Abstract
Autism spectrum disorders are developmental disorders characterized by impairments in social and communication abilities and repetitive behaviours. Converging neuroscientific evidence has suggested that the neuropathology of autism spectrum disorders is widely distributed, involving impaired connectivity throughout the brain. Here, we evaluate the hypothesis that decreased connectivity in high-functioning adolescents with an autism spectrum disorder relative to typically developing adolescents is concentrated within domain-specific circuits that are specialized for social processing. Using a novel whole-brain connectivity approach in functional magnetic resonance imaging, we found that not only are decreases in connectivity most pronounced between regions of the social brain but also they are selective to connections between limbic-related brain regions involved in affective aspects of social processing from other parts of the social brain that support language and sensorimotor processes. This selective pattern was independently obtained for correlations with measures of social symptom severity, implying a fractionation of the social brain in autism spectrum disorders at the level of whole circuits.
Keywords: autism spectrum disorders, functional connectivity, resting state functional MRI, limbic system, cluster analysis
Introduction
Autism spectrum disorders are developmental disorders characterized by impairments in social and communication abilities, along with restricted interests and repetitive behaviours. Converging neuroscientific evidence has shown that autism spectrum disorder-related neuropathology is not localized to one brain region but involves brain networks over a larger spatial scale, suggesting that autism spectrum disorders reflect alterations in connectivity at the level of whole brain circuits (Castelli et al., 2002; Belmonte et al., 2004; Just et al., 2004). Consistent with this, evidence from neuroimaging studies of anatomical connectivity using diffusion tensor imaging and ‘functional’ connectivity that examine patterns of correlated activity between brain regions have shown reduced connectivity in autism spectrum disorders over a wide range of ages from toddlers to adults and involving a variety of brain regions in frontal, temporal and parietal cortex (Booth et al., 2011; Müller et al., 2011; Vissers et al., 2012, for recent reviews).
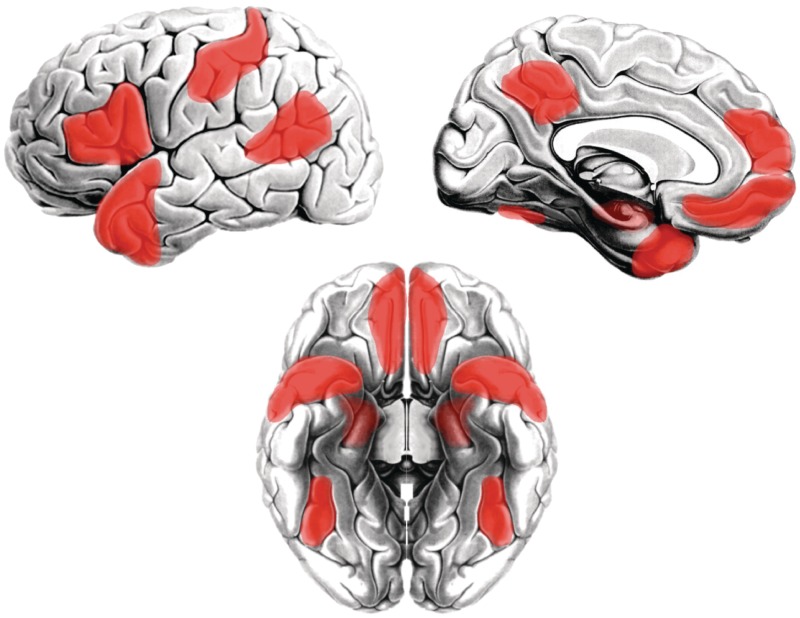
Parallel research in social neuroscience has revealed a set of brain regions that appear to be selectively involved in social processing in typically developing humans, as well as in other social animal species such as monkeys (Frith and Frith, 2007; Adolphs, 2009; Mitchell, 2009; Sallet et al., 2011) (Fig. 1). These include classic limbic areas (e.g. amygdala: Adolphs and Spezio, 2006; Adolphs, 2010; and anterior hippocampus: Fanselow and Dong, 2010) and related cortex such as the more ventral and medial aspects of prefrontal cortex (Carmichael and Price, 1995; Amodio and Frith, 2006; Frith, 2007) and the anterior temporal lobes (Olson et al., 2007; Saleem et al., 2008; Simmons and Martin, 2009, 2011), the posterior cingulate and precuneus (Cavanna and Trimble, 2006; Andrews-Hanna et al., 2010b), posterior temporal regions involved in representing form, motion and conceptual information about animate entities (lateral fusiform gyrus: Kanwisher et al., 1997; Chao et al., 1999; Wiggett et al., 2009; posterior superior temporal sulcus and temporo-parietal junction: Castelli et al., 2002; Beauchamp et al., 2003; Samson et al., 2004; Deen and McCarthy, 2010), the left inferior frontal gyrus involved in social communication (for review, see Turken and Dronkers, 2011), as well as somatosensory and anterior intraparietal cortices involved in action understanding (Hamilton and Grafton, 2006; Adolphs, 2009) and parts of the insula involved in representing visceral/emotive responses to social stimuli (Singer et al., 2004; von dem Hagen et al., 2009). The idea of the ‘social brain’, originally proposed based on studies in monkey (Brothers, 1990), has gained increasing support based on the common co-activation of these areas during performance of a wide range of social tasks (Frith and Frith, 2007; Blakemore, 2008; Adolphs, 2009; Mitchell, 2009).
Figure 1.
Areas of the ‘social brain’. A set of brain regions are commonly co-activated across a range of social tasks: the medial and ventromedial prefrontal cortex, the posterior cingulate/precuneus, the amygdala and anterior hippocampus, the anterior temporal lobes, the posterior superior temporal sulcus and temporo-parietal junction, the lateral portion of the fusiform gyrus, the left inferior frontal gyrus, somatosensory and anterior intraparietal cortices, and the anterior insula (not shown). These are often referred to collectively as the ‘social brain’ (for reviews, see Frith and Frith, 2007; Olson et al., 2007; Blakemore, 2008; Adolphs, 2009; Mitchell, 2009).
A basic question arises from a joint examination of this literature: To what extent is abnormal connectivity in autism spectrum disorders limited to the ‘social brain’? The phenotype among individuals diagnosed with an autism spectrum disorder can vary considerably, including co-morbid general intellectual impairments, epilepsy and/or known genetic disorders (Jeste, 2011). However, pronounced social impairments are common to all of these individuals. If one were to consider a relatively homogenous subsample of high-functioning [i.e. intelligent quotient (IQ) scores in the average or above average range] individuals with an autism spectrum disorder without the presence of additional neurological conditions or known genetic disorders, then proponents of the ‘social brain’ should predict: (i) abnormal connectivity will preferentially involve social brain areas and (ii) the magnitude of deviation from control levels of connectivity in these brain areas should predict the severity of social symptoms measured behaviourally. On the other hand, proposals that are focused instead on how connectivity problems can affect information processing more generally would be equally compatible with finding effects outside of the social brain. Two such prominent proposals include a disconnection between the hemispheres via the corpus callosum, with further disconnection between frontal and more posterior brain regions (Just et al., 2007; Schipul et al., 2011), as well as the proposal that strong but non-selective local connectivity leads to decreases in long-range connectivity (Belmonte et al., 2004; see also Markram and Markram, 2010; Vattikuti and Chow, 2010).
The extent to which abnormal connectivity in autism spectrum disorders is limited to domain-specific social brain areas has not been directly evaluated to date. In part, this has been due to methodological limitations. Connectivity differences in functional MRI, assessed using correlation or related methods, are much more difficult to measure in a whole-brain manner than differences in mean activity level because these methods operate over pairs of voxels or larger regions of interest. The problem of searching systematically through all possible combinations of voxels/regions of interest, along with the accompanying problem of adjusting the statistics for multiple comparisons, renders systematic examination of connectivity differences between groups impractical if not impossible (however, cf. Anderson et al., 2011b). In the face of this complexity, most researchers have anchored their correlation analyses around a small number of ‘seed’ regions of interest. Although the choice of regions of interest can arguably be determined in reasonable ways (e.g. using separate functional localizer scans or standard comparisons of group/condition means in task-based connectivity studies), it is still unclear how comprehensively these regions of interest will represent the full set of connectivity differences that might be present in the data. This situation can be influenced by the choice of stimuli and tasks and potentially by the presence of overall performance differences on those tasks.
Recently, a number of studies have analysed connectivity differences between subjects with autism spectrum disorders and typically developing control subjects in the absence of an overt task, while subjects were at rest (e.g. Müller et al., 2011). In resting-state functional connectivity studies (for review, see Fox and Raichle, 2007), correlations across brain regions reflect the covariation of spontaneous or internally generated brain activity. Spatially specific patterns of correlation in such studies are strongly, although not exclusively, influenced by anatomical connectivity (Honey et al., 2009) and exist independently of explicit thought in so far as they can be observed in anaesthetized monkeys and humans during sleep (Fransson et al., 2007; Vincent et al., 2007; Larson-Prior et al., 2009; Margulies et al., 2009). However, like task-based functional connectivity studies, resting-state studies of autism spectrum disorders have only evaluated a handful of seed locations in any individual study, such as the posterior cingulate cortex (Kennedy and Courchesne, 2008; Monk et al., 2009; Weng et al., 2010), medial prefrontal cortex and left angular gyrus (Kennedy and Courchesne, 2008), anterior versus posterior insula (Ebisch et al., 2011), left intraparietal sulcus, right superior precentral sulcus and left posterior middle temporal cortex (Kennedy and Courchesne, 2008; Ebisch et al., 2011), and structures in the striatum (caudate and putamen, Di Martino et al., 2011). Several studies have demonstrated reduced correlation in autism spectrum disorders among regions of the so-called ‘default’ network (Kennedy and Courchesne, 2008; Monk et al., 2009; Assaf et al., 2010; Weng et al., 2010), which overlap largely, but not completely, with social brain regions (Simmons and Martin, 2011). These findings are consistent with the predictions of the social brain outlined above. However, the use of such a small number of seed locations, 16 in total in the above studies and no more than seven in any individual study, has left us with a piecemeal understanding of the full pattern of connectivity differences. For comparison, the number of possible seed locations in a typical whole-brain functional MRI scan is >50 000 when considering single voxels and >1500 when considering non-overlapping spherical regions of interest with a standard 6-mm radius.
In this study, we combined resting-state functional connectivity with a novel, data-driven analysis approach that permits a comprehensive, whole-brain characterization of the altered functional connectivity in high-functioning participants with an autism spectrum disorder. This approach allows us to address two predictions of the domain-specific neural systems view of social processing: (i) differences in functional connectivity should be concentrated preferentially among social brain areas and (ii) the severity of autism spectrum disorder social symptoms should be predicted preferentially by connectivity levels among social brain areas.
Materials and methods
Participants
Twenty-nine typically developing participants (28 males, one female) between 12 and 23 years of age, and 31 high-functioning participants (29 males, two females) with an autism spectrum disorder, between 12 and 23 years of age, took part in the study. Participants with autism spectrum disorders were recruited from the Washington, DC, metropolitan area and all met Diagnostic and Statistical Manual-IV diagnostic criteria as assessed by an experienced clinician (20 Asperger’s syndrome, seven high-functioning autism and four pervasive developmental disorder-not otherwise specified). Thirty participants with autism spectrum disorders received the autism diagnostic interview (ADI or ADI-R) (Le Couteur et al., 1989; Lord et al., 1994) and the autism diagnostic observation schedule (ADOS, modules 3 or 4; Lord et al., 2000), administered by a trained, research-reliable clinician. All scores from participants with autism spectrum disorders met cut-off for the category designated as ‘broad autism spectrum disorders’ according to criteria established by the National Institute of Child Health and Human Development/National Institute on Deafness and Other Communication Disorders Collaborative Programs for Excellence in Autism (Lainhart et al., 2006). As the ADI and ADOS do not provide an algorithm for Asperger’s syndrome, Lainhart et al. (2006) developed criteria that include an individual on the broad autism spectrum if she/he meets the ADI cut-off for ‘autism’ in the social domain and at least one other domain, or meets the ADOS cut-off for the combined social and communication score. Additionally, to be conservative, group comparisons were reanalysed using only participants with autism spectrum disorders who met research criteria on the ADOS (27 of 31 participants with autism spectrum disorders’ ADOS combined social and communication scores met the ‘autism spectrum disorders’ cut-off of 7). The results from this additional analysis were comparable with the results for the entire sample of 31 participants with autism spectrum disorders. Scores on the Social Responsiveness Scale (Constantino, 2002), an informant-based rating scale used to assess autism spectrum disorders social and communication traits quantitatively over the full range of severity, were obtained from parents for 29 participants with autism spectrum disorders. IQ scores were obtained for all participants, and all full-scale IQ scores were ≥85 as measured by the Wechsler Abbreviated Scale of Intelligence (26 with autism spectrum disorders, 29 typically developing), the Wechsler Adult Intelligence Scale-III (three with autism spectrum disorders), or the Wechsler Intelligence Scale for Children-IV (two with autism spectrum disorders). Participant groups did not differ in terms of full-scale IQ, age, or sex ratio (Table 1). Informed assent and consent were obtained from all participants and/or their parent/guardian when appropriate in accordance with a National Institutes of Health Institutional Review Board approved protocol.
Table 1.
Demographic characteristics of study participants
| Autism spectrum disorders | Typically developing | |
|---|---|---|
| Age | 16.92 (2.66) | 17.86 (3.00) |
| Full scale IQ | 112.87 (15.81) | 114.17 (10.43) |
| Sex (male:female) | 29:2 | 28:1 |
| ADI social | 19.53 (4.99) | |
| ADI communication | 14.73 (4.27) | |
| ADI-restricted/repetitive behaviours | 5.67 (2.63) | |
| ADOS communication + social interaction | 11.47 (4.62) | |
| Social Responsiveness Scale total score | 82.53 (29.09) |
Data are mean (SD).
Functional magnetic resonance imaging
Functional MRI data were collected using a GE Signa 3 T whole-body MRI scanner at the NIH Clinical Centre NMR Research Facility using standard imaging procedures. For each participant, a high-resolution T1-weighted anatomical image (MPRAGE) was obtained (124 axial slices, 1.2 mm slice thickness, field of view = 24 cm, 224 × 224 acquisition matrix). Spontaneous, slowly fluctuating brain activity was measured during functional MRI using a gradient-echo echo-planar series with whole-brain coverage while participants maintained fixation on a central cross and were instructed to lie still and rest quietly (repetition time = 3500 ms, echo time = 27 ms, flip angle = 90°, 42 axial contiguous interleaved slices per volume, 3.0 mm slice thickness, field of view = 22 cm, 128 × 128 acquisition matrix, single-voxel volume = 1.7 mm × 1.7 mm × 3.0 mm). Each resting scan lasted 8 min, 10 s for a total of 140 consecutive whole-brain volumes. Independent measures of nuisance physiological variables (cardiac and respiration) were recorded during the resting scan for later removal. A GE 8-channel send-receive head coil was used for all scans, with a SENSE factor of 2 used to reduce gradient coil heating during the session.
Functional magnetic resonance imaging preprocessing
Prior to statistical analyses, image preprocessing was conducted using the AFNI software package (Cox, 1996). The first four echo-planar image volumes were removed from the resting scan and large transients in the remaining volumes were removed through interpolation (using AFNI’s 3dDespike). Volumes were then slice-time corrected, co-registered to the anatomical scan, resampled to 2.0 mm isotropic voxels, smoothed with an isometric 6-mm full-width half-maximum Gaussian kernel, normalized by the mean signal intensity in each voxel to reflect per cent signal change, and transformed into the standardized Talairach and Tournoux (1988) volume for the purposes of group analyses. We applied the basic ANATICOR procedure (Jo et al., 2010) for removing nuisance physiological and non-physiological artefacts from the echo-planar image data as follows: the anatomical scan was segmented into tissue compartments using Freesurfer (Fischl et al., 2002). Ventricle and white-matter masks were created, eroding the white matter masks to prevent partial volume effects with grey matter. Masks were then applied to the volume-registered echo-planar image data prior to smoothing to yield pure nuisance time series for the ventricles, as well as local estimates of the blood oxygen level-dependent signal in white matter that were averaged within a 15-mm radius sphere. Retroicor (Glover et al., 2000) and respiration volume per time (Birn et al., 2008) regressors were created from the cardiac and respiration measures. All nuisance time series were detrended with fourth-order polynomials prior to least-squares model fitting to each voxel’s time series. Nuisance variables for each voxel included the following: an average ventricle time series, a local average white-matter time series, six parameter estimates for head motion and nine respiration regressors from Retroicor and respiration volume per time (for slice time 0, applied after slice-time correction; Supplementary Fig. 1). The predicted time course of these nuisance variables was then subtracted from the full voxel time series to yield a residual time series to be used in correlation analyses. Large artefacts in the cardiac measures were present in most participants due to poor skin contact, preventing their inclusion. Global brain signal was also not included among the nuisance variables, as whole-brain correlation analyses revealed substantial group-level differences in global signal distribution (Supplementary Fig. 2). Removing global signal would therefore be expected to alter the group-level analyses qualitatively, introducing group differences where none existed in the original data (Murphy et al., 2009; Fox and Greicius, 2010; Saad et al., 2012).
Functional magnetic resonance imaging analyses
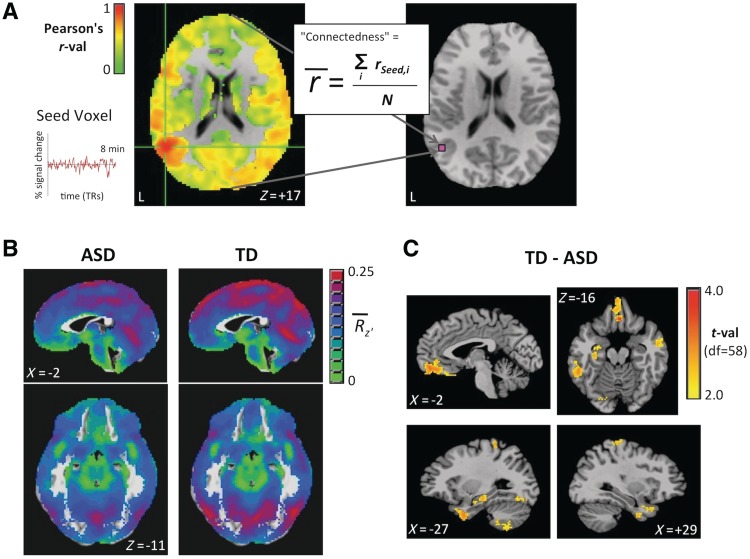
Following functional MRI preprocessing and the removal of nuisance variables, brain masks were created for each participant that excluded the ventricles, white matter, and other non-brain tissue (Fig. 2A). Then functional ‘connectedness’ maps were created by calculating the correlation (Pearson’s r) of the residual time series in each voxel with every other voxel in their brain mask and storing the mean correlation back in the voxel (using the AFNI function 3dTcorrMap). These connectedness values were then transformed using Fisher’s z to yield normally distributed values. Group mean connectedness was then compared between the autism spectrum disorders and typically developing groups using two-sample t-tests (two-tailed) in each voxel in Talairach coordinates to identify candidate seeds. Only voxels present in >85% of single-participant brain masks in both the autism spectrum disorders and typically developing groups were included in the group analyses to prevent any potential gross differences in brain anatomy from biasing the results. Clusters of voxels with a significant t-value (P < 0.05, uncorrected) and at least 100 contiguous voxels in the brain volume then served as seed regions of interest (a total of 13) for the next step of the analysis. The rationale for choosing P < 0.05 (uncorrected) at this early step was to be as inclusive as possible in considering candidate seeds (with a cluster-size criterion of 100 voxels intended to reduce the number of Bonferroni corrections that would be necessary at the next analysis step). We also considered it likely that comparing whole-brain connectedness would dilute or attenuate the magnitude of correlation differences, because these comparisons are intrinsically averaged across correlated and uncorrelated locations. The seeds for the left hippocampus and amygdala were originally contiguous, and given the prior empirical evidence for abnormality of both structures in autism spectrum disorders (Schumann et al., 2004; Schumann and Amaral, 2006), these were broken into two regions of interest to evaluate their separate contributions by first making an independent anatomical mask of the left amygdala from a Talairach version of MNI group brain template (AFNI TT_N27 brain). Voxels that were part of the original cluster but lay outside of the amygdala seed region of interest served as the hippocampus seed region of interest. Prior to seed testing, the reliability of the connectedness comparisons was evaluated by breaking each participant’s data into two halves (first half = 68 time points; second half = 68 time points) and then randomly assigning these halves in a counterbalanced manner across participants into two new data sets, each with an equal number of first and second half segments. Connectedness was recalculated separately for both of these data sets and compared through t-tests in the same manner. Voxels for which typically developing > autism spectrum disorders was significant in both halves at P < 0.05 (both one- and two-tailed; Supplementary Fig. 3) were then identified. All 13 seeds showed good agreement across the two halves of data in this manner (compare Supplementary Fig. 3 with Fig. 3).
Figure 2.
Using ‘connectedness’ to identify seed regions of interest that yield differences in functional connectivity between autism spectrum disorders (ASD) and typically developing (TD) groups. For each participant, in A, functional connectedness was calculated for each voxel within the brain mask by first extracting its specific time series (i.e. the ‘seed’), then correlating it with all of the other voxels’ time series. These correlation values were then averaged over the brain mask, storing the mean value back into the seed voxel, and this process was then repeated for all voxels. For group analyses, in B, individual connectedness maps were transformed to normally distributed values (Fisher’s z) and averaged by group (autism spectrum disorders versus typically developing) (shown are the group-average z-transformed connectedness values,  ; see colour bar). (C) The normally distributed group maps could then be compared using t-tests (P < 0.05, two-tailed, minimum cluster size = 100 contiguous voxels). Data for this and subsequent figures are plotted in standard anatomical coordinates (Talairach-Tournoux).
; see colour bar). (C) The normally distributed group maps could then be compared using t-tests (P < 0.05, two-tailed, minimum cluster size = 100 contiguous voxels). Data for this and subsequent figures are plotted in standard anatomical coordinates (Talairach-Tournoux).
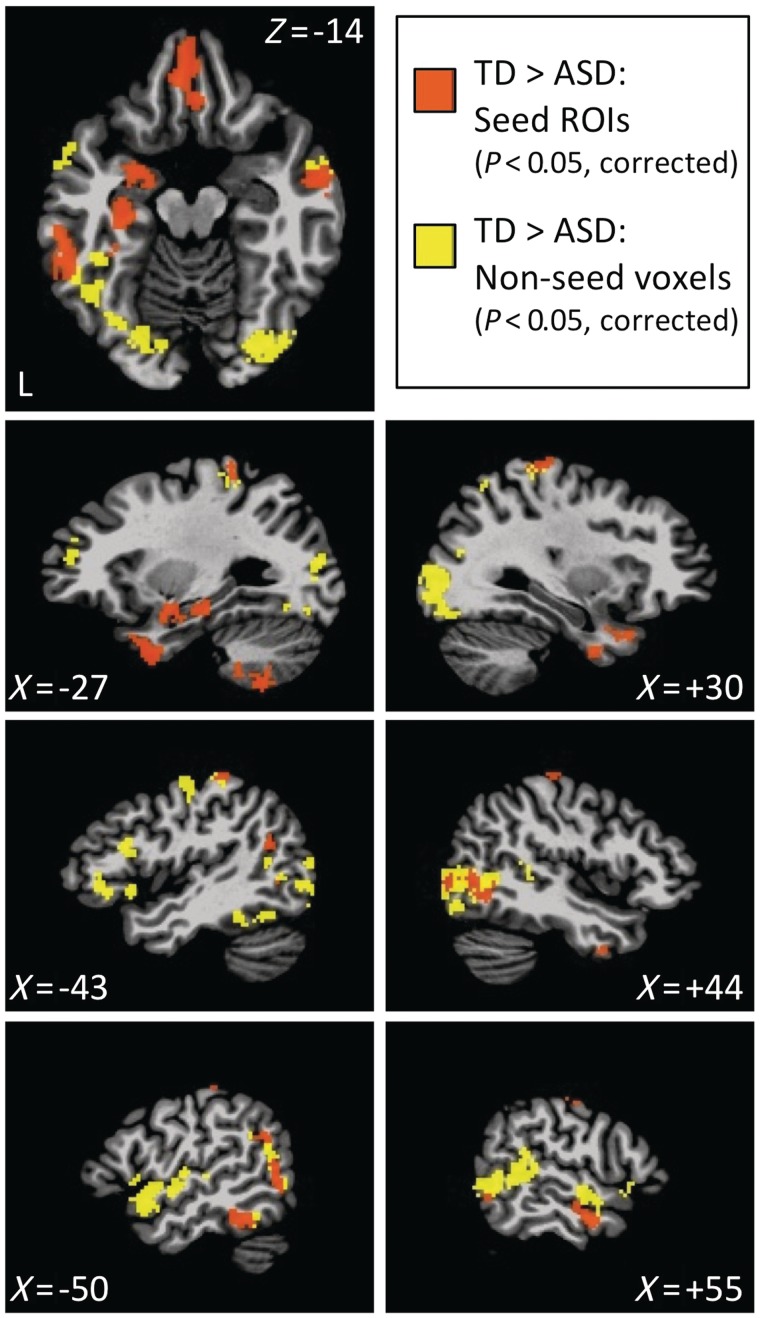
Figure 3.
Seed and non-seed regions of interest showing significant differences between the autism spectrum disorders (ASD) group and the typically developing (TD) group. Seed regions of interest from Fig. 2 that yielded significant differences in functional connectivity between groups (all typically developing > autism spectrum disorders) are shown in orange. Contiguous non-seed voxels that appeared in ≥2 of the individual seed maps were included as additional non-seed regions of interest (shown in yellow). All results are corrected for multiple comparisons with P < 0.05 both for whole-brain tests, as well as the number of seeds tested (see Supplementary Fig. 4 and Table 2 for complete details).
Correlation maps were created for each of the 13 seeds and for each participant, transformed using Fisher’s z, and then compared with additional two-sample t-tests (two-tailed) at P < 0.001, correcting for whole-brain comparisons by cluster size (using AFNI’s AlphaSim) and the number of seed tests (Bonferroni) to P < (0.05/13) (cluster size threshold = 464 mm3). Lieberman and Cunningham (2009) have suggested that voxel-wise alpha levels of P < 0.005 result in a good trade-off of type I and type II statistical errors in functional MRI research, and the group comparisons reported in this study are qualitatively similar using either P < 0.001 or P < 0.005 (results not shown). The Bonferroni corrections applied to the whole-brain corrected P-value were found to accurately reduce the expected family-wise type I error of finding even a single chance seed result to P < 0.05 when the full set of seed selection criteria were checked using Monte Carlo simulation. In the corrected group-difference maps, clusters of voxels (>20 contiguous) detected in more than one seed test, but were distinct from the seed regions of interest, were combined with seed regions of interest yielding corrected results for region of interest–level group correlation analyses (a total of 27 regions of interest; Table 2 and Supplementary Fig. 4 for further details). Region X Region correlation matrices were constructed for each participant, averaged by group, and then submitted to K-means clustering and a metric version of multi-dimensional scaling, both implemented in Matlab (http://www.mathworks.com), to identify regions of interest with similar patterns of functional connectivity with respect to the other regions of interest (Supplementary material). Group comparisons of functional connectivity could then be carried out at the cluster level by finding the median Region X Region correlation both within and between clusters for each participant. Non-parametric permutation tests (Maris and Oostenveld, 2007; Kravitz et al., 2010) were conducted for the cluster-level analyses by jointly shuffling rows/columns of the Region X Region matrix (i.e. if one region of interest was randomly picked to be the third row, this region of interest also served as the third column), permitting the same row/column interdependencies in the actual data to occur in the randomly permuted data. The number of times that a difference greater than or equal to the magnitude observed in the actual data occurred in the shuffled data was tabulated, and this frequency divided by the total number of shuffles (10 000 for these analyses) corresponded to the likelihood of a Type I error (e.g. P < 0.05 corresponded to fewer than 500/10 000 occurrences).
Table 2.
Regions of interest showing differences in functional connectivity (typically developing > autism spectrum disorders)
| Regions of interest in matrix row/column order | Talairach coordinates of region of interest ‘peak’ |
Volume (mm3) | Regions of interest with seed | Overlapping seed maps (max) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 Ventromedial prefrontal | 3 | 27 | −16 | 3864 | 17 | 1 |
| 2 Left amygdala | −23 | −5 | −16 | 1032 | 1 | 1 |
| 3 Left hippocampus | −31 | −19 | −12 | 1048 | 11 | 1 |
| 4 Right ventromedial anterior temporal | 21 | 1 | −36 | 1448 | 14 | 1 |
| 5 Left ventromedial anterior temporal | −23 | 5 | −38 | 1424 | 9 | 0 |
| 6 Right anterior middle temporal gyrus/superior temporal gyrus | 53 | −5 | −16 | 1792 | 6 | 2 |
| 7 Right dorsomedial frontal pole | 9 | 61 | 16 | 376 | 2 | |
| 8 Left inferior frontal gyrus (pars triangularis) | −43 | 17 | 20 | 424 | 3 | |
| 9 Left inferior frontal gyrus (pars orbitalis) | −41 | 31 | 2 | 800 | 3 | |
| 10 Left inferior frontal gyrus (pars opercularis) | −41 | 15 | −2 | 544 | 3 | |
| 11 Right inferior frontal gyrus (pars opercularis) | 53 | 13 | −6 | 496 | 2 | |
| 12 Left inferior temporal gyrus | −59 | −37 | −8 | 2208 | 1 | 2 |
| 13 Left temporo-parietal junction | −47 | −59 | 12 | 2016 | 2 | 3 |
| 14 Left anterior superior temporal | −53 | 9 | −6 | 5424 | 4 | |
| 15 Right superior temporal sulcus | 55 | −45 | 6 | 2280 | 5 | |
| 16 Left anterior superior frontal | −23 | 41 | 24 | 1360 | 3 | |
| 17 Left occipital | −9 | −83 | 36 | 6176 | 3 | |
| 18 Left cuneus | −3 | −93 | 12 | 232 | 2 | |
| 19 Right precuneus | 15 | −63 | 54 | 2736 | 3 | |
| 20 Left fusiform gyrus | −45 | −45 | −18 | 744 | 2 | |
| 21 Right posterior middle temporal gyrus/occipital | 49 | −73 | −2 | 10 208 | 10 | 6 |
| 22 Right dorsal occipital | 23 | −75 | 28 | 3280 | 4 | |
| 23 Left postcentral gyrus | −45 | −31 | 58 | 4408 | 2 | 4 |
| 24 Right postcentral gyrus | 21 | −27 | 68 | 2128 | 3 | 2 |
| 25 Left supplementary motor area | −3 | −3 | 64 | 208 | 2 | |
| 26 Right supplementary motor area | 5 | −9 | 64 | 296 | 2 | |
| 27 Left cerebellum | −35 | −59 | -48 | 1856 | 13 | 0 |
Regions of interest are listed in order of their row/column presentation in Figs. 4 and 5, where r Regions 1–7 are members of Cluster 1 (red), Regions 8–16 are members of Cluster 2 (blue), and Regions 17–27 are members of Cluster 3 (green). Bold text indicates that a region of interest was one of the 12 seed regions of interest that produced significant (corrected) results. Peak Talairach coordinates are for the peak t-value in seed regions of interest for the group comparisons of functional connectedness (Fig. 2), and for the spatial centroid of non-seed regions of interest. The rightmost two columns show: (i) the total number of regions of interest (out of 27) containing at least one voxel in the corrected t-test maps for each seed; and (ii) the maximum overlap of the corrected t-test maps for each seed (out of 12) in any single voxel within the region of interest (Supplementary Fig. 4).
Correlations with autism spectrum disorders symptom severity
Correlations of autism spectrum disorder symptom severity with Region X Region functional connectivity and whole-brain functional connectedness were carried out, removing the effects of age and IQ using partial correlation. These analyses were statistically independent of the autism spectrum disorders/typically developing group comparisons because they utilized the variance of Region X Region functional connectivity measures around the mean solely within the autism spectrum disorders group and did not depend in any way on the mean differences between the two groups. Measures of symptom severity were taken from the ADI (social, communication and restricted/repetitive behaviours subscales), ADOS (communication + social interaction subscale) and Social Responsiveness Scale (total score). Each of these scales was first correlated with the cluster-level median Region X Region correlations that were extracted for each of the participants with an autism spectrum disorder. As Social Responsiveness Scale total score was significantly correlated with median Region X Region functional connectivity between Cluster 1 and Clusters 2/3, follow-up whole-brain correlations of Social Responsiveness Scale with the single-participant connectedness maps were carried out to identify the brain regions most responsible for driving the significant correlation with Social Responsiveness Scale. As with the cluster-level analyses, effects of age and IQ were removed through partial correlation. Voxel-wise alpha levels of the partial correlation coefficients were set at P < 0.01 (although similar results are obtained for P < 0.05), corrected for whole-brain comparisons with P < 0.05 using cluster size (AFNI AlphaSim) (cluster size threshold = 912 mm3). Less stringent voxel-wise alpha levels were used for the functional MRI-behaviour correlations than for the group comparisons due to the relatively small number of degrees of freedom (df) available for the partial correlation analysis [n = 29; df = 25 (N-2-2 covariates)]. For df = 25, the voxel-level alpha of P < 0.01 corresponds to a threshold r-value ≥ 0.49, in the range of functional MRI–behaviour correlations that are typically reported in social neuroscience studies (∼0.4–0.6) (Vul et al., 2009). Both the group comparisons and correlations with autism spectrum disorder symptom severity were corrected for multiple comparisons to the same family-wise alpha (P < 0.05).
Results
Using functional MRI, we scanned 31 subjects with autism spectrum disorders and 29 typically developing control participants who were matched on age, IQ and sex. As discussed earlier, we sought to limit the heterogeneity of our autism spectrum disorders sample and rule out basic confounds of overall intellectual ability by restricting our study to high-functioning participants with autism spectrum disorders (IQ > 85) without epilepsy, other neurological or known genetic disorders (Table 1). We examined functional connectivity of slowly fluctuating, spontaneous brain activity while participants were at rest. Rather than exhaustively testing all possible seed locations, our analysis method (Fig. 2) employed a simplification that is capable of yielding the same kind of information. We first assessed the average level of functional connectivity of each functional MRI voxel with the rest of the brain for each participant (Fig. 2A) (see also Buckner et al., 2009; Cole et al., 2010; Salomon et al., 2011). This participant-specific, average ‘connectedness’ measure can then be averaged over participants within the autism spectrum disorders and typically developing groups and compared statistically with t-tests after first transforming the correlation maps to yield normally distributed values (Fig. 2B and C). Brain locations with low connectedness in one of the two groups are likely to have corresponding correlation maps that differ significantly between the groups, allowing us to determine effective seed locations empirically. Comparison of these connectedness maps led to the identification of 13 seed regions of interest, all of which showed lower connectedness for the autism spectrum disorders group relative to the typically developing group (Fig. 2C) and which were found to be internally consistent when checked with split-half replication (Supplementary Fig. 3). Of note, with respect to the experimental predictions articulated earlier, nearly all these 13 seed regions are components of the ‘social brain’ shown in Fig. 1, such as the ventromedial prefrontal cortex, left anterior hippocampus and amygdala, bilateral anterior temporal lobes, left temporo-parietal junction, bilateral postcentral gyrus, right lateral occipital cortex extending into the right temporo-parietal junction and posterior middle temporal gyrus. Additional seed regions were identified in the left posterior inferior temporal gyrus, the posterior parahippocampal gyrus, as well as the cerebellum.
Although this procedure identified seed regions of interest in an unbiased manner, it did not identify the corresponding brain locations that were driving the differences in connectedness. To evaluate this question, we extracted the voxel-averaged blood oxygen level-dependent time series for each seed region of interest for each participant and determined its correlation with all brain voxels. These resulting correlation maps were then transformed to normal distributions and compared between the autism spectrum disorders and typically developing groups with t-tests (corrected for multiple comparisons). Twelve of 13 seeds yielded significant results (orange voxels in Fig. 3; Table 2), with all results indicating greater functional connectivity for the typically developing group than the autism spectrum disorders group, and no clusters surviving correction that showed the opposite pattern. Only the posterior parahippocampal gyrus seed failed to yield corrected results. An additional control seed placed in the region of the posterior cingulate cortex typically used to identify the ‘default’ network (Fox et al., 2005) failed to produce significant results at the same statistical thresholds (Supplementary material). Many of the 12 remaining seed regions of interest produced results that overlapped spatially with one another, suggesting circuit-like interrelationships among the regions of interest. To identify more completely the brain regions that consistently showed functional connectivity differences resulting from the seed regions of interest, we identified voxels that were present in the correlation difference maps of at least two of the seed regions of interest but were not seed voxels themselves (Supplementary Fig. 4 and yellow voxels in Fig. 3). When combined with the 12 significant seed regions of interest, this yielded a total set of 27 regions of interest showing a consistent pattern of functional connectivity differences among set members that distinguished between the autism spectrum disorders and typically developing groups (Table 2).
Organization of region of interest interrelationships
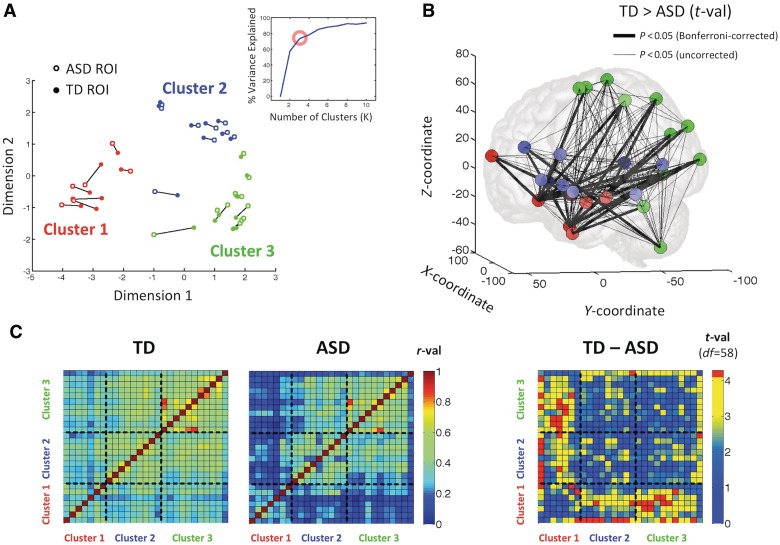
We visualized and quantified the interrelationships among the 27 regions of interest by first calculating the Region X Region correlation matrices for each single participant and then averaging across participants within each group to yield group mean autism spectrum disorders and typically developing matrices. We then identified regions of interest in the group matrices that had similar patterns of correlation with the entire set of regions using multidimensional scaling and K-means cluster analysis. A three-cluster solution provided the best trade-off of variance explained to model complexity according to a simple ‘elbow’ criterion (Fig. 4A, inset), and the three clusters are shown superimposed on the multidimensional scaling plot in Fig. 4A using colour. Cluster 1 (red points in Fig. 4A) corresponded to limbic-related brain areas that are associated with more affective aspects of social processing such as ventromedial prefrontal cortex, left amygdala and the anterior portion of the hippocampus, bilateral anterior ventral temporal cortex, right anterior middle temporal gyrus and right anterior medial frontal cortex. Cluster 2 (blue points) was composed of brain areas involved in linguistic and communicative aspects of social processing in the left hemisphere (for review, see Turken and Dronkers, 2011) such as the three divisions of the inferior frontal gyrus, anterior superior temporal gyrus, posterior temporal cortex near the temporo-parietal junction, posterior inferior temporal gyrus, as well as left anterior middle frontal gyrus and right posterior superior temporal sulcus. Cluster 3 (green points) was composed of brain regions involved in visuospatial, somatosensory and motor aspects of social processing in the left extrastriate cortex, bilateral precuneus, left fusiform gyrus, right middle occipital gyrus, right superior parietal, bilateral postcentral gyrus, bilateral supplementary motor area and left cerebellum.
Figure 4.
Autism spectrum disorders (ASD) and typically developing (TD) groups differ in the functional relationships among regions of interest (ROI) at the level of whole circuits. Functional relationships among the regions of interest were visualized both through multidimensional scaling and K-means clustering with good agreement between the methods in A. Points in the 2D scatter plot represent the 27 regions of interest, shown separately for the autism spectrum disorders group and the typically developing group, with near points in the plot indicating similarity in the pattern of correlation with respect to the entire set of regions of interest. A three-cluster solution (shown in red, blue and green) served as the best trade-off of variance explained to model complexity (inset), with Cluster 1 (red) identifying limbic-related regions of interest involved in emotional/affective aspects of social processing, Cluster 2 (blue) identifying regions of interest related to linguistic aspects, and Cluster 3 (green) identifying regions of interest related to visuospatial and somatomotor aspects. The 3D anatomical locations of these regions of interest are shown in B by cluster membership in Talairach coordinates. Thick lines connecting regions of interest correspond to Bonferroni-corrected group differences (t-tests) in the correlation values between those regions of interest, whereas thin lines denote group differences that failed to survive correction. The full Region X Region correlation matrices are shown in C for the autism spectrum disorders and typically developing groups, sorted by cluster. The corresponding t-tests, which highlight the reliable differences between the groups, are shown to the right (see Table 2 for row/column order of region of interest identities). Correlation values are selectively lower in the autism spectrum disorders group between limbic-related regions of interest in Cluster 1 and those in Clusters 2 and 3, but are relatively preserved within Cluster 1 (see colour bars for corresponding r- and t-values; red t-values indicate a Bonferroni-corrected level of significance).
Although all these regions of interest were selected based on the fact that they showed significant differences in functional connectivity between the two groups, it remained unclear which combinations of regions showed the largest functional connectivity differences. One simple approach was to identify which Region X Region combinations showed differences in functional connectivity that survived Bonferroni corrections for the number of combinations tested. This is shown in Fig. 4B in the context of an anatomical rendering of the regions of interest in Talairach coordinates, with region of interest colours matching the cluster designations in Fig. 4A. Thick lines between two regions of interest indicate that the correlation values between those two regions of interest are significantly larger in the typically developing group than in the autism spectrum disorders group at a level that survives correction. Thin lines indicate a significant difference that fails to survive correction. There are two striking aspects of the distribution of the thick lines: (i) they occur almost exclusively between clusters (23/25 compared to 2/25 within-cluster) and (ii) virtually all involve Cluster 1 regions of interest (24/25; Cluster 1 with 2: 9/25, Cluster 1 with 3: 13/25). The region of interest showing the largest number of Bonferroni-corrected group differences in functional connectivity was the ventromedial prefrontal region of interest in Cluster 1 (11/25). These results are clarified further by viewing the full Region X Region correlation matrices by group but with the rows and columns sorted by cluster (Fig. 4C). The largest differences between the groups occur between Cluster 1 regions of interest and those in Clusters 2 and 3, with the autism spectrum disorders group showing decreased correlations between regions (corresponding t-tests shown in Fig. 4C). In contrast, the group differences among regions of interest in Cluster 1 and in Clusters 2 and 3 were less pronounced, with positive correlations observed in both groups at more comparable levels. These issues were evaluated statistically at the cluster level using the median within- versus between-cluster correlation value for each participant. Group t-tests within and across clusters revealed differences in functional connectivity between Cluster 1 regions of interest and regions of interest in Clusters 2 and 3 [Cluster 1 with Cluster 2 regions of interest: t(df 58) = 4.17, P < 0.001, two-tailed, Bonferroni-corrected; Cluster 1 with Cluster 3 regions of interest: t(df 58) = 4.38, P < 0.001, two-tailed, corrected], as well as among Cluster 1 regions of interest [t(df 58) = 3.59, P < 0.01, two-tailed, corrected]. Group differences between Cluster 1 and Clusters 2/3 were also found to be significantly larger than differences in other portions of the Region X Region matrix (versus within-Cluster 1, permutation test: P < 0.05; versus within- and across Clusters 2 and 3, permutation test: P < 0.04). Although group differences were found to be significant among Cluster 1 regions of interest, correlation values were still substantially above chance for the autism spectrum disorders group, indicating that low correlation values across clusters were not due to the absence of coordinated activity within Cluster 1 (average median correlation = 0.249, SEM = 0.011, P < 10–5; average median correlation for typically developing group = 0.341, SEM = 0.025, P < 10–5). Simple alternative explanations of these results in terms of group-level differences in head motion or local blood oxygen level-dependent signal amplitudes were evaluated but failed to account for the observed patterns (Supplementary Figs. 5–11, Supplementary Table 1). Taken together, these results suggest that not only are the largest differences in functional connectivity in autism spectrum disorders situated within brain areas that are involved in aspects of social processing, but also there is a pronounced decoupling of the activity in limbic-related brain regions in Cluster 1 from the activity in other social brain regions.
Correlations with autism spectrum disorders social behaviours
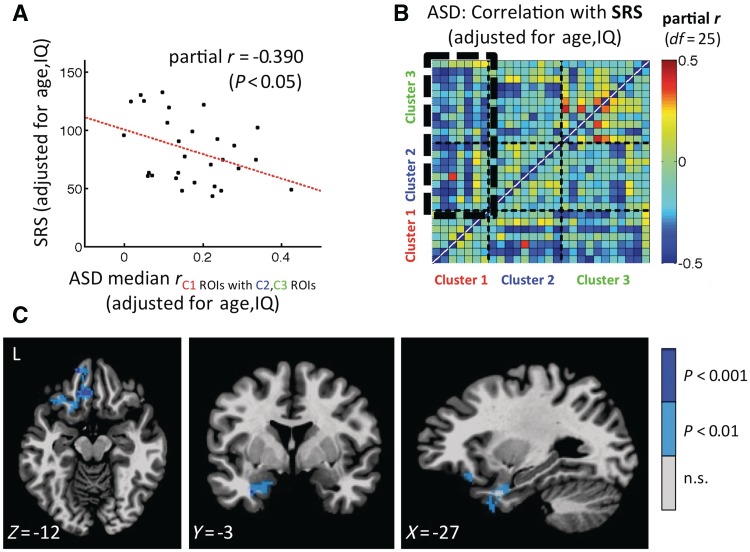
We next asked whether the functional decoupling of brain regions in Cluster 1 from Clusters 2 and 3 observed in the autism spectrum disorders/typically developing group differences could independently predict the severity of the social and communication impairments within the autism spectrum disorders group. Autism spectrum disorders social symptoms were assessed with three of the most commonly applied behavioural scales: the Social Responsiveness Scale, the ADOS and the ADI or ADI-R. The median Region X Region correlation between Cluster 1 and Clusters 2 and 3 for each participant with autism spectrum disorders was significantly associated with symptom severity only for Social Responsiveness Scale (total score) after partialling the effects of age and IQ [partial r (df 25) = -0.39, P < 0.05], with weaker across-cluster correlations related to greater severity of symptoms (Fig. 5A). The full pattern of partial correlations with Social Responsiveness Scale for the entire autism spectrum disorders Region X Region matrix further revealed that symptom severity was selectively predicted by these particular across-cluster relationships (Fig. 5B), demonstrating a mirror pattern to that observed for the group functional differences in Fig. 4C (see also Supplementary Fig. 12). Indeed, within the autism spectrum disorders group, partial correlations with Social Responsiveness Scale were significant for both of the across-cluster relationships involving Cluster 1 [with Cluster 2: partial r (df 25) = −0.406, P < 0.04; with Cluster 3: partial r (df 25) = −0.393, P < 0.05], whereas none of the other partial correlations approached significance (P > 0.2 for all). Although the choice of regions of interest was determined independently based on the brain regions showing group differences in functional connectivity with the typically developing participants, we also conducted a whole-brain search within the autism spectrum disorders group connectedness maps (Fig. 2B) to determine which brain regions were most responsible for driving the correlations with Social Responsiveness Scale. Two clusters of voxels (Fig. 5C), one in the ventromedial prefrontal cortex and one extending from the left amygdala into the left anterior ventral temporal cortex, showed significant correlations with Social Responsiveness Scale after partialling out the effects of age and IQ and correcting for whole-brain comparisons. These clusters overlapped substantially with three of the regions of interest in Cluster 1 showing group-level differences between participants with autism spectrum disorders and typically developing participants (ventromedial prefrontal, left amygdala and left anterior ventral temporal cortex regions of interest; Fig. 4), despite the fact that they were discovered using only the autism spectrum disorders group data. As with the region of interest–level analyses, lower levels of functional connectedness were associated with higher Social Responsiveness Scale total scores indicating that decreases in functional connectivity involving these brain areas predict higher symptom severity in autism spectrum disorders.
Figure 5.
Correlations with autism spectrum disorders (ASD) symptom severity reveal the same circuit-level organization as the group comparisons. The median correlation value between regions of interest (ROI) in Cluster 1 and those in Clusters 2 and 3 for each participant with an autism spectrum disorders is negatively related to Social Responsiveness Scale (SRS) total score after controlling age and IQ (lower median correlation > higher severity) is shown in A. The x- and y-axes show adjusted values after removing the linear effects of age and IQ from each variable using multiple regression. The partial correlation values between functional connectivity (region of interest–region of interest correlation) and Social Responsiveness Scale total score are shown in B for all region of interest combinations, revealing the mirror pattern to that observed in C. A thick, dashed line denotes the portion of the matrix used to calculate the median correlations in A. In a whole-brain search, negative partial correlations of autism spectrum disorders functional connectedness with Social Responsiveness Scale total score are observed in left ventromedial prefrontal cortex, amygdala and anterior ventral temporal cortex (adjusted for age, IQ) is shown in C. Results are corrected for multiple comparisons to P < 0.05 and overlap with three of the seed region of interest identified independently in the group comparisons (see colour bar for uncorrected significance levels in single voxels).
Discussion
The domain-specific neural systems view of social processing produced two central predictions that were evaluated in this study: (i) differences in interregional correlations between high-functioning participants with an autism spectrum disorder and typically developing participants should be concentrated preferentially among social brain regions and (ii) the severity of autism spectrum disorders social impairments should be predicted preferentially by correlation levels among social brain regions. Utilizing a novel data-driven approach to analysing spontaneous, resting brain activity in functional MRI, we found strong support for both predictions. Indeed, the observed pattern of correlation differences was strikingly selective to a smaller subset of social brain regions. The strongest decreases in autism spectrum disorders were observed between limbic-related brain regions thought to process affective aspects of social behaviour (Cluster 1) and the remaining brain regions that are more involved in language/communication-related (Cluster 2) and visuospatial/motor-related (Cluster 3) aspects. Although a number of the brain regions found to exhibit group differences in correlations are clearly involved in non-social functions (e.g. occipital, parietal and somatosensory regions of interest in Cluster 3), these regions appear to have been identified primarily through their lack of correlation in the autism spectrum disorders group with the limbic regions of interest in Cluster 1, all of which are more selectively engaged in social task contexts (Mitchell, 2009; Simmons and Martin, 2009; Fanselow and Dong, 2010). Combined with the relatively preserved levels of correlation among limbic regions in autism spectrum disorders, these results indicate a fractionation of the activity dynamics between the limbic circuit and other regions of the social brain, rather than a complete degeneration of limbic circuit activity. This same pattern of fractionation was independently obtained for the autism spectrum disorders group when examining the quantitative relationship between Region X Region correlation values and scores on the Social Responsiveness Scale, a behavioural instrument specifically designed to measure impaired social functioning on a quantitative scale (Constantino, 2002). A separate whole-brain correlation analysis with Social Responsiveness Scale scores using the autism spectrum disorders connectedness maps confirmed that the effects were driven by reduced overall correlations of activity with Cluster 1 regions of interest. Aside from replicating the overall pattern of Region X Region interrelationships present in the group comparisons, the correlation with Social Responsiveness Scale scores solely within the autism spectrum disorders group strongly suggests that the group differences in correlation observed relative to the typically developing participants reflect something about the social aspects of autism spectrum disorders rather than some of the non-social characteristics that can accompany these disorders. The selective pattern of correlation differences we observed is consistent with a decoupling of brain regions that are more involved in affective aspects of social processing from regions that mediate language/communication abilities and the perception of socially relevant form and action. This pattern could not be anticipated by more general information processing views of impaired connectivity in autism spectrum disorders that do not explicitly appeal to domain specific social brain regions.
There are at least three distinct mechanisms that are capable of explaining our pattern of results. The first, and perhaps the most straightforward, is that there is a gross-disruption of anatomical connections between limbic and non-limbic brain regions in autism spectrum disorders. Previous post-mortem anatomical studies of the autistic brain, although not always able to control for co-morbid conditions such as epilepsy and intellectual disability, have also highlighted abnormalities within limbic structures (Kemper and Baumann, 1993; Schumann et al., 2004; Amaral et al., 2008). In non-human primates, the amygdala and hippocampus and other brain regions that make up our Cluster 1 regions of interest (medial and ventromedial prefrontal cortex, anterior aspects of the temporal lobes) are known to be monosynaptically connected and therefore form an anatomical circuit (Carmichael and Price, 1995; Saleem et al., 2008). In typically developing humans, these same limbic-related areas show distinct growth trajectories across age relative to the rest of neocortex, implying that distinct genetic mechanisms guide their development (Shaw et al., 2008; Voineagu et al., 2011). Subsets of the regions of interest in Clusters 2 and 3 are also likely to form distinct anatomical circuits, such as the language-related regions in Cluster 2 (Turken and Dronkers, 2011) and the occipital, occipitotemporal, parietal regions in Cluster 3 (Felleman and Van Essen, 1991). Activity coupling within the limbic circuit could be mediated through preserved monosynaptic connections, whereas activity decoupling with non-limbic neocortical circuits could result from weak or lacking long-distance projections. A second possibility is that the long-range anatomical connections are fully intact, but that local structural or cellular abnormalities within the limbic circuit create aberrant local activity dynamics that undermine effective synchronization with more distal anatomical circuits that are separated by longer conduction delays [see Yizhar et al. (2011) as a recent example of this type of proposal]. This view makes clear predictions for autism spectrum disorders in neuroimaging methods with high temporal and spatial resolution such as magnetoencephalography that the power spectrum of neural activity should be altered locally in limbic-related brain regions, and this in turn should lead to reduced coherence/phase-locking of activity with non-limbic areas. A third possibility that we cannot entirely rule out is that some explicit differences in mental state between participants with autism spectrum disorders and typically developing participants during the rest scans explain our selective pattern of correlation differences. There are several points to be made with respect to this possibility: (i) to the extent that there are real brain differences between these two groups, perhaps along the lines of either of the first two mechanisms discussed above, then we would certainly expect some differences in mental state; so our main concern should be whether these are due to uninteresting differences in mental state that are not central to the distinction between autism spectrum disorders and control participants; (ii) if the selective pattern of correlation differences observed here corresponds to a different mental state, it is a mental state that predicts the severity of social impairment in participants with autism spectrum disorders, and therefore, it is clearly of interest to us; and (iii) this pattern of correlation differences has not been reported previously in resting-state or task-based functional connectivity studies; if these results reflect differences in explicit thought, they are providing novel information about the circuit-level functional organization of brain and mind that is relevant for the understanding of autism spectrum disorders. All of that said, there are a variety of reasons to discount this third possibility in favour of the first two. The dynamics of resting blood oxygen level-dependent activity are concentrated at very low frequencies (∼0.01–0.05 Hz) (Cordes et al., 2001), with single cycles of fluctuation taking up as much as 100 s. This would appear to be too slow to correspond to the dynamic changes in explicit thought that can occur on the timescale of a few seconds. Studies that have attempted to evaluate the contribution of explicit thought and/or mind-wandering to resting-state functional MRI correlation patterns have observed some modest effects within the so-called ‘default’ network (Mason et al., 2007; Andrews-Hanna et al., 2010a), but they are certainly not identical to the profile of changes observed in this study, nor do they appear to be of sufficient magnitude to explain the autism spectrum disorders-typically developing differences. Similar, spatially specific correlation patterns in spontaneous activity have also been observed in monkeys under anaesthesia (Vincent et al., 2007; Margulies et al., 2009) and in humans during sleep (Fransson et al., 2007; Larson-Prior et al., 2009), conditions that eliminate the possibility of explicit thought.
Limitations and relationship to existing studies
Our study examined the extent to which functional connectivity differences in high-functioning participants with an autism spectrum disorder without epilepsy or other co-morbid neurological/genetic conditions are concentrated in regions of the social brain, as well as the extent to which the severity of their social impairments are preferentially predicted by correlation levels among the same social brain regions. Although our results may help to clarify the neural bases of social impairments in this subgroup of participants with autism spectrum disorders, the degree to which our results generalize to the larger autism spectrum disorders population is unclear. For example, participants with pronounced language delays were under-represented in this study due to our weighting towards participants with Asperger’s syndrome. One might expect a stronger concentration of correlation differences in more classic language areas if this selection constraint were relaxed. Similarly, if participants with lower levels of intellectual functioning were included, then one might expect the results to be more distributed among frontal brain regions that are associated with IQ test performance (such as lateral prefrontal and anterior cingulate cortices; Duncan et al., 2000; Geake and Hansen, 2005). Additional studies are needed in order to confirm the applicability of our current results to these and other autism spectrum disorders subgroups. We would speculate that if social behaviours are indeed mediated by domain-specific neural circuitry, then the results regarding the severity of autism spectrum disorders social impairments should generalize to the autism spectrum disorders population at large, whereas the results in group comparisons would hinge on the inclusion of appropriately matched control groups. However, appropriate control groups selected from the typically developing population may not exist in some cases (e.g. with a general intellectual impairment and/or severe language delay), perhaps requiring selection from other clinical populations with relatively spared social and communication abilities.
It is also unclear to what extent the decoupling of limbic from non-limbic social brain circuits that we observe is causally responsible for poor social functioning in autism spectrum disorders. We cannot rule out the possibility that this decoupling resulted indirectly from a long history of abnormal social functioning and the under- or mis-utilization of the limbic circuit. In other words, it is possible that this phenomenon is more of an experience-dependent effect rather than a cause. Age-stratified and/or longitudinal developmental studies might help to clarify this relationship, perhaps in conjunction with studies of the accompanying anatomical changes (Raznahan et al., 2010; Wallace et al., 2010).
To our knowledge, only one other study to date has explored functional connectivity differences in autism spectrum disorders and typically developing populations in a whole-brain fashion during rest. Anderson et al. (2011b) spatially sampled seed locations over an entire grey matter brain mask (7266 total seed regions of interest), and they used the results to classify subjects either as having an autism spectrum disorder or as typically developing. They controlled for multiple comparisons using a mixture of permutation tests and chance estimates derived from the binomial distribution, allowing them to identify which brain regions contained the largest number of informative pairwise connections for the autism spectrum disorders-typically developing classification. While their approach diverges from ours in a number of respects, it nevertheless identified many of the same brain regions (e.g. bilateral fusiform gyrus, medial prefrontal cortex, posterior cingulate, temporo-parietal junction, anterior temporal cortex, anterior insula, superior parietal cortex and right orbitofrontal cortex; compare with Supplementary Fig. 4). The effect size of group differences present at individual Region X Region connections has less of an influence on their estimate of which brain regions are most relevant than it does in our approach (using group t-tests), which may account for some of the quantitative differences between our two studies. Their study was also more targeted toward classification than toward detailed analyses of the circuit-like interrelationships among the regions of interest, which is the most novel contribution of our study. Another recent study of naturally sleeping toddlers (1- to 3.5-years old), either autistic, language-delayed or typically developing, utilized patterns of correlation across corresponding locations in the two cerebral hemispheres while the toddlers were passively exposed to auditory stimuli such as words, pseudo-words, sentences, tones or environmental sounds (Dinstein et al., 2011). This approach does not provide a whole-brain view of correlation differences per se [for N voxels, ∼ N/2 interrelationships out of a possible N(N − 1)/2], but it does provide a much more systematic view of differences throughout the brain than is afforded by a small handful of isolated seeds (see also Anderson et al., 2011a). Dinstein et al. (2011) observed weaker correlations in the autistic toddlers relative to language delayed or typically developing toddlers between homologous locations of the superior temporal gyrus/superior temporal sulcus and the inferior frontal gyrus. Despite numerous differences with our study (the age range and severity of subjects with autism spectrum disorders, direct exposure to auditory stimuli, etc.), we also observed weaker correlations in the participants with autism spectrum disorders throughout the entire extent of the superior temporal gyrus/superior temporal sulcus bilaterally, as well as the inferior frontal gyrus on the left (Supplementary Fig. 4). The restriction of their findings to the superior temporal gyrus/superior temporal sulcus and inferior frontal gyrus, which are involved in higher order auditory and linguistic processing (for review see Turken and Dronkers, 2011), may have resulted at least in part, from the use of overt auditory stimuli and preserved stimulus-level variation around the mean response (‘Discussion’ section) and/or from the focus on point-to-point cross-hemispheric correlations.
More generally, the relationship between resting-state functional connectivity studies of autism spectrum disorders such as ours and task-based connectivity studies that utilize similar participant selection criteria is unclear (for review, see Müller et al., 2011). In task-based studies, only the condition means are removed through regression, leaving the trial-level or stimulus-level variation around the mean fully intact. Indeed, this variation is what connectivity methods such as psycho-physiological interaction (Friston et al., 1997) and dynamic causal modelling (Friston et al., 2003) rely on to show differences in connectivity between task conditions within an experimental session. One might therefore expect results in studies that employ overt behavioural tasks or the presentation of sensory stimuli to be biased towards stimulus/task-responsive brain regions. Consistent with this, Just et al. (2004) employed a sentence comprehension task and found decreases in their autism group in both mean activity and interregional correlations in left hemisphere language areas (see also Dinstein et al., 2011). Kleinhans et al. (2008) presented blocks of face and house pictures to subjects with autism spectrum disorders and control subjects in a one-back task. Using a right fusiform face area seed, both subjects with autism spectrum disorders and controls showed greater correlation with the right posterior superior temporal sulcus and bilateral amygdala during the face blocks relative to the house blocks, with controls showing significantly larger effects with the left amygdala and the posterior cingulate/precuneus. Mostofsky et al. (2009), using a sequential finger-tapping task, found greater correlations in typically developing subjects relative to subjects with autism spectrum disorders throughout the network of brain regions involved in motor execution (e.g. primary motor cortex, thalamus, supplementary motor area and cerebellum). Studies of autism spectrum disorders that have utilized inherently social or ‘theory of mind’ tasks have tended instead to find results in multiple areas of the social brain (Fig. 1) such as the medial prefrontal cortex, the posterior superior temporal sulcus and temporo-parietal junction, the amygdala, the insula, and the lateral anterior temporal lobes (Castelli et al., 2002; Lombardo et al., 2010; see also Di Martino et al., 2009). Although we do not view this bias towards stimulus/task-responsive brain regions as inherently problematic, it does suggest that the choice of task/stimuli can have a direct impact on the results and that results across different tasks/stimuli may not be directly comparable. The virtue of the task-based approach is that mental state is more explicitly controlled. In our view, an integrated approach that combines resting-state and task-based functional connectivity studies in the same subjects, utilizing a wide range of social and non-social tasks with whole-brain analyses, would combine the strengths of both approaches. Such an integrated approach might help to isolate the set of behavioural circumstances that require the intact coordination of limbic and non-limbic brain circuitry highlighted by our study.
Funding
This study was supported by the National Institute of Mental Health, NIH, Division of Intramural Research.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Lauren Kenworthy, John Strang and Rafael Oliveras-Rentas for guidance and aid in autism spectrum disorder (ASD) participant testing; Hang Joon Jo, Ziad Saad and Kelly Barnes for helpful methodological advice; and members of the Laboratory of Brain and Cognition, NIMH, for helpful feedback and discussions.
Glossary
Abbreviations
- ADI
autism diagnostic interview
- ADOS
autism diagnostic observation schedule
References
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Prog Brain Res. 2006;156:363–78. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cereb Cortex. 2011a;21:1134–46. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011b doi: 10.1093/brain/awr263. doi:10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010a;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010b;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53:247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–54. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Booth R, Wallace GL, Happe F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011;189:303–17. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–9. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. Neuroimage. 2010;49:3132–48. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deen B, McCarthy G. Reading about the actions of others: biological motion imagery and action congruency influence brain activity. Neuropsychologia. 2010;48:1607–15. doi: 10.1016/j.neuropsychologia.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatr. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–25. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, et al. A neural basis for general intelligence. Science. 2000;289:457–60. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–28. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci USA. 2007;104:15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671–8. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–32. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Geake JG, Hansen PC. Neural correlates of intelligence as revealed by fMRI of fluid analogies. Neuroimage. 2005;26:555–64. doi: 10.1016/j.neuroimage.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. J Neurosci. 2006;26:1133–7. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–40. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste SS. The neurology of autism spectrum disorders. Curr Opin Neurol. 2011;24:132–9. doi: 10.1097/WCO.0b013e3283446450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–82. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Baumann ML. The contribution of neuropathological studies to the understanding of autism. Neurol Clin. 1993;11:175–87. [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Kriegeskorte N, Baker CI. High-level visual object representations are constrained by position. Cereb Cortex. 2010;20:2916–25. doi: 10.1093/cercor/bhq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140:2257–74. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489–94. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview—a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–87. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. 2010;133:611–24. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–3. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;25:2862–74. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory—a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Social psychology as a natural kind. Trends Cogn Sci. 2009;13:246–51. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–25. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–40. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012 doi: 10.1089/brain.2012.0080. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–93. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O'Reilly JX, Jbabdi S, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Salomon R, Bleich-Cohen M, Hahamy-Dubossarksy A, Dinstien I, Weizman R, Poyurovsky M, et al. Global functional connectivity deficits in schizophrenia depend on behavioral state. J Neurosci. 2011;31:12972–81. doi: 10.1523/JNEUROSCI.2987-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–9. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc. 2009;15:645–9. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A. Spontaneous resting-state BOLD fluctuations reveal persistent domain-specific neural networks. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr018. doi:10.1093/scan/nsr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;1:20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC. A computational model of cerebral cortical dysfunction in autism spectrum disorders. Biol Psychiatry. 2010;67:672–8. doi: 10.1016/j.biopsych.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Behav Rev. 2012;36:604–25. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Beaver JD, Ewbank MP, Keane J, Passamonti L, Lawrence AD, et al. Leaving a bad taste in your mouth but not in my insula. Soc Cogn Affect Neurosci. 2009;4:379–86. doi: 10.1093/scan/nsp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–54. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S-J, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–14. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggett AJ, Pritchard IC, Downing PE. Animate and inanimate objects in human visual cortex: evidence for task-independent category effects. Neuropsychologia. 2009;47:3111–7. doi: 10.1016/j.neuropsychologia.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.