Abstract
The majority of the human population becomes infected early in life by the gammaherpesvirus Epstein Barr Virus (EBV). Some findings suggest that there is an association between EBV infection and the appearance of pathogenic antibodies found in Lupus. Gammaherpesvirus 68 infection of adult mice (an EBV model) has been shown to induce polyclonal B cell activation and hypergammaglobulinemia, as well as increased production of autoantibodies. Here we explored the possibility that this breach of tolerance reflects loss of B cell anergy. Our findings show that although anergic B cells transiently acquire an activated phenotype early during infection, they do not become responsive to autoantigen as measured by the ability to mobilize Ca2+ following antigen receptor crosslinking or mount antibody responses following immunization. Indeed, naïve B cells also acquire an activated phenotype during acute infection, but are unable to mount antibody responses to either T-dependent or T-independent antigens. In acutely infected animals, antigen stimulation leads to upregulation of costimulatory molecules and relocalization of antigen-specific B cells to the B-T cell border, however, these cells do not proliferate or differentiate into antibody secreting cells. Adoptive transfer experiments show that the suppressed state is reversible and is dictated by the environment in the infected host. Finally, B cells in infected mice deficient of CD4+ T cells are not suppressed, suggesting a role for CD4+ T cells in enforcing unresponsiveness. Thus rather than promoting loss of tolerance, gammaherpesvirus 68 infection induces an immunosuppressed state, reminiscent of Compensatory Anti-inflammatory Response Syndrome (CARS).
Introduction
Autoimmunity is caused by destructive interplay between genetic predisposition and environmental factors. Among environmental factors that have been associated with the development of autoimmunity are bacterial and viral infections (1, 2). Infectious agents promote autoimmunity by various mechanisms, including molecular mimicry, wherein the response to pathogen-associated antigens crossreacts with self-antigens, as well as by activation of bystander lymphocytes. For example, recent studies have demonstrated a role for gut flora in promotion of rheumatoid arthritis via activation of Th17 cells (3).
Infection by a number of agents causes polyclonal B cell activation, often accompanied by an increase in total serum immunoglobulin (4–10). Depending on the pathogen, increases in serum autoantibody titers have also been reported (9–12). One such pathogen is the murine gammaherpesvirus 68 (γHV68)2 (9). A member of the gammaherpes viridea family of dsDNA viruses, γHV68 infects mice in the wild and is often used as a model for EBV infection (13). Infection is characterized by an acute/lytic phase lasting about 14 days during which a number of cell types become infected, including B cells and dendritic cells. The acute phase is followed by a life-long latent infection, primarily in B cells.
During the acute phase of γHV68 infection there is a several fold increase in B cells as well as CD4+ and CD8+ T cells in the spleen, with most displaying an activated phenotype (14). Serum IgG is 10-fold increased and remains elevated for an extended period, serum IgM is also elevated though to a lesser extent. Sangster et al. showed that γHV68-infected mice spontaneously produce IgG anti-DNA and IgG anti-collagen II, suggesting bystander activation of autoreactive lymphocytes (9).
At the outset of our studies we hypothesized that anergic B cells might be the source of the autoantibodies produced during γHV68 infection. Anergic B cells are autoreactive B cells that persist in the periphery in an antigen unresponsive state (15). They are characterized by a shortened life span and a biochemical signature of previous activation (elevated basal calcium and pErk), while being unresponsive to further stimulation through their BCR (as measured by a severely reduced calcium mobilization, phosphorylation of downstream signaling proteins and initiation of antibody responses). Many anergic B cells have a transitional 3 (T3) B cell phenotype, i.e. B220+ CD93+ CD23hi IgMlo, also referred to as anergic 1 (An1) (16, 17). Their unresponsive state is consequent to chronic B cell receptor occupancy by self-antigen and continuous signaling. Unresponsiveness is rapidly reversible upon dissociation of cognate self-antigen (18, 19). This reversibility sets anergy apart from the other extant mechanisms of tolerance, i.e. clonal deletion and receptor editing. Reversibility of unresponsiveness, coupled with persistence of anergic cells in the periphery where they may be exposed to inflammatory cytokines and innate immune stimuli, make anergic B cells likely participants in development of autoimmunity.
In this report we tested the hypothesis that the production of autoantibodies during γHV68 infection is due loss of unresponsiveness of anergic B cells in the infected hosts. While we found that anergic B cells transiently lose the anergy-associated cell surface phenotype, acquire an activated phenotype and spontaneously produce some antibody during γHV68 infection, we found no evidence that at any point they regain responsiveness to antigen. Interestingly, naive B cells in infected mice also transiently acquire an activated phenotype and, in addition, are inhibited in their ability to mount antibody responses. This unresponsiveness is reversible and depends on CD4+ T cells.
Thus rather than overtly promoting autoimmunity, γHV68 infection induces an immunosuppressed state reminiscent of Compensatory Anti-inflammatory Response Syndrome (CARS). CARS occurs after severe trauma and infections, resulting in inability to control secondary infections (20, 21).
Material and Methods
Mice
Except where otherwise indicated six to sixteen week old mice were used for all experiments. Ars/A1 (22), MD4 (23), MD4×ML5 (23), MyD88−/− (24), I-Ab−/− (B6.129S2-H2dlAb1-Ea/J) (25), T C Rα−/− (B6.129S2-Tcratm1Mom/J) (26), IFNγ−/− (B6.129S7-Ifngtm1Ts/J) (27) and IL10−/− (C57BL/6-IL-10(tm1Cgn) (28) mice have been described previously.
Bone marrow chimeric mice were created by reconstituting sublethally irradiated C57BL/6 mice (600 rad) with 2×106 Ars/A1 bone marrow cells. The mice were allowed to reconstitute for at least 6 weeks. Mice were housed and bred in the Biologic Resource Center at National Jewish Health, with the exception of C57BL/6 mice, which were purchased from Jackson Laboratories. All experiments with mice were performed in accordance with the regulations and with approval of National Jewish Health (Denver, CO) and Institutional Animal Care and Use Committee.
Viruses and infections
γHV68 clone WUMS (ATCC VR1465) (WT), M1-deficient γHV68 (γHV68-M1Delta511) (29) and M3-deficient γHV68 (γHV68-M3.stop) (30) were passaged, grown and titered as previously described (31). Unless mentioned differently, mice were infected with 106 PFU by i.p. injection in 0.5 ml of DMEM or with 104 PFU administered intranasally in 40 μl of DMEM or sham-infected with DMEM alone.
Antigens and immunization
To study T-independent antibody responses mice were immunized with 5μg LPS-NP0.6 or 25μg Ficoll-NP24 (Biosearch Technologies) in 200 μl PBS by i.p. injection. To study T cell dependent antibody responses mice were immunized with 100 μg OVA-Ars7 or 100 μg KLH-Ars14 in Freunds Incomplete Adjuvant (Sigma), 50 μg OVA-NP4.5 in 0.5mg alhydrogel (alum; Brenntag) or SRBC. SRBC were purchased from the Colorado Serum Company and stored in sterile Alsever's solution at 4 °C. The cells were washed three times in PBS before use and mice were injected i.p. with 200 μl of 1% SRBC suspension. OVA-Ars, KLH-Ars and OVA-NP were conjugated in-house.
For experiments with MD4 B cells HEL was chemically coupled to SRBC. One ml packed SRBC were resuspended in 15 ml PBS containing 5 mg/ml HEL (Sigma). To cross-link, 1ml of 50 mg/ml 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma) in PBS was added, mixed and incubated for 1 h at RT, with occasional mixing. Afterwards the cells were washed four times in PBS and the conjugation of HEL to the SRBC cell surface was confirmed by flow cytometry.
Hemagglutination assay
Serum was serially diluted in HBSS in V-bottomed microtiter plates in a 50μl volume. Twenty-five μl 1% SRBC suspension in HBSS and 25 μl of HBSS was added to each well and plates were incubated at 37°C for one hour. The hemagglutination titer was defined as the highest dilution where hemagglutination of SRBC was still detected.
Plaque forming cell (PFC) assay
A modified version (32) of the Jerne hemolytic plaque forming cell assay (33) was used. Briefly, agarose, SRBC, guinea pig serum (as source of C) and spleen cells were plated on a glass slide and incubated at 37°C for 3 h. Subsequently, hemolytic plaques were counted “blindly”.
Adoptive transfers
To study the effects of γHV68 infection on antigen specific B cells, B cells from Ars/A1 or MD4 mice were enriched by depletion of CD43+ cells with anti-CD43-conjugated magnetic beads (MACS anti-mouse CD43; Miltenyi Biotec). B cells were >97% pure by B220 staining followed by FACS analysis. 2–5×106 B cells in 200 μl PBS were adoptively transferred by i.v. injection. For some experiments B cells were labeled with CFSE (2.5μM for 3 min) prior to adoptive transfer.
To exclude that defects in T cell priming could account for the observed suppression, in some experiments we adoptively transferred purified 106 CD4 T cells (MACS anti-mouse CD4; Miltenyi Biotec), isolated from mice immunized with 0.005% SRBC 4 days earlier.
Flow cytometry
Cells were resuspended in PBS containing 1% FCS and 0.05% sodium azide and incubated with an optimal amount of biotinylated or directly labeled antibodies. Antibodies directed against the following molecules were used: B220 (RA3-6B2, BD), CD93 (493, Gift of A. Rolink), CD86 (GL1, BD), CD23 (B3B4, BD), CD69 (H1.2F3, BD), CD138 (281-2, BD), IgMb (AF6.78) and IgMa (RS3.1). RS3.1, AF6.78 and 493 were produced in our own laboratory and were biotinylated (Pierce) or directly conjugated to Alexa (Invitrogen) or DyLight (Pierce) fluorchromes, according to the manufacturer's protocol. Anti-CD93 was used biotinylated and was detected with SAFITC. For intracellular detection of anti-HEL splenocytes were first stained with B220-PerCP and CD138-PE, fixed and permeabilized with BD Cytofix/Cytoperm™ and stained with HEL-Dylight649 (conjugated in our laboratory). Events were collected on a FACScaliber or LSRII flow cytometer (BD) and analyzed using FlowJo software (Tree Star).
Analysis of viral loads
DNA was prepared from whole spleen tissue or isolated B cells (MACS anti-biotin; Miltenyi Biotec, after staining with anti-B220 biotin) isolated from mock-infected or γHV68-infected mice using the Qiagen DNeasy Blood and Tissue Kit (Valencia, CA). Genomic DNA concentrations were measured in triplicates using 260/280nm measurements (NanoDrop, Biolabs). Viral loads were measured using qPCR by detecting a 70 bp region of the γHV68 gB gene (34). 10 ng of extracted genomic DNA was added to reactions containing SYBR Green/Rox PCR master mix (SABiosciences), forward (5'GGCCCAAATTCAATTTGCCT3') and reverse primers (5'CCCTGGACAACTCCTCAAGC3') in a 25 μl total reaction volume. Each reaction was supplemented with 30 ng of carrier DNA (pBR322, Sigma). qPCR analysis was done on a 7300 Fast Real-Time PCR System with SDS Ver. 1.4 (Applied Biosystems, Foster City, CA) consisted of 40 cycles of 15 s at 90°C and 60 s at 60°C. Standard curves were generated using known amounts of plasmid containing the γHV68 gB gene, while total DNA input was kept constant at 40 ng per reaction using a plasmid (pBR322, Sigma). Cycle threshold values for experimental samples were converted to copy numbers of the gB gene. Copy numbers were standardized to the amount of input DNA and were expressed as copies of viral geneome per 100 ng DNA. Triplicate measurements were made for each sample.
Analysis of calcium mobilization
For measurements of free intracellular calcium concentration ([Ca2+]i), splenocytes from chimeric mice were first stained with anti-B220 and anti-IgMa or anti-B220 and anti-IgMb, washed and subsequently loaded with Indo-1 acetoxymethyl (Indo1-AM) (Molecular Probes) as described previously (18). The cells were resuspended at 107 cells/ml in warm IMDM with 2% FCS in a 500μl volume. After the baseline was established for 30 seconds, cells were stimulated with 5 μg rabbit anti-mouse IgM (H+L) F(ab')2 (Zymed) for 3.5 minutes. Mean [Ca2+]i was measured over time using an LSR II flow cytometer (BD) and analyzed using FlowJo software (Tree Star). To determine the calcium mobilization in Ars/A1 B cells, we gated on the B220+IgMb− population of cells stained against B220 and IgMb. To determine the calcium mobilization in C57BL/6 B cells, we gated on the B220+IgMa− population of cells stained against B220 and IgMa.
Enzyme linked immunosorbent assay
For detection of anti-chromatin IgG antibodies from serum of mice, 96-well microtitre plates (Costar, Corning, NY) were coated with calf chromatin (10 μg/mL) in PBS with 1mM EDTA, followed by incubation with blocking buffer solution (2 mg/mL bovine serum albumin (BSA), 1 mM EDTA, 0.05% Tween-20 in PBS). Mouse serum was serially diluted in PBS with 1mM EDTA and 0.1% Tween-20 and incubated in the 96 well plates overnight at 4°C. Between all steps the plates were washed 4 times with PBS-0.05% Tween-20. IgG anti-chromatin antibodies were detected with a HPR-conjugated goat anti-mouse IgG (γ chain-specific) antibody (Southern Biotechnology). The ELISA was developed with TMB single solution (Invitrogen) and the reaction was stopped with 1N H2PO4 (Sigma).
For detection of anti-p-Azophenylarsonate (Ars) IgM antibodies microtiter plates were coated with 10 μg/mL Ars-BSA in PBS. For detection of anti-NP IgM and IgG antibodies microtiter plates were coated with 10 μg/mL NP-BSA in PBS. For detection of anti-HEL IgM microtiter plates were coated with 10 μg/mL HEL (Sigma) in PBS. Serial dilutions of mouse serum in PBS 0.1% Tween-20 were added and incubated overnight at 4°C. Ars/A1-derived IgM anti-Ars antibodies and MD4-derived IgM anti-HEL antibodies were detected with biotinylated RS3.1 (anti-IgMa), followed by Streptavidin-HRP (Pierce). IgM and IgG were detected with rat anti-mouse IgM-HPR (Invitrogen) and goat anti-mouse IgG-HPR (Southern Biotechnologies), respectivly. Plates were developed as described above. The OD was determined at 450 nm using a VERSAMax plate reader (Molecular Devices) and the data were analyzed with Softmax software.
ANA Detection
Mouse sera were diluted to 1:100 in PBS and incubated with HEp-2 antigen substrate slides (BION) for 1 hr at RT. The slides were then washed in PBS, stained with anti-mouse IgG Alexa488 antibody (Molecular Probes) for 1 hr at room temperature, washed, and mounted in fluoromount G (Southern Biotech). The slides were analyzed using a Leica DMRXA microscope (Carl Zeiss) under a 10× objective and further analyzed using Slidebook software.
Immunohistochemistry
Spleens were stored in buffered formalin overnight, paraffin-embedded and cut in 6 μm sections. The sections were deparaffinized, rehydrated and subjected to antigen retrieval (25 min incubation in citrate buffer (10 mM citric acid, 0.05% Tween 20, pH 6.0) at 96°C). The slides were washed twice in PBS, blocked with 5% FCS in PBS and stained with anti-fluorescein rabbit IgG fraction (Molecular Probes, Invitrogen) (for detection of CFSE stained cells) followed by goat anti-rabbit IgG-Alexa488 (Molecular Probes, Invitrogen) and B220-PE (clone RA3-6B2, eBioscience). The slides were washed twice and mounted in fluoromount G (Southern Biotech). Images were obtained at room temperature using an inverted Zeiss 200M microscope (Carl Zeiss) under a 10× objective and further analyzed using Slidebook software.
Statistics
Statistical analyses were performed with the unpaired Student's t test. P-values <0.05 (*) were considered statistically significant.
Results
Anergic B cells transiently acquire an activated phenotype during γHV68 infection, but do not break tolerance
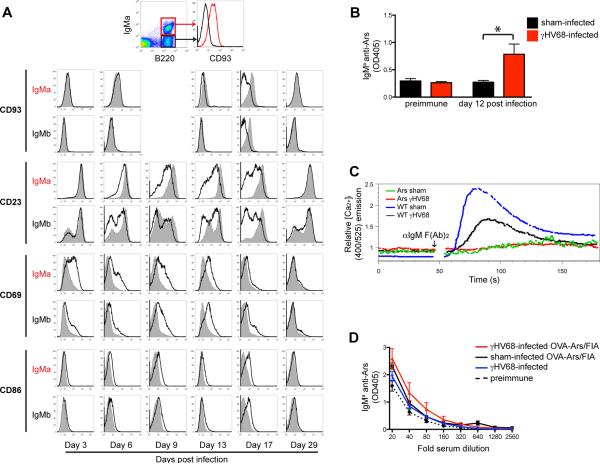
We began our studies by confirming the previous report that there is an increase in autoantibody production in wild type C57BL/6 following infection with γHV68 (Fig S1A) (9). Increased IgG anti-chromatin antibody was detected by ELISA at 14 and 28 days following infection, and this response also manifested as anti-nuclear antibody staining. To test whether this increase could be due to a disruption in B cell anergy we assessed the effect of infection in a transgenic model of B cell anergy, the Ars/A1 mouse (22). These mice express B cell receptors that have high affinity for the hapten arsonate, and low affinity for a crossreactive self-antigen, apparently ssDNA, that renders them anergic. We produced bone marrow chimeric mice containing a wild-type C57BL/6 B cell population (IgMb+) and an anergic Ars/A1 B cell population (IgMa+), and infected them with γHV68. Despite the fact that <1% of B cells were infected in normal mice (36, 37) and based on recovery of viral genomes anergic B cells are similar (Fig S2B), during the course of acute infection the majority of normal IgMb+ B cells acquired an activated phenotype detectable based on CD69 expression (Fig 1A). Interestingly, anergic B cells (IgMa+) also acquired an activated phenotype, upregulating CD69 and CD86, and down regulating CD23 and CD93. The transient loss of CD93 was particularly interesting as we and others have identified the CD93+ CD23hi IgMlo surface phenotype as characteristic of anergic murine B cells (16, 17). Activation marker expression was lost by 29 days following infection, as anergic cells regained their original phenotype. Thus infection does not lead to permanent loss of the anergic phenotype, even among cells whose autoantigen is an agonist for TLR7 and TLR9. In addition, during infection we detected an ~2 fold increase in Ars/A1 derived antibodies in the serum of chimeric mice (Fig 1B). We confirmed that during γHV68 infection in another model (MD4 × ML5), anergic B cells or their descendents also produce more Ig (Fig S1B).
Figure 1. Anergic B cells acquire an actived phenotype during γHV68 infection but do not break tolerance.
A–D) Ars/A1-C57BL/6 chimeric mice were infected with γHV68 or sham-infected (n=5/group). A) On the indicated days post infection the cell-surface phenotype was determined of the Ars/A1 B cells (B220+IgMa+) and the C57BL/6 B cells (B220+IgMa−). Data from infected mice is presented as thick lines, data from uninfected control mice as filled histograms, representative histograms are shown. B) Ars/A1 derived serum antibody (IgMa anti-Ars) at day 12 post infection (OD405 at 1:40 serum dilution). C) At day 13 post infection spleen cells from chimeric mice were stained with anti-IgMa/B220 or anti-IgMb/B220, loaded with indo-1 and stimulated with 5μg F(Ab)2 anti-IgM. Changes in intracellular calcium in Ars/A1 B cells was determined by gating on B220+IgMb− events, C57BL/6 (WT) by gating B220+IgMa− events. D) chimeric mice were immunized i.p. with 100μg OVA-Ars/FIA 3 days post infection, 13 days later serum was collected and the IgMa anti-Ars response was determined. Error bars show SEM, * p<0.05. All data is representative of at least 2 independent experiments.
Given these findings we wanted to determine whether infection affects responsiveness of anergic Ars/A1 B cells to antigen. We determined the ability of wild type and Ars/A1 B cells to mobilize calcium upon IgM crosslinking (Fig 1C). Ars/A1 B cells in infected mice did not regain the ability to mobilize calcium. Surprisingly, wild type B cells also displayed reduced calcium mobilization upon BCR stimulation. To determine if the Ars/A1 B cells were functionally anergic despite their activated phenotype, we immunized infected animals with Ars-OVA in Freund's incomplete adjuvant (FIA). Ars/A1 B cells bind Ars with high affinity, but under normal circumstances they are unable to mount an antibody response to arsonate-conjugate immunogens (22). In γHV68-infected mice, Ars/A1 B cells did not acquire responsiveness to immunization with Ars-OVA (Fig 1D). Thus, while during γHV68 infection anergic B cells transiently lose surface markers of anergy, acquire an activated phenotype and produce more immunoglobulin, they remain unresponsive to immunogen stimulation.
γHV68 infection causes a global suppression of antibody responses
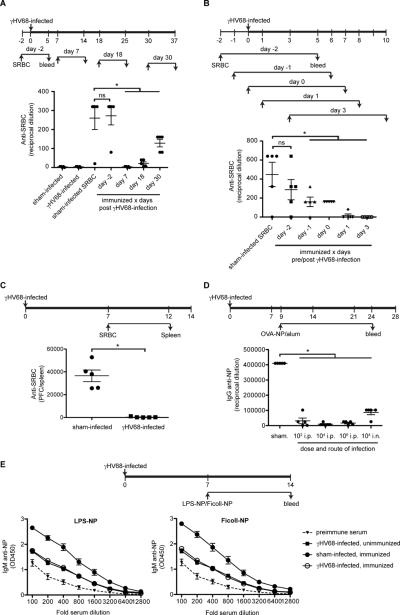
In control experiments we made the unexpected observation that there is general inhibition of antibody responses in γHV68 infected animals. This seems counterintuitive as γHV68-infected mice have elevated serum IgM and IgG levels. Intrigued, we investigated this further by immunizing mice at different time points following infection (Fig 2A). Wild type mice failed to mount detectable antibody responses to sheep red blood cells (SRBC) when immunized 7 days post γHV68 infection. When immunized at day 18 post infection subsequent anti-SRBC responses were still ~85% suppressed, but at later times the ability to mount antibody responses recovered. Next we determined the effect of infection on antibody responses when immunization occurs at the time of, prior to or immediately following infection. While antibody responses initiated 2 days prior to infection were equivalent to those of uninfected mice (Fig 2A and 2B), responses initiated one day prior to infection or on the day of infection were 65% inhibited (Fig 2B). Responses to immunization following infection were suppressed 95% when initiated at day 1 and undetectable at day 3-post infection (Fig 2B), though they varied slightly among experiments. The partial inhibition observed when animals were immunized at the time of infection could explain the observed delay and initially poor antibody response to γHV68 (14), assuming that the γHV68-specific B cells also encounter their antigen at the time of infection.
Figure 2. γHV68 infection causes a global suppression of antibody responses.
A–B) C57BL/6 mice were infected with γHV68 or sham-infected. At the indicated days the mice (n=5/ group) were immunized with SRBC or left unimmunized. Seven days later blood was collected and the IgM anti-SRBC response was determined by hemagglutination. C) C57BL/6 mice (n=5/ group) were infected with γHV68 or sham-infected and immunized with SRBC seven days later. Five days post immunization spleens were collected and the number of IgM anti-SRBC producing B cells was determined by plaque assay. D) C57BL/6 mice (n=5/ group) were infected with different doses and different routes as indicated or sham-infected. On day 9 post infection the mice were immunized with 50 μg OVA-NP/alum i.p. and 15 days later the serum IgG anti-NP response was determined by ELISA. E) C57BL/6 mice (n=5/ group) were infected with γHV68 or sham-infected. Seven days later mice were immunized with 25μg Ficoll-NP, 5μg LPS-NP or left unimmunized. Seven days later serum was collected and the IgM anti-NP response was determined by ELISA. Error bars show SEM, * p<0.05. Statistics were calculated with a student t-test, comparing the response of each individual group with that of the sham infected control group. All data is representative of at least 2 independent experiments.
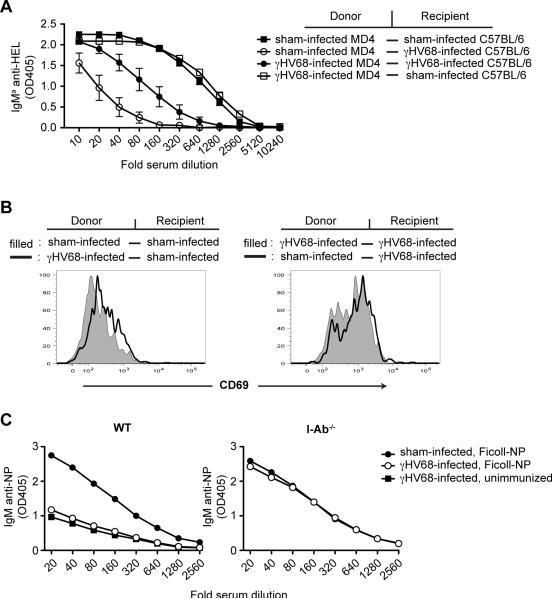
Suppression of antibody responses was also reflected in greatly reduced generation of antibody forming cells (Fig 2C). Using a different antigen, OVA-NP in alum, we tested the effect on antibody responses of infection with different doses of virus and a different route of infection (i.p. vs i.n.) (Fig 2D). Responses were suppressed regardless of route, and when as few as 100 virus PFU were injected. The immunogens employed in these experiments were T cell-dependent. To address the possibility that the observed suppression is due to failure to generate T cell help, we tested the ability of infected mice to respond to two different T cell independent antigens, Ficoll-NP and LPS-NP (Fig 2E). For both immunogens the anti-NP titers in infected mice did not rise above the ~2 fold increased titers induced by infection alone. To further address the possibility that the observed suppression is due to a defect in T cell priming when using T-dependent antigens, we adoptively transferred purified CD4+ T cells isolated from mice previously immunized with the same antigen. Transfer of primed T cells did not restore the ability of the infected recipients to initiate an antibody response upon immunization (Fig 4A). Collectively these data demonstrate that γHV68 infection causes a transient suppression of antibody responses and this is due to inhibition of the B cell response and not a secondary consequence of an inhibition of T helper cell responses.
Figure 4. Suppression of antibody responses in caused by the environment in the infected host.
A) MD4 and C57BL/6 mice were infected with γHV68 or sham infected. Six days later B cells from γHV68-infected or sham-infected MD4 mice were purified and adoptively transferred into γHV68-infected or sham-infected C57BL/6 mice by i.v. injection. All mice also received CD4+ T cells isolated from mice primed 4 days earlier with SRBC. At the time of transfer the mice were immunized with 100μl 10% SRBCHEL by i.p. injection. Seven days later serum was collected and the IgMa anti-HEL response was determined by ELISA (n=5/ group). B) Isolated B cells from mice infected 8 days earlier or from sham-infected mice were CFSE labeled and adoptively transferred into γHV68-infected or sham-infected C57BL/6 mice. Sixteen hours later spleens were collected and the CD69 expression was determined on B220+ CFSE+ cells (n=4/ group). Representative histograms are shown. C) C57BL/6 and I-Ab−/− mice (n=5/ group) were infected with γHV68 or sham infected. Seven days later the mice were immunized with Ficoll-NP or left unimmunized. After seven days blood was collected and the IgM anti-NP response was determined by ELISA. Error bars show SEM. All data is representative of at least 2 independent experiments.
While the early stages of antigen-specific B cell responses are unchanged in γHV68-infected mice, later stages such as proliferation and differentiation are inhibited
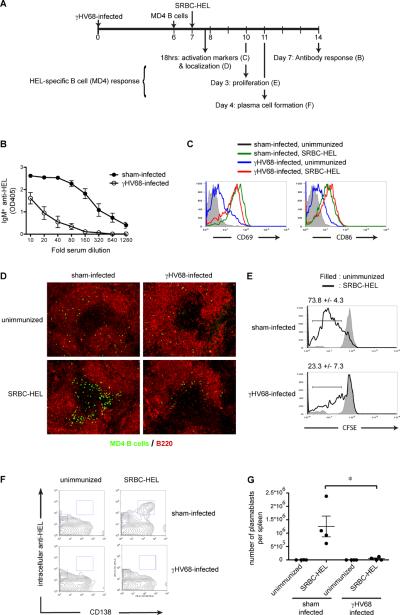
To gain more insight into the stage following infection at which B cell responses are inhibited, we used another B cell receptor transgenic model (MD4) in which the B cells are specific for hen egg lysozyme (11). Purified MD4 B cells were adoptively transferred into γHV68 or sham infected C57BL/6 mice at day 6 post infection. Twenty-four hours later the mice were immunized with HEL conjugated SRBC and HEL-specific B cell responses measures thereafter. As observed for in situ B cells in intact mice, purified MD4 B cells transferred into mice infected with γHV68 failed to mount an antibody response (Fig 3B). Transferring CFSE-labeled MD4 B cells allowed us to monitor upregulation of activation markers, cell localization and proliferation to determine if these stages were affected by the infection process. When adoptively transferred into γHV68 recipients, MD4 B cells upregulated CD69 and CD86 comparably to B cells in infected mice (Figs. 3C and 1A). In immunized mice, however, expression of CD69 and 86 on MD4 cells was increased at least ten fold higher regardless of infection (Fig 3C). Similarly, by 18 hrs post antigen stimulation the MD4 B cells migrated to the B-T cell boundary regardless of γHV68 infection (Fig 3D). Fewer cells are detectable in follicles of γHV68 infected mice, but this is likely due to the fact that infection caused a 3-fold increase lymphocyte numbers diluting the transferred cells. While the early stages of the B cell response appear to be intact despite infection, major differences were observed at later stages. Antigen-induced proliferation measured by dye dilution at 3 days post immunization was almost completely inhibited by infection (Fig 3E), as was plasma cell differentiation measured at 4 days (Fig 3F).
Figure 3. While early stages of antigen-specific B cell responses are intact during γHV68 infection, proliferation and differentiation are inhibited.
A) Experimental design. B–G) B cells isolated from MD4 mice (CFSE labeled in C–E) were adoptively transferred into γHV68 or sham-infected C57BL/6 mice on day 6 post infection. Twenty-four hours later the mice were immunized with 100μl 10% SRBC-HEL. B) Seven days later serum was collected and the IgMa anti-HEL response was determined by ELISA (n=5/ group). C) 18 hrs after immunization the cell surface expression of CD69 and CD86 on MD4 B cells transferred into sham-infected (black, unimmunized; green, immunized) or gHV68-infected mice (blue, unimmunized; red, immunized) (n=5/ group) was determined by gating on B220+ CFSE+ cells. Representative histograms are shown. D) 18 hrs after immunization spleens were collected, sectioned and stained for MD4 B cells (green) and B cells (red) (n=5/ group) Representative sections are shown. E) 72 hrs after immunization spleens were collected and CFSE dilution in MD4 B cells was determined in unimmunized mice (filled) and SRBC-HEL immunized mice (thick line) by gating on B220+IgMa+ cells. Representative histograms are shown. The average % ± SEM of cells which have undergone >1 round of division is show. The difference between the γHV68-infected and sham-infected group is statistically significant (p<0.05) F) 106 hrs after immunization spleens were collected and stained for B220, CD138 and intracellular anti-HEL. The displayed plots are gated on B220+ cells (n=4/ group). Representative plots are shown. G) The number of plasmablasts/spleen are plotted, calculated as % CD138+ intracellular HELhi cells (F) × total splenocytes. Error bars show SEM. All data is representative of at least 2 independent experiments.
These data indicate that despite a partial inhibition of BCR-mediated calcium responses, B cells are induced by antigen to upregulate CD69 and CD86, and move to the T/B boundary in infected animals, but they do not receive signals needed to drive proliferation and differentiation into plasma cells. Alternatively, they may be confused by collateral signals induced by infection.
Environmental factors induced by γHV68 infection cause a suppressed B cell state that is reversible
While we know that the suppressive state is rapidly induced (Fig 2B), we know little regarding how it is induced and maintained. To begin to address these questions we adoptively transferred MD4 B cells from infected or sham-infected mice into infected or sham-infected C57BL6 recipients. The recipients were immunized with SRBC-HEL at the time of the cell transfer, and 7 days later IgMa anti-HEL responses (derived from MD4 B cells) were measured (Fig 4A). As expected, MD4 B cells from infected mice that were transferred into infected recipients mounted greatly reduced responses compared to those of MD4 B cells from sham-infected mice transferred into sham-infected recipients. However, MD4 B cells from infected mice were perfectly able to respond to immunization after transfer into sham-infected mice. Conversely, B cells from non-infected mice became unresponsive to immunogen upon transfer to infected animals. In the experiment shown in Fig 4A, B cells from sham-infected animals transferred into infected mice mounted poorer responses than B cells from infected mice transferred into infected mice. This relationship varied among experiments, however the transferred B cells always adopted the phenotype of the B cells in the recipient. This phenomenon extends to expression of activation markers. Within 18 hrs of adoptive transfer to non-infected mice, B cells isolated from infected mice lost their activated phenotype and, conversely, B cells isolated from non-infected mice acquired an activated phenotype within 18 hours of transfer to infected mice (Fig 4B). These results indicate that the suppressive state of the B cells is rapidly reversible and dictated by factors in the environment (presence of virus and mediators produced in response to the virus infection) in which the B cells reside.
Previous work by others has demonstrated that some changes in B cells that occur during γHV68 infection (polyclonal B cell activation, expansion and elevated serum Ig) are dependent on CD4+ T cells (9, 38). To test whether the affects described here are also dependent on CD4+ T cells we analyzed responses in MHC class II deficient mice (I-Ab−/−), which lack CD4+ T cells. As expected, responses to Ficoll-NP, a classical TI type II antigen, were robust in I-Ab−/− mice (Fig 4C). These responses were not affected by γHV68 infection, suggesting involvement of CD4+ T cells in γHV68-induced suppression of antibody responses. Since infection of CD4+ T cell deficient mice leads to lower virus production (39) it was important to exclude the possibility that lack of suppression is merely a consequence of lower viral titer. We compared viral loads between wild type and I-Ab−/− mice, and found that while at day 7 post infection splenic viral load is lower in I-Ab−/− mice compared to wild type mice, viral titers in wild type mice during the first days of infection are lower or comparable to those in I-Ab−/− mice at day 7 post infection (Fig S2C). Since the antibody responses initiated in wild type mice at these early time points are completely suppressed (Fig 2B) while I-Ab−/− mice immunized at day 7 post infection are not suppressed (Fig 4C), lower viral loads per se are an unlikely explanation for the lack of suppression. Rather following infection CD4+ T cells help to establish the suppressive environment. A role for T cells was further confirmed by demonstration of lack of suppression in infected mice lacking T cells due to disruption of the TCR alpha genes (Fig S3A). While the manner by which CD4 T cells mediate their effects remains a subject of further study, preliminary experiments have excluded a role for IL10 (Fig S3B) and IFNγ (Fig S3C).
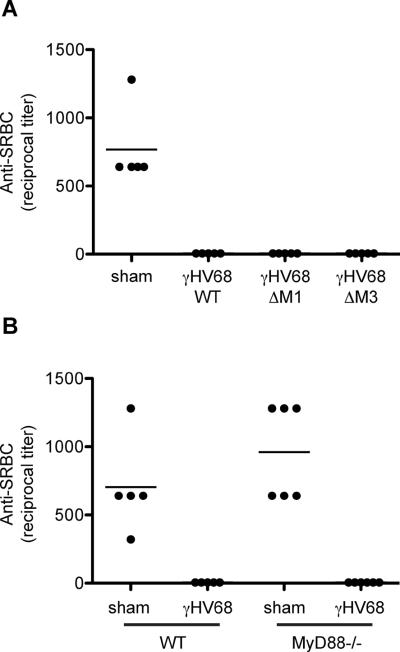
No role for MyD88 or the virally encoded M1 and M3 proteins in the suppression of antibody responses
The suppressive environment generated by infection could be a consequence of the host innate response to the infection or due to viral proteins produced during infection. Two virally encoded proteins in particular have been shown to have immune modulatory activity that could affect B cell immune responses. The γHV68-encoded M1 protein acts as a superantigen for a subset of CD8+ T cells (35) and affects their production of cytokines. The M3 protein is a broad-spectrum chemokine binding protein (40), which binds CXCL13 and many other chemokines that could affect B cell localization, and has been demonstrated to affect B cell localization (41). To test the role of these proteins in suppression we assessed the response to SRBC of mice infected with γHV68 lacking either M1 or M3. All mice infected with γHV68, sufficient or deficient in M1 or M3, displayed severely inhibited antibody responses (Fig 5A), indicating that these proteins play no essential role in suppression of the antibody response.
Figure 5. No role for MyD88 or the virally encoded M1 and M3 proteins during γHV68-induced suppression.
A) C57BL/6 mice we infected with wild type γHV68 or γHV68 deficient in M1 or M3 (n=5/group). Seven days later the mice were immunized with 200μl of 1% SRBC i.p. . After seven days blood was collected and the IgM anti-SRBC response was determined by hemagglutination. B) C57BL/6 and MyD88−/− mice were infected with γHV68 or sham infected (n=5/group). Seven days later the mice were immunized with 200μl of 1% SRBC i.p.. After seven days blood was collected and the IgM anti-SRBC response was determined by hemagglutination. All data is representative of at least 2 independent experiments.
To explore whether the host innate immune response to γHV68 participates in generation of the suppressive environment we assessed dependence of suppression on MyD88 expression. TLR signaling reportedly plays a role in the host response to γHV68 (42, 43) and a reduction in polyclonal B cell activation has been reported in infected MyD88−/− mice (44). However, as shown in figure 5B, we detected no difference in the ability of γHV68 infection to suppress antibody responses in MyD88−/− mice. Similarly, a preliminary experiment in mice deficient in STING, a.k.a. MPYS, MITA and TMEM173, a transducer of signals generated upon sensing of intracellular DNA (45–47), showed antibody responses to be >95% suppressed after infection. Collectively these results suggest no or only a minor role for signaling by TLRs or intracellular DNA sensing pathways in γHV68-induced suppression of antibody responses.
Discussion
Gammaherpesvirus 68 infection results in polyclonal B cell activation and hypergammaglobulinemia, including elevated autoantibody levels. The data presented in this paper confirm these findings, but suggest based on a model system of B cell anergy that this is not attributable to loss of B cell anergy. They further show that γHV68 infection causes mice to become transiently but profoundly unresponsive to immunization as measured by antigen receptor signaling, clonal expansion and differentiation to antibody secreting cells.
Increased occurrence of total serum immunoglobulins and autoantibodies seems inconsistent with concurrent unresponsiveness to immunization. The two most commonly proposed mechanisms by which autoreactive B cells break tolerance during infection are molecular mimicry and bystander activation. Diversity of the specificity of autoantibodies produced during acute γHV68 infection, which ranges from nuclear antigens to collagen (9)(Fig S1A), argue against crossreactivity between self-antigens and viral antigens. The data presented herein shows that, at least for anergic B cells, bystander activation does not result in breakage of tolerance per se. It remains possible that anergic anti-DNA B cells, which could bind DNA that has virus nucleic acid-binding proteins attached to it, would be helped by anti-viral CD4 T cells, which then could promote breakage of anergy. However, Ars/A1 B cells (ssDNA binding) remain anergic during infection. We favor the possibility that the appearance of autoantibodies is a reflection of the general increase in serum IgG. It is noteworthy that work from the Pelanda group has demostrated a striking correlation between levels of autoantibodies and levels of total serum IgG in γHV68 infected autoimmune prone mouse models (48).
An alternative to bystander activation and mimicry is cell infection driven differentiation and antibody production. The elevated serum IgG produced in infected mice is reactive with a wide variety of antigens, self and foreign. Though the mechanism by which γHV68 infects B cells is unknown, it seems reasonable to assume that the virus infects B cells regardless of their BCR specificity. This includes autoreactive ignorant and anergic B cells present in the periphery. Recently it was shown that transitional B cells, including transitional 3 B cells, which are autoreactive and anergic (16), are readily infected (49). Once infected, virally encoded proteins reportedly drive these B cells to participate in the germinal center reaction and become plasma cells (50, 51), resulting in increased (auto)antibody production. Several studies have shown a striking enrichment of γHV68-infected B cells in the germinal center B cell and plasma cell pool (36, 37). Infection driven differentiation of infected B cells into plasma cells could also explain the discrepancy between the observed hypergammaglobemia and suppression of antigen-driven antibody responses during infection as reported here. γHV68-encoded M2 protein drives B cell proliferation and plasma cell differentiation (50, 51), both of which are inhibited in antigen-stimulated B cells in infected hosts (Fig 3E–G). This suggests a mechanism by which infected B cells are induced to develop into antibody secreting cells in an otherwise suppressive environment.
To begin to understand how antibody responses are inhibited by infection, we analyzed what stage of the B cell response is inhibited. Despite the fact that BCR-mediated calcium signaling is compromised in cells from infected animals, the ability of antigen-specific B cells to upregulate activation markers after immunization (Fig 3C) suggests that they respond to antigen. Therefore it is unlikely that there is a defect in antigen transport. In other viral infection models a distortion of splenic architecture and cellular location has been reported due to disruption of homeostatic chemokine expression (52, 53). While the splenic architecture gradually changes during infection possibly as a consequence of M3 function (40), at day 7 we could still detect largely normal follicular architecture and proper localization of lymphocytes. Antigen-stimulated B cells still localize at the B-T cell border after infection (Fig 3D). We have not studied the CD4 T cell response during infection, so at this time we do not know if antigen-stimulated B cells receive cognate T cell help. However, since T-cell independent responses are suppressed and the transfer of primed T cells did not rescue T cell-dependent responses during infection, it is unlikely that the defect in antibody responses is due to a problem in receiving T cell help. Similarly, we cannot exclude the possibility that antigen specific T cells acquire suppressive properties due to the infection. However, once again the fact that T independent antibody responses also are suppressed argues against this possibility. It appears that the suppressive mechanism must occur early in B cell activation and proliferation because antibody responses initiated 2 days before infection develop normally (Fig 2A). The finding that calcium mobilization is reduced in B cells from infected mice (Fig 1C, WT traces) suggests that BCR signal processing is altered in these B cells. It is possible that some pathways are still functional, e.g.. those resulting in CD69, CD86 upregulation after BCR stimulation, while others are not. We are currently further exploring this possibility.
Inhibition of antibody responses is not unique to γHV68 infection. A number of viruses (54–57) and other pathogens (58–61) cause suppression of antibody responses during infection. The degree and duration of suppression varies between pathogens and a number of mechanisms have been suggested, ranging from B cell intrinsic effects (56, 59) to suppressor T cells (58) and myeloid cells (60). A common denominator for all these pathogens is that they cause polyclonal B cell activation and that suppression seems to coincide with the polyclonal activation (56–58). Previous work done on γHV68 has shown that the observed polyclonal B cell activation is dependent on T cells (38) and is mediated via CD40-CD40L interaction (9). The finding that MHC class II deficient mice, which lack CD4+ T cells, do not suppress antibody responses, is consistent with the possibility that polyclonal B cell activation induces a suppressed state.
It is possible that polyclonal activation of B cells renders them anergic-like. In addition to being unable to mount an antibody response, B cells in γHV68-infected mice display several other characteristics of anergic B cells (15), including elevated basal intracellular free Ca2+ levels, dampened Ca2+ influx upon antigen receptor crosslinking (Fig 1C) and rapid reversibility of unresponsiveness (Fig 4). Classical B cell anergy is thought to be the consequence of chronic B cell signaling through the B cell receptor (signal 1) in the absence of a second activating signal from T cells or TLR ligands. Could receiving an activating signal 2 without signal 1 also result in an anergic-like state? Some experimental data suggests this could be the case. For example, exposure of B cells to LPS prior to antigen stimulation has been shown to inhibit antibody responses, both in vitro (62) and in vivo (63). As mentioned above, B cells reportedly become activated during γHV68 infection through CD40-CD40L interactions (9). Although this interaction is thought to have an activating effect, several reports show that CD40 ligation can inhibit B cell antibody responses (64, 65). CD40 ligation on B cells in the absence of antigen stimulation can induce generation of IL10 producing regulatory B cells (66). However, preliminary results indicate that while during acute γHV68 infections numbers of IL10 producing B cells and CD4 T cells increase, CD4+ Foxp3+ cell numbers are unchanged (67). Since antibody responses are suppressed in IL10−/− mice (Fig S3B), it is also unlikely that regulatory B cells play a large role in the effect.
Suppression of B cell/antibody responses due to infection is likely to be a widespread phenomenon with significant clinical implications. Generalized immune unresponsiveness has been observed in the clinic after trauma and severe infections, and termed Compensatory Anti-inflammatory Response Syndrome (CARS). CARS adversely affects the ability of patients to control secondary infections (20, 21). While B cells have not been carefully studied in the context of CARS, an anergic-like T cell phenotype has been described (68). Our data suggest a similar effect on B cells during infection.
Defects in B cell priming during and shortly after infection could affect the efficacy of vaccination. The majority of the population is infected with EBV during childhood (69). While in older children and adults EBV infection often cause infectious mononucleosis, infection of young children causes no or very mild symptoms. If EBV infection causes immune suppression similar to its murine counterpart, with duration of over 30 days, it could have important implications for effectiveness of vaccination. This is especially true since most vaccinations are given during the time of life when EBV infections are usually acquired. Specifically, vaccination may be contraindicated for a period following EBV infection.
On the other hand, emerging data indicate that there can be protective effects of infection on the development of autoimmunity. Infection of NOD mice with γHV68 causes a delay in disease onset (70) and infection of B6.Sle123 mice with gHV68 inhibits the development and progression of lupus-like disease (48). Given the critical roles of B cells in these autoimmune diseases, suppression of B cell function by viral infections, as described in this study, could mediate beneficial functions for the host.
Supplementary Material
Acknowledgements
The authors would like to thank Drs. Thiago Detanico and Lawrence Wysocki for calf chromatin, Drs. Ross Kedl, Yoseph Refaeli, Erwin Gelfand and Pippa Marrack for mice and Drs. Roberta Pelanda, Jennifer Larson and Pippa Marrack for helpful discussion, suggestions regarding the manuscript and sharing unpublished data.
Footnotes
This work was funded by NIH grants PO1AI22295 (Project 2) and RO1AI077597 to J.C.C. A.G was supported by fellowships from the Swedish Medical Society and the Swedish Research Council. I.K was supported by NIH training grant T32 AI007405. The authors have no conflicting financial interests.
Abbreviations: murine gammaherpes virus 68 (γHV68), p-Azophenylarsonate (Ars), Freund's incomplete adjuvant (FIA)
References
- 1.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–1104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Herrath MG, Oldstone MB. Virus-induced autoimmune disease. Curr Opin Immunol. 1996;8:878–885. doi: 10.1016/S0952-7915(96)80019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HJ, Ivanov, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen A, Gergely P, Jondal M, Klein G, Britton S. Polyclonal Ig production after Epstein-Barr virus infection of human lymphocytes in vitro. Nature. 1977;267:52–54. doi: 10.1038/267052a0. [DOI] [PubMed] [Google Scholar]
- 5.Lardans V, Godfraind C, van der Logt JT, Heessen WA, Gonzalez MD, Coutelier JP. Polyclonal B lymphocyte activation induced by mouse hepatitis virus A59 infection. J Gen Virol. 1996;77(Pt 5):1005–1009. doi: 10.1099/0022-1317-77-5-1005. [DOI] [PubMed] [Google Scholar]
- 6.Coutelier JP, Coulie PG, Wauters P, Heremans H, van der Logt JT. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64:5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutt-Fletcher LM, Balachandran N, Elkins MH. B cell activation by cytomegalovirus. J Exp Med. 1983;158:2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods A, Monneaux F, Soulas-Sprauel P, Muller S, Martin T, Korganow AS, Pasquali JL. Influenza virus-induced type I interferon leads to polyclonal B-cell activation but does not break down B-cell tolerance. J Virol. 2007;81:12525–12534. doi: 10.1128/JVI.00839-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangster MY, Topham DJ, D'Costa S, Cardin RD, Marion TN, Myers LK, Doherty PC. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol. 2000;164:1820–1828. doi: 10.4049/jimmunol.164.4.1820. [DOI] [PubMed] [Google Scholar]
- 10.Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather D, Lawson CM, Chapman AJ, Brown CM, Booth TW, Papadimitriou JM, Shellam GR. Wild isolates of murine cytomegalovirus induce myocarditis and antibodies that cross-react with virus and cardiac myosin. Immunology. 1998;94:263–270. doi: 10.1046/j.1365-2567.1998.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu PA, Gomez KA, Coutelier JP, Retegui LA. Sequence similarity and structural homologies are involved in the autoimmune response elicited by mouse hepatitis virus A59. J Autoimmun. 2004;23:117–126. doi: 10.1016/j.jaut.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton E, Mandal P, Speck SH. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol. 2011;29:351–397. doi: 10.1146/annurev-immunol-072710-081639. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson PG, Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol. 1998;72:943–949. doi: 10.1128/jvi.72.2.943-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Teague BN, Pan Y, Mudd PA, Nakken B, Zhang Q, Szodoray P, Kim-Howard X, Wilson PC, Farris AD. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J Immunol. 2007;178:7511–7515. doi: 10.4049/jimmunol.178.12.7511. [DOI] [PubMed] [Google Scholar]
- 18.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 20.Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–625. viii. doi: 10.1016/j.ccm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 22.Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 23.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 25.Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci U S A. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 27.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Clambey ET, Virgin H. W. t., Speck SH. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J Virol. 2000;74:1973–1984. doi: 10.1128/jvi.74.4.1973-1984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Berkel V, Levine B, Kapadia SB, Goldman JE, Speck SH, Virgin H. W. t. Critical role for a high-affinity chemokine-binding protein in gamma-herpesvirus-induced lethal meningitis. J Clin Invest. 2002;109:905–914. doi: 10.1172/JCI14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virgin H. W. t., Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Getahun A, Heyman B. Studies on the mechanism by which antigen-specific IgG suppresses primary antibody responses: evidence for epitope masking and decreased localization of antigen in the spleen. Scand J Immunol. 2009;70:277–287. doi: 10.1111/j.1365-3083.2009.02298.x. [DOI] [PubMed] [Google Scholar]
- 33.Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140:405. [PubMed] [Google Scholar]
- 34.Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology. 2008;380:182–190. doi: 10.1016/j.virol.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans AG, Moser JM, Krug LT, Pozharskaya V, Mora AL, Speck SH. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J Exp Med. 2008;205:669–684. doi: 10.1084/jem.20071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collins CM, Boss JM, Speck SH. Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. J Virol. 2009;83:6484–6493. doi: 10.1128/JVI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flano E, Kim IJ, Woodland DL, Blackman MA. Gamma-herpesvirus latency is preferentially maintained in splenic germinal center and memory B cells. J Exp Med. 2002;196:1363–1372. doi: 10.1084/jem.20020890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson PG, Doherty PC. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J Virol. 1999;73:1075–1079. doi: 10.1128/jvi.73.2.1075-1079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usherwood EJ, Ross AJ, Allen DJ, Nash AA. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. The J. Gen Virol. 1996;77(Pt 4):627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 40.Parry CM, Simas JP, Smith VP, Stewart CA, Minson AC, Efstathiou S, Alcami A. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J Exp Med. 2000;191:573–578. doi: 10.1084/jem.191.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol. 2006;177:7296–7302. doi: 10.4049/jimmunol.177.10.7296. [DOI] [PubMed] [Google Scholar]
- 42.Guggemoos S, Hangel D, Hamm S, Heit A, Bauer S, Adler H. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol. 2008;180:438–443. doi: 10.4049/jimmunol.180.1.438. [DOI] [PubMed] [Google Scholar]
- 43.Michaud F, Coulombe F, Gaudreault E, Kriz J, Gosselin J. Involvement of TLR2 in recognition of acute gammaherpesvirus-68 infection. PLoS One. 2010;5:e13742. doi: 10.1371/journal.pone.0013742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gargano LM, Moser JM, Speck SH. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J Virol. 2008;82:3853–3863. doi: 10.1128/JVI.02577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Larson JD, Thurman JM, Rubtsov AV, Claypool D, Marrack P, van Dyk LF, Torres RM, Pelanda R. Murine gammaherpesvirus 68 infection protects lupus-prone mice from the development of autoimmunity. Proc Natl Acad Sci U S A. 2012;109:E1092–1100. doi: 10.1073/pnas.1203019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman CB, Nealy MS, Tibbetts SA. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J Virol. 2010;84:13045–13052. doi: 10.1128/JVI.01455-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel AM, Herskowitz JH, Speck SH. The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog. 2008;4:e1000039. doi: 10.1371/journal.ppat.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog. 2009;5:e1000677. doi: 10.1371/journal.ppat.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, Ware CF. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2006;2:e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 54.Howard RJ, Najarian JS. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974;18:109–118. [PMC free article] [PubMed] [Google Scholar]
- 55.McChesney MB, Fujinami RS, Lampert PW, Oldstone MB. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986;163:1331–1336. [PMC free article] [PubMed] [Google Scholar]
- 56.Mosier DE, Yetter RA, Morse HC., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceglowski WS, Campbell BP, Friedman H. Immunosuppression by leukemia viruses. Effect of Friend leukemia virus on humoral immune competence of leukemia-resistant C57BL/6 mice. J Immunol. 1975;114:231–236. [PubMed] [Google Scholar]
- 58.Reed SG, Roters SB, Goidl EA. Spleen cell-mediated suppression of IgG production to a non-parasite antigen during chronic Trypanosoma cruzi infection in mice. J Immunol. 1983;131:1978–1982. [PubMed] [Google Scholar]
- 59.Albright JW, Albright JF, Dusanic DG. Mechanisms of trypanosome-mediated suppression of humoral immunity in mice. Proc Natl Acad Sci U S A. 1978;75:3923–3927. doi: 10.1073/pnas.75.8.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki Y, Kobayashi A. Suppression of unprimed T and B cells in antibody responses by irradiation-resistant and plastic-adherent suppressor cells in Toxoplasma gondii-infected mice. Infect Immun. 1983;40:1–7. doi: 10.1128/iai.40.1.1-7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valdez JC, Meson OE, de Valdez GA, Sirena A. Suppression of humoral response during the course of Candida albicans infection in mice. Mycopathologia. 1984;88:61–63. doi: 10.1007/BF00439297. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann MK, Weiss O, Koenig S, Hirst JA, Oettgen HF. Suppression and enhancement of the T cell-dependent production of antibody to SRBC in vitro by bacterial lipopolysaccharide. J Immunol. 1975;114:738–741. [PubMed] [Google Scholar]
- 63.Rearte B, Landoni V, Laborde E, Fernandez G, Isturiz M. Differential effects of glucocorticoids in the establishment and maintenance of endotoxin tolerance. Clin Exp Immunol. 2009;159:208–216. doi: 10.1111/j.1365-2249.2009.04052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyashita T, McIlraith MJ, Grammer AC, Miura Y, Attrep JF, Shimaoka Y, Lipsky PE. Bidirectional regulation of human B cell responses by CD40-CD40 ligand interactions. J Immunol. 1997;158:4620–4633. [PubMed] [Google Scholar]
- 65.Callard RE, Herbert J, Smith SH, Armitage RJ, Costelloe KE. CD40 cross-linking inhibits specific antibody production by human B cells. Int Immunol. 1995;7:1809–1815. doi: 10.1093/intimm/7.11.1809. [DOI] [PubMed] [Google Scholar]
- 66.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gasper-Smith N, Marriott I, Bost KL. Murine gamma-herpesvirus 68 limits naturally occurring CD4+CD25+ T regulatory cell activity following infection. J Immunol. 2006;177:4670–4678. doi: 10.4049/jimmunol.177.7.4670. [DOI] [PubMed] [Google Scholar]
- 68.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 70.Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179:7325–7333. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.