.19 (0.03) | −10.27 (0.02) | 5.95 (0.42) |
| 4-Hydroxylation pathway / parent estrogens | 0.31 | 0.14 | 0.64 | −6.36 (0.04) | −10.32 (0.03) | 5.92 (0.45) |
| 16-Hydroxylation pathway / parent estrogens | 2.6 | 1.3 | 4.8 | −5.74 (0.05) | −8.82 (0.05) | 5.81 (0.42) |
| 2-Hydroxylation pathway / 16-hydroxylation pathway | 0.79 | 0.4 | 1.21 | −0.81 (0.79) | −2.23 (0.63) | 0.42 (0.96) |
| 2-Hydroxyestrone / 16-α hydroxyestrone | 1.14 | 2.47 | 4.43 | −2.37 (0.44) | −5.10 (0.29) | 7.77 (0.31) |
| 4-Hydroxylation pathway / 2-hydroxylation pathway | 0.16 | 0.12 | 0.22 | −0.61 (0.85) | −0.31 (0.95) | <0.01 (1.00) |
| 4-Pathway catechol / 4-pathway methylated catechols | 2.7 | 1.3 | 5.6 | −0.69 (0.83) | 0.01 (1.00) | −2.10 (0.80) |
| 2-Pathway catechols / 2-pathway methylated catechols | 2.6 | 1.4 | 5.1 | −0.76 (0.81) | 0.41 (0.93) | 3.15 (0.69) |
Derived measures of estrogen metabolism and statistically significant estimates are presented in bold font.
For linear regression modeling, each measure of estrogen metabolism was log transformed to a base corresponding to the observed ratio of the 90th to the 10th percentile. Therefore the regression coefficient associated with each estrogen, metabolite, group or ratio corresponds to the average change in density across the interdecile range of that estrogen measure.
Adjusted for continuous age and BMI, ever use of combination hormone therapy, parity / age at first birth (nulliparous, first birth at age ≤21 y, 22-29 y, and 30+ y).
Multivariable-adjusted linear associations of log-transformed measures of EM with percent density, dense area, and total area are also presented in Table 2. Estrone and estradiol were not statistically significantly associated with any measure of MD. While most EM were not significantly associated with percent density or dense area, in general, parent estrogens were positively associated, and estrogen metabolites in the 2-, 4-, and 16-hydroxylation pathways were inversely associated with percent density. Across the interdecile range of 2-methoxyestrone, percent MD declined significantly by 7.7 percentage points (p=0.01) and dense area declined by 8.7 cm2 (p=0.09); similar associations were noted for 4-methoxyestrone.
An increased ratio of parent estrogens to estrogen metabolites was associated with higher MD; across the interdecile range of this ratio, mean percent MD increased by 6.8 percentage points (p=0.02) and mean dense area increased by 10.3 cm2 (p=0.03) (Table 2). After adjusting for covariates, the ratio of parent estrogens to estrogen metabolites accounted for 2.1% of the variation in percent density, and 2.4% of the variation in dense area. Ratios of 2-, 4-, and 16-hydroxylation pathways to parent estrogens were each inversely associated with percent density (p=0.03, 0.04, 0.05, respectively) and dense area (p=0.02, 0.03, and 0.05, respectively). Ratios of 2- to 16-pathways, and of 2-hydroxyestrone to 16α-hydroxyestrone were not significantly associated with any measure of MD. No statistically significant associations were observed between any EM measure and total area (cm2) of the breast. Observed multivariable-adjusted associations were similar in direction and magnitude to univariate associations (data not shown).
BMI was directly correlated with urinary estrone (Pearson’s r=0.24, p=0.0008) and inversely correlated with percent MD (r=−0.39, p<0.0001, not shown). Results from adjusted models that did and did not include BMI showed similar results (data not shown). Regression coefficients for statistically significant findings in Tables 2 and 3 did not vary by >10% based upon the decision to include or exclude BMI, nor did the decision to include or exclude BMI modify their statistical significance.
Table 3.
Linear associations between log-transformed urinary estrogens and estrogen metabolites (EM, in pmol/mg creatinine)* and percent mammographic density in postmenopausal women, stratified by body mass index (BMI) and by years since menopause.
| BMI | Years Since Menopause | |||||
|---|---|---|---|---|---|---|
| < 30 | ≥ 30 | ≤8 | >8 | |||
| N=127 | N=67 | N=102 | N=92 | |||
| Estrogens and measures of estrogen metabolism | Beta (P) † | Beta (P) † | P int‡ | Beta (P) † | Beta (P) † | P int § |
| Total Estrogens and Metabolites | −6.08 (0.07) | 6.50 (0.27) | 0.41 | 1.31 (0.75) | −6.72 (0.11) | 0.04 |
| Parent Estrogens | −3.81 (0.24) | 15.0 (0.005) | 0.03 | 8.42 (0.02) | −6.78 (0.11) | 0.004 |
| Estrone | −3.47 (0.26) | 15.3 (0.005) | 0.02 | 7.38 (0.03) | −5.86 (0.17) | 0.01 |
| Estradiol | −3.90 (0.28) | 12.5 (0.03) | 0.19 | 9.61 (0.02) | −7.78 (0.08) | 0.004 |
|
| ||||||
| 2-Hydroxylation pathway | −5.49 (0.17) | −0.91 (0.88) | 0.75 | −6.28 (0.16) | −3.28 (0.49) | 0.96 |
| 2-Pathway catechols | −4.01 (0.34) | −2.02 (0.74) | 0.97 | −5.37 (0.24) | −3.48 (0.49) | 0.96 |
| 2-Hydroxyestrone | −4.35 (0.30) | −3.03 (0.63) | 0.84 | −6.70 (0.15) | −3.60 (0.48) | 0.83 |
| 2-Hydroxyestradiol | −0.82 (0.83) | 2.50 (0.65) | 0.55 | 0.05 (0.99) | −0.83 (0.87) | 0.94 |
| 2-Pathway methylated catechols | −6.58 (0.09) | 1.01 (0.86) | 0.43 | −6.15 (0.17) | −2.91 (0.50) | 0.87 |
| 2-Methoxyestrone | −8.77 (0.02) | −2.96 (0.60) | 0.64 | −8.52 (0.05) | −6.25 (0.16) | 0.82 |
| 2-Methoxyestradiol | −3.03 (0.48) | 6.86 (0.21) | 0.19 | −0.42 (0.93) | 2.58 (0.59) | 0.80 |
| 2-Hydroxyestrone-3-methyl ether | −1.43 (0.70) | −2.02 (0.74) | 0.88 | −1.98 (0.66) | −3.39 (0.42) | 0.65 |
|
| ||||||
| 4-Hydroxylation pathway EM | −6.17 (0.14) | −1.50 (0.81) | 0.91 | −7.11 (0.13) | −4.09 (0.40) | 0.95 |
| 4-Pathway catechol: 4-Hydroxyestrone | −5.07 (0.21) | −1.11 (0.85) | 0.93 | −5.62 (0.20) | −3.99 (0.40) | 0.85 |
| Methylated catechols | −3.99 (0.30) | −2.05 (0.73) | 0.98 | −5.35 (0.26) | −2.45 (0.58) | 0.62 |
| 4-Methoxyestrone | −5.56 (0.15) | −5.66 (0.34) | 0.99 | −9.47 (0.04) | −4.25 (0.34) | 0.95 |
| 4-Methoxyestradiol | −1.49 (0.72) | 5.34 (0.29) | 0.58 | 2.11 (0.62) | 1.33 (0.78) | 0.31 |
|
| ||||||
| 16- Hydroxylation pathway | −5.75 (0.09) | 2.59 (0.65) | 0.99 | 0.89 (0.84) | −6.75 (0.09) | 0.045 |
| 16α-Hydroxyestrone | −7.58 (0.08) | 9.19 (0.15) | 0.21 | 0.80 (0.87) | −5.00 (0.34) | 0.22 |
| Estriol | −4.42 (0.20) | 1.35 (0.81) | 0.73 | 1.52 (0.72) | −6.80 (0.10) | 0.047 |
| 17-Epiestriol | −5.20 (0.17) | 5.49 (0.32) | 0.31 | 1.84 (0.65) | −5.51 (0.28) | 0.047 |
| 16-Ketoestradiol | −6.48 (0.06) | 0.25 (0.96) | 0.92 | −1.72 (0.67) | −6.19 (0.15) | 0.09 |
| 16-Epiestriol | −3.80 (0.34) | −2.20 (0.72) | 0.74 | −2.37 (0.58) | −5.85 (0.26) | 0.26 |
|
| ||||||
| Ratios | ||||||
| Parent estrogens / estrogen metabolites | 0.40 (0.91) | 16.82 (0.002) | 0.02 | 13.95 (<0.0001) | −2.48 (0.59) | 0.02 |
| 2-Hydroxylation pathway / parent estrogens | 0.87 (0.81) | −16.39 (0.002) | 0.0496 | −15.50 (<0.0001) | 5.04 (0.26) | 0.0006 |
| 4-Hydroxylation pathway / parent estrogens | 0.33 (0.93) | −17.36 (0.002) | 0.03 | −15.68 (<0.0001) | 4.13 (0.38) | 0.002 |
| 16-Hydroxylation pathway / parent estrogens | −0.49 (0.89) | −13.22 (0.009) | 0.02 | −10.67 (0.005) | 0.53 (0.90) | 0.14 |
| 4-Hydroxylation pathway / 2- hydroxylation pathway | −1.43 (0.73) | −1.43 (0.82) | 0.59 | −1.07 (0.82) | −2.26 (0.64) | 0.76 |
| 2-Hydroxylation pathway / 16-hydroxylation pathway | 1.74 (0.64) | −3.14 (0.54) | 0.63 | −7.29 (0.07) | 5.50 (0.21) | 0.02 |
| 2-Hydroxyestrone / 16-α hydroxyestrone | −9.61 (0.07) | 2.61 (0.50) | 0.11 | 1.26 (0.81) | −6.41 (0.12) | 0.13 |
| 4-Pathway catechols / 4-Pathway methylated catechols | −1.20 (0.77) | 0.61 (0.92) | 0.93 | −1.55 (0.72) | −1.85 (0.73) | 0.55 |
| 2-Pathway catechols / 2-Pathway methylated catechols | 2.22 (0.57) | −4.98 (0.38) | 0.33 | −0.84 (0.85) | −1.56 (0.74) | 0.80 |
Derived measures of estrogen metabolism and statistically significant estimates are presented in bold font.
For linear regression modeling, each measure of estrogen metabolism was log transformed to a base corresponding to the observed ratio of the 90th to the 10th percentile. Therefore the regression coefficient associated with each estrogen, metabolite, group or ratio corresponds to the average change in density across the interdecile range of that estrogen measure.
n=194. Linear regression models adjusted for continuous age and BMI, parity / age at first birth (nulliparous, first birth at age ≤21 y, 22-29 y, and 30+ y) and never/ever use of combination hormone therapy.
Statistical significance for each potential interaction was assessed by the Wald p-value of the interaction term EM*BMI added to the model.
Statistical significance for each potential interaction was assessed by the Wald p-value of the interaction term for EM*years since menopause when added to the model.
Associations of EM profiles with percent MD were significantly modified by BMI and years since menopause (Table 3). When results were stratified on BMI (>30.0 or <30.0), MD increased by 15.3 percentage points across the interdecile range of estrone (p=0.005) and by 12.5 percentage points across the interdecile range of estradiol (p=0.03) among obese women , but estrone and estradiol were not associated with density in non-obese women (pinteraction=0.02, and 0.19, respectively). Accordingly, in obese women MD increased by 16.8 percentage points across the interdecile range of the ratio of parent estrogens to estrogen metabolites (p<0.002); this ratio accounted for 12.6% of the variation in percent MD. Accordingly, in obese women MD declined by 16.4, 17.4, and 13.2 percentage points across the interdecile ranges of ratios of 2-, 4-, and 16-pathways to parent estrogens (with p<0.002, p<0.002, and p=0.009, respectively). These associations were not observed in non-obese women (pinteraction =0.0496, pinteraction=0.03, and pinteraction=0.02, respectively) (Table 3). Associations of the ratio of the 2-hydroxylation pathway to the parent estrogens in obese women and their non-obese counterparts are illustrated in Supplementary Figure 1.
Among women with menopause <8 years before baseline, MD increased by 7.4 percentage points across the interdecile range of estrone (p=0.03), and 9.6 percentage points across the interdecile range of estradiol (p=0.02); these associations were not observed in women with a more distant menopause (pinteraction=0.01, and 0.004, respectively) (Table 3). In women with more recent menopause, MD increased by 14.0 percentage points across the interdecile range of the ratio of parent estrogens to estrogen metabolites (p<0.0001); the ratio of parent estrogens to estrogen metabolites accounted for 9.6% of the variation in percent MD. In the same group, MD declined by 15.5, 15.7, and 10.7 percentage points across the interdecile ranges of the ratios of 2-, 4-, and 16-pathways to parent estrogens (with p<0.0001, p<0.0001, and p=0.005, respectively). These associations were not observed in women with menopause >8 years prior (pinteraction=0.02, 0.0006, 0.002, and 0.14, respectively) (Table 3).
Associations of 2- to 16-pathways, and of 2-hydroxyestrone to 16α-hydroxyestrone with percent density were not significantly modified by BMI; however, there was some suggestion that the association of the ratio of 2- to 16 pathways with percent MD was modified by years since menopause (pinteraction= 0.02). In women with more recent menopause, the ratio of 2- to 16-pathway estrogen metabolites was associated with non-significant lower percent density (β=−7.3, p=0.07), while among women with more distant menopause, the same ratio was non-significantly associated with higher percent density (β=5.5, p=0.21).
While groups defined by BMI and years since menopause overlap, no statistically significant association between BMI and years since menopause was observed (data not shown). Although the subgroup findings could suggest that the associations are present only in postmenopausal women with more recent or sustained exposure to higher circulating estrogens, we observed no statistically significant differences in total EM across subgroups defined by years since menopause and obesity (data not shown). No statistically significant modification of these associations was noted by latency of hormone therapy use (data not shown).
No significant interactions were observed for other factors investigated (data not shown), including age, parity/age at first birth, type of menopause, previous use of menopausal hormone therapy and years since last use of menopausal hormone therapy.
DISCUSSION
Among postmenopausal women, we observed no overall associations for individual estrogens or for most estrogen metabolites. However, urinary concentrations of the most prevalent methylated catechols, 2-methoxyestrone and 4-methoxyestrone, were each inversely associated with MD. We observed statistically significant direct associations between percent MD and the ratio of parent estrogens to all estrogen metabolites, suggesting that less extensive hydroxylation may be associated with higher MD. Similar associations were observed between this ratio and dense area, suggesting that the association between percent density and estrogen metabolism is mediated through differences in dense area. The inverse associations between ratios of the 2-, 4-, and 16-hydroxylation pathways to parent estrogens with percent MD and dense area did not differ markedly by metabolic pathway, suggesting a protective role for hydroxylation by any pathway. In addition, these associations remained apparent in models with and without adjustment for BMI, suggesting that adiposity is neither a confounder of the association nor is it on the causal pathway of estrogen metabolism to MD.
Nine previous studies have cross-sectionally assessed associations between circulating estrogens (including estrone, estradiol, estrone sulfate, and free estradiol) and MD in postmenopausal women (9-17, 19). In these studies, immunoassays were used to measure estrogens in serum or plasma, and quantitative or semi-quantitative measures were used to characterize MD. The studies have diverse results; in six studies (9, 10, 12, 15, 17, 19) inverse associations between circulating estrone or estradiol and percent MD were observed; but, in three of these, the associations were attenuated and no longer statistically significant following adjustment for a measure of adiposity (12, 15, 19). A single study found no statistically significant associations of MD with plasma estrogens (13). In three studies, investigators found positive associations between estrogens and MD after adjustment for BMI (11, 14, 16).
These inconsistent finding s may be attributable to sampling variation but alternatively, could reflect an underlying heterogeneity in the association. In the present study, associations of MD with parent estrogens and with the ratios of parent estrogens to their metabolites were strongly apparent in two overlapping but uncorrelated subgroups. The ratio of parent estrogens to all metabolites was stronger in obese women and those with a recent menopause. These subgroup findings may be due to chance but should be considered in future studies. It is notable that in one previous study which found a direct association of estrogens with MD, the participants reported very recent menopause (with a mean of 15 months prior to their mammogram) (16).
In a previous study of estrogen metabolites and MD in postmenopausal women, Riza and colleagues (27) considered urinary 2-hydroxyestrone, 16α-hydroxyestrone, and their ratio in association with qualitatively-assessed low- (n=70) and high-risk (n=70) Wolfe mammographic parenchymal patterns They observed strong direct associations of risk with 2-hydroxyestrone and the ratio of 2-hydroxyestrone to 16-hydroxyestrone. In contrast, we observed no associations with MD for 2-hydroxyestrone, 16-α hydroxyestrone, their ratio, or the ratio of their corresponding pathways. The study by Riza et al. relied on an immunoassay to measure estrogen metabolites; recognized limitations in the specificity, sensitivity, and reproducibility of this type of assay could bias results towards the null but would not explain the distinct pattern of their findings (34).
In our study, MD decreased significantly as urinary concentrations of some methylated catechols increased. Methylation of catechol estrogens reduces their estrogenicity and prevents their conversion to reactive quinones (35). This finding is consistent with that from the first prospective study of postmenopausal breast cancer to study serum EM using the LC-MS/MS method, which suggested that greater 2-hydroxylation of parent estrogens was associated with reduced risk of breast cancer (36). In the same study, investigators found increasing risk of breast cancer associated with the ratio of the 4-hydroxylated catechol to the methylated catechols (36). In contrast, we did not observe any significant associations between measures of MD and ratios of catechols to methylated catechols in the 4-hydroxylation pathways. Differences between these findings could be attributable to sampling variation, use of an intermediate marker rather than a breast cancer endpoint, or differences between urinary and circulating EM profiles.
While MD has been shown to be highly heritable (37), only a few genetic variants are strongly and consistently associated with this phenotype. In a meta-analysis of five genome-wide association studies of MD, polymorphic variants in ZNF365 (rs10995190), ESR1 (rs2046210), and LSP1 (rs3817198) were associated with percent density (38). A recent pooled analysis of 19 studies from 10 countries identified associations of LSP1 (rs3817198) with both percent density and dense area, and of RAD51L1 (rs10483813) with percent density (39). Although numerous studies have examined genes in pathways that regulate estrogen synthesis and metabolism in relation to MD, these have produced largely conflicting results (37). Ongoing consortial efforts will provide better powered tests of hypotheses about the genes involved in estrogen metabolism and MD.
Our study has a number of strengths, including urinary EM profiles representing a detailed assessment of metabolic phenotypes that are accurate and reproducible in postmenopausal women. MD was measured quantitatively, showed good reproducibility and was associated with covariates as expected. Continuous exposures and outcomes result in good power to detect associations with even modest sample sizes. Study limitations, however, include the concern that urinary EM may not represent relative or absolute levels in circulation or in breast tissue, and that some EM may be excreted via bile rather than urine. Our MD measures were based on a 2-dimensional area; volumetric density measures may yet prove to be more accurate predictors of breast cancer risk (40). However, quantitative, computer-assisted measures of percent MD have previously been shown to be a reproducible and robust marker of breast cancer risk (41). We had limited power to detect interactions, particularly by multilevel covariates. Further, the exclusion of current users of hormone therapy, although customary in studies of endogenous hormones, may limit the generalizability of findings (42). We have not adjusted for multiple comparisons due to the exploratory nature of the study.
In summary, our findings suggest that greater urinary excretion of parent estrogens compared with metabolites may be associated with higher MD in postmenopausal women. This suggests that increased hydroxylation of parent estrogens may protect against breast cancer through a causal pathway that includes MD. Our data also suggest that the association of estrogens and MD is maintained only among postmenopausal women with recent or sustained exposure to higher levels of circulating estrogens (such as occurs close to the time of menopause or among obese women). Future larger studies are needed to explore these potential interactions in more detail.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute (NCI), National Institutes of Health (NIH) and contract # HHSN261200800001E to SAIC, Inc. from NCI, NIH, DHHS. The views expressed are those of the authors and do not reflect the official policy or position of the USUHS, the Department of Defense, or the United States Government.
Abbreviations
- BMI
body mass index
- EM
estrogens and estrogen metabolites
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MD
mammographic density
- PD
percent density
- RIA
radioimmunoassay
References
- 1.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–92. doi: 10.1002/1097-0142(197605)37:5<2486::aid-cncr2820370542>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87:670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 4.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. The journal of the British Menopause Society. 2006;12:186–93. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 6.Boyd NF, Melnichouk O, Martin LJ, Hislop G, Chiarelli AM, Yaffe MJ, et al. Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol. 2011;29:2985–92. doi: 10.1200/JCO.2010.33.7964. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst. 2011;103:744–52. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 8.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 9.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, et al. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87:876–82. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, et al. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:1411–7. doi: 10.1158/1055-9965.EPI-04-0920. [DOI] [PubMed] [Google Scholar]
- 11.Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, et al. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162:826–34. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 12.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–7. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- 13.Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:1502–8. doi: 10.1158/1055-9965.EPI-05-0828. [DOI] [PubMed] [Google Scholar]
- 14.Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121:2506–11. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- 15.Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast cancer research : BCR. 2007;9:R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson H, Gandini S, Bonanni B, Mariette F, Guerrieri-Gonzaga A, Serrano D, et al. Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res Treat. 2008;108:57–67. doi: 10.1007/s10549-007-9577-9. [DOI] [PubMed] [Google Scholar]
- 17.McCormack VA, Dowsett M, Folkerd E, Johnson N, Palles C, Coupland B, et al. Sex steroids, growth factors and mammographic density: a cross-sectional study of UK postmenopausal Caucasian and Afro-Caribbean women. Breast cancer research : BCR. 2009;11:R38. doi: 10.1186/bcr2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker S, Kaaks R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: can mammographic density be considered an intermediate marker of risk? Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2009;181:135–57. doi: 10.1007/978-3-540-69297-3_14. [DOI] [PubMed] [Google Scholar]
- 19.Sprague BL, Trentham-Dietz A, Gangnon RE, Buist DS, Burnside ES, Bowles EJ, et al. Circulating sex hormones and mammographic breast density among postmenopausal women. Hormones & cancer. 2011;2:62–72. doi: 10.1007/s12672-010-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–50. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 21.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. Journal of the National Cancer Institute Monographs. 2000:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 22.Zhu BT, Liehr JG. Inhibition of the catechol-O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by catecholamine: implications for the mechanism of estrogen-induced carcinogenesis. Archives of biochemistry and biophysics. 1993;304:248–56. doi: 10.1006/abbi.1993.1346. [DOI] [PubMed] [Google Scholar]
- 23.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–8. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Keefer LK, Ziegler RG, Veenstra TD. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat Protoc. 2007;2:1350–5. doi: 10.1038/nprot.2007.176. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–21. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 26.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104:326–39. doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riza E, dos Santos Silva I, De Stavola B, Bradlow HL, Sepkovic DW, Linos D, et al. Urinary estrogen metabolites and mammographic parenchymal patterns in postmenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:627–34. [PubMed] [Google Scholar]
- 28.Fuhrman BJ, Teter BE, Barba M, Byrne C, Cavalleri A, Grant BJ, et al. Equol status modifies the association of soy intake and mammographic density in a sample of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:33–42. doi: 10.1158/1055-9965.EPI-07-0193. [DOI] [PubMed] [Google Scholar]
- 29.Fuhrman BJ, Xu X, Falk RT, Hankinson SE, Veenstra TD, Keefer LK, et al. Stability of 15 estrogens and estrogen metabolites in urine samples under processing and storage conditions typically used in epidemiologic studies. The International journal of biological markers. 2010;25:185–94. [PMC free article] [PubMed] [Google Scholar]
- 30.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. Automated analysis of mammographic densities. Physics in medicine and biology. 1996;41:909–23. doi: 10.1088/0031-9155/41/5/007. [DOI] [PubMed] [Google Scholar]
- 31.Kabat GC, Chang CJ, Sparano JA, Sepkovie DW, Hu XP, Khalil A, et al. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:505–9. [PubMed] [Google Scholar]
- 32.Ursin G, London S, Stanczyk FZ, Gentzschein E, Paganini-Hill A, Ross RK, et al. Urinary 2-hydroxyestrone/16alpha-hydroxyestrone ratio and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91:1067–72. doi: 10.1093/jnci/91.12.1067. [DOI] [PubMed] [Google Scholar]
- 33.Muti P, Bradlow HL, Micheli A, Krogh V, Freudenheim JL, Schunemann HJ, et al. Estrogen metabolism and risk of breast cancer: a prospective study of the 2:16alpha-hydroxyestrone ratio in premenopausal and postmenopausal women. Epidemiology. 2000;11:635–40. doi: 10.1097/00001648-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Faupel-Badger JM, Fuhrman BJ, Xu X, Falk RT, Keefer LK, Veenstra TD, et al. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Current drug metabolism. 2002;3:321–49. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- 36.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen Metabolism and Risk of Breast Cancer in Postmenopausal Women. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelemen LE, Sellers TA, Vachon CM. Can genes for mammographic density inform cancer aetiology? Nat Rev Cancer. 2008;8:812–23. doi: 10.1038/nrc2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindstrom S, Vachon CM, Li J, Varghese J, Thompson D, Warren R, et al. Common variants in ZNF365 are associated with both mammographic density and breast cancer risk. Nature genetics. 2011;43:185–7. doi: 10.1038/ng.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vachon CM, Scott CG, Fasching PA, Hall P, Tamimi RM, Li J, et al. Common Breast Cancer Susceptibility Variants in LSP1 and RAD51L1 Are Associated with Mammographic Density Measures that Predict Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shepherd JA, Kerlikowske K, Ma L, Duewer F, Fan B, Wang J, et al. Volume of mammographic density and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1473–82. doi: 10.1158/1055-9965.EPI-10-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102:1224–37. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan RM, Crespo CJ, Wactawski-Wende J. Health behaviors and other characteristics of women on hormone therapy: results from the Third National Health and Nutrition Examination Survey, 1988-1994. Menopause. 2004;11:536–42. doi: 10.1097/01.gme.0000119982.77837.c4. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler RG, Rossi SC, Fears TR, Bradlow HL, Adlercreutz H, Sepkovic D, et al. Quantifying estrogen metabolism: an evaluation of the reproducibility and validity of enzyme immunoassays for 2-hydroxyestrone and 16alpha-hydroxyestrone in urine. Environmental health perspectives. 1997;105(Suppl 3):607–14. doi: 10.1289/ehp.97105s3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.