Abstract
Clinical trials in Parkinson’s disease have shown that transplants of embryonic mesencephalic dopamine neurons form new functional connections within the host striatum, but the therapeutic benefits have been highly variable. One obstacle has been poor survival and integration of grafted dopamine neurons. Activation of Akt, a serine/threonine kinase that promotes cell survival and growth, increases the ability of neurons to survive after injury and to regenerate lost neuronal connections. Because the lipid phosphatase, phosphatase and tensin homolog (PTEN) inhibits Akt, we generated a mouse with conditional knock-out of PTEN in dopamine neurons, leading to constitutive expression of Akt in these neurons. Ventral mesencephalic tissue from dopamine phosphatase and tensin homologue knock-out or control animals was then transplanted bilaterally into the dopamine depleted striata of MitoPark mice that express a parkinsonian phenotype because of severe respiratory chain dysfunction in dopamine neurons. After transplantation into MitoPark mice, PTEN-deficient dopamine neurons were less susceptible to cell death, and exhibited a more extensive pattern of fibre outgrowth compared to control grafts. Voltammetric measurements demonstrated that dopamine release and reuptake were significantly increased in the striata of animals receiving dopamine PTEN knock-out transplants. These animals also displayed enhanced spontaneous and drug-induced locomotor activity, relative to control transplanted MitoPark mice. Our results suggest that disinhibition of the Akt-signalling pathway may provide a valuable strategy to enhance survival, function and integration of grafted dopamine neurons within the host striatum and, more generally, to improve survival and integration of different forms of neural grafts.
Keywords: transplantation, substantia nigra, striatal innervation, Akt, mTOR
Introduction
Parkinson’s disease is a degenerative neurological disorder that typically affects the patient’s motor skills, speech, writing, as well as other cognitive functions and is characterized by the progressive loss of dopamine neurons in the substantia nigra pars compacta. These midbrain neurons modulate motor and cognitive functions through dopamine innervation of basal ganglia and forebrain regions. Pharmacological enhancement of dopamine signalling through l-DOPA or agonist therapy is beneficial early in the disease, but becomes less effective as the disease progresses (Marsden, 1994; Rajput et al., 2002). Intrastriatal transplantation of foetal dopamine neurons in affected individuals aims to normalize dopamine levels and release of dopamine in striatum. Embryonic dopamine neurons are capable of establishing a new terminal network within the host striatum, augmenting both afferent input to striatal projection neurons and information flow through the damaged motor circuitry (Lindvall et al., 1990a, b, 1994; Freed et al., 1992, 2001; Mendez et al., 2000; Piccini et al., 2000). However, the poor survival of implanted dopamine neurons remains an issue in animal models and clinical trials alike and is considered a major factor in the incomplete and variable recovery seen in clinical trials (Brundin et al., 2010).
Recovery of motor function by grafted dopamine neurons appears dependent on several crucial parameters, including dopamine neuron survival and integration with the host tissue. Several protocols have been developed to improve cell survival, such as preincubation of embryonic cells with trophic factors, or substances that inhibit apoptosis (Bjorklund et al., 1998; Tornqvist et al., 2000; Ahn et al., 2005; Andereggen et al., 2009; Chou et al., 2011). However, the success of these protocols has been limited and cell survival after transplantation remains an obstacle in the neural transplantation field. One possible way to promote extended survival of transplanted embryonic neurons is to enhance the activity of cellular pathways known to mediate cell survival signals, and the ability of neurons to extend neurites into surrounding tissues. PI3K/Akt enhances activity of intracellular cell survival pathways and also exhibits anti-apoptotic effects under a variety of conditions such as withdrawal of trophic factors, oxidative stress and ischaemic shock (Brunet et al., 1999, 2001; Datta et al., 1999; Chang et al., 2007). Phosphatase and tensin homologue (PTEN) is a lipid phosphatase that antagonizes PI3K activity and therefore is the main inhibitor of the Akt-signalling pathway (Stiles et al., 2004). Utilizing Cre-loxP technology to specifically inactivate the Pten gene in dopamine neurons (DA-PTEN-KO mice), we and others found that PTEN ablation in dopamine neurons enhances Akt signalling, suppresses apoptosis and preserves striatal innervation following nigrostriatal lesions (Diaz-Ruiz et al., 2009; Domanskyi et al., 2011). Subsequent studies have shown that constitutive forms of the kinase Akt in dopamine neurons induce regrowth of axons after damage by neurotoxic lesions (Domanskyi et al., 2011; Kim et al., 2011). Thus, several lines of evidence suggest a role for the PI3K/Akt pathway in the survival of different neuronal populations. However, it is not clear if activation of this pathway can also enhance the outcome of cell replacement therapies, in particular those affecting the survival and function of dopamine neurons. To examine whether Akt activation can protect vulnerable dopamine neurons after transplantation, we grafted ventral mesencephalic embryonic tissue from conditional DA-PTEN-KO and control mice bilaterally into the dopamine depleted striata of MitoPark mice (Ekstrand et al., 2007; Harvey et al., 2008; Ekstrand and Galter, 2009; Beal, 2010; Dawson et al., 2010; Galter et al., 2010; Good et al., 2011). The MitoPark mouse provides a valuable model to examine the benefit of dopamine cell replacement therapy, since this animal model of mitochondrial dysfunction closely mimics the progressive degeneration of dopamine neurons in Parkinson’s disease (Beal, 2010; Galter et al., 2010), and presents a much less variable phenotype than that seen with toxin models.
Materials and methods
All studies were conducted according to standards outlined in the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the NIDA Intramural Research Programme. Mice were bred to a congenic c57bl/6 background. All surgical procedures were conducted under general anaesthesia using ketamine (100 mg/kg) and xylazine (10 mg/kg body weight).
Transplantation of ventral mesencephalic embryonic tissue into MitoPark mice
Timed pregnant mice (day of vaginal plug designated as embryonic Day 0.5) were generated by crossing (i) PtenloxP/loxP DAT (dopamine transporter) Cre/Cre with PtenloxP/loxP and (ii) Ptenwt/wt DATCre/Cre with c57bl/6. At embryonic Day 16.5 experimental Ptenloxp/loxp DATCre/wt (DA-PTEN-KO), and control Ptenwt/wt DATCre/wt (control) embryos were removed from pregnant mothers after lethal exposure to isoflurane. Tissue blocks from the ventral mesencephalon containing dopamine neurons were dissected free from each embryo taking care to remove the meninges. Each tissue block, corresponding to one embryo, was divided in the midline into two pieces to provide material for bilateral grafting into the striata of one MitoPark mouse. Tissue blocks were stored in tissue culture media (Glasgow Minimum Essential Medium) and placed on ice prior to transplantation. MitoPark mice (Tfam mitochondrial transcription factor Aloxp/loxp DATCre/wt) were bred on a c57bl/6 congenic background, to avoid transplant rejection. Each embryo was genotyped to verify the mutation. Male MitoPark mice used in this study were heterozygous for DAT-Cre expression and homozygous for the loxp flanked Tfam gene. They were single-housed and received an unlimited diet of ground mouse chow starting at 19 weeks of age (1 week prior to the transplantation procedure), and for the duration of the study. The transplantation procedure was performed using a 22-gauge Chiba needle attached to a 10 µl Hamilton syringe. The ventral mesencephalon corresponding to one embryo, dissected into two tissue blocks as described above, was grafted bilaterally into the striatum of 20-week-old MitoPark mice at the following stereotaxic coordinates: anterior–posterior +0.5, median–lateral +2.3, dorsal–ventral −3.5 (flat skull position). Tissue blocks were injected over 2 min and the needle was left in place for another 2 min before slow withdrawal. For behavioural and morphological studies, animals were divided into five groups. Groups 1 (n = 17) and 2 (n = 13) consisted of MitoPark animals grafted with ventral mesencephalic tissue from DA-PTEN-KO or control embryos, respectively. Group 3 (n = 6) consisted of sham-operated MitoPark mice. Group 4 (n = 9) consisted of naïve MitoPark animals. Group 5 (n = 8) was the baseline control group and consisted of aged-matched DAT-Cre heterozygous animals. As control Groups 3 and 4 did not show any significant differences, their data were pooled into one group referred to as ‘MitoPark mice’.
Behavioural testing
MitoPark mice receiving bilateral control or DA-PTEN-KO transplants were behaviourally evaluated to determine the impact of the grafts on specific behavioural tasks including open field and nomifensine-induced locomotion, motoric circadian rhythm and also on execution of locomotor tasks highly dependent on dopamine such as body posture (rearing, vertical movement). Figure 1 represents an overview of the animals and timing of experimental procedures.
Figure 1.
Overview of the present experiment. Behavioural measurements were obtained for all animal groups included in the study starting at 16 weeks of age. After recording baseline behavioural activity at 20 weeks, MitoPark mice were grafted with control or DA-PTEN-KO ventral mesencephalon tissue. This was followed by a sequence of behavioural tests during the subsequent 16 weeks post-grafting.
Spontaneous locomotor activity
Spontaneous ambulatory activity (total distance) and vertical movements of mice were recorded using activity chambers placed into analysers, where total distance and vertical movements were monitored through a grid of infrared light beams (Versamax, AccuScan Intruments). Behavioural recordings started at 16 weeks of age and continued every 4 weeks, until the end point of the study at 36 weeks of age. Recording sessions lasted 60 min. After behavioural recordings at 20 weeks of age, MitoPark mice received bilateral striatal transplants. Behavioural recordings continued after the animals recovered from surgery at 24 weeks of age. At 36 weeks of age, and after recording spontaneous locomotor activity, mice were injected with saline (intraperitoneally) and the measurements continued for another 60 min. After habituation to the injection procedure, mice received an injection of nomifensine (15 mg/kg intraperitoneally) and locomotor activity was recorded for an additional 60 min. During each recording session animals were placed randomly in one of eight experimental chambers.
Circadian locomotor activity
Circadian locomotor activity was recorded when the animals were 35-weeks-old. Animals were placed in activity cages (Versamax, AccuScan Instruments) with water and food available and distances travelled were monitored for a period of 24 h.
Immunocytochemistry
Adult mice were deeply anaesthetized with chloral hydrate (30 mg/kg, intraperitoneal) and perfused transcardially with saline followed by 4% paraformaldehyde. Ptenloxp/loxp DATCre/wt (DA-PTEN-KO), and control Ptenwt/wt DATCre/wt (control) embryonic Day 16.5 embryos were removed from pregnant mothers after lethal exposure to isoflurane. Brains were quickly removed and post-fixed in 4% paraformaldehyde for 4 h, rinsed and cryoprotected overnight in 18% sucrose in 0.1 M phosphate buffer. Coronal 40 µm cryostat sections were collected through striatum and the ventral midbrain area in two series for embryonic Day 16.5 embryos and four series for adult animals. Sections were rinsed with phosphate buffer (3 × 10 min), permeabilized and blocked with 0.25% Triton™ X-100 and 4% bovine serum albumin in phosphate buffer. Sections were incubated overnight at 4°C with a rabbit polyclonal antibody against tyrosine hydroxylase (1:1000, Chemicon). Sections were then rinsed (3 × 10 min) in phosphate buffer and incubated for 1 h with a biotinylated anti-rabbit antibody (1:200, Vector Labs). Sections were rinsed (3 × 10 min) in phosphate buffer and incubated with avidin-biotinylated horseradish peroxidase for 2 h. Sections were again rinsed and the peroxidase reaction was developed with 0.05% 3,3′-diaminobenzidine-4-HCl (DAB) and 0.003% H2O2. Sections from adult animals, stained as described above, were mounted on coated slides, dehydrated and cover slipped. As sections from Day 16.5 embryos were frail, they were mounted on coated slides after sectioning and prior to the staining procedure.
Western blot analysis
Tyrosine hydroxylase and β-actin protein levels were assayed by western blot in ventral mesencephalon tissues from Day 16.5 embryos (n = 3 controls, n = 3 knock-out) and adult mice (n = 3 controls, n = 3 knock-out). Protein was isolated using the total protein extraction kit (Chemicon,) following the manufacturer’s instructions. Isolated protein (15 µg) was transferred to a polyvinylidene-difluoride membrane and incubated with an anti-tyrosine hydroxylase antibody (Chemicon, 1:1000) and β-actin (Sigma, 1:10 000) overnight at 4°C. Membranes were then incubated with anti-rabbit (1:5000) and anti-mouse 1:5000 (Roche) secondary antibodies for 2 h at room temperature. Chemiluminescent signal detection on the polyvinylidene-difluoride membranes was performed using the Lumi-Light western blotting kit (Roche). Membranes for embryonic and adult tissues were developed separately. Band densities were analysed using NIH ImageJ software. Quantification was performed by measuring the intensity of the tyrosine hydroxylase-specific band and comparing with that of β-actin in adult and embryonic tissues.
Cell counting
Unbiased stereological counts of tyrosine hydroxylase-positive neurons within the substantia nigra pars compacta of adult and Day 16.5 embryos were performed using stereological principles and analysed with Stereo Investigator software (Microbrightfield). The number of tyrosine hydroxylase-positive neurons was estimated in (i) adult control (n = 4), naïve Mitopark mice (n = 4), and Mitopark mice receiving control grafts (n = 4) and DA-PTEN-KO grafts (n = 4); and (ii) Day 16.5 control (n = 3) and DA-PTEN-KO (n = 3) embryos. The optical fractionator probe was used to generate an estimate of neuronal tyrosine hydroxylase-positive numbers. The entire substantia nigra pars compacta region in adult animals was outlined under a low magnification objective (× 5) following landmarks from the Franklin and Paxinos mouse atlas (Franklin and Paxinos, 1997). For Day 16.5 embryos, three consecutive sections within a tissue series were analysed, and therefore do not represent cell counts for the entire substantia nigra pars compacta region. Counting started on the first and most rostral tissue section containing both the substantia nigra pars compacta and ventral tegmental area. The medial border of substantia nigra pars compacta was delineated with a vertical line passing through the medial tip of the cerebral peduncle and the medial terminal nucleus of the accessory nucleus of the optic tract. All the stereological analyses were performed under the × 40 objective of a Leica DM5000B microscope and only one hemisphere was quantified per animal (Leica Microsystems). For each tissue section analysed, section thickness was assessed in each sampling site and guard zones of 2.5 µm were used at the top and bottom of each section. Systematic random sampling design was performed and generated with the following stereological parameters: grid size = 131 µm, counting frame = 123 µm and dissector height = 25 µm. Our criterion for counting an individual tyrosine hydroxylase-positive neuron was the presence of its nucleus either within the counting frame, or touching the right or top frame lines (green), but not touching the left or bottom lines (red). The area of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta was estimated using the nucleator probe. Co-efficients of error were calculated and values <0.10 were accepted.
The small and heterogeneous size of the grafts allowed for identification and manual counting of all tyrosine hydroxylase-positive neurons present in each analysed section of striatum, thus avoiding the need for stereological counting procedures. The total number of tyrosine hydroxylase-positive cells in the intrastriatal grafts was estimated for each animal through extrapolation of the number of tyrosine hydroxylase-positive cells counted in every fourth section. The method of Abercrombie (1946) was used to correct for changes in cell size and double counting caused by cells spanning more than one section.
Relative optical density
To determine density of tyrosine hydroxylase-positive fibres, the mean optical density was measured in striatum. Optical density is a sensitive and reliable tool to measure density of innervation and to detect changes caused by experimental manipulations (Burke et al., 1990). Sections were scanned using a Nikon Coolscan 9000, under identical exposure conditions and digital images were then transformed to 8-bit grey scale images. The optical density quantification was performed using NIH ImageJ software. Optical density measures were determined in every fourth striatal section from each mouse brain, within +1.1 mm and +0.14 mm relative to bregma. Non-specific background was determined by subtracting readings made from the adjacent cortex. To measure staining density in the striatum, optical density measures included the entire striatal region in each section. In the grafted MitoPark animals, the mean optical density values from (i) striatal sections containing transplanted tyrosine hydroxylase-positive neurons and (ii) striatal sections devoid of transplanted neurons, were collected separately. By differentiating striatal sections containing grafted embryonic neurons, from those solely containing tyrosine hydroxylase-positive fibres, we were able to determine differences in reinnervation of striatum and cell densities among the transplanted animals.
Recordings from brain slices
Slice preparation
Thirty-six-week old MitoPark mice grafted with control (n = 5) or DA-PTEN-KO tissue (n = 5), as well as non-grafted MitoPark (n = 3) and control mice (n = 3) were sacrificed by cervical dislocation and brains rapidly removed and placed in a modified, high-sucrose containing ice-cold artificial CSF medium. Coronal hemisections (280 µm) containing striatum were cut using a vibratome (Leica VT1000S). Slices were incubated in standard oxygenated artificial CSF at 34–35°C for 20–30 min, then allowed to stabilize at room temperature for >30 min prior to initiating recordings. During recordings, slices were continuously superfused with artificial CSF at a rate of 2 ml/min, and maintained at 28–30°C.
Voltammetric recordings
Fast scan cyclic voltammetry was performed according to previously published protocols (Good et al., 2011). Carbon fibres (7 µm diameter) were vacuum-aspirated into borosilicate pipette glass. Pipettes were pulled using a conventional patch-pipette puller, and the ends of the carbon fibre were cut to allow ∼25–30 µm exposed length protruding from the pipette tip. Pipettes were back filled with a 4 M potassium acetate/150 mM KCl solution and connected to a standard patch pipette holder/headstage assembly. A patch clamp amplifier (HEKA EVA-8) was used to deliver voltage and measure current from the headstage. Voltammetric scan and stimulation-timing protocols were performed using PCI-based A/D boards (National Instruments) and custom software (courtesy of Dr Mark Wightman, University of North Carolina). Scans consisted of sweeps from −0.4 to 1.3 V and back to −0.4 V, at a rate of 400 V/s, and were obtained every 100 ms. A 5 s (50 scan) control period preceded each electrically-evoked response, and was used to obtain a background current that was digitally subtracted from the current obtained during the peak of the response. Currents were converted to concentration by generation of linear in vitro calibration curves for each electrode using 1–5 µm dopamine. Electrode sensitivities did not differ between groups. All signals used in analyses matched the expected voltammetric profile for dopamine.
Electrically evoked dopamine signals in brain slices
Under stereoscopic magnification, carbon fibres were lowered to a depth of ∼100 µm in the dorsal striatum. A bipolar stimulating electrode was positioned ∼75–100 µm from the carbon fibre. Input–output curves (stimulus intensity versus dopamine release) were constructed using single, constant current pulses (0–250 µA, 1 ms duration) delivered between voltammetric scans. Because of heterogeneity of release, four to five sites were sampled and averaged within each slice. The transplanted tissue could be visualized in some sections. In these sections, we sampled from sites both proximal (∼100 µm) and distal (>500 µm) to the implanted tissue. However, in order to assess the overall innervation of striatum, we sampled from sections both anterior and posterior (±560 µm) to the transplant site. Dopamine uptake was assessed by fitting the decay portion of each signal to a single exponential function. The obtained tau values are independent of the signal amplitude, and have previously been demonstrated to be related to the efficiency of dopamine transporter-mediated uptake of dopamine (Vmax/Km), and are sensitive to dopamine transporter inhibitors (Good et al., 2011).
Statistical analyses
Data are expressed as means ± SEM. Two group comparisons were analysed by a Student’s t-test and multiple group comparisons were performed by analyses of variance (ANOVA) followed by post hoc analyses. Differences were considered significant at P < 0.05. Statistical analyses and curve fits were carried out using Prism (v. 5.0; GraphPad Scientific).
Results
Tyrosine hydroxylase expression levels and number of tyrosine hydroxylase-positive neurons are similar in the ventral mesencephalon of control and DA-PTEN-KO embryos at the time of transplantation
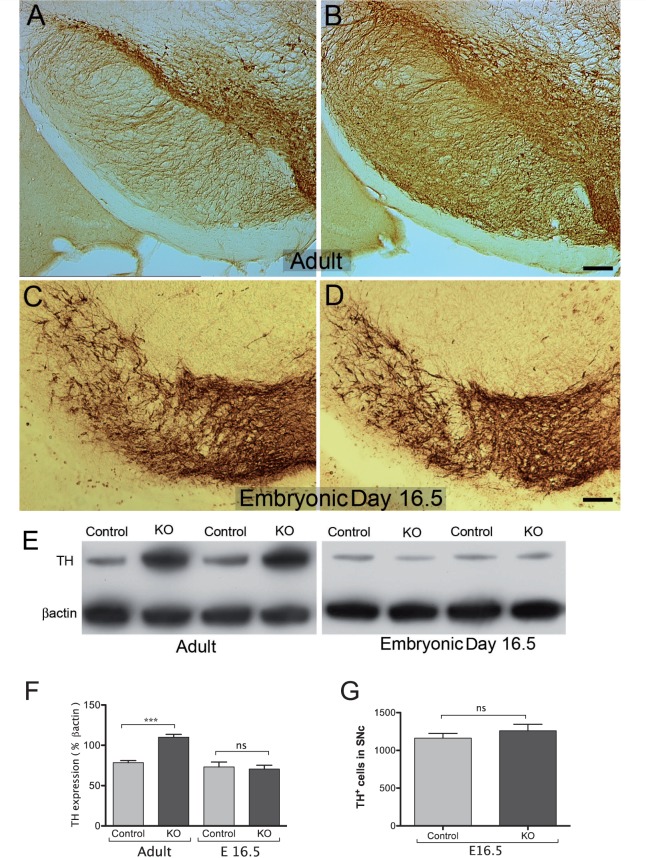
Dopamine midbrain neurons are first generated near the midbrain-hindbrain junction and migrate radially to their final position in the ventral midbrain. Tyrosine hydroxylase, the rate limiting enzyme in the biosynthetic pathway of cathecholamines, can be first detected in the mouse at embryonic Day 11.5, suggesting initiation of dopamine differentiation. In contrast, gene transcripts for the dopamine transporter are not detected in the mouse ventral mesencephalon until dopamine axons reach the target at about Day 15. This suggests that during ontogeny, dopamine synthesis and high-affinity uptake develop asynchronously and in a non-correlated fashion. In DAT-Cre conditional transgenic mice, Cre recombinase activity, and therefore Pten deletion is induced shortly after DAT induction, about Day 15, and just prior to the time of transplantation in this study (Backman et al., 2006). Since we have previously shown that Pten deletion in dopamine neurons induces a significant increase in the number of dopamine neurons in adult animals (Diaz-Ruiz et al., 2009), we considered it important to determine whether the number of tyrosine hydroxylase-positive neurons in the ventral mesencephalon of control and DA-PTEN KO embryos was similar at the time of transplantation. Stereological cell counts were performed in the substantia nigra pars compacta from Day 16.5 embryos and showed no significant differences in the number of tyrosine hydroxylase-positive neurons between control and DA-PTEN-KO mice (Fig. 2C, D and G). It must be noted that in Day 16.5 embryos, most tyrosine hydroxylase-positive neurons in the ventral tegmental area did not show an identifiable nucleus, and existed in immunoreactive clusters, so this region was not included in the stereological analyses. However, immunocytochemical analyses determined the area occupied by tyrosine hydroxylase-positive cell bodies in the substantia nigra pars compacta and ventral tegmental area were similar between control (n = 4, 0.19 ± 0.006 mm2) and DA-PTEN-KO (n = 4, 0.2 ± 0.006 mm2) Day 16.5 embryos (Fig. 2C and D). Additionally, the cell size of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta did not significantly differ between the groups. The average cell area for tyrosine hydroxylase-positive neurons in control embryos was 71.37 ± 3.46 µm2, and 77.50 ± 3.84 µm2 in DA-PTEN-KO embryos (two-tailed t-test, P > 0.05). Western blot analyses further confirmed the presence of similar amounts of tyrosine hydroxylase protein levels in control and mutant Day 16.5 embryos (Fig. 2E and F, one-way ANOVA, P > 0.05). These data suggest that embryonic tissue from control and DA-PTEN-KO animals contained the same number of tyrosine hydroxylase-positive cells at the time of transplantation.
Figure 2.
Tyrosine hydroxylase immunocytochemistry in the substantia nigra of adult control (A) and DA-PTEN-KO animals (B). Adult DA-PTEN-KO animals contain a significantly greater number of tyrosine hydroxylase-positive neurons and fibres in the ventral midbrain region. Significant differences in tyrosine hydroxylase-positive immunocytochemistry were not observed in embryonic Day 16.5 DA-PTEN-KO embryos (D) compared with controls (C). These findings were verified by western blot analyses of tyrosine hydroxylase (TH) protein in ventral mesencephalon tissue homogenates from control and DA-PTEN-KO adult and embryonic Day 16.5 (E16.5) mice (E and F, one-way ANOVA, ***P < 0.001), and (G) by quantification of tyrosine hydroxylase-positive (TH+) neurons in the substantia nigra pars compacta (SNc) from Day 16.5 control and DA-PTEN-KO embryos (two-tailed t-test). Data presented as mean ± SEM. Scale bars: A and B = 150 µm, C and D = 100 µm. ns = non-significant.
Effects of bilateral embryonic transplants on motor decline in MitoPark mice
Spontaneous locomotor activity
As shown in previous studies, MitoPark mice begin showing motor impairment symptoms by 12 weeks of age (Ekstrand et al., 2007; Ekstrand and Galter, 2009) and are severely debilitated by 36 weeks due to the significant degeneration of dopamine neurons in the substantia nigra pars compacta, and loss of dopaminergic striatal innervation (Ekstrand et al., 2007; Ekstrand and Galter, 2009). In the present study, we observed a similar progressive decline in non-grafted MitoPark mice. As shown in Fig. 3, untreated MitoPark mice demonstrated significantly reduced spontaneous locomotor activity at 16 weeks of age, when compared to age-matched controls. At 36 weeks of age, these mice displayed symptoms similar to advanced Parkinson’s disease with respect to their spontaneous locomotor behaviour. These deficits were not significantly improved in MitoPark animals that received control grafts (Fig. 3A). However, MitoPark mice grafted with DA-PTEN-KO tissue showed a significant increase in locomotor activity at 36 weeks of age (or 16 weeks after transplantation), relative to both non-transplanted MitoPark mice and MitoPark mice receiving control grafts (Fig. 3A). Vertical activity in MitoPark animals grafted with DA-PTEN-KO tissue showed a trend towards better performance, when compared to control grafts (Fig. 3B); however, the difference was not statistically significant.
Figure 3.
Spontaneous and drug-induced locomotor activity following transplants in MitoPark mice. (A and B) Spontaneous locomotor behavior was examined every 4 weeks, starting at ∼16 weeks of age. Mitopark animals received control and DA-PTEN-KO intrastriatal grafts at 20 weeks of age, after baseline behavioural recordings, and behavioural evaluations continued for an additional 16 weeks. MitoPark mice showed reduced spontaneous locomotor activity at 16 weeks of age. Total distance (A) and vertical activity (B) of MitoPark mice continued to decline, and animals were severely impaired at 36 weeks of age (two-way ANOVA; Bonferroni post hoc analysis *P < 0.05, **P < 0.01). MitoPark animals grafted with DA-PTEN-KO tissue showed a significant improvement in spontaneous ambulatory activity at 36 weeks, relative to control grafted and naïve MitoPark mice. Vertical activity did not change significantly in MitoPark animals grafted with either DA-PTEN-KO or control tissue, although there was a trend to increased vertical activity in the former group. (C) At 36 weeks of age (or 16 weeks after grafting), animals were evaluated for nomifensine (15 mg/kg, intraperitoneal)-induced locomotor behaviour. Thirty-six-week old MitoPark mice did not respond to nomifensine treatment, while locomotor activity of DA-PTEN-KO grafted MitoPark mice was similar to that from age-matched controls, and significantly higher when compared with both control-grafted MitoPark animals and naïve MitoPark mice (one-way ANOVA; Dunn’s post hoc analysis **P < 0.01). (D) At 35 weeks of age, locomotor activity was measured every hour for 24 h, starting at 11:00 am, during the light phase. All groups showed a normal circadian rhythm by moving less during the light phase, after habituation to the new environment and more during the dark phase. MitoPark animals grafted with DA-PTEN-KO tissue showed a significant increase in the total distance in the nocturnal cycle as compared with all other groups. Significant differences shown compared to MitoPark control graft group (two-way ANOVA; Bonferroni post hoc analyses for all groups: *P < 0.05, **P < 0.01, ***P < 0.001). Data presented as mean ± SEM; ns = non-significant.
Nomifensine-induced locomotor activity
The partial restoration of spontaneous motor activity in MitoPark mice receiving DA-PTEN-KO tissue suggests a partial normalization of dopamine input following transplantation. In order to further evaluate this, we examined the locomotor stimulating effects of the dopamine uptake inhibitor nomifensine (15 mg/kg). Thirty-six-week-old control mice responded to nomifensine by significantly increased locomotor activity after habituation to a saline injection (Fig. 3C). In contrast, 36-week-old MitoPark mice did not respond to nomifensine treatment, presumably due to the lack of functional dopamine transporter-containing axon terminals in the striatum. Interestingly, after nomifensine treatment, the mean total distance activity of DA-PTEN-KO grafted MitoPark mice was similar to that from aged-matched controls, and significantly higher when compared to both control-grafted MitoPark animals and naïve/sham MitoPark mice (Fig. 3C). Overall, these behavioural results suggest dopamine availability, and distribution of dopamine axon terminals, are significantly augmented in the striatum of MitoPark mice receiving DA-PTEN-KO grafts, when compared with control grafts.
Circadian motor activity
All groups showed a normal circadian rhythm by moving less during the light phase, once they habituate to the new environment, and more during the dark phase. Interestingly, MitoPark animals grafted with DA-PTEN-KO tissue showed a significant and robust increase in the total distance associated with the nocturnal cycle compared with non-grafted MitoPark mice, MitoPark mice with control grafts and control mice (Fig. 3D).
Dopamine neuron survival and neurite outgrowth
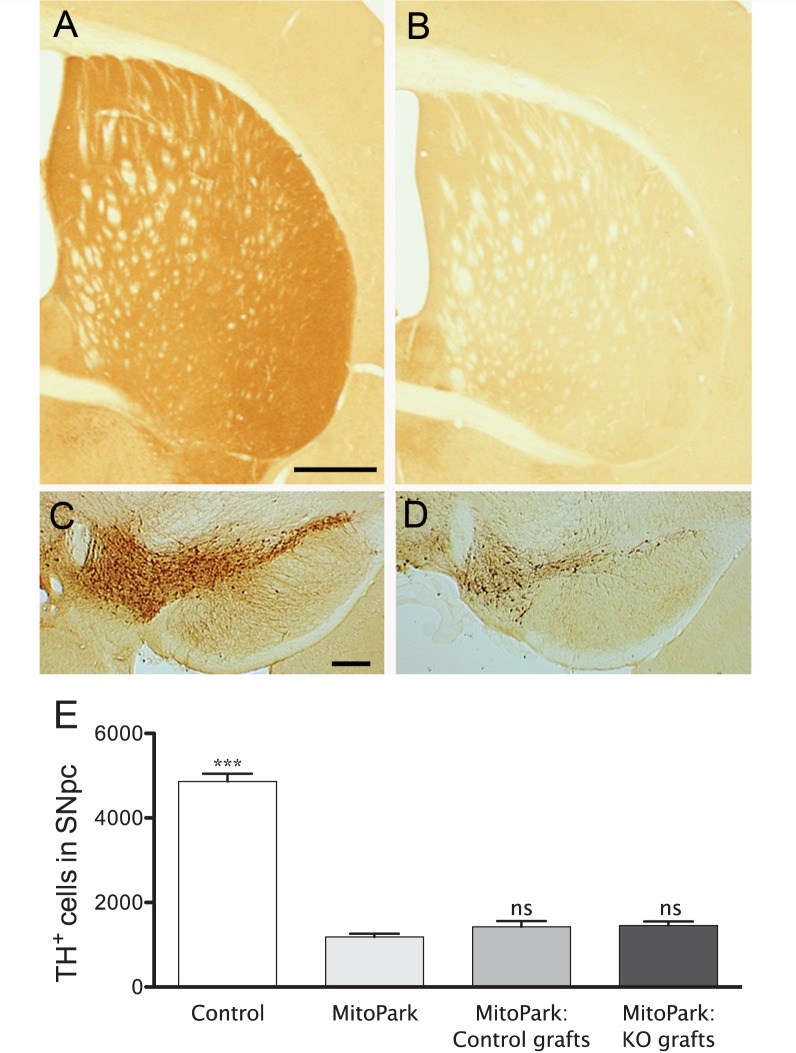
The pattern of tyrosine hydroxylase expression in striatum of 36-week-old MitoPark and sham operated MitoPark animals illustrates the extensive denervation induced by the lack of TFAM in dopamine neurons, compared with levels in age-matched control mice (Fig. 4A and B). Consistent with previous studies (Ekstrand et al., 2007), at the level of the midbrain there was a near complete loss of tyrosine hydroxylase-positive dopamine neurons in the substantia nigra pars compacta, while tyrosine hydroxylase-positive neurons in the ventral tegmental area were partially spared (Fig. 4C and D). While stereological cell counts for the substantia nigra pars compacta revealed a large reduction in the number of tyrosine hydroxylase-positive neurons in all MitoPark animals when compared to aged-matched controls, no significant differences were found between naïve and transplanted MitoPark mice (Fig. 4E), thereby suggesting that dopamine neuron degeneration is not affected by the transplantation procedure in MitoPark animals.
Figure 4.
Tyrosine hydroxylase immunoreactivity in striatum of a 36-week-old control (A) and naïve MitoPark (B) mouse illustrates the extensive degeneration following TFAM deletion in dopamine neurons. At the level of the midbrain, there was a near complete loss of tyrosine hydroxylase-positive dopamine neurons in the substantia nigra pars compacta of MitoPark mice (D) when compared with controls (C). Tyrosine hydroxylase-positive neurons in the ventral tegmental area were partially spared in MitoPark mice (C and D). (E) Quantification of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta of control and MitoPark mice confirmed a significant and comparable loss of tyrosine hydroxylase-positive neurons in both naïve and transplanted MitoPark mice (one-way ANOVA; Bonferroni post hoc analyses for all groups: ***P < 0.001). Significant differences are shown compared to the MitoPark group. Scale bars: A = 250 µm, B = 200 µm. ns = non-significant. KO = knock-out; SNpc = substantia nigra pars compacta; TH = tyrosine hydroxylase; ns = non-significant.
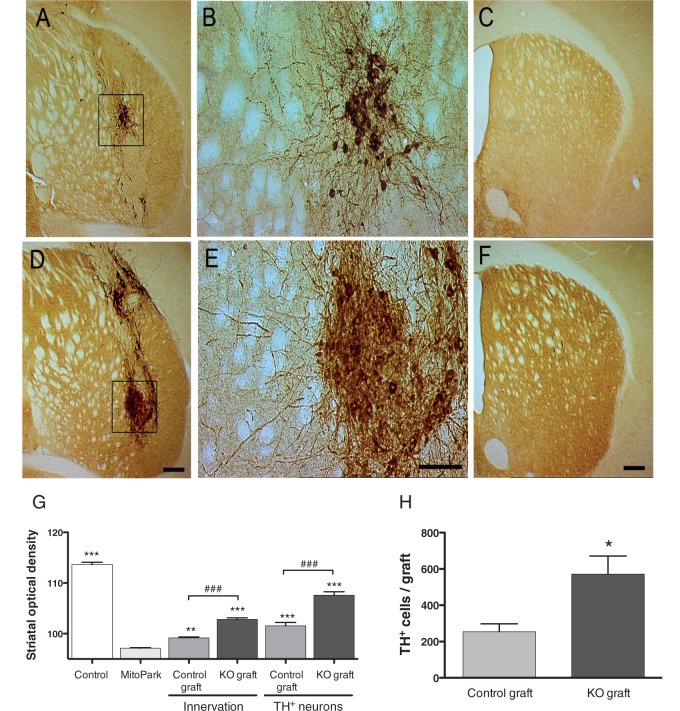
Four months after transplantation, immunohistochemistry for tyrosine hydroxylase showed 33/34 (97%) and 20/24 (83%) surviving grafts in MitoPark animals transplanted with DA-PTEN-KO or control tissue, respectively. Non-surviving grafts were not included in cell count and optical density analyses. Cell counts (Fig. 5H) showed that an average of 254 ± 42.91 tyrosine hydroxylase-positive neurons survived in control grafts, while a significantly higher number was found in DA-PTEN-KO grafts (567 ± 93.21, P < 0.01). Consistent with our previous observations in adult DA-PTEN-KO mice (Diaz-Ruiz et al., 2009), morphological analyses of the transplants demonstrated larger and more densely packed tyrosine hydroxylase-positive neurons in the DA-PTEN-KO transplants compared with control transplants (Fig. 5B and E). The average cell area for tyrosine hydroxylase-positive neurons in control grafts was 184 ± 11.31 µm2, compared with 333 ± 18.33 µm2 in DA-PTEN-KO tyrosine hydroxylase grafts (two-tailed t-test, P < 0.001). Tyrosine hydroxylase immunoreactivity was found in cell bodies and throughout the dendritic and axonal processes of the grafted dopamine neurons. Immunohistochemistry for tyrosine hydroxylase revealed a clear difference in the ability of the PTEN-KO dopamine neurons to innervate the host striatum compared with control grafts (Fig. 5G). Average tyrosine hydroxylase optical density levels corresponding to striatal sections containing graft-derived innervation, and no tyrosine hydroxylase-positive cell bodies (thereby away from the graft core, Fig. 5G ‘innervation’) demonstrated a 2-fold increase in tyrosine hydroxylase-positive innervation in the DA-PTEN-KO grafted striatum. These data suggest that neurites extending from PTEN-deficient transplanted dopamine neurons reinnervate larger areas of the striatum and/or provide a denser fibre network than control transplants. Likewise, striatal optical density of the tyrosine hydroxylase-positive cell body containing compartment of the grafts was twice as high in grafts lacking PTEN, than in control dopamine grafts (Fig. 5G ‘TH+ neurons’).
Figure 5.
Photomicrographs illustrating a control (A, B and C) and DA-PTEN-KO (D, E and F) graft in the striatum of 36-week-old MitoPark mice. For each group, one section through the core of the graft and one section from the same animal, rostral to the graft placement site, are shown. Boxed areas in A and D are enlarged and illustrate the appearance of tyrosine hydroxylase-positive neurons in control (B) and DA-PTEN-KO transplants (E). Tyrosine hydroxylase-positive neurons in DA-PTEN-KO transplants are more densely packed and are larger in size. Neurites extending from DA-PTEN-KO grafts are increased and extend into more distal areas of the MitoPark striatum, when compared with control grafts (C and F). (F) To determine differences in innervation among the transplanted animals, optical density values were obtained separately from striatal sections containing tyrosine hydroxylase-positive cell bodies (see A and D: TH+ neurons), and sections containing only fibres and no cell bodies (see C and F: innervation). (G) While MitoPark mice transplanted with both control and DA-PTEN-KO embryonic tissue showed a significant increase in striatal tyrosine hydroxylase density when compared with naïve MitoPark mice (two-way ANOVA; Bonferroni post hoc analysis, *P < 0.05, ***P < 0.001), MitoPark mice transplanted with DA-PTEN-KO grafts showed significantly higher tyrosine hydroxylase density values when compared with control grafts (two-way ANOVA; Bonferroni post hoc analysis, ###P < 0.001). Data presented as mean ± SEM. (H) Quantification of tyrosine hydroxylase-positive neurons in the striatum of grafted MitoPark mice confirmed a significant increase in the number of tyrosine hydroxylase-positive neurons present in DA-PTEN-KO grafts as compared with control grafts. Two-tailed t-test, *P < 0.05. Data presented as mean ± SEM. Scale bars: D and F = 200 µm, E = 100 µm, KO = knock-out.
Striatal dopamine signalling in naïve and transplanted MitoPark mice
At 36 weeks of age, naïve (non-grafted) MitoPark mice exhibited minimal dopamine release compared with age-matched controls (Fig. 6A). Dopamine transporter-mediated uptake could not be quantified reliably in these MitoPark mice, as signals appeared to exhibit diffusion-like characteristics, consistent with marked axonal terminal loss in these animals (Fig. 6A). We next compared the extent of dopamine transmitter recovery in transplanted MitoPark mice. Coronal sections (280 µm) were prepared as for standard striatal recordings. In order to minimize variability arising from the exact location/size of the grafted tissue, we sampled multiple sites from slices that contained a visible transplant, as well as two to three additional slices anterior/posterior to the graft core. In sections that contained a visible transplant, we sampled from sites both proximal (<200 µm) and distal (>500 µm) from the grafted tissue, but did not observe any systematic differences (data not shown). When averaged across all recording sites throughout the striatum, the amplitude of dopamine signals was significantly greater across a range of stimulus intensities in the DA-PTEN-KO grafted striatum (Fig. 6C). In addition, the decay time constants of the observed signals from DA-PTEN-KO grafts more closely matched those found in control striata (Fig. 6D), and were significantly faster than those observed from control-grafted tissue. These data suggest a greater capacity for dopamine transporter-mediated uptake as well as release in the DA-PTEN-KO transplanted striatum.
Figure 6.
Voltammetric assessment of graft function in 36-week-old MitoPark mice. (A) Fast scan cyclic voltammetry pseudocolour plots of signals obtained in a striatal slice obtained from a 36-week-old control mouse and a MitoPark mouse. Release was elicited by a single, 1 ms pulse (100 µA). Time and applied potential are indicated on the x- and y-axes, respectively; current is represented in pseudocolour. Dashed white line indicates the potential at which voltammograms below each colour plot were obtained. Average current versus time plots (mean ± SEM, indicated by dashed line) are shown for all slices tested (control, nine slices; MitoPark, nine slices). (B) Representative signals taken from the striatum of a control-grafted (top) and a DA-PTEN-KO grafted (bottom) MitoPark mouse. Following recording, sections were processed for tyrosine hydroxylase immunohistochemistry. Numbers indicate the approximate location of the recording site within each slice. Note the clear difference in innervation density (left), corresponding to the differences in the signals (right). (C) Summary of input–output curves in 36-week-old MitoPark mice grafted with control (pooled data from 60 recording sites in 15 slices) or DA-PTEN-KO tissue (65 recording sites in 14 slices). Signals are averaged (SEM indicated by dashed lines) responses to 100 µA, single pulse stimulation (arrow). Significantly greater release was observed in slices from DA-PTEN-KO grafted MitoPark mice [two-way repeated measures ANOVA, genotype effect; F(1,27) = 19.46, P < 0.001]. (D) Summary of decay time constants for voltammetric signals. Upper panel shows the averaged signals elicited by 100 µA stimulation, in DA-PTEN-KO grafted striatum (red trace, n = 14) and control-grafted striatum (blue trace, n = 15) normalized to the amplitude of control, non-MitoPark mice (control, green, n = 9). Decay time constants from DA-PTEN-KO grafted striatum did not significantly differ from control striatum (one-way ANOVA, Tukey post hoc analysis P > 0.05). Note that decay time constants could not be calculated for age-matched, non-grafted MitoPark mice owing to the minimal nature of the signal (A). DA = dopamine.
Discussion
Our results indicate that Pten deletion from embryonic dopamine neurons enhances their intrinsic growth capacity, and results in significant improvements in graft integration. Morphological analyses show that Pten deletion from embryonic dopamine neurons enhances the overall reinnervation of the dopamine depleted striatum after grafting and leads to a markedly increased capacity for neurons to extend functional axons into areas distal from the graft placement site. Behavioural results demonstrated the enhancement of both spontaneous and drug-induced locomotor activity in MitoPark mice following DA-PTEN-KO transplantation. These anatomical and behavioural findings were further confirmed by voltammetric recordings demonstrating improved dopamine release and uptake throughout the striatum in DA-PTEN-KO grafted MitoPark mice. Together, these data suggest that strategies aimed at targeting PTEN signalling in dopamine neurons could be exploited to improve graft survival and function.
For more than 20 years, embryonic dopamine cell transplantation has been performed in humans and a number of open clinical trials have described benefits from transplantation (Lindvall et al., 1988, 1989; Olanow et al., 2009; Freed et al., 2011; Tsui and Isacson, 2011). However, up to now, transplant efficacy has been variable, presumably because the majority of grafted dopamine cells die early after transplantation and because grafted dopamine neurons demonstrate a limited ability to properly integrate with the host striatum. Paucity of trophic factor support by adult host tissue, hypoxia and other aspects of the grafting procedure have all been suggested to be involved in death of many grafted dopamine neurons. In addition, most cell replacement protocols evaluated in animal models of Parkinson’s disease have utilized post-mitotic, differentiated dopamine neurons as grafting material. It has been documented that once neurons differentiate and neural circuits are established during development, intrinsic neuronal growth signals are gradually repressed (Abe and Cavalli, 2008). It is possible the robust growth mode typical of immature neurons is repressed in differentiated dopamine neurons at the time of transplantation, thereby limiting the ability of grafted neurons to interact and form robust connections with the host tissue. In accordance with this hypothesis, previous studies in rodents have shown that young donor age ventral mesencephalic grafts (Day 12) appear to retain a larger capacity to yield greater numbers of surviving dopamine neurons and reinnervate the host striatum more effectively, compared with older donor tissues (Day 14; Torres et al., 2007, 2008). In this study we grafted ventral mesencephalon tissues around the time of Pten deletion at Day 16.5. This approach allowed us to evaluate whether Pten deletion protects embryonic dopamine neurons despite grafting during a developmental stage that is not optimal for either survival or integration with host tissue. Moreover, grafting at this suboptimal stage allowed a larger response window by reducing the efficacy of PTEN-containing control transplants.
Inactivation of PTEN enhances intrinsic growth signals and survival potential of mature neurons, by permitting the constitutive activation of the serine–threonine Akt-signalling pathway, also known as the protein kinase B pathway. Akt blocks cell death by regulating the activity of several proteins directly involved in cell death/survival pathways, such as the regulation of apoptosis (Datta et al., 1999; Brunet et al., 2001; Chang et al., 2007). In addition, Akt phosphorylation enhances the activity of the mammalian target of rapamycin (mTOR) complex 1 (Park et al., 2008, 2010), which in turn leads to an increment in the overall activity of the cell by increasing protein synthesis, mitochondrial function, energy production, metabolism and cell growth (Chong et al., 2010; Bove et al., 2011; Perier et al., 2011). Thus, emerging evidence suggests that Akt is a master regulator of cell growth, survival and bioenergetic signalling. We and others have previously demonstrated the resistance of PTEN knock-out dopamine neurons to neurotoxic insult (Diaz-Ruiz et al., 2009; Domanskyi et al., 2011). In order to further evaluate the therapeutic potential of cell-specific Pten deletion, in this communication we transplanted ventral mesencephalic tissue from DA-PTEN-KO or control mice into the dopamine depleted striatum of MitoPark mice. Sixteen weeks following transplantation, MitoPark animals that received DA-PTEN-KO grafts exhibited increased dopamine neuron survival rates and reinnervation of the host striatum compared with control transplants. Since we transplanted post-mitotic and phenotypically differentiated dopamine neurons, and in our knockout model Pten is only deleted in differentiated dopamine neurons, it is unlikely that the observed increase in tyrosine hydroxylase-positive neurons in DA-PTEN-KO grafts reflects an increase in newly formed neurons. In addition, we determined by western blot analyses and immunocytochemistry that at embryonic Day 16.5, DA-PTEN-KO and control mice contained equal levels of tyrosine hydroxylase protein in the ventral mesencephalon, and that the number of tyrosine hydroxylase-positive neurons in the substantia nigra pars compacta is similar between the groups, thereby suggesting that at the time of transplantation all grafts would contain approximately the same number of dopamine neurons. It is thus likely that Pten ablation preserves dopamine neurons after transplantation by suppressing the initiation of apoptotic pathways.
Beyond improved dopamine neuron survival; an additional factor to consider for functional effects of intrastriatal grafts is the establishment of sufficient amounts of new synapses providing dopamine release in the host striatum. While Akt signalling is known as a mediator of axon regeneration in the mature nervous system (Namikawa et al., 2000; Read and Gorman, 2009; Burke, 2010; Christie et al., 2010; Cheng et al., 2011; Kim et al., 2011), it is not known if Akt-signalling enhancement in embryonic dopamine neurons can facilitate axonal growth after transplantation into an adult host brain. Our morphological results suggest that grafted DA-PTEN-KO neurons exhibit more axonal projections away from the graft location into distal sites of the dopamine deficient host striatum, when compared with control grafts. These morphological findings were further confirmed by voltammetric recordings performed in transplanted striatal slices. Since 36-week-old MitoPark mice show minimal release and reuptake (Fig. 6A), the signals obtained in grafted animals at this time point are primarily indicative of graft function. On average, we observed more robust dopamine release throughout the striatum in DA-PTEN-KO grafted MitoPark mice than in mice grafted with control tissue.
At 36 weeks of age (16 weeks after grafting), MitoPark animals receiving DA-PTEN-KO grafts responded more strongly to nomifensine, and were also more active during the dark cycle, when compared not only with control grafted animals, but also with age-matched controls with an intact dopamine system. In the spontaneous locomotor activity test, MitoPark animals containing DA-PTEN-KO grafts showed a tendency towards improvement starting at 12 weeks post-grafting, which became significant at 16 weeks after transplantation. This recovery curve for correction of spontaneous motor function may reflect continued growth and maturation of the DA-PTEN-KO transplants between 12 and 16 weeks to finally establish a threshold level of graft-derived dopamine innervation in the host striatum. We also hypothesize that the enhanced behavioural effects may reflect the well-known denervation supersensitivity that follows dopamine cell degeneration (Rinne et al., 1990; Antonini et al., 1997; Berke et al., 1998; Gerfen et al., 2002). Supersensitivity would not be expected to be reversed by the transplants, since the PTEN-KO grafts normalized dopamine to ∼10% of that observed in intact striatum (Fig. 6A and C). However, these grafts may release sufficient dopamine to stimulate supersensitive dopamine receptors, thereby resulting in enhanced locomotor activity. Indeed, we found that dopamine uptake was normalized following transplantation of DA-PTEN-KO neurons. This implies that, although the number of functional release sites and/or releasable dopamine content is still greatly reduced in the DA-PTEN-KO MitoPark transplanted striatum when compared with the intact striatum, these sites maintain the capacity for normal, dopamine transporter-mediated control of extracellular dopamine levels. The recovery of dopamine transporter function was further confirmed in behavioural studies. Elevation of motor behaviour by nomifensine, a dopamine reuptake blocker, was only observed in DA-PTEN-KO transplanted MitoPark mice. By comparison, control transplants demonstrate reduced clearance of dopamine, confirming earlier observations in 6-hydroxydopamine lesioned animals models of Parkinson’s disease (Cragg et al., 2000). Further studies will be necessary to determine if the use of optimized transplantation protocols in combination with Pten deletion in embryonic, induced pluripotent and/or stem cell-derived dopamine neurons can normalize dopamine function in the MitoPark striatum to levels comparable with age-matched controls. Although the MitoPark model has many unique advantages, such as a uniform and long temporal window of endogenous dopamine neuron degeneration, it must be remembered that there are no data showing a TFAM polymorphism in Parkinson’s disease. In this study, therefore, MitoPark mice are used mainly as a platform for studies on transplant survival.
Taken together, the results presented here demonstrate that selective deletion of Pten in dopamine neurons facilitates the survival and integration of grafted neurons with the host tissue. These studies further support the importance of the PTEN/Akt-signalling pathway in determining the intrinsic survival and growth responsiveness of neurons intended for transplantation. Activation of the master growth signalling pathway, Akt, in embryonic dopamine neurons induces survival and extensive axonal growth after transplantation, suggesting that retaining active protein synthesis during the grafting procedure is important for initiating a robust neuronal regenerative programme for axon growth in the adult host tissue. The improved survival and function of genetically modified dopamine neurons upon transplantation into this animal model suggests a promising strategy aimed at enhancing graft viability and function in cell replacement therapies.
Funding
This work was supported by the U.S. National Institutes of Health, the National Institute on Drug Abuse Intramural Research Program and The Swedish Research Council, Swedish Brain Power, the Karolinska Distinguished Professor Award.
Acknowledgements
The authors thank Sumayyah Abiff for technical assistance.
Glossary
Abbreviations
- DA-PTEN-KO
mice with inactive phosphatase and tensin homologue (Pten) gene in dopamine neurons
References
- Abe N, Cavalli V. Nerve injury signaling. Curr Opin Neurobiol. 2008;18:276–83. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YH, Bensadoun JC, Aebischer P, Zurn AD, Seiger A, Bjorklund A, et al. Increased fiber outgrowth from xeno-transplanted human embryonic dopaminergic neurons with co-implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor. Brain Res Bull. 2005;66:135–42. doi: 10.1016/j.brainresbull.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Andereggen L, Meyer M, Guzman R, Ducray AD, Widmer HR. Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson's disease. Brain Res. 2009;1276:39–49. doi: 10.1016/j.brainres.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson's disease: a study with positron emission tomography and [11C]raclopride. Mov Disord. 1997;12:33–8. doi: 10.1002/mds.870120107. [DOI] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, et al. Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–90. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Beal MF. Parkinson's disease: a model dilemma. Nature. 2010;466:S8–10. doi: 10.1038/466S8a. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–10. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund L, Vidal N, Stromberg I. Lazaroid-enhanced survival of grafted dopamine neurons does not increase target innervation. Neuroreport. 1998;9:2815–9. doi: 10.1097/00001756-199808240-00024. [DOI] [PubMed] [Google Scholar]
- Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–52. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson's disease Problems and possibilities. Prog Brain Res. 2010;184:265–94. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Burke RE. Intracellular signalling pathways in dopamine cell death and axonal degeneration. Prog Brain Res. 2010;183:79–97. doi: 10.1016/S0079-6123(10)83005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Cadet JL, Kent JD, Karanas AL, Jackson-Lewis V. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. J Neurosci Methods. 1990;35:63–73. doi: 10.1016/0165-0270(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Chang N, El-Hayek YH, Gomez E, Wan Q. Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 2007;30:581–6. doi: 10.1016/j.tins.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Kim SR, Oo TF, Kareva T, Yarygina O, Rzhetskaya M, et al. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J Neurosci. 2011;31:2125–35. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Greig NH, Reiner D, Hoffer BJ, Wang Y. Enhanced survival of dopaminergic neuronal transplants in hemiparkinsonian rats by the p53 inactivator PFT-alpha. Cell Transplant. 2011;20:1351–9. doi: 10.3727/096368910X557173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci. 2010;30:9306–15. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Clarke DJ, Greenfield SA. Real-time dynamics of dopamine released from neuronal transplants in experimental Parkinson’s disease. Exp Neurol. 2000;164:145–53. doi: 10.1006/exnr.2000.7420. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–61. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz O, Zapata A, Shan L, Zhang Y, Tomac AC, Malik N, et al. Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PLoS One. 2009;4:e7027. doi: 10.1371/journal.pone.0007027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanskyi A, Geissler C, Vinnikov IA, Alter H, Schober A, Vogt MA, et al. Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson's disease models. FASEB J. 2011;25:2898–910. doi: 10.1096/fj.11-181958. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Galter D. The MitoPark Mouse - an animal model of Parkinson's disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat Disord. 2009;15(Suppl 3):S185–8. doi: 10.1016/S1353-8020(09)70811-9. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci USA. 2007;104:1325–30. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327:1549–55. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Freed CR, Zhou W, Breeze RE. Dopamine cell transplantation for Parkinson's disease: the importance of controlled clinical trials. Neurotherapeutics. 2011;8:549–61. doi: 10.1007/s13311-011-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galter D, Pernold K, Yoshitake T, Lindqvist E, Hoffer B, Kehr J, et al. MitoPark mice mirror the slow progression of key symptoms and l-DOPA response in Parkinson's disease. Genes Brain Behav. 2010;9:173–81. doi: 10.1111/j.1601-183X.2009.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–54. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, Backman CM, et al. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. FASEB J. 2011;25:1333–44. doi: 10.1096/fj.10-173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BK, Wang Y, Hoffer BJ. Transgenic rodent models of Parkinson's disease. Acta Neurochir. 2008;101(Suppl):89–92. doi: 10.1007/978-3-211-78205-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, et al. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011;70:110–20. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990a;247:574–7. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Neural transplantation in Parkinson's disease: the Swedish experience. Prog Brain Res. 1990b;82:729–34. doi: 10.1016/s0079-6123(08)62666-7. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46:615–31. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, et al. Fetal dopamine-rich mesencephalic grafts in Parkinso’s disease. Lancet. 1988;2:1483–4. doi: 10.1016/s0140-6736(88)90950-6. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann Neurol. 1994;35:172–80. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Problems with long-term levodopa therapy for Parkinson's disease. Clin Neuropharmacol. 1994;17(Suppl 2):S32–44. [PubMed] [Google Scholar]
- Mendez I, Dagher A, Hong M, Hebb A, Gaudet P, Law A, et al. Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line–derived neurotrophic factor in patients with Parkinson’s disease. Report of two cases and technical considerations. J Neurosurg. 2000;92:863–9. doi: 10.3171/jns.2000.92.5.0863. [DOI] [PubMed] [Google Scholar]
- Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, et al. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20:2875–86. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Kordower JH, Lang AE, Obeso JA. Dopaminergic transplantation for Parkinson's disease: current status and future prospects. Ann Neurol. 2009;66:591–6. doi: 10.1002/ana.21778. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–6. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bove J, Vila M. Mitochondria and programmed cell death in Parkinson's disease: apoptosis and beyond. Antioxid Redox Signal. 2011;16:883–95. doi: 10.1089/ars.2011.4074. [DOI] [PubMed] [Google Scholar]
- Piccini P, Lindvall O, Bjorklund A, Brundin P, Hagell P, Ceravolo R, et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–95. [PubMed] [Google Scholar]
- Rajput AH, Fenton ME, Birdi S, Macaulay R, George D, Rozdilsky B, et al. Clinical-pathological study of levodopa complications. Mov Disord. 2002;17:289–96. doi: 10.1002/mds.10031. [DOI] [PubMed] [Google Scholar]
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–84. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne UK, Laihinen A, Rinne JO, Nagren K, Bergman J, Ruotsalainen U. Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson's disease. Mov Disord. 1990;5:55–9. doi: 10.1002/mds.870050114. [DOI] [PubMed] [Google Scholar]
- Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273:175–84. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Tornqvist N, Bjorklund L, Almqvist P, Wahlberg L, Stromberg I. Implantation of bioactive growth factor-secreting rods enhances fetal dopaminergic graft survival, outgrowth density, and functional recovery in a rat model of Parkinson's disease. Exp Neurol. 2000;164:130–8. doi: 10.1006/exnr.2000.7411. [DOI] [PubMed] [Google Scholar]
- Torres EM, Dowd E, Dunnett SB. Recovery of functional deficits following early donor age ventral mesencephalic grafts in a rat model of Parkinson's disease. Neuroscience. 2008;154:631–40. doi: 10.1016/j.neuroscience.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Torres EM, Monville C, Gates MA, Bagga V, Dunnett SB. Improved survival of young donor age dopamine grafts in a rat model of Parkinson's disease. Neuroscience. 2007;146:1606–17. doi: 10.1016/j.neuroscience.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Tsui A, Isacson O. Functions of the nigrostriatal dopaminergic synapse and the use of neurotransplantation in Parkinson’s disease. J Neurol. 2011;258:1393–405. doi: 10.1007/s00415-011-6061-6. [DOI] [PubMed] [Google Scholar]