Summary
Purpose
Early surgical intervention can be advantageous in the treatment of refractory temporal lobe epilepsy (TLE). The success of TLE surgery relies on accurate lateralization of the seizure onset. The purpose of this study was to determine whether resting functional MRI (fMRI) connectivity mapping of the hippocampus has the potential to complement conventional presurgical evaluations in distinguishing left from right TLE. In addition, we sought to determine whether this same network might separate patients with favorable from unfavorable postoperative outcomes.
Methods
Resting fMRI acquisitions were performed on 21 TLE patients and 15 healthy control subjects. The patients included 7 left TLE and 7 right TLE patients with seizure free post-operative outcome, and 5 left TLE and 2 right TLE patients with recurring seizures after surgery. Functional connectivity maps to each hippocampus were determined for each subject and were compared between the controls and the seizure free left and right TLE patients. The one network identified was then quantified in the TLE patients with recurring seizures.
Key Findings
The resting functional connectivity between the right hippocampus and the ventral lateral nucleus of the right thalamus was the most statistically significant network to distinguish between seizure free left and right TLE patients with high sensitivity and specificity. This connectivity was also significantly greater in the seizure free left TLE patients than the healthy controls. Finally, six of the seven patients where seizures recurred after surgery had connectivity values in this network unlike those who were seizure free.
Significance
This study identified a region in the ventral lateral nucleus of the right thalamus whose connectivity to the hippocampi separates left from right TLE subjects. This suggests that the quantification of resting state functional MRI connectivity across this network may be a potential indicator of lateralization of TLE that may be added to other presurgical MRI assessments. Further validation in a larger, independent cohort is required.
Keywords: temporal lobe epilepsy, brain, functional MRI, connectivity, hippocampus, thalamus
Introduction
Early surgical intervention can be advantageous in the treatment of refractory temporal lobe epilepsy (TLE) (Wiebe, et al. 2001). Uncontrolled seizures themselves result in an array of psychological consequences including isolation, anxiety and depression (de Boer, et al. 2008). In addition, chronic TLE is associated with increased cognitive decline and progressive memory impairment (Helmstaedter, et al. 2003). The success of TLE surgery relies on accurate lateralization of the seizure onset. Conventional presurgical evaluations (Duncan 2010, Jobst 2009, McKhann, et al. 2002, Siegel 2004) can be effective for this purpose. When hippocampal sclerosis is present on Magnetic Resonance Imaging (MRI), there have been reports ranging from 66% to 87% (Janszky, et al. 2005, Ozkara, et al. 2008, Ramos, et al. 2009) of patients being seizure free with or without auras at 3 years post-surgery. In patients without MRI lesions, seizure free rates drop to approximately 45% (Bien, et al. 2009) to 60% (Bell, et al. 2009).
Functional MRI (fMRI) can provide complementary information to aid in the lateralization of TLE seizure onset. This MRI method creates images that are sensitive to blood oxygenation level (Logothetis, et al. 2001, Ogawa, et al. 1990). Blood oxygenation increases in brain regions employed or “activated” during a specific task, and so these regions can be localized non-invasively when the task is performed during fMRI image acquisition. Jokeit et al. prompted TLE patients to envision a walk through their hometown including specific landmarks along the route (Jokeit, et al. 2001). Asymmetric activation in the mesial temporal regions induced by this long-term memory retrieval task was quantified and compared between left and right TLE patients. The results showed increased activation contralateral to the seizure onset in 90% of patients.
In addition to localizing regions of activation, synchronous low frequency fluctuations in blood oxygenation measured by fMRI can be used to identify brain networks (Biswal, et al. 1995, Rogers, et al. 2007). Correlation of the fMRI signal across brain regions is referred to as functional connectivity. Measured during the resting state in subjects awake with eyes closed, increases in functional connectivity across regions of the mesial temporal lobe contralateral to seizure onset, and decreases ipsilateral to the seizure onset were detected (Bettus, et al. 2010, Bettus, et al. 2009). These fMRI studies suggest that functional connectivity is altered with progressive seizures; and functional connectivity mapping may potentially identify these changes in order to discriminate between left and right TLE patients.
The primary objective of this study was to identify resting fMRI connectivity differences between left TLE, right TLE and control subjects, specifically of networks involving the hippocampus across the whole brain. We hypothesized that there are distinct differences between left and right TLE hippocampal networks that can potentially lateralize TLE. A homogenous cohort of patients with confirmed lateralization who became seizure free after mesial temporal resection was studied. The secondary objective of this study was to investigate the identified networks in a small cohort of TLE patients that had recurring seizures after mesial temporal surgery. While our sample size does not allow statistical evaluation of this small group, we hoped to gain insight as to whether the identified networks could potentially distinguish between patients who would become seizure free and those that would not. Note that we did not combine the seizure free patients and those with recurring seizures in the first analysis, because our assumption is that those with recurring seizures may not have the same pathology or connectivity patterns as those that became seizure free, even if other presurgical evaluations are unable to distinguish them.
Methods
Subjects
From a population of 57 TLE patients recruited from the Vanderbilt University Epilepsy Program for imaging, 21 patients were included in this study. All patients underwent presurgical inpatient video-EEG monitoring for localization of the epileptogenic zone, high resolution structural MRI, fluorodeoxyglucose positron emission tomography (FDG-PET), neuropsychological testing, and in select patients, Wada test. Inclusion criteria for this study were: 1) temporal lobe epilepsy determined by presurgical evaluation, 2) no foreign tissue lesions, and 3) underwent temporal resection as epilepsy surgery. Seven subjects with left TLE (5F/2M, 1 left handed, 38.2 ± 9.8 years) and seven subjects with right TLE (5F/2M, 1 left handed, 36.8 ± 11.6 years) became seizure free after surgery. These subjects formed the left and right TLE patients with seizure freedom groups (“sz free”). Five left TLE patients and two right TLE patients experienced post-surgical seizures. These subjects formed the left and right TLE seizure recurring groups (“sz recur”). See Table 1 for all patient characteristics. Finally, we also recruited 15 right-handed healthy control subjects for comparison (12F/3M, 31.3 ± 10.8 years). There is no statistically significant difference in age between the right TLE (n=9), left TLE (n=12), and control (n=15) groups. Similarly, there was no statistical difference in duration of disease between the right TLE and left TLE groups.
Table 1.
Patient Characteristics
| ID | Gender/Handedness | Age (yrs) | Age of onset (yrs) | Seizure types | Ictal EEG | MRI-hippocampus + temporal lobe | PET hypometabolism | Surgery | Time since surgery (months) |
|---|---|---|---|---|---|---|---|---|---|
| Right TLE sz free | |||||||||
| 16 | M/L | 53 | 28 | SPS, CPS, SGTC | R TEMP | Normal | R MT | R Sel AH | 16 |
| 25 | M/R | 33 | 7 | SPS, CPS | R TEMP | R MTS | R MT | R Sel AH | 24 |
| 37 | F/R | 46 | 38 | SPS, CPS, SGTC | R >> L TEMP | Normal | Normal | R Sel AH | 16 (had only 3 CPS with AED withdrawal) |
| 49 | F/R | 29 | 23 | CPS, SGTC | R TEMP | R MTS | R MT | R Sel AH | 17 |
| 51 | F/R | 43 | 23 | SPS, CPS, SGTC | R TEMP | R MTS | R MT | R Sel AH | 13 |
| 58 | F/R | 36 | 0.6 | CPS, SGTC | R TEMP | R MTS | R MT | R Sel AH | 14 |
| 10 | F/R | 18 | 15.5 | SPS, CPS | R TEMP | Normal | R MT | R St TL | 20 |
| Left TLE sz free | |||||||||
| 3 | F/R | 35 | 18 | SPS, CPS, SGTC | L TEMP | L MTS + lateral encephalomalacia | L MT | L St TL after subdural grids | 25 |
| 13 | F/R | 49 | 27 | SPS, CPS, SGTC | L TEMP | Normal | Normal | L Sel AH after subdural strips | 28 (had two seizures with AED withdrawal) |
| 17 | F/R | 31 | 26 | SPS, CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 16 |
| 21 | F/L | 47 | 1.4 | SPS, CPS, SGTC | L TEMP | Normal | L MT | L St TL | 17 |
| 34 | F/R | 46 | 5 | SPS, CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 15 |
| 42 | M/R | 38 | 7 | SPS, SGTC | L TEMP | L MTS + Severe global left temporal lobe atrophy | Global left temporal lobe | L Sel AH after subdural grids | 10 |
| 46 | M/R | 22 | 16 | CPS, SGTC | L TEMP | Normal | L MT | L St TL after subdural grids | 14 |
| Right TLE sz recur | |||||||||
| 52 | M/R | 33 | 25 | CPS, SGTC | R TEMP | R MTS, encephalomalacia in the R insula | R MT | R St TL | 11 |
| 50 | M/L | 44 | 14 | SPS, CPS, SGTC | R TEMP | R MTS | R MT | R St TL after subdural grids | 14 |
| Left TLE sz recur | |||||||||
| 11 | F/R | 22 | 0.5 | SPS, CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 31 |
| 30 | M/R | 44 | 1.5 | SPS, CPS, SGTC | L TEMP | bilateral MTS, L>R | L MT | L Sel AH | 12 |
| 53 | F/R | 19 | 1.4 | SPS, CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 12 |
| 2 | M/R | 23 | 17 | CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 31 |
| 14 | M/R | 46 | 43 | SPS, CPS, SGTC | L TEMP | L MTS | L MT | L Sel AH | 28 |
F = female, M = male, sz = seizure, SPS = simple partial seizures, CPS = complex partial seizures, SGTC= secondary generalized tonic clonic seizures, R = right, L = left, TEMP = temporal, MTS = mesial temporal sclerosis, MT = mesial temporal lobe hypometabolism on PET; Sel AH-selective amygdalohippocampectomy; St TL- standard temporal lobectomy.
Imaging
All MRI imaging was performed using a Philips Achieva 3T MRI scanner (Philips Healthcare, Inc., Best, Netherlands) using an 8-channel head coil. Informed consent was obtained prior to scanning per Institutional Review Board guidelines. The images analyzed for this study were part of a more comprehensive protocol and included the following scans: 1) Three-dimensional, T1-weighted high-resolution image series across the whole brain for inter-subject normalization (1 × 1 × 1 mm3), 2) Two-dimensional, T1-weighted high-resolution axial full brain image series in the same slice locations as the fMRI scan for functional to 3D data registration (1 × 1 × 5 mm3), 3) fMRI Blood Oxygenation Level Dependent (BOLD) image series at rest with eyes closed – 64x64, FOV = 240 mm, 30 axial slices, TE = 35 ms, TR = 2 sec, slice thickness = 4.5 mm/ 0.5 mm gap, 300 volumes. The controls underwent the same protocol with 200 volumes for the fMRI scan. For this study, 200 volumes were analyzed for each subject.
Hippocampal Volumes
While we attempted to maximize homogeneity across the groups by including only those patients with the similar surgical treatments and outcomes, there remains variability in the volumes of the hippocampus within the groups. Of the “sz free” patients, three with left TLE and three with right TLE have hippocampi and temporal lobes determined as normal by MRI (see Table 1). The small number of patients prohibited those with hippocampal sclerosis from being separated into unique groups. We, therefore, needed to quantify these volumes and determine the effect of volume on the functional connectivity.
The estimation of the gray matter volume of the left and right hippocampus of an individual was determined in the following way using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). First, the high spatial resolution T1-weighted MRI scan (scan 1 above) was spatially normalized to the Montreal Neurological Institute (MNI) template. Second, this image set was segmented into gray matter, white matter and cerebrospinal fluid. Next, hippocampal masks were created on the template in the left (LH, 7360 mm3) and right hippocampus (RH, 7616 mm3) individually using WFU Pickatlas (Maldjian, et al. 2004, Maldjian, et al. 2003). Finally, the hippocampal masks were applied to the individual's segmented gray matter image to obtain the gray matter volume of each hippocampus in cm3. To normalize these volumes across patients, the right hippocampus volume was divided by the left hippocampus volume to determine a hippocampal volume fraction. To validate this procedure, the normalize volume fractions were compared between all right TLE, left TLE and controls with a one-way ANOVA with post-hoc, pair-wise Scheffe tests using SPSS v20 (IBM Corp., Somer, NY,USA). The expectation was that the right TLE patients should have lower values than the left TLE patients, indicating smaller right hippocampal volumes on average. However, those without hippocampal sclerosis may have values closer to 1.0 (equal volumes) as in the controls.
Whole Brain Hippocampal Functional Connectivity
The fMRI image analyses were performed using SPM8 software. The fMRI images were corrected for slice timing effects and motion and spatially normalized to the MNI template using co-registration to the three-dimensional and two-dimensional T1-weighted structural image sets as intermediate steps. The images were then spatially smoothed with a 7 × 7 × 7 mm FWHM kernel. This resulted in functional image series of 46 × 55 × 46 voxels (4 × 4 × 4 mm3).
Seed regions of interest for all subjects were created in the left (LH) and right (RH) hippocampus using the hippocampal masks described above from the WFU Pickatlas (Maldjian, et al. 2004, Maldjian, et al. 2003). The fMRI images from each subject were then processed as follows to set up the general linear model to create functional connectivity maps to each of the two seed regions. The time series from the LH from each subject's fMRI dataset were high pass filtered at 0.001 Hz to remove low frequency drifts and then averaged to create a seed time series. The signal averaged from all the voxels in the gray matter (GM) and in the cerebrospinal fluid (CSF) in the brain was also determined. These two signals were considered estimates of physiological noise in the fMRI images (Weissenbacher, et al. 2009). The GM signal, the CSF signal, as well as the time series from the six motion parameters determined by SPM8, were linearly regressed from the seed time series. The seed time series was then low pass filtered at 0.01 Hz (Cordes, et al. 2001). The fMRI images for all subjects were also low pass filtered at 0.01 Hz. Finally, a general linear model (GLM) (Friston, et al. 1995) was created using the processed LH seed time series as the regressor and the GM and CSF signal and motion time series as confounds. By performing the voxel-wise GLM on the low pass filtered fMRI images, a functional connectivity map to the seed region was created. The process was repeated using the RH seed region in each patient and control subject.
To identify any regions of significant difference in functional connectivity to the LH between left TLE “sz free” subjects (n=7), right TLE “sz free” subjects (n=7) and controls subjects (n=15), an ANOVA test was performed in SPM8 using the LH connectivity maps determined above for each of the subjects in these three groups. The same process was then repeated with the RH connectivity maps to determine regions of significant difference of RH connectivity between the groups. In order to verify that any regions detected using the ANOVAs are not related to the variations in hippocampal volume fraction, we repeated each of the ANOVAs with the hippocampal volume fractions as a covariate. Finally, the hippocampal connectivity of regions of significance determined in the above ANOVA analyses were linearly correlated with duration of disease, maximum translational motion during the acquisition and hippocampal volume fraction using SPSS to determine whether any of these parameters are potential other confounds in the analysis.
Results
All subjects had translational motion less than one voxel length throughout the fMRI scanning and were included in the analyses. The right TLE group (1.55 ± 1.00 mm) had significantly higher motion than the left TLE group (0.76 ± 0.39 mm), which had higher motion than the controls (0.32 ± 0.16 mm). However, this motion was not linearly correlated with the functional connectivity in the network described below.
The calculated hippocampal volume fraction was found to be significantly different across the three groups via ANOVA (right TLE = 0.958 ± 0.131, left TLE = 1.137 ± 0.175, controls = 0.975 ± 0.045; p=0.002). Post-hoc Scheffe tests showed left TLE patients had significantly higher volume fraction than right TLE patients (p=0.010) and controls (p=0.008). These values were not linearly correlated with the functional connectivity in the network described below. Similarly, the duration of disease was not linearly correlated with the functional connectivity of the identified network.
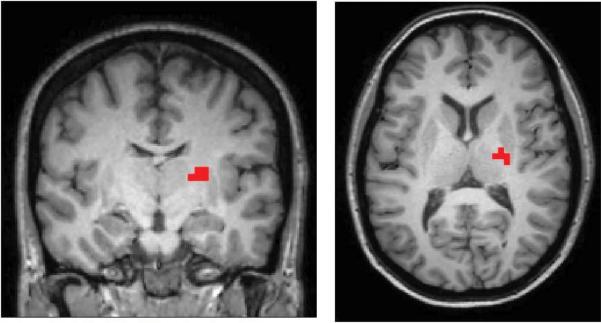
The ANOVA (F-Test) in SPM8 testing for group differences showed two regions that met significance at p<0.0001 (uncorrected) cluster size 5 in the RH connectivity maps. One region included part of the right thalamus with separation between the right TLE and the left TLE patients (MNI coordinate 22, -14, 12 mm, F = 19.41, volume = 576 mm3, RTHAL) (Figure 1). A second region in the white matter adjacent to the right precuneus indicated most difference between the right TLE patients and the controls (MNI coordinate 26, -54, 20 mm, F = 16.30, volume = 576 mm3). We focused our interest on the RTHAL region because it had the highest statistical significance from the ANOVA and showed greater separation between the two patient groups. No regions of connectivity to the LH were significantly different between the three groups (left TLE “sz free”, right TLE “sz free”, and controls) at p<0.0001 (uncorrected) cluster size 5. The ANOVAs performed using the hippocampal volume fraction as a covariate showed the same peak regions of activation as those without the covariate.
Figure 1.
Region whose connectivity to the RH was significantly different between the three groups – right TLE “sz free” (n=7), left TLE “sz free” (n=7), and controls (n=15) by ANOVA (p<0.0001 uncorrected, cluster size 5). (MNI coordinate 22, -14, 12 mm, F = 19.41 volume = 576 mm3, RTHAL)
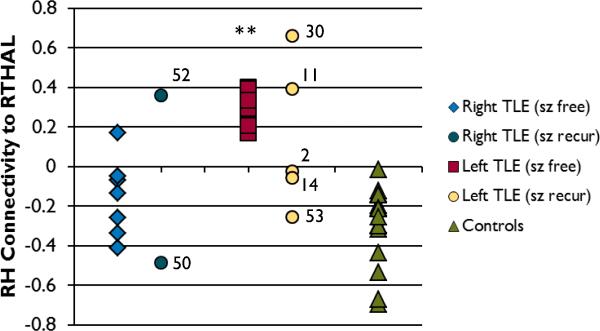
The mean connectivity value between the RH and the RTHAL cluster in each subject is shown in Figure 2 (right TLE = -0.155 ± 0.197, left TLE = 2.910 ± 0.091, controls = -0.302 ± 0.200). Unpaired T-Tests between the “sz free” groups and the controls showed that the left TLE “sz free” patients had higher connectivity between the RH and RTHAL than both right TLE “sz free” (p < 0.0001) and controls (p < 0.0001). Next, the sensitivity [true positive/(true positive + false negative)] and specificity [true negative/(true negative + false positive)] was calculated for a cutoff value to correctly categorize the right TLE “sz free” patients from the left TLE “sz free” patients based on the connectivity from the RH to the RTHAL. Using a cutoff value of 0.0, the sensitivity of correctly categorizing the right TLE less than the cutoff is 1.0, while the specificity is 0.875. Using a cutoff value of 0.170, the sensitivity and specificity is 1.0.
Figure 2.
The RH connectivity to RTHAL in each subject. The right TLE “sz free”, left TLE “sz free” and controls were used in the ANOVA to identify the RTHAL region of interest. The other subjects are shown for comparison. The asterisks indicates the left TLE “sz free” group has significantly higher connectivity to the RTHAL region than the right TLE “sz free” group and the controls (unpaired T-Test p<0.0001). The number adjacent to the “sz recur” data indicates the subject ID in Table 1.
No statistics were performed on separate “sz recur” groups due to low numbers of patients. However, it is clear in Figure 2 that the range of connectivity values in the “sz recur” patients is much larger than in the “sz free” patients in both the right and left TLE groups. Furthermore, the connectivity values of the “sz recur” patients fall outside the range of the corresponding “sz free” patients in all but one case.
Discussion
This study identifies a resting state functional network in TLE patients that distinguishes between those patients with confirmed left hippocampal seizure onset and those with right hippocampal seizure onset with high sensitivity and specificity. The network involves the right hippocampus and a small region in the right thalamus. The primary advantage of this fMRI method is that it is measured non-invasively at rest, without the confounding factor of task performance. In addition, in this case only 400 sec of fMRI imaging was required, which may be practical to include in a clinical MRI examination.
The best sensitivity and specificity in categorizing the right TLE and left TLE patients was found when using a cutoff of 0.17 for connectivity between RH and RTHAL. We found that the right TLE subjects have functional connectivity to the RH less than this, and the left TLE subjects have connectivity that is greater (see Figure 2). The controls had significantly lower values of RH to RTHAL connectivity than the left TLE group, but were similar to the right TLE patients.
In order to verify that the differences in functional connectivity between the right hippocampus and the right thalamus were not due primarily to difference in hippocampal volume between the patients, we repeated the ANOVA analyses including the hippocampal volume fraction as a covariate. The results of these analyses (one from right hippocampus and one from left hippocampus) revealed the same peak voxels of significant connectivity difference as in the analyses without the volume fraction covariate. This suggests that the network between the RTHAL and right hippocampus is different between groups, regardless of differences in hippocampal volume fractions. In addition we validated our measures of hippocampal volume fraction by comparing the values to our expectations, i.e. that the right TLE will have mean values less than 1.0, the left TLE will have mean values greater than 1.0 and the controls will have values close to 1.0.
Prior studies have reported increased functional connectivity in spatially limited networks involving the hippocampus contralateral to the seizure focus and decreased functional connectivity ipsilateral to the seizure focus (Bettus, et al. 2010, Bettus, et al. 2009). Our findings partially support these studies in that we detected increased functional connectivity contralateral to the seizure focus (high functional connectivity between right hippocampus and right thalamus in left TLE) and decreased or the same as controls in ipsilateral to the seizure focus (low functional connectivity between right hippocampus and right thalamus in right TLE). However, we were unable to identify a region in the left thalamus that would separate the two patient groups, even at reduced statistical levels, to fully investigate this hypothesis.
Our secondary aim was to investigate the same network in those patients with seizures recurring after surgery. We found that those patients had functional connectivity from the RH to RTHAL region that was outside the range defined by the seizure free patients in 6 of 7 cases. This may suggest that this measure might distinguish between seizure free and seizure recur subjects, however, the functional connectivity of the seizure recur patients was both higher and lower than the seizure free patients. These findings may indicate heterogeneous reasons for seizure recurrence.
While not part of specific hypothesis testing, the RH and LH ANOVAs were performed including all right TLE (“sz free” and “sz recur”) vs. all left TLE (“sz free” and “sz recur”) vs. controls. As in the previous ANOVA with only “sz free” subjects, no regions were detected using the LH seed. Using the RH seed three regions were detected at the p<0.0001 (uncorrected) with cluster size 5 level, but none which distinguished left from right TLE (only TLE vs. control) including the region near the right precuneus found in the ANOVA of “sz free” patients only.
Role of Thalamus in TLE
There is a large body of evidence supporting the role of the thalamus in TLE, specifically in the secondary generalization of seizures (Englot, et al. 2010, Norden & Blumenfeld 2002, Yu & Blumenfeld 2009). Studies using intracerebral electrical recordings found synchronous electrical activity in the thalamus and temporal lobes during temporal lobe seizures (Arthuis, et al. 2009, Guye, et al. 2006); and that the degree of synchrony was related to loss of consciousness. Low coupling between these regions was also related to better surgical outcome (Guye, et al. 2006). Using single photon emission computed tomography (SPECT) during secondary generalization in a combined group of left and right sided onset tonic-clonic seizures, increases in cerebral blood flow were detected primarily in the left thalamus, bilateral superior cerebellum and left caudate (Blumenfeld, et al. 2009). In a comparison between left TLE patients and healthy controls, differences in fMRI functional connectivity between the left anterior hippocampus and right thalamus were reported (Morgan, et al. 2010). Recent work in brain stimulation therapy for the treatment of refractory epilepsy has found that bilateral stimulation of the anterior thalamus can significantly reduce seizures, with most effectiveness in those patients with temporal lobe seizure onset (Fisher, et al. 2010).
Structural MRI imaging has also confirmed the involvement of the thalamus in TLE seizures. Studies quantifying the changes in gray matter volume between TLE and healthy controls using voxel-based morphometry methods have found reductions in bilateral thalamus (Labate, et al. 2008) and the thalamus ipsilateral to the seizure onset (McMillan, et al. 2004, Mueller, et al. 2010). Furthermore, volume loss in bilateral thalamic regions was positively correlated with the thickness of the entorhinal cortex in these patients (Mueller, et al. 2010).
Negative Functional Connectivity in Right TLE
The mechanism underlying the negative vs. positive functional connectivity we detected across this network is not clear. Mathematically, an increased negative connectivity value represents an increased negative linear correlation between the two time series, which is generally caused by phase differences between them. This can arise physiologically through accumulated phase delay across long path lengths between two nodes in a network (Cabral, et al. 2011, Chen, et al. 2011). In neurophysiological terms, negative connectivity could be the result of inhibition of an excitatory neuron across two or more synapses.
In functional connectivity mapping, negative functional connectivity can be the result of linearly regressing the global signal (average signal time series across the whole brain) from the seed region (Murphy, et al. 2008, Weissenbacher, et al. 2009). This regression shifts the set of connectivities across the brain towards a mean of zero. This can cause low connectivity values close to zero to be shifted to negative values. In this study, the global signal was not linearly regressed from the data, instead the average white matter and cerebrospinal fluid signals were used to approximate fluctuations due to physiological noise (Weissenbacher, et al. 2009). However, these signals may be similar to the global signal and may be partially responsible for the negative values.
Conclusions
This study identified a region in the ventral lateral nucleus of the right thalamus whose connectivity to the hippocampi separates left TLE “sz free” subjects from right TLE “sz free” subjects with high specificity and sensitivity. The results from a small group of patients where seizures recurred after surgery had connectivity values in this network unlike those who were seizure free. Therefore, the resting state functional MRI connectivity across this network may be a potential indicator of lateralization of TLE. If validated in a larger independent cohort, this measure may be added to other presurgical assessments of these patients.
Acknowledgements
This work was supported in part by NIH R01 NS055822 and UL1 RR024975-01.
Footnotes
Disclosure
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
References
- Arthuis M, Valton L, Regis J, Chauvel P, Wendling F, Naccache L, Bernard C, Bartolomei F. Impaired consciousness during temporal lobe seizures is related to increased long-distance corticalsubcortical synchronization. Brain. 2009;132:2091–2101. doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- Bell ML, Rao S, So EL, Trenerry M, Kazemi N, Stead SM, Cascino G, Marsh R, Meyer FB, Watson RE, Giannini C, Worrell GA. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50:2053–2060. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry. 2010;81:1147–1154. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and Surgical Outcomes of Patients With Refractory Magnetic Resonance Imaging-Negative Epilepsies. Arch Neurol. 2009;66:1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J, Hugues E, Sporns O, Deco G. Role of local network oscillations in resting-state functional connectivity. Neuroimage. 2011;57:130–139. doi: 10.1016/j.neuroimage.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen G, Xie C, Li S-J. Negative functional connectivity and its dependence on the shortest path length of positive network in the resting-state human brain. Brain Connect. 2011;1:195–206. doi: 10.1089/brain.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Ajnr. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- de Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12:540–546. doi: 10.1016/j.yebeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat. Rev. Neurol. 2010;6:537–550. doi: 10.1038/nrneurol.2010.131. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai XX, Marfeo A, Yu L, Gordon A, Purcaro MJ, Motelow JE, Agarwal R, Ellens DJ, Golomb JD, Shamy MCF, Zhang HP, Carlson C, Doyle W, Devinsky O, Vives K, Spencer DD, Spencer SS, Schevon C, Zaveri HP, Blumenfeld H. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 2010;133:3764–3777. doi: 10.1093/brain/awq316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Krishnamurthy KB, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human brain mapp. 1995;2:189–210. [Google Scholar]
- Guye M, Regis J, Tamura M, Wendling F, McGonigal A, Chauvel P, Bartolomei F. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, Ebner A. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2005;128:395–404. doi: 10.1093/brain/awh358. [DOI] [PubMed] [Google Scholar]
- Jobst BC. Treatment algorithms in refractory partial epilepsy. Epilepsia. 2009;50(Suppl 8):51–56. doi: 10.1111/j.1528-1167.2009.02236.x. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Okujava M, Woermann FG. Memory fMRI lateralizes temporal lobe epilepsy. Neurology. 2001;57:1786–1793. doi: 10.1212/wnl.57.10.1786. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Gambardella A, Aguglia U, Quattrone A. Hippocampal and thalamic atrophy in mild temporal lobe epilepsy: a VBM study. Neurology. 2008;71:1094–1101. doi: 10.1212/01.wnl.0000326898.05099.04. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Bourgeois BFD, Goodman RR. Epilepsy surgery: Indications, approaches, and results. Semin. Neurol. 2002;22:269–278. doi: 10.1055/s-2002-36653. [DOI] [PubMed] [Google Scholar]
- McMillan AB, Hermann BP, Johnson SC, Hansen RR, Seidenberg M, Meyerand ME. Voxel-based morphometry of unilateral temporal lobe epilepsy reveals abnormalities in cerebral white matter. Neuroimage. 2004;23:167–174. doi: 10.1016/j.neuroimage.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Morgan VL, Gore JC, Abou-Khalil B. Functional epileptic network in left mesial temporal lobe epilepsy detected using resting fMRI. Epilepsy res. 2010;88:168–178. doi: 10.1016/j.eplepsyres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Finlay D, Garcia P, Cardenas-Nicolson V, Weiner MW. Involvement of the thalamocortical network in TLE with and without mesiotemporal sclerosis. Epilepsia. 2010;51:1436–1445. doi: 10.1111/j.1528-1167.2009.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2008;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic-resonance image of rodent brain at high magnetic-fields. Magnet Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ozkara C, Uzan M, Benbir G, Yeni N, Oz B, Hanoglu L, Karaagac N, Ozyurt E. Surgical outcome of patients with mesial temporal lobe epilepsy related to hippocampal sclerosis. Epilepsia. 2008;49:696–699. doi: 10.1111/j.1528-1167.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- Ramos E, Benbadis S, Vale FL. Failure of temporal lobe resection for epilepsy in patients with mesial temporal sclerosis: results and treatment options Clinical article. J. Neurosurg. 2009;110:1127–1134. doi: 10.3171/2009.1.JNS08638. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC. Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AM. Presurgical evaluation and surgical treatment of medically refractory epilepsy. Neurosurg rev. 2004;27:1–18. doi: 10.1007/s10143-003-0305-6. discussion 19-21. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Yu L, Blumenfeld H. Theories of impaired consciousness in epilepsy. Ann N Y Acad Sci. 2009;1157:48–60. doi: 10.1111/j.1749-6632.2009.04472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]