Abstract
Recent studies suggest that delirium is associated with risk of dementia and also acceleration of decline in existing dementia. However, previous studies may have been confounded by incomplete ascertainment of cognitive status at baseline. Herein, we used a true population sample to determine if delirium is a risk factor for incident dementia and cognitive decline. We also examined the effect of delirium at the pathological level by determining associations between dementia and neuropathological markers of dementia in patients with and without a history of delirium. The Vantaa 85+ study examined 553 individuals (92% of those eligible) aged ≥85 years at baseline, 3, 5, 8 and 10 years. Brain autopsy was performed in 52%. Fixed and random-effects regression models were used to assess associations between (i) delirium and incident dementia and (ii) decline in Mini-Mental State Examination scores in the whole group. The relationship between dementia and common neuropathological markers (Alzheimer-type, infarcts and Lewy-body) was modelled, stratified by history of delirium. Delirium increased the risk of incident dementia (odds ratio 8.7, 95% confidence interval 2.1–35). Delirium was also associated with worsening dementia severity (odds ratio 3.1, 95% confidence interval 1.5–6.3) as well as deterioration in global function score (odds ratio 2.8, 95% confidence interval 1.4–5.5). In the whole study population, delirium was associated with loss of 1.0 more Mini-Mental State Examination points per year (95% confidence interval 0.11–1.89) than those with no history of delirium. In individuals with dementia and no history of delirium (n = 232), all pathologies were significantly associated with dementia. However, in individuals with delirium and dementia (n = 58), no relationship between dementia and these markers was found. For example, higher Braak stage was associated with dementia when no history of delirium (odds ratio 2.0, 95% confidence interval 1.1–3.5, P = 0.02), but in those with a history of delirium, there was no significant relationship (odds ratio 1.2, 95% confidence interval 0.2–6.7, P = 0.85). This trend for odds ratios to be closer to unity in the delirium and dementia group was observed for neuritic amyloid, apolipoprotein ε status, presence of infarcts, α-synucleinopathy and neuronal loss in substantia nigra. These findings are the first to demonstrate in a true population study that delirium is a strong risk factor for incident dementia and cognitive decline in the oldest-old. However, in this study, the relationship did not appear to be mediated by classical neuropathologies associated with dementia.
Keywords: delirium, dementia, neuropathology, population-based, epidemiology
Introduction
Delirium is a severe, acute neuropsychiatric syndrome that affects at least 15% of hospitalized older adults (Inouye, 2006; Siddiqi et al., 2006; Young and Inouye, 2007; MacLullich and Hall, 2011). There has been much interest in whether delirium may be a marker for future dementia risk. In a population of memory clinic patients already diagnosed with dementia, delirium was associated with faster decline in cognitive test scores (Fong et al., 2009). Delirium was associated with future dementia in a follow-up study of hospital patients aged ≥65 years [odds ratio (OR) 6.0, 95% confidence interval (CI) 1.8–20] (Rockwood et al., 1999). Higher rates of dementia diagnosis were also observed in subjects with postoperative delirium following elective hip surgery (relative risk 1.9, 95% CI 1.1–3.3) (Kat et al., 2008). These results are consistent with a systematic review of dementia outcomes following hospitalization with delirium (Witlox et al., 2010). However, because dementia itself is a major risk factor for delirium, and around half of dementia is undiagnosed (Sampson et al., 2009), the key question of whether delirium is a risk factor for new onset dementia remains unanswered (MacLullich et al., 2009). Moreover, studies of selected hospital and memory clinic samples may be biased toward more severe disease. Capturing the full range of dementia risk following delirium within a population-based design would provide more generalizable risk estimates.
The Vantaa 85+ study is a true population-based cohort study of 553 individuals (92% of those eligible) aged ≥85 years at baseline, 3, 5, 8 and 10 years (Polvikoski et al., 2001, 2006). Vantaa 85+ is one of only six population-based cohorts with neuropathology information (Zaccai et al., 2006), and is the only one to have ascertained delirium. In addition, using autopsy data, the burden of standard dementia-related neuropathology markers in individuals with and without a history of delirium were examined.
The present study addressed two main questions. First, does delirium increase the risk of incident dementia? Second, in those with dementia, is a history of delirium associated with an increased burden of standard neuropathology markers of dementia? We also determined if delirium was associated with accelerated cognitive decline and increased severity of dementia.
Materials and methods
Sample characteristics
The Vantaa 85+ cohort study methods have previously been reported in detail (Polvikoski et al., 2006). Briefly, the study population comprised 553 individuals, representing 92% of the 601 adults aged ≥85 years living in Vantaa, Finland in 1991. Participants were recruited from the whole population, unrestricted by residential or health status. Follow-up for incident dementia and other markers of health status occurred at 3 (n = 277), 5 (n = 155), 8 (n = 65) and 10 (n = 25) years. The study received approval from the Ethics Committee of the City of Vantaa.
Clinical assessments
Dementia diagnosis by DSM-III-R (Diagnostic and Statistical Manual of Mental Disorders, third edition, revised) criteria (APA, 1987) was agreed by two neurologists simultaneously examining each participant. Dementia subtypes were classified using National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for Alzheimer’s dementia (McKhann et al., 1984) and National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences for vascular dementia (Roman et al., 1993). Cognition was assessed at every wave using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), the Short Portable Mental Status Questionnaire (Pfeiffer, 1975), and the Clinical Dementia Rating Scale (Morris, 1993). Depression was assessed using the Depression Status Inventory (Zung, 1972). Functional abilities were measured with the Personal and Instrumental Activities of Daily Living scales (Katz et al., 1963; Lawton and Brody, 1969). Hospital, primary care and social work records were also used to help identify incident dementia in participants between last assessment and death.
At each interview, the examining neurologists assessed participants and informant(s) for a history of any episodes of delirium, specifically assessing: changes in cognitive functioning, level of alertness, psychotic and perceptual symptoms, with reference to a checklist of DSM-III-R criteria for delirium diagnosis (Rahkonen et al., 2001). The reported history and number of episodes of delirium were corroborated with hospital case notes that were available at the time of assessment, and any additional likely episodes of delirium not recalled by participants or informants were ascertained through detailed inspection of hospital and primary care case notes. Therefore, the study ascertainment of delirium was retrospectively derived from multiple sources and the overall diagnosis accepted if the examining neurologists judged there was sufficient evidence from participant and informant recall and/or indication in the medical notes.
At baseline and at each subsequent wave, the presence of the following conditions was assessed through interview and medical records: myocardial infarction; congestive heart failure; peripheral vascular disease, cerebrovascular disease; chronic lung disease; connective tissue disease; hemiplegia; diabetes mellitus, diabetes with complications; tumours; leukaemia; and lymphoma.
Mortality
Dates of death were collected through Statistics Finland.
Neuropathology
Brains were fixed in phosphate-buffered 4% formaldehyde solution for at least 2 weeks. All specimens were macroscopically examined by one pathologist (Tuomo Polvikoski), blind to all clinical data, using a standardized dissection and sampling protocol. Cerebral infarcts and lacunes visible to the naked eye were identified by examination of the surface of the brain and from 1-cm thick coronal slices of the cerebral hemispheres, from 5-mm thick transverse slices of the brainstem and sagittal slices of the cerebellum. These lesions were subsequently histologically verified to be infarcts. In addition, a standardized set of samples were obtained from the middle frontal, superior temporal and middle temporal gyri, inferior parietal lobule, uncal region, hippocampal body, cingulate gyrus, occipital lobe (including the primary visual cortex) and midbrain. The protocols for quantifying Alzheimer-type [Braak stage (0 to 6); neuritic amyloid plaque (none 0 to severe 3)] (Polvikoski et al., 1995, 2006), infarcts (present or absent) (Rastas et al., 2007; Ahtiluoto et al., 2010) and Lewy body [neuronal loss in substantia nigra (none 0 to severe 3); α-synucleinopathy (none 0 to severe 3) (Oinas et al., 2009)] pathologies have been described in detail previously (Supplementary material).
Genetic testing
Apolipoprotein E (ApoE) genotyping was performed using both PCR and solid-phase mini-sequencing techniques (Syvanen et al., 1993; Polvikoski et al., 2006).
Statistical analyses
STATA 11.1 (StataCorp) was used for all analyses. Logistic regression was used to determine if episodes of delirium were associated with new onset of dementia. Because dementia neuropathology tends to be mixed in unselected populations (Matthews et al., 2009), we did not attempt to assess the associations of delirium with clinical dementia subtypes. Only episodes of delirium occurring at least one wave prior to participants last known as having no dementia were regarded as an exposure; controls were subjects in whom dementia was never ascertained using the thorough methods described above. Logistic regression was also used to assess worsening in Clinical Dementia Rating score in relation to a history of delirium prior to that wave. Similar analyses were conducted for functional sequelae, where outcomes in logistic models represented worsening in global function score. The association between delirium history at baseline and mortality was determined using a Cox proportional hazards model. All models were adjusted for age, sex and co-morbidities (using equivalent weightings from the Charlson co-morbidity index) (Charlson et al., 1987). CIs of 95% were employed, and are reported in the results. Post-model testing included examination of Pearson residuals for logistic models and Schoenfeld residuals, and log–log survival plots for proportional hazards models.
Longitudinal change in MMSE was modelled using random-effects linear regression for both MMSE at study entry (intercept) and rate of change in MMSE (slope), having first compared model fit for fixed intercepts and slopes using maximum likelihood estimates. ‘Time in study’ was used as the time metric. Covariance matrices were unstructured. The effect of delirium history at baseline, mean-centred age at baseline, sex, baseline functional status on intercept and slope was considered, and model fit assessed using likelihood ratio tests. The final model included adjustment for these variables for MMSE at study entry with an additional term adjusting for the influence of delirium history at baseline on rate of MMSE change. Finally, a quadratic term for the time metric was tested. After fitting models, assumptions were checked by constructing Q–Q plots of the standardized residuals.
In keeping with previous methods, neuropathological variables were dichotomized into ‘high’ or ‘low’ values (Savva et al., 2009; Brayne et al., 2010). This approach allows for simpler interpretation and is more likely to be robust. The relationships between these markers (exposure) and dementia (outcome) were evaluated using logistic regression models, adjusted for sex and age at death (Savva et al., 2009). These associations were then assessed, stratified by delirium history, to determine if the OR for the dementia–pathology association differed between those with and without a history of delirium. The possibility of a statistical interaction was also tested using a multiplicative interaction term (delirium × pathology) (Supplementary material).
Results
Participant characteristics
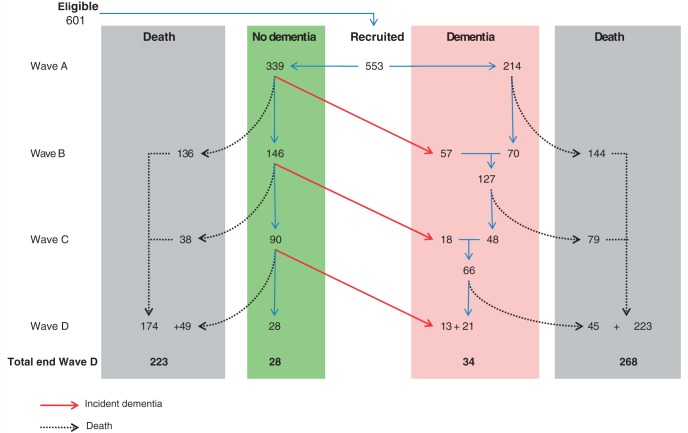
Participant characteristics are summarized in Table 1. Figure 1 shows the flow diagram for the study. At baseline, there were 71 subjects (13%) with a history of delirium. There were no differences in age, sex or years of education between those with and without a history of delirium. However, subjects with a history of delirium were more likely to have prevalent dementia (77% versus 33%, P < 0.01) and lower MMSE scores (15/30 versus 21/30, P < 0.01). A delirium episode was recorded at least once during the study in 121 subjects (22%). Brain autopsy data were available in similar proportions of individuals with and without an episode of delirium (54% and 48%, respectively, P = 0.26).
Table 1.
Clinical characteristics of participants at baseline
| No history of delirium | ≥ 1 episode of delirium | P-value | |
|---|---|---|---|
| n at baseline (%) | 482 (87) | 71 (13) | |
| Person years | 1901 | 164 | |
| Mean age (SD) | 88 (2.9) | 90 (3.1) | 1.00 |
| Sex (% females) | 385 (80) | 55 (77) | 0.64 |
| Proportion with >4 years education (%)a | 98 (23) | 10 (17) | 0.31 |
| Mean time in study (years, IQR) | 3.2 (1.6–5.9) | 1.9 (0.9–3.2) | <0.01 |
| Co-morbidity score at baseline (IQR)b | 3 (1–4) | 3 (2–5) | <0.01 |
| Functionally independent at baseline (%) | 321 (67) | 24 (34) | <0.01 |
| Prevalent dementia | 159 (33) | 55 (77) | <0.01 |
| MMSE | |||
| Baseline (IQR) | 21 (17–26) | 15 (10–19) | <0.01 |
| Last follow-up (IQR) | 19 (11–24) | 13 (9–17) | <0.01 |
A total of 121 participants experienced delirium at any time during the study (22%). Of these, 58 were brain donors (48%) and 232 brain donors had no history of delirium (54%) (P = 0.26).
a Co-morbidity index uses the same weightings as the Charlson index. The maximum score is 19.
‘Functionally independent’ refers to those who reported being fully independent or needing minor assistance to complete activities of daily living.
b Years of education undetermined in 71 participants.
IQR = interquartile range.
Figure 1.
Flow diagram of follow-up in the Vantaa study. Illustration enumerating dementia and mortality events in Vantaa over time. Wave A = 1991; Wave B = 1994; Wave C = 1996 and Wave D = 1999.
Delirium and dichotomous outcomes
A history of delirium at any wave in subjects with no dementia was associated with a significantly higher risk of new dementia at the following wave (OR 8.7, 95% CI 2.1–35) (Table 2). For all participants, delirium was also associated with a worse Clinical Dementia Rating score at follow-up (OR 3.1, 95% CI 1.5–6.3) as well as deterioration in global function scores (OR 2.8, 95% CI 1.4–5.5) (Table 2). A history of delirium at study entry was associated with increased mortality, even after adjustment for co-morbidities [hazard ratio 1.6, 95% CI 1.2–2.1] (full models in Supplementary Table 1)
Table 2.
The association of between delirium and clinical outcomes
| Outcome | Delirium (n) | No delirium (n) | LCI | UCI | P-value | ||
|---|---|---|---|---|---|---|---|
| Dementiaa | 10 | 311 | OR | 8.65 | 2.13 | 35.12 | <0.01 |
| Dementia worseningb | 38 | 226 | OR | 3.06 | 1.49 | 6.29 | <0.01 |
| Functional worseningb | 42 | 230 | OR | 2.76 | 1.38 | 5.52 | <0.01 |
| Mortalityc | 71 | 469 | HR | 1.61 | 1.25 | 2.10 | <0.01 |
The results of four separate models where delirium is the exposure of interest, adjusted by age, sex and co-morbidity, given with 95% CIs [lower confidence interval (LCI), upper confidence interval (UCI)].
a The dementia outcome gives the OR that a person with a history of delirium but no dementia was then diagnosed with incident dementia at the following wave.
b The OR of worsening in dementia (at least one point decline in clinical dementia rating scale) or function (at least one category decline in five-point scale from independent to fully dependent for all care needs) between baseline and first follow-up in individuals also experiencing delirium.
c Association between co-morbidity and mortality is also significant in this model (HR 1.24, 95% CI 1.18–1.30) per point on co-morbidity index.
All Pearson and Schoenfeld residuals P > 0.1.
The full models are given in the Supplementary material.
HR = hazard ratio.
Delirium and decline in Mini-Mental State Examination score
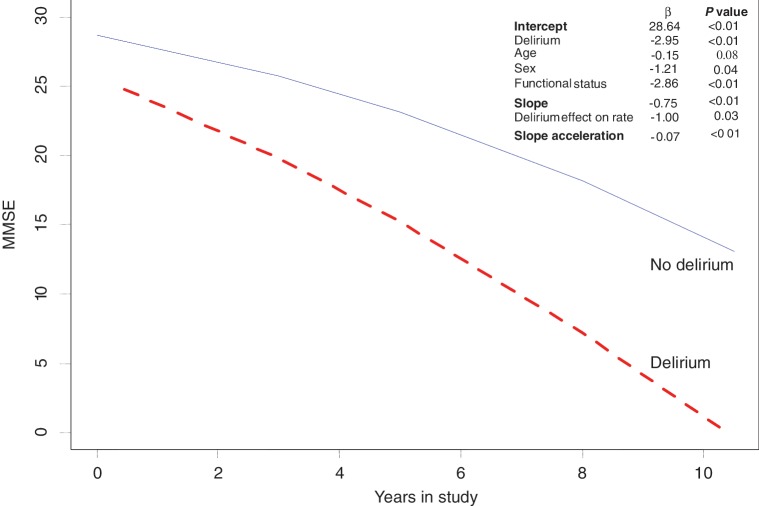
MMSE trajectory was best described by a quadratic model when contrasted with a linear model (Supplementary Fig. 1). Figure 2 shows the predicted trajectories from the model fitted (Supplementary Table 2). MMSE scores at baseline were estimated at 28.6 (95% CI 26.5–30.8), representing cognitive function for an individual with zero value on all covariates. In the whole population, cognitive function declined at 0.75 points per year (95% CI 0.49–1.0), with a change in annual rate of decline of 0.07 points (95% CI 0.4–0.1). Baseline MMSE scores of individuals with history of delirium were 3.0 points (95% CI 1.4–4.5) lower than MMSE scores of individuals without any delirium. A history of delirium was associated with a significantly faster rate of decline in MMSE scores with decline of 1.0 (95% CI 0.11–1.89) MMSE point per year compared to those without delirium.
Figure 2.
Longitudinal trajectory of change in MMSE score over time. Predicted trajectory of MMSE change for those with or without a history of delirium at baseline. Co-efficients and P-values are shown. The estimates for the intercept and slope are given when all covariates = 0. The estimate changes with the addition of each covariate, subtracting the appropriate β co-efficient where: delirium = yes; age per year; sex = female; functional status per increase in five-point scale. The full model, along with 95% CIs for each estimate, and related graphs are given in the Supplementary material.
Delirium, dementia and neuropathological markers of dementia
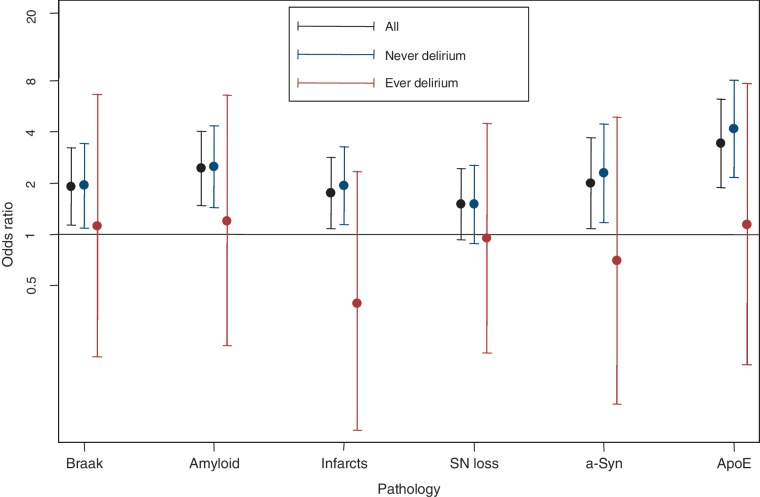
All neuropathological markers were significantly associated with dementia. However, when stratifying the group by history of delirium, the relationship between dementia and all markers became stronger in individuals with no history of delirium (all ORs consistently larger) (Fig. 3 and Supplementary Table 3). Conversely, in the group with delirium there were no significant associations between dementia and neuropathology and genotype markers (all ORs closer to unity). For example, higher Braak stage was associated with dementia but no delirium (OR 2.0, 95% CI 1.1–3.5, P = 0.02), but in those with a history of delirium, there was no significant relationship (OR 1.2, 95% CI 0.2–6.7, P = 0.85). This pattern was observed consistently with neuritic amyloid, ApoE status, presence of infarcts, α-synucleinopathy and neuronal loss in substantia nigra. While this raises the possibility that the relationship between dementia and neuropathological markers is modified by a history of delirium, the sample size was under-powered to conclude this statistically using an interaction term (Supplementary Table 4). Delirium history was not itself associated with any of the neuropathological markers of dementia or ApoE status among the brain donors (Supplementary Table 5).
Figure 3.
Relationship between delirium, dementia and neuropathology/genotype. Display of logistic regression models, with 95% CIs. The y-axis is log-scaled. Models show association between dementia and pathology (or genotype), adjusted by age at death and sex. Markers were treated as dichotomous variables (high/low). For each marker, the relationship is given for the whole population, and then stratified by delirium history (n = 58 with history of delirium; n = 232 no history of delirium). SN = substantia nigra; Syn = synucleinopathy.
Discussion
This is the first study to examine the hypothesis that delirium is a risk factor for dementia using a true population-based sample of older individuals. The results definitively confirm this hypothesis. Additionally, in individuals with existing dementia, delirium was associated with worsening dementia severity, worsening global functional status and higher mortality. Moreover, in the whole population, a history of delirium was significantly associated with an accelerated decline in MMSE scores. This is also the first prospective cohort study to examine the potential effects of delirium history on the relationships between dementia and its neuropathological markers. Individuals with dementia and no history of delirium had strong associations with Alzheimer-type, infarcts and Lewy body pathology. In contrast, those with dementia and a history of delirium showed no such relationships. Though this is an intriguing finding, the study was not powered to determine if delirium is genuinely associated with an altered pattern of pathology.
The present results are consistent with studies reporting cognitive decline after delirium or inter-current illness where there have been pre-morbid assessments of cognition. As mentioned above, follow-up of memory-clinic patients showed delirium was subsequently associated with greater decline in cognitive test scores (Fong et al., 2009). In addition, a report from the Adult Changes in Thought study found that critical illness (without specifically considering delirium) was associated with incident dementia (hazard ratio 1.4, 95% CI 1.1–1.7) (Ehlenbach et al., 2010). Participants in the Health and Retirement Study who had an inter-current episode of severe sepsis also had a higher risk of being subsequently diagnosed with severe cognitive impairment (OR 3.4, 95% CI 1.5–7.3) (Iwashyna et al., 2010). The larger effect size in the present study may reflect the older age in this cohort.
The results are also consistent with the emerging evidence from animal models of delirium demonstrating that in vulnerable animals, systemic inflammatory insults can cause transient, reversible deterioration in cognition and significant acceleration in disease progression after the transient impairments have resolved (Cunningham, 2011). A single, moderate dose of lipopolysaccharide (as a bacterial mimic) or polyinosinic:polycytidylic acid (as a viral mimic), consistent with the level of inflammatory insult that typically induces delirium in vulnerable humans, has been shown to induce de novo neuronal death in animals with existing neurodegenerative disease (Cunningham et al., 2005; Field et al., 2010), and to accelerate the progression of disease without obvious effects on extracellular amyloidosis (Cunningham et al., 2009). In this context, it is of note that a case-control autopsy study of individuals who died with delirium showed differential increases in IL6 and CD68-positive microglia (Munster et al., 2011). Consistent with these findings, the present study suggests the possibility that dementia following delirium may not be as strongly linked with classical dementia neuropathological markers as dementia in those without a history of delirium, but further work is needed.
This study has several strengths. This cohort has high generalizability for the oldest-old, and has a high rate of brain autopsy (Zaccai et al., 2006). The characteristics of the brain donors show no evidence of systematic bias (Brayne et al., 2010). While it has been shown that neuropathological assessments can reliably be made by a single rater (Mirra et al., 1994), it is an advantage that all scoring was interpreted by the same neuropathologist. There were multiple waves of measurement over a decade; this allows accurate assessment of longitudinal change.
Some limitations of the present study should be acknowledged. Only changes from age ≥85 years could be studied and this resulted in substantial losses to follow-up due to mortality. There is likely to be a survivor effect and this may result in selective differences in clinical and genetic characteristics. Depression also has a complex relationship with cognitive assessment and dementia, and no attempt was made to address this in the present analysis. The results of the random-effects models produced estimated parameters comparable to other population-based studies of general cognitive decline (Terrera et al., 2008). However, similar to many prospective studies of ageing, attrition was significant and data missing-not-at-random was not accounted for. Though autopsy rates were high in our study, the absolute number of cases in each category of delirium exposure was relatively low.
Self-reported (or informant-reported) delirium may be subject to recall bias, though this is mitigated by corroborating the history with medical records during the interview. Though the history of delirium was specifically assessed at each wave, this approach is not as accurate as clinician assessment during delirium and is likely to under-detect delirium given that diagnosis rates in routine clinical practice are generally considerably below the true prevalence (Flaherty et al., 2007). In the absence of robust delirium ascertainment being embedded in routine hospital care, only a prospective study in which researchers could assess every patient for delirium during every hospital admission could overcome this issue. This is impractical, however, and combining patient and informant interviews with inspection of case notes is a pragmatic alternative. Indeed, medical records have been validated for the diagnosis of delirium history (Inouye et al., 2005), and the diagnostic accuracy for past episodes is likely to be higher if case notes are reviewed in conjunction with clinical interview as is the case in the present study.
This study confirmed that delirium is associated with general cognitive decline, with an 8-fold increase in incident dementia and accelerated decline in MMSE scores. Previous investigations for other dementia risk factors (Daviglus et al., 2011) have often been dwarfed by the relationship of dementia with older age itself. The strong association with delirium, even after adjusting for age, in a general population underscores the clinical importance of delirium in relation to dementia risk. Future research should seek to include prospective delirium measures in cohort studies of dementia, correlating these with neuroimaging and neuropathology findings. Up to 30% of delirium is preventable (Inouye et al., 1999) and definitive data would come from intervention trials where the outcome is secondary prevention of dementia. The present study suggests that this would be a plausible approach.
Funding
Supported by the Wellcome Trust (D.D., C.C.), Alzheimer Society (G.M.T.), National Health and Medical Research Council, Australia (H.K.), Alzheimer Foundation of Finland (T.R.), Emil Aaltonen Foundation (T.R.), Uulo Arhio Foundation (T.R., M.O.), Foundation of Signe and Anu Gyllenberg, Helsingin Sanomat Centennial Foundation, University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology—UK Biotechnology and Biological Sciences Research Council, Engineering and Physical Research Council, Economics and Social Research Council, and Medical Research Council (A.M.). The funders had no role in study design; data collection and analysis, the decision to publish, or preparation of this article.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank the participants and their families their long involvement with the study and particularly for their agreement to participate in the brain-donation program. D.D. had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviation
- MMSE
Mini-Mental State Examination
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd edn. revised (DSM-III-R). Washington: American Psychiatric Association; 1987. [Google Scholar]
- Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Polvikoski T, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–6. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cunningham C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem Soc Trans. 2011;39:945–53. doi: 10.1042/BST0390945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–12. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, et al. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011;68:1185–90. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–70. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field RH, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty JH, Rudolph J, Shay K, Kamholz B, Boockvar KS, Shaughnessy M, et al. Delirium is a serious and under-recognized problem: why assessment of mental status should be the sixth vital sign. J Am Med Dir Assoc. 2007;8:273–5. doi: 10.1016/j.jamda.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state - practical method for grading cognitive state of patients for clinicians. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, et al. Delirium accelerates cognitive decline in alzheimer disease. Neurology. 2009;72:1570–5. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65+16. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–8. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kat MG, Vreeswijk R, De Jonghe JFM, Van Der Ploeg T, Van Gool WA, Eikelenboom P, et al. Long-term cognitive outcome of delirium in elderly hip surgery patients: A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord. 2008;26:1–8. doi: 10.1159/000140611. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- MacLullich AMJ, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- MacLullich AMJ, Hall RJ. Who understands delirium? Age Ageing. 2011;40:412–4. doi: 10.1093/ageing/afr062. [DOI] [PubMed] [Google Scholar]
- Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Gearing M, McKeel DW, Jr, Crain BJ, Hughes JP, van Belle G, et al. Interlaboratory comparison of neuropathology assessments in Alzheimer's disease: a study of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) J Neuropathol Exp Neurol. 1994;53:303–15. doi: 10.1097/00005072-199405000-00012. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR) - current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res. 2011;14:615–22. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinas M, Polvikoski T, Sulkava R, Myllykangas L, Juva K, Notkola IL, et al. Neuropathologic findings of dementia with lewy bodies (DLB) in a population-based Vantaa 85+ study. J Alzheimers Dis. 2009;18:677–89. doi: 10.3233/JAD-2009-1169. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. Short Portable Mental Status Questionnaire for assessment of organinc brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–7. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56:1690–6. doi: 10.1212/wnl.56.12.1690. [DOI] [PubMed] [Google Scholar]
- Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinisto L, Notkola IL, et al. Incidence of dementia in very elderly individuals: a clinical, neuropathological and molecular genetic study. Neuroepidemiology. 2006;26:76–82. doi: 10.1159/000090252. [DOI] [PubMed] [Google Scholar]
- Rahkonen T, Eloniemi-Sulkava U, Halonen P, Verkkoniemi A, Niinisto L, Notkola IL, et al. Delirium in the non-demented oldest old in the general population: risk factors and prognosis. Int J Geriatr Psychiatry. 2001;16:415–21. doi: 10.1002/gps.356. [DOI] [PubMed] [Google Scholar]
- Rastas S, Verkkoniemi A, Polvikoski T, Juva K, Niinisto L, Mattila K, et al. Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38:1454–60. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–6. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195:61–6. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: A systematic literature review. Age Ageing. 2006;35:350–64. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- Syvanen AC, Sajantila A, Lukka M. Identification of individuals by analysis of biallelic DNA markers, using PCR and solid-phase minisequencing. Am J Hum Genet. 1993;52:46–59. [PMC free article] [PubMed] [Google Scholar]
- Terrera GM, Matthews F, Brayne C. A comparison of parametric models for the investigation of the shape of cognitive change in the older population. BMC Neurol. 2008;8:16. doi: 10.1186/1471-2377-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- Young J, Inouye SK. Delirium in older people. BMJ. 2007;334:842–6. doi: 10.1136/bmj.39169.706574.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation–a systematic review. BMC Neurol. 2006;6:2. doi: 10.1186/1471-2377-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW. The Depression Status Inventory: an adjunct to the self-rating depression scale. J Clin Psychol. 1972;28:539–43. doi: 10.1002/1097-4679(197210)28:4<539::aid-jclp2270280427>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.