Abstract
Mucolipidosis II is a neurometabolic lysosomal trafficking disorder of infancy caused by loss of mannose 6-phosphate targeting signals on lysosomal proteins, leading to lysosomal dysfunction and accumulation of non-degraded material. However, the identity of storage material and mechanisms of neurodegeneration in mucolipidosis II are unknown. We have generated ‘knock-in’ mice with a common mucolipidosis II patient mutation that show growth retardation, progressive brain atrophy, skeletal abnormalities, elevated lysosomal enzyme activities in serum, lysosomal storage in fibroblasts and brain and premature death, closely mimicking the mucolipidosis II disease in humans. The examination of affected mouse brains at different ages by immunohistochemistry, ultrastructural analysis, immunoblotting and mass spectrometric analyses of glycans and anionic lipids revealed that the expression and proteolytic processing of distinct lysosomal proteins such as α-l-fucosidase, β-hexosaminidase, α-mannosidase or Niemann–Pick C2 protein are more significantly impacted by the loss of mannose 6-phosphate residues than enzymes reaching lysosomes independently of this targeting mechanism. As a consequence, fucosylated N-glycans, GM2 and GM3 gangliosides, cholesterol and bis(monoacylglycero)phosphate accumulate progressively in the brain of mucolipidosis II mice. Prominent astrogliosis and the accumulation of organelles and storage material in focally swollen axons were observed in the cerebellum and were accompanied by a loss of Purkinje cells. Moreover, an increased neuronal level of the microtubule-associated protein 1 light chain 3 and the formation of p62-positive neuronal aggregates indicate an impairment of constitutive autophagy in the mucolipidosis II brain. Our findings demonstrate the essential role of mannose 6-phosphate for selected lysosomal proteins to maintain the capability for degradation of sequestered components in lysosomes and autophagolysosomes and prevent neurodegeneration. These lysosomal proteins might be a potential target for a valid therapeutic approach for mucolipidosis II disease.
Keywords: mucolipidosis, lysosomal storage disease, trafficking of lysosomal proteins, ganglioside, impaired autophagy
Introduction
Mucolipidosis type II (also known as I-cell disease) is a rare lysosomal storage disorder characterized by progressive psychomotor retardation, skeletal abnormalities, facial dysmorphism, cardiorespiratory defects and early death between 5 and 8 years of age (Leroy and Demars, 1967; Spranger and Wiedemann, 1970; Kornfeld and Sly, 2001). The disease results from defective intracellular targeting of multiple lysosomal enzymes (Kollmann et al., 2010). These acid hydrolases play a critical role in cellular and tissue homeostasis and function in degradation and recycling of macromolecules, including proteins, polysaccharides, lipids, nucleic acids, glyco- and phosphoconjugates, reaching the lysosomes through the endocytic or autophagic pathway (Cox and Cachon-Gonzalez, 2012).
The efficient transport of more than 50 newly synthesized acid hydrolases to lysosomes depends on the formation of mannose 6-phosphate residues on N-linked oligosaccharides of these proteins that are recognized by mannose 6-phosphate-specific receptors (Hickman and Neufeld, 1972; Kaplan et al., 1977; Sleat et al., 2006; Braulke and Bonifacino, 2009; Lübke et al., 2009). The first step in the synthesis of the mannose 6-phosphate recognition marker is catalyzed by a hexameric (α2β2γ2) N-acetylglucosamine (GlcNAc)-1-phosphotransferase complex encoded by the genes GNPTAB and GNPTG. GNPTAB encodes an enzymatically inactive precursor protein of 1256 amino acids that spans the membrane twice (Kudo et al., 2005; Tiede et al., 2005a) and is proteolytically cleaved by the site-1 protease to form catalytically active α- and β-subunits (Marschner et al., 2011). GNPTG encodes the functionally undefined soluble γ-subunits of the phosphotransferase complex (Raas-Rothschild et al., 2000).
Homozygous or compound heterozygous nonsense or frameshift mutations in GNPTAB leading to mucolipidosis II are characterized by the total loss of GlcNAc-1-phosphotransferase activity and of a mannose 6-phosphate recognition marker on lysosomal hydrolases, whereas missense mutations in GNPTAB are often associated with residual phosphotransferase activity causing a milder more slowly progressive disease, mucolipidosis III (Tiede et al., 2005b; Kudo et al., 2006; Cathey et al., 2010). The subsequent missorting and hypersecretion of lysosomal enzymes is detectable in sera of patients with mucolipidosis II, and the intracellular enzyme deficiency results in lysosomal dysfunction and accumulation of non-degraded material.
A few animal models of mucolipidosis II have been described that exhibit distinct biochemical symptoms and pathological features of the human disease. Domestic short-hair cats carrying a mutation in GNPTAB (c.2655C > T) have been discovered, exhibiting facial dysmorphism, dysostosis multiplex, behavioural dullness and ataxia as leading symptoms and early death. Fibroblasts show excessive missorting of multiple lysosomal hydrolases, which were elevated in the serum (Bosshard et al., 1996; Mazrier et al., 2003). A second model of mucolipidosis II, GNPTAB-depleted zebrafish embryos, exhibits skeletal abnormalities, craniofacial defects and impaired motility allowed studies during early developmental stages and revealed changes in timing and expression of chondrogenic factors associated with altered chondrocyte differentiation and intracellular matrix homeostasis (Flanagan-Steet et al., 2009; Petrey et al., 2012). Recently, a Gnptab gene trap mouse model was described displaying elevated levels of serum acid hydrolases, reduced size, retinal degeneration and vacuolization in secretory epithelial cells of exocrine glands (Gelfman et al., 2007; Vogel et al., 2009). The mice, however, exhibited a relatively normal lifespan and failed to develop severe skeletal abnormalities.
To explore the mechanism behind neurodegenerative processes in mucolipidosis II, we generated a Gnptab-defective mouse by single base insertion corresponding to a mutation detected in a patient with mucolipidosis II (Tiede et al., 2005a). These ‘knock-in’ mice are severely affected and show all of the clinical and biochemical symptoms and features of the mucolipidosis II disease in humans. The detailed analysis of storage material and lysosomal components in the brain led to the identification of a distinct group of lysosomal proteins such as α-l-fucosidase, β-hexosaminidase, α-mannosidase and Niemann–Pick C2 protein that require mannose 6-phosphate residues more than others to maintain lysosomal and autophagic functions and prevent neurodegeneration in mucolipidosis II.
Materials and methods
Antibodies and lectins
The following antibodies were used: anti-CD68 antibody (clone FA-11), AbD (Serotec); anti-p62 antibody (ENZO Life Sciences); glycerinaldehyde-3-phosphate dehydrogenase and beclin1 antibodies (Santa Cruz Biotechnology); anti-GM2 antibody (hydridoma clone 10-11; Progenics Pharmaceuticals); anti-GM3 antibody (clone DH2; GlycoTech); anti-β-tubulin (clone 9E10) and anti-Lamp1 (1D4B) antibody (Developmental Studies Hybridoma Bank); anti-microtubule-associated protein 1 light chain 3 antibody (LC3; clone 2G6), nanotools; anti-neuronal nuclei, anti-myelin binding protein and anti-manganese superoxide dismutase antibodies (Millipore); anti-cathepsin B antibody (Acris Antibodies); anti-glial fibrillary acidic protein (clone G-A-5), anti-calbindin (clone CB-955), anti-myc antibodies and filipin complex from Streptomyces filipinensis (F9765; Sigma-Aldrich). Biotinylated Lotus tetragonolobus and Aleuria aurantia lectins were purchased from Vector laboratories. Rabbit Niemann–Pick C2 protein anti-serum was a kind gift from Dr S. C. Patel (Ong et al., 2004). The polyclonal antibody against cathepsin D and the myc-tagged single-chain antibody fragment against mannose 6-phosphate residues have been described previously (Claussen et al., 1997; Müller-Loennies et al., 2010). Secondary antibodies and streptavidin coupled to Alexa® Fluor 488 and 546 or horseradish peroxidase were purchased from Invitrogen and Jackson ImmunoResearch.
Generation of Gnptabc.3082insC mice
Targeting vector construction and ‘knock-in’ strategy have been designed and performed by genOway (Supplementary Fig. 1). The generation and genotyping procedure of the Gnptabc.3082insC mice are described in Supplementary Fig. 2 and in the Supplementary methods. After deletion of the neomycin cassette by crossing with FLPe-expressing mice (Rodriguez et al., 2000), the resulting heterozygous Gnptabc.3082insC mice were inbred to yield homozygous Gnptabc.3082insC. Experiments were performed with mice in a mixed C57Bl/6–129/SvJ genetic background, always using littermates as controls. Mice were housed in a pathogen-free animal facility at the University Medical Center, Hamburg-Eppendorf, and experimental procedures were performed according to the institutional guidelines.
Immunohistochemistry and ultrastructural analysis
Methods for tissue fixation and subsequent immunohistochemistry, including lectin staining and filipin labelling, were performed as described previously (Micsenyi et al., 2009; Müller-Loennies et al., 2010; Damme et al., 2011). Ultrastructural analyses were conducted as described previously (Weinert et al., 2010).
Analysis of lipids and oligosaccharides
Oligosaccharides from brain tissue were extracted as described previously (Roces et al., 2004), identified by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and gas chromatography–mass spectrometry linkage analysis (Morelle et al., 2005a, b) and quantified by high-performance liquid chromatography (Blanz et al., 2008).
Total lipids were extracted and separated into a neutral and an anionic fraction by reversed-phase C18 columns (Varian), as described previously (Jabs et al., 2008). Aliquots of the neutral and anionic lipid fractions were analysed by electrospray ionization–mass spectrometry also using tandem mass spectrometry and lipid class-specific detection modes (Käkelä et al., 2003; Jabs et al., 2008).
Further methods
The enzymatic activity of lysosomal enzymes was assayed by estimation of 4-nitrophenol, 4-nitrocatechol, 4-methylumbelliferone or 7-amino-4-methylcoumarin liberated from the hydrolase-specific substrates. Details about the substrates, the standard assay mixture, incubation times and preparation of protein extracts are described in the Supplementary material. Real-time PCR and western blot analysis were described previously (Müller-Loennies et al., 2010; Marschner et al., 2011). Details about preparation of detergent-insoluble protein fractions are described in the Supplementary material.
Statistical analysis
Data were analysed by two-tailed Student t-test. For all graphs, data are represented as mean ± standard deviation.
Results
Generation of Gnptabc.3082insC mice and functional analysis of Gnptab gene inactivation
To explore the pathogenetic course and mechanisms of neurodegeneration in mucolipidosis II, we generated ‘knock-in’ mice in which the patient mutation GNPTAB c.3145insC (Tiede et al., 2005a) was introduced into the highly homologous exon 16 of the orthologous Gnptab gene (Supplementary Figs 2, 3A and B). The insertion of a cytosine at position c.3082 interrupts the open reading frame of the Gnptab gene and changes the C-terminal conserved sequence distal to the α/β subunit precursor cleavage site, leading to a truncated GlcNAc-1-phosphotransferase protein (p.G1028RfsX16; Supplementary Fig. 3C). Western blotting of human embryonic kidney cells transfected with complementary DNA encoding wild-type and mutant α/β-subunit precursor constructs of human GlcNAc-1-phosphotransferase showed that the expression of mutant truncated α/β-subunit precursor is reduced and exhibits a slightly reduced electrophoretic mobility compared with the mature wild-type α-subunit (Supplementary Fig. 4). The data indicate that the mutant α/β-subunit precursor is not cleaved to the mature α-subunit and cannot exit the endoplasmic reticulum (Kudo and Canfield, 2006; Kollmann et al., 2010).
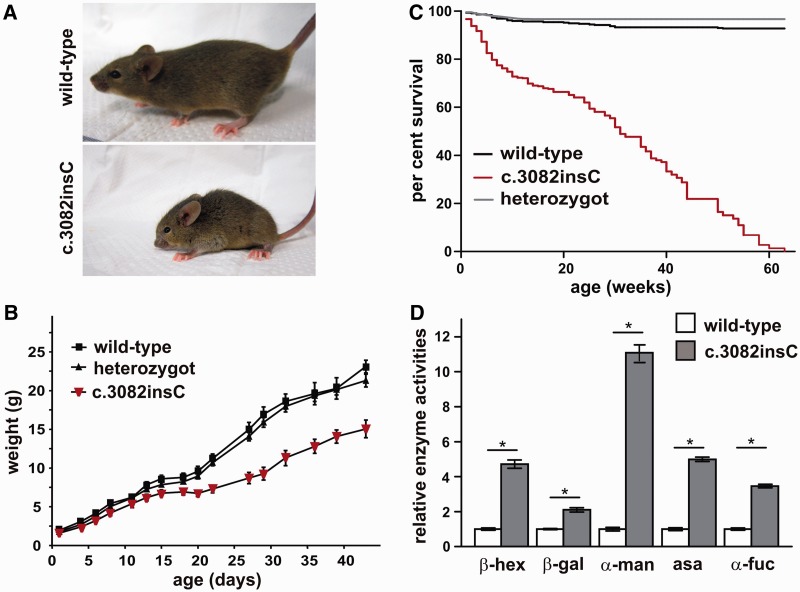
Heterozygous crossings revealed a reduced frequency of 13% homozygous mutant mice. Mating of homozygously affected mice was successful in 10% of inter-crossings, suggesting a high infertility rate. The mutant mice were considerably smaller in size compared with wild-type siblings, and showed back deformities, additional prominent skeletal abnormalities and a flat facial profile (Fig. 1A). Growth was impaired during development, accompanied by significantly lower weight gain (Fig. 1B). Heterozygous animals did not show differences in morbidity and fertility. Compared with unaffected littermates, Gnptabc.3082insC mice displayed an increased mortality rate with a lifespan of ∼64 weeks (Fig. 1C). Gnptabc.3082insC mice developed a progressive abnormality of gait and showed limb-clasping reflexes at 6 months. Early-onset photoreceptor cell degeneration in Gnptabc.3082insC mice was observed by 3–4 months of age (Supplementary Fig. 5).
Figure 1.
Phenotype of Gnptabc.3082insC mice. (A) One-month-old Gnptabc.3082insC mice show reduced body size and skeletal abnormalities like facial dysmorphism and curvature of the spine. (B) Body weight progression of Gnptabc.3082insC mice is reduced compared with wild-type and heterozygous littermates (n = 8, mean ± SD). (C) Kaplan–Meier analysis of male and female wild-type, heterozygous and homozygous Gnptabc.3082insC mice (0–20 weeks: n = 160, 20–40 weeks: n = 70, 40–60 weeks: n = 25). (D) The relative enzyme activities of the lysosomal hydrolases β-hexosaminidase (β-hex), β-galactosidase (β-gal), α-mannosidase (α-man), α-l-fucosidase (α-fuc) and arylsulphatase A (asa) were measured in sera of 6-month-old Gnptabc.3082insC mice. The specific activities of the wild-type were set to 1 (mean ± SD, n = 3, *P < 0.05).
The clinical diagnosis of human patients with mucolipidosis II is confirmed by (i) elevated lysosomal enzyme activities in serum; (ii) loss of GlcNAc-1-phosphotransferase activity leading to the loss of the mannose 6-phosphate targeting signal; (iii) decreased lysosomal enzyme activities in cultured fibroblasts accompanied by elevated enzyme level in the culture medium; and (iv) light microscopic presentation of prominent inclusions in patient fibroblasts (Kornfeld and Sly, 2001). In serum of Gnptabc.3082insC mice, activities of the lysosomal hydrolases β-hexosaminidase, β-galactosidase, α-mannosidase, arylsulphatase A and α-l-fucosidase were increased 2- to 11-fold compared with wild-type controls (Fig. 1D). Western blot analysis using single-chain anti-mannose 6-phosphate antibody fragments (Müller-Loennies et al., 2010) revealed the total loss of mannose 6-phosphate-containing proteins in cultured Gnptabc.3082insC mouse embryonic fibroblasts. These fibroblasts exhibited characteristic cytoplasmic inclusion bodies (Supplementary Fig. 6A and B). The activities of several lysosomal glycosidases were found to be reduced by 50–95% in Gnptabc.3082insC fibroblasts and increased in the supernatant 3- to 18-fold. In contrast, the activities of the lysosomal proteinases cathepsin B and D were not altered in Gnptabc.3082insC compared with wild-type fibroblasts (Supplementary Fig. 6C). No alterations in the messenger RNA expression of lysosomal proteins were detected between Gnptabc.3082insC and wild-type fibroblasts (Supplementary Fig. 6C). For comparison, the transcript level of Gnptab was reduced in Gnptabc.3082insC mice by 75% compared with wild-type mice, whereas the expression of Gnptg encoding the γ-subunit of the GlcNAc-1-phosphotransferase was not changed (Supplementary Fig. 6C). In addition, the steady-state expression of cathepsin D and the lysosomal mannose 6-phosphate-containing Niemann–Pick C2 protein required for the lysosomal export of cholesterol (Deffieu and Pfeffer, 2011), was examined by western blotting. The major 40-kDa cathepsin D immunoreactive band in cell extracts represents the precursor polypeptide, which is proteolytically processed, generating a 39-kDa intermediate and a 27-kDa mature form. In Gnptabc.3082insC fibroblasts, an increased amount of the complex oligosaccharide-decorated precursor form of cathepsin D is secreted into the medium within 24 h (Supplementary Fig. 6A). Pulse-chase experiments indicate that the rate of cathepsin D synthesis is similar in Gnptabc.3082insC fibroblasts compared with wild-type fibroblasts (Supplementary Fig. 6E, lanes 1 and 3), whereas 5 h after synthesis, the amount of cathepsin D retained intracellularly was reduced owing to higher secretion into the medium (Supplementary Fig. 6E, lanes 2 and 4–6). Furthermore, the proteolytic processing of the intracellularly retained cathepsin D precursor form is altered (Supplementary Fig. 6E, lanes 2 and 4). The expression of Niemann–Pick C2 protein is strongly reduced in Gnptabc.3082insC fibroblasts in comparison with wild-type fibroblasts and missorted into the medium (Supplementary Fig. 6A). The data indicate variations in the extent of mannose 6-phosphate-dependent sorting of different lysosomal proteins and the existence of alternative protein-specific mannose 6-phosphate-independent transport routes to lysosomes. Few studies report on activities of lysosomal enzymes in human mucolipidosis II liver autopsy material showing normal or increased values (Tondeur et al., 1971; Owada and Neufeld, 1982; Waheed et al., 1982). In the liver homogenates of Gnptabc.3082insC mice, lysosomal enzyme activities, except cathepsin B, were significantly increased compared with wild-type liver tissue (Supplementary Fig. 7A), whereas their messenger RNA levels were unchanged (Supplementary Fig. 7B). Taken together, these data clearly demonstrate that fibroblasts and liver of Gnptabc.3082insC mice exhibit the characteristic biochemical phenotype observed in patients with mucolipidosis II.
Expression of lysosomal enzymes in the brain
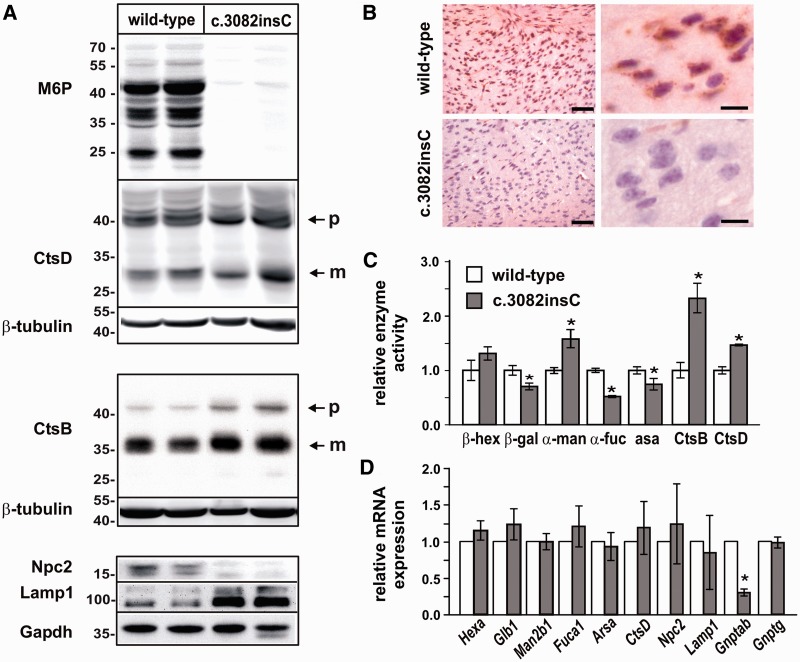
As shown for fibroblasts, no mannose 6-phosphate-containing proteins are detectable in the brain of Gnptabc.3082insC mice (Fig. 2A and B). Western blot analysis and densitometric evaluation revealed that the lysosomal proteases cathepsin B and cathepsin D are expressed to a similar extent (1.1- to 1.6-fold of wild-type controls) and exhibit the same proteolytic pattern in whole-brain extracts of 4-month-old wild-type and Gnptabc.3082insC mice (Fig. 2A). In contrast, Niemann–Pick C2 protein was not detectable in Gnptabc.3082insC brain tissue (Fig. 2A). Furthermore, 6.5-fold higher amounts of the lysosomal-associated membrane protein Lamp1 were found in brain extracts of Gnptabc.3082insC mice after densitometric evaluation of western blots, which is an indication of an increased number and/or size of lysosomes (Karageorgos et al., 1997). When the activities of other lysosomal enzymes were measured in homogenates of perfused whole brains of 4-month-old mice, significant increased activities of α-mannosidase (1.6-fold), cathepsin D (1.5-fold) and cathepsin B (2.3-fold) were found in Gnptabc.3082insC mice, whereas mean β-hexosaminidase activity was only slightly (1.3-fold) increased (Fig. 2C). The activities of β-galactosidase, α-l-fucosidase and arylsulphatase A were significantly reduced by 25, 50 and 25%, respectively, in Gnptabc.3082insC brain (Fig. 2C). To analyse whether altered messenger RNA expression levels contribute to changes in protein expression or enzyme activities of lysosomal proteins, quantitative real-time PCR measurements were performed on the brains of 4-month-old wild-type and Gnptabc.3082insC mice. Similar to fibroblasts or liver tissue, neither the messenger RNA expression of lysosomal enzymes nor Gnptg was significantly affected, whereas the transcript level of Gnptab was reduced in Gnptabc.3082insC mice by 75% compared with wild-type mice (Fig. 2D).
Figure 2.
Expression of lysosomal enzymes in brain of Gnptabc.3082insC mice. (A) Brain homogenates from 4-month-old wild-type and Gnptabc.3082insC mice were analysed by western blotting using antibodies against mannose 6-phosphate residues (M6P), cathepsin D (CtsD), cathepsin B (CtsB), Npc2 and Lamp1. β-Tubulin and Gapdh were used as loading controls. p = precursor; m = mature form. (B) Immunodetection of M6P residues on lysosomal proteins demonstrate lack of M6P in cortical brain sections of 1-month-old Gnptabc.3082insC mice. Scale bars = 50 µm (left panel), 10 µm (right panel). (C) The relative enzyme activities of the lysosomal hydrolases β-hexosaminidase (β-hex), β-galactosidase (β-gal), α-mannosidase (α-man), α-l-fucosidase (α-fuc), arylsulphatase A (asa), cathepsin B (CtsB) and cathepsin D (CtsD) were measured in homogenates of perfused wild-type and Gnptabc.3082insC whole-brain tissue of 4-month-old mice. The specific activities of the wild-type were set to 1 (mean ± SD, n = 3, *P < 0.05). (D) The relative messenger RNA level of the lysosomal hydrolases β-hexosaminidase (Hexa), β-galactosidase (Glb1), α-mannosidase (Man2b1), α-l-fucosidase (Fuca1) and arylsulphatase A (Arsa), cathepsin D (Ctsd), Npc2, Lamp1 and the phosphotransferase subunits encoding genes Gnptab and Gnptg were determined by real-time PCR and normalized to β-actin messenger RNA expression (mean ± SD, n = 3, *P < 0.05).
Progressive neurodegeneration in Gnptabc.3082insC mice
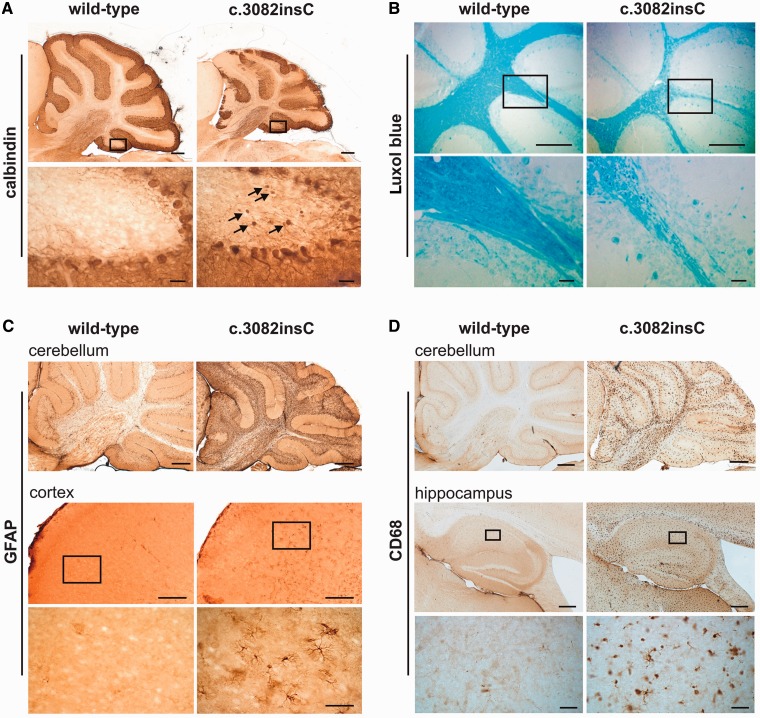
Macroscopic inspection of 4-month-old Gnptabc.3082insC mouse brain revealed a significant reduction in size of the cerebellum, with a reduced granular cell layer, compared with wild-type siblings (Supplementary Fig. 8A and B). Immunohistochemical staining for calbindin showed a substantial loss of Purkinje cells in 9-month-old Gnptabc.3082insC mice (Fig. 3A, top). Purkinje cells often exhibited severe axonal spheroid formation (Fig. 3A, arrows), a characteristic feature in several lysosomal storage diseases (Walkley et al., 2010). The loss of neuronal cells and atrophy of brain tissue were progressive (Supplementary Fig. 8C) and accompanied by cerebellar demyelination with considerably thinned white matter tracts (Fig. 3B). Strong glial fibrillary acidic protein immunoreactivity was observed throughout the entire brain of Gnptabc.3082insC mice with prominent astrogliosis of the cerebellum and cerebral cortex (Fig. 3C). In the cerebellum, astrogliosis was obvious in the white matter, granular and Purkinje cell layer. Hypertrophy of the Bergmann glia was most pronounced in regions of Purkinje cell loss (Fig. 3C). Microglia activation visualized by CD68 immunoreactivity was also detected in all brain regions (Fig. 3D), with the highest intensity found in the cerebellum, cerebral cortex, hippocampus and the thalamus. Microgliosis in the cerebellum was most accented in white matter tracts and the molecular cell layer evident as early as 10 weeks of age (data not shown).
Figure 3.
Pathological alterations in the brain of 9-month-old Gnptabc.3082insC mice. (A) Purkinje cells in cerebellar sections were stained for calbindin. Higher-magnification images of the regions marked by the black rectangles revealed axonal spheroids of Purkinje cells (arrows) in Gnptabc.3082insC mice. Scale bars: upper panel = 500 µm; insets = 50 µm. (B) Luxol fast blue staining of the cerebellar sections indicates decreased myelination and degeneration of the subcortical white matter in the Gnptabc.3082insC mouse. Scale bars: upper panel = 500 µm; insets = 50 µm. (C) Strong immunoreactivity for glial fibrillary acidic protein (GFAP) throughout the Gnptabc.3082insC brain. Insets represent higher-magnification images of the cortical region marked by the black rectangles. Scale bars: cerebellum = 500 µm; cortex = 250 µm; insets = 50 µm. (D) Activated microglial cells immunoreactive for CD68 are observed in the cerebellum and the hippocampus of Gnptabc.3082insC mice. Insets reveal the activated phagocytic shape of the microglial cells. Scale bars: cerebellum = 500 µm; hippocampus = 500 µm; insets = 50 µm.
Ultrastructural analysis of the Gnptabc.3082insC mouse brain
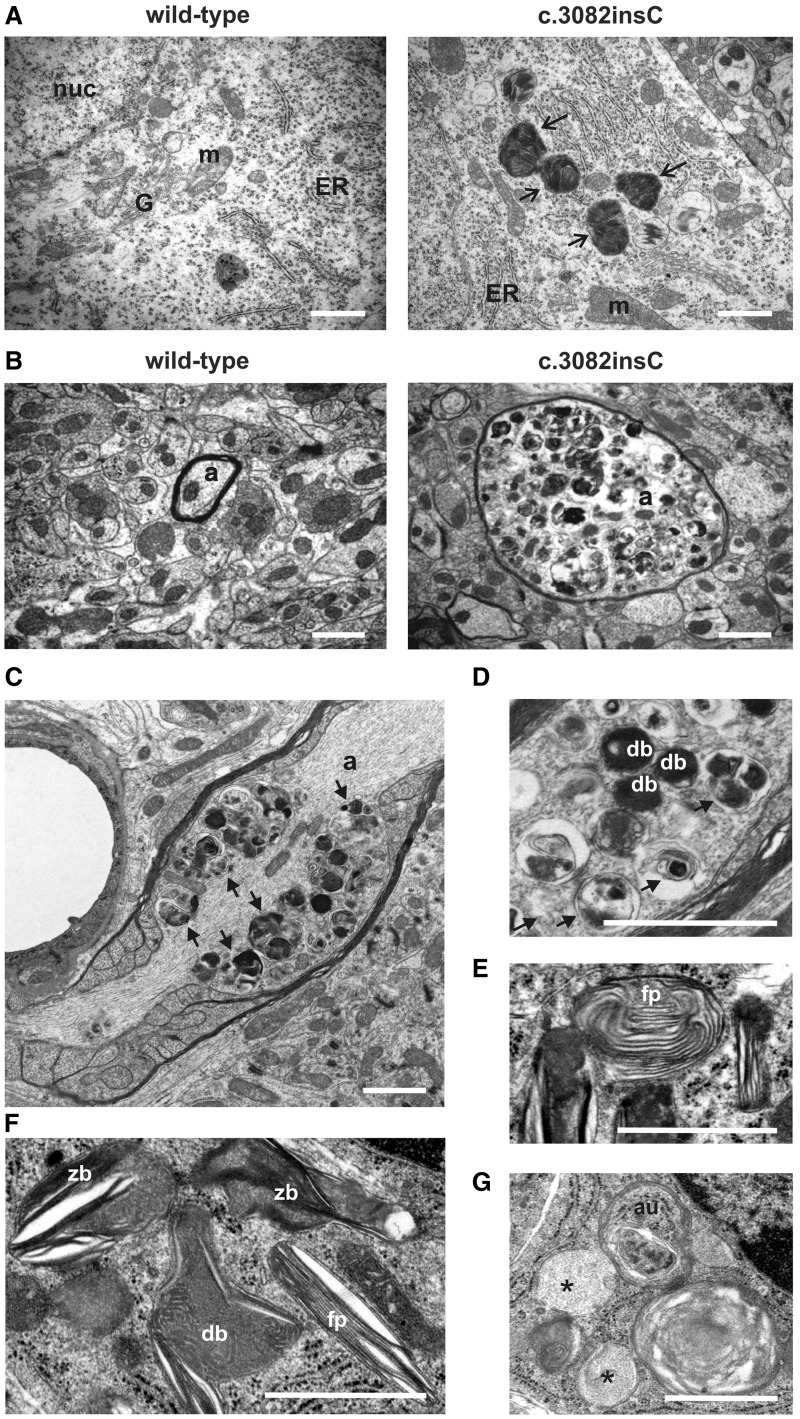
Transmission electron microscopy was carried out to investigate the identity of lysosomal storage material accumulating in the brain of Gnptabc.3082insC mice. Abnormally increased amounts of dense bodies were found to accumulate in the majority of cortical, hippocampal and cerebellar neurons of 4-month-old Gnptabc.3082insC mice (Fig. 4A). Numerous Gnptabc.3082insC neurons exhibited swollen axons (spheroids) filled with heterogeneously appearing membrane-bound storage vacuoles (Fig. 4B–D), ranging from electron-dense to electron-lucent structures. Higher-magnification images of storage material revealed a mixture of dense and multi-lamellar bodies, electron-lucent floccular bodies, zebra bodies and fingerprint-like storage material, characteristic for accumulation of lipofuscin, glycans or glycogen and gangliosides (Fig. 4D–G). In addition, autophagosomes were found (Fig. 4G).
Figure 4.
Ultrastructural analysis of storage material in Gnptabc.3082insC brain. (A) Large electron-dense inclusions (arrows) are found in the somata of hippocampal neurons from 4-month-old Gnptabc.3082insC mice. (B) Cross-sections of myelinated axons (a) of the cerebella from wild-type and Gnptabc.3082insC mice revealed swollen axons filled with pleomorphic granular and membranous dense bodies in 4-month-old Gnptabc.3082insC mice. (C and D) In longitudinal sections of myelinated axons (a) from 11-month-old Gnptabc.3082insC mice, the multi-vesicular accumulations (black arrows) and dense bodies (db) were clustered near the node of Ranvier. (E and F) The storage material in the cerebellar granule cells of 11-month-old Gnptabc.3082insC mice forms fingerprint bodies (fp), zebra bodies (zb) and dense bodies (db). (G) Vesicular structures with floccular storage material (black asterisks), and autophagosomes (au) are also found in granule cells. nuc = nucleus; m = mitochodrium; G = Golgi apparatus; ER = endoplasmatic reticulum; a = axon. Scale bars = 1 µm.
Accumulation of fucosylated N-glycans, gangliosides and bis(monoacylglycero) phosphate in the brain of Gnptabc.3082insC mice
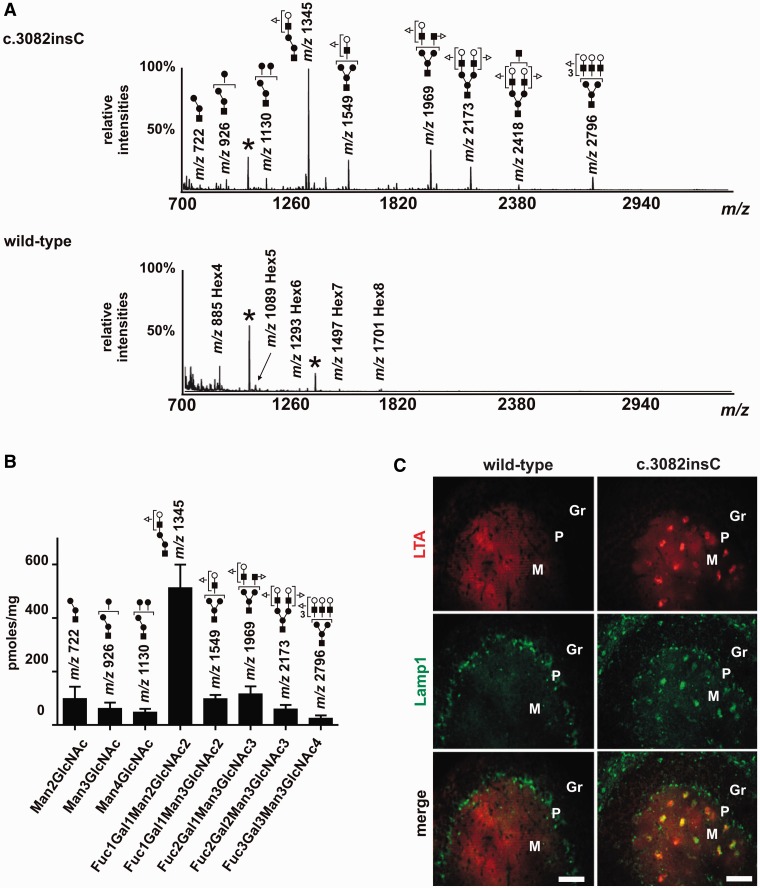
Analysis and identification of storage material allow conclusions on lysosomal enzymes responsible for metabolic defects in lysosomal disorders. Therefore, oligosaccharide species extracted from brain of 6-month-old wild-type and Gnptabc.3082insC mice were identified by MALDI-TOF mass spectrometry. Data presented in Fig. 5A and Supplementary Fig. 9 clearly indicate that in wild-type brain, glycogen (Hex4 to Hex8) is the only detectable glycan species. In Gnptabc.3082insC brain, however, the accumulation of high mannose structures (Hex2-8HexNAc) with variable proportions of less abundant bi- and triantennary structures was observed. In addition, fucosylated branched complex-type glycans were detected in Gnptabc.3082insC mouse brain dominated by the Fuc1Gal1Man2GlcNAc2 (m/z 1345) structure. Quantitative evaluation estimated ∼500 pmol of Fuc1Gal1Man2GlcNAc2/mg brain tissue (Fig. 5B). Using L. tetragonolobus lectin, the accumulation of fucose-containing glycostructures was confirmed on cerebellar brain sections of 10-month-old Gnptabc.3082insC mice (Fig. 5C) showing a co-localization with the lysosomal marker protein Lamp1 in particular in the molecular layer. The data are in agreement with a currently accepted model of lysosomal N-glycan degradation that after release of N-glycans from peptide moiety by β-endo-N-acetylglucosaminidase, hydrolysis of terminal fucose residues by acid α-l-fucosidase initiates the stepwise cleavage of complex-type glycans (Winchester, 2005). Therefore, the loss or reduced level of α-l-fucosidase and α-mannosidase in lysosomes may contribute to the accumulation of fucosylated branched complex-type glycans and high mannose-type oligosaccharides in the brain of Gnptabc.3082insC mice.
Figure 5.
Analysis of the accumulating free sugar species in Gnptabc.3082insC brain. (A) Mass spectrometric identification of sugar species from brain extracts of 6-month-old Gnptabc.3082insC mice. Glycans were permethylated and subjected to Sep-Pak clean-up. Permethylated derivates were then analysed by MALDI-TOF mass spectrometry in the positive ion reflective mode as [M + Na]+. Only the structures of the major glycans are given. In wild-type mice, glycogen (Hex4 to Hex8) is the only detectable sugar species. Black asterisks indicate contaminants from the MALDI-TOF 2,5-dihydroxybenzoic acid solution. The monosaccharides of the detected glycans are symbolically represented as follows: fucose (triangle), galactose (white circle), mannose (black circle) and N-acetylglucosamine (black square). (B) Quantification of 2-aminobenzamide-labelled oligosaccharides by high-performance liquid chromatography. (C) Fucosylated N-glycans were detected with the L. tetragonolobus lectin (LTA, red) by lectin fluorescence microscopy in cerebellar sections of 10-month-old Gnptabc.3082insC mice. Lysosomal membranes were stained for Lamp1 (green). M = molecular layer; P = Purkinje cell layer; Gr = granule cell layer. Scale bars = 50 µm.
These data are supported by A. aurantia lectin staining of fucose-containing glycostructures in lysosomes of cultured embryonic Gnptabc.3082insC fibroblasts. Strong reactivity of A. aurantia lectin was observed co-localizing with Lamp1 (Supplementary Fig. 10). In wild-type embryonic fibroblasts, a weak A. aurantia lectin staining in Lamp1-positive structures was detectable.
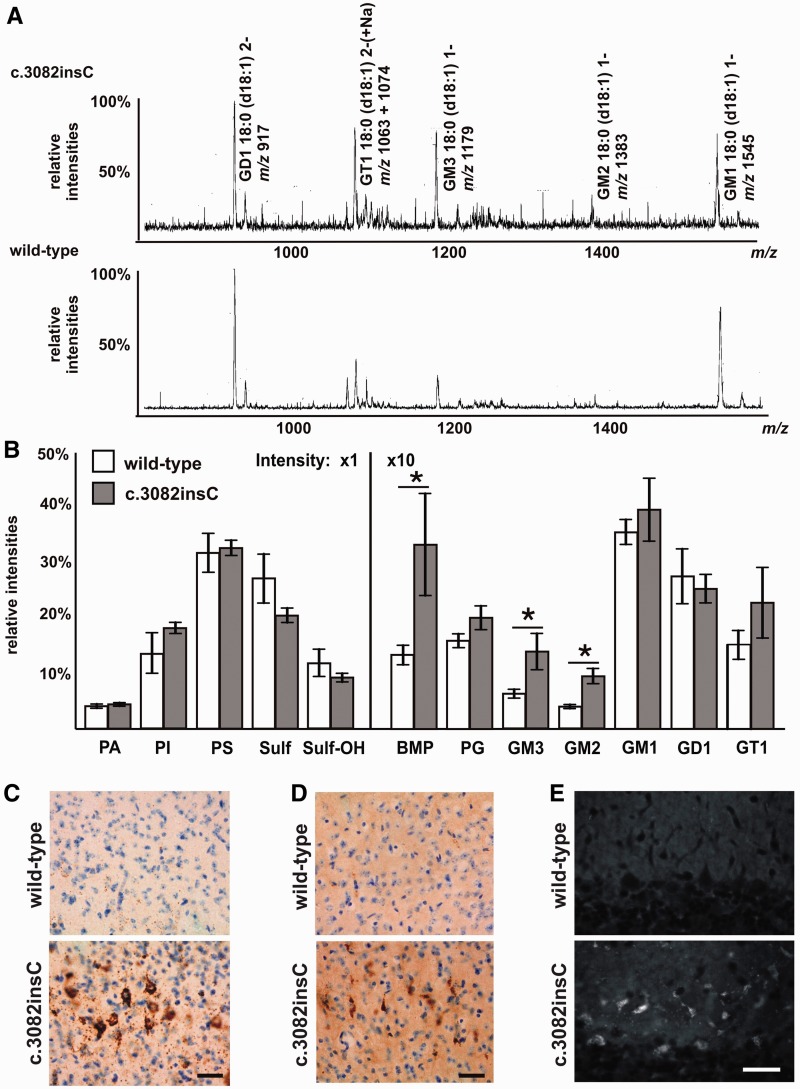
In a second approach, anionic lipids were prepared from total lipid extracts of 5- and 10-month-old mice and analysed by electrospray ionization mass spectrometry. The gangliosides were detected as precursors of m/z 290. All major ganglioside species had an 18:0 acyl and a sphingosine (d18:1) backbone. Multiple minor cationized species were present, especially for GT1. In wild-type mice, the monosialogangliosides GM2 and GM3 were barely detectable but were 1.8-fold increased in 5-month-old Gnptabc.3082insC mice (Fig. 6A and Supplementary Table 1). In addition, 1.3–1.9-fold increased concentrations of bis(monoacylglycero)phosphate (BMP) were found in Gnptabc.3082insC mice compared with wild-type mice. The most abundant BMP species was dipolyunsaturated 44:12 (22:6 n-3/22:6 n-3), constituting two-third of this lipid class (Supplementary Table 1). At the age of 10 months, the contents of GM2, GM3 and BMP increased further in Gnptabc.3082insC brain to approximately 2.3-fold higher levels than those in wild-type mice (Fig. 6B and Supplementary Table 1). The electrospray ionization mass spectrometry analyses of the neutral glycerolipids showed no significant differences between the wild-type and Gnptabc.3082insC mice.
Figure 6.
Analysis of the lipid profile stored in Gnptabc.3082insC. (A) The ganglioside-containing anionic lipid fractions isolated from brain of 5-month-old Gnptabc.3082insC and wild-type mice were analysed by mass spectrometry. The gangliosides were detected as precursors of m/z 290. The gangliosides were detected either as singly charged (1-) or doubly charged (2-) ions. Multiple minor cationized species are present especially for GT1. Intensities of the spectra were normalized to the main GD1 species. (B) The relative changes in the totals of anionic phospholipid classes in brain extracts of 10-month-old Gnptabc.3082insC and wild-type mice. The total intensity of all anionic species detected in each sample was set to 100 (*P < 0.05). PA = phosphatidic acid; PI = phosphatidylinositol; PS = phosphatidylserine; Sulf = sulphatide; Sulf-OH = alpha-hydroxysulphatide; BMP = bis(monoaclylglycero)phosphate; PG = phosphatidylglycerol. (D and E) Strong GM2 immunoreactivity (C) and moderate GM3 immunoreactivity (D) were detected in the retrosplenal cortex of 8-month-old Gnptabc.3082insC mice counterstained with Nissl staining. Scale bars = 20 µm. (E) Cholesterol accumulation was stained by filipin in Purkinje cells and granule layer of the cerebellum of 8-month-old Gnptabc.3082insC mice. Scale bars = 20 µm.
Immunohistochemical staining for GM2 and GM3 gangliosides confirmed their significant accumulation in vesicular structures in many neurons in the cerebral cortex as well as in hippocampal, subcortical, cerebellar and brainstem areas of Gnptabc.3082insC mice. Although neurons often appeared positive for accumulating gangliosides at 4 months of age, staining increased and was more intense and widespread by 8 months. Staining for GM2 tended to exceed that of GM3 at all ages (Fig. 6C and D). An accumulation of free cholesterol detectable by filipin histochemistry became evident coincident with ganglioside storage, with the pattern of staining appearing to most closely follow that of GM3 ganglioside. In 8-month-old Gnptabc.3082insC mice, neuronal storage of cholesterol was observed in widespread areas of the CNS. Strong filipin staining was detectable in Purkinje cells and the granular layer of the cerebellum (Fig. 6E).
When cultured embryonic mouse fibroblasts were analysed by double immunofluorescence microscopy, filipin staining was observed in Gnptabc.3082insC cells, which partially co-localized with Lamp1-positive lysosomal membranes (Supplementary Fig. 11). Significantly less staining for Lamp1 and cholesterol was found in fibroblasts of wild-type mice. The accumulation of fucosylated and high mannose-type oligosaccharide branches as well as sialic acid-containing GM2, GM3 gangliosides and cholesterol in the Gnptabc.3082insC brain indicates that α-l-fucosidase, α-mannosidase, β-galactosidase, sialidase and the Niemann–Pick C2 protein involved in cholesterol release from lysosomes are more affected than other lysosomal proteins by the absence of the mannose 6-phosphate recognition marker and their subsequent lysosomal localization.
Impaired autophagy and accumulation of p62 aggregates in neuronal tissue of Gnptabc.3082insC mice
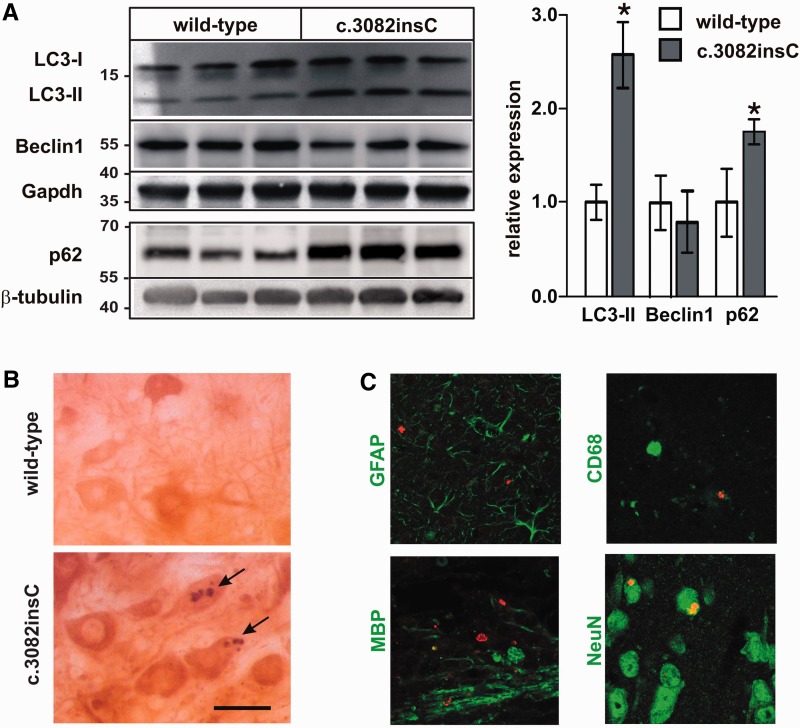
To identify other cellular processes affected by dysfunctional lysosomes, the autophagy–lysosome pathway responsible for removing effete organelles and some proteins was examined in Gnptabc.3082insC brain. Disruption of autophagy is known to result in accumulation of polyubiquitinylated protein aggregates and neurodegeneration (Jansen et al., 2007; Komatsu et al., 2007). Western blotting showed that in brain tissue of Gnptabc.3082insC mice, the amounts of the autophagic markers lipidated LC3-II and the detergent-insoluble p62 were increased by 2.5- and 1.8-fold, respectively, compared with wild-type brain (Fig. 7A). Autophagy, however, did not appear to be activated, as evident from unchanged expression of beclin1. Immunohistochemical analysis of brain sections of 11-month-old mice revealed p62-positive aggregates throughout the brain (Fig. 7B and Supplementary Fig. 12), which were not observed in wild-type brain. Double immunofluorescence microscopy showed that p62-positive aggregates were found exclusively in anti-neuronal nuclei-immunoreactive neuronal cells of Gnptabc.3082insC mice and not in astroglial, microglial or oligodendroglial cells (Fig. 7C). These data suggest that the lysosomal dysfunction in Gnptabc.3082insC mice led to accumulation of autolysosomes in neuronal cells, which subsequently resulted in formation of p62 aggregates.
Figure 7.
Accumulation of autophagosomes and p62-positive aggregates in the brain of Gnptabc.3082insC mice. (A) Western blot analyses and densitometric evaluation for LC3-II and beclin1 in extracts and in Triton™ X-100-insoluble fractions from cerebella of 10-month-old wild-type and Gnptabc.3082insC mice (mean ± SD, n = 3, *P < 0.05). (B) Immunohistochemistry for p62 showing p62 aggregates (arrows) in the soma of large neurons of the pons from 11-month-old Gnptabc.3082insC mice. Scale bars = 5 µm. (C) Double immunofluorescence staining reveals the co-localization of p62 (red) and the neuronal marker NeuN in Gnptabc.3082insC brain sections. No co-localization of p62 was found with marker proteins for astroglial cells (GFAP), microglial cells (CD68) and oligodendroglial cells (MBP).
Discussion
The insertion of a single cytosine in exon 16 of Gnptab, corresponding to a mutation found in patients with mucolipidosis II, resulted in a clinical disease course in mice with ultrastructural, histological and biochemical findings that were highly similar to those reported in patients (Kornfeld and Sly, 2001). Birth weight and length were below normal, and affected pups revealed facial dysmorphism, severe skeletal abnormalities and premature death. In Gnptabc.3082insC mice, we observed (i) greatly increased levels of lysosomal hydrolases in serum; (ii) typical inclusion bodies in fibroblasts, as well as storage material in other tissues, including brain; and (iii) characteristic alterations in transport and activities of lysosomal enzymes in fibroblasts and liver tissue. If the progressive neurodegeneration and gliosis observed in Gnptabc.3082insC mice also occurred in human patients with mucolipidosis II, they might be the cause for the psychomotor retardation that characterizes the human disease. These clinical features and symptoms found in human and cat mucolipidosis II and Gnptabc.3082insC mice differ from those reported for Gnptab−/− mice (Gelfman et al., 2007, Vogel et al., 2009). Although the liver, brain and muscle tissue appeared grossly unaffected, immunohistochemical analysis of brain sections of Gnptab−/− mice showed age-dependent lesions and widespread reactive microgliosis. In addition, the limb-clasping response observed in 4–6-month-old Gnptab−/− mice indicates neurological abnormalities (P. Vogel and S. Kornfeld, personal communication). These data suggest that at least in the Gnptab−/− brain, the pathogenic alterations represent a ‘milder’ course of the disease than observed in Gnptabc.3082insC mice. It is unclear whether the differences between Gnptab−/− and Gnptabc.3082insC mice are due to different mouse substrains (129S5/SvEvBrd versus 129/SvJ, respectively) used to generate the mice or secondary effects caused by the complete absence of the α/β-subunits of GlcNAc-1-phosphotransferase in the Gnptab−/− mice in comparison with low amounts of the C-terminal truncated α/β-subunit precursor localized in the endoplasmic reticulum of Gnptabc.3082insC mouse cells. It will be important to resolve this question in future studies.
In human and mouse mucolipidosis II disease, the targeting efficiency of lysosomal enzymes is variable in a cell-type- and tissue-specific manner (Owada and Neufeld, 1982, Waheed et al., 1982, Boonen et al., 2011). Here, we showed that the lack of mannose 6-phosphate residues in Gnptabc.3082insC mice led to a loss and missorting of the Niemann–Pick C2 protein involved in the lysosomal export of low-density lipoprotein-derived unesterified cholesterol in fibroblasts and brain. The activities of several glycosidases were strongly reduced in cultured Gnptabc.3082insC fibroblasts, whereas the activities of cathepsin B and D were comparable with wild-type cells. Furthermore, western blot and pulse-chase experiments demonstrated that distinct lysosomal enzymes, e.g. cathepsin D, can partly reach lysosomes in a mannose 6-phosphate-independent manner. Moreover, uptake of circulating lysosomal enzymes into the liver of Gnptabc.3082insC mice by various carbohydrate-specific receptors may explain the normal or increased activities of several lysosomal enzymes (Köster et al., 1994). In contrast, in whole-brain extracts of Gnptabc.3082insC mice, the specific activities of e.g. β-galactosidase, α-l-fucosidase and arylsulphatase A were found to be reduced by 25–50% of wild-type brain, whereas others such as α-mannosidase, cathepsin B and cathepsin D were significantly increased. Total activity measurements as well as western blot analysis cannot distinguish between intra- and extracellular localization, which might explain the discrepancies between reduced or even increased activities of lysosomal enzymes and accumulation of non-degraded storage material. The role of alternative mannose 6-phosphate-independent transport systems with compensatory and potential selective properties for soluble proteins in lysosomal trafficking in Gnptabc.3082insC brain remains to be studied. Candidates for alternative receptor proteins are sortilin, the lysosome-integrated membrane protein 2 (Limp-2), low-density lipoprotein receptor-related protein or megalin (Hiesberger et al.; 1998; Lefrancois et al., 2003; Nielsen et al., 2007; Reczek et al., 2007; Braulke and Bonifacino, 2009). The variable content of acid hydrolases and proteins in lysosomes as well as alterations in their activity due to impaired proteolytic maturation in lysosomes appear to be responsible for the clinical phenotype, which combines features of mucopolysaccharidoses and sphingolipidoses (Spranger and Wiedemann, 1970). Therefore, in mucolipidosis II, the accumulation of a variety of compounds can be expected, depending on the extent and deficiency of single or multiple proteins or enzymes, the substrate specificity of the latter and the presence of tissue-specific substrates (Ballabio and Gieselmann, 2009). This is further revealed by the ultrastructural variability of storage material in Gnptabc.3082insC mouse brain ranging from floccular and electron-opaque to electron-dense material, to membranous and zebra bodies and curvilinear and fingerprint inclusions, as have been described in the brains of patients with mucolipidosis II and cats (Martin et al., 1984; Bosshard et al., 1996). The appearance of these inclusions is characteristic for storage of oligosaccharides, glycosaminoglycans and glycolipids. Furthermore, the accumulation of fucosylated branched oligosaccharides in the brain and the prominent staining pattern of L. tetragonolobus and A. aurantia lectin, mainly detecting fucose in α1,3- and α1,6-linked oligosaccharides, suggest that α-l-fucosidase activity within lysosomes is significantly reduced in the brain of Gnptabc.3082insC mice. Similarly, the detection of various high mannose-type oligosaccharides in whole-brain extracts indicates decreased activity of α-mannosidase in brain lysosomes of Gnptabc.3082insC mice. Thus, the identification and compositional analysis of mucolipidosis II-associated storage material in the brain allow conclusions by which mannose 6-phosphate-containing enzymes/proteins could be thought as limiting for overall function of lysosomes.
In this study, we also found 2-fold elevated levels of GM2 and GM3 gangliosides in brain tissue of Gnptabc.3082insC mice. This might be due to reduced levels of β-hexosaminidase A and sialidase in lysosomes, respectively, or the partial loss of the mannose 6-phosphate-containing GM2 activator proteins or saposin B, which are required for the hydrolysis of GM2 and GM3 (Schulze et al., 2009). Alternatively, the absence of Niemann–Pick C2 protein, which is closely associated with the storage of these gangliosides in Niemann–Pick C disease, might indirectly cause these accumulations (Walkley and Vanier, 2009). On the other hand, there are numerous other lysosomal diseases, such as mucopolysaccharidoses, neuronal ceroid lipofuscinoses and glycoproteinoses, that exhibit significant secondary accumulation of GM2 and GM3 (Walkley and Vanier, 2009), which have been implicated as possible factors causing neuron dysfunction and neurodegeneration, e.g. by the generation of toxic lyso-GM2 derivatives. In addition, the accumulation of different species of the anionic lipid BMP, required for degradation of distinct sphingolipids in brain tissue of Gnptabc.3082insC mice, support the idea that the hydrolytic functions of lysosomes are impaired. Increased levels of BMP in association with alterations in the intracellular distribution of cholesterol have also been reported in the brain of cathepsin D-deficient mice (Jabs et al., 2008) and may play a critical role in efficient sorting of lysosomal hydrolases (Kobayashi et al., 1999). The accumulation of BMP in lysosomal membranes may also affect the interaction and function of lysosomal proteins as well as the degradation of distinct lipids (Gallala and Sandhoff, 2011).
The increase in LC3-II and the ubiquitin- and LC3-binding protein p62 was found to be associated with the accumulation of cytoplasmic inclusions in neuronal cells of Gnptabc.3082insC mice. An accumulation of autophagosomes containing undigested cytoplasmic material has been reported in several exocrine glands but not in the brain of Gnptab−/− mice (Boonen et al., 2011). Together with the prevalence of autophagic structures, our data suggest that the constitutive autophagy responsible for the turnover of long-lived proteins, damaged organelles or removal of aggregate-prone proteins is severely impaired and might have irreversible cytotoxic effects in Gnptabc.3082insC brain. The aberrant accumulation of organelles and storage material in focally swollen axons of Gnptabc.3082insC mice, characteristic for a progressive dystrophy of axons, is similar to observations in mice with Purkinje-cell-specific loss of autophagy. The local control of axonal membrane transport and turnover by autophagy may be important in preventing axonopathy in association with neurodegeneration (Komatsu et al., 2007). At present, it is not clear whether the maturation of autophagosomes into autolysosomes is inhibited in neurons of Gnptabc.3082insC mice due to the loss of hydrolytic capability of lysosomes, alterations in lysosomal membranes prohibiting the fusion between autophagosomes and lysosomes or both. Unlike the accumulation seen in neuronal cells, p62 aggregates have not been observed in glial cells of Gnptabc.3082insC mice, suggesting cell-type-specific differences in the autophagic activity.
Conclusion
The complete loss of mannose 6-phosphate residues on lysosomal enzymes resulted in a progressive neurodegeneration in Gnptabc.3082insC mice. The identification of storage material in the brain of mucolipidosis II mice demonstrated differences among lysosomal proteins in their susceptibility to the loss of mannose 6-phosphate residues. In mouse brain, the mannose 6-phosphate recognition marker is clearly more important for the targeting and/or activities of Niemann–Pick C2 protein, α-l-fucosidase, α-mannosidase or β-hexosaminidase A than for cathepsin D or cathepsin B, which can use mannose 6-phosphate-independent transport pathways to reach lysosomes in Gnptabc.3082insC brain cells. Further investigation is needed to understand these differences and to identify receptors involved. Furthermore, the efficiency of constitutive autophagic degradation appears to decline with progression of the disease, leading to neuronal accumulation of p62-associated degradation products. Thus, the generated novel mouse model of mucolipidosis II shows biochemical and clinical features of human disease and provides new insight in the role of lysosomes in degradation of brain-cell-specific substrates and into molecular mechanisms of neurodegeneration.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (STO761/2-1; FOR885; GRK1459; SFB877 to K.K., S.M., A.K.R., S.P. and T.B.) and the NIH–HD045561 (S.U.W.). The mass spectrometry facility used in this study for the analysis of glycans was funded by the European Community (FEDER), the Région Nord-Pas de Calais (France) and the Université des Sciences et Technologies de Lille I.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank the Microscopic Imaging Facility of the University Medical Center Hamburg-Eppendorf for using the spinning disk microscope, and Johannes Brand for technical assistance.
Glossary
Abbreviations
- BMP
bis(monoacylglycero)phosphate
- GlcNAc
N-acetylglucosamine
References
- Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta. 2009;1793:684–96. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Blanz J, Stroobants S, Lüllmann-Rauch R, Morelle W, Ludemann M, D’Hooge R, et al. Reversal of peripheral and central neural storage and ataxia after recombinant enzyme replacement therapy in alpha-mannosidosis mice. Hum Mol Genet. 2008;17:3437–45. doi: 10.1093/hmg/ddn237. [DOI] [PubMed] [Google Scholar]
- Boonen M, van Meel E, Oorsschot V, Klumperman J, Kornfeld S. Vacuolization of mucolipidosis type II mouse exocrine gland cells represents accumulation of autolysosomes. Mol Biol Cell. 2011;22:1135–47. doi: 10.1091/mbc.E10-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard NU, Hubler M, Arnold S, Briner J, Spycher MA, Sommerlade HJ, et al. Spontaneous mucolipidosis in a cat: an animal model of human I-cell disease. Vet Pathol. 1996;33:1–13. doi: 10.1177/030098589603300101. [DOI] [PubMed] [Google Scholar]
- Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–14. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Cathey SS, Leroy JG, Wood T, Eaves K, Simensen RJ, Kudo M, et al. Phenotype and genotype in mucolipidosis II and III alpha/beta: a study of 61 probands. J Med Genet. 2010;47:38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M, Kübler B, Wendland M, Neifer K, Schmidt B, Zapf J, et al. Proteolysis of insulin-like growth factors (IGF) and IGF binding proteins by cathepsin D. Endocrinology. 1997;138:3797–803. doi: 10.1210/endo.138.9.5418. [DOI] [PubMed] [Google Scholar]
- Cox TM, Cachon-Gonzalez MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226:241–54. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- Damme M, Stroobants S, Walkley SU, Lüllmann-Rauch R, D’Hooge R, Fogh J, et al. Cerebellar alterations and gait defects as therapeutic outcome measures for enzyme replacement therapy in alpha-mannosidosis. J Neuropathol Exp Neurol. 2011;70:83–94. doi: 10.1097/NEN.0b013e31820428fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieu MS, Pfeffer SR. Niemann-Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc Natl Acad Sci USA. 2011;108:18932–6. doi: 10.1073/pnas.1110439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Steet H, Sias C, Steet R. Altered chondrocyte differentiation and extracellular matrix homeostasis in a zebrafish model for mucolipidosis II. Am J Pathol. 2009;175:2063–75. doi: 10.2353/ajpath.2009.090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallala HD, Sandhoff K. Biological function of the cellular lipid BMP-BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem Res. 2011;36:1594–600. doi: 10.1007/s11064-010-0337-6. [DOI] [PubMed] [Google Scholar]
- Gelfman CM, Vogel P, Issa TM, Turner CA, Lee WS, Kornfeld S, et al. Mice lacking alpha/beta subunits of GlcNAc-1-phosphotransferase exhibit growth retardation, retinal degeneration, and secretory cell lesions. Invest Ophthalmol Vis Sci. 2007;48:5221–8. doi: 10.1167/iovs.07-0452. [DOI] [PubMed] [Google Scholar]
- Hickman S, Neufeld EF. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972;49:992–9. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Huttler S, Rohlmann A, Schneider W, Sandhoff K, Herz J. Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP) EMBO J. 1998;17:4617–25. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs S, Quitsch A, Käkelä R, Koch B, Tyynelä J, Brade H, et al. Accumulation of bis(monoacylglycero)phosphate and gangliosides in mouse models of neuronal ceroid lipofuscinosis. J Neurochem. 2008;106:1415–25. doi: 10.1111/j.1471-4159.2008.05497.x. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–57. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Käkelä R, Somerharju P, Tyynelä J. Analysis of phospholipid molecular species in brains from patients with infantile and juvenile neuronal-ceroid lipofuscinosis using liquid chromatography-electrospray ionization mass spectrometry. J Neurochem. 2003;84:1051–65. doi: 10.1046/j.1471-4159.2003.01602.x. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Fischer D, Achord D, Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977;60:1088–93. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karageorgos LE, Isaac EL, Brooks DA, Ravenscroft EM, Davey R, Hopwood JJ, et al. Lysosomal biogenesis in lysosomal storage disorders. Exp Cell Res. 1997;234:85–97. doi: 10.1006/excr.1997.3581. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–18. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kollmann K, Pohl S, Marschner K, Encarnacao M, Sakwa I, Tiede S, et al. Mannose phosphorylation in health and disease. Eur J Cell Biol. 2010;89:117–23. doi: 10.1016/j.ejcb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, et al. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci. USA. 2007;104:14489–94. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster A, von Figura K, Pohlmann R. Mistargeting of lysosomal enzymes in Mr 46000 mannose 6-phosphate receptor-deficient mice is compensated by carbohydrate-specific endocytotic receptors. Eur J Biochem. 1994;224:685–9. doi: 10.1111/j.1432-1033.1994.00685.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Sly WS. I-cell disease and pseudo-Hurler polydystrophy: disorders of lysosomal enzyme phosphorylation and localization. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill Inc.; 2001. pp. 3421–52. [Google Scholar]
- Kudo M, Bao M, D’Souza A, Ying F, Pan H, Roe BA, et al. The alpha- and beta-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J Biol Chem. 2005;280:36141–9. doi: 10.1074/jbc.M509008200. [DOI] [PubMed] [Google Scholar]
- Kudo M, Brem MS, Canfield WM. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-Hurler polydystrophy) are caused by mutations in the GlcNAc-phosphotransferase α/β-subunits precursor gene. Am J Hum Genet. 2006;78:451–63. doi: 10.1086/500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Canfield WM. Structural requirements for efficient processing and activation of recombinant human UDP-N-acetylglucosamine:lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase. J Biol Chem. 2006;281:11761–8. doi: 10.1074/jbc.M513717200. [DOI] [PubMed] [Google Scholar]
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22:6430–7. doi: 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy JG, Demars RI. Mutant enzymatic and cytological phenotypes in cultured human fibroblasts. Science. 1967;157:804–6. doi: 10.1126/science.157.3790.804. [DOI] [PubMed] [Google Scholar]
- Lübke T, Lobel P, Sleat DE. Proteomics of the lysosome. Biochim Biophys Acta. 2009;1793:625–35. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner K, Kollmann K, Schweizer M, Braulke T, Pohl S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science. 2011;333:87–90. doi: 10.1126/science.1205677. [DOI] [PubMed] [Google Scholar]
- Martin JJ, Leroy JG, van Eygen M, Ceuterick C. I-cell disease. A further report on its pathology. Acta Neuropathol. 1984;64:234–42. doi: 10.1007/BF00688114. [DOI] [PubMed] [Google Scholar]
- Mazrier H, Van Hoeven M, Wang P, Knox VW, Aguirre GD, Holt E, et al. Inheritance, biochemical abnormalities, and clinical features of feline mucolipidosis II: the first animal model of human I-cell disease. J Hered. 2003;94:363–73. doi: 10.1093/jhered/esg080. [DOI] [PubMed] [Google Scholar]
- Micsenyi MC, Dobrenis K, Stephney G, Pickel J, Vanier MT, Slaugenhaupt SA, et al. Neuropathology of the Mcoln1(-/-) knockout mouse model of mucolipidosis type IV. J Neuropathol Exp Neurol. 2009;68:125–35. doi: 10.1097/NEN.0b013e3181942cf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle W, Jimenez JC, Cieniewski-Bernard C, Dei-Cas E, Michalski JC. Characterization of the N-linked glycans of Giardia intestinalis. Glycobiology. 2005a;15:549–59. doi: 10.1093/glycob/cwi035. [DOI] [PubMed] [Google Scholar]
- Morelle W, Page A, Michalski JC. Electrospray ionization ion trap mass spectrometry for structural characterization of oligosaccharides derivatized with 2-aminobenzamide. Rapid Commun Mass Spectrom. 2005b;19:1145–58. doi: 10.1002/rcm.1900. [DOI] [PubMed] [Google Scholar]
- Müller-Loennies S, Galliciotti G, Kollmann K, Glatzel M, Braulke T. A novel single-chain antibody fragment for detection of mannose 6-phosphate-containing proteins: application in mucolipidosis type II patients and mice. Am J Pathol. 2010;177:240–7. doi: 10.2353/ajpath.2010.090954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, et al. Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci USA. 2007;104:5407–12. doi: 10.1073/pnas.0700330104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Sundaram RK, Huang E, Ghoshal S, Kumar U, Pentchev PG, et al. Neuronal localization and association of Niemann Pick C2 protein (HE1/NPC2) with the postsynaptic density. Neuroscience. 2004;128:561–70. doi: 10.1016/j.neuroscience.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Owada M, Neufeld EF. Is there a mechanism for introducing acid hydrolases into liver lysosomes that is independent of mannose 6-phosphate recognition? Evidence from I-cell disease. Biochem Biophys Res Commun. 1982;105:814–20. doi: 10.1016/0006-291x(82)91042-7. [DOI] [PubMed] [Google Scholar]
- Petrey AC, Flanagan-Steet H, Johnson S, Fan X, De la Rosa M, Haskins ME, et al. Excessive activity of cathepsin K is associated with cartilage defects in a zebrafish model of mucolipidosis II. Dis Model Mech. 2012;5:177–90. doi: 10.1242/dmm.008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raas-Rothschild A, Cormier-Daire V, Bao M, Genin E, Salomon R, Brewer K, et al. Molecular basis of variant pseudo-hurler polydystrophy (mucolipidosis IIIC) J Clin Invest. 2000;105:673–81. doi: 10.1172/JCI5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, Hughes H, Blanz J, Jin X, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–83. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Roces DP, Lüllmann-Rauch R, Peng J, Balducci C, Andersson C, Tollersrud O, et al. Efficacy of enzyme replacement therapy in alpha-mannosidosis mice: a preclinical animal study. Hum Mol Genet. 2004;13:1979–88. doi: 10.1093/hmg/ddh220. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–40. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Schulze H, Kolter T, Sandhoff K. Principles of lysosomal membrane degradation: cellular topology and biochemistry of lysosomal lipid degradation. Biochim Biophys Acta. 2009;1793:674–83. doi: 10.1016/j.bbamcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Zheng H, Qian M, Lobel P. Identification of sites of mannose 6-phosphorylation on lysosomal proteins. Mol Cell Proteomics. 2006;5:686–701. doi: 10.1074/mcp.M500343-MCP200. [DOI] [PubMed] [Google Scholar]
- Spranger JW, Wiedemann HR. The genetic mucolipidoses. Diagnosis and differential diagnosis. Humangenetik. 1970;9:113–39. doi: 10.1007/BF00278928. [DOI] [PubMed] [Google Scholar]
- Tiede S, Storch S, Lübke T, Henrissat B, Bargal R, Raas-Rothschild A, et al. Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase. Nat Med. 2005a;11:1109–12. doi: 10.1038/nm1305. [DOI] [PubMed] [Google Scholar]
- Tiede S, Muschol N, Reutter G, Cantz M, Ullrich K, Braulke T. Missense mutations in N-acetylglucosamine-1-phosphotransferase alpha/beta subunit gene in a patient with mucolipidosis III and a mild clinical phenotype. Am J Med Genet A. 2005b;137A:235–40. doi: 10.1002/ajmg.a.30868. [DOI] [PubMed] [Google Scholar]
- Tondeur M, Vamos-Hurwitz E, Mockel-Pohl S, Dereume JP, Cremer N, Loeb H. Clinical, biochemical, and ultrastructural studies in a case of chondrodystrophy presenting the I-cell phenotype in tissue culture. J Pediatr. 1971;79:366–78. doi: 10.1016/s0022-3476(71)80143-9. [DOI] [PubMed] [Google Scholar]
- Vogel P, Payne BJ, Read R, Lee WS, Gelfman CM, Kornfeld S. Comparative pathology of murine mucolipidosis types II and IIIC. Vet Pathol. 2009;46:313–24. doi: 10.1354/vp.46-2-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A, Pohlmann R, Hasilik A, von Figura K, van Elsen A, Leroy JG. Deficiency of UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase in organs of I-cell patients. Biochem Biophys Res Commun. 1982;105:1052–8. doi: 10.1016/0006-291x(82)91076-2. [DOI] [PubMed] [Google Scholar]
- Walkley SU, Sikora J, Micsenyi M, Davidson C, Dobrenis K. Lysosomal compromise and brain dysfunction: examining the role of neuroaxonal dystrophy. Biochem Soc Trans. 2010;38:1436–41. doi: 10.1042/BST0381436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley SU, Vanier MT. Secondary lipid accumulation in lysosomal disease. Biochim Biophys Acta. 2009;1793:726–36. doi: 10.1016/j.bbamcr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, et al. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl- accumulation. Science. 2010;328:1401–3. doi: 10.1126/science.1188072. [DOI] [PubMed] [Google Scholar]
- Winchester B. Lysosomal metabolism of glycoproteins. Glycobiology. 2005;15:1R–15R. doi: 10.1093/glycob/cwi041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.