Abstract
The neural substrates that enable individuals to achieve their fastest and strongest motor responses have long been enigmatic. Importantly, characterization of such activities may inform novel therapeutic strategies for patients with hypokinetic disorders, such as Parkinson’s disease. Here, we ask whether the basal ganglia may play an important role, not only in the attainment of maximal motor responses under standard conditions but also in the setting of the performance enhancements known to be engendered by delivery of intense stimuli. To this end, we recorded local field potentials from deep brain stimulation electrodes implanted bilaterally in the subthalamic nuclei of 10 patients with Parkinson’s disease, as they executed their fastest and strongest handgrips in response to a visual cue, which was accompanied by a brief 96-dB auditory tone on random trials. We identified a striking correlation between both theta/alpha (5–12 Hz) and high-gamma/high-frequency (55–375 Hz) subthalamic nucleus activity and force measures, which explained close to 70% of interindividual variance in maximal motor responses to the visual cue alone, when patients were ON their usual dopaminergic medication. Loud auditory stimuli were found to enhance reaction time and peak rate of development of force still further, independent of whether patients were ON or OFF l-DOPA, and were associated with increases in subthalamic nucleus power over a broad gamma range. However, the contribution of this broad gamma activity to the performance enhancements observed was only modest (≤13%). The results implicate frequency-specific subthalamic nucleus activities as substantial factors in optimizing an individual’s peak motor responses at maximal effort of will, but much less so in the performance increments engendered by intense auditory stimuli.
Keywords: subthalamic nucleus, local field potentials, peak force, paradoxical kinesia, motor vigor
Introduction
To what extent might the basal ganglia dictate the speed and the force of our maximal motor responses? Some contribution might be expected given that the basal ganglia have recently been implicated in the scaling of movement (Turner et al., 2003; Thobois et al., 2007; Muthukumaraswany, 2010; Turner and Desmurget, 2010; Grafton and Tunik, 2011; Brücke et al., 2012), although whether the same relationship holds true at the limits of voluntary performance remains unclear. Moreover, what is achieved by our maximal ‘effort of will’ is known to vary according to the context, with paradoxical enhancements of ‘best’ performance reported in response to intense stimuli both in healthy subjects (Woodworth, 1938; Angel, 1973; Anzak et al., 2011a) and, most notably, in the form of the brief amelioration of motor impairment, termed ‘paradoxical kinesis’ (Souques, 1921), described in patients with Parkinson’s disease (Valldeoriola et al., 1998; Anzak et al., 2011b). So, if activity in the basal ganglia is involved in achieving our ‘best’ performance, is it also modulated by experimental challenges like the introduction of intense stimuli? Or, are the performance improvements associated with such challenges achieved relatively independently of the basal ganglia? Indeed, understanding how fastest and strongest movements are achieved, and how their performance can be enhanced still further, are important goals which may inform the search for treatments for movement disorders such as Parkinson’s disease.
One of the difficulties in determining which brain activities may be instrumental in achieving optimal motor performance relates to the confound of peripheral afference, so that techniques like electrophysiological recording, which have high temporal resolution, have a unique contribution to make. Here, we recorded directly from deep brain stimulation electrodes implanted bilaterally in the region of the subthalamic nucleus of patients with Parkinson’s disease whilst they executed handgrips as fast and as strongly as possible in response to a visual cue. By delivery of a loud auditory tone simultaneous with the visual cue, on random trials, we further aimed to relate subthalamic nucleus local field potential (LFP) activity to any changes in force parameters and reaction time observed in response to intense stimuli. Finally, by testing patients ON and OFF dopaminergic medication, we could investigate the role, if any, of dopaminergic processes in performance of the task.
Materials and methods
Subjects
Ten patients with Parkinson’s disease (mean disease duration 10 years, mean age 58 years, range 42–65 years; seven males) gave informed consent to take part in this study, which was approved by the local ethics committees at our recording sites in Oxford and London, UK. Patients underwent bilateral implantation of deep brain stimulation electrodes into the subthalamic nucleus, as a prelude to therapeutic high-frequency stimulation for advanced idiopathic Parkinson’s disease with motor fluctuations and/or dyskinesias. Techniques to target and implant electrodes in the subthalamic nucleus have previously been described (Foltynie and Hariz, 2010). No microelectrode recordings were made, although the effects of direct stimulation were confirmed intra-operatively at surgical sites 1 and 3 (Table 1). The locations of the electrodes were additionally confirmed with immediate postoperative stereotactic imaging. Nonetheless, in acknowledgement of the fact that not all electrode contacts could be expected to lie in the subthalamic nucleus per se, we term the area sampled by the contact pairs as the subthalamic nucleus region. Electrodes were attached to extension cables ‘externalized’ through the scalp to permit recordings before connection to a subcutaneous deep brain stimulation pacemaker, implanted in a second operative procedure up to 7 days later. Clinical details of the patients are given in Table 1. The mean percentage improvement in the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) ON treatment with l-DOPA was 59.9 ± 5.7% (P < 0.0001) across subjects, indicating good responsiveness to l-DOPA in our study participants.
Table 1.
Patient details
| Site | Patient number | Age (years) | Disease duration (years) | Daily l-DOPA equivalent dose (mg) | Preoperative UPDRS part III |
|
|---|---|---|---|---|---|---|
| OFF | ON | |||||
| 1 | 1 | 64 | 13 | 500 | 33 | 6 |
| 1 | 2 | 65 | 17 | 150 | 29 | 8 |
| 1 | 3 | 42 | 6 | 500 | 50 | 38 |
| 1 | 4 | 63 | 7 | 400 | 76 | 16 |
| 2 | 5 | 62 | 8 | 950 | 64 | 25 |
| 2 | 6 | 46 | 7 | 600 | 43 | 10 |
| 2 | 7 | 57 | 9 | 625 | 60 | 27 |
| 3 | 8 | 62 | 9 | 750 | 38 | 20 |
| 3 | 9 | 57 | 7 | 100 | 40 | 20 |
| 3 | 10 | 59 | 15 | 750 | 33 | 16 |
Surgical sites: (1) John Radcliffe Hospital, Oxford; (2) National Hospital for Neurology and Neurosurgery, London; (3) Kings College Hospital, London, United Kingdom.
Experimental paradigm
Subjects were presented with a series of imperative visual cues, separated by 11–13 s, and instructed to squeeze a force dynamometer ‘as fast and hard as you possibly can when the light comes on and maintain this for the duration of the light’ (red light-emitting diode illuminated for 5 s). In half of these trials, which were randomly selected, a loud auditory stimulus (0.3 s duration, 1 kHz, 96 dB) was delivered binaurally through headphones, with onset simultaneous with that of the visual cue (auditory–visual cue). However, subjects were asked to just focus on responding to the visual cues.
Twenty trials were collected in each experimental run. Trials were approximately equally divided (allowing for the randomization process in each session) into those with visual and auditory–visual cues. Trials were carried out in a blocked design, and left- and right-hand recordings were counterbalanced across subjects. The rationale for the number of trials executed, and the intertrial interval, as well as stimulus intensity and decision to include a reaction time component into our paradigm has previously been described (Anzak et al., 2011b). Stimulus intensity was measured with a Brüel and Kjaer 2260 Observer.
Grip force was measured one hand at a time in each subject using an isometric dynamometer with standard Jamar design, and its handle set in the second of the five discrete grip diameter adjustments possible (G100; Biometrics Ltd, Sancho-Bru et al., 2008). Subjects were seated with their shoulders adducted (so that elbows rested against the trunk), their elbows flexed at ∼90° and their forearms in neutral, as recommended by the American Association of Hand Therapists (Fess, 1992).
Recordings and analysis
Recordings were made 3–6 days after surgery. To complete the recordings in one morning, and limit intrusion on our easily fatigable postoperative patients, recordings were always made first after overnight withdrawal of anti-parkinsonian medication (OFF l-DOPA), and then again ∼1 h after taking their usual morning dose [average l-DOPA dose administered, 155 ± (SEM) 25 mg, although Cases 4 and 10 also received subcutaneous apomorphine]. Improvement with medication was confirmed through assessment of finger tapping, wrist rigidity and tremor (using the corresponding items of the motor UPDRS). Although this sequence of recordings may have introduced a confound in that ON medication performance may have been affected by fatigue, our results suggest that any such effects were relatively minimal, as medication was still able to reduce reaction time.
LFPs were recorded bipolarly from adjacent contacts of each deep brain stimulation electrode (0–1, 1–2, 2–3) using either a D360 amplifier (Digitimer Ltd) in combination with a 1401 A/D converter (Cambridge Electronic Design) and sampled onto a computer using Spike2 software (Cambridge Electronic Design), or TMSi porti (TMS international) and its respective software. All recordings were originally sampled at 2048 Hz. Analogue correlates of the visual and auditory stimuli and dynamometer output were recorded and digitized in a similar way.
Analyses of both behavioural and LFP data were performed in Matlab (version 7.10). Peak yank (where yank is defined as the rate of change of force, calculated by differentiation of the force signal) and peak force were the chosen biomechanical variables of interest, and had the advantage that they could be measured trial by trial without realignment to compensate for differences in premotor reaction times. Premotor reaction time was operationally defined as the time interval between cue onset and the point at which force exceeded 5% of peak force (taken as response onset). We acknowledge that premotor reaction time is more usually considered to be the interval between cue presentation and EMG onset (Botwinick and Thompson, 1966). However, we found the use of EMG to be suboptimal in the context of maximal grips because of movement artefact and sampling error, given that many muscles are activated in this task. The process of derivation of average induced frequency-specific LFP power, over discrete frequency bands, from contralateral subthalamic nuclei to the gripping hand, is described in the online Supplementary material.
Statistics
For behavioural data, grand averages of peak force, peak yank and premotor reaction time for visual and auditory–visual trials were calculated after deriving each of these variables from the individual grips made by a subject, and calculating averages for that subject, before averaging across study participants. Group mean percentage changes in variables were calculated as the average of the mean percentage changes in each subject.
Our approach to statistical analysis of subthalamic nucleus region LFP power relationships with motor performance is summarized in Fig. 1. We first sought to characterize frequency band-specific oscillatory activities in the subthalamic nucleus region that might independently predict between-subject differences in maximal handgrip performance under standard/baseline conditions. This was achieved by means of multiple regression analysis applied to behavioural and LFP responses to visual cues alone, retrieved from experimental runs where visual and auditory–visual cues were delivered at random, when patients were ON medication. The continuous black regression line, labelled ‘RL’ in Fig. 1, describes the expected relationship of motor performance with any given frequency band-specific activity identified as a significant independent predictor of peak motor parameters, across subjects, in this baseline (visual cue, ON l-DOPA) condition.
Figure 1.
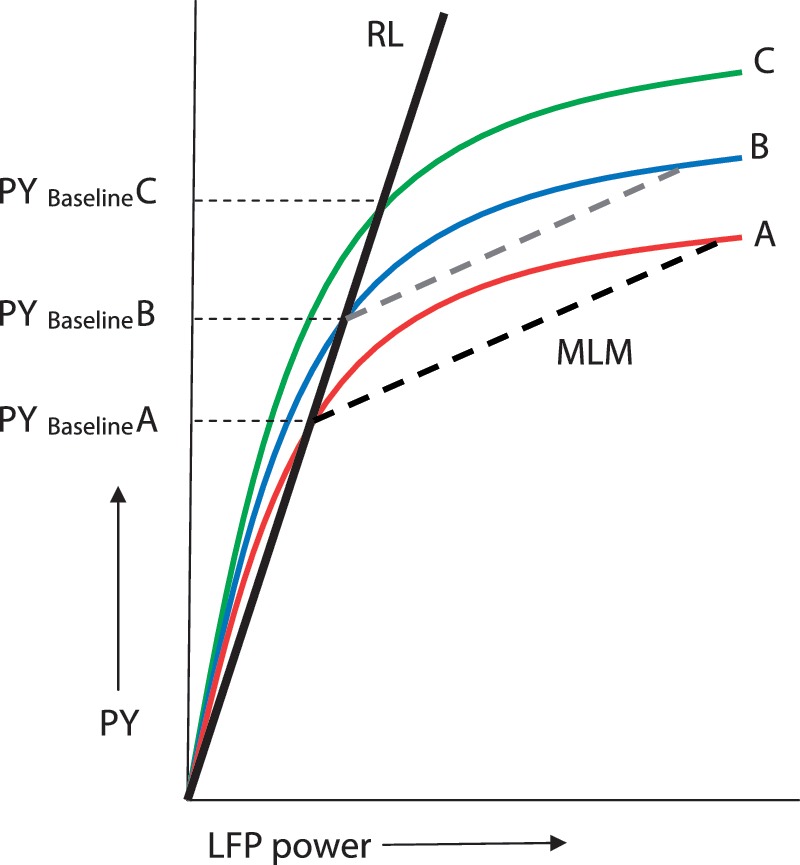
Putative relationship between frequency-specific LFP power and peak yank (PY), shown for three hypothetical individuals (Cases A, B and C). The continuous black line labelled ‘RL’ is the regression line fitting the LFP power relationship with peak yank, under baseline conditions (response to visual cues, when patients ON l-DOPA), across subjects. The interrupted black line, labelled ‘MLM’ is estimated by multi-level modelling on the basis of experimental condition-specific regression coefficients between frequency-specific LFPs and peak yank, corrected to each individual’s baseline performance. It thus models ‘within-subject’ effects of experimental manipulations from standard conditions. An average experimental condition-specific intercept shift of the MLM line from baseline would implicate increments or decrements in motor performance independent of frequency-specific subthalamic nucleus LFP power. Such an average shift would equate to the shift of curve A to curve B, whilst maintaining the gradient of the MLM line (translation of interrupted black line to the position of the interrupted grey line).
Next, we used a multi-level multivariate regression modelling approach (Hox, 2002) to identify any contribution of frequency-specific subthalamic nucleus region LFP activity to the change in motor performance with experimental manipulations, relative to our baseline condition (thus effectively removing the baseline relationship between LFP amplitude and performance given by the line labelled ‘RL’, and concentrating on those dependencies described by the interrupted black line, labelled ‘MLM’ in Fig. 1). The multi-level multivariate regression modelling approach used here had the advantage that each trial executed by every subject under the different experimental conditions was considered. The resulting high degrees of freedom (Supplementary Table 1) meant that very small effects could be identified with relatively narrow confidence limits. Further details of both the multiple regression and multi-level modelling technique are described in the Supplementary material and Supplementary Fig. 1.
Statistical analyses were performed in SPSS Statistics 19 (SPSS Inc.) and R (for multi-level modelling; R Development Core Team, version 2.13.2). Kolmogorov–Smirnov tests were applied to confirm that both transformed LFP data (Supplementary material) and behavioural measures were normally distributed, before further parametric testing. Where Mauchly’s test of sphericity was significant (P < 0.05) in repeated-measures ANOVAs, Greenhouse–Geisser corrections were applied. Mean ± SEM are presented throughout the text, unless otherwise specified.
Results
Our aim was to identify the role of basal ganglia activity, as indexed by the subthalamic nucleus region LFP, in the generation of grips made as fast and as strongly as possible, under standard conditions and in the setting of the performance enhancements engendered by intense auditory stimuli (Anzak et al., 2011a, b). To this end, we first identified those frequency bands in the subthalamic nucleus region LFP that underwent a change when grips were triggered by simple visual cues. We chose to consider recordings made in the ON l-DOPA state as our starting point and then define how LFP responses differed when triggered by intense stimuli (combined auditory and visual cues) and with or without overnight withdrawal of anti-parkinsonian medication.
Maximal effort grips are associated with frequency-specific changes in subthalamic nucleus region local field potential activity that correlate with peak motor performance across subjects
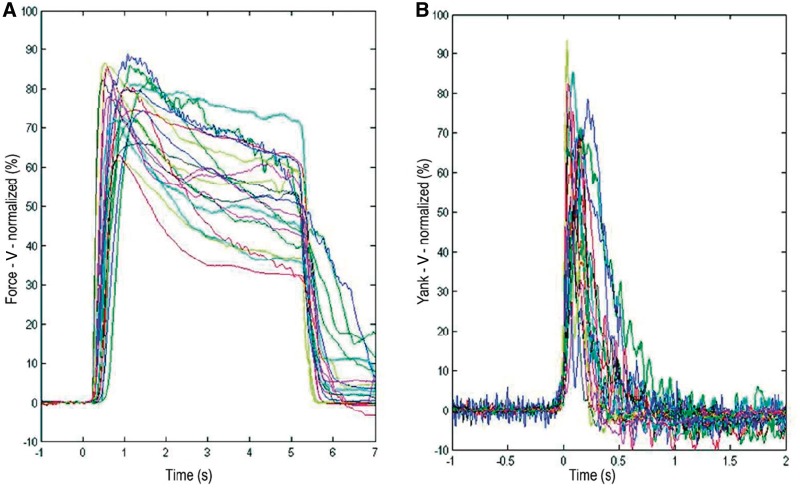
Three motor parameters of interest were derived from handgrips executed as quickly and strongly as possible, in response to visual cues in the ON l-DOPA state: premotor reaction time, peak yank and peak force. Average reaction time across subjects was 358 ± 21 ms. Average peak force and peak yank were 12.3 ± 1.5 kg and 72.8 ± 13.6 kg/s, respectively (n = 20 hands). The average force and yank traces for the hand contralateral to each subthalamic nucleus are represented in Fig. 2.
Figure 2.
(A) Sustained maximal force grips, in response to a visual (V) cue illuminated for 5 s, when patients with Parkinson’s disease were ON dopaminergic medication. In this panel, time zero represents cue onset, so that each individual’s average reaction times can be discerned. Average grip traces for left and right hands are presented and are normalized as a percentage of the maximal voluntary contraction achieved, by each hand, respectively, under this condition. Note, as can be seen in the figure, those trials in which subjects were slow in releasing the maximal grip were not excluded from further analysis, as the motor parameters of interest in this study fell at much earlier latencies. (B) Yank (rate of development of force), averaged after realignment to response onset following a visual cue, when patients with Parkinson’s disease were ON dopaminergic medication. Traces have been normalized as in A. Each patient is colour-coded with the same colour in A and B.
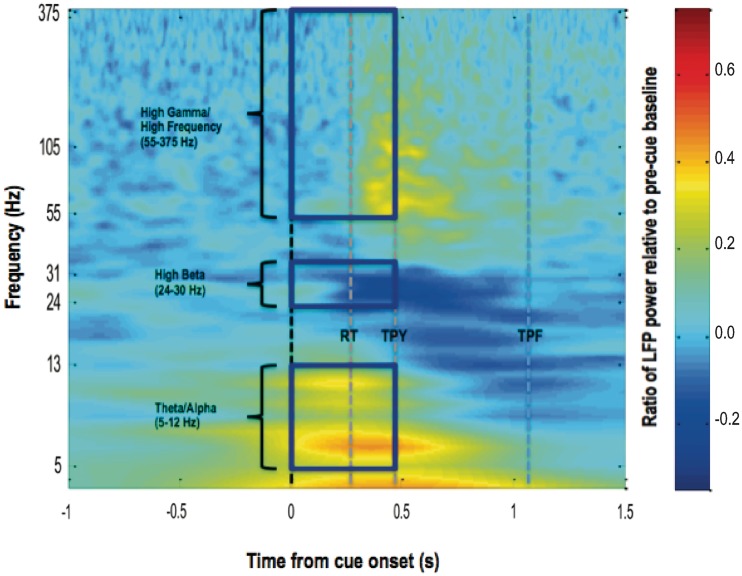
Time-evolving power spectra of changes in subthalamic nucleus region LFPs induced by visual cues, relative to a pre-cue baseline, were derived by averaging across all visual-cued trials in an experimental run, for each patient undertaking the task when ON medication. These were then averaged across subjects (Fig. 3). In this way, three frequency bands were identified over which increases or decreases in power, in the period from cue onset to average time to peak yank, could be distinguished from the pre-cue baseline and from frequency bands with an absence of reactivity to cue. These were the theta/alpha (5–12 Hz), high-beta (25–30 Hz) and high-gamma/high-frequency (55–375 Hz) ranges.
Figure 3.
Average time–frequency plot of change in induced spectral power in 20 subthalamic nuclei contralateral to sustained maximal handgrips, relative to a pre-cue baseline. Time zero represents onset of the imperative visual (V) cue. Patients were recorded ON their normal dopaminergic medication. Colour gradient represents ratio of post-cue LFP power to average LFP power 1–2 s before cue onset. Lines labelled RT, TPY and TPF demarcate the average premotor reaction time, time to peak yank and time to peak force, under this condition, respectively. Frequency is plotted on a log axis.
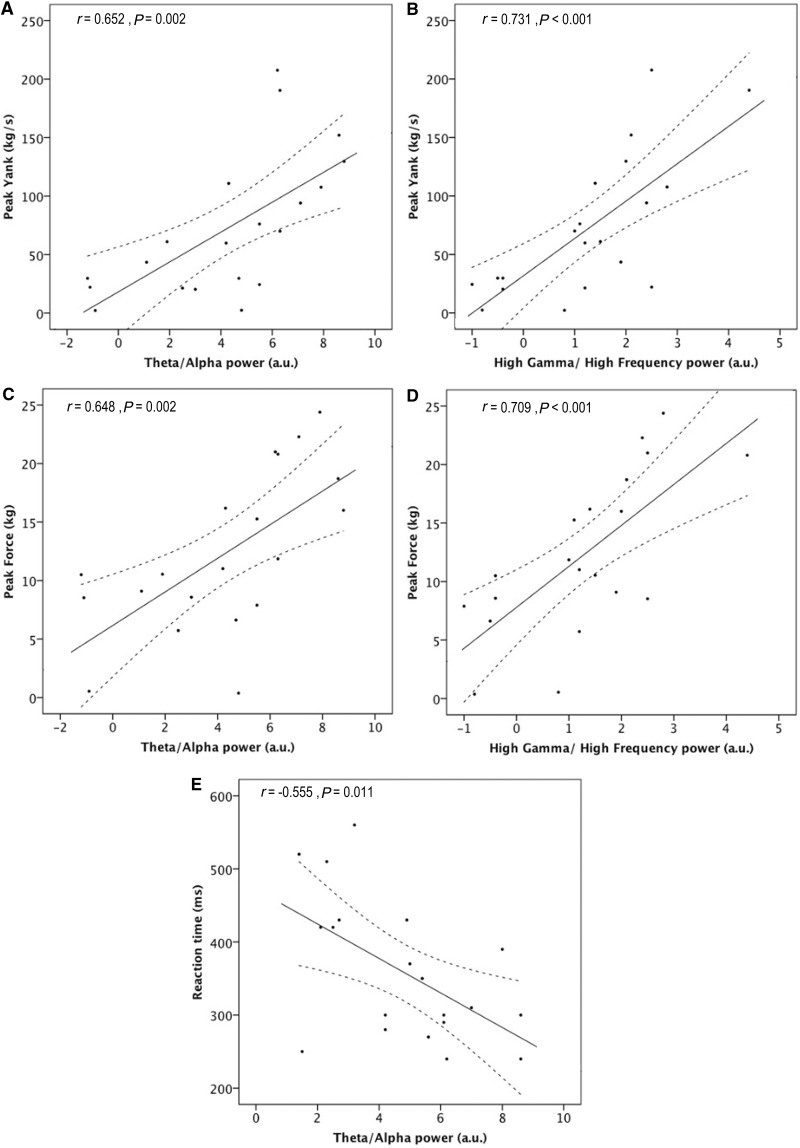
Evidence of a relationship between performance and average LFP power in the earlier defined frequency bands, was then sought. Simple linear regression analysis identified significant correlations between performance and LFP activity in theta/alpha and high gamma/high frequency, but not beta bands. The significant associations are shown as scatter plots fitted by simple regression in Fig. 4. However, whether activity in these frequency bands made an independent contribution to the motor parameters of interest, over and above the influence of other frequency-specific activities, remained to be clarified. To address this question, multiple regression analysis was performed, using each subject’s mean LFP in the three reactive frequency bands identified earlier as the predictive variables, and each motor parameter of interest as the response variable.
Figure 4.
Scatter-graphs relating LFP power to performance. Continuous and interrupted lines represent the best fit and corresponding 95% confidence limits estimated by linear regression. Significant correlations with peak yank, peak force and reaction time were found in the theta/alpha (5–12 Hz) band, and with peak yank and peak force in the high-gamma/high-frequency (55–375 Hz) range. No correlations were found in the beta (13–30 Hz) band. Non-significant correlations are not illustrated.
Both increases in theta/alpha and high-gamma/high-frequency LFP power were found to be predictive of (i.e. could explain some of the variance in) increases in peak yank (β = 0.486, P = 0.003 and β = 0.594, P = 0.001, respectively). Beta activity was of no independent predictive value. The overall fit of the regression model was excellent (F = 15.662, P < 0.001, R2 = 0.698) and the intercept was non-significant.
The overall model fit for the multiple regression model for peak force was also very strong (F = 13.700, P < 0.001, R2 = 0.667), and similarly identified significant contributions of theta/alpha (β = 0.505, P = 0.003) and high-gamma/high-frequency (β = 0.600, P = 0.001) power in predicting peak force. The intercept was non-significant and beta activity was of no independent predictive value. Thus, the results suggest that had a given subject been able to achieve a greater mean increase in LFP activity in either the theta/alpha or high-gamma/high-frequency bands, their mean peak yank or peak force would also have improved. Furthermore, the strong fits of the regression models, as indexed by large R2 values, and the insignificant intercepts suggest that subthalamic nucleus region LFPs were able to predict the majority of the variance in peak force and peak yank between subjects.
Conversely, only increases in theta/alpha LFP power were found to be predictive of reductions in reaction time (β = −0.490, P = 0.035). Neither beta nor high-gamma/high-frequency activity made an independent predictive contribution. The model fit was moderate, only approaching significance (F = 3.106, P = 0.056, R2 = 0.250), and the regression model had a significant intercept of 465 ms (P < 0.001), suggesting that the greater part of the reaction time was dictated by processes that were independent of, at the very least, any linear relationship with induced subthalamic nucleus region LFP activity.
Earlier in the text, we considered LFP power changes from cue onset to response onset in the case of reaction time, and from cue onset to the moment of development of peak yank in the case of peak yank and peak force. However, the latter allows sufficient time for peripheral afference to contribute to the correlations. We therefore repeated the previous simple and multiple regression analyses for peak yank and peak force taking LFP power changes from cue onset to response onset and confirmed the same relationships (Supplementary material and Supplementary Fig. 2), despite the fact that the greatest gamma increases developed after response onset (Fig. 3). Finally, in a small number of cases, we were also able to identify a scaling effect of frequency band-specific LFP power with performance at the within-subject level (Supplementary material and Supplementary Fig. 3).
Intense auditory stimuli enhance mean peak yank and reaction time, whereas l-DOPA only reduces reaction time and does so without interacting with the effects of loud auditory cues
The delivery of brief 96-dB auditory tones at the same time as visual cues, improved average peak yank and reaction time in our subjects. A repeated-measures ANOVA applied to peak yank data identified an effect of cue type [F(1,19) = 6.464, P = 0.020], but no effect of dopaminergic medication [F(1,19) = 2.695, P = 0.117], or dopaminergic medication × cue-type interaction [F(1,19) = 3.333, P = 0.084]. Peak yank increased by 11.8 ± 3.5% with auditory–visual cues (82.0 ± 16.1 kg/s) as compared with visual cues (71.8 ± 13.0 kg/s), when averaging across OFF and ON medication recordings.
A repeated-measures ANOVA applied to reaction time data identified an effect of cue type [F(1,19) = 64.919, P < 0.001] and dopaminergic medication [F(1,19) = 5.597, P = 0.029], but no dopaminergic medication × cue-type interaction [F(1,19) = 3.465, P = 0.078]. Reaction time reduced by 35.4 ± 2.2% with auditory–visual cues (239.3 ± 13.8 ms) compared with visual cues (380.6 ± 26.3 ms), when averaging across OFF and ON medication recordings. Reaction time decreases from 322.5 ± 22.2 ms OFF l-DOPA to 297.4 ± 17.2 ms ON l-DOPA were further observed (P = 0.029, paired t-test averaged across cue types). The lack of dopaminergic medication × cue-type interactions for both peak yank and reaction time data suggested that improvements in performance with auditory–visual cues, were independent of dopaminergic therapy (Anzak et al., 2011a, b).
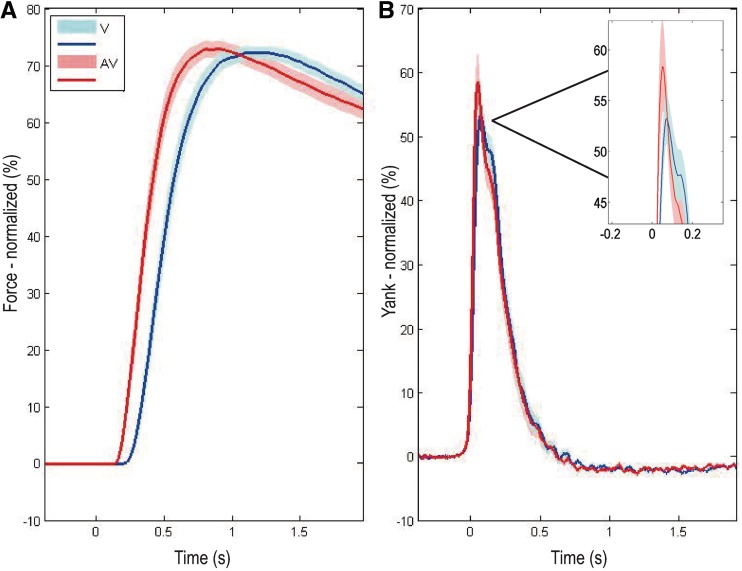
In the current study, however, there were no significant effects of cue or drugs on peak force so that this measure of performance was not considered further [cue type, F(1,19) = 0.212, P = 0.650; medication state, F(1,19) = 0.985, P = 0.333; medication × cue-type interaction. F(1,19) = 2.462, P = 0.133]. Group average force and yank traces, in response to visual and auditory–visual cues, are shown in Fig. 5.
Figure 5.
(A) Group average grip force achieved in visual (V) and auditory–visual (AV) trials across patients (n = 20 hands), averaged across OFF and ON l-DOPA conditions. Time zero represents cue onset. For the purposes of graphical representation only (Anzak et al., 2011a, b), group average traces have been derived as the mean of the average responses of each gripping hand to visual and auditory–visual cues, normalized as a percentage of the average maximal voluntary contraction achieved in the visual condition when patients were ON and OFF l-DOPA (Fig. 2). Shaded area represents SEM. of the trace. (B) Group average yank (rate of development of force) achieved in visual and auditory–visual trials, averaged across OFF and ON l-DOPA conditions. Traces have been averaged after realignment to response onset. Significant reductions in reaction time and enhancements in peak yank (see boxed expansion of the peak difference), but no change in peak force, are evident in response to auditory–visual as compared with visual cues.
Changes in peak yank and reaction time are accompanied by frequency-specific mean changes in local field potential power with experimental condition
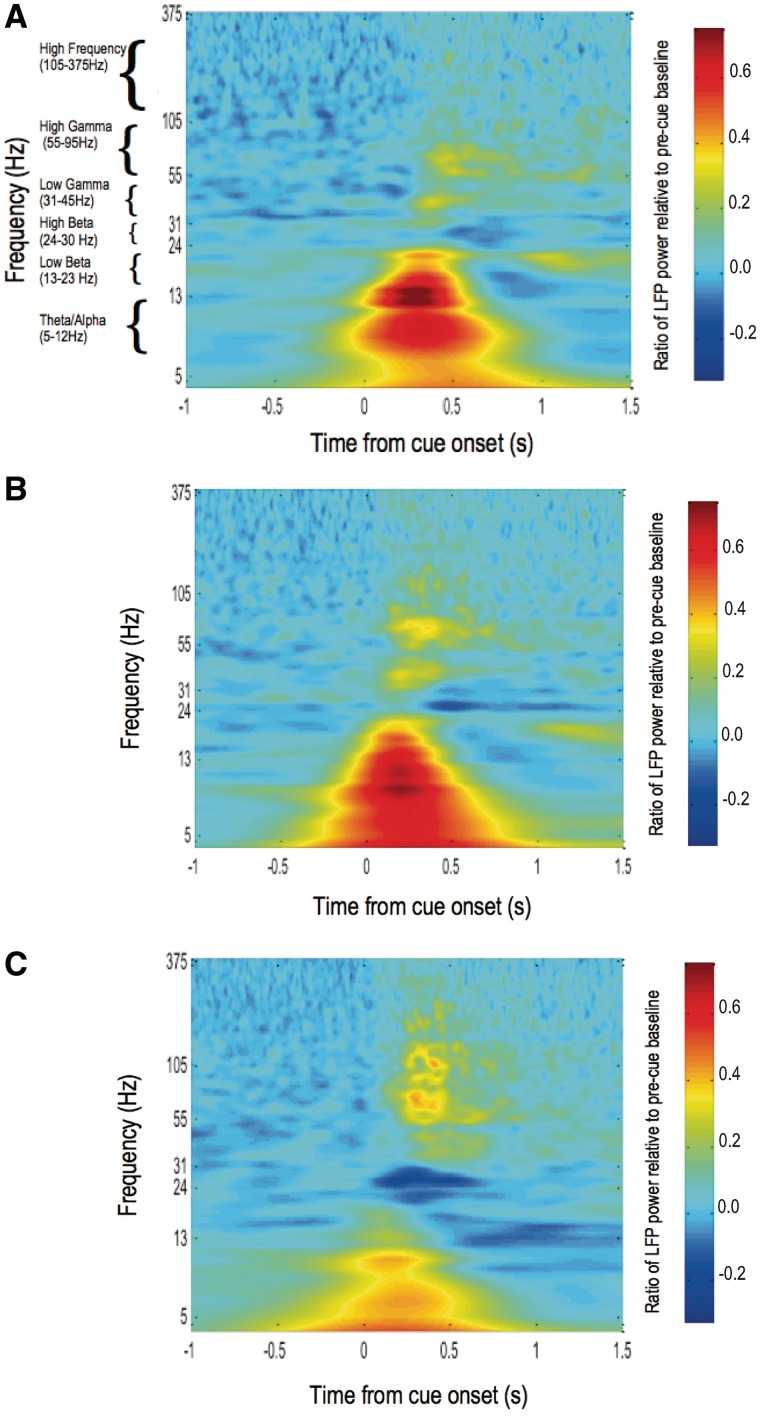
We next defined how LFP responses differed when triggered by combined auditory and visual cues with or without overnight withdrawal of anti-parkinsonian medication. To this end, we aimed to group activity over frequencies with similar reactivity to either cue type or l-DOPA into discrete bands, contiguous with neighbouring bands which differed in their average response to experimental condition. Time–frequency spectra of cue-induced subthalamic nucleus region LFPs in the three additional experimental conditions (OFF l-DOPA visual cue, OFF l-DOPA auditory–visual cue, and ON l-DOPA auditory–visual cue) revealed prominent differences in power at frequencies ≤30 Hz between OFF and ON l-DOPA recordings, and at frequencies >30 Hz between visual and auditory–visual cues (Fig. 6). We therefore performed separate ANOVAs of percentage change in LFP power (relative to a pre-cue baseline) in activities across the three frequency bands ≤30 Hz (5–12, 13–23 and 24–30 Hz) and for the three frequency bands >30 Hz (31–45, 55–95 and 105–375 Hz), to see which, if any of these bands, might be influenced by cue type and medication status (refer to the online Supplementary material for rationale for selection of frequency bands). The results of these ANOVAs are presented in Table 2. Post hoc exploration of significant main effects and interactions is described below.
Figure 6.
Matrices of average time–frequency plots of change in induced spectral power in 20 subthalamic nuclei contralateral to sustained maximal hand grips, relative to a pre-cue baseline, under different experimental manipulations. Time zero represents onset of the imperative visual or loud auditory–visual cue. Colour gradient represents ratio of post-cue LFP power to average LFP power 1–2 s before cue onset. Spectral changes under three different experimental conditions are represented as (A) OFF L-DOPA, visual cue; (B) OFF l-DOPA, auditory-visual cue and (C) ON l-DOPA, auditory–visual cue. The spectral changes in the baseline condition, ON l-DOPA, visual cue state are shown in Fig 2. Power changes common to each experimental condition occurred over six frequency bands: theta/alpha (5–12 Hz), low beta (13–23 Hz), high beta (24–30 Hz), low gamma (31–45 Hz), high gamma (55–95 Hz), and high frequency (105–375 Hz). Frequency is plotted on a log axis.
Table 2.
Separate repeated-measures ANOVAs of percentage change in LFP power (relative to a pre-cue baseline) applied to activities ≤30 Hz (5–12, 13–23, 24–30 Hz bands) and >30 Hz (31–45, 55–95, 105–375 Hz bands)
| Within-subject effects | ≤30 Hz frequency bands | >30 Hz frequency bands |
|---|---|---|
| Frequency | F(2,38) = 56.099, P < 0.001 | F(2,38) = 11.493, P < 0.001 |
| Cue type | F(1,19) = 0.282, P = 0.602 | F(1,19) = 15.000, P = 0.001 |
| Medication | F(1,19) = 3.585, P = 0.074 | F(1,19) = 0.059, P = 0.811 |
| Frequency × medication | F(2,38) = 3.503, P = 0.041 | F(2,38) = 7.777, P = 0.002 |
| Cue type × frequency | F(2,38) = 0.094, P = 0.865 | F(2,38) = 0.632, P = 0.500 |
| Cue type × medication | F(1,19) = 0.047, P = 0.830 | F(1,19) = 0.658, P = 0.427 |
| Frequency × cue type × medication | F(2,38) = 0.160, P = 0.843 | F(2,38) = 0.785, P = 0.432 |
Additional factors included cue type (visual or auditory–visual) and medication (OFF or ON l-DOPA). Significant results are in bold.
The repeated-measures ANOVA applied to activities ≤30 Hz identified a significant effect of frequency and a significant medication × frequency interaction. Post hoc paired t-tests confirmed a significant reduction in power in the low beta band (P = 0.020) and trend towards a similar effect on high-beta activity (P = 0.077), but no effect on theta/alpha power (P = 0.774) with L-DOPA, when averaging across responses to different cue types.
Conversely, the ANOVA of percentage change in activities >30 Hz identified a significant effect of cue type, but no cue type × frequency interaction, suggesting a similar magnitude of response to auditory–visual cues in each frequency band. An overall effect of frequency reflected only differences in the average power across each frequency band relative to baseline. A significant medication × frequency interaction, resulted solely from an increase in low gamma activity (P = 0.015, paired t-test when averaging across cue type; high gamma, P = 0.284; high frequency, P = 0.180). Given these results recordings from OFF and ON dopaminergic medication were averaged across the three frequency bands, and post hoc paired t-tests were performed that demonstrated higher power in a broad gamma (31–375 Hz) band following auditory–visual as opposed to visual cues (low gamma, P = 0.027; high gamma, P = 0.006; high frequency, P ≪ 0.001). Thus, auditory–visual cues promoted cue-related power increases in frequency bands >30 Hz. This broad gamma band was used for all subsequent analyses.
Frequency-specific subthalamic nucleus region local field potential activity contributes to the facilitation of peak motor performance with experimental condition
Multi-level modelling was used to derive general regression equations for percentage increments/decrements in peak yank and reaction time, relative to our baseline condition of maximal effort grips executed in response to visual cues when patients were ON medication. Within the model, regression coefficients afford an estimate of the degree to which the change in performance from baseline scales with LFP activity, whereas intercepts represent the portion of the change from baseline performance that is independent of subthalamic nucleus region LFPs.
The models only identified broad gamma power (31–375 Hz) as a significant predictor of the response variables (Table 3 and Supplementary Table 1). Auditory–visual cues were found to induce a scaling of broad gamma activity with peak yank, in both OFF and ON l-DOPA states, which was not present in response to visual cues alone. On the other hand, a consistent scaling of broad gamma activity with reaction time was present independent of experimental condition. There were also large shifts in intercepts relative to the baseline condition; those due to withdrawal from dopamine included an improvement in peak yank that most probably related to the lack of fatigue rather than drug state, as OFF drug recordings were necessarily conducted before ON drug experiments. Moreover, no significant difference was identified between peak yank attained in OFF and ON drug recordings, in an earlier analysis of the performance data alone (see earlier text).
Table 3.
Experimental condition specific regression coefficients (β) and intercept shifts (μ) for regression models derived for peak yank and reaction time
| Experimental condition | Peak yank |
Reaction time |
|||||
|---|---|---|---|---|---|---|---|
| Broad gamma regression coefficient (β) | Intercept shift (μ) |
Broad gamma regression coefficient (β) | Intercept shift (μ) |
||||
| AV cue | OFF l-DOPA | AV cue | OFF l-DOPA | ||||
| OFF l-DOPA | V cue | – | – | 8.57 | −0.101 | – | 4.38 |
| AV cue | 0.223 | 11.38 | 8.57 | −0.101 | −36.46 | 4.38 | |
| ON l-DOPA | V cue | – | – | – | −0.101 | – | – |
| AV cue | 0.223 | 11.38 | – | −0.101 | −36.46 | – | |
Intercept shifts represent the % increments/decrements from maximal grips made in response to visual (V) cues when patients were ON l-DOPA, which also formed the baseline condition for our multi-level multivariate regression modelling approach.
AV = auditory–visual.
Given the relatively large intercept shifts with auditory–visual cues, we lastly evaluated the quantitative contribution of broad gamma activity in predicting auditory–visual-induced changes in peak yank and reaction time from the baseline visual ON l-DOPA condition. This was achieved by multiplying coefficients by the mean level of broad gamma band activity in the corresponding condition. Averaging across drug states, broad gamma activity contributed to 13.2 ± 8.0% (95% confidence interval) of the enhancement in peak yank, but only 1.1 ± 0.1% of the reduction in reaction time with loud auditory cues. Thus, changes in broad gamma activity contributed to improvements in peak yank, but barely affected reaction time. Even in the case of peak yank, the larger proportion of motor enhancement with auditory–visual cuing was independent of synchronous oscillatory activities in the subthalamic nucleus, or, at the very least, independent of a linear relationship with the LFPs.
Local field potential activity reflects local processing in the subthalamic nucleus region
LFP recordings from the subthalamic region were highly focal, as indexed by steep percentage drops in power when comparing the ‘best’ contact pair, with the highest absolute power, to the mean power recorded by the two remaining contact pairs on each electrode. Figure 7 shows the power drop for each frequency band used in multi-level modelling analysis. In this, we have averaged across experimental conditions and drug states. There is a clear drop in all frequency bands, albeit less pronounced in the theta–alpha band.
Figure 7.
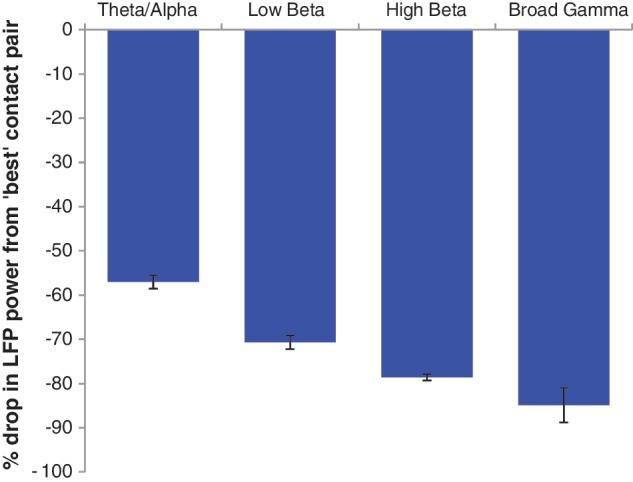
Mean ± SEM percentage drop in LFP power from the contact pair recording the greatest power change from baseline, for each frequency band, averaged across experimental condition.
Discussion
The findings of our study are 2-fold. We first report a striking correlation between both theta/alpha (5–12 Hz) and high-gamma/high-frequency (55–375 Hz) activities in the subthalamic nucleus region LFP and average peak force measures attained, in response to visual cues when patients were ON l-DOPA (baseline condition). These spectral features were able to independently predict close to 70% of the variance in peak yank and peak force across subjects. The relationship with reaction time, however, was limited to the theta/alpha band, and less substantial, suggesting a larger influence of a different neural source, or at the very least a non-linear effect of LFP activity, on this parameter. Second, we describe an increase in broad gamma (31–375 Hz) subthalamic nucleus region LFP power, over and above those activities encoding maximal effort grips in the baseline condition, which was found to make a modest but significant 13.2% contribution to enhancements in peak yank, but only a trivial contribution to reaction time shortening, with auditory–visual cues. Although reaction time shortening was also observed in response to administration of l-DOPA, independent of cue type delivered, no frequency-specific components of subthalamic nucleus region LFP activity could be attributed to this effect.
Theta/alpha and high-gamma/high-frequency subthalamic nucleus region local field potential activity predicts average peak force measures attained by an individual
The results of our study ascribe a novel function to frequency-specific subthalamic nucleus region LFP activity, as having substantial explanatory influences on the peak motor parameters attainable by a subject at maximal effort. A similar, albeit weaker, phenomenon has previously been described whereby subthalamic nucleus region LFP measures have been found to correlate with motor UPDRS across patients (Chen et al., 2010; Pogosyan et al., 2010). These subthalamic nucleus LFP-force correlations are in line with the hypothesized role of the basal ganglia in the scaling of movement, although this role is largely deduced from activities related to a range of submaximal motor responses (Turner and Desmurget, 2010). Indeed, both PET (Turner et al., 2003) and functional MRI studies (Vaillancourt et al., 2004, 2007; Prodoehl et al., 2009; Grafton and Tunik, 2011) have identified correlations between basal ganglia activity and a range of amplitudes, velocities and forces with which healthy subjects were required to execute movement. In particular, subthalamic nucleus activity has been associated with the generation of force pulses in precision grip tasks (Vaillancourt et al., 2007).
With respect to the correlation between high-gamma/high-frequency (55–375 Hz) activity in the subthalamic nucleus LFP and force measures reported in our study, high-gamma (35–100 Hz) activity in the globus pallidus interna LFP has also recently been implicated in the scaling of movement in patients with segmental dystonia, although the correlations with movement velocity were much weaker than those shown here (Brücke et al., 2012). In addition, a similar role has been suggested for gamma (60–90 Hz) synchronization in the motor cortex of healthy subjects (Muthukumaraswamy, 2010). A prokinetic function of subthalamic nucleus LFP activity at very high frequencies (>100 Hz), has further been proposed, on the basis of the increase in amplitude of high-frequency oscillations accompanying voluntary movements, and following treatment with dopaminergic medication in patients with Parkinson’s disease (Foffani et al., 2003). However, the latter is contentious as Lopez-Azcarate and colleagues (2010) reported high-frequency oscillations of a similar amplitude both OFF and ON dopaminergic medication, but showed marked movement-related amplitude modulation of this activity when liberated from beta coupling when ON dopaminergic medication. A further study has suggested that it is the ratio of slower (200–300 Hz) to faster (300–400 Hz) high-frequency oscillations that correlates with UPDRS motor scores (Özkurt et al., 2011). As with Lopez-Azcarate et al. (2010), we found that our high-gamma/high-frequency band (which included oscillatory activity >100 Hz) was of similar amplitude with or without dopaminergic medication, although in considering a wide frequency range we could not address the question of a frequency shift in the high-frequency activity. The very wide spectral range of the high-frequency oscillations in this and other studies also raises the possibility that some of the activity could reflect neuronal spiking in the electrode vicinity, as has been suggested at the cortical level (Ray and Maunsell, 2011).
Oscillatory activity in the theta/alpha range, which was found to correlate with our peak force measures, has generally been associated with mechanisms of attention (for review see Palva and Palva, 2007). In particular, alpha activity (7–13 Hz) in the subthalamic nucleus of patients with Parkinson’s disease at rest is coherent with parietotemporal cortex in a circuit that has been proposed to subserve attentional functions (Hirschmann et al., 2011; Litvak et al., 2011). More apposite to the present results, LFP recordings from the subthalamic nucleus of patients with Parkinson’s disease and from the globus pallidus interna of patients with dystonia have shown that contralateral alpha activity increases in fast movements compared with rest, and passive or active slow repetitive extension and flexion of the elbow (Singh et al., 2011). In the latter study, the increased synchronization in the alpha range was concurrent with the period of elevated acceleration in the fast movement. As this was seen in both patient groups, it was considered to be primarily physiological and task specific, rather than disease related.
The lack of an independent contribution of beta band oscillations to the prediction of force measures was conspicuous. Elsewhere, it has been argued that suppression of population synchrony in the beta frequency range is necessary to allow task-related rate coding and more focal neuronal assemblies to engage in task-specific processing related to voluntary movement (Brown and Williams, 2005). Indeed, recordings in non-human primates confirm an inverse relationship between oscillatory LFP activity in the beta band and local task-related rate coding in the striatum (Courtemanche et al., 2003). Our finding of a suppression of beta activity following imperative visual cues, which did not scale with behavioural performance, is thus consistent with a binary gating function of beta (Kempf et al., 2007; Brücke et al., 2012).
Finally, whether theta/alpha and high-gamma/high-frequency activities in the basal ganglia relate to a phenomenon that parameterizes force or a higher order process that regulates the scaling of movement (Turner and Desmurget, 2010) is unclear. It has previously been suggested that movement parameters are essentially ‘selected’ from an underlying range of physiological capabilities, so as to optimize the use of neuromuscular energy; the likelihood of selection of faster or stronger movement parameters, based on such implicit cost-benefit assessments, has been termed ‘motor vigor’ (Mazzoni et al., 2007). In the current paradigm, it is plausible that even under instruction to execute maximal handgrips, individuals continued to select grips from the previously described distribution, and it is, thus, the motor vigor or effort invested in selection of the optimal motor parameters, that frequency-specific oscillatory activity in the basal ganglia encodes. Further work in this area would be of great interest.
Broad gamma subthalamic nucleus region local field potential activity makes a significant but modest contribution to motor enhancement with intense stimuli
Our multi-level multivariate regression modelling approach identified the induction of a novel (over and above that in the baseline state) but small scaling factor between broad gamma (31–375 Hz) activity and peak yank, with auditory–visual cuing. Such an influence of frequency-specific subthalamic nucleus LFP activity on motor enhancement with intense stimuli provides further support for the previously proposed role of the subthalamic nucleus in ‘energizing’ movement. Indeed, it has previously been proposed that ‘motor vigor’ itself can be subject to modulation by temporally pressing situations, whereby the system is forced to adopt a more expensive trade-off, thus leading to a more consistent optimum performance (Ballanger et al., 2006).
Nonetheless, it was the contribution of experimental condition-specific ‘intercept shifts’ of the best-fit line relating broad gamma LFP power to motor performance, which dominated over changes in regression coefficients for both performance measures. Broad gamma activity was found to account for only 13.2 ± 8.0% of the enhancement in peak yank, when considering the induced scaling factor together with the increase in amplitude of LFP activity in this frequency band, following auditory–visual cues. Moreover, the effect of changes in broad gamma LFP power on the marked shortening of reaction time with auditory–visual cuing was near inconsequential. Thus, we conclude that enhancement of peak motor performance with auditory–visual cues was achieved largely independently of frequency-specific subthalamic nucleus region LFP activity, or at the very least, independent of a linear relationship of this activity with the performance increments observed.
What then might be the neural substrate mediating performance enhancements with auditory–visual cuing? We have previously posited that the intense or arousing nature of our auditory–visual cue implicates a role of phasic arousal (Sturm and Willmes, 2001) in driving the phenomenon (Anzak et al., 2011b). The brainstem reticular activating system, in particular, is a largely non-dopaminergic network that has been implicated in cortical activation or arousal (Dringenberg and Vanderwolf, 1998). Its involvement would fall in line with the dopamine independence of the behavioural effect observed. Within this network, a role of the pedunculopontine nucleus, known to be tightly coupled to the subthalamic nucleus (Aravamuthan et al., 2009), is conceivable, but whether alerting cholinergic projections from the brainstem reticular activating system (Steriade et al., 1990, 1991; Munk et al., 1996) can bypass those basal ganglia components dependent on dopaminergic input deserves further investigation. In addition, a role of the left parasagittal and lateral cerebellar hemisphere, as well as bilateral sensory motor cortices, has also been suggested in sensory stimulus-elicited improvements in performance (Thobois et al., 2007).
Caveats and concluding remarks
Two possible limitations of the present study are worth highlighting. First, our study participants were necessarily patients with Parkinson’s disease, so inferences regarding normal functioning must be circumspect (Williams et al., 2002). That said, and as discussed previously, the core relationship between subthalamic nucleus region LFPs and force measures in our baseline task had much in common with the behaviour of cortical and pallidal activities in healthy subjects and patients with cranial dystonia, without obvious upper limb involvement (Muthukumaraswamy, 2010; Brücke et al., 2012). The latter, however, introduces the second issue—could the power changes picked up at the bipolar contacts of the deep brain stimulation electrode be the product of volume conduction from another, possibly cortical, source? Against this, we report a steep gradient in LFP power between those bipolar contact pairs recording the highest absolute power and the two remaining contact pairs, independent of the frequency of the oscillatory activity, which is consistent with a local generator (Kühn et al., 2004, 2006). Moreover, a number of studies have now demonstrated the locking of discharge of neurons in the subthalamic nucleus to the LFP (Levy et al., 2002; Kühn et al., 2005; Pogosyan et al., 2006; Trottenberg et al., 2006; Weinberger et al., 2006). Finally, it should be pointed out that involvement of the subthalamic nucleus in motor control has been suggested to extend well beyond the scaling of movement, as discussed here, to include motor learning, action selection and response inhibition, and the online correction of motor error (Turner and Desmurget, 2010).
To conclude, we provide strong correlative evidence (accounting for ∼70% of the intersubject variance) linking subthalamic nucleus region oscillatory activity over the theta/alpha and high-gamma/high-frequency ranges to the average peak force and peak rate of development of force attained by individuals, in voluntary grips performed as fast and as strongly as possible in response to a visual cue. Frequency-specific oscillatory activity, however, was found to make only a small contribution to the additional improvement in peak yank and reaction times engendered by intense auditory stimuli, independent of dopaminergic state. Our findings provide insight into the relationship between oscillatory activity in the subthalamic nucleus region and contractions made with maximal effort and raise the possibility that such activity encodes motor vigor. At the same time the suggestion that non-dopaminergic processes (which are also independent of frequency-specific oscillatory activity in the subthalamic nucleus region) underscore the additional performance improvements following intense stimuli, encourages the search for alternative, non-dopaminergic, systems that may beneficially influence motor behaviour.
Funding
This work was funded by the Cure Parkinson’s trust and the National Institute of Health Research, Oxford Biomedical Research Centre.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors would like to thank the patients for their participation and Dr Daniel Lunn (Department of Statistics, University of Oxford) for his advice regarding statistical analysis.
Glossary
Abbreviations
- LFP
local field potential
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- Angel A. Input-output relations in simple reaction time experiments. J Exp Psychol. 1973;25:193–200. doi: 10.1080/14640747308400338. [DOI] [PubMed] [Google Scholar]
- Anzak A, Tan H, Pogosyan A, Djamshidian A, Ling H, Lees A, et al. Improvements in rate of development and magnitude of force with intense auditory stimuli in patients with Parkinson's disease. Eur J Neurosci. 2011a;34:124–32. doi: 10.1111/j.1460-9568.2011.07735.x. [DOI] [PubMed] [Google Scholar]
- Anzak A, Tan H, Pogosyan A, Brown P. Doing better than your best: loud auditory stimulation yields improvements in maximal voluntary force. Exp Brain Res. 2011b;208:237–43. doi: 10.1007/s00221-010-2474-1. [DOI] [PubMed] [Google Scholar]
- Aravamuthan BR, McNab JA, Miller KL, Rushworth M, Jenkinson N, Stein JF, et al. Cortical and subcortical connections within the pedunculopontine nucleus of the primate Macaca mulatta determined using probabilistic diffusion tractography. J Clin Neurosci. 2009;16:413–20. doi: 10.1016/j.jocn.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Ballanger B, Thobois B, Batusche P, Turner RS, Brouselle E, Desmurget M. “Paradoxical kinesis” is not a hallmark of Parkinson’s disease but a general property of the motor system. Mov Disord. 2006;21:1490–5. doi: 10.1002/mds.20987. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Willi M, Jancke L. Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: a transcranial magnetic stimulation study. Neuroreport. 2007;18:261–5. doi: 10.1097/WNR.0b013e328012272e. [DOI] [PubMed] [Google Scholar]
- Botwinick J, Thompson LW. Premotor and motor components of reaction time. J Exp Psychol. 1966;71:9–15. doi: 10.1037/h0022634. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–63. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–9. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Brücke C, Huebl J, Schönecker T, Neumann WJ, Yarrow K, Küpsch A, et al. Scaling of movement is related to pallidal gamma oscillations in patients with dystonia. J Neurosci. 2012;32:1008–19. doi: 10.1523/JNEUROSCI.3860-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Hsu YT, Chan HL, Chiou SM, Tu PH, Lee ST, et al. Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson's disease. Exp Neurol. 2010;224:234–40. doi: 10.1016/j.expneurol.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Tandonnet C, Fujiyama H, Janelle CM, Cauraugh JH, Summers JJ. Emotion and motor preparation: a transcranial magnetic stimulation study of corticospinal motor tract excitability. Cogn Affect Behav Neurosci. 2009;9:380–8. doi: 10.3758/CABN.9.4.380. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–52. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22:243–57. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Fess EE. Grip strength. In: Casanova JS, editor. Clinical assessment recommendations. Chicago: American Society of Hand Therapists; 1992. pp. 41–5. [Google Scholar]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E, et al. 300 Hz subthalamic oscillations in Parkinson’s disease. Brain. 2003;126:2153–63. doi: 10.1093/brain/awg229. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Hariz MI. Surgical management of Parkinson's disease. Expert Rev Neurother. 2010;10:903–14. doi: 10.1586/ern.10.68. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Tunik E. Human basal ganglia and the dynamic control of force during on-line corrections. J Neurosci. 2011;3:1600–5. doi: 10.1523/JNEUROSCI.3301-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hirschmann J, Özkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, et al. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. NeuroImage. 2011;55:1159–68. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Multilevel analysis: techniques and applications. Mahwah NJ: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Kempf F, Kühn AA, Kupsch A, Brücke C, Weise L, Schneider GH, et al. Premovement activities in the subthalamic area of patients with Parkinson's disease and their dependence on task. Eur J Neurosci. 2007;25:3137–45. doi: 10.1111/j.1460-9568.2007.05536.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, et al. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–46. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Exp Neurol. 2005;194:212–20. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Doyle L, Pogosyan A, Yarrow K, Kupsch A, Schneider GH, et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain. 2006;129:695–706. doi: 10.1093/brain/awh715. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity. J Neurosci. 2002;22:2855–61. doi: 10.1523/JNEUROSCI.22-07-02855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's Disease. Brain. 2011;134:359–74. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Lopez-Azcarate J, Tainta M, Rodriguez-Oroz M, Valencia M, Gonzalez R, Guirdi J, et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 2010;30:6667–77. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don’t we move faster? Parkinson’s disease, movement vigor and implicit motivation. J Neurosci. 2007;27:7105–116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–85. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Munk MH, Roelfoma PR, Konig P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–4. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Özkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wotjeckl L, et al. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp Neurol. 2011;229:324–31. doi: 10.1016/j.expneurol.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–8. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Kühn AA, Trottenberg T, Schneider GH, Kupsch A, Brown P. Elevations in local gamma activity are accompanied by changes in the firing rate and information coding capacity of neurons in the region of the subthalamic nucleus in Parkinson's disease. Exp Neurol. 2006;202:271–9. doi: 10.1016/j.expneurol.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Yoshida F, Chen CC, Torres I, Foltynie T, Limousin P, et al. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronisation. Neuroscience. 2010;171:245–57. doi: 10.1016/j.neuroscience.2010.08.068. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–8. doi: 10.1016/j.neubiorev.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Clery-Melin ML, Larfargue G, Valabregue R, Fossati P, Dubois B, et al. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci. 2009;29:9450–7. doi: 10.1523/JNEUROSCI.1951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Bru JL, Vergara M, Rodriguez-Cervantes PJ, Giurintano DJ, Perez- Gonzalez A. Scalability of the muscular action in a parametric 3D model of the index finger. Ann Biomed Eng. 2008;36:102–7. doi: 10.1007/s10439-007-9395-6. [DOI] [PubMed] [Google Scholar]
- Singh A, Levin J, Mehrkens JH, Bötzel K. Alpha frequency modulation in the human basal ganglia is dependent on motor task. Eur J Neurosci. 2011;33:960–7. doi: 10.1111/j.1460-9568.2010.07577.x. [DOI] [PubMed] [Google Scholar]
- Souques A. Rapports sur les syndromes parkinsoniens. Rev Neurol (Paris) 1921;37:534–715. [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–90. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Paré D, Oakson G, Curró Dossi RC. Neuronal activities in brain-stem cholinergic nucleus related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Paré D, Oakson G. Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nucleus in the cat. Proc Natl Acad Sci USA. 1991;88:4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, Wilmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:876–84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Thobois S, Ballanger B, Bardac P, Le Bars D, Lavenine F, Broussole E, et al. Functional anatomy of motor urgency. Neuroimage. 2007;37:243–52. doi: 10.1016/j.neuroimage.2007.04.049. [DOI] [PubMed] [Google Scholar]
- Trottenberg T, Fogelson N, Kühn AA, Kivi A, Kupsch A, Schneider G-H, et al. Subthalamic gamma activity in patients with Parkinson’s disease. Exp Neurol. 2006;200:56–65. doi: 10.1016/j.expneurol.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–16. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Gtethe J, Crutcher MD, Grafton ST. Motor sub-circuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3598–966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. NeuroImage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valldeoriola F, Valls-Solé J, Tolosa E, Ventura PJ, Nobbe FA, Martí MJ. Effects of a startling acoustic stimulus on reaction time in different parkinsonian syndromes. Neurology. 1998;51:1315–20. doi: 10.1212/wnl.51.5.1315. [DOI] [PubMed] [Google Scholar]
- Wasson P, Prodoehl J, Coombes SA, Corcos DM, Vaillancourt DE. Predicting grip force amplitude involves circuits in the anterior basal ganglia. NeuroImage. 2010;49:3230–8. doi: 10.1016/j.neuroimage.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V, et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–69. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental psychology. New York: H. Holt and company; 1938. Reaction time; pp. 317–326. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.