Abstract
Amyotrophic lateral sclerosis is a devastating neurodegenerative disorder that is more prevalent in males than in females. A similar gender difference has been reported in some strains of transgenic mouse models of familial amyotrophic lateral sclerosis harbouring the G93A mutation in CuZn superoxide dismutase. Mitochondrial damage caused by pathological alterations in Ca2+ accumulation is frequently involved in neurodegenerative diseases, including CuZn superoxide dismutase-related amyotrophic lateral sclerosis, but its association with gender is not firmly established. In this study, we examined the effects of genetic ablation of cyclophilin D on gender differences in mice expressing G93A mutant CuZn superoxide dismutase. Cyclophilin D is a mitochondrial protein that promotes mitochondrial damage from accumulated Ca2+. As anticipated, we found that cyclophilin D ablation markedly increased Ca2+ retention in brain mitochondria of both males and females. Surprisingly, cyclophilin D ablation completely abolished the phenotypic advantage of G93A females, with no effect on disease in males. We also found that the 17β-oestradiol decreased Ca2+ retention in brain mitochondria, and that cyclophilin D ablation abolished this effect. Furthermore, 17β-oestradiol protected G93A cortical neurons and spinal cord motor neurons against glutamate toxicity, but the protection was lost in neurons lacking cyclophilin D. Taken together, these results identify a novel mechanism of oestrogen-mediated neuroprotection in CuZn superoxide dismutase-related amyotrophic lateral sclerosis, whereby Ca2+ overload and mitochondrial damage are prevented in a cyclophilin D-dependent manner. Such a protective mechanism may contribute to the lower incidence and later onset of amyotrophic lateral sclerosis, and perhaps other chronic neurodegenerative diseases, in females.
Keywords: amyotrophic lateral sclerosis, calcium, cyclophilin D, oestrogen, mitochondria, SOD1, transgenic mice
Introduction
Amyotrophic lateral sclerosis is a fatal disease characterized by progressive degeneration of upper and lower motor neurons (Rowland and Shneider, 2001). Epidemiological studies indicate that males are at a higher risk to develop amyotrophic lateral sclerosis than females, and that this gender difference declines among older patients (Haverkamp et al., 1995). Approximately 10% of amyotrophic lateral sclerosis cases are familial 20% of which are associated with dominantly inherited mutations in CuZn superoxide dismutase (SOD1) (Rosen et al., 1993), which cause a toxic gain of function of the protein (Gurney et al., 1994). Consistent with the gender difference observed in humans, female G93A mutant SOD1 transgenic mice have a slower disease course than males, while ovariectomy accelerates disease progression, an effect that can be reversed by chronic oestrogen treatment (Choi et al., 2008). This evidence suggests that oestrogen plays a protective role in amyotrophic lateral sclerosis.
A potential link between oestrogen and amyotrophic lateral sclerosis involves mitochondria and intracellular Ca2+ homeostasis. Mutant SOD1 causes mitochondrial dysfunction, resulting in abnormal Ca2+ handling (reviewed in Kawamata and Manfredi, 2010). Furthermore, in mitochondria from brain and spinal cord of G93A mutant SOD1 transgenic mice, Ca2+ capacity decreases before disease onset (Damiano et al., 2006), suggesting that abnormal Ca2+ homeostasis is a primary pathogenic event. Oestrogen regulates mitochondrial Ca2+ handling (Lobaton et al., 2005), and mitochondrial Ca2+ uptake is higher in male than in female rat heart mitochondria (Arieli et al., 2004), suggesting that lower mitochondrial Ca2+ uptake may be protective in females. Oestrogen also modulates the mitochondrial permeability transition pore (Moro et al., 2010), which follows excessive Ca2+ accumulation in the matrix (Bernardi, 1999). Inhibition of cyclophilin D (CypD), a mitochondrial matrix peptidylprolyl cis/trans isomerase, which translocates to the inner membrane and promotes mitochondrial permeability transition pore opening (Connern and Halestrap, 1994), is protective in certain disease models (Schinzel et al., 2005; Du et al., 2008; Thomas et al., 2012). It was reported that cyclophilin D knockout (CypDKO) extended the survival of G93A mice (Martin et al., 2009), but the effects of CypD deficiency on mitochondrial Ca2+ handling were not investigated.
Here, we show that genetic ablation of CypD (now known as Ppid in mice) abolishes the phenotypic advantage in female G93A mice. We determine that this effect is associated with a loss of gender difference in mitochondrial Ca2+ handling. CypDKO eliminated the oestrogen-mediated ability of mitochondria to be protected from Ca2+ overload, resulting in increased sensitivity to excitotoxicity. Based on these findings, we propose a novel mechanism of regulation, whereby oestrogen decreases mitochondrial Ca2+ uptake in a CypD-dependent manner, affording neuroprotection in amyotrophic lateral sclerosis, and perhaps other diseases, by preventing Ca2+ overload in mitochondria.
Materials and methods
Genetically modified mice
CypDKO mice have been previously described (Schinzel et al., 2005). Mice were backcrossed into the C57BL/6J background for eight generations. G93A mutant human SOD1 mice in a C57BL/6J genetic background, strain B6.Cg-Tg(SOD1-G93A)1Gur/J, were from Jackson Laboratories. Both mouse lines were bred at the Weill Medical College of Cornell University animal facility. G93A male mice were crossed with female knockouts (CypDKO) to generate CypDKO–G93A mice and controls, as outlined in Supplementary Fig. 1 (the procedure for mouse genotyping is described in the Supplementary material).
All experiments were approved by the Institutional Animal Care and Use Committee of the Weill Medical College of Cornell University and carried out in compliance with the National Institutes of Health guide for the care and use of laboratory animals.
Mouse disease phenotypes
Age of death was scored as the time when mice became unable to right themselves within 20 s after being placed on their side. Rotarod activity was assessed using an accelerating Rotarod every 2 weeks starting at 80 days of age. Mice were placed on the rod with an accelerating rotating speed (2 rpm/s), and the time that mice stayed on the rod until falling was recorded. To become acquainted with the apparatus (Columbus Instruments), mice were trained for 2 days. After training, mice were tested in three trials, and the best result was recorded. A time of 2 min was recorded as a normal score.
Muscle strength was assessed by wire hang test every 2 weeks, starting at 108 days of age. Following the placement of the mouse on top of a cage wire lid, the lid was gently waved in the air three times to make the mouse firmly grip, and then, turned upside down and held at 30 cm above the home cage for a maximum time of 2 min. Latency to fall was measured and averaged for three consecutive trials, with 5-min intervals between trials.
Isolation of brain and spinal cord mitochondria
All reagents were from Sigma, unless otherwise indicated. Isolation and purification of mouse brain and spinal cord mitochondria were performed as previously described (Damiano et al., 2006), with some modifications (Supplementary material).
Western blots
Brain mitochondria proteins were denatured in Laemmli buffer containing β-mercaptoethanol at 100°C for 8 min. Proteins were separated by SDS-PAGE on 15% Tris-acrylamide/bis-acrylamide gels then transferred to polyvinylidene fluoride membranes. The membranes were probed overnight with primary antibodies at 4°C. The primary antibodies used were sheep polyclonal anti-SOD1 (1:1000, Calbiochem), mouse monoclonal anti-CypD (1:1000, Mitoscience) and mouse monoclonal anti-Tim23 (1:1000, BD Transduction Laboratories). Membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (1:10 000) for 1 h, followed by detection of peroxidase substrate chemiluminescence (GE Healthcare).
Mitochondrial Ca2+ uptake and membrane potential
Mitochondrial Ca2+ uptake was estimated fluorometrically with the calcium-sensitive fluorescent dye Fura-6F. Mitochondrial membrane potential was estimated using safranin O. Both procedures were performed as described previously (Damiano et al., 2006), with modifications (Supplementary material).
Primary motor and cortical neuron cultures and glutamate exposure
Enriched motor neuron fraction from embryonic day 12.5 mouse embryo spinal cords and cortical neurons from embryonic day 18 mouse embryo cortices were prepared as described (Kim and Magrane, 2011; Magrane et al., 2012). Neurons were cultured for 2 weeks before use. Motor neurons were plated in a 48-well plate (10 000 cells/well) for 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and on glass cover-slips (10 000 cells/cover-slip) precoated with polyornithine–laminin for immunofluorescence staining. Cortical neurons were plated in a 96-well plate precoated with polyornithine and laminin at a density of 20 000 cells/well for MTT and lactate dehydrogenase assays. For glutamate toxicity assays, neurons were exposed to 100 µM glutamate for 24 h, after 1 h of pretreatment with or without 10 µM 17β-oestradiol or vehicle (ethanol). 17β-Oestradiol was maintained in the culture for the duration of glutamate exposure. For motor neurons, five embryos for each genotype were pooled, and the neurons plated in three wells for treatments and measurements of cell viability. For cortical neurons, nine individual embryos were used for each genotype, and the neurons plated in three wells for treatments and measurements of cell viability.
Cell viability determination
Neuronal viability was determined by MTT and lactate dehydrogenase assays. MTT (5 mg/ml in PBS) was added to culture medium in 48-well or 96-well plates at a final concentration of 0.5 mg/ml. The plates were incubated for 3 h, and then cells were solubilized in dimethyl sulphoxide. Absorbance was measured at 595 nm. Lactate dehydrogenase assay was conducted with the CytoTox 96® Non-Radioactive Cytotoxicity assay kit (Promega) according to the manufacturer’s protocol. Maximum lactate dehydrogenase release was determined by incubating cells with 0.8% Triton™ X-100.
Statistical analyses
Survival data were analysed by Kaplan–Meier survival curves by Prism 5.0 software (GraphPad Software). All other statistical comparisons among groups were performed by one-way ANOVA followed by Newman–Keuls post hoc test.
Results
Generation of cyclophilin D-deficient G93A mutant SOD1 mice
CypD-deficient G93A (CypDKO–G93A) mice were generated as illustrated in Supplementary Fig. 1. PCR of the CypD wild-type allele amplified a 250-bp band, while the KO allele yielded a 450-bp band (Fig. 1A, left). PCR also detected the presence or absence of a human SOD1 transgene-specific 236-bp DNA fragment (Fig. 1A, right) An interleukin 2-specific 324-bp PCR product was used as an internal positive control.
Figure 1.
Genetic and molecular characterization of CypDKO and CypDKO–G93A mice. (A) PCR banding pattern corresponding to wild-type (WT), heterozygote CypDKO (Het) and homozygote CypDKO (DKO) mice (left panel); PCR banding pattern of mice containing the human SOD1 G93A transgene and interleukin 2 gene (non-Tg) as internal positive control (right panel). (B) Immunoblots of purified brain mitochondria using anti-CypD and anti-SOD1 antibodies. CypD was undetectable in the mitochondria of CypDKO and CypDKO–G93A mice. Human transgenic SOD1 was detected in the mitochondria of G93A, CypDHet–G93A and CypDKO–G93A mice. A monoclonal antibody against the inner membrane protein Tim23 was used as a loading control for mitochondria. mSOD1 = mouse SOD1; hSOD1 = human SOD1.
By immunoblot, CypD was undetectable in brain mitochondria from CypDKO and CypDKO–G93A mice, while it was reduced by ∼50% in CypD heterozygotes compared with CypD wild-type mitochondria (Fig. 1B). The ablation of CypD did not affect the content of SOD1 in G93A brain mitochondria (Fig. 1B).
Cyclophilin D ablation abolishes the phenotypic advantage in female G93A mice
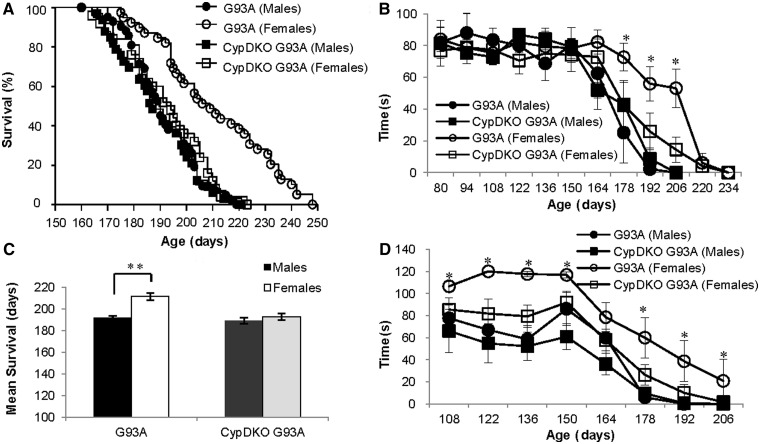
To investigate the effects of CypD ablation on disease, we compared lifespan, motor performance, muscle strength and body weight. Of note, the mean survival of both male and female G93A control mice (i.e. containing both CypD alleles, herein referred as G93A mice) obtained by crossing the pure C57BL/6J with the strain reported by Schinzel et al. (2005) was longer than that of G93A mice in the pure C57BL/6J genetic background (157 days). We determined that the cumulative survival percentage (Fig. 2A) and the mean survival (Fig. 2B) in female G93A mice were higher than that in males by ∼10% [211 ± 3.4 days, n = 40 (female G93A) versus 192 ± 2.0 days, n = 42 (male G93A); P < 0.01]. CypD ablation caused a decrease in the mean survival of female, but not male, G93A mice [193 ± 3.2 days, n = 25 (female CypDKO–G93A) versus 189 ± 2.6 days, n = 33 (male CypDKO–G93A)].
Figure 2.
CypD ablation accelerates disease in female G93A mice. (A) Kaplan–Meier survival curve and (B) mean survival of G93A (males: n = 42; females: n = 40) and CypDKO–G93A (males: n = 33; females: n = 25) mice. CypDKO significantly shortened survival of female G93A mice. (C) Rotarod performance expressed as seconds spent on the accelerating rod (2 rpm/s) for G93A (males: n = 5; females: n = 7) and CypDKO–G93A (males: n = 9; females: n = 10) mice. (D) Muscle strength in G93A (males: n = 5; females: n = 6) and CypDKO–G93A (males n = 9, females n = 9) mice expressed as hanging time in seconds. CypDKO accelerated motor performance impairment and muscle weakness in female G93A mice. Data are presented as mean ± SEM. **P < 0.01, *P < 0.05.
The onset of motor impairment, when a decline in Rotarod performance started, was at 150 days in male and 164 days in female G93A mice (Fig. 2C). Muscle strength, assessed by wire hang test, started to decline at 150 days in both male and female G93A mice (Fig. 2D). However, female G93A mice had a slower rate of motor decline than males. CypD ablation accelerated the decline in Rotarod performance and muscle strength in female G93A mice, thereby eliminating the gender difference in motor phenotype.
Body weight of G93A mice started to decline in both G93A and CypDKO–G93A mice at 164 days of age. There was no difference in the rate of decline in male (Supplementary Fig. 2A) and female (Supplementary Fig. 2B) G93A and CypDKO–G93A mice.
Cyclophilin D ablation abolishes the gender difference in mitochondrial Ca2+ uptake threshold and Ca2+-induced membrane potential decrease
To determine the effects of CypD ablation on mitochondrial Ca2+ capacity, we measured Fura-6F fluorescence after bolus Ca2+ additions to purified brain mitochondria at 140 days of age prior to the onset of disease symptoms. We expressed the Ca2+ uptake threshold as the amount of Ca2+ (nmol Ca2+/mg protein) at which mitochondria become incapable of completely taking up the Ca2+ added (examples of Ca2+ uptake measurements are in Supplementary Fig. 3). Ca2+ uptake thresholds in male and female G93A mitochondria were significantly lower, compared with non-transgenic mitochondria [Fig. 3A and B; 1351.2 ± 54.0 nmol Ca2+/mg (male non-transgenic) versus 1068.9 ± 28.6 (male G93A), P < 0.01; 1106.9 ± 32.6 (female non-transgenic) versus 872.5 ± 71 (female G93A), P < 0.01; n = 6 mice per group]. In both non-transgenic and G93A mice, females had significantly lower mitochondrial Ca2+ uptake than males (Fig. 3A and B, P < 0.01 in both groups). CypDKO and CypDKO–G93A mice mitochondria accumulated more Ca2+ than that of non-transgenic and G93A ones (Fig. 3A and B). CypDKO–G93A mice mitochondria had less Ca2+ uptake than CypDKO mice mitochondria, consistent with mitochondrial impairment caused by mutant SOD1. However, in CypDKO mice, the differences between mitochondria of males and females were abolished (male CypDKO: 2058.4 ± 58.9 nmol Ca2+/mg; female CypDKO: 1965.7 ± 63.3; male CypDKO–G93A: 1573.8 ± 87.0; female CypDKO–G93A: 1729.6 ± 51.0; n = 6 mice per group).
Figure 3.
CypD ablation abolishes gender differences in brain mitochondrial Ca2+ uptake and Ca2+-induced mitochondrial depolarization. (A) Kinetics of Ca2+ uptake in brain mitochondria measured by monitoring the change of Fura-6F fluorescence ratio (340/380 nm excitation, 510 nm emission) on Ca2+ loading (250 nmol of Ca2+/mg protein in each addition, indicated by arrows). The fluorescence peak corresponds to increase in extra-mitochonrial Ca2+, whereas the decrease in fluorescence reflects mitochondrial Ca2+ uptake. The sustained increase at the end of trace shows that mitochondria are unable to further accumulate Ca2+. In the example, control (WT) mitochondria took up six Ca2+ additions. However, G93A mice mitochondria only took five Ca2+ additions. CypDKO and CypDKO–G93A mice mitochondria took up significantly more Ca2+ than their WT and G93A counterparts, but CypDKO–G93A mice mitochondria took up less Ca2+ than CypDKO mice mitochondria. (B) Average brain mitochondrial Ca2+ uptake threshold in nmol Ca2+/mg of mitochondria protein (n = 6 mice per group). (C) ΔΨm response to 75 nmol Ca2+ load (750 nmol of Ca2+/mg protein) calculated as (ΔΨm 75 nmol Ca2+/ΔΨm-ini) × 100%. Mitochondria from males had higher Ca2+ uptake threshold and less ΔΨm sensitivity to Ca2+ loads than that from females, both in WT and G93A mice (n = 5 mice per group). CypDKO abolished the gender differences for both parameters. Error bars indicate mean ± SEM. *P < 0.01: statistically significant differences between male and female mice; #P < 0.01: statistically significant differences between non-transgenic and G93A mice; n.s = not significant.
Saturation of mitochondrial Ca2+ uptake is accompanied by a loss of mitochondrial membrane potential (ΔΨm) in brain mitochondria (Chalmers and Nicholls, 2003). Thus, we measured the sensitivity of ΔΨm to increasing concentrations of Ca2+ (Damiano et al., 2006). For comparison among the various groups, we used the change in ΔΨm after addition of 75 nmol Ca2+ (Supplementary Fig. 4). ΔΨm decrease in G93A mice brain mitochondria in response to 75 nmol Ca2+ was more pronounced than in non-transgenic mice mitochondria, in both males and females (Fig. 3C). In male non-transgenic mice mitochondria, average residual ΔΨm after Ca2+ loading ( ) was 81 ± 2.3% versus 65 ± 1.2% in G93A males (P < 0.01, n = 5 mice per group). In female non-transgenic mice mitochondria, it was 70 ± 1.5% versus 57 ± 1.3% in G93A female mice mitochondria (P < 0.01, n = 5 mice per group). ΔΨm was decreased more in mitochondria of females than of males, in both non-transgenic and G93A groups (P < 0.01). There were significantly less ΔΨm decreases in response to 75 nmol Ca2+ in CypDKO and CypDKO–G93A mice mitochondria. However, both male and female CypDKO–G93A mice mitochondria showed a more severe ΔΨm decline than CypDKO controls [94 ± 2.1% residual ΔΨm (male CypDKO) versus 82 ± 1.2% (male CypDKO–G93A); 92 ± 2.5% (female CypDKO) versus 85 ± 1.7% (female CypDKO–G93A); P < 0.01, n = 5 mice per group]. Consistent with the Ca2+ uptake results, CypD ablation abolished the gender difference in sensitivity of ΔΨm to Ca2+ load.
) was 81 ± 2.3% versus 65 ± 1.2% in G93A males (P < 0.01, n = 5 mice per group). In female non-transgenic mice mitochondria, it was 70 ± 1.5% versus 57 ± 1.3% in G93A female mice mitochondria (P < 0.01, n = 5 mice per group). ΔΨm was decreased more in mitochondria of females than of males, in both non-transgenic and G93A groups (P < 0.01). There were significantly less ΔΨm decreases in response to 75 nmol Ca2+ in CypDKO and CypDKO–G93A mice mitochondria. However, both male and female CypDKO–G93A mice mitochondria showed a more severe ΔΨm decline than CypDKO controls [94 ± 2.1% residual ΔΨm (male CypDKO) versus 82 ± 1.2% (male CypDKO–G93A); 92 ± 2.5% (female CypDKO) versus 85 ± 1.7% (female CypDKO–G93A); P < 0.01, n = 5 mice per group]. Consistent with the Ca2+ uptake results, CypD ablation abolished the gender difference in sensitivity of ΔΨm to Ca2+ load.
Since motor neuron degeneration in G93A mice affects the spinal cord more severely than the brain, we also investigated the Ca2+ capacity of mitochondria isolated from pools of spinal cords. Consistent with the result in brain mitochondria, spinal cord mitochondrial Ca2+ capacity was decreased in both non-transgenic and G93A females, but CypD ablation increased Ca2+ capacity in all groups and eliminated the gender differences (Fig. 4).
Figure 4.
Effect of CypD ablation on spinal cord mitochondrial Ca2+ uptake. Kinetics of Ca2+ uptake in spinal cord mitochondria assayed as in Fig. 3A. Experiments were performed on mitochondria isolated from a pool of three spinal cords for each genotype.
Taken together, these data demonstrate that female mitochondria take up less Ca2+ than male and depolarize faster in response to Ca2+ accumulation. This may represent a protective mechanism against Ca2+ overload, which is abolished by CypD ablation.
Oestrogen protects mitochondria against Ca2+-induced damage by regulating Ca2+ handling in a cyclophilin D-dependent manner
The gender differences in disease phenotype and neural mitochondrial Ca2+ handling suggest a role for oestrogen regulation. Mitochondrial Ca2+ uptake is modulated by 17β-oestradiol (Batra, 1973; Horvat et al., 2000). To assess the role of oestrogen in mitochondrial Ca2+ handling in G93A mice, brain mitochondria were treated with 10 µM 17β-oestradiol or its stereoisomer 17α-oestradiol, which has 200-fold less affinity for the oestrogen receptor. 17β-oestradiol decreased Ca2+ capacity in both male and female non-transgenic mice (male non-transgenic: 71 ± 3.8% of untreated; female non-transgenic mice: 75 ± 5.8% of untreated, n = 5; P <0.01; Fig. 5A) and of male G93A mice (74 ± 8% of untreated, n = 5; P < 0.01; Fig. 5B), but not of female G93A mice (101 ± 8.9% of untreated, n = 5; Fig. 5B). CypDKO completely abolished 17β-oestradiol regulation of Ca2+ handling in non-transgenic and G93A mice mitochondria, in both genders (male CypDKO: 95 ± 3.5% of untreated; female CypDKO: 95 ± 2.6% of untreated; n = 5; Fig. 5C; male CypDKO–G93A: 95 ± 6.5% of untreated; female CypDKO–G93A: 90 ± 7.5% of untreated; n = 5; Fig. 5D). These results were reproduced in spinal cord mitochondria (Supplementary Fig. 5). Mitochondrial Ca2+ uptake threshold was unaffected by 17α-oestradiol treatment (Supplementary Fig. 6A–D), indicating that the effect on mitochondrial Ca2+ handling of oestradiol is mediated through the oestrogen receptor.
Figure 5.
CypD ablation abolishes oestrogen effect on mitochondrial Ca2+ handling. Kinetics of Ca2+ uptake in brain mitochondria in 140-day-old mice was performed on purified brain mitochondria treated with 10 µM 17β-oestradiol (E2β), as in the experiment in Fig. 3A. 17β-Oestradiol was added to mitochondria for 100 s before Ca2+ loading. Averaged absolute Ca2+ uptake thresholds (nmol Ca2+/mg of protein) are shown (n = 5 mice for each genotype, using mitochondria preparation from independent animals; *P < 0.01).
To assess the gender-specific protection against mitochondrial damage induced by Ca2+ overload, we measured mitochondrial membrane swelling (Supplementary material). Non-transgenic and, more prominently, G93A male mitochondria swelled in response to Ca2+ load female mitochondria did not swell (Supplementary Fig. 7A). 17β-Oestradiol completely protected both non-transgenic and G93A male mice mitochondria from Ca2+-induced swelling (Supplementary Fig. 7B), indicating that oestrogen confers protection against Ca2+-induced damage.
Taken together, these results indicate that oestrogen decreases neural mitochondrial Ca2+ accumulation and protects against mitochondrial damage, in an oestrogen receptor- and CypD-dependent manner.
Cyclophilin D ablation abolishes oestrogen protection against glutamate toxicity in G93A neurons
17β-Oestradiol was shown to protect motor neurons expressing mutant SOD1 against the toxic effects of glutamate-induced intracellular Ca2+ rise (Kruman et al., 1999). To test the role of CypD in the mechanisms of oestrogen protection against neuronal Ca2+-mediated toxicity, we treated embryonic cortical neurons (embryonic Day 18) and spinal cord motor neurons (embryonic Day 12.5) from G93A or CypDKO–G93A mice with glutamate (100 µM) for 24 h, in the presence or absence of 17β-oestradiol (10 µM), administered 1 h before and throughout glutamate exposure.
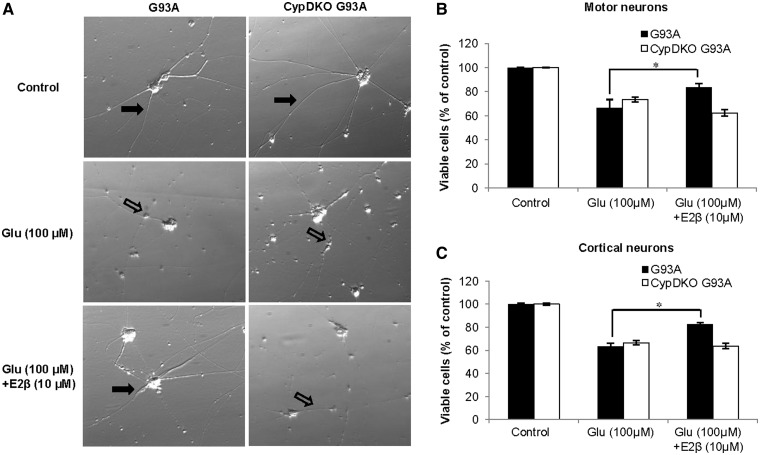
17β-Oestradiol protected G93A motor neurons against glutamate toxicity, but the protection was completely abolished in CypDKO–G93A neurons, as assessed by phase contrast microscopy (Fig. 6A) and by immunostaining with an SMI32 antibody, a motor neuron marker (as outlined in Supplementary material and Supplementary Fig. 8A). The treatment of neurons with glutamate (100 µM) induced significant loss of neuronal survival in both motor neurons (66 ± 7.1% of untreated; P < 0.01; Fig. 6B) and cortical neurons (63 ± 2.7% of untreated; P < 0.01; Fig. 6C), by the MTT assay. Treatment of neurons with 17β-oestradiol, administered before and during glutamate exposure, decreased glutamate-induced cell death (motor neurons: 84 ± 3.2% of untreated; cortical neurons: 83 ± 1.4% of untreated). However, CypDKO–G93A neurons treated with 17β-oestradiol and glutamate showed only 62 ± 2.3% (motor neuron) and 64 ± 2.4% (cortical neuron) survival (Fig. 6B and C). The loss of protection by 17β-oestradiol in CypDKO–G93A cortical neurons was confirmed with an independent cell death assay that measures lactate dehydrogenase release (Supplementary Fig. 8B).
Figure 6.
Effect of CypD ablation on glutamate toxicity in cultured spinal cord motor neurons and cortical neurons. Embryonic motor neurons (E12.5) and cortical neurons (E18) were cultured in vitro for 2 weeks. Where indicated, neurons were pretreated with 10 µM 17β-oestradiol (E2β) for 1 h, followed by 100 μM glutamate (Glu) exposure for 24 h in the presence of 17β-oestradiol. (A) Phase contrast images of spinal cord motor neurons. Viable neurons (filled arrows) had intact long neuritic processes, whereas dead or dying neurons (unfilled arrows) had shorter neuritis with beaded appearance. Cell viability was assessed using an MTT-based assay (cell survival) in motor neurons (B) and cortical neurons. (C) 17β-Oestradiol attenuated the loss of cell viability caused by glutamate in G93A neurons, but not in CypDKO–G93A neurons. Data are presented as mean ± SEM (n = 3 for motor neurons; n = 9 for cortical neurons, *P < 0.01).
These data support the hypothesis that CypD is necessary for oestrogen-mediated protection of mitochondria against Ca2+ toxicity induced by glutamate.
Discussion
Gender differences in amyotrophic lateral sclerosis
Gender is one of the risk factors in amyotrophic lateral sclerosis (Traynor et al., 1999). Males are almost twice as likely to be affected as females, but the difference is less significant when the disease onset is at an older age (Haverkamp et al., 1995; Chio et al., 2011), suggesting that declining oestrogen levels may result in loss of neuroprotection. Furthermore, females with amyotrophic lateral sclerosis have a history of earlier menopause than controls. Taken together these epidemiological observations imply oestrogen as a protective factor against amyotrophic lateral sclerosis (Chio et al., 1991). This protection has to be prolonged and start early in life, since studies in postmenopausal females did not show neuroprotective effects of oestrogen replacement on incidence or progression of amyotrophic lateral sclerosis (Rudnicki, 1999). The gender differences are well characterized in some of the G93A–SOD1 mouse lines. Female G93A mice have slower disease course and longer survival than males (Kirkinezos et al., 2003). Ovariectomy in female G93A mice leads to disease acceleration, which becomes identical to that of males (Groeneveld et al., 2004), while treatment with 17β-oestradiol delays disease progression of ovariectomized females (Groeneveld et al., 2004; Choi et al., 2008). These observations strongly suggest that oestrogen plays a role in modulating SOD1-familial amyotrophic lateral sclerosis outcomes.
In this study, we crossed G93A mice in the C57BL/6J background with the CypDKO mouse line described by Schinzel et al. (2005). There was no gender-specific phenotypic difference in the G93A mice in pure C57BL/6J background (Supplementary Fig. 9A–C), but on crossing with the CypDKO line, the average lifespan was increased in both genders, and females had later disease onset and longer survival (Fig. 2). This is in agreement with the observation that gender differences in the SOD1 mice depend on modifying genetic factors (Heiman-Patterson et al., 2005).
The effects of oestrogen on mitochondrial Ca2+ handling
Oestrogen can directly modulate mitochondrial function through the oestrogen receptor in the mitochondria (Yang et al., 2004). Consistently, we found that 17α-oestradiol, with low oestrogen receptor affinity, did not affect mitochondrial Ca2+ handling (Supplementary Fig. 6A–D), 17β-oestradiol affected Ca2+ handling almost instantaneously, indicating that it acts directly on the mitochondrial oestrogen receptor.
To address the contribution of mitochondrial Ca2+ handling and CypD to the phenotypic gender differences in G93A mice, we used a genetic approach to completely eliminate CypD. CypD ablation worsened the phenotype of female G93A mice, in contrast to a previous study, where CypD ablation increased lifespan in G93A mice more prominently in females than in males (Martin et al., 2009). Reasons for this discrepancy may include the mouse genetic background since in some lines, the gender differences are lost due to mutations of yet unidentified genes (Heiman-Patterson et al., 2005). In particular, the gender effects on mitochondrial Ca2+ handling were not investigated in that study.
In liver mitochondria, modulation of the Ca2+ uniporter is responsible for lower female Ca2+ uptake, independent of mitochondrial permeability transition pore (Arieli et al., 2004). Here, we show for the first time that both normal and SOD1 mutant female brain and spinal cord mitochondria have lower Ca2+ uptake than male mitochondria. Since ablation of CypD abolished the gender differences (Figs 3 and 4), we now propose that in the brain CypD plays a crucial role in the gender difference in mitochondrial Ca2+ handling.
The role of cyclophilin D in the oestrogen regulation of mitochondrial Ca2+ handling
CypD increased brain and spinal cord mitochondria Ca2+ capacity in non-transgenic and G93A mice, but CypDKO–G93A mitochondria still had less Ca2+ capacity than CypDKO. This is explained by the bioenergetic defects in G93A mitochondria (Jung et al., 2002; Mattiazzi et al., 2002). CypD ablation raises the Ca2+ threshold for mitochondrial permeability transition pore in both groups but does not eliminate the bioenergetic defects in G93A mitochondria. We propose that lower Ca2+ uptake in females is a defence mechanism that prevents Ca2+ overload in neural mitochondria, and that this mechanism depends on CypD, as it is lost on its ablation.
CypD may control a ‘low conductance’ permeability pore, allowing for regulated mitochondrial Ca2+ release (Novgorodov and Gudz, 1996). This putative Ca2+ release pathway is dependent on oestrogen and mitochondrial oestrogen receptor, in agreement with earlier observations in myometrium and heart mitochondria, where 10 µM 17β-oestradiol inhibited mitochondrial Ca2+ uptake (Batra, 1973) and increased Ca2+ efflux (Horvat et al., 2000). In brain, we showed that 17β-oestradiol decreased mitochondrial Ca2+ capacity (Fig. 5), in males more than females. The stronger effect of 17β-oestradiol in male mitochondria may be explained by the fact that female mitochondria are naturally exposed to high oestrogen concentration, which may already occupy the mitochondrial oestrogen receptor.
The interplay between oestrogen and cyclophilin D in mitochondrial neuroprotection
The novel neuroprotective mechanism that we propose in G93A female mice may apply to diverse disease conditions, where mitochondrial Ca2+ overload plays a neurotoxic role. A paradigmatic example is glutamate excitotoxicity, one of the potential mechanisms in amyotrophic lateral sclerosis pathogenesis (Rothstein, 1996). Excitotoxic glutamate causes massive cytosolic Ca2+ influx and excessive mitochondrial Ca2+ accumulation in neurons, followed by mitochondrial dysfunction and cell death. 17β-Oestradiol protected neurons from G93A mice against glutamate toxicity (Kruman et al., 1999) and excessive mitochondrial Ca2+ accumulation in cells exposed to H2O2 (Wang et al., 2006). However, consistent with our hypothesis, 17β-oestradiol failed to protect G93A neurons lacking CypD from glutamate toxicity (Fig. 6).
The neuroprotective effects of oestrogen could be in part mediated through decreased mitochondrial Ca2+ accumulation and dependent of the mitochondrial oestrogen receptor and CypD. This hypothesis assumes that oestrogen binds to oestrogen receptor in mitochondria and promotes a functional interaction with CypD. This interaction allows for the opening of a controlled Ca2+ release pathway that decreases net Ca2+ uptake, thereby protecting mitochondria against Ca2+ overload. This protection alleviates the stress on mitochondria that are burdened by bioenergetic defects caused by aberrant interactions of mutant SOD1 with mitochondrial protein, such as Bcl2 (Pedrini et al., 2010) and VDAC (Israelson et al., 2010), and by protein misfolding in the intermembrane space (Igoudjil et al., 2011). The interaction between CypD and oestrogen receptor could be direct or otherwise mediated by other protein complexes. Intriguingly, it was shown that both CypD (Giorgio et al., 2009; Chinopoulos et al., 2011) and oestrogen receptor (Alvarez-Delgado et al., 2010) bind to the mitochondrial ATPase complex, suggesting that ATPase could be a hub for oestrogen receptor–CypD interaction.
Conclusion
Additional studies are needed to elucidate the molecular characteristics of these interactions and potential requirements for other components of the regulatory pathway. Nevertheless, this pathway may become a target for neuroprotection in chronic neurodegenerative diseases and open new windows of opportunity to test pharmacological approaches.
Funding
The Robert Packard Center for ALS Research, the Muscular Dystrophy Association and the National Institutes of Health (RO1-NS051419 and RO1-NS062055 to G.M.).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CypD
cyclophilin D
- KO
knockout
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ΔΨm
mitochondrial membrane potential
References
- Alvarez-Delgado C, Mendoza-Rodriguez CA, Picazo O, Cerbon M. Different expression of alpha and beta mitochondrial estrogen receptors in the aging rat brain: interaction with respiratory complex V. Exp Gerontol. 2010;45:580–5. doi: 10.1016/j.exger.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol. 2004;37:507–13. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Batra SC. Effect of some estrogens and progesterone on calcium uptake and calcium release by myometrial mitochondria. Biochem Pharmacol. 1973;22:803–9. doi: 10.1016/0006-2952(73)90359-6. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–55. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–70. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Konrad C, Kiss G, Metelkin E, Torocsik B, Zhang SF, et al. Modulation of F0F1-ATP synthase activity by cyclophilin D regulates matrix adenine nucleotide levels. FEBS J. 2011;278:1112–25. doi: 10.1111/j.1742-4658.2011.08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A, Calvo A, Moglia C, Mazzini L, Mora G. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82:740–6. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- Chio A, Meineri P, Tribolo A, Schiffer D. Risk factors in motor neuron disease: a case-control study. Neuroepidemiology. 1991;10:174–84. doi: 10.1159/000110267. [DOI] [PubMed] [Google Scholar]
- Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268:40–7. doi: 10.1016/j.jns.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302(Pt 2):321–4. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, et al. Neural mitochondrial Ca2 + capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–61. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, et al. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284:33982–8. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld GJ, Van Muiswinkel FL, Sturkenboom JM, Wokke JH, Bar PR, Van Den Berg LH. Ovariectomy and 17beta-estradiol modulate disease progression of a mouse model of ALS. Brain Res. 2004;1021:128–31. doi: 10.1016/j.brainres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118(Pt 3):707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, et al. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Horvat A, Petrovic S, Nedeljkovic N, Martinovic JV, Nikezic G. Estradiol affect Na-dependent Ca2+ efflux from synaptosomal mitochondria. Gen Physiol Biophys. 2000;19:59–71. [PubMed] [Google Scholar]
- Igoudjil A, Magrane J, Fischer LR, Kim HJ, Hervias I, Dumont M, et al. In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space. J Neurosci. 2011;31:15826–37. doi: 10.1523/JNEUROSCI.1965-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelson A, Arbel N, Da Cruz S, Ilieva H, Yamanaka K, Shoshan-Barmatz V, et al. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 2010;67:575–87. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Higgins CMJ, Xu Z. Mitochondrial electron transport chain complex dysfunction in a transgenic mouse model for amyotrophic lateral sclerosis. J Neurochem. 2002;83:535–45. doi: 10.1046/j.1471-4159.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev. 2010;131:517–26. doi: 10.1016/j.mad.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Magrane J. Isolation and culture of neurons and astrocytes from the mouse brain cortex. Methods Mol Biol. 2011;793:63–75. doi: 10.1007/978-1-61779-328-8_4. [DOI] [PubMed] [Google Scholar]
- Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53:804–7. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- Lobaton CD, Vay L, Hernandez-Sanmiguel E, Santodomingo J, Moreno A, Montero M, et al. Modulation of mitochondrial Ca(2+) uptake by estrogen receptor agonists and antagonists. Br J Pharmacol. 2005;145:862–71. doi: 10.1038/sj.bjp.0706265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Sahawneh MA, Przedborski S, Estevez AG, Manfredi G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci. 2012;32:229–42. doi: 10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–46. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–33. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Hsieh JT, Ford J, Simpson ER, Hajibeigi A, et al. Aromatase deficiency inhibits the permeability transition in mouse liver mitochondria. Endocrinology. 2010;151:1643–52. doi: 10.1210/en.2009-1450. [DOI] [PubMed] [Google Scholar]
- Novgorodov SA, Gudz TI. Permeability transition pore of the inner mitochondrial membrane can operate in two open states with different selectivities. J Bioenerg Biomembr. 1996;28:139–46. doi: 10.1007/BF02110644. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH Jr, Naniche N, et al. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19:2974–86. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity hypothesis. Neurology. 1996;47(4 Suppl 2):S19–25. doi: 10.1212/wnl.47.4_suppl_2.19s. discussion S6. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Rudnicki SA. Estrogen replacement therapy in women with amyotrophic lateral sclerosis. J Neurol Sci. 1999;169:126–7. doi: 10.1016/s0022-510x(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Banerjee R, Starkova NN, Zhang SF, Calingasan NY, Yang L, et al. Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson's Disease. Antioxid Redox Signal. 2012;16:855–68. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Incidence and prevalence of ALS in Ireland, 1995-1997: a population-based study. Neurology. 1999;52:504–9. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, et al. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against H2O2 toxicity in human neuroblastoma SK-N-SH cells. Mol Pharmacol. 2006;70:395–404. doi: 10.1124/mol.106.022384. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101:4130–5. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.