Abstract
Background
Soy consumption may protect against breast cancer through modification of estrogen metabolism.
Objective
We examined the effect of soy foods on urinary estrogens and the 2-hydroxy (OH)/16α-OH estrone (E1) ratio in 2 dietary interventions with premenopausal women.
Methods
BEAN1 was a 2-year randomized trial and BEAN2 a 13-month randomized crossover study. In both interventions, study participants consumed a high-soy diet with 2 soy food servings/day and a low-soy diet with <3 servings of soy/week. Urine samples were collected at baseline and at the end of the diet periods, analyzed for 9 estrogen metabolites by liquid chromatography mass spectrometry, and adjusted for creatinine levels. For BEAN1, 2 samples for 188 participants and for BEAN2, 3 samples for 79 women were analyzed. We applied mixed-effects regression models with log-transformed values of estrogen metabolites and soy intake as the exposure variable.
Results
In BEAN1, no effect of the high-soy diet on individual estrogen metabolites or hydroxylation pathways was observed. The median 2-OH/16α-OH E1 ratio decreased non-significantly in the intervention group from 6.2 to 5.2 as compared to 6.8 and 7.2 in the control group (p=0.63). In BEAN2, only 4-OHE1 was significantly lower after the high-soy diet. Interaction terms of the high-soy diet with equol producer status, ethnicity, and weight status revealed no significant effect modification.
Conclusions
Contrary to our hypothesis and some previous reports, the results from 2 well controlled dietary interventions do not support an effect of a high-soy diet on a panel of urinary estrogen metabolites and the 2-OH/16α-OHE1 ratio.
Keywords: Soy foods, estrogen metabolites, isoflavones, breast cancer risk, dietary intervention, premenopausal women
Introduction
Soy beans and isoflavones have been investigated as cancer protective agents for a long time; a meta-analysis described a 15% lower breast cancer risk associated with soy intake, but the association is primarily found among women of Asian background.1 Although the estrogen-like isoflavones appear to exert, depending on the hormonal milieu, estrogenic or antiestrogenic effects through competitive binding to the estrogen receptors (ER),2 there is little evidence for an effect of soy foods on circulating estrone (E1) and estradiol (E2) levels.3 The protective action of soy isoflavones against breast cancer may be due to the modulation of cytochrome P450 enzymes, including CYP1A1, CYP1A2, CYP1B1, and the 3A family, which play a role in estrogen hydroxylation and person-to-person differences in estrogen action.4 Three major competing pathways result in the more carcinogenic 4-hydroxy (OH) and 16α-OH metabolites and the less harmful 2-OH metabolites.5 Thus, women who metabolize a larger proportion of endogenous estrogens as 16α-OHE1 and have a lower 2-OH/16α-OHE1 ratio may be at greater breast cancer risk,6 but a recent report from the Nurses’ Health Study did not detect a lower breast cancer risk with a higher 2/16α-OH metabolite ratio.7 An influence of isoflavones on several P450 enzymes has been shown in experimental settings8;9 and a number of intervention studies in women suggested beneficial changes in the 2-OH/16α-OHE1 ratio after administration of soy foods or supplements.10-12 Whereas previous reports relied on ELISA assays and on gas chromatography mass spectrometry (GCMS) to assess 2-OHE1 and 16α-OHE1, new liquid chromatography mass spectrometry (LCMS) methods allows for the detection of multiple estrogen metabolites in a faster, less expensive and more efficient manner.13-15 Based on the hypothesis that soy intake may result in a decrease of the more carcinogenic 4-OH and 16α-OH metabolites and an increase in the 2-OH pathway, we explored the effect of 2 daily servings of soy on the formation of urinary, 2, 4, and 16α-OH estrogen metabolites in 2 randomized soy trials with premenopausal women. One consisted of 93 intervention and 95 control participants16 and the other investigation used a cross-over design among 79 women.17;18
Methods
Study design and recruitment
The first Breast, Estrogens, And Nutrition (BEAN1) study was designed as a randomized clinical trial to examine the effects of 2 daily soy servings on sex steroid hormones and mammographic densities.16;19 Overall, 10,022 women with normal mammograms were contacted, 975 (9.73%) replied, and 352 were found eligible. Women were excluded from the study due to pregnancy or breast-feeding, consumption of estrogen-containing oral contraceptives or supplements containing isoflavones, cancer diagnosis, breast implants, hysterectomy, lack of a regular menstrual period, or intake of >5 soy servings per week. After a run-in period, 220 women were randomized to a soy diet or to the control group and 189 subjects completed 2 years of intervention. The number of dropouts did not differ by group; 17 (15.6%) women in the intervention group and 14 (12.6%) controls dropped out of the study prematurely (p=0.53).
BEAN2 used a crossover design; 82 participants completed a 6-month high-soy diet and a 6-month low-soy diet, which were separated by a 1-month washout period. The participants were recruited through multiple sources, as described elsewhere.17 After mailing 16,306 invitations, 825 interested women replied, 310 women were screened, 96 women were randomized, and 14 women dropped out of the study. The exclusion criteria were the same as in BEAN1 except for the mammogram requirement.
In both studies, all subjects completed a baseline questionnaire asking for demographic, anthropometric, reproductive, and dietary information. The protocols of the two studies were approved by the University of Hawaii Committee on Human Studies and by the institutional review boards of the participating hospitals. All women signed an informed consent form before entry into the trial and gave written permission to use frozen samples for future analyses. A Data Safety Monitoring Committee reviewed the progress of the studies, reasons for dropouts, and any reported symptoms annually. During the high-soy diet, women consumed 2 daily servings of soy containing approximately 25 mg aglycone equivalents of isoflavones per serving. Participants chose between tofu, soy milk, roasted soy nuts, soy bars, and soy protein powder. Dietitians provided dietary counseling on how to replace common dishes with soy foods. During the low-soy diet, the women were instructed to maintain their regular diet and to consume <3 soy food servings per week. Adherence to the study protocol as assessed by unannounced 24-hour dietary recalls and urinary isoflavonoid excretion was high in both studies.16;17
Urine collection and analysis
In both studies, repeated overnight urine samples were collected during the luteal phase, aliquoted into 2 mL containers, and stored at -80°C. Ascorbic and boric acid were added to the urine collection containers to control bacterial growth. For the BEAN1 study, the baseline and the final samples were analyzed for 188 women after 8-11 years of storage. If no sample was available at month 24, the month 12 sample was used instead. For the 79 BEAN2 participants, 3 samples (baseline and at the end of low-soy [month 6] and high-soy [month 13] diets) were analyzed after 1-4 years of storage. For one woman, only months 0 and 13 samples were available for this analysis.
In both studies, the most predominant steroidal estrogens in premenopausal women,14 namely E1, E2, 2-OHE1, 2-OHE2, 2-MeOE1, 4-OHE1, E3, 16keto-E2, 16α-OHE1 were measured by LCMS (model Exactive, Thermo Fisher Scientific, Waltham, MA) using 5 labeled internal standards as described in detail previously.15 Ascorbic acid was added during hydrolysis and during derivatization to prevent artificial oxidation of sensitive analytes.15 Analysis of an external urine pool from premenopausal women repeated on 9 different days revealed coefficients of variation of 4-21% depending on the analyte concentrations. Urinary creatinine concentrations were measured using a Roche-Cobas MiraPlus clinical chemistry autoanalyzer (Roche Diagnostics, Switzerland). All estrogen and isoflavonoid measurements were expressed per mg creatinine to adjust for urine volume.
Urinary isoflavonoids as a biomarker for soy intake were measured previously by high-pressure liquid chromatography in BEAN116 and by LCMS in BEAN2.17 The isoflavonoid equol was assessed by LCMS in both studies, but in BEAN1, it was measured at the same time as the estrogen metabolites in 2 urine samples only, whereas in BEAN2 equol was measured together with genistein and dadzein in 8 urine samples per woman. Since equol producers are thought to experience more protective effects of isoflavones than non-producers,20 equol producer status was determined based on 2 criteria: urinary daidzein excretion ≥2 nmol/mg creatinine and a urinary equol to daidzein ratio ≥0.018.21;22 In BEAN1, 23 women met the criteria at least once and were considered equol producers: 7 were of Asian ethnicity and 16 were non-Asian. In BEAN2, 41 women were equol producers, of which 10 were of Asian ethnicity.
Statistical analysis
The SAS statistical software package version 9.2 (SAS Institute Inc., Cary, NC) was used for the statistical analysis. We calculated the sum of the 9 urinary metabolites measured in both studies and the relative percentages for the 3 pathways (2, 4, and 16α) based on molar concentrations. The 2/16α-OHE1 ratio was computed as the ratio of the 2 metabolites. Urinary estrogen metabolite variables were log-transformed due to non-normal distributions. Student’s t-tests of the log-transformed values and chi-square tests were performed to assess differences in the baseline values of urinary estrogen metabolites and demographic characteristics between randomization groups. To examine the effect of the high-soy diet on urinary estrogen metabolites, we applied mixed-effects regression models with log-transformed values of estrogen metabolites;23 the models also included the randomization group and the time of urine collection (baseline vs. end of diet period).
Results
For BEAN1, 188 participants were included in the analysis (Table 1); 95 women were in the control group and 93 women in the intervention group. In both groups, nearly half of the women were Asians. At baseline, the 2 groups did not differ in age, body mass index, dietary isoflavone intake, urinary isoflavonoid excretion, total estrogen metabolites, and the 2/16α-OHE1 ratio. The number of equol producers was low in both groups (7 in the control and 17 in the intervention group; p=0.29). The median total estrogen metabolite levels were 53.9 and 54.8 ng/mg creatinine for the control and the intervention group, while the median 2/16α-OHE1 ratios were 6.8 and 6.2. BEAN2 included 79 participants (Table 1); 40 women were in group A and 39 women in group B. Women in group B were significantly younger than women in group A with mean age of 37.6±5.8 versus 41.3±5.6 (p=0.01). The mean urinary isoflavonoid excretion was lower in group B than in group A (3.0±6.3 vs. 7.3±11.3; p=0.04). Except for 4-OHE1 (p=0.03), levels of estrogen metabolites (43.4 vs. 48.7 ng/mg creatinine) and the 2/16α-OHE1 ratio (3.8 vs. 5.3) were similar across randomization groups. In both studies, 2-OH metabolites constituted the largest proportion and were closely followed by 16-OH metabolites, but the 4-OH pathway constituted less than 10% (Figure 1).
Table 1.
Baseline characteristics of BEAN1 and BEAN2 participantsa
| BEAN1
|
BEAN2
|
||||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | p value | Group A (high to low-soy) | Group B (low to high-soy) | p value | ||
| Number of participants | 95 | 93 | 40 | 39 | |||
| Ethnicity | Non-Asian | 57 (60) | 57 (61) | 0.86 | 29 (72) | 29 (74) | 0.85 |
| Asian | 38 (40) | 36 (39) | 11 (28) | 10 (26) | |||
| Equol producer statusb | No | 45 (47) | 66 (71) | 0.29 | 17 (43) | 21 (54) | 0.31 |
| Yes | 7 (7) | 17 (18) | 23 (57) | 18 (46) | |||
| Age (years) | 42.8±2.9 | 43.2±2.8 | 0.40 | 41.3±5.6 | 37.6±5.8 | 0.01 | |
| Body mass index (kg/m2) | 26.0±6.1 | 26.2±5.6 | 0.82 | 25.8±5.2 | 26.0±6.1 | 0.83 | |
| Dietary isoflavone intake (mg/d) | 5.0±7.5 | 4.2±4.8 | 0.38 | 16.3±38.8 | 21.7±37.3 | 0.53 | |
| Urinary isoflavonoids (nmol/mg creatinine) | 5.5±15.1 | 8.4±18.6 | 0.24 | 7.3±11.3 | 3.0±6.3 | 0.04 | |
| Total estrogen metabolites (ng/mg creatinine) | 53.9 (35.2, 80.8) | 54.8 (35.3, 73.5) | 0.53 | 43.4 (28.8, 61.7) | 48.7 (33.8, 76.1) | 0.21 | |
| Estrone (E1) | 10.0 (6.8, 12.9) | 10.3 (6.9, 14.5) | 0.81 | 7.4 (5.4, 12.6) | 9.2 (6.6, 15.7) | 0.11 | |
| 2-OHE1 | 13.8 (6.1, 23.3) | 10.2 (4.0, 20.2) | 0.14 | 9.6 (4.3, 21.6) | 11.6 (5.3, 21.5) | 0.78 | |
| 4-OHE1 | 2.5 (1.5, 3.8) | 2.1 (1.2, 3.7) | 0.36 | 0.8 (0.5, 2.0) | 1.3 (0.8, 2.2) | 0.03 | |
| 16α-OHE1 | 2.2 (1.0, 3.4) | 1.9 (1.0, 3.2) | 0.70 | 2.5 (1.3, 4.2) | 2.2 (1.2, 3.4) | 0.92 | |
| 2-MeOE1 | 4.6 (2.3, 9.2) | 4.6 (1.8, 7.7) | 0.45 | 2.1 (0.9, 4.0) | 2.6 (1.4, 4.5) | 0.39 | |
| Estradiol (E2) | 3.2 (2.5, 4.7) | 3.9 (2.5, 5.2) | 0.57 | 2.7 (2.0, 4.4) | 3.3 (2.5, 5.1) | 0.24 | |
| 2-OHE2 | 0.9 (0.5, 2.4) | 1.2 (0.3, 2.1) | 0.99 | 1.2 (0.3, 2.9) | 1.3 (0.4, 3.8) | 0.73 | |
| 16-keto E2 | 2.1 (1.1, 2.9) | 2.1 (1.2, 2.7) | 0.92 | 1.6 (1.1, 2.7) | 2.0 (1.0, 2.6) | 0.83 | |
| Estriol (E3) | 9.7 (5.7, 15.9) | 10.9 (7.1, 16.0) | 0.64 | 9.9 (5.7, 15.1) | 11.0 (6.6, 17.9) | 0.53 | |
| 2-OH pathway (%)c | 40.1 (26.1, 50.9) | 34.6 (25.2, 46.5) | 0.09 | 31.2 (21.9, 45.8) | 30.0 (19.8, 45.7) | 0.50 | |
| 4-OH pathway (%)d | 4.5 (3.2, 6.2) | 4.5 (2.9, 6.3) | 0.77 | 2.2 (1.6, 3.0) | 3.1 (1.7, 4.4) | 0.05 | |
| 16-OH pathway (%)e | 25.2 (19.0, 36.3) | 30.3 (19.9, 41.5) | 0.32 | 32.3 (22.1, 46.9) | 28.2 (19.9, 42.7) | 0.80 | |
| 2/16α-OHE1 ratio | 6.8 (3.6, 12.4) | 6.2 (2.1, 12.3) | 0.60 | 3.8 (2.2, 8.5) | 5.3 (1.8, 8.3) | 0.74 | |
Data are shown as N (%) or mean ±std for all but the estrogen metabolites, which are shown as medians (Q1, Q3); P-values were calculated from χ2 and Student’s t tests; for the estrogen metabolites log transformed estrogen values were used. N=94 for total estrogen metabolites for the BEAN1 control group.
Missing if urinary daidzein excretion ≥2 nmol/mg; yes if daidzein ≥2 nmol/mg and equol/daidzein ≥0.018; no if daidzein ≥2 nmol/mg and equol/daidzein <0.018.
(2-OHE1 + 2-OHE2 + 2-MeOE1)/total estrogen metabolites after conversion to molar concentrations
(4-OHE1)/total estrogen metabolites after conversion to molar concentrations
(E3 + 16α-OHE1 + 16-ketoE2)/total estrogen metabolites after conversion to molar concentrations
Figure 1.
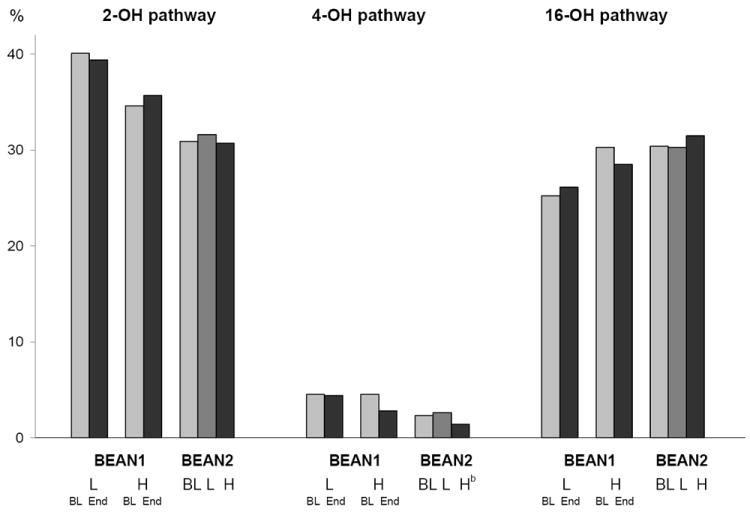
Urinary estrogen metabolite excretion by pathway in BEAN1 and BEAN2a
- 2-OH pathway = (2-OHE1 + 2-OHE2 + 2-MeOE1)/total estrogen metabolites
- 4-OH pathway = (4-OHE1)/total estrogen metabolites
- 16-OH pathway = (E3 + 16α-OHE1 + 16-ketoE2)/total estrogen metabolites
BL = baseline; End = 2 years;
L=control group or low-soy diet (month 6 or 13); H=intervention group or high-soy diet (month 6 or 13)
bP value = 0.01 for the high-soy diet
In BEAN1, no significant effect of the high-soy diet on any estrogen metabolite was observed (Table 2); there was only a tendency for lower 4-OHE1 (p=0.06). For example, the respective medians of total estrogen metabolites in the control versus the intervention group were 53.9 and 54.8 and 45.9 and 49.7 ng/mg creatinine at baseline and at the end of the study (p=0.81). The decrease over time in median total estrogen metabolites of 8 ng/mg creatinine for the controls and 5 ng/mg creatinine for the intervention group was not statistically significant (p=0.15). For the 2/16α-OHE1 ratio, the median was similar between the 2 groups at baseline (6.2 and 6.8), whereas at the end of the study the ratio decreased in the intervention but not in the control group (5.2 vs. 7.5). However, neither the dietary intervention effect (p=0.63) or its interaction with time were significant (p=0.93).
Table 2.
Effect of the high-soy diet on urinary estrogen metabolite excretion in BEAN1 and BEAN2a
| BEAN 1 (randomized 2-year study) | BEAN 2 (randomized 13-month crossover study) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Time | Control | Intervention | p valueb | Baseline | Low-soy (month 6 or 13) | High-soy (month 6 or 13) | p valueb | |
| Total estrogen metabolites (ng/mg creatinine) | Baseline | 53.9 (35.2, 80.8) | 54.8 (35.3, 73.5) | 0.81 | 47.1 (31.8, 66.5) | 55.8 (38.4, 76.3) | 53.2 (33.6, 80.4) | 0.99 |
| After 2 yrs | 45.9 (28.9, 70.9) | 49.7 (33.2, 74.4) | ||||||
| Estrone (E1) | Baseline | 10.0 (6.8, 12.9) | 10.3 (6.9, 14.5) | 0.53 | 8.8 (6.3, 13.6) | 10.7 (7.4, 15.6) | 12.5 (6.8, 17.6) | 0.45 |
| End | 8.5 (5.1, 12.0) | 8.4 (5.5, 13.6) | ||||||
| 2-OHE1 | Baseline | 13.8 (6.1, 23.3) | 10.2 (4.0, 20.2) | 0.45 | 10.0 (4.8, 21.5) | 14.6 (5.6, 23.8) | 11.5 (5.6, 18.6) | 0.76 |
| End | 10.5 (3.9, 20.3) | 9.6 (4.1, 22.0) | ||||||
| 4-OHE1 | Baseline | 2.5 (1.5, 3.8) | 2.1 (1.2, 3.7) | 0.06 | 1.1 (0.6, 2.1) | 1.3 (0.6, 2.3) | 0.8 (0.3, 1.9) | <0.01 |
| End | 2.1 (1.1, 3.6) | 1.2 (0.5, 2.7) | ||||||
| 16α-OHE1 | Baseline | 2.2 (1.0, 3.4) | 1.9 (1.0, 3.2) | 0.69 | 2.3 (1.3, 4.0) | 3.0 (1.6, 5.1) | 2.6 (1.4, 6.7) | 0.65 |
| End | 1.4 (0.7, 2.8) | 1.6 (0.7, 2.9) | ||||||
| 2-MeOE1 | Baseline | 4.6 (2.3, 9.2) | 4.6 (1.8, 7.7) | 0.66 | 2.3 (1.1, 4.4) | 2.5 (1.1, 4.7) | 2.7 (1.2, 4.5) | 0.66 |
| End | 3.9 (1.7, 6.8) | 4.5 (1.8, 6.8) | ||||||
| Estradiol (E2) | Baseline | 3.2 (2.5, 4.7) | 3.9 (2.5, 5.2) | 0.22 | 2.9 (2.1, 4.8) | 3.7 (2.6, 5.2) | 3.5 (2.3, 6.3) | 0.49 |
| End | 3.0 (2.0, 4.5) | 3.2 (2.1, 4.7) | ||||||
| 2-OHE2 | Baseline | 0.9 (0.5, 2.4) | 1.2 (0.3, 2.1) | 0.90 | 1.3 (0.4, 3.2) | 1.4 (0.6, 3.2) | 1.4 (0.5, 3.0) | 0.89 |
| End | 1.1 (0.3, 2.2) | 0.9 (0.4, 2.1) | ||||||
| 16-keto E2 | Baseline | 2.1 (1.1, 2.9) | 2.1 (1.2, 2.7) | 0.57 | 1.8 (1.0, 2.7) | 2.1 (1.3, 3.0) | 1.7 (1.0, 4.1) | 0.89 |
| End | 1.5 (0.9, 2.6) | 1.6 (1.0, 2.8) | ||||||
| Estriol (E3) | Baseline | 9.7 (5.7, 15.9) | 10.9 (7.1, 16.0) | 0.29 | 9.9 (5.8, 16.2) | 12.0 (6.5, 17.2) | 10.7 (5.8, 22.1) | 0.73 |
| End | 8.9 (4.8, 14.1) | 9.3 (5.1, 16.5) | ||||||
| 2-OH pathway (%)c | Baseline | 40.1 (26.1, 50.9) | 34.6 (25.2, 46.5) | 0.16 | 30.9 (21.3, 45.7) | 31.6 (22.4, 43.5) | 30.7 (22.0, 46.7) | 0.81 |
| End | 39.4 (23.8, 49.1) | 35.7 (21.3, 47.5) | ||||||
| 4-OH pathway (%)d | Baseline | 4.5 (3.2, 6.2) | 4.5 (2.9, 6.3) | 0.12 | 2.3 (1.7, 3.8) | 2.6 (1.4, 3.7) | 1.4 (0.7, 2.6) | 0.01 |
| End | 4.4 (3.2, 6.7) | 2.8 (1.4, 5.0) | ||||||
| 16-OH pathway (%)e | Baseline | 25.2 (19.0, 36.3) | 30.3 (19.9, 41.5) | 0.37 | 30.4 (21.2, 43.3) | 30.3 (23.3, 45.6) | 31.5 (19.9, 43.2) | 0.50 |
| End | 26.1 (18.5, 39.4) | 28.5 (18.1, 42.9) | ||||||
| 2/16α-OHE1 ratio | Baseline | 6.8 (3.6, 12.4) | 6.2 (2.1, 12.3) | 0.63 | 5.1 (2.1, 8.3) | 4.5 (1.9, 8.4) | 4.4 (1.9, 8.7) | 0.24 |
| End | 7.5 (2.9, 14.5) | 5.2 (2.4, 14.2) | ||||||
Numbers are medians (Q1, Q3)
p-values for the effect of high-soy diet were calculated from mixed-effects models using log-transformed values of urinary estrogen metabolites as exposure variables.
(2-OHE1 + 2-OHE2 + 2-MeOE1)/total estrogen metabolites after conversion to molar concentrations
(4-OHE1)/total estrogen metabolites after conversion to molar concentrations
(E3 + 16α-OHE1 + 16-ketoE2)/total estrogen metabolites after conversion to molar concentrations.
In BEAN2, the high-soy diet significantly affected 4-OHE1 but not any other metabolite (Table 2); 4-OHE1 was lower at the end of the high-soy diet than at baseline or at the end of the low-soy diet (p<0.01). The respective medians for total estrogen metabolite levels were 47.1, 55.8, and 53.2 ng/mg creatinine at baseline, low-soy and high-soy (p=0.99). For the 2/16α-OHE1 ratio, the respective medians were 5.1, 4.5, and 4.4 (p=0.24). No significant time effects were found for total estrogen metabolites (p=0.31) and the 2/16α-OHE1 ratio (p=0.47). Interaction terms of the dietary assignment with ethnicity, equol producer status, and overweight indicated no significant effect modification.
Grouping the metabolites by pathway confirmed the lack of an intervention effect on the relative proportions of 2, 4, and 16-OH metabolites in both studies (Figure 1); only the 4-OH pathway in BEAN2 reached statistical significance (p=0.01).
Discussion
Contrary to our hypothesis that soy may increase the 2/16α-OHE1 ratio, the current analysis showed little effect of long-term, daily soy food consumption on urinary estrogen metabolite excretion. In both trials, estrogen metabolites, individually or grouped by pathway, and the 2/16α-OHE1 ratio, did not change significantly. Only 4-OHE1, a metabolite with a very low concentration, decreased at the end of the high-soy diet in BEAN2, probably a chance finding given the multiple testing. In BEAN1, total estrogen metabolites decreased over time in both groups and the 2/16α-OHE1 ratio was slightly lower at the end in the intervention than in the control group without reaching statistical significance. Furthermore, the data on equol producer status need to be interpreted with caution. Equol production was not adequately assessed in BEAN1, especially in the control group, because it was only measured in 2 urine samples and not all participants were actually exposed to isoflavones. As shown before, a soy challenge is needed to assess the ability to produce equol.24
The current study conflicts with a cross-sectional study among 430 Asian American women that found no association of 15 estrogen metabolites assessed by LCMS with soy intake but reported a higher 2/16α-OHE1 ratio among women with high soy intake.25 The association was of similar size for pre-and postmenopausal women but only statistically significant in the entire population. Interventions among premenopausal women reported discrepant findings. An investigation with a soy beverage26 and with an isoflavone supplement27 detected no change in urinary estrogen metabolites and the 2/16α-OH ratio. However, a GCMS-based crossover trial in 12 women consuming 10, 65, and 129 mg of isoflavones from soy protein powder for 3 months each described significantly lower 16α-OHE1, 4-OHE1, and 4-OHE2 and a higher 2/16α-OHE1 ratio after supplement intake.10 In a similar investigation with 8 women, soy milk with high isoflavone content (113-202 mg/day) 11 was associated with a higher urinary excretion of 2-OHE1 and a higher 2/16α-OHE1 ratio.11 Our previous analysis using GCMS in 82 BEAN2 participants also found a higher 2/16α-OHE1 ratio at the end of the high-soy diet (p=0.05), but the individual metabolites did not differ significantly.28
Findings in postmenopausal women are of interest because the weak estrogenic effects of isoflavones are more likely to be physiologically important in women with low endogenous production,2;29 but conflicting results have also been reported. In a trial with >100 mg isoflavones from protein isolates, soy and seaweed plus soy significantly increased 2-OHE1 and the 2/16α-OHE1 ratio,12 but the 2/16α-OHE1 ratio only increased among women with detectable serum equol levels in another investigation.30 No association between urinary isoflavonoid excretion and the 2/16α-OH ratio was detected in a cross-sectional study31 and in 46 women consuming a soy flour or flaxseed muffin.32
Possible explanations for the conflicting literature include the relatively long-term duration, the larger sample size, and the moderate isoflavone dosages in the BEAN studies as compared to short trials with high doses of isoflavones.10-12 There is also a possibility that soy drinks as administered in some trials lead to higher isoflavone uptake than soy foods.33 The discrepancy with the previously published BEAN2 results based on GCMS measurements of 2-OHE1 and 16α-OHE1 28 may be due to analytical issues. As reported previously, 2-OHE1 levels measured by LCMS were approximately twice as high as the GCMS results.15 This may be a result of different internal standards that were used for the 2 methods; the GCMS procedure included only one internal standard (5-α-Androstan-3-α) that was not well related to 2-OHE1, whereas 5 labeled estrogen metabolites were used as internal standards in the LCMS assay.15 Nevertheless, using 4-OHE2-d5 as internal standard for 2-OHE1 in the LCMS assay was suboptimal, as most other methods are, for 2-OHE1 determination and may have led to less than perfect values. For future studies, it may be advisable to use exact matches of internal standards for each measured estrogen metabolite. Alternatively, a loss of the relatively unstable analyte 2-OHE1 might have occurred during derivatization in the GCMS procedure that did not include ascorbic acid for preservation.15
The current analysis had a number of limitations. The multiple testing for many different urinary estrogen measures may have resulted in false positive findings. The possible selection bias due to the strict participant eligibility criteria for the intervention studies likely limited the generalizability of the observed findings to all women. In BEAN2, a major weakness of the study is that the randomization did not lead to perfectly balanced groups as indicated by the baseline difference in age. On the other hand, this study had several strengths. The exposure to isoflavones by traditional soy foods represented soy intake in Asian countries more closely than the administration of oral supplements used in other studies. In our studies, urine collection was timed according to menstrual cycle and most samples were collected during the midluteal phase as confirmed by progesterone testing in BEAN116 or subsequent menstruation dates in BEAN2.17 The repeated measures for urinary estrogens reduced concerns about intra-individual variability over time. The use of LCMS provided excellent assessments of multiple estrogen metabolites.13;15 Adherence to the dietary intervention was carefully monitored by 2 methods (dietary recalls and urinary isoflavonoid excretion) and remained high throughout the trial.17
In conclusion, no beneficial influence of a high-soy diet on the 2-OH/16α-OHE1 ratio and a panel of urinary estrogen metabolites was detected at the end of 2 well controlled dietary interventions among premenopausal women, but the analytic challenges of measuring these metabolites may have prevented the detection of an effect. Since the potential of isoflavones to modulate estrogen metabolizing enzymes is primarily based on limited evidence from experimental studies8 and on urinary excretion profiles in humans, this research area needs further elucidation.
Acknowledgments
Support for this study was obtained by grants from the National Cancer Institute R01 CA 80843 and P30 CA71789 and from the National Center for Research Resources S10 RR020890.
Abbreviations
- E1
Estrone
- 2-OHE1
2-hydroxy estrone
- 4-OHE1
4-hydroxy estrone
- 16α-OHE1
16α-hydroxy estrone
- 2-MeOE1
2-methoxy estrone
- E2
Estradiol
- 2-OHE2
2-hydroxy estradiol
- 16-keto E2
16-keto estradiol
- E3
Estriol
- GCMS
Gas chromatography mass spectrometry
- LCMS
Liquid chromatography mass spectrometry
Footnotes
Conflict of Interest None of the authors has any conflicts of interest.
References
- 1.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setchell KD. Soy isoflavones--benefits and risks from nature’s selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–362S. doi: 10.1080/07315724.2001.10719168. [DOI] [PubMed] [Google Scholar]
- 3.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 6.Obi N, Vrieling A, Heinz J, Chang-Claude J. Estrogen metabolite ratio: Is the 2-hydroxyestrone to 16alpha-hydroxyestrone ratio predictive for breast cancer? Int J Womens Health. 2011;3:37–51. doi: 10.2147/IJWH.S7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliassen AH, Spiegelman D, Xu X, Keefer LK, Veenstra TD, Barbieri RL, Willett WC, Hankinson SE, Ziegler RG. Urinary Estrogens and Estrogen Metabolites and Subsequent Risk of Breast Cancer among Premenopausal Women. Cancer Res. 2012;72:696–706. doi: 10.1158/0008-5472.CAN-11-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28:801–808. doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:1101–1108. [PubMed] [Google Scholar]
- 11.Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16alpha- hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000;60:1299–1305. [PubMed] [Google Scholar]
- 12.Teas J, Hurley TG, Hebert JR, Franke AA, Sepkovic DW, Kurzer MS. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J Nutr. 2009;139:939–944. doi: 10.3945/jn.108.100834. [DOI] [PubMed] [Google Scholar]
- 13.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG. A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer Epidemiol Biomarkers Prev. 2008;17:3411–3418. doi: 10.1158/1055-9965.EPI-08-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Anal Bioanal Chem. 2011;401:1319–1330. doi: 10.1007/s00216-011-5164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy SP, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–1744. [PubMed] [Google Scholar]
- 17.Maskarinec G, Morimoto Y, Conroy SM, Pagano IS, Franke AA. The volume of nipple aspirate fluid is not affected by 6 months of treatment with soy foods in premenopausal women. J Nutr. 2011;141:626–630. doi: 10.3945/jn.110.133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maskarinec G, Ollberding NJ, Conroy SM, Morimoto Y, Pagano IS, Franke AA, Gentzschein E, Stanczyk FZ. Estrogen levels in nipple aspirate fluid and serum during a randomized soy trial. Cancer Epidemiol Biomarkers Prev. 2011;20:1815–1821. doi: 10.1158/1055-9965.EPI-11-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–3094. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 21.Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Br J Nutr. 2011:1–6. doi: 10.1017/S0007114511004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke AA, Lai JF, Halm BM, Pagano I, Kono N, Mack WJ, Hodis HN. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.03.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc.; 1996. [Google Scholar]
- 24.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 25.Fuhrman BJ, Pfeiffer R, Xu X, Wu AH, Korde L, Gail MH, Keefer LK, Veenstra TD, Hoover RN, Ziegler RG. Soy intake is associated with increased 2-hydroxylation and decreased 16alpha-hydroxylation of estrogens in Asian-American women. Cancer Epidemiol Biomarkers Prev. 2009;18:2751–2760. doi: 10.1158/1055-9965.EPI-09-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martini MC, Dancisak BB, Haggans CJ, Thomas W, Slavin JL. Effects of soy intake on sex hormone metabolism in premenopausal women. Nutr Cancer. 1999;34:133–139. doi: 10.1207/S15327914NC3402_2. [DOI] [PubMed] [Google Scholar]
- 27.Maskarinec G, Williams AE, Stanczyk FZ, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- 28.Morimoto Y, Conroy SM, Pagano IS, Isaki M, Franke AA, Nordt FJ, Maskarinec G. Urinary estrogen metabolites during a randomized soy trial. Nutr Cancer. 2012;64:307–314. doi: 10.1080/01635581.2012.648819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:3095S–3108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 30.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J Nutr. 2005;135:603–608. doi: 10.1093/jn/135.3.603. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson C, Skor HE, Dawn FE, Scholes D, Chen C, Wahala K, Schwartz SM, Lampe JW. Urinary equol excretion in relation to 2-hydroxyestrone and 16alpha-hydroxyestrone concentrations: an observational study of young to middle-aged women. J Steroid Biochem Mol Biol. 2003;86:71–77. doi: 10.1016/s0960-0760(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 32.Brooks JD, Ward WE, Lewis JE, Hilditch J, Nickell L, Wong E, Thompson LU. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am J Clin Nutr. 2004;79:318–325. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- 33.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]


