Abstract
Background
The blood-brain-barrier, formed by specialized brain endothelial cells that are interconnected by tight junctions, strictly regulates paracellular permeability to maintain an optimal extracellular environment for brain homeostasis. Diabetes is known to compromise the blood-brain-barrier although the underlying mechanism remains unknown.
Objective
The aim of this study was to elucidate the molecular mechanisms underlying disruption of the blood-brain-barrier in diabetes and to determine whether activation of AMP-activated protein kinase prevents diabetes-induced blood-brain-barrier dysfunction
Methods and Results
Exposure of human brain microvascular endothelial cells to high glucose (25 mmol/L D-glucose), but not to high osmotic conditions (20 mmol/L L-glucose plus 5 mmol/L D-glucose), for 2 h to 1 week significantly increased the permeability of blood-brain-barrier in parallel with lowered expression levels of zonula occludens-1, occludin, and claudin-5, three proteins that are essential to maintain endothelial cell tight junctions. In addition, high glucose significantly increased generation of superoxide anions. Adenoviral overexpression of superoxide dismutase or catalase significantly attenuated the high glucose-induced reduction of endothelial cell tight junction proteins. Furthermore, administration of apocynin reversed the effects of high glucose on endothelial cell tight junction proteins. Finally, activation of AMP-activated protein kinase with 5-amino-4-imidazole carboxamide riboside (AICAR) or adenoviral overexpression of constitutively active AMP-activated protein kinase mutants (Ad-AMPK-CA) abolished both induction of NAD(P)H oxidase-derived superoxide anions and tight junction protein degradation induced by high glucose.
Conclusions
We conclude that high glucose increases blood-brain-barrier dysfunction in diabetes through induction of superoxide anions and that the activation of AMP-activated protein kinase protects the integrity of the blood-brain-barrier by suppressing the induction of NAD(P)H oxidase-derived superoxide anions.
Keywords: AMPK, NAD(P)H oxidase, blood brain barrier, tight junctions, diabetes
INTRODUCTION
The blood-brain barrier protects the brain from potentially neurotoxic substances and facilitates the exchange of nutrients and waste products between the brain and blood, thus maintaining an optimal extracellular environment for neuronal function. The first line of defense is the endothelium, which presents a dynamic and highly regulated interface between the blood and central nervous system. The endothelial cells of the blood-brain barrier are distinguished from peripheral endothelial cells by their lack of fenestrations, minimal pinocytotic activity, and the presence of endothelial cell tight junctions (1). A major role of the blood-brain barrier is strict regulation of paracellular permeability. This is primarily mediated by the tight junction between endothelial cells of the capillary, which restrict paracellular movement of solutes, ions, and water.
Tight junction is dynamic and highly regulated structures, and decreased tight junction protein expression or variations in subcellular localization are associated with alterations in paracellular permeability. Preservation of the tight junction is governed by three essential transmembrane proteins: occludin, claudins, and junction adhesion molecules. Occludin is a tetraspaning membrane protein (~60–65 kDa) containing two extracellular loops separated by a short cytosolic loop with both amino and carboxy-terminal domains within the cytosol. Occludin expression is correlated with increased electrical resistance across the membrane and decreased paracellular permeability. Occludin may act as a primary shock-absorber, mediating tight junction responses to acute changes in vascular dynamics (2). Claudins (~20–24 kDa) are a family of at least 24 proteins, among which claudins-3, -5, and -12 have been shown to be present within blood-brain barrier endothelial cells. Claudin-5 is a critical component of the blood-brain barrier as it closes the blood-brain barrier to small molecules up to 800 Da (3). Junctional adhesion molecules (JAMs) are a family of immunoglobulin superfamily proteins (~40 kDa) that have a single transmembrane domain localized within the intercellular cleft of tight junction. The cytoplasmic regions of occludin, claudins, and JAMs have been shown to bind to the zonula occludens protein family via their carboxy terminals; in turn the zonula occludens proteins bind to the actin cytoskeleton, localizing them to the cellular membrane(1). Tight junction proteins exist in various isoforms depending on tissue origin. Generally, movement of these proteins away from the cellular borders or decreased expression at the tight junction cleft indicates a loss of junction integrity and increased paracellular permeability. Multiple factors regulate the expression, trafficking, protein-protein interactions, and integrity of tight junction proteins over the course of development and in disease(1). Previous studies suggest that tight junction proteins can be modulated by numerous signaling pathways such as PI3 kinase, protein kinase C, Ca2+, cAMP, and phospholipase C. Moreover, tight junction themselves may play a role in signal transduction and participate in the regulation of growth, differentiation, and gene expression(4). Blood-brain barrier dysfunction has been proposed to play a role in the pathophysiology of neurological diseases such as inflammatory, infectious, and neurodegenerative diseases(5) and accumulating evidence indicates blood-brain barrier disruption in diabetic patients(6). In addition, blood-brain barrier compromise worsens with the progression of diabetes. Increased blood-brain barrier permeability to small molecules has been observed in the streptozotocin model of type 1 diabetic rats, along with decreased occludin and zonula occludens-1 content in brain microvessels(7;8). However, the molecular mechanism by which diabetes leads to alterations of tight junction proteins is not clear.
Endothelial dysfunction in diabetes is characterized by enhanced production of reactive oxygen species, which are important factors in the pathogenesis of diabetic vascular disease(9;10). Evidence indicates that oxidative stress can disrupt endothelial tight junction, resulting in increased paracellular permeability(11;12). Suppression of reactive oxygen species production or an increase in reactive oxygen species scavengers have been shown to effectively reduce blood-brain barrier hyperpermeability(13). However, the targets and underlying mechanisms by which abnormal production of reactive oxygen species disrupts blood-brain barrier functions remains unknown.
AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase involved in the regulation of cellular and organismal metabolism. Our previous studies indicate that AMPK activation counteracts oxidative stress by suppressing NAD(P)H oxidase-derived reactive oxygen species production in endothelial cells(14). At present, the specific signaling pathways within brain endothelial cells that target the tight junction to disengage remain elusive. We hypothesized that AMPK activation via its suppression of reactive oxygen species contributes to maintenance of blood-brain barrier integrity. Here we provide evidence that hyperglycemia regulates tight junction dynamics in brain endothelial cells via NAD(P)H oxidase-derived superoxide anions, and show that AMPK activation protects tight junction proteins, and consequently blood-brain barrier integrity, by suppressing the expression of NAD(P)H oxidase.
METHODS AND MATERIALS
Reagents
Human brain microvascular endothelial cells (HBMECs), human astrocytes, cell culture media, and growth supplements were obtained from Sciencell (Carlsbad, CA). Antibodies against phospho-AMPKα (Thr-172), AMPKα, and β-actin, and all secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA); antibodies against occludin, zonula occludens-1, and Junctional adhesion molecules-1 were from Invitrogen Life Technologies (Carlsbad, CA); anti-claudin-5 was from Abcam (Cambridge, MA); antibodies against the NAD(P)H oxidase subunits (p47phox, p67phox, gp91phox, NOX1, NOX4, and Rac1) were from Santa Cruz Biotechnology (Santa Cruz, CA); FITC-dextran and antibodies against catalase were from Sigma-Aldrich (St. Louis, MO).
Cell culture
HBMECs were cultured in endothelial cell media mixed with astrocyte-conditioned medium (1:1). All cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Confluent cells were maintained in 1% fetal calf serum and exposed to normal glucose (NG, 5 mmol/l) or high glucose (HG, D-glucose 25 mmol/l) for up to 1 week, with a media change every 2 days. To account for media hyperosmolarity, control groups were exposed to L-glucose (LG, 20 mmol/l) in normal medium containing D-glucose (5 mmol/l). In all experiments, cells were used between passages 3 and 8.
Adenovirus infection
HBMECs were infected with adenoviruses encoding catalase, Cu-Zn-SOD, green fluorescence protein (GFP), constitutively active AMPK (AMPK-CA), or a dominant negative AMPK (AMPK-DN) (multiplicity of infection 50) as described previously (15). Under these conditions, the infection efficiency was typically >80% as determined by green fluorescence protein expression.
Measurement of intracellular superoxide anions
Intracellular superoxide anions was measured using the dihydroethidium fluorescence/HPLC assay with minor modification(16). Briefly, the growth medium was replaced by cell culture media containing no glucose and HBMECs were incubated with 0.5 μmol/l dihydroethidium for 30 min. After incubation, cells were harvested and extracted with methanol. Oxyethidium (a product of dihydroethidium and superoxide anions) and ethidium (a product of dihydroethidium auto-oxidation) were separated and quantified by C-18 HPLC column (mobile phase: gradient of acetonitrile and 0.1% trifluoroacetic acid) coupled with fluorescent detector. Superoxide anion production was determined by the conversion of dihydroethidium into oxyethidium.
Permeability of the blood-brain barrier in vitro
HBMECs were cultured on Costar Transwell filters (pore size 0.4 μm; Corning, NY) that were coated on the upper side with fibronectin (Sigma-Aldrich) in endothelial cell growth medium mixed with astrocyte-conditioned medium at a 1:1 ratio(17;18). Permeability for FITC-dextran (150 kDa) was assayed as described previously(19). At various time points after HG treatment, samples were collected from the acceptor chambers for measurement of fluorescence intensity at excitation 485 nm and emission 520 nm using a microplate reader Synergy HT (BioTek Instruments, Inc, Winooski, VT).
Determination of trans-endothelial electrical resistance
Determination of trans-endothelial electrical resistance across the monolayers of HBMECs was measured by the Millicell-ERS (Millipore). Briefly, 250 μl cell suspension (8×105 cells per ml) was seeded in each collagen-coated well. When confluent monolayers reached maximum resistance (~150 Ω·cm2) the endothelial integrity of HBMECs treated with or without HG was measured in real-time as described previously(20). Values are presented as Ω·cm2 of culture well. The trans-endothelial electrical resistance of cell-free wells was subtracted from the obtained values.
Western blotting
Cells were washed with PBS and homogenized in RIPA lysis buffer (Cell Signaling Technology). Cell lysates were sonicated twice for 10 s in an Ultrasonic Dismemberator with 10% output (Model 500; Fisher Scientific) and centrifuged at 14000 × g for 20 min at 4°C. The supernatants were subjected to western blot analysis, as described preiously (14, 15). β-actin was used as an internal control.
Measurement of NAD(P)H oxidase activity
NAD(P)H oxidase activity was measured as described previously(21). Briefly, 20 μg protein was incubated with dihydroethidium (10 μM) and DNA (1.25 μg/ml) in PBS with the addition of NAD(P)H (50 μM), in a final volume of 120 μl. Incubations were performed for 30 min at 37°C in the dark. Fluorescence intensity was recorded in a microplate reader (excitation 490 nm and emission 590 nm).
Statistical analysis
Data are expressed as mean ± standard error of the mean unless otherwise noted. Statistical comparison was performed using a one-way analysis of variance followed the Student-Newman-Keuls test by SPSS 10.0 software. Differences were considered significant at P < 0.05.
RESULTS
High glucose disrupts blood-brain barrier integrity
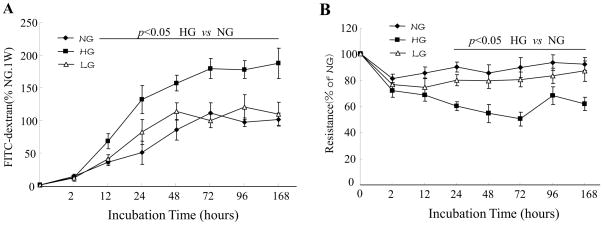
We first determined the paracellular permeability toward large hydrophilic molecules. Exposure of a HBMEC monolayer on transwell filters to HG, but not the osmotic control, increased leakage of FITC-dextran (150 kDa). As depicted in Fig. 1A, the leakage of FITC-detran increased by 67±15.1% at 72 h and by 85±23.3% at 1 week (P < 0.05).
Figure 1. High glucose decreases blood-brain barrier integrity.
A: Confluent monolayers of human brain microvascular endothelial cells (HBMECs) were cultured on Transwell filters and the diffusion of FITC-conjugated dextran (150 kDa; 100μg/ml) in 5 mmol/l D-glucose (normal glucose, NG), 25 mmol/l D-glucose (HG), or 5 mmol/l D-glucose plus 20 mmol/l L-glucose (LG) was measured at various time-points. Data are expressed as the mean fluorescence intensity ± SD of three individual Transwell filters where 100% corresponds to 6.7±0.5μg/ml FITC dextran in NG for 1 week. The graph is a representative example of results from three independent experiments. B: Trans-endothelial electrical resistance was measured in real-time in confluent monolayers of HBMECs. Data are expressed as percentage of the mean resistance ± SD of three independent experiments performed in triplicate; 100% corresponds to 143 ± 19.6 Ω·cm2 in NG for 1 week.
To investigate the influence of HG on the integrity of the brain endothelial barrier in vitro, trans-endothelial electrical resistance of confluent human brain endothelial monolayers was measured at different time points after HG exposure. As shown in Fig. 1B, HG reduced trans-endothelial electrical resistance across a monolayer of human brain microvascular endothelial cell in a time-dependent manner. The maximal effect of HG was seen at 72 h (39±5.3% decrease, P < 0.05).
High glucose reduces expression of tight junction proteins
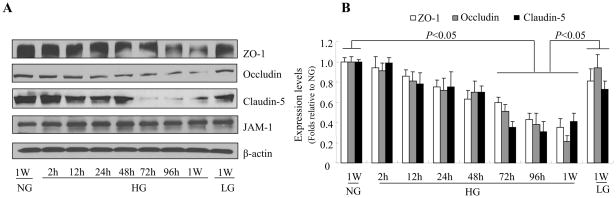
Blood-brain barrier integrity is mainly dependent on the presence of tight junction. Therefore, HG-induced alterations in tight junction may be responsible for the increased blood-brain barrier permeability. To determine the effect of HG on tight junction in brain endothelium, we measured real-time dynamics of the expression of the tight junction protein zonula occludens -1, and JAM-1, occludin, and claudin-5, three proteins that are essential for tight junction maintenance. As shown in Fig. 2A and 2B, expression levels of zonula occludens -1, claudin-5, and occludin were significantly decreased by HG, but not LG, although expression of JAM-1 was not significantly affected. These results suggest that blood-brain barrier leakage under HG conditions was due to the loss of tight junction proteins.
Figure 2. High glucose reduces the expression of tight junction proteins in human brain microvascular endothelial cells (HBMEC).
Confluent HBMECs were exposed to 5 mmol/l D-glucose (NG), 25 mmol/l D-glucose (HG), or 5 mmol/l D-glucose plus 20 mmol/l L-glucose (LG) for the time indicated. A: Representative western blot of tight junction proteins showing that HG (but not the osmotic control LG) significantly reduced expression of zonula occludens (ZO)-1, occludin, and claudin-5 after 72 h exposure, with a further decrease after 1 week. JAM-1 expression was not affected. B: Quantification of zonula occludens (ZO)-1, occludin, and claudin-5 expression in HBMECs at various time points after exposure to HG. β-actin was used as a control for protein loading. The blot is representative of three blots from three independent experiments.
High glucose increases production of superoxide anions
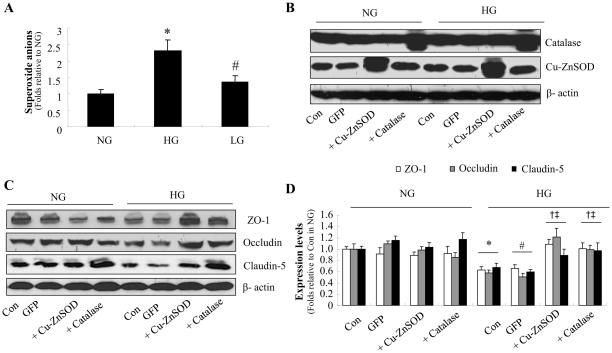
There is evidence suggesting that reactive oxygen species disrupt endothelial cell tight junction resulting in increased paracellular permeability(12). To determine whether reactive oxygen species caused the tight junction alterations induced by HG, we next assayed superoxide anion levels in HBMECs after exposure to NG, HG, or LG for 72 h. Exposure to HG, but not LG, caused a greater than two-fold increase in superoxide anions in HBMECs (Fig. 3A).
Figure 3. Adenoviral overexpression of superoxide dismutase or catalase suppresses high glucose-induced reactive oxygen species generation and maintains tight junction proteins in human brain microvascular endothelial cell (HBMECs).
A: Superoxide anion production induced by 72 h exposure to HG. *P < 0.05 for HG vs. NG; #P < 0.05 for LG vs. HG. n=3. B: Western blot analysis of catalase or Cu-Zn SOD expression after adenoviral overexpression. n=3. C: Cu-ZnSOD or catalase overexpression prevents the decrease in zonula occludens (ZO)-1, occludin, and claudin-5 expression induced by 72 h exposure to HG. D: Quantification of zonula occludens (ZO)-1, occludin, and claudin-5 expression. n=3, *P < 0.05 for con/HG vs. con/HG, #P < 0.05 for GFP/HG vs. GFP/NG, †P < 0.05 for Cu-Zn SOD overexpression/HG vs. con/HG, ‡P < 0.05 for catalase overexpression/HG vs. GFP/HG. β-actin was used as a control for protein loading. The blot is representative of three blots from three independent experiments. NG: 5 mmol/l D-glucose; HG: 25 mmol/l D-glucose; LG: 5 mmol/l D-glucose plus 20 mmol/l L-glucose.
Overexpression of SOD or catalase prevents high glucose-induced reduction of tight junction proteins
To further confirm whether the loss of tight junction proteins was induced by reactive oxygen species, HBMECs were injected with adenoviruses encoding Cu-ZnSOD or catalase, two major antioxidant enzymes. Adenoviral overexpression of Cu-ZnSOD or catalase increased the levels of SOD or catalase respectively in HBMECs (Fig. 3B). Overexpression of Cu-ZnSOD or catalase did not alter the expression of zonula occludens -1, occludin, and claudin-5 in HBMECs exposed to NG but did abolish the reduction of zonula occludens -1, occludin, and claudin-5 expression in HG-treated HBMECs (Fig. 3C and 3D). Taken together, these results suggest that the suppression of tight junction proteins by HG is mediated by superoxide anions.
Inhibition of NAD(P)H oxidase attenuates high glucose-induced reduction of tight junction proteins
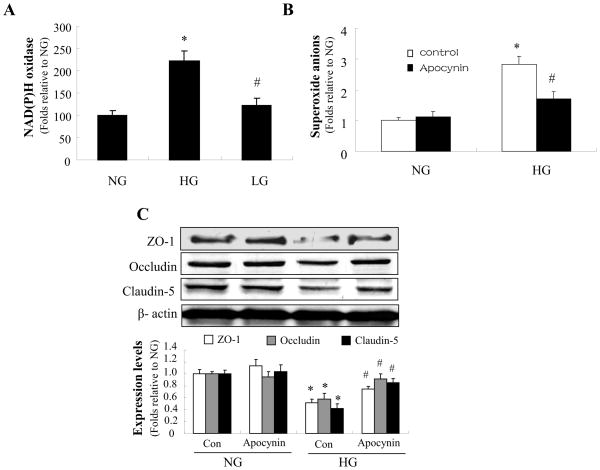
NAD(P)H oxidase is the major source of reactive oxygen species in endothelial cells. To study the role of NAD(P)H oxidase in reactive oxygen species -induced tight junction alterations, we measured NAD(P)H oxidase activity in HBMECs following exposure to HG. As shown in Fig. 4A, the activity of NAD(P)H oxidase was markedly increased by HG but not by the osmotic control. Apocynin, a potent pharmacologic inhibitor of NAD(P)H oxidase, significantly reduced the HG-induced increase in reactive oxygen species (Fig. 4B).
Figure 4. NAD(P)H oxidase mediates high glucose-induced reduction of tight junction proteins.
Confluent HBMECs were exposed to HG for 72 h. A: The activity of NAD(P)H oxidase was markedly increased by HG but not by the osmotic control. *P < 0.05 for HG vs. NG; #P < 0.05 for LG vs. HG; n=3. B: Apocynin (10 μmol/l) significantly reduced the induction of reactive oxygen species production by HG. *P < 0.05 for con/HG vs. con/NG; #P < 0.05 for apocynin/HG vs. con/HG; n=3. C: Apocynin (10 μmol/l) significantly attenuated the reduction of zonula occludens (ZO)-1, occludin, and claudin-5 expression under HG conditions. *P < 0.05 for con/HG vs. con/NG; #P < 0.05 for apocynin/HG vs. con/HG; n=3; β-actin was used as a control for protein loading. The blot is representative of three blots from three individual experiments. NG: 5 mmol/l D-glucose; HG: 25 mmol/l D-glucose.
We further determined whether pharmacological inhibition of NAD(P)H oxidase affected the level of tight junction proteins. As expected, apocynin significantly attenuated the reduction of zonula occludens -1, occludin, and claudin-5 caused by HG (Fig. 4C). Taken together, these results suggest that HG reduces the level of tight junction proteins via NAD(P)H oxidase.
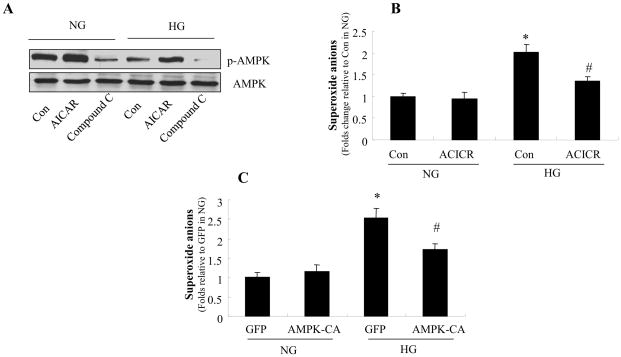
High glucose decreases AMPK phosphorylation
AMPK is reported to play a key role in maintaining redox homeostasis in endothelial cells and inhibition of AMPK causes aberrant oxidative stress(14). We next determined the effects of HG on AMPK Thr172 phosphorylation, a key index of AMPK activity. As shown in Fig. 5A, 72-h treatment of HBMECs with HG significantly decreased AMPK Thr172 phosphorylation. AICAR, a potent AMPK activator, markedly increased the level of AMPK- Thr-172 phosphorylation in HBMECs exposed to NG or HG (Fig. 5A). Conversely, Compound C, a potent AMPK inhibitor, further decreased AMPK Thr172 phosphorylation in HBMECs exposed to NG or LG (Fig. 5A).
Figure 5. AMPK reduces high glucose-enhanced the production of reactive oxygen species in HBMECs.
Confluent HBMECs were exposed to HG for 72 h in the presence of AICAR (0.5 mmol/l) or compound C (10 μmol/l). A: AMPK activity in HBMECs. AMPK-Thr-172 phosphorylation was increased by AICAR and decreased by compound C. The blot is a representative of three blots from three independent experiments. B: Effect of AICAR on O2·− levels in HBMECs. *P < 0.05 for con/HG vs. con/NG, #P < 0.05 for AICAR/HG vs. con/HG, n=3. C: Overexpression of constitutively active AMPK (AMPK-CA) decreased superoxide anions levels in HBMECs. *P < 0.05 for GFP/HG vs. GFP/NG, #P < 0.05 for AMPK-CA/HG vs. GFP/HG, n=3. NG: 5 mmol/l D-glucose; HG: 25 mmol/l D-glucose
AMPK inhibits reactive oxygen species production induced by high glucose in HBMECs
A recent study suggested that AMPK activation counteracts oxidative stress in endothelial cells by suppressing the expression and activation of NAD(P)H oxidase (14). We reasoned that AMPK activation might prevent the HG-induced reduction of tight junction. We therefore examined whether alteration of AMPK activity affected reactive oxygen species production following exposure of HBMECs to HG for 72 h. As expected, activation of AMPK by AICAR significantly attenuated the HG-induced elevation of reactive oxygen species (Fig. 5B). Consistently, adenoviral overexpression of constitutively active AMPK mutants (AMPK-CA), but not the GFP control, significantly decreased the induction of superoxide anions by HG (Fig. 5C).
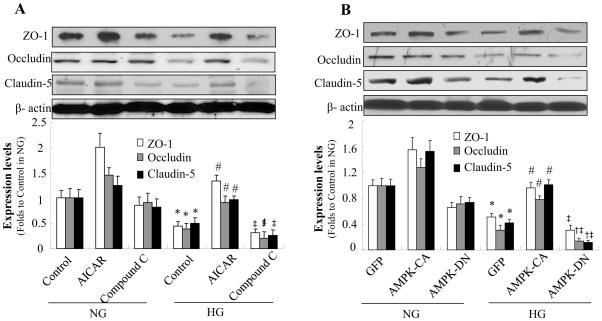
AMPK activation prevents high glucose-induced reduction of tight junction proteins
Next, we examined the effects of AMPK activation on tight junction proteins. To determine whether AMPK activation can prevent loss of tight junction proteins, we tested the effects of AICAR and compound C on the expression of zonula occludens -1, occludin, and claudin-5 after HG exposure. As expected, AICAR significantly alleviated the HG-induced loss of tight junction proteins (Fig. 6A). Conversely, inhibition of AMPK with compound C further reduced tight junction protein expression (Fig. 6A).
Figure 6. AMPK activation prevents high glucose-induced loss of tight junction proteins.
Confluent HBMECs were exposed to HG for 72 h. A: HG decreased expression of zonula occludens (ZO)-1, occludin, and claudin-5. Tight junction protein levels were increased by AICAR (0.5 mmol/l) and further decreased by compound C (10 μmol/l). *P < 0.05 for con/HG vs. con/NG; #P < 0.05 for AICAR/HG vs. con/HG; ‡ P < 0.05 for Compound C/HG vs. AICAR/HG, n=3. B: Degradation of zonula occludens (ZO)-1, occludin, and claudin-5 under HG conditions was reversed by overexpression of constitutively active AMPK (AMPK-CA) and enhanced by overexpression of dominant negative AMPK (AMPK-DN). *P < 0.05 for GFP/HG vs. GFP/NG; #P < 0.05 for AMPK-CA/HG vs. GFP/HG; †P < 0.05 for AMPK-DN/HG vs. GFP/HG; ‡P < 0.05 for AMPK-DN/HG vs. AMPK-CA/HG, n=3. β-actin was used as a control for protein loading. The blot is a representative of three blots from three independent experiments. NG: 5 mmol/l D-glucose; HG: 25 mmol/l D-glucose.
To exclude potential off-target effects of AICAR or compound C, we also investigated the effects of AMPK on tight junction proteins using genetic approaches. Adenoviral overexpression of constitutively active AMPK mutants (AMPK-CA) accentuated the reduction of tight junction proteins caused by HG, whereas overexpression of dominant negative mutants (AMPK-DN) enhanced tight junction protein degradation (Fig. 6B).
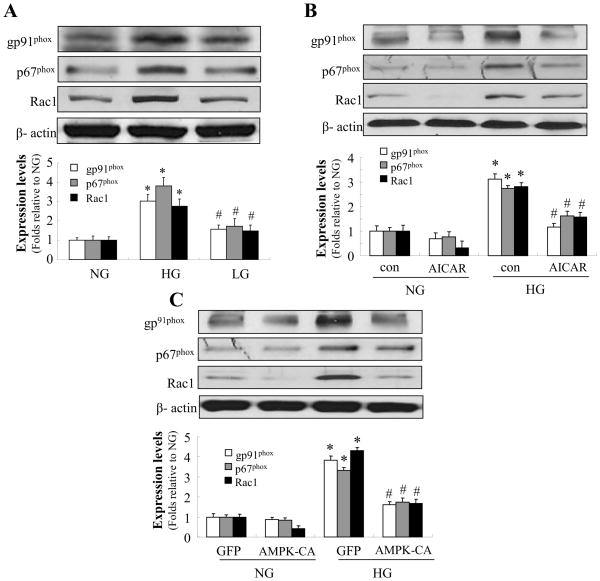
AMPK activation prevents high glucose-induced upregulation of NAD(P)H oxidase
NAD(P)H oxidase consists of membrane-bound gp91phox and p22phox as well as cytosolic subunits such as p47phox, p67phox and small GTPase Rac. Further analysis was performed to define the subunits of NAD(P)H oxidase involved in tight junction alterations induced by HG. As shown in Fig. 7A, expression of the essential subunits, p67phox, gp91phox, and the small GTPase Rac1 was markedly increased by HG exposure compared with normal conditions and osmotic control.
Figure 7. AMPK activation prevents high glucose-induced upregulation of NAD(P)H oxidase subunits.
Confluent HBMECs were exposed to HG for 72 h. A: Expression of NAD(P)H oxidase subunits p67phox, gp91phox, and Rac1 was markedly increased by HG but not by the osmotic control. *P < 0.05 for HG vs. NG; #P < 0.05 for LG vs. HG; n=3. B: AICAR (0.5 mmol/l) significantly alleviated the increased expression of p67phox, gp91phox, and Rac1 caused by HG. *P < 0.05 for con/HG vs. con/NG; #P < 0.05 for AICAR/HG vs. con/HG. C: Adenoviral overexpression of AMPK-CA significantly abolished the increased expression of p67phox, gp91phox, and Rac1 caused by HG. *P < 0.05 for GFP/HG vs. GFP/NG; #P < 0.05 for AMPK-CA/HG vs. GFP/HG. β-actin was used as a control for protein loading. The blot is representative of three blots from three individual experiments. NG: 5 mmol/l D-glucose; HG: 25 mmol/l D-glucose.
As AMPK activation suppressed HG-induced reactive oxygen species production (Fig. 4) and the loss of tight junction in HBMECs (Fig. 5), we next determined whether these effects of AMPK were mediated by decreasing NAD(P)H oxidase activity and by inhibiting HG-induced upregulation of these NAD(P)H oxidase subunits. As expected, activation of AMPK with AICAR significantly alleviated the increased expression of p67phox, gp91phox, and Rac1 caused by HG in HBMECs (Fig. 7B). Consistently, overexpression of AMPK-CA prevented HG-induced upregulation of p67phox, gp91phox, and Rac1 (Fig. 7C). Taken together, these data suggest that AMPK activation maintains the levels of tight junction protein in HBMECs by inhibiting HG-enhanced NAD(P)H oxidase expression.
DISCUSSION
In the present study, we demonstrated that HG reduces the levels of zonula occludens -1, occludin, and claudin-5, tight junction proteins in HBMECs that are essential for maintaining the integrity of the blood-brain barrier. With respect to the underlying mechanism, we have found that HG significantly increased generation of superoxide anions and that adenoviral overexpression of SOD or catalase significantly attenuated HG-induced tight junction protein reduction. Moreover, administration of the NAD(P)H oxidase inhibitor apocynin reversed the reduction of tight junction protein expression. Finally, AMPK activation by AICAR or by adenoviral overexpression of AMPK abolished HG-induced superoxide anions, upregulation of NAD(P)H oxidase subunits, and the reduction of tight junction protein expression. Taken together, our results suggest that HG causes blood-brain barrier dysfunction via NAD(P)H oxidase-derived superoxide anions and that AMPK activation might be effective in preventing blood-brain barrier dysfunction in diabetes by inhibiting HG-enhanced NAD(P)H oxidase expression in endothelial cells.
The cerebral vasculature is particularly susceptible to oxidative stress. Reactive oxygen species can rapidly overwhelm endogenous scavenging mechanisms and damage cellular macromolecules such as lipids, proteins, and nucleic acids(30–33). Reactive oxygen species are also mediators in signaling involving mitochondria, DNA repair enzymes, and transcription factors(34). Compromise of the blood-brain barrier is a complication of neurodegenerative disease and brain injury(5). One of the most important findings of the present study is that HG reduces the level of tight junction proteins. Additionally, we found that HG significantly increased levels of superoxide anions and that adenoviral overexpression of SOD or catalase significantly attenuated the HG-induced suppression of tight junction proteins. Thus, it is likely that the HG-induced reduction in tight junction proteins is mediated by endogenous superoxide anions. Our observations are consistent with published studies indicating that oxidative stress disrupts endothelial tight junction resulting in increased paracellular permeability that is associated with occludin and zonula occludens -1 and mediated through the Src or Rho/PI3K/Akt pathway(11;12;26–29).
Generally, brain microvascular endothelial cells containing tight junction are characterized by a high trans-endothelial electrical resistance and low permeability(24;25). High trans-endothelial electrical resistance values reflect the integrity of the junctional complexes of cultured brain endothelial cells. Paracellular transport of hydrophilic and relative large molecules across the blood-brain barrier is limited by the tight junction. In this study, we found that administration of HG to monolayers of HBMECs induced a time-dependent decrease in the trans-endothelial electrical resistance. The drop in trans-endothelial electrical resistance caused by HG was accompanied by an enhanced permeability of the monolayers to the hydrophilic model compound FITC-dextran (150 kDa). Tight junction proteins are the main determinants of low permeability and high electrical resistance in vitro. Consistently, our studies revealed that treatment of HBMEC monolayers with HG resulted in a time-dependent decrease in tight junction protein expression, which paralleled the decreased trans-endothelial electrical resistance and the increased amount of FITC-dextran that crossed the monolayers. This verified that the enhanced leakage across the HBMEC monolayers was due to loss of the tight junction. Indeed, published studies (22;23) have shown that diseases of the periphery, such as diabetes, have deleterious effects on the blood-brain barrier that may contribute to the increased risk of cerebrovascular disease and neurodegeneration associated with these conditions. Thus, the blood-brain barrier may prove to be a key therapeutic target in the long-term management of diabetes.
Another important finding of the present study is that AMPK activation prevents HG-induced blood-brain barrier dysfunction. AMPK is known to play a key role in endothelial cell biology(35). In the current study, we found that AMPK activity declined upon long-term exposure to HG. Activation of AMPK by a pharmacologic activator (AICAR) or by genetic means (overexpression of constitutively active AMPK) suppressed reactive oxygen species production and reversed the tight junction protein levels, whereas AMPK inhibition by compound C or overexpression of AMPK-DN exacerbated the loss of tight junction proteins. Moreover, we have identified NAD(P)H oxidase as the main source of reactive oxygen species induced by HG. Our data showed that expression of NAD(P)H oxidase subunits was upregulated in the presence of HG. Accordingly, inhibition of NAD(P)H oxidase with apocynin abolished reactive oxygen species production and consequently significantly reversed the level of tight junction proteins. Importantly, AMPK activation markedly decreased the upregulated expression of NAD(P)H oxidase subunits, suggesting that the effects of AMPK on tight junction protein levels are directly mediated by inhibition of NAD(P)H oxidase-derived reactive oxygen species. In addition, previous studies suggest that the ubiquitin-proteasome pathway might affect tight junction stability and there is in vivo evidence that occludin is ubiquitinated and its degradation is retarded by a proteasome inhibitor(39). We have previously shown that loss of AMPK activity leads to increased 26S proteasome activity(14), which may further explain the reactive oxygen species -induced reduction in tight junction proteins. Whether such a pathway is involved in HG-induced blood-brain barrier disruption needs to be further determined. Indeed, recent studies suggest that AMPK activation improves endothelial function by counteracting oxidative stress in endothelial cells(36). It has been reported that the AMPK activator metformin decreases reactive oxygen species production in aortic endothelial cells, an effect partially attributed to a decrease in reactive oxygen species derived from the mitochondrial respiratory chain(37). Kukidome et al. have showed that activation of AMPK reduces hyperglycemia-induced mitochondrial reactive oxygen species production(38). Our previous study also indicated that AMPK promotes endothelial function by suppressing NAD(P)H oxidase- derived reactive oxygen species production(14).
In summary, our results suggest that HG disrupts the expression of tight junction proteins in the blood-brain barrier via production of reactive oxygen species and that activation of AMPK helps to maintain the integrity of blood-brain barrier by suppression of NAD(P)H oxidase and reactive oxygen species production. Thus, AMPK might be important in maintaining the integrity of blood-brain barrier in patients with diabetes and AMPK dysfunction might play an important role in the initiation and progression of neurological disorders in diabetes.
Highlights.
hyperglycemia disrupts blood-brain barrier
hyperglycemia causes tight junction dysfunctions
AMPK reverses the effects of hyperglycemia
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81070646), Basic Research Programs of Science and Technology Commission Foundation of Xianning City and Hubei Province. Work in Dr. Ming-Hui Zou’s laboratory is supported by grants from the National Institutes of Health. Dr. Ming-Hui Zou is a recipient of the Established Investigator Award of American Heart Association.
Abbreviations
- Ad-AMPK-CA
AMPK-constitutively active AMP-activated protein kinase mutants
- AICAR
5-amino-4-imidazole carboxamide riboside
- AMPK
AMP-activated protein kinase
- GFP
Green fluorescence protein
- HG
High glucose
- HBMECs
Human brain microvascular endothelial cells
- LG
L-glucose
- NG
Normal glucose
- SOD
Superoxide dismutase
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2008;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 5.Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 8.Chehade JM, Haas MJ, Mooradian AD. Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res. 2002;27:249–252. doi: 10.1023/a:1014892706696. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 10.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 11.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreibelt G, Kooij G, Reijerkerk A, van DR, Gringhuis SI, van der PS, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 13.Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes Metab. 2009;11:480–490. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachschmid M, Thurau S, Zou MH, Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J. 2003;17:914–916. doi: 10.1096/fj.02-0530fje. [DOI] [PubMed] [Google Scholar]
- 16.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 17.De Groot CJ, Langeveld CH, Jongenelen CA, Montagne L, Van DV, Dijkstra CD. Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: immunophenotypical and functional characterization. J Neurosci Res. 1997;49:342–354. doi: 10.1002/(sici)1097-4547(19970801)49:3<342::aid-jnr9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Kuo YC, Lu CH. Effect of human astrocytes on the characteristics of human brain-microvascular endothelial cells in the blood-brain barrier. Colloids Surf B Biointerfaces. 2011;86:225–231. doi: 10.1016/j.colsurfb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 19.de Vries HE, Blom-Roosemalen MC, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J Pharmacol Exp Ther. 1996;277:1418–1423. [PubMed] [Google Scholar]
- 20.Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci U S A. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes DC, Wosniak J, Jr, Pescatore LA, Bertoline MA, Liberman M, Laurindo FR, Santos CX. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 22.Pasquier F, Boulogne A, Leys D, Fontaine P. Diabetes mellitus and dementia. Diabetes Metab. 2006;32:403–414. doi: 10.1016/s1262-3636(07)70298-7. [DOI] [PubMed] [Google Scholar]
- 23.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 24.Cereijido M, Gonzalez-Mariscal L, Contreras RG, Gallardo JM, Garcia-Villegas R, Valdes J. The making of a tight junction. J Cell Sci Suppl. 1993;17:127–132. doi: 10.1242/jcs.1993.supplement_17.18. [DOI] [PubMed] [Google Scholar]
- 25.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 27.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 28.Lehner C, Gehwolf R, Tempfer H, Krizbai I, Hennig B, Bauer HC, Bauer H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der GA, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez J, Ballinger SW, rley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 31.Zmijewski JW, Landar A, Watanabe N, Dickinson DA, Noguchi N, rley-Usmar VM. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33:1385–1389. doi: 10.1042/BST20051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 34.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 36.Shirwany NA, Zou MH. AMPK in cardiovascular health and disease. Acta Pharmacol Sin. 2010;31:1075–1084. doi: 10.1038/aps.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 39.Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem. 2002;277:10201–10208. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]