Abstract
Magnetic resonance imaging sequences such as diffusion and spectroscopy have been well studied in X-linked adrenoleukodystrophy, but no data exist on magnetic resonance perfusion imaging. Since inflammation is known to modulate the microcirculation, we investigated the hypothesis that changes in the local perfusion might be one of the earliest signs of lesion development. Twenty patients with different phenotypes of adrenoleukodystrophy and seven age-matched controls were evaluated between 2006 and 2011. Fluid attenuated inversion recovery, post-contrast T1-weighted and normalized dynamic susceptibility contrast magnetic resonance perfusion cerebral blood volume maps were co-registered, segmented when cerebral lesion was present, and normalized cerebral blood volume values were analysed using a Food and Drug Association approved magnetic resonance perfusion software (NordicICE). Clinical and imaging data were reviewed to determine phenotype and status of progression. All eight patients with cerebral adrenoleukodystrophy had an average 80% decrease in normalized cerebral blood volume at the core of the lesion (P < 0.0001). Beyond the leading edge of contrast enhancement cerebral perfusion varied, patients with progressive lesions showed an average 60% decrease in normalized cerebral blood volume (adults P < 0.05; children P < 0.001), while one child with arrested progression normalized cerebral blood volume in this region. In six of seven patients with cerebral adrenoleukodystrophy lesions and follow-up imaging (2–24 month interval period), we found progression of contrast enhancement into the formerly hypoperfused perilesional zone. Asymptomatic, adrenomyeloneuropathy and female heterozygote patients had no significant changes in cerebral perfusion. Our data indicate that decreased brain magnetic resonance perfusion precedes leakage of the blood–brain barrier as demonstrated by contrast enhancement in cerebral adrenoleukodystrophy and is an early sign of lesion progression.
Keywords: MRI perfusion, demyelination, neuroinflammation, adrenoleukodystrophy, leukodystrophy
Introduction
X-linked adrenoleukodystrophy is a genetic disorder that leads to accumulation of very long-chain fatty acids in the brain, spinal cord and adrenal glands (Moser et al., 2001, 2004). Thirty-five to 40% of male patients carrying the mutation develop normally until 4–8 years of age and then suffer progressive dementia and profound neurological decline, a product of catastrophic inflammatory demyelination known as childhood cerebral adrenoleukodystrophy. In late adulthood, males inevitably develop adrenomyeloneuropathy, a slowly progressive paraparesis resulting from a non-inflammatory chronic axonopathy of the long tracts of the spinal cord. Twenty percent of these patients will also suffer a rapidly progressive inflammatory demyelination (adult cerebral adrenoleukodystrophy) with a pattern similar to the childhood cerebral form (Eichler et al., 2007; van Geel et al., 2011). In contrast, female heterozygotes can display symptoms similar to adrenomyeloneuropathy but are not known to develop cerebral adrenoleukodystrophy.
Previous pathological studies of the inflammatory process in adrenoleukodystrophy demarcate three concentric zones (Schaumburg et al., 1975). The first or inner zone consists of a dense mesh of glial fibrils and scattered astrocytes where the disease process has run its course, and there is no longer evidence of an active process; here, oligodendroglia, axons and myelin sheaths are absent. Moving outward, the leading edge of the inflammatory process consists of two immediately abutting zones: the first of these, Zone 2, is populated by many perivascular macrophages; in Zone 3, the peripheral edge of the process, there is ongoing myelin destruction but axons are spared. Immediately beyond the actively demyelinating lesion edge, there is a zone lacking microglia within the perilesional white matter (Eichler et al., 2008). The precise sequence leading to this distinct distribution of pathological zones is not completely understood.
Neuroimaging studies are exquisitely sensitive in detecting the initial plaque of the expanding confluent lesion (Moser et al., 2000; Loes et al., 2003). Use of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) demonstrates a fringe of accumulated contrast material at the leading edge of the demyelinating process (Melhem et al., 2000). This contrast enhancement appears to correspond with the histologically mapped zone of active inflammation (van der Voorn et al., 2011). Furthermore, contrast enhancement appears to predict lesion progression (Melhem et al., 2000), suggesting a role of blood–brain barrier disruption in the pathophysiology of adrenoleukodystrophy.
Microvascular changes in white matter can be assessed by dynamic susceptibility perfusion MRI following administration of contrast material. Using this technique, the haemodynamic changes in normal-appearing white matter and pathological conditions have been explored. So far there exists no data on cerebral magnetic resonance perfusion in patients with adrenoleukodystrophy. The purpose of this study is to provide a first assessment of magnetic resonance perfusion abnormalities in patients with adrenoleukodystrophy and their relationship to conventional MRI findings.
Materials and methods
Patients
Patients with X-linked adrenoleukodystrophy were evaluated at Massachusetts General Hospital between 2006 and 2011. In adult patients, the clinical status was evaluated with the Expanded Disability Status Scale (Kurtzke, 1983) and, in children, with the Neurologic Function Score (Moser et al., 2000). Asymptomatic patients older than 7 years underwent magnetic resonance scanning without sedation, whereas younger children and symptomatic patients required general anaesthesia. Imaging was performed for clinical monitoring of disease progression. IRB approval for image analysis was obtained (MGH protocol 2005-P-001112/14). We also studied seven age-matched controls that underwent magnetic resonance perfusion imaging during routine clinical care. None of the four adults (mean age 45.7 years, range 32–67 years) or the three children (mean age 12 years, range 4–17 years) had evidence of perfusion or structural abnormalities within the regions of interest.
Imaging
Magnetic resonance imaging
Magnetic resonance studies were predominantly performed on 1.5 T magnetic resonance units (GE Signa® HDx, GE healthcare) using eight-channel head coils. A few perfusion magnetic resonance data sets (6 out of 27 baseline studies, similarly distributed between the different groups) were obtained using a 3 T magnetic resonance unit (MAGNETOM Tim Trio, Siemens) using a 12-channel head coil. The magnetic resonance protocol included axial FLAIR (repetition time/echo time/inversion time, 10 000/124/2200 ms), pre- and post-contrast axial T1-weighted spin-echo (repetition time/echo time, 767/13 ms) and dynamic susceptibility contrast magnetic resonance perfusion sequences.
Structural magnetic resonance imaging
The severity of cerebral abnormalities was assessed on sagittal T1- and axial T2-weighted brain MRI at baseline and on most recent follow-up examination using the adrenoleukodystrophy MRI Loes scale (Loes et al., 1994). This 34-point scale quantifies the extent of white matter abnormalities in the cerebrum, brainstem and cerebellum. Higher scores represent greater lesion burden and degree of damage. The investigator who performed the MRI scoring was blinded to the results of the magnetic resonance perfusion.
Lesion segmentation
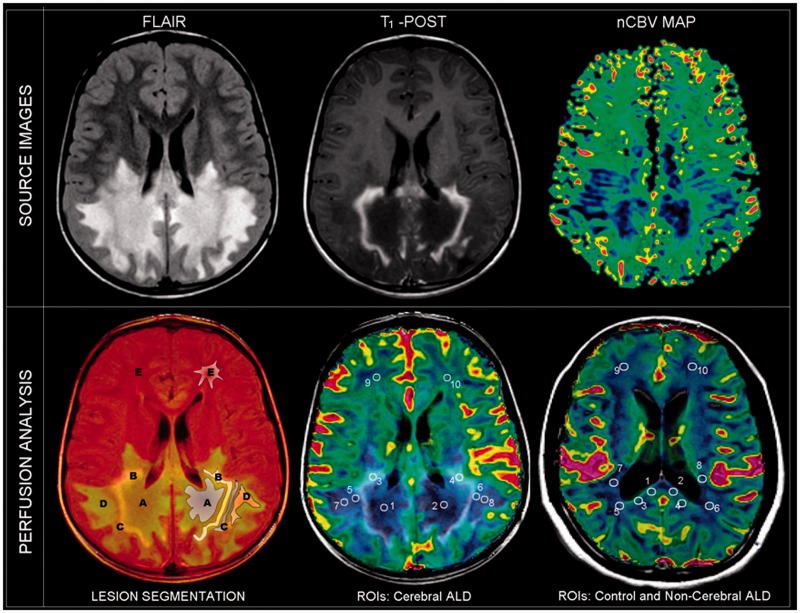
In patients with cerebral adrenoleukodystrophy, we co-registered post-contrast T1 weighted and FLAIR images and defined five different concentric zones based on abnormal FLAIR and T1-post contrast signal. Figure 1 illustrates the five consecutive segmented zones in a patient with cerebral adrenoleukodystrophy: Zone A (central FLAIR hyperintense, non-enhancing), Zone B (FLAIR hyperintense, with abnormal enhancement), Zone C (FLAIR isointense, non-enhancing), Zone D (FLAIR hyperintense, non-enhancing) and Zone E (distant normal appearing white matter). In each zone, a region of interest (18 mm × 18 mm, area = 3.29 cm3) was selected for analysis. For patients without brain lesions on conventional MRI (adrenomyeloneuropathy, female heterozygotes and asymptomatic males), five corresponding regions of interest of representative white matter were selected, including the splenium of the corpus callosum, the periatrial white matter of the posterior horn of the lateral ventricle, the occipital centrum semiovale, parietal retrolenticular white matter and frontal centrum semiovale.
Figure 1.
Nine-year-old male patient with childhood cerebral adrenoleukodystrophy (ALD). Top: FLAIR, post-contrast axial T1-weighted (T1-POST), and normalized cerebral blood volume (nCBV) map. Bottom: Co-registered images with regions of interest (ROIs). Five different zones of involvement are distinguished in the white matter (Zones A–E; see main text). In patients with non-cerebral disease (adrenomyeloneuropathy, female heterozygotes, asymptomatic males and controls) representative regions of normal-appearing white matter were assessed.
Perfusion imaging
Dynamic susceptibility contrast magnetic resonance perfusion was performed with gradient-echo sequences targeting the supratentorial white matter. Specific acquisition parameters in GE scanners included a repetition time of 1500 ms, echo time of 40 ms, flip angle of 60°, acquisition matrix of 128 × 128, field of view of 22 cm and slice thickness of 5 mm. Sixty time points were typically acquired before, during and after Gd-DTPA administration. For Siemens scanners, the acquisition parameters included a repetition time of 1500, echo time of 32, echo time of 90°, matrix of 128 × 128, field of view of 22 cm and a slice thickness of 5 mm. Eighty phases were acquired before, during and after Gd-DTPA administration. The gadolinium dose was 0.1 mM/kg. Contrast was injected at 5 cc/s for older patients using a power injector. Paediatric patients were manually injected using maximal permissible rates depending on their intravenous access (up to 5 ml/s). Magnetic resonance perfusion datasets significantly degraded by motion artefacts or incomplete were excluded from the analysis.
The included dynamic susceptibility contrast perfusion magnetic resonance datasets were post-processed using an FDA-approved magnetic resonance perfusion analysis software (NordicICE Perfusion Analysis module, NordicNeuroLab AS) and normalized T1/T2 leakage corrected cerebral blood volume maps were generated using a multislice spin-echo echo-planar imaging sequence (repetition time/echo time, 1500/40 ms) before, during and after the passage of a bolus of Gd-DTPA. The contrast agent leakage correction performed by this software is based on a method previously described (Boxerman et al., 2006). Normalized cerebral blood volume maps, representing fractional cerebral blood volume values relative to the global mean of ‘normal appearing’ brain parenchyma, were obtained and co-registered with post contrast T1 and FLAIR images using the same software. This normalization procedure has previously been described (Emblem and Bjornerud, 2009). Since there was no significant difference in mean normalized cerebral blood volume between corresponding regions of interest of the left and right hemispheres, the average normalized cerebral blood volume values in each zone were compared.
Statistical analysis
We performed descriptive statistics to compare characteristics (age, clinical score, MRI score) of our adrenoleukodystrophy cohort to controls as well as to compare subtypes (childhood cerebral adrenoleukodystrophy, adult cerebral adrenoleukodystrophy, asymptomatic, adrenomyeloneuropathy and female heterozygotes) to controls. In patients with cerebral disease, normalized cerebral blood volume (Fig. 1) was compared to corresponding anatomical white matter regions of age-matched controls. In patients without cerebral disease, corresponding brain regions were analysed. A multivariate two-way ANOVA with Bonferroni post hoc test was performed to compare white matter perfusion of patients with cerebral and non-cerebral adrenoleukodystrophy, in children and adults separately. In patients who had follow-up MRIs, progression was assessed, and patients were dichotomized into ‘progressive’ (change of >1 points on Loes score) and ‘non-progressive’ (change of ≤1 points on Loes score) by MRI score. To measure the association between changes in perfusion and lesion progression, we performed a univariate analysis using a Wilcoxon rank sum test.
Results
Magnetic resonance imaging and clinical data
Clinical characteristics and the severity of MRI abnormalities, as assessed by the Loes score, of patients with adrenoleukodystrophy with cerebral disease are shown in Table 1. In addition, seven controls were studied: four adults (mean age 45.7 years, range 32–67 years) and three children (mean age 12 years, range 4–17 years).
Table 1.
Clinical and MRI characteristics of patients with X-linked adrenoleukodystrophy
| No. | Phenotype | Age (years) | Follow-up interval (months) | First NFS | Second NFS | First EDSS | Second EDSS | First MRI Loes | Second MRI Loes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CCALD | 5 | 2 | 5 | 19 | – | – | 16 | 19 |
| 2 | CCALD | 6 | 13 | 8 | 24 | – | – | 20 | 30 |
| 3 | CCALD* | 6 | 11 | 2 | 21 | – | – | 23 | 28 |
| 4 | CCALD* | 9 | 12 | 12 | 24 | – | – | 21 | 22 |
| 5 | CCALD | 10 | 4 | 0 | 0 | – | – | 3 | 3 |
| 6 | CCALD* | 12 | 24/29 | 1 | 4/4 | – | – | 12 | 15/15 |
| 7 | Asymptomatic | 3 | 17 | 0 | 0 | – | – | 0 | 0 |
| 8 | Asymptomatic | 4 | 28 | 0 | 0 | – | – | 0 | 0 |
| 9 | Asymptomatic | 6 | 6 | 0 | 0 | – | – | 0 | 0 |
| 10 | Asymptomatic | 8 | 36 | 0 | 0 | – | – | 0 | 0 |
| 11 | Asymptomatic | 16 | 14 | 0 | 0 | – | – | 0 | 0 |
| 12 | ACALD | 19 | 0 | – | – | 6 | – | 5 | – |
| 13 | ACALD | 29 | 13 | – | – | 2 | 2 | 7 | 9 |
| 14 | AMN | 47 | 40 | – | – | 3 | 3 | 1 | 1 |
| 15 | AMN | 50 | 0 | – | – | 2 | – | 0 | – |
| 16 | AMN | 53 | 0 | – | – | 8 | – | 0 | – |
| 17 | Heterozygotes | 28 | 20 | – | – | 3 | 3 | 0 | 0 |
| 18 | Heterozygotes | 39 | 0 | – | – | 0 | – | 0 | – |
| 19 | Heterozygotes | 39 | 0 | – | – | 0 | – | 0 | – |
| 20 | Heterozygotes | 74 | 0 | – | – | 4 | – | 0 | – |
*Post-haematopoietic stem cell transplantation. Only Patient 6 was studied before and after haematopoietic stem cell transplantation took place 15 months after first evaluation. There were two follow-up evaluations 9 and 14 months thereafter. Clinical and MRI characteristics of 20 patients with X-linked adrenoleukodystrophy. ACALD = adult cerebral adrenoleukodystrophy; AMN = adrenomyeloneuropathy; CCALD = childhood cerebral adrenoleukodystrophy; EDSS = Expanded Disability Status Scale; NFS = Neurologic Function Score. The severity of cerebral abnormality was assessed on sagittal T1- and axial T2-weighted brain MRI at baseline and on most recent follow-up examination with the adrenoleukodystrophy MRI (Loes) scale.
Eight patients had cerebral lesions (six with childhood cerebral adrenoleukodystrophy, two with adult cerebral adrenoleukodystrophy). Their average Loes score at baseline was 13.4 (range 3–23). Eleven patients had normal brain MRIs (four female heterozygotes, two adrenomyeloneuropathy and five asymptomatic boys). One patient with adrenomyeloneuropathy had bilateral T2-weighted hyperintense lesions in the corona radiata and posterior limb of the internal capsule (Patient 14), consistent with corticospinal tract axonopathy, and received a Loes score of 1.
Fourteen patients had repeat brain imaging and clinical evaluation with a mean duration of follow-up of 16.3 months (range 2–40 months). Of the six children with cerebral adrenoleukodystrophy, five progressed both by clinical and radiographic criteria. One patient with a frontal lobe lesion remained stable (Patient 5). Of the two adults with cerebral adrenoleukodystrophy, we had follow-up on only one (Patient 13). He remained clinically stable but progressed radiographically. In patients with progressive lesions, the Loes score increased by an average of 4.3 points (range 1–10).
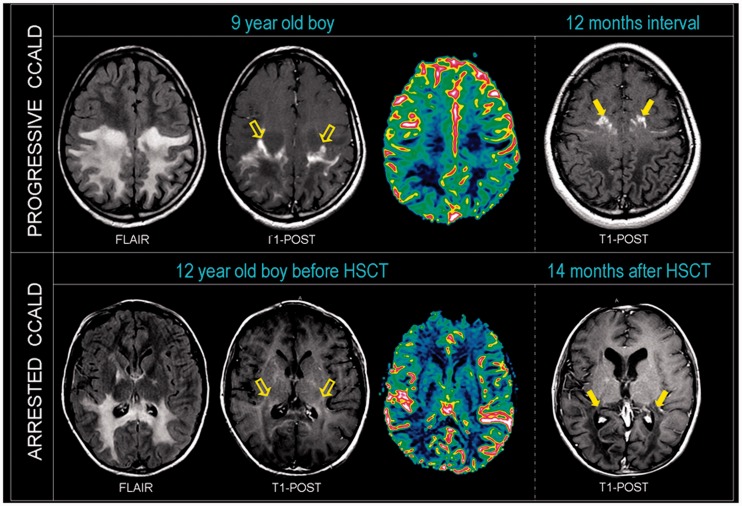
In our cohort, three children with childhood cerebral adrenoleukodystrophy underwent haematopoietic stem cell transplantation. Two patients had been transplanted at advanced stage prior to our study and continued to progress during our study (Patients 3 and 4). Only one patient had magnetic resonance perfusion available for analysis before and after transplant (Patient 6). In this patient, disease progression arrested by Loes score and Neurologic Function Score (NFS) 9 months post-haematopoietic stem cell transplantation. He remained stable up to his last follow-up 14 months after transplant.
Dynamic susceptibility contrast magnetic resonance perfusion
Dynamic susceptibility contrast magnetic resonance perfusion was obtained in all 20 patients. Significant decreases in normalized cerebral blood volume were recorded in all patients with cerebral adrenoleukodystrophy but none of the patients with normal conventional brain imaging. Segmentation revealed distinct zonal differences within the lesion. In one child with a frontal lobe lesion (Patient 5), segmentation of the lesion was not possible due to the small size of the lesion.
In adrenomyeloneuropathy, female heterozygote and asymptomatic patients, normalized cerebral blood volume values were not significantly different when compared with corresponding regions of interest from age-matched controls (Fig. 2). Age-matched controls did not show alterations in normalized cerebral blood volume values across the different white matter regions or over a 6-month interval period (follow-up only available in two adult and one paediatric case).
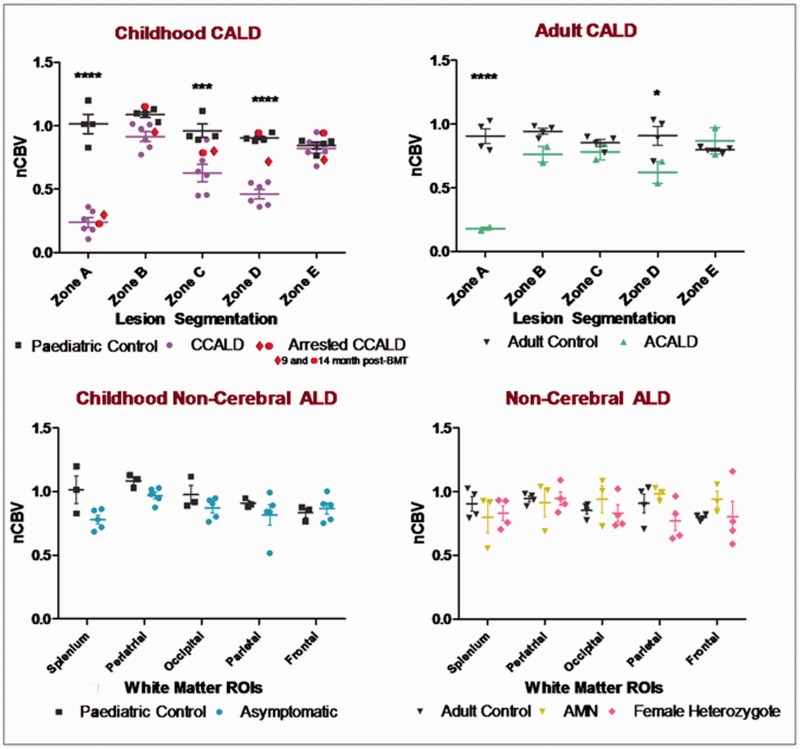
Figure 2.
Normalized cerebral blood volume (nCBV) measured by dynamic susceptibility contrast magnetic resonance perfusion imaging was significantly decreased in zones A, C and D in patients with cerebral adrenoleukodystrophy (CALD) but not in patients without lesions (non-cerebral). In the core of the lesion (Zone A), normalized cerebral blood volume was 80% lower than that in corresponding white matter of control brain. Beyond the region of contrast enhancement (Zones C and D), normalized cerebral blood volume was up to 60% lower in patients with cerebral adrenoleukodystrophy compared with controls. ACALD = adult cerebral adrenoleukodystrophy; CCALD = childhood cerebral adrenoleukodystrophy; HSCT = haematopoetic stem cell transplantation; ROIs = regions of interest.
Perfusion measurements in the five cerebral adrenoleukodystrophy segmented zones
In all eight patients with cerebral adrenoleukodystrophy, we were able to analyse regional normalized cerebral blood volume and found a characteristic pattern (Fig. 2). In children, in comparison with their age-matched controls, we found an 80% decrease in normalized cerebral blood volume within the lesion core where the disease process had begun but was no longer active [Zone A: mean 0.26; 95% confidence interval (CI) of difference: −0.99 to −0.51; P < 0.0001], relatively preserved normalized cerebral blood volume in the region of contrast enhancement (Zone B: mean 0.94; 95% CI of difference: −0.38 to 0.096; P > 0.05), a significant decrease in normalized cerebral blood volume immediately beyond the region of contrast enhancement (Zone C: mean 0.63; 95% CI of difference: −0.58 to −0.11, P < 0.001; and Zone D: mean 0.47; 95% CI of difference: −0.67 to −0.20; P < 0.0001) and normal normalized cerebral blood volume in distant normal-appearing white matter (Zone E: mean 0.82, 95% CI of difference: −0.24 to 0.23; P > 0.05). Two adult patients with cerebral adrenoleukodystrophy showed significantly decreased perfusion in Zones A (mean 0.18, 95% CI of difference: −1.01 to −0.450; P < 0.0001) and D (mean 0.62, 95% CI of difference: −0.57 to −0.01; P < 0.05) (Fig. 2).
In six out of seven patients with cerebral adrenoleukodystrophy lesions and follow-up imaging (2–24 month interval period), conventional MRI demonstrated progression of contrast enhancement into the formerly T2-hyperintense hypoperfused Zone D (Fig. 3). One patient with arrested progression following haematopoietic stem cell transplantation (Patient 6) showed normalization of previously decreased perfusion values in Zone D and lack of progression of contrast enhancement into the T2 hyperintense region 9 and 14 months post-transplant (Figs 2 and 3).
Figure 3.
Decreased brain magnetic resonance perfusion in cerebral adrenoleukodystrophy precedes lesion progression. Top: T2, T1 post-contrast weighted and T1-post contrast images co-registered with normalized cerebral blood volume images of a 9-year-old child with progressive cerebral adrenoleukodystrophy shows decreased magnetic resonance perfusion beyond the contrast enhancing region (empty arrows; Zone D). Follow-up T1-post contrast weighted imaging after 12 months shows lesion extension and advancement of contrast material (solid arrows) into prior hypoperfused region. Bottom: T2, T1 post-contrast weighted and T1-post contrast images co-registered with normalized cerebral blood volume images of a 12-year-old child with cerebral adrenoleukodystrophy (CCALD) shows normal normalized cerebral blood volume beyond the contrast enhancing region (empty arrows; Zone D) 9 months after the engraftment of haematopoietic stem cell transplantation (HSCT). No lesion progression was observed up to 14 months post-transplant. Contrast material (solid arrows) has not advanced.
Discussion
Since the seminal paper by Schaumburg et al. (1975), the prominent perivascular inflammation at the leading edge of demyelination is known to occur in cerebral adrenoleukodystrophy. This study demonstrates that distinct regional magnetic resonance perfusion changes correspond to these previously recognized pathological zones. The premise underlying these zonal changes is one of concentric and centrifugal disease progression. The zonal sequence as evidenced by advanced magnetic resonance techniques is one of decreased perfusion at the necrotic demyelinated core, normalized perfusion at the inflammatory leading edge, and significantly decreased yielding to normal perfusion beyond the contrast enhancing region.
Overall, significant perfusion abnormalities only occurred in patients with brain lesions demonstrable with conventional imaging. In both children (n = 6) and adults (n = 2) with brain lesions, we found 80% lower normalized cerebral blood volume in the lesion core, while the contrast enhancing zone demonstrated normalized cerebral blood volume (Fig. 2). Surprisingly, the area beyond the contrast enhancement showed moderately decreased perfusion in all patients with cerebral adrenoleukodystrophy who had evidence of disease progression in follow-up MRIs. This hypoperfused region matches the full extent of the T2-hyperintense zone that lies between the contrast enhancing and the normal-appearing white matter, suggesting abnormal distribution of extracellular fluid.
In patients with progressive cerebral disease, the region of contrast enhancement invariably moved into areas of previously decreased perfusion on follow-up examination. This may represent shunting of perfusion from adjacent areas to sites of active inflammation, but it may also be evidence of early tissue injury or dysfunction of the neurovascular unit. In contrast, patients with normal brain MRI (adrenomyeloneuropathy, female heterozygote and asymptomatic adrenoleukodystrophy) did not show significant perfusion changes. Although there was a tendency towards decreased perfusion within the affected corticospinal tracts in patients with adrenomyeloneuropathy, this did not reach statistical significance.
Schaumburg et al. (1975) partitioned the disease process of cerebral adrenoleukodystrophy into three concentric zones where the core Zone 1 corresponded to the quiescent initial focus where disease activity had run its course, adjacent area Zone 2 populated with lipid-laden macrophages and the outer Zone 3, the leading edge of demyelination, where there was an ongoing myelinolytic–macroglial process marked by prominent perivascular inflammation (Schaumburg et al., 1975). This pattern of perfusion abnormalities maps upon the known zonal pathology at autopsy of cerebral adrenoleukodystrophy. It suggests a disease process during which mildly decreased perfusion anticipates an inflammatory surge in perfusion followed by decreased perfusion once the acute process is spent. It further invites speculation on the contribution of blood volume and vascular density to the pathogenesis of demyelination as it has for decades in the field of multiple sclerosis (Putnam, 1933, 1935; Arnold et al., 1984; Lightman et al., 1987). In both cerebral adrenoleukodystrophy and multiple sclerosis, the decreased perfusion at the innermost core may be the result of severe tissue destruction and gliosis (Schaumburg et al., 1975; Melhem et al., 2000). Decreases in brain perfusion have also been observed in patients with relapsing-remitting multiple sclerosis preceding the appearance of T2-hyperintense lesions, at times without signs of clinical decline (Law et al., 2004; Wuerfel et al., 2004). It is possible that inflammatory cytokines produced in the active demyelinating zone trigger vasoconstriction and/or cytotoxicity of perivascular elements without leaving lasting structural damage.
The observation of decreased perfusion preceding lesion extension in cerebral adrenoleukodystrophy adds to a list of known magnetic resonance abnormalities predictive of disease progression. The most well-recognized marker is the presence of contrast enhancement on conventional imaging (Melhem et al., 2000). Proton magnetic resonance spectroscopy data obtained from patients with X-linked adrenoleukodystrophy show elevation in the choline-to-total creatine ratio in regions of normal-appearing white matter prior to progression (Eichler et al., 2002). Magnetization transfer imaging and diffusion tensor imaging differentiate regions of T2 hyperintensity but have not been shown to be predictive of lesion progression (Melhem et al., 1996; Ito et al., 2001).
Although our study suggests that perfusion imaging is a promising and likely clinically relevant technique to investigate patients with adrenoleukodystrophy, there are some reservations at this stage. Manual selection of the regions of assessment was necessary and precluded the implementation of possibly more reliable, semi-automated, computer-based methods for quantification of cerebral blood volume within zonal pathology. While perfusion in one patient stabilized after haematopoietic stem cell transplantation, no patients during our study period converted from non-cerebral to the cerebral form of adrenoleukodystrophy, preventing us from drawing conclusions about this key transition and its potential relationship to perfusion changes. Future prospective studies are needed to address the role of perfusion in the conversion from asymptomatic to symptomatic cerebral adrenoleukodystrophy.
In summary, this is the first report of in vivo brain magnetic resonance perfusion abnormalities that correspond with the known zonal pathology of patients with cerebral adrenoleukodystrophy. Our study suggests that dynamic susceptibility contrast perfusion could be a powerful biomarker of lesion progression and could help elucidate the pathophysiology of inflammatory demyelination in adrenoleukodystrophy.
Funding
This work was supported by the National Institutes of Health: K08NS52550 and R01 NS072446 to F.S.E. and R25NS065743 to P.L.M.
Glossary
Abbreviations
- Gd-DTPA
gadolinium-diethylenetriamine pentaacetic acid
References
- Arnold AC, Pepose JS, Hepler RS, Foos RY. Retinal periphlebitis and retinitis in multiple sclerosis. I. Pathologic characteristics. Ophthalmology. 1984;91:255–62. doi: 10.1016/s0161-6420(84)34296-8. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–67. [PMC free article] [PubMed] [Google Scholar]
- Eichler FS, Barker PB, Cox C, Edwin D, Ulug AM, Moser HW, et al. Proton MR spectroscopic imaging predicts lesion progression on MRI in X-linked adrenoleukodystrophy. Neurology. 2002;58:901–7. doi: 10.1212/wnl.58.6.901. [DOI] [PubMed] [Google Scholar]
- Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Nagpal S, Moser AB, et al. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol. 2008;63:729–42. doi: 10.1002/ana.21391. [DOI] [PubMed] [Google Scholar]
- Eichler F, Van Haren K. Immune response in leukodystrophies. Pediatr Neurol. 2007;37:235–44. doi: 10.1016/j.pediatrneurol.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Emblem KE, Bjornerud A. An automatic procedure for normalization of cerebral blood volume maps in dynamic susceptibility contrast-based glioma imaging. AJNR Am J Neuroradiol. 2009;30:1929–32. doi: 10.3174/ajnr.A1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Melhem ER, Mori S, Eichler FS, Raymond GV, Moser HW. Diffusion tensor brain MR imaging in X-linked cerebral adrenoleukodystrophy. Neurology. 2001;56:544–7. doi: 10.1212/wnl.56.4.544. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Law M, Saindane AM, Ge Y, Babb JS, Johnson G, Mannon LJ, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231:645–52. doi: 10.1148/radiol.2313030996. [DOI] [PubMed] [Google Scholar]
- Lightman S, McDonald WI, Bird AC, Francis DA, Hoskins A, Batchelor JR, et al. Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain. 1987;110(Pt 2):405–14. doi: 10.1093/brain/110.2.405. [DOI] [PubMed] [Google Scholar]
- Loes DJ, Hite S, Moser H, Stillman AE, Shapiro E, Lockman L, et al. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol. 1994;15:1761–6. [PMC free article] [PubMed] [Google Scholar]
- Loes DJ, Fatemi A, Melhem ER, Gupte N, Bezman L, Moser HW, et al. Analysis of MRI patterns aids prediction of progression in X-linked adrenoleukodystrophy. Neurology. 2003;61:369–74. doi: 10.1212/01.wnl.0000079050.91337.83. [DOI] [PubMed] [Google Scholar]
- Melhem ER, Breiter SN, Ulug AM, Raymond GV, Moser HW. Improved tissue characterization in adrenoleukodystrophy using magnetization transfer imaging. AJR Am J Roentgenol. 1996;166:689–95. doi: 10.2214/ajr.166.3.8623652. [DOI] [PubMed] [Google Scholar]
- Melhem ER, Loes DJ, Georgiades CS, Raymond GV, Moser HW. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000;21:839–44. [PMC free article] [PubMed] [Google Scholar]
- Moser H, Smith K, Watkins P, Powers J, Moser A. X-linked adrenoleukodystrophy. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw Hill; 2001. pp. 3257–301. [Google Scholar]
- Moser HW, Loes DJ, Melhem ER, Raymond GV, Bezman L, Cox CS, et al. X-Linked adrenoleukodystrophy: overview and prognosis as a function of age and brain magnetic resonance imaging abnormality. A study involving 372 patients. Neuropediatrics. 2000;31:227–39. doi: 10.1055/s-2000-9236. [DOI] [PubMed] [Google Scholar]
- Putnam T. The pathogenesis of multiple sclerosis: a possible vascular factor. NEJM. 1933;209:786–90. [Google Scholar]
- Putnam T. Evidences of vascular occlusion in multiple sclerosis and encephalomyelitis. Arch Neurol Neuropsychol. 1935;32:1298–321. [Google Scholar]
- Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP., Jr Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975;32:577–91. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- van der Voorn JP, Pouwels PJ, Powers JM, Kamphorst W, Martin JJ, Troost D, et al. Correlating quantitative MR imaging with histopathology in X-linked adrenoleukodystrophy. AJNR Am J Neuroradiol. 2011;32:481–9. doi: 10.3174/ajnr.A2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel BM, Bezman L, Loes DJ, Moser HW, Raymond GV. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol. 2001;49:186–94. doi: 10.1002/1531-8249(20010201)49:2<186::aid-ana38>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wuerfel J, Bellmann-Strobl J, Brunecker P, Aktas O, McFarland H, Villringer A, et al. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain. 2004;127:111–9. doi: 10.1093/brain/awh007. [DOI] [PubMed] [Google Scholar]