Abstract
TCF-1 and LEF-1 transcription factors have redundant roles in promoting thymocyte maturation. TCF-1 has been recently shown to critically regulate memory CD8+ T cell differentiation and persistence. The complete spectrum of regulatory roles for TCF-1 and LEF-1 in CD8+ T cell responses are yet unknown. We conditionally targeted LEF-1, and by combination with germline deletion of TCF-1, we found that loss of both factors completely abrogated the generation of KLRG1loIL-7Rα+ memory precursors in effector CD8+ T cell population in response to Listeria monocytogenes infection. Whereas CD8+ effectors deficient for TCF-1 and LEF-1 retained the capacity to express IFN-γ, granzyme B, and perforin, they were defective in TNF-α production. In the memory phase, the antigen-specific CD8+ T cells lacking TCF-1 and LEF-1 exhibited an effector phenotype and were severely impaired in secondary expansion upon re-challenge. Thus, TCF-1 and LEF-1 cooperatively regulate generation of memory precursors and protective memory CD8+ T cells.
Introduction
Cytotoxic CD8+ T cells are essential for controlling intracellular pathogens including viruses and some bacteria. Upon encountering their cognate antigen, naïve CD8+ T cells are activated and expanded into clonal effector T cells. A small fraction of CD8+ effectors then give rise to memory T cells which confer enhanced protections against the same pathogen (1, 2). Both effector and memory CD8+ T cells are heterogeneous. Effector CD8+ T cells with increased expression of killer cell lectin-like receptor G1 (KLRG1) and decreased expression of interleukin-7 receptor α chain (IL-7Rα) are considered to be short-lived and terminally differentiated; in contrast, KLRG1loIL-7Rα+ effectors demonstrate increased potential of generating long-lasting memory CD8+ T cells and are therefore proposed to be memory precursors (3, 4). Among memory CD8+ T cells, CD62L+CCR7+ central memory T cells exhibit a greater capacity of homeostatic proliferation, whereas CD62L−CCR7− effector memory T cells decay over time (5).
Transition of naïve into effector and memory CD8+ T cells is accompanied by diversification of the T cell transcriptome, and several transcription factors are known to direct effector and memory T cell differentiation. T-bet, Blimp-1, and Id2 are highly induced upon T cell activation, and their expression is more enriched in the terminally differentiated CD8+ effectors than the KLRG1loIL-7Rα+ memory precursors (3, 6–8). On the other hand, the memory precursors express higher levels of Bcl-6, TCF-1 and Id3 than the terminally differentiated effectors (8).
TCF-1 and LEF-1, encoded respectively by Tcf7 and Lef1, are transcription factors that act downstream of the canonical Wnt signaling pathway (9, 10). These two factors have known redundant roles in T cell development (11, 12). We and others have recently demonstrated that TCF-1 deficiency compromised memory CD8+ T-cell differentiation and persistence (13, 14). To address possible compensatory roles between TCF-1 and LEF-1, we conditionally targeted the Lef1 locus. By combination with germline deletion of Tcf7, here we demonstrate that TCF-1 and LEF-1 are essential for generation of memory precursors and functional memory CD8+ T cells.
Materials and Methods
Mice
Tcf7−/− and Gzmb-Cre transgenic mice were from Hans Clevers and Joshy Jacob, respectively (15, 16). Rosa26-EGFP reporter mice were from Jackson Laboratory. The Lef1-floxed strain was generated in-house (17). All the animals were housed and handled following protocols approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Infections
Mice were initially i.v. infected with ~ 0.5 LD50 of actA− Listeria monocytogenes expressing ovalbumin (LM-OVA) (18). For recall response, the immune mice were i.v. infected with 10 LD50 of virulent LM-OVA, and CFUs in the spleen and liver were determined (19).
Flow cytometry and cell sorting
Cell surface staining, intracellular staining for cytokines, intranuclear staining for Eomes and T-bet, and Ova257–264 (SIINFEKL)-MHC I tetramer staining were performed as previously described (14, 19). The flow data were analyzed using FlowJo software (TreeStar). Tetramer-stained CD8+ effector or memory T cells from the spleens were sorted on a FACSAria.
Chromatin immunoprecipitation (ChIP) and quantitative RT-PCR
A LEF-1 antibody (C18A7, Cell signaling) was used in ChIP on CD8+ T cells as described (14). RNA extraction, reverse-transcription, and quantitative PCR were performed as described (14).
Results and Discussion
TCF-1 and/or LEF-1 deficiency diminished expansion of effector CD8+ T cells
TCF-1 and LEF-1 have redundant roles during T cell development, with TCF-1 exhibiting a more dominant role (9, 10). Whereas LEF-1 deficiency alone did not have detectable impact on thymocyte maturation, deletion of LEF-1 in Tcf7−/− fetuses exacerbated T cell developmental defects (12). By adoptive transfer of WT or Tcf7−/− OT-I CD8+ T cells followed by infection with actA− LM-Ova, we previously demonstrated that TCF-1 deficiency reduced effector CD8+ T cell expansion by approximately 50% (14). We confirmed this finding when we directly infected Tcf7−/− mice without adoptive transfer and detected the endogenous Ova-specific effector CD8+ T cells (Fig. 1A and 1B). To address possible compensatory roles between TCF-1 and LEF-1 in regulating CD8+ responses, we investigated the impact of deficiency in both factors.
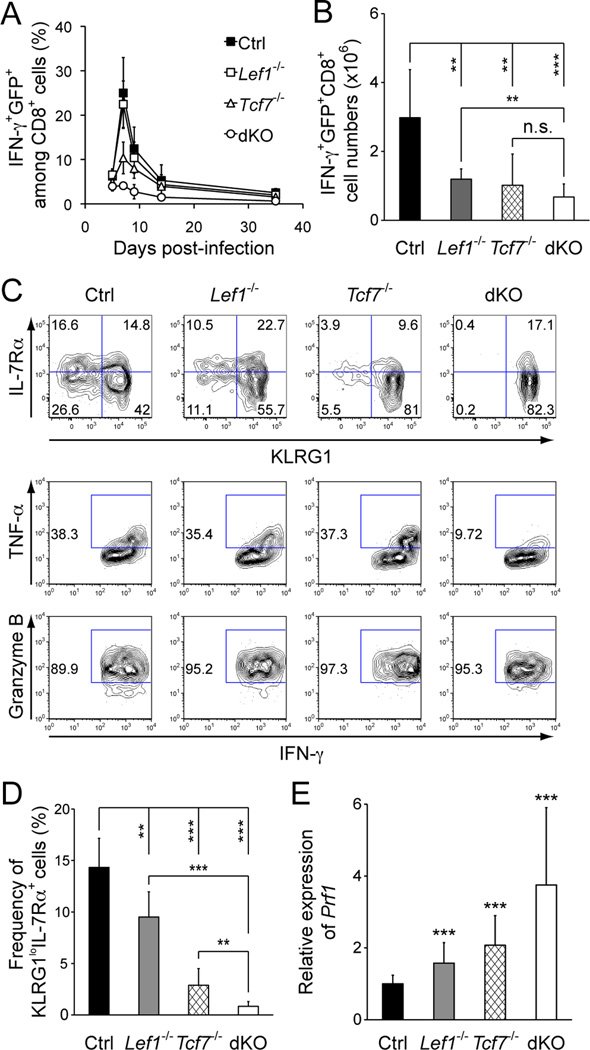
Figure 1.
Deficiency in TCF-1 and/or LEF-1 impaired expansion and function of effector CD8+ T cells and generation of memory precursors. A. Kinetics of CD8+ T cell responses in PBLs. Mice were infected with actA− LM-Ova, and Ova-specific CD8+ T cells in the PBLs were detected by peptide-stimulated IFN-γ production. The frequency of IFN-γ+GFP+ cells among CD8+ T cells was shown. Data are representative of 3 independent experiments (n ≥ 4 for each time point). B. Numbers of Ova-specific CD8+ effectors in the spleen, as determined on day 7 post-infection. C. Characterization of CD8+ effectors in the absence of TCF-1 and/or LEF-1. On day 7 post-infection, Ova-specific IFN-γ+GFP+CD8+ T cells were detected in the spleen, and fractionated based on KLRG1 and IL-7Rα expression or detected for TNF-α and granzyme B induction. The gating is based on isotype control staining, and the frequency of each subset is shown in representative contour plots from 3 independent experiments (n ≥ 6). D. Cumulative frequency of KLRG1loIL-7Rα+ memory precursors in IFN-γ+GFP+ CD8+ effector T cells. E. Perforin expression in CD8+ effectors. Tetramer+GFP+CD8+ effector T cells were sorted from the spleens on day 7 post-infection, and Prf1 expression was measured by quantitative RT-PCR. The primers are 5’-gatgtgaaccctaggccaga and 5’-ggtttttgtaccaggcgaaa. Data in B, D, and E are means ± s.d. from 3–4 independent experiments (n ≥ 6). **, p<0.01; ***, p<0.001 by Student’s t-test. n.s., not significant.
To avoid perinatal lethality, we conditionally targeted the Lef1 gene by inserting 2 LoxP sites to flank exons 7 and 8, which encode the DNA-binding HMG domain of LEF-1 (17). To circumvent the impact of TCF-1 and LEF-1 double deficiency on T cell development and to specifically investigate their roles in mature T cells, we use a Cre transgene driven by human Granzyme B promoter (Gzmb-Cre) (16). In this system, LEF-1 expression remains intact in mature CD8+ T cells, and excision of the floxed Lef1 allele (Lef1FL/+) occurs only after the naïve T cells are activated (8). We also used a Rosa26-EGFP reporter strain in which GFP is only expressed after Cre-mediated excision of an intervening floxed STOP sequence (20). This reporter thus permanently marks antigen-experienced CD8+ T cells with effective excision of the floxed alleles. Upon proper crossing, we obtained Gzmb-Cre+Rosa26-EGFP+Lef1FL/FL and Gzmb-Cre+Rosa26-EGFP+Lef1FL/FLTcf7−/− mice (hereafter designated as Lef1−/− and dKO mice, respectively). For control and Tcf7−/− mice, we used those positive for Gzmb-Cre and Rosa-EGFP reporter genes for a consistent comparison. On day 7 post-infection with LM-Ova, 70–95% of the Ova-specific effector CD8+ T cells, detected either with Ova peptide-stimulated IFN-γ production or SIINFEKL-MHC I tetramer, were positive for GFP in all genotypes examined (Fig. S1A and S1B). We also sorted tetramer+GFP+ CD8+ effectors and found that the Lef1 transcripts were decreased by approximately 80% in Lef1−/− and dKO mice (Fig. S1D). Assuming the remaining Lef1 transcripts were all derived from one undeleted “floxed” Lef1 allele in a single cell, ~80% of Lef1−/− and dKO CD8+ effectors should have complete inactivation of LEF-1.
To determine the impact of TCF-1 and LEF-1 deficiency on CD8+ T cell responses, we tracked Ova-specific IFN-γ+GFP+ CD8+ T cells in both peripheral blood lymphocytes (PBLs) and spleens. In the PBLs, whereas TCF-1 and/or LEF-1 deficiency affected the magnitude of CD8+ effector expansion to different extent, the peak expansion in all genotypes occurred on day 7 post-infection (Fig. 1A). Consistent with our previous findings (14), TCF-1 deficiency reduced frequency of CD8+ effectors in the PBLs as well as their numbers in the spleen by > 50% (Fig. 1A and 1B). Although ablation of LEF-1 only minimally affected the frequency of CD8+ effectors in the PBLs (Fig. 1A), it significantly reduced the number of effector CD8+ T cells in the spleen (Fig. 1B). These results suggest that LEF-1 is also required for optimal expansion of CD8+ effectors, and this is in contrast to the observation that LEF-1 is dispensable for normal T cell development (12). On the other hand, deletion of both TCF-1 and LEF-1 substantially reduced the frequency of Ova-specific CD8+ effectors detected in PBLs (Fig. 1A). In the spleen, albeit there was a trend of further reduction of CD8+ effectors in dKO mice compared with Lef1−/− or Tcf7−/− mice, the additive effect of TCF-1 and LEF-1 double deficiency was not evident (Fig. 1B). We also used SIINFEKL-MHC I tetramer to detect Ova-specific CD8+ effectors and confirmed those findings using IFN-γ-based functional measurements (Fig. S1C). These data collectively suggest that TCF-1 and LEF-1 transcription factors are necessary for optimal expansion of effector CD8+ T cells, and that deletion of both factors did not completely abrogate CD8+ effector differentiation.
Loss of TCF-1 and LEF-1 abrogated generation of memory precursors
In addition to quantitative measurements, we next performed in-depth phenotypic and functional analysis of effector CD8+ T cells lacking TCF-1 and/or LEF-1. When fractionated based on KLRG1 and IL-7Rα expression, the KLRG1loIL-7Rα+ memory precursors were diminished by either LEF-1 or TCF-1 deficiency (Fig. 1C and 1D). Strikingly, the memory precursors were almost completely abrogated in dKO mice (Fig. 1C and 1D), indicating that the generation of memory precursors depends on TCF-1 and LEF-1. Additionally, although loss of either TCF-1 or LEF-1 alone exhibited little effect, the double deficiency substantially diminished production of TNF-α in CD8+ effectors (Fig. 1C). In contrast, TCF-1 and/or LEF-1 deficiency did not compromise the ability of CD8+ effectors in acquiring the cytolytic effector molecules such as granzyme B (Fig. 1C). In fact, the expression of perforin (encoded by Prf1) was most evidently increased in dKO CD8+ effectors (Fig. 1E).
TCF-1 and LEF-1 deficiency impaired maturation and functions of memory CD8+ T cells
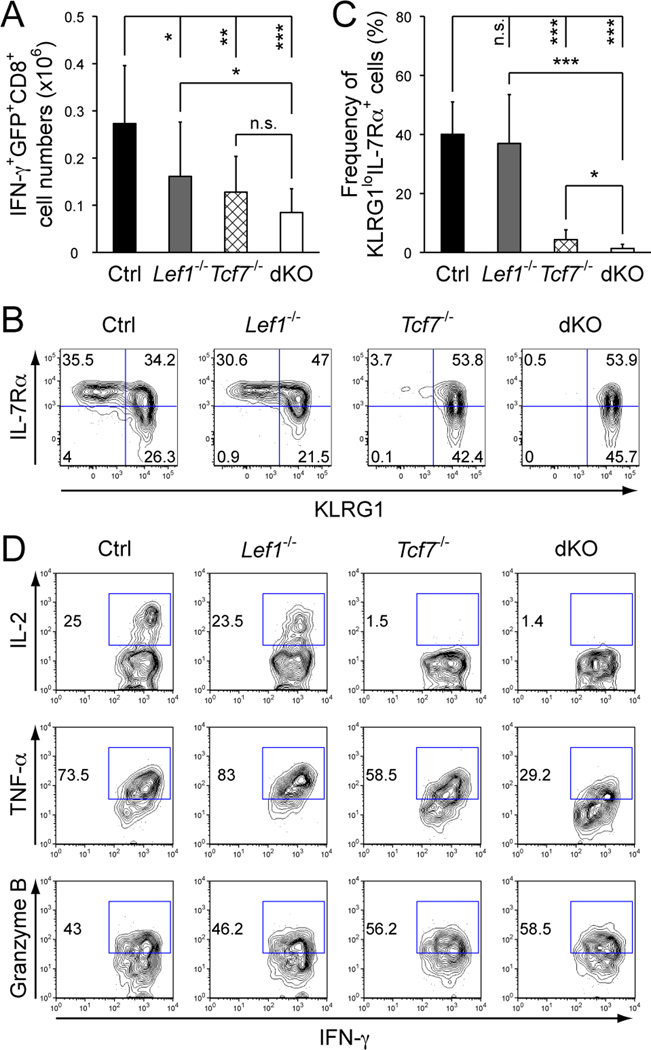
After the peak response, a fraction of the CD8+ effector T cells survive the contraction and transition into memory phase. On day 35 post-infection, the numbers of Ova-specific memory CD8+ T cells were reduced in the Tcf7−/− spleens, as detected by Ova peptide-stimulated IFN-γ production or MHC I tetramer (Fig. 2A, S1E to S1G). Double deletion of TCF-1 and LEF-1 further reduced the numbers of memory CD8+ T cells, albeit the reduction did not reach a statistical significance compared with TCF-1 single deficiency (Fig. 2A and S1G). It is of note that loss of both TCF-1 and LEF-1 abrogated generation of KLRG1loIL-7Rα+ memory precursors in the effector clonal expansion phase (Fig. 1C and 1D). Although the memory precursors are considered to have increased potential to give rise to memory CD8+ T cells, in the context of TCF-1 and LEF-1 double deficiency, the dKO Ova-specific CD8+ T cells detected at the early memory phase are most likely derived from the KLRG1+IL-7Rα− effector CD8+ T cells. In line with this notion, the dKO memory CD8+ T cells exhibited almost exclusively a KLRG1+ effector phenotype (Fig. 2B and 2C). Although not affected in granzyme B expression, the dKO memory CD8+ T cells manifested decreased production of TNF-α and failed to produce IL-2 (Fig. 2D). We also sorted tetramer+GFP+CD8+ memory T cells to assess Lef1 excision and found that the cells with complete deletion of Lef1 transcripts were reduced to approximately 60% at the memory stage, compared with 80% excision in effectors (compare Fig. S1H with Fig. S1D). This suggests that the cells that escaped Lef1 excision may have had growth/survival advantage during effector-to-memory transition, and hence the defects of memory CD8+ T cells in Lef1−/− and dKO mice may have been underestimated in this experimental system.
Figure 2.
Deficiency in TCF-1 and LEF-1 compromised maturation and function of memory CD8+ T cells. A. Numbers of memory CD8+ T cells. Ova-specific IFN-γ+GFP+CD8+ T cells were detected in the spleens on day 35 post-infection. Data are means ± s.d. from 4 independent experiments (n ≥ 9). B. Phenotypic characterization of memory CD8+ T cells. KLRG1 and IL-7Rα expression was determined on splenic Ova-specific IFN-γ+GFP+ memory CD8+ T cells. The percentage of each subset is shown in representative contour plots from 2 experiments (n ≥ 4). C. Cumulative frequency of KLRG1loIL-7Rα+ memory CD8+ T cells. For A and C, *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant. D. Functional characterization of memory CD8+ T cells. IL-2, TNF-α or granzyme B was detected by intracellular staining in IFN-γ+GFP+ memory CD8+ T cells on day 35 post-infection. The percentages of positive subsets are shown. Data are representative of 3 independent experiments (n ≥ 6).
We previously showed that Eomes is a direct TCF-1 target gene in memory CD8+ T cells (14). Eomes is upregulated in CD8+ effectors and retained at high levels in memory T cells (21). At the effector phase, Eomes was only minimally affected by loss of TCF-1 or LEF-1 alone, but was evidently reduced in dKO CD8 effectors; in contrast, T-bet expression was not affected by loss of TCF-1 and/or LEF-1 (Fig. S2A). Thus, deficiency in TCF-1 and LEF-1 had an early impact on proper upregulation of Eomes in CD8+ effectors, impairing generation of functional memory CD8+ T cells. At the memory phase, TCF-1 deficiency reduced Eomes expression as expected, and interestingly, loss of LEF-1 alone also modestly reduced Eomes expression (Fig. S2B). We previously reported direct binding of TCF-1 to four upstream regulatory regions in the Eomes gene (14). By ChIP on CD8+ T cells, we found that LEF-1 occupied the same regulatory sequences (Fig. S2C). These data indicate that both TCF-1 and LEF-1 contribute to positive regulation of Eomes during CD8+ T cell response.
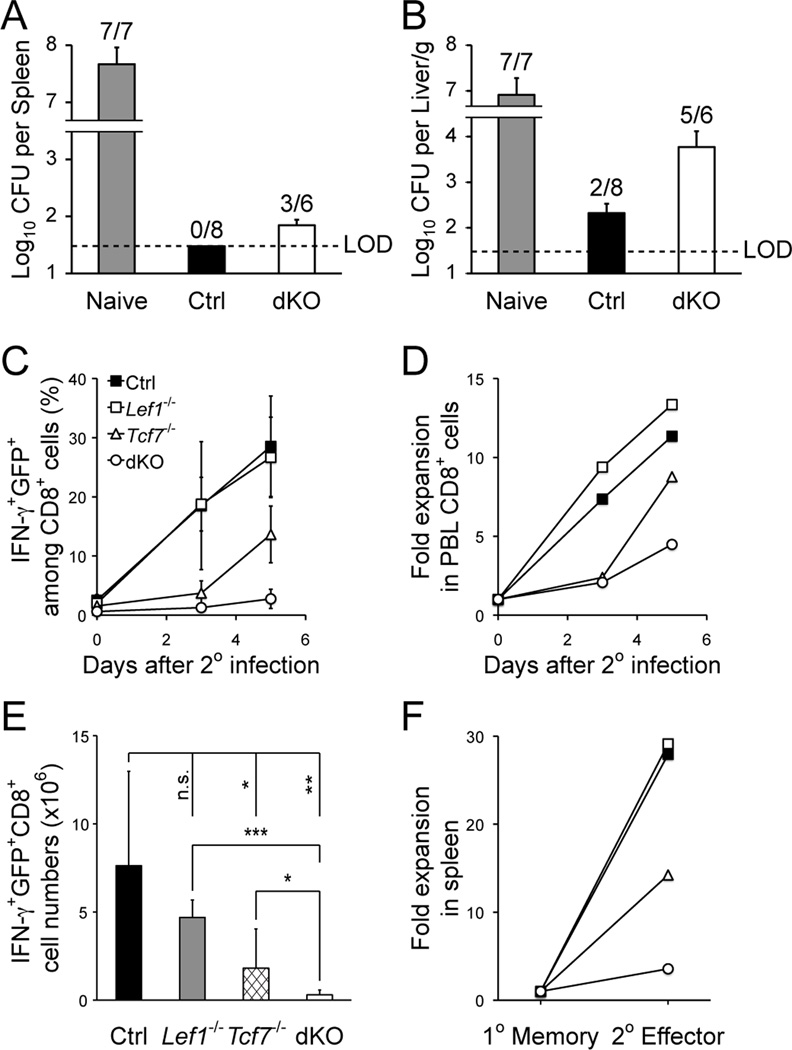
Loss of TCF-1 and LEF-1 compromised recall response of memory CD8+ T cells
Memory T cells confer enhanced protection upon re-encountering the same antigen. When challenged with virulent LM-Ova, whereas naïve mice showed uncontrolled bacteria growth, control immune mice completely cleared LM in the spleen and largely in the liver (Fig. 3A and 3B). In contrast, the bacteria were detected in the spleen of 50% of dKO mice; and in the liver of dKO mice, the bacteria burden was more than one order of magnitude higher than the control mice (Fig. 3A and 3B). To further investigate the less efficient bacterial clearance in the absence of TCF-1 and LEF-1, we tracked the recall response of memory CD8+ T cells. In PBLs, the secondary expansion of dKO memory CD8+ T cells was greatly diminished, as measured by absolute frequency or relative expansion after normalizing to the starting point (Fig. 3C and 3D). When examined in the spleen, the Ova-specific dKO memory CD8+ T cells were greatly impaired in generating secondary effectors, in terms of absolute counts as well as relative expansion (Fig. 3E and 3F). Collectively, most of these defects in dKO CD8+ memory were more severe than those observed in Tcf7−/− memory CD8+ T cells, indicating that TCF-1 and LEF-1 have redundant roles in regulating CD8+ responses, in addition to their well-known roles in cooperatively promoting thymocyte maturation (12).
Figure 3.
Loss of TCF-1 and LEF-1 greatly impaired recall response by the primary memory CD8+ T cells. A and B. Clearance of virulent LM-Ova by memory CD8+ T cells. Naïve or immune mice (day 35 post-infection) were challenged with virulent LM-Ova, and 3 days later, CFUs were determined in the liver and spleen. Shown are cumulative data from 2 experiments. Data are reported as CFU numbers (means ± s.d.) per spleen (A) or per gram of liver (B), from each organ with positive detection of LM-Ova. Frequency of animals with positive detection of the bacteria is marked on top of the bar. LOD, limit of detection. C and D. Secondary CD8+ T cell expansion in PBLs. Ova-specific CD8+ T cells were tracked in the PBLs after secondary challenge. (C) shows the frequency of IFN-γ+GFP+ cells among CD8+ T cells. (D) shows the relative expansion of secondary CD8+ effectors after normalization to starting memory CD8+ frequency. Data are representative of 3 independent experiments (n ≥ 7). E. Numbers of secondary effector CD8+ T cells in the spleens, as determined on day 5 after the re-challenge. Data are means ± s.d. from 3 independent experiments (n ≥ 5). *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant. F. Relative expansion of secondary CD8+ effectors in the spleen. The numbers of secondary CD8+ effectors (as in E) was normalized to those of primary memory CD8+ T cells (as in Fig. 2A) to calculate the relative expansion.
In summary, using the newly established LEF-1 conditional knockout mouse model, our study revealed unique requirements of TCF-1 and LEF-1 transcription factors in regulating mature CD8+ T cell responses. To our knowledge, this is the first demonstration that deletion of transcription factors (i.e., TCF-1 and its relative LEF-1) in activated T cells completely abrogates the generation of KLRG1loIL-7Rα+ memory precursors. This study further uncovers essential roles of TCF-1 and LEF-1 in TNF-α production and proper upregulation of Eomes in CD8+ effectors. Upon transitioning to the memory phase, the antigen-specific CD8+ T cells lacking both factors exhibit an effector phenotype and fail to effectively acquire functions characteristic of memory T cells, such as rapid secondary expansion and effective control of pathogens. These observations reveal that deficiency in TCF-1/LEF-1 causes the most profound defects in CD8+ responses among all the transcription regulators studied in vivo thus far. Our data indicate that TCF-1 and LEF-1 are necessary for optimal expansion of CD8+ effectors, and are indispensable for generation of memory precursors as well as further maturation and acquisition of CD8+ memory functionality. These findings thus identify TCF-1 and LEF-1 as key regulatory nodes in enhancing T-cell immunity against infectious agents and malignant cells.
Supplementary Material
Acknowledgements
We thank Drs. John Harty and Vladimir Badovinac for providing LM-Ova and SIINFEKL tetramers and critical reading of this manuscript. We thank Drs. Hans Clevers (Hubrecht Institute, the Netherlands) and Joshy Jacob (Emory University) for providing the Tcf7−/− and Gzmb-Cre transgenic mice, respectively.
This work was supported by NIH grants (HL095540 and AI080966) and funds from the American Cancer Society (RSG-11-161-01-MPC).
References
- 1.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefrancois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–218. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 7.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 8.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue HH, Zhao DM. Regulation of mature T cell responses by the Wnt signaling pathway. Ann N Y Acad Sci. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- 10.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 12.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 13.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 16.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Zhou X, Steinke FC, Liu C, Chen SC, Zagorodna O, Jing X, Yokota Y, Meyerholz DK, Mullighan CG, Knudson CM, Zhao DM, Xue HH. TCF-1 cooperates with LEF-1 to promote β-selection and restrains LEF-1 expression to suppress T-cell malignancy. Immunity. doi: 10.1016/j.immuni.2012.08.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tvinnereim AR, Hamilton SE, Harty JT. CD8(+)-T-cell response to secreted and nonsecreted antigens delivered by recombinant Listeria monocytogenes during secondary infection. Infect Immun. 2002;70:153–162. doi: 10.1128/IAI.70.1.153-162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- 21.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.