Abstract
Evolutionary theories of aging suggest that trade-offs between longevity and fitness should be found under certain conditions. In C. elegans, there is little evidence for the existence of such tradeoffs. We asked if fertility/longevity trade-offs exist in populations of randomly mating males and hermaphrodites. We set up a large population of young males and 5-day-old hermaphrodites that were no longer self-fertile. We then allowed them to mate for one day with an equal number young males and then separated hermaphrodites to individual plates and determined daily fertility of individual hermaphrodites. There was a significant negative relationship between late-life fertility and individual longevity.
Keywords: Genetics, Nematodes, Fertility, Demography, Longevity, Evolutionary Theory, Antagonistic Pleiotropy
1. Introduction
One of the central predictions of the evolutionary theory of aging is that there should be a strong tradeoff between longevity and reproduction (Rose, 1991). In particular, under the antagonistic pleiotropy theory of aging, natural selection operating on early reproduction should lead to the preferential fixation alleles that have positive effects on reproduction but negative effects latter in life, especially for genes that act post-reproductively (Williams, 1957). Somewhat surprisingly, such tradeoffs have largely not been observed within the model nematode, Caernorhabditis elegans (reviewed in Anderson et al. 2011). Starting with the first analyses of natural variation (Johnson, 1987) and mutant lines (Klass, 1983; Friedman and Johnson, 1988a,b), C. elegans has become one of the premiere systems for studying the genetics of aging, with several hundred genes having been identified as affecting aging, with most of these being long-lived (Tissenbaum and Johnson, 2008; Kenyon, 2010). When raised in the laboratory, C. elegans exhibits an unusually long post-reproductive period, and most age-related mutants tend to further extend this period. Starting with the first longevity variants in C. elegans, Johnson (1987) found “no correlation between the length of the reproductive period and life span.” Similarly, most longevity-extending mutants appear to have, at most, mild effects on reproduction (e.g., Gems et al., 1998; Jenkins et al., 2004; Kenyon, 2010), although this might be partially the result of being raised in a resource-rich environment (e.g., Walker et al., 2000). Anderson et al. (2011) used evolutionary approaches to select for increased early reproduction in a diverse set of natural isolates in C. elegans and failed to detect the correlated decrease in longevity that would be expected under the tradeoff hypothesis, although they did find an antagonistic relationship in the timing of early and late reproduction. Similarly, Knight et al. (2001) failed to find a trade-off between body size and fertility. Overall, then, C. elegans would appear to be something of an enigma with respect to reproductive patterns expected under the evolutionary theory of aging.
An important component of each of these studies, however, is that reproduction was measured via self-fertility. C. elegans is an androdioecious nematode with hermaphrodites and rare males (reviewed in Anderson et al., 2010). Although genomic data suggests that outcrossing is rare in nature (Anderson et al., 2010), hermaphrodites that mate with males can produce several-fold more offspring than self-fertilizing individuals (Hughes et al., 2007; Luo et al., 2009; Mendenhall et al., 2011). Importantly, females retain their fertility nearly three weeks after reaching sexual maturity, suggesting that the “post-reproductive” period of a hermaphrodite’s life has the potential to be fully reproductive in the presence of males. What are the effects of this late-life reproduction on eventual hermaphrodite longevity? Will tradeoffs between reproduction and longevity that have yet to be observed in C. elegans appear when reproduction is extended over the entire lifetime of an individual? Here, we pursue these questions using life-history analysis of both mutant and wildtype individuals, raised in the presence and absence of males.
2. Materials and methods
2.1 Strains and Growth Conditions
All strains were grown and results obtained at 20°C , using standard NGM plates and media under standard laboratory conditions (Johnson and Wood, 1982). Strains used in these experiments were: N2(CGCb), TJ1052 [age-1(hx546)], and CB1370 [daf-2(e1370)]. Mating protocols were as previously published (Mendenhall et al., 2011). Three replicates were generated under each condition, and the results from each replicate are displayed separately to illustrate the strong consistency observed across trials. Males were maintained by serial mating of ten males and four hermaphrodites. Progeny production was recorded each day via the number of hatched larvae detected on the plate.
2.2 Late-life mating
For late-life mating, 89 hermaphrodites on day 5 of adult life were mated with 89 young males on a large plate, with the ratio of 1:1. Males were allowed to mate for 24 hours, and then hermaphrodites were transferred to 89 individual small NGM plates with one worm on each plate. Progeny were counted every day until the end of life.
2.3 Statistics
A t-test was used to compare differences in mean number of progeny between mated worms and unmated worms. A linear regression model was used to fit the relationship between lifespan and number of progeny. All statistics were performed using S-Plus. Ages refer to adult age following the first day of adulthood, which was termed “Age 0”.
3. Results
3.1. No detectable trade-offs within self-fertilizing populations of hermaphrodites
We first asked if we could replicate the many earlier studies that found little evidence for trade-offs between number of progeny produced by a hermaphrodite and its subsequent life span (reviewed in Anderson et al., 2010). We similarly found no detectable relationship between total reproduction and longevity within populations of self-fertilizing hermaphrodites (Fig. 1). Self-fertilizing hermaphrodites were typically sterile after the fifth day of adult life and then spent another 15 days in a post-reproductive state before eventually dying at roughly 19 days of age.
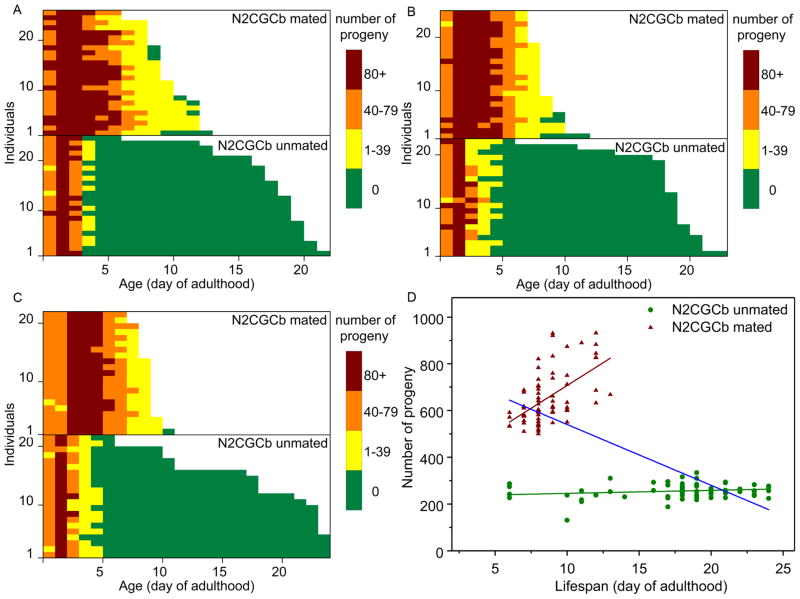
Fig. 1. Lifespans and daily progeny production of mated and unmated wild-type hermaphrodites (N2CGCb).
Individual hermaphrodites were separated at the fourth larval stage. About half were never mated with males, while the other half were mated with five males on the first day of adulthood, which we called day 0. (A–C) Graphs of individual experiments. Daily progeny production of each individual hermaphrodite is shown on a different horizontal line, using different colors to depict progeny production on each day. Mated animals live significantly shorter lives (P < 0.0001) and produce significantly more progeny (P < 0.0001) than unmated. (D) Scatterplots and linear fit of the data from Figures 1A–C, with lifespan (x axis) plotted against progeny production (y axis). Regression coefficient between lifespan and progeny production of each group are as follows: (A) Experiment 1, unmated: beta = −0.82, p = 0.62; mated: beta = 35.98, p = 0.01; both: beta = −38.36, p = 0. (B) Experiment 2, unmated: beta = 2.43, p = 0.22; mated: beta = 7.74, p = 0.42; both: beta = −28.19, p = 0. (C) Experiment 3, unmated: beta =1.71, p = 0.17; mated: beta = 15.18, p = 0.2; both: beta = −18.05, p = 0. All unmated (beta = 1.28, p = 0.13), all mated (beta = 39.05, p = 0), both (beta = −25.99, p = 0).
3.2. Negative trade-offs from mating hermaphrodites with males
Mating wild-type (N2CGCb) hermaphrodites with males increases their fertility dramatically and extends the fertile period well beyond that found in unmated hermaphrodites (Fig. 2A, B; see also Ward and Carrel 1979; Mendenhall et al., 2011). We found an average of 600 – 800 offspring could be produced under the mating conditions utilized here (5 males per individual hermaphrodite, mating begins at day 0 of adult life). Male mating greatly extended the fertile period but significantly decreased longevity of the mated hermaphrodites (P < 0.0001; Figs. 1, 2). Male mating also dramatically shortened the sterile period following the end of reproduction, such that most hermaphrodites died while they were still fertile. Out of a total of 70 N2 worms mated, 42 individuals died as a result of internal hatching of embryos (bagging), while only 3 of 67 unmated worms died by bagging. Within the mated population there was a positive relationship between length of life and the number of offspring (Fig. 1D, beta = 39.05, p < 0.0001). The relationship is likely driven by the combination of the reduction of longevity generated by mating and the fact that, when hermaphrodites die early (while still fertile), they cannot lay as many eggs. Simply put: dead worms lay no eggs.
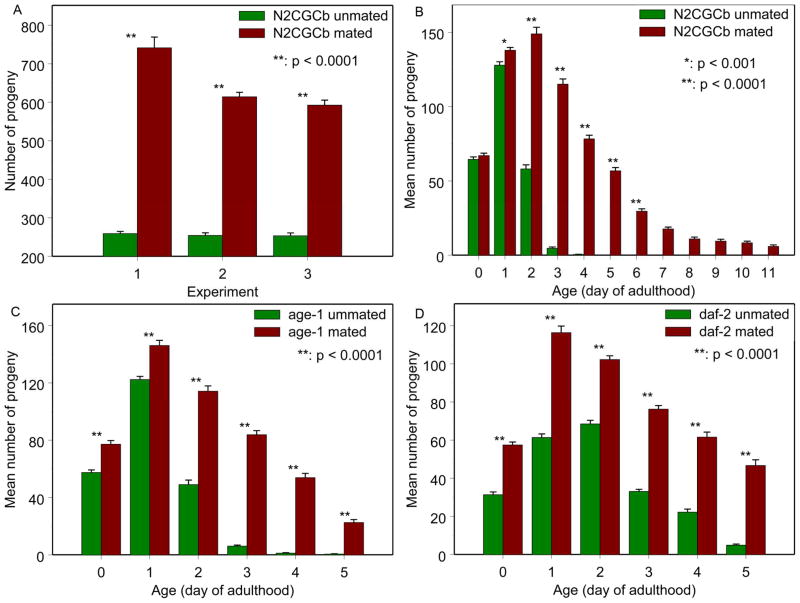
Fig. 2. Total number of progeny, mated or unmated, as a function of adult age.
The mating scheme was five males to one hermaphrodite, mating starts at the first day of adulthood. (A) Summary of total fertility, all experiments with N2CGCb hermaphrodites. (B-D) Number of progeny per day for N2CGCb, age-1(hx546) and daf-2(e137). P values are shown.
3.3. Negative trade-offs in random mating hermaphrodites with males
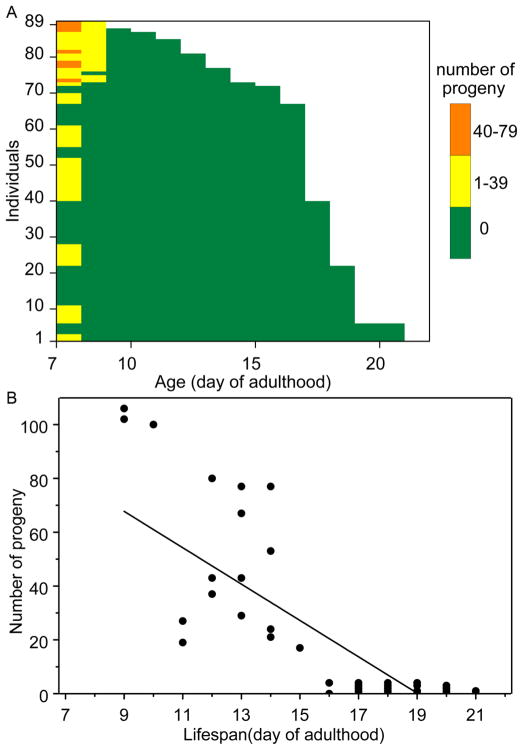
Many natural isolates of C. elegans support high frequencies of males in lab populations (Anderson et al., 2010). We asked if fertility/longevity trade-offs might emerge under random mating of post-self-reproductive hermaphrodites and males in a mixed population. There was indeed a significant negative relationship between late-life fertility and individual longevity in hermaphrodites (Fig. 3), with individuals with high reproduction dying after as little as nine days and presumably unmated individuals with no-reproduction displaying lifespans that are very similar to self-fertilization only hermaphrodites (up to 21 days).
Fig. 3. Relationship between cross-progeny production and lifespan.
In a mass mating (89 males and 89 hermaphrodites, N2CGCb), animals were allowed to mate for 24 hours (large NGM, OP50). (A) Horizontal bar graph of lifespans (x axis) of individual worms (y axis). Different colors indicate number of progeny produced per day for each individual. (B) Scatterplot of individual animal lifespans (x axis) and progeny production (y axis). There is a negative correlation between progeny production and lifespan (p < 0.0001).
3.4. Longevity mutants show no trade-offs
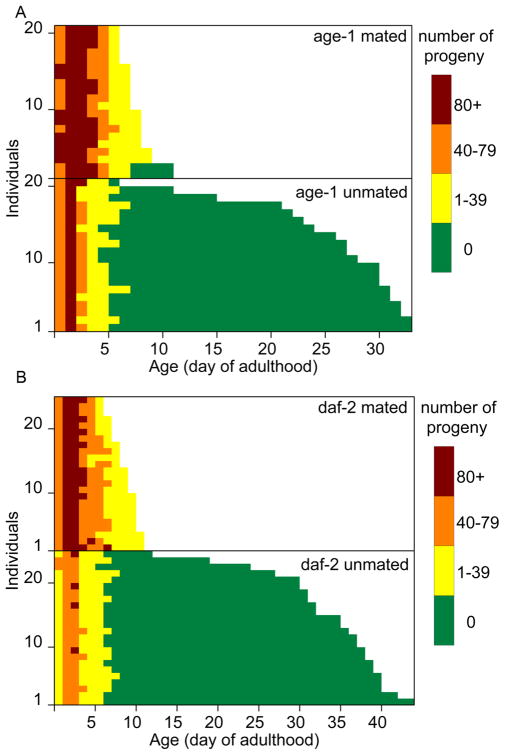
Longevity can be increased dramatically in C. elegans with up to a ten-fold increase in longevity (Ayyadevara et al., 2008). Similar to our results for wildtype hermaphrodites, when Age hermaphrodites were mated with wild-type males there was a highly significant decrease in lifespan for both age-1 and daf-2 (Fig. 4). In contrast, unmated populations displayed no significant trade-off between longevity and fertility.
Fig. 4. Lifespan and progeny production of mated and unmated individual hermaphrodites.
(Mating as in Figure 2). Horizontal bar graph of lifespans (x axis) of individual worms (y axis). Different colors indicate number of progeny produced per day for each individual. (A) age-1(hx546). (B) daf-2(e137). In both, animals that are mated live significantly shorter lives (P < 0.0001) and produce significantly more progeny (P < 0.0001).
4. Discussion
Many studies have looked for negative relationships between longevity and fertility in C. elegans (Chen et al., 2007; see Fontana, 2010; Anderson et al., 2011; and Mendenhall et al., 2011 for extensive reviews). A motivating factor for these studies is that one would expect competition for energy between the distinct physiological systems underlying reproduction and long-term survival, which should result in a tradeoff between offspring production and longevity. In general, there has been little evidence at the level of the individual for the existence of such trade-offs, although there is support for trade-offs between different genotypes; e.g., Age mutants often have reduced or altered fertility (Tissenbaum and Johnson, 2008; Gems and Riddle, 1996) and/or fail to compete with wild-type under conditions of changing environment (Jenkins et al., 2004). Here, we find that mating with males dramatically increases total reproduction in hermaphrodites and dramatically lowers female lifespan (Gems and Riddle, 1996). However, when females are “forced” to mate, there is no tradeoff between reproduction and longevity. In fact, the opposite relationship is observed in which longer-lived hermaphrodites actually produce more offspring than short-lived individuals. Well-characterized Age mutants behave much like wildtypes in this respect (Fig. 4). In contrast, when hermaphrodites and males are permitted to mate in an “open field” environment, a strong tradeoff emerges (Fig. 3).
It seems clear, however, that this tradeoff is not driven by a physiological tradeoff within hermaphrodites but is instead a behavioral tradeoff driven by the negative impacts of mating on longevity on the one hand (Gems and Riddle, 1996) and the positive effects of additional male sperm on hermaphrodite productivity on the other (Mendenhall et al., 2011). Because mated females tend to die while still fertile, there is a linear increase in reproductive output with age. In other words, there is no post-reproductive period in intensively mated hermaphrodites, and because dead worms cannot reproduce, “older” hermaphrodites produce more offspring. Yet these hermaphrodites are not old on the scale of unmated hermaphrodites (living less than half as long), so there is unrealized potential for even more reproduction within self-fertilizing hermaphrodites that must be caused by a tradeoff between early and late reproduction (Anderson et al. 2011). When there is variance in the tendency to become mated among hermaphrodites, then male-induced mortality drives a tradeoff between sperm limitation and longevity. It is therefore behavioral ecology and not physiology that shapes the late-life demography of this species.
The question, then, is what is the appropriate ecological and evolutionary context within which one should evaluate the shape of C. elegans life histories; particularly post-reproductive longevity? Population genomic data suggest that mating within natural populations of C. elegans may be rare (Anderson et al., 2010, 2012), although certain strains maintain sizeable populations of males within the laboratory (Anderson et al., 2010). Further, C. elegans hermaphrodites have retained the capacity for increasing their reproductive success via late life outcrossing with males (Mendenhall et al., 2011) and there is evidence that hermaphrodites change their mating behavior in response to the depletion of their own self sperm (Morsci et al., 2011). This capacity must therefore either be a relic maintained after their transition from an outcrossing dioecious/gonochoristic species or be still actively maintained within populations for its direct reproductive benefits. Either way, interpretation of the demographic consequences and potential tradeoffs of aging, longevity extension, and natural patterns of reproduction within this species must clearly be considered within the context of both self fertilization and outcrossing.
Highlights.
Evolutionary theories predict fertility/longevity trade-offs in certain conditions but little evidence in C. elegans
Observed negative trade-offs in randomly mating male/hermaphrodite populations
Significant positive relationship between overall fertility and longevity in highly mated hermaphrodites
Age mutants show similar patterns of trade-offs
Acknowledgments
This work has been supported by the NIH (PO1-AG08761), the Glenn Foundation, and the Ellison Medical Research Foundation (PCP). The authors declare no competing financial interests.
Footnotes
Author Contributions DW, PT and TEJ designed all experiments. DW and PT conducted all experiments. DW performed statistical analysis on data. TEJ, PT, PCP and DW generated the manuscript. All authors concurred on the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JL, Morran LT, Phillips PC. Outcrossing and the maintenance of males within C. elegans populations. J Hered. 2010;101(Suppl 1):S62–S74. doi: 10.1093/jhered/esq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Reynolds RM, Morran LT, Tolman-Thompson J, Phillips PC. Experimental evolution reveals antagonistic pleiotropy in reproductive timing but not life span in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2011;66:1300–1308. doi: 10.1093/gerona/glr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EC, Gerke JP, Shapiro JA, Crissman JR, Ghosh R, Bloom JS, Félix MA, Kruglyak L. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet. 2012;44:285–90. doi: 10.1038/ng.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Senturk D, Wang JL, Muller HG, Carey JR, Caswell H, Caswell-Chen EP. A demographic analysis of the fitness cost of extended longevity in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2007;62:126–135. doi: 10.1093/gerona/62.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988a;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, Johnson TE. Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. J Gerontol A Biol Sci Med Sci. 1988b;43:B102–B109. doi: 10.1093/geronj/43.4.b102. [DOI] [PubMed] [Google Scholar]
- Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996;379:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e2. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NL, McColl G, Lithgow GJ. Fitness cost of extended lifespan in Caenorhabditis elegans. Proc Biol Sci. 2004;271:2523–2526. doi: 10.1098/rspb.2004.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Aging can be genetically dissected into component processes using long- lived lines of Caenorhabditis elegans. Proc Natl Acad Sci USA. 1987;84:3777–3781. doi: 10.1073/pnas.84.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1982;79:6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983;22:279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Knight CG, Azevedo RB, Leroi AM. Testing life-history pleiotropy in Caenorhabditis elegans. Evolution. 2001;55:1795–1804. doi: 10.1111/j.0014-3820.2001.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Luo S, Shaw WM, Ashraf J, Murphy CT. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 2009;5:e1000789. doi: 10.1371/journal.pgen.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall AR, Wu D, Park SK, Cypser JR, Tedesco PM, Link CD, Johnson TE. Genetic dissection of late-life fertility in C. elegans. J Gerontol: A Biol Sci Med Sci. 2011;66:842–854. doi: 10.1093/gerona/glr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsci NS, Haas LA, Barr MM. Sperm status regulates sexual attraction in Caenorhabditis elegans. Genetics. 2011;189:1341–1346. doi: 10.1534/genetics.111.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR. Evolutionary Biology of Aging. New York: Oxford Univ. Press; 1991. [Google Scholar]
- Tissenbaum HA, Johnson TE. Aging Processes in Caenorhabditis elegans. In: Guarente L, Partridge L, Wallace DC, editors. Molecular Biology of Aging. Cold Spring Harbor Press; Cold Spring Harbor, N.Y: 2008. pp. 153–183. [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Walker DW, McColl G, Jenkins NL, Harris J, Lithgow GJ. Natural selection - evolution of lifespan in C. elegans. Nature. 2000;405:296–297. doi: 10.1038/35012693. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]