Abstract
Empathy refers to the ability to perceive and share another person’s affective state. Much neuroimaging evidence suggests that observing others’ suffering and pain elicits activations of the anterior insular and the anterior cingulate cortices associated with subjective empathetic responses in the observer. However, these observations do not provide causal evidence for the respective roles of anterior insular and anterior cingulate cortices in empathetic pain. Therefore, whether these regions are ‘necessary’ for empathetic pain remains unknown. Herein, we examined the perception of others’ pain in patients with anterior insular cortex or anterior cingulate cortex lesions whose locations matched with the anterior insular cortex or anterior cingulate cortex clusters identified by a meta-analysis on neuroimaging studies of empathetic pain perception. Patients with focal anterior insular cortex lesions displayed decreased discrimination accuracy and prolonged reaction time when processing others’ pain explicitly and lacked a typical interference effect of empathetic pain on the performance of a pain-irrelevant task. In contrast, these deficits were not observed in patients with anterior cingulate cortex lesions. These findings reveal that only discrete anterior insular cortex lesions, but not anterior cingulate cortex lesions, result in deficits in explicit and implicit pain perception, supporting a critical role of anterior insular cortex in empathetic pain processing. Our findings have implications for a wide range of neuropsychiatric illnesses characterized by prominent deficits in higher-level social functioning.
Keywords: anterior cingulate cortex, anterior insular cortex, empathy, meta-analysis, necessity
Introduction
Humans are social animals. We constantly perceive and share the affective state of others, while we are still able to distinguish between self and others’ feelings. This ability, referred to as empathy, is critical for effective social interaction (Preston and de Waal, 2002). Human empathy has long fascinated philosophers and social scientists, and has gained increasing interest in neuroscience research (Morrison et al., 2004; Singer et al., 2004; Gu et al., 2010; Caruana et al., 2011). Techniques such as functional MRI have been used to identify brain regions that may subserve empathy for pain (Morrison et al., 2004; Singer et al., 2004; Gu and Han, 2007; Singer and Lamm, 2009; Gu et al., 2010). Such structures include the anterior insular cortex and the dorsal anterior cingulate cortex (Singer and Lamm, 2009; Gu et al., 2010). The anterior insular cortex is related to the integration of polymodal sensory information (Critchley et al., 2004; Craig, 2009) as well as the integration of emotional and cognitive processes (Gu et al., 2012), whereas the dorsal anterior cingulate cortex is known to participate in the monitoring and voluntary control of behaviours (Shackman et al., 2011). However, the relationship between brain regions and empathetic pain inferred from neuroimaging studies is correlational and does not establish causality (Robertson et al., 1993; Damasio et al., 1994; Fellows et al., 2005). A recent study showed that intracortical microstimulation of the ventral insular cortex elicits affiliative behaviours in macaque monkeys, supporting the sufficiency of insular activation in social functions (Caruana et al., 2011). Despite the contribution of these methods of investigation, it remains unknown whether anterior insular cortex, anterior cingulate cortex or both are necessary for empathetic pain perception.
Studying patients with focal lesions in anterior insular or anterior cingulate cortices provides a critical test of the necessity of these regions in empathetic pain perception (Robertson et al., 1993; Damasio et al., 1994, Schoenfeld et al., 2002; Fellows et al., 2005). Due to the rarity of selective brain damage in these regions, only a handful of studies have explored the consequences of insular damage to human behaviours (Naqvi et al., 2007; Clark et al., 2008; Khalsa et al., 2009; Jones et al., 2010), yet none have studied empathetic pain perception in patients with lesions strictly limited to the anterior portion of the insular cortex, or have compared the effects of anterior insular cortex lesions to anterior cingulate cortex lesions. Despite the common observation that anterior insular and anterior cingulate cortices are often jointly activated in brain imaging studies (Craig, 2009; Medford and Critchley, 2010), we previously reported that anterior insular cortex, rather than dorsal anterior cingulate cortex, shows increased activation when a subject sees others’ pain when controlling for cognitive load, suggesting that the anterior insular cortex is more domain-specific than the dorsal anterior cingulate cortex in processing empathetic pain (Gu et al., 2010).
We hypothesized that the anterior insular cortex, rather than anterior cingulate cortex, is necessary for empathetic pain processing. To test this hypothesis, we first conducted a quantitative meta-analysis of 28 functional MRI studies on empathetic pain to localize the exact locations of anterior insular and anterior cingulate cortices associated with empathetic pain perception. We then assessed empathetic pain processing using a perceiving-other-person’s pain paradigm (Gu et al., 2010) in three rare patients with focal anterior insular cortex lesions and three patients with focal dorsal anterior cingulate cortex lesions whose lesion locations overlapped with the anterior insular cortex and anterior cingulate cortex clusters identified in the meta-analysis, and compared them with six patients with focal lesions in areas other than anterior insular or anterior cingulate cortices (brain-damaged controls) and 14 neurologically intact matched controls. We examined both explicit and implicit pain perception because previous studies suggest that explicit and implicit emotional processes might be subserved by different neural substrates (Critchley et al., 2000). We measured explicit pain perception by requiring the subject to attend to and evaluate the emotional state of another individual, while we assessed implicit pain perception by instructing the subject to attend to a pain-irrelevant dimension of the event when watching others’ suffering. We predicted that patients with anterior insular cortex lesions, but not patients with dorsal anterior cingulate cortex lesions, would show deficits in both explicit and implicit empathetic pain processing.
Materials and methods
Activation likelihood estimation meta-analysis
To localize the key brain regions associated with empathetic pain processing, we first conducted a coordinate-based meta-analysis (Salimi-Khorshidi et al., 2009) on 28 functional MRI studies on empathetic pain using activation likelihood estimation (ALE) (Turkeltaub et al., 2002; Laird et al., 2005; Eickhoff et al., 2009) to achieve an unbiased quantification of the neural substrates underlying empathetic pain in healthy adults (see Supplementary Table 1 for included studies). ALE assesses the overlap among all foci included in a meta-analysis by modelling them as 3D Gaussian distributions (Turkeltaub et al., 2002; Laird et al., 2005; Eickhoff et al., 2009). During the data extraction stage, studies were grouped by different spatial normalization schemes according to GingerALE coordinate transformation (http://brainmap.org, Research Imaging Center of the University of Texas Health Science Center): using a Talairach native template; using FSL (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl), SPM (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm) or other programs to report Montreal Neurological Institute (MNI) coordinates; or using Brett methods to convert MNI into Talairach space (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). Coordinate transformations were needed for the latter two groups. For studies using MNI, we converted the MNI coordinates into the Talairach space (icbm2tal); for studies using Brett–Talairach transformation, the coordinates were back-transformed to MNI (tal2icbm) and then transformed to Talairach again. A master list of all studies (foci and experiments/contrasts) was created by combining all coordinates in the Talairach space that could be readily entered into GingerALE 2.0 for ALE meta-analysis. The original ALE method implemented in earlier GingerALE versions (Laird et al., 2005) treats activated foci of brain regions as 3D Gaussian probability distributions centred at the given coordinates instead of points. ALE maps are obtained through computing the activation probability for each voxel. Permutation tests are then conducted to differentiate true activations from random clusters. The distribution of ALE scores obtained from thousands of permutation tests then generates P-values to the experimental values. The revised version of ALE implemented in GingerALE 2.0 (Eickhoff et al., 2009) used in the current meta-analysis has improved the meta-analysis algorithm by incorporating the size of the probability distributions by taking into account the sample size of each study and by utilizing random-effect rather than fixed-effect inference by testing the above-chance clustering between experiments/contrasts rather than the above-chance clustering among foci. We used a threshold of false-discovery rate P < 0.05 and cluster size > 30 voxels (2 × 2 × 2 mm each voxel, 240 mm3) to protect against false positives of multiple comparisons for the analysis.
Four investigators independently conducted an exhaustive literature search of functional MRI studies examining the neural bases of empathetic pain in healthy adults in PubMed and Web of Science through August 2010 (start date of the present study), using one of the key words ‘empathy’, ‘empathetic’, ‘altruism’, ‘sympathy’, ‘emotional contagion’ or ‘compassion’ in conjunction with ‘pain’ and ‘functional MRI’. We included studies on either (i) explicitly instructing subjects to evaluate another person’s pain (e.g. ‘in how much pain do you think s/he is’) or (ii) implicitly inducing affective sharing in the subjects showing another person’s suffering (e.g. button press) or without a control task (e.g. passive observation). Importantly, we excluded studies that focused on the understanding others’ beliefs and intentions (theory of mind, ToM) rather than emotional feelings. We then applied several exclusion criteria to eliminate articles that did not fall into the scope of the current study: (i) non-first hand empirical studies such as review articles; (ii) studies that did not report results in standard stereotactic coordinate space either Talairach or MNI; (iii) studies using tasks unrelated to empathetic processes and with no measurement of trait empathy; (iv) studies of structural brain analyses such as voxel-based morphometry or diffusion tensor imaging; (v) studies purely reporting results from region of interest analysis, for instance, using anatomical masks or coordinates from other studies, or functional or effective connectivity analysis; and (vi) studies of special populations whose brain functions might deviate from those of normal healthy adults, including children, aging adults, psychiatric patients or preselected participants with certain personality traits such as alexithymia, or with certain expertise in inhibiting or enhancing empathy such as physicians and Buddhist mediators. This yielded 28 studies in total. We then selected contrasts in following categories in the analysis: (i) direct comparison between emotional stimuli and baseline [neutral stimuli, self emotion or blank screen with fixation; e.g. ‘pain > no pain in others’ (Singer et al., 2004)]; (ii) direct comparison between an empathy task and a control task [e.g. brain activations shown in the contrast between rating and counting painful stimuli (Gu and Han, 2007)]; (iii) correlation analysis with trait empathy measured by self-report questionnaires (e.g. the Empathy Quotient; Baron-Cohen and Wheelwright, 2004) as covariates; and (iv) correlation analysis with valence rating (e.g. pain or unpleasantness ratings as covariates). Contrasts from connectivity analyses were excluded from the current analysis. This yielded 948 foci from 98 contrasts in the 28 included studies.
Brain lesions patients and control groups
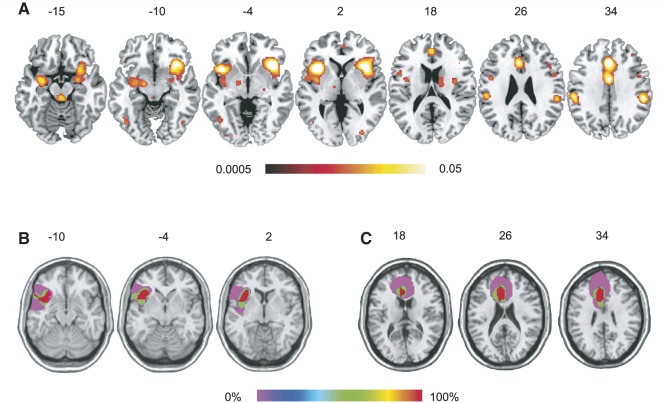
Three patients with focal unilateral anterior insular cortex lesions and three patients with focal dorsal anterior cingulate cortex lesions participated in the study (see Table 1 for all participants’ characteristics). All lesions resulted from surgical removal of low-grade gliomas. Among the three patients with anterior insular cortex lesions, two had left lesions and one had a right lesion. Reconstructed anterior insular and anterior cingulate cortex lesions are shown in Fig. 1B and C. The locations of these lesions were in close proximity with the peak coordinates of anterior insular and anterior cingulate cortex activation associated with empathetic pain perception localized in the meta-analysis (Fig. 1A). Six patients with focal lesions in regions other than the anterior insular and anterior cingulate cortices (e.g. lateral prefrontal cortex and temporal pole) were recruited as brain-damaged controls and 14 neurologically intact participants were recruited as normal controls. All patients (anterior insular cortex, anterior cingulate cortex and brain-damaged controls) were recruited from the Patient’s Registry of Tiantan Hospital, Beijing, China. Neurologically intact controls were recruited in local Beijing communities and were matched with the patients for age, gender, education and ethnicity (Table 1). All subjects had normal colour vision and reported no previous or current neurological or psychiatric conditions. Neither patients with anterior insular cortex nor those with dorsal anterior cingulate cortex lesions differed from neurologically intact or brain-damaged control subjects in age, education or chronicity (P > 0.05). All three anterior insular cortex patients were considered cognitively intact, measured by Mini-Mental State Examination, a measurement of cognitive impairment (Folstein et al., 1975), although the raw scores of patients with anterior insular cortex lesions were slightly lower than controls (P < 0.05). None of the anterior cingulate cortex patients showed cognitive impairment either, and their raw scores on Mini-Mental State Examination were not statistically different from controls (P > 0.05). Patients with anterior insular cortex lesions did not show alteration in Beck Depression Inventory score, a measurement of baseline mood (Knight, 1984) compared with neurologically intact controls (P > 0.05), although they scored higher than brain-damaged controls (P < 0.05). Anterior cingulate cortex patients did not show statistical difference on Beck Depression Inventory score compared to controls (P > 0.05). All subjects were informed of the study requirements and provided written consent prior to participation. The study was approved by the Institutional Review Boards of Mount Sinai School of Medicine in New York and the Tiantan Hospital of the Capital Medical University in Beijing.
Table 1.
Participant characteristics
| Lesion laterality | Lesion size (ml) | Chronicity (months)a | Age (years)b | Gender | Handedness | Education (years) | MMSE | BDI | |
|---|---|---|---|---|---|---|---|---|---|
| AIC1 | Left | 15 | 32 | 42 | M | Right | 9 | 22 | 7 |
| AIC2 | Left | 36 | 20 | 46 | F | Right | 9 | 21 | 8 |
| AIC3 | Right | 28 | 3 | 51 | F | Right | 9 | 24 | 5 |
| ACC1 | Left | 14 | 16 | 37 | M | Left | 9 | 28 | 6 |
| ACC2 | Left | 54 | 6 | 47 | F | Right | 12 | 26 | 3 |
| ACC3 | Bilateral | 38 | 18 | 35 | F | Left | 9 | 23 | 18 |
| BDC | 2 Left/4 right | 46 ± 21 | 23 ± 5 | 43 ± 12 | 3 F/3 M | 0 Left/6 right | 12 ± 4 | 26 ± 3 | 3 ± 4 |
| NC | N/A | N/A | N/A | 45 ± 4 | 6 F/8 M | 0 Left/14 right | 10 ± 1 | 26 ± 3 | 5 ± 6 |
AIC1, AIC2 and AIC3 are patients with anterior insular cortex lesion; ACC1, ACC2 and ACC3 are patients with anterior cingulate cortex lesion.
BDC = brain damaged controls; BDI = short form of the Beck Depression Inventory, a measure of baseline mood (Knight, 1984); MMSE = Mini-Mental State Examination, a test for cognitive impairment (Folstein et al., 1975); N/A = not available; NC = neurologically intact controls.
a Chronicity: the time length between surgery date and testing date.
b Age: the age at the testing date.
Figure 1.
(A) Common brain activation related to empathetic pain perception as revealed by a meta-analysis on 28 functional MRI studies on empathetic pain (false-discovery rate P < 0.05 and cluster size > 240 mm3). Anterior insular and anterior cingulate cortices showed the most consistent activation among all 28 studies. (B) Reconstruction of anterior insular cortex lesions of three patients and (C) reconstruction of anterior cingulate cortex lesions of another three patients. Lesions were mapped on the same hemisphere to show degree of overlap. The brain template used in (B) and (C) was created by a neurologist (R.T.K.) and its reference line is tilted 12 degrees from the anterior commissure—posterior commissure plane. Red colour indicates 100% overlap.
Lesion reconstruction
A neurologist (R.T.K.), blind to the behavioural results, identified and entered the lesions for each subject onto templates derived from a digital MRI volume of a normal control (ch2.nii) created by Christopher Rorden and provided for use with his MRIcron program (http://www.cabiatl.com/mricro/mricro/index.html). In each case, lesions evident on MRI were transcribed onto corresponding sections of the template in order to create a volume of interest file. This was used to measure the location (MNI coordinates) and volume (in ml) of individual lesions and to create within group overlaps of multiple lesions using the MRIcron program.
Stimulus, task design and procedure
The stimulus set included 216 digital colour photographs showing another person’s left or right hand or foot in painful or non-painful situations (Fig. 2; see also Gu et al., 2010). Photographs showing painful events and those showing non-painful events were identical in physical properties (i.e. context, brightness and contrast). For the explicit pain condition, subjects were instructed to judge whether the person in the photograph was suffering from pain or not [task pain (TP)]; in the implicit pain condition, they were told to judge the laterality of the hand/foot [task laterality (TL)]. Subjects responded through button press within a time window of 4 s (2.5 s of stimulus display and 1.5 s of post-stimulus fixation period). All instructions and choices, as implemented in our previous experiment (Gu et al., 2010), were translated from English to Chinese by a native Chinese speaker (X.G.). There were two TP sessions and two TL sessions in total. Each session included 27 trials of painful photographs, 27 trials of non-painful photographs and 27 null trials of blank screen (with a fixation in the centre) to jitter the intertrial intervals. This yielded a factorial design with 2 (task: TP versus TL) × 2 (pain: painful versus non-painful) × 2 (laterality: left versus right) conditions. Subjects were instructed to respond as quickly and accurately as possible. Response accuracy and reaction time (RT) were recorded.
Figure 2.
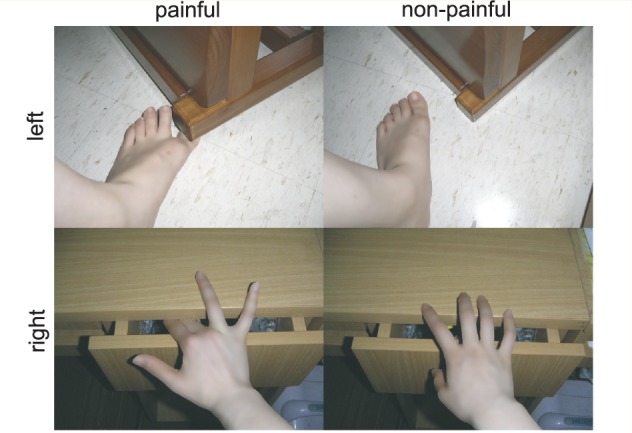
Sample stimuli of the experimental stimulus set of 216 digital colour photographs showing another person’s left or right hand/foot in painful or non-painful situations. Subjects were instructed to choose between ‘non-painful’ and ‘painful’ for the task pain and ‘left’ and ‘right’ for the task laterality through button press within a time window of 4 ms (2.5 ms of stimulus display and 1.5 s of fixation).
Data analysis
We used signal detection theory to analyse the behavioural responses. Signal detection theory, a method that discerns signal from noise, assumes that the perceiver has a distribution of internal responses for both signal and noise (Snodgrass and Corwin, 1988; Stanislaw and Todorov, 1999). A major advantage of signal detection theory is the dissociation between discrimination accuracy (distance between signal and noise distributions) and decision bias (tendency to respond ‘signal’ or ‘noise’). Subjects’ discrimination accuracy d′ is defined as d′ = (μs − μn)/σ, where μs is the mean of signal distribution and μn is the mean of noise distribution, and σ is the common standard deviation of both distributions. It is calculated as d′ = Zhit rate − Zfalse alarm rate, with four possible outcomes: hit (pain present and subject’s response is ‘yes’), miss (pain present and subject’s response is ‘no’), false alarm (pain absent and subject’s response is ‘yes’), and correct rejection (pain absent and subject’s response is ‘no’). Decision bias β is defined as β = fs(λ)/fn(λ), where fs(λ) is the height of the signal distribution at a given criterion λ and fn(λ) is the height of the noise distribution at the same λ. It is calculated as β = exp(d′ × C), where C = −(Zhit rate + Zfalse alarm rate)/2.
For TP index d′ is the distance between the mean of the probability distribution for ‘pain’ (signal) and the mean of the probability distribution for ‘no pain’ (noise), measured in units of the common standard deviation; index β, representing the position of the subject’s decision criterion, is the ratio of the height of the ‘pain’ distribution to that of the ‘no pain’ distribution at the subject’s decision criterion, and a value of 1 indicates no bias. For TL, we first computed discrimination accuracy index d′ and decision bias β of laterality judgment (‘left’ was arbitrarily defined as signal and ‘right’ as noise) for painful and non-painful conditions separately and then calculated the difference scores between the two conditions as the interference effect of pain on laterality (TL) judgment (d′TL-painful − d′TL-non-painful). Negative scores indicate a normal interference effect of empathetic pain on laterality judgment as shown in our previous study (Gu et al., 2010).
We assessed overall RT of correct trails under each task condition, defined as (RTTP-pain + RTTP-no pain)/2 for TP and (RTTL-pain + RTTL-no pain)/2 for TL. We also computed cost of pain, defined as RTTP-pain – RTTP-no pain for TP and RTTL-pain – RTTL-no pain for TL. Error trials were excluded from calculations of RT because mental processes associated with incorrect trials are noisy. Overall accuracy and RT data are shown in Supplementary Fig. 1.
Because the current data set does not meet the assumptions of parametric tests and all comparisons were based on a priori hypotheses in small samples, we used the non-parametric bootstrapping method (Mooney, 1993; Hasson et al., 2003) to assess the probability of observing a difference between two groups (anterior insular cortex versus neurologically intact controls, anterior insular cortex versus brain-damaged controls, anterior cingulate cortex versus neurologically intact controls and anterior cingulate cortex versus brain-damaged controls) by chance. The bootstrapping procedure was conducted with 10 000 iterations as follows (e.g. the comparison between three anterior insular cortex patients and 14 neurologically intact controls): (i) 14 subjects were selected randomly as the surrogate neurologically intact controls group, from the whole group of 17 subjects including both anterior insular cortex and neurologically intact controls subjects; (ii) three subjects were selected randomly as the surrogate anterior insular cortex group from the whole group of 17 subjects; and (iii) the t-value of the difference between the two surrogate groups was calculated. After 10 000 iterations, the distribution of the t-values was obtained. The observed t-value (e.g. between anterior insular cortex and neurologically intact controls groups) was then calculated and compared along the t distribution. If the probability of obtaining the observed t-value along the permutated distribution of t-value is <5% (one tailed), we considered the difference between the patient and control groups to be significant.
Results
Anterior insular cortex–anterior cingulate cortex common activation during empathetic pain processes revealed by meta-analysis
The main analysis based on all activation foci related to empathetic pain perception from 28 functional MRI studies using the ALE method showed involvement of bilateral anterior insular cortex, anterior cingulate cortex, supplementary and premotor areas, somatosensory cortex, inferior frontal gyrus, inferior temporal gyrus, inferior parietal lobule and mid occipital gyrus (Fig. 1A and Supplementary Table 2). Subcortical structures involved in empathetic pain perception included amygdala, globus pallidus, thalamus and cerebellum. It is worth noting that although we adopted a relatively stringent threshold of false-discovery rate, the anterior cingulate cortex cluster covered the anterior rostral cingulate zone, posterior rostral cingulate zone and caudal cingulate zone (see the coordinates defined in Fan et al., 2008) and the insular clusters on both hemispheres spanned from anterior to posterior insula.
Deficits in explicit empathetic pain processing associated with anterior insular cortex lesions
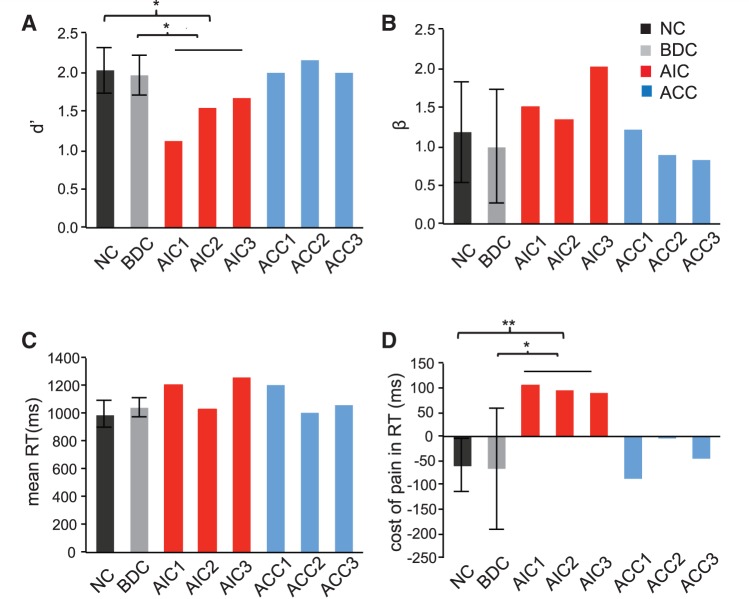
For the lesion study, we first examined behavioural performance during explicit empathetic pain processing under task pain. Patients with anterior insular cortex lesions had significantly smaller d′ compared to both neurologically intact controls and brain-damaged control subjects (P < 0.05; Fig. 3A), indicating diminished ability to discriminate painful from non-painful stimuli. In comparison, patients with anterior cingulate cortex lesions did not show abnormality in d′ compared to either neurologically intact controls or brain-damaged controls (P > 0.05; Fig. 3A). Neither patients with anterior insular cortex lesions nor those with anterior cingulate cortex lesions showed significant alternation in β during pain judgment (P > 0.05; Fig. 3B). It is noteworthy that d′ and β are two independent measures, that is, discrimination accuracy does not correlate with decision bias. Our results demonstrate significant impairment in discrimination accuracy to others’ pain indexed by d′, yet no significant deficit in likelihood ratio decision bias measured by β, during explicit empathetic processing in patients with anterior insular cortex lesions and sparing of both measures in patients with anterior cingulate cortex lesions.
Figure 3.
Behavioural performance on task pain (TP). (A) Patients with anterior insular cortex (AIC) lesions (P < 0.05), but not anterior cingulate cortex patients (P > 0.05), had significantly smaller d′ compared with neurologically intact controls and brain-damaged controls, indicating impaired discrimination accuracy to empathetic pain in anterior insular cortex patients. (B) Neither patients with anterior insular cortex lesions nor those with anterior cingulate cortex lesions showed any significant alternation in decision bias indexed by β during task pain (P > 0.05). (C) Neither patients with anterior insular cortex lesions nor anterior cingulate cortex lesions showed any significant alternation in overall reaction time (RT) [(RTTP-pain + RTTP-no pain)/2] (P > 0.05). (D) Patients with anterior insular cortex lesions (P < 0.01 versus neurologically intact controls and P < 0.05 versus brain-damaged controls), but not those with anterior cingulate cortex lesions (P > 0.05), had greater cost of pain (RTTL-pain – RTTL-no pain). Error bar represents 95% confidence interval (CI). Statistical inference was not based on 95% confidence interval but on the bootstrapping method. All reaction times were calculated based on correct trials only. *P < 0.05; **P < 0.01.
We then assessed response speed in patients during explicit empathetic pain judgment. Neither patients with anterior insular cortex lesions nor those with anterior cingulate cortex lesions showed significant abnormality in overall RT (P > 0.05; Fig. 3C). However, anterior insular cortex patients had greater cost of pain in RT compared with controls (P < 0.05), while the anterior cingulate cortex group did not display such abnormality (P > 0.05; Fig. 3D). We speculate that anterior insular cortex patients needed to depend on deliberative reasoning (slow and cognitive) rather than intuitive (fast and emotional) responses (Kahneman, 2003; Morewedge and Kahneman, 2010) to make judgment of others’ pain, resulting in longer RT cost of pain. This result provides additional evidence that deficits in explicit empathetic pain processing were associated with anterior insular cortex, but not anterior cingulate cortex, lesions.
Deficits in implicit empathetic pain processing associated with anterior insular cortex lesions
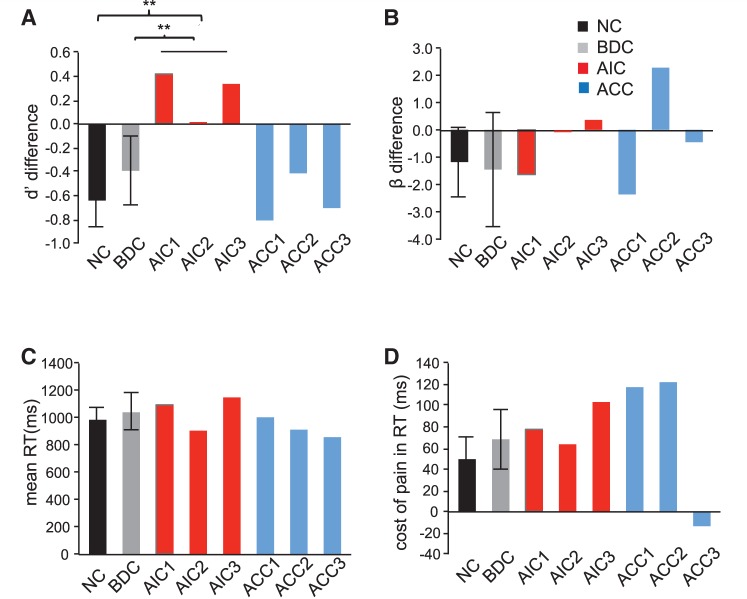
We then examined implicit empathetic processing by assessing the interference effect of empathetic pain on judgment (d′TL-painful – d′TL-non-painful). Consistent with our previous findings (Gu et al., 2010), normal controls and brain-damaged control patients both displayed a negative difference score, suggesting that they performed worse on the TL painful than the TL-non-painful condition and that empathetic pain interfered with laterality judgment. However, patients with anterior insular cortex lesions had slightly positive difference scores in d′ and lacked the normal interference effect of pain on laterality judgment compared to brain-damaged controls and neurologically intact controls (P < 0.05; Fig. 4A). Interestingly, patients with anterior cingulate cortex lesions, in contrast to those with anterior insular cortex lesions, displayed a normal interference effect (P > 0.05; Fig. 4A). Anterior insular cortex and anterior cingulate cortex patients did not show significant alternation in interference effect of empathy for pain on decision bias, mean RT [(RTTL-pain + RTTL-no pain)/2] or RT cost of pain (RTTL-pain − RTTL-no pain) (P > 0.05; Fig. 4B–D) during laterality judgment. These results suggest that anterior insular cortex lesions, but not anterior cingulate cortex lesions, disrupted the typical interference effect of empathy for pain on discriminability of laterality, indicating that implicit processing of empathetic pain is also impaired in patients with anterior insular cortex lesions but not in those with anterior cingulate cortex lesions.
Figure 4.
Behavioural performance on task laterality (TL). (A) Patients with anterior insular cortex lesions (P < 0.01), but not those with anterior cingulate cortex lesion patients (P > 0.05), lacked the normal interference effect of pain on laterality discriminability. (B) Neither patients with anterior insular cortex lesions nor those with anterior cingulate cortex lesions showed significant difference in interference effect of pain on task laterality decision bias (P > 0.05). (C) Neither the anterior insular cortex lesions nor the anterior cingulate cortex lesions showed any significant alternation during laterality judgment in overall reaction time (RT) of task laterality (TL) [(RTTL-pain + RTTL-no pain)/2] (P > 0.05). (D) Neither patients with anterior insular cortex lesions nor those with anterior cingulate cortex lesions showed significant differences in RT cost of pain during TL (RTTL-pain − RTTL-no pain) (P > 0.05). Error bar represents 95% confidence interval. Statistical inference was not based on 95% confidence interval but on the bootstrapping method. All RTs were calculated based on correct trials only. **P < 0.01.
Discussion
We provide the neuropsychological evidence that anterior insular cortex lesions, but not anterior cingulate cortex lesions, disrupt both explicit and implicit empathetic pain perception, while both the anterior insular and anterior cingulate cortices are activated in empathetic processes as shown by our meta-analysis of functional MRI studies. These findings argue for a necessary role of the anterior insular cortex in empathetic social processes.
A necessary role of the anterior insular cortex in empathetic pain perception
The insular cortex is traditionally considered as a limbic sensory region (Critchley et al., 2004; Craig, 2009) and is characterized by the occurrence, in layer V of the anterior insular cortex, of a particular neuronal population, the von Economo neurons (Allman et al., 2010), which has been proposed to participate in the intuitive processing of complex situations (Allman et al., 2005). Notably, abnormalities in von Economo neurons have been observed in children with autism (Santos et al., 2011) and patients with frontotemporal dementia and callosal agenesis (Seeley et al., 2006; Kaufman et al., 2008; Kim et al., 2012), both conditions characterized by prominent deficits in empathy. Grounded in such unique anatomy, anterior insular cortex is well suited for intuitive and ‘effortless’ information processing (Kuo et al., 2009) and for effectively switching between task states (Sridharan et al., 2008). Therefore, we propose that the behavioural deficits observed in anterior insular cortex patients are due to the failure of anterior insular cortex to effectively link sensory inputs with the abstract representation of subjective feelings and emotional awareness and, consequently, failure to discriminate between emotionally salient information from neutral events. This proposal is supported by observed behavioural deficits that anterior insular cortex lesion worsened patients’ discrimination accuracy of perception of another person’s pain but did not significantly interfere with their likelihood ratio decision criterion (patients were neither too liberal nor too stringent toward a ‘pain’ response and adopted similar decision criterion as control subjects).
The brain network involved in social cognition, generally referred to as the ‘social brain’, consists primarily of anterior insular cortex, the medial wall of prefrontal cortex (including anterior cingulate cortex), inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule, areas along the intraparietal sulcus and amygdala (Brothers, 1990; Adolphs, 2003; Frith, 2007; Blakemore, 2008). Although this view is facing great conceptual challenges (Adolphs, 2010), our meta-analysis shows that the main neural network subserving empathetic pain largely overlaps with the ‘social brain’ as defined by Brothers (1990) and others (Adolphs, 2003; Frith, 2007; Blakemore, 2008). The anterior insular cortex, in particular, is necessary for such processes as shown in the present lesion study. The anterior insular cortex is also known to participate in a wide range of processes other than empathy or social function. For instance, it has been reported that patients with anterior insular cortex lesions display deficits in a large variety of functions including speech, sensorimotor functions, bodily awareness, perceptual processes and high-level decision-making processes (see Jones et al., 2010 for a review). A recent functional MRI study has shown that anterior insular cortex serves as a site for cognition–emotion integration (Gu et al., 2012). Therefore, it is likely that a shared mechanism underlying these processes exists and that the anterior insular cortex represents a crucial neural substrate of that shared component. We speculate that these seemingly independent processes may all inevitably involve a somatic signal, which is essential for coordinating between an individual’s homeostatic states and the external environment and for reaching subjective awareness (Damasio, 1996; Craig, 2009, 2011). We propose that anterior insular cortex is necessary for conveying this somatic marker. This interpretation would also explain the involvement of the insula in a wide range of psychiatric and neurological disorders (Allman et al., 2005; Naqvi et al., 2007; Santos et al., 2011; Butti et al., 2012; Kim et al., 2012) marked with abnormalities in complex emotional and social abilities.
Distinctions between the roles of the anterior insular and anterior cingulate cortices
Our meta-analysis results suggest that anterior insular cortex and dorsal anterior cingulate cortex are both activated during empathetic pain processing. Both regions encode the affective-motivational, rather than the sensory-discriminative aspect of pain experienced either in oneself (Wager et al., 2004) or by another person (Singer et al., 2004; Jackson et al., 2005). In other words, the activation of the anterior insular and anterior cingulate cortices does not require direct somatosensory stimulation. Therefore, their activation in empathetic pain paradigms is indicative of similar affective-motivational processes involved in witnessing another person’s suffering and in experiencing pain in oneself.
However, the present study shows that only anterior insular cortex, but not anterior cingulate cortex, lesions result in prominent deficits in both explicit and implicit empathetic pain perception. In a previous functional MRI study (Gu et al., 2010), we also found significant increase in activation of the anterior insular cortex, rather than the anterior cingulate cortex, for painful compared to non-painful images after controlling for cognitive load. These findings suggest there are clear distinctions between the roles of the anterior insular and anterior cingulate cortices. As previously proposed (Craig, 2009; Medford and Critchley, 2010), the anterior insular cortex serves as the input region of the system, which translates sensations into subjective feelings and awareness, whereas the anterior cingulate cortex functions as the output region that exerts volitional control and is related to cognitive processing load but not the property of the input itself. The joint action of the anterior insular and anterior cingulate cortices allows an integrated awareness of sensory, affective and cognitive processes involved in empathetic pain. However, only damage to the input structure (i.e. anterior insular cortex) that implements feelings, but not damage to the output region (i.e. anterior cingulate cortex) that implements control, resulted in impaired empathetic processing. This suggests that, first, the anterior insular cortex might be the only input region whereas the anterior cingulate cortex may be one of the several output regions and, second, that affective feelings, instead of volitional control, constitute the essential and core component of ‘true empathy’ (Preston and de Waal, 2002).
Funding
The National Institute of Health (NIH) (grants R21 MH083164 to J.F. and NS21135 to R.T.K), the James S. McDonnell Foundation (grant 22002078 to P.R.H.), and a Brain and Behavior Research Foundation NARSAD young investigator award (to X.L.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Dr Michael I. Posner for constructive comments on the manuscript, Dr Leon Deouell for statistical advice, Clay Clayworth for help with lesion reconstruction, Nicholas Van Dam for helpful discussion, Thaddeus Daniel for proofreading, and David Fan, Ji Young Kim and Gabrielle Frenkel for help with literature search and data extraction of the meta-analysis.
Glossary
Abbreviation
- ALE
activation likelihood estimate
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–67. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, et al. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–73. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Butti C, Santos M, Uppal N, Hof PR. Von Economo neurons: clinical and evolutionary perspectives. Cortex. 2012 doi: 10.1016/j.cortex.2011.10.004. Advance Access published on March 21, 2012, doi:10.1016/j.cortex.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:195–9. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–22. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann NY Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage—clues about the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17:850–8. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007;362:671–8. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–44. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-emotion integration in the anterior insular cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr367. Advance Access published on January 23, 2012, doi:10.1093/cercor/bhr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. J Cogn Neurosci. 2003;15:419–31. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jones CL, Ward J, Critchley HD. The neuropsychological impact of insular cortex lesions. J Neurol Neurosurg Psychiatry. 2010;81:611–8. doi: 10.1136/jnnp.2009.193672. [DOI] [PubMed] [Google Scholar]
- Kahneman D. A perspective on judgment and choice: mapping bounded rationality. Am Psychol. 2003;58:697–720. doi: 10.1037/0003-066X.58.9.697. [DOI] [PubMed] [Google Scholar]
- Kaufman JA, Paul LK, Manaye KF, Granstedt AE, Hof PR, Hakeem AY, et al. Selective reduction of Von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathol. 2008;116:479–89. doi: 10.1007/s00401-008-0434-7. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–6. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof PR, Miller BL, et al. Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia. Cereb Cortex. 2012;22:251–9. doi: 10.1093/cercor/bhr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RG. Some general population norms for the short form Beck Depression Inventory. J Clin Psychol. 1984;40:751–3. doi: 10.1002/1097-4679(198405)40:3<751::aid-jclp2270400320>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Sjostrom T, Chen YP, Wang YH, Huang CY. Intuition and deliberation: two systems for strategizing in the brain. Science. 2009;324:519–22. doi: 10.1126/science.1165598. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney CZ. Bootstrapping: a nonparametric approach to statistical inference. Thousand Oaks, CA: Sage; 1993. [Google Scholar]
- Morewedge CK, Kahneman D. Associative processes in intuitive judgment. Trends Cogn Sci. 2010;14:435–40. doi: 10.1016/j.tics.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4:270–8. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 71. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Knight RT, Rafal R, Shimamura AP. Cognitive neuropsychology is more than single-case studies. J Exp Psychol Learn Mem Cogn. 1993;19:710–7. doi: 10.1037/0278-7393.19.3.710. discussion 8–34. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage. 2009;45:810–23. doi: 10.1016/j.neuroimage.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, et al. von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res. 2011;1380:206–17. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Noesselt T, Poggel D, Tempelmann C, Hopf JM, Woldorff MG, et al. Analysis of pathways mediating preserved vision after striate cortex lesions. Ann Neurol. 2002;52:814–24. doi: 10.1002/ana.10394. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–7. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann NY Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–49. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.