Synopsis
Brain injury is a frequent co-morbidity in chronically ventilated preterm infants. However, the molecular basis of the brain injury remains incompletely understood. The focus of this paper is the subtler (diffuse) form of brain injury that has white matter and gray matter lesions, without germinal matrix hemorrhage-intraventricular hemorrhage, posthemorrhagic hydrocephalus, or cystic periventricular leukomalacia. The purpose of this review is to synthesize data that suggest diffuse lesions to white matter and gray matter are collateral damage related to ventilator strategy. Evidence is introduced from the two large-animal, physiological models of evolving neonatal chronic lung disease that suggest an epigenetic mechanism may underlie the collateral damage.
Keywords: White matter injury, gray matter injury, bronchopulmonary dysplasia, neonatal chronic lung disease
Introduction
The brain of chronically ventilated preterm infants is vulnerable to injury during the days, weeks, or months of ventilation support with oxygen-rich gas that are necessary to keep them alive 98. Familiar lesions are germinal matrix hemorrhage-intraventricular hemorrhage, posthemorrhagic hydrocephalus, or periventricular leukomalacia, particularly with parenchymal cysts 6. These gross histopathological lesions are not the focus of this review. Instead, we focus on more subtle diffuse lesions that lead to abnormal neural function and subsequent suboptimal neurodevelopmental outcome.
Diffuse lesions to white matter and gray matter are recognized among chronically ventilated preterm infants 72. Diffuse white matter lesions within the first week of life are characterized histopathologically at autopsy as palely-stained and soft regions of degeneration of white matter and thinning of the corpus callosum. Gray matter lesions also occur 75. Gray matter lesions are characterized by diffuse neuronal loss in deeper cerebral cortical layers, the hippocampus, thalamus, globus pallidus, and cerebellar Purkinje cell layers in the dentate nucleus 75. A mixture of diffuse white and gray matter lesions presumably contributes to subtler delays and/or deficits in neurodevelopment and impairments in motor skills, learning disabilities, attention deficit/hyperactivity disorders, and/or anxiety disorders in former preterm children 72, 90.
Subtler adverse neurodevelopmental outcomes impact health and quality of life of the survivors and their families. The outcomes also increase the cost for health care borne by the families and society. Therefore, brain injury in chronically ventilated preterm neonates should be viewed as a significant national public health issue.
In spite of increasing recognition of diffuse lesions to white matter and gray matter in chronically ventilated preterm infants, the molecular basis of the lesions remains incompletely understood. The purpose of this review is to synthesize data that suggest ventilator strategy leads to collateral white and gray matter lesions. Evidence will be introduced to suggest an epigenetic mechanism may underlie the collateral damage.
Prematurity as the setting for collateral damage to the brain
Prematurity contributes to about a third of all infant deaths in the United States 59. Mortality rate is greatest among infants born at or before 25 weeks of gestation (http://www.nichd.nih.gov/about/org/cdbpm/pp/prog_epbo/epbo_case.cfm).
Infants born prematurely are at risk of acute respiratory distress or failure because the future gas-exchange regions of the lung are not developed structurally. The relative or absolute absence of surfactant contributes to collapse of the distal airspaces (atelectasis), which contributes to functional mismatch of ventilation and perfusion that exacerbates gas exchange 4.
Two treatments are used routinely for anticipated preterm birth and subsequent respiratory distress. One treatment is antenatal corticosteroid administration to the mother who is in premature labor. The objective of administering corticosteroids antenatally is to stimulate production of endogenous surfactant in the fetus 50. The other treatment is postnatal surfactant replacement to the preterm infant 62. The intended consequence of these treatments is to reduce surface tension and thereby increase lung compliance and gas exchange 21, 36, 39, 48 Preterm infants with larger surfactant pools are likely to be supported by nasal continuous positive airway pressure (nasal CPAP). The rationale for using nasal CPAP is to avoid or minimize endotracheal intubation and positive-pressure ventilation support 5, 17, 26, 92. However, when nasal CPAP is insufficient, the remedy is endotracheal intubation and positive-pressure ventilation using an oxygen-rich gas mixture. High inflation pressure and mean airway pressure may be necessary to recruit the collapsed distal airspaces to achieve ventilation and oxygenation targets. Infants who do not recover from acute respiratory distress and require prolonged positive-pressure ventilation with oxygen-rich gas are predisposed to develop neonatal chronic lung disease (also called bronchopulmonary dysplasia, BPD or the “new” BPD) 2, 68.
Vulnerability of the immature brain in chronically ventilated preterm neonates
Vulnerability for collateral damage to the brain is related in part to the width of the developmental window and types of developmental processes that occur within the window. The developmental window of vulnerability is 22 to 36 weeks of gestation (Table 1). The types of developmental processes during this window include proliferation, differentiation, and migration of neurons and glia. During the same period, neuronal circuits develop as synapses form and synaptic connections are optimized through a process of synaptic stabilization. Functional circuits become evident as spontaneous electroencephalographic bursts 10, 30, 43, 45, 63. A structural manifestation of these processes occurs in the subplate layer of neurons, which attains maximal thickness around the 36th week of gestation. Cortical folding also occurs during this developmental window. From 28 weeks of gestation to postnatally after birth at term gestation, the earlier exuberant proliferation of neurons and glia is pruned by apoptosis to optimize their numbers. Myelination occurs from the 36th week of gestation to 2 to 3 years postnatally.
Table 1.
Timing of Structural Development of the Human Brain
| Time from conception | Developmental Process | References |
|---|---|---|
| 3–4 weeks | Formation of neuroectoderm | 96 |
| 3–4 weeks | Primary neurulation | 15,86,96 |
| 5–10 weeks | Formation of prosencephalon and hemispheres | 96 |
| 5–10 weeks to ? years postnatally | Cerebral angiogenesis and formation of the blood-brain barrier | 66 |
| 7–10 weeks | Generation of the subplate layer of neurons (from germinal matrix) | 46 |
| 10–15 weeks to ? years postnatally | Neurogenesis | 96 |
| 12–24 weeks | Neuronal migration | 27,28,78,79,85,96 |
| 16–24 weeks | Blood vessel density in subcortical white matter is low | 65 |
| 20 weeks to ? years postnatally | Synaptogenesis and synaptic stabilization | 12,16,71 |
| 20 weeks to ? years postnatally | Gliogenesis | 9,14,95,96 |
| 22–36 weeks | Maximal thickness of the subplate layer of neurons (from germinal matrix) is reached | 46 |
| 23–29 weeks | Cortical folding and spontaneous EEG bursts; delta brushes | 10,30,43,45,63 |
| 28–36 weeks | Blood vessel density in deep white matter is low | 37,65 |
| 28 weeks to ? years postnatally | Neuronal and glial apoptosis | 96 |
| 36 weeks to ? years postnatally | Myelination | 9,96 |
Details regarding development of the cerebral circulation are incomplete. The cerebral circulation expands during the period of 22 to 36 weeks of gestation (Table 1). However, blood vessel formation lags in deep regions, including the germinal matrix, periventricular white matter, and corpus callosum, and deep gray matter structures such as the basal ganglia and hippocampus 32. The lag in blood vessel formation in deep regions of the brain is part of the basis of the ‘watershed’ explanation of vulnerability of germinal matrix, periventricular white matter, and deep cortical gray matter. Developmental immaturity of the blood-brain barrier also creates vulnerability for circulating toxic molecules to access the brain parenchyma 64Stonestreet, 2000 #9358.
The immature brain is vulnerable to fluctuations in systemic blood pressure 31, 55, 74. Fluctuations are dangerous because of the immaturity of autoregulation, which may expose the brain to increased or decreased blood pressure, a characteristic that is referred to as “pressure-passive”. For example, systemic hypotension combined with a pressure-passive cerebral circulation may lead to hypoxic ischemia (hypoperfusion) of white matter and/or gray matter 23, 69, 99. A subsequent vulnerability may be reperfusion injury, particularly in association with systemic hypertension 3, 24, 31, 42, 53, 55, 56, 59, 72, 76, 77, 91, 102. Another cerebrovascular vulnerability is increased intrathoracic pressure in preterm infants who are intubated and ventilated or who develop a pneumothorax. Increased intrathoracic pressure may affect cerebral perfusion pressure and/or flow 16, 71.
Molecules that participate in brain injury are numerous. Inflammatory cytokines and chemokines, with or without infection, are participants 18, 20, 34, 81. Their participation, in part, is mediated by platelet and neutrophil adhesion in cerebral blood vessels 35, 51. Other molecular participants are reactive species of oxygen or nitrogen that are generated during reperfusion following hypoxia-ischemia 35, 70. In addition, expression of growth factors is reduced, notably, insulin-like growth factor-1 (IGF-1) 8, 29. IGF-1 is emphasized here because its regulation of expression is relevant to an epigenetic hypothesis that will be proposed subsequently in this review.
Developmental processes that are vulnerable in the brain are summarized in Table 2. The vulnerabilities include germinal matrix injury, diffuse white matter injury, and diffuse gray matter injury.
Table 2.
Vulnerability of the Brain of Preterm Neonates
| Time from conception | Developmental Process | Vulnerability | References |
|---|---|---|---|
| 10–15 weeks to ? years postnatally | Neurogenesis |
Germinal matrix injury1 |
|
| 12–24 weeks | Neuronal migration | ||
| 16–24 weeks | Low vessel density in subcortical white matter | ||
| 20 weeks to ? years postnatally | Synaptogenesis and synaptic stabilization | 1,60,93,95 | |
| 20 wk to ? years postnatally | Gliogenesis | Diffuse white matter lesions (periventricular leukomalacia)2 |
2,19,33,37,41,60,61,65,73,82,87,90,94 |
| 22–36 weeks | Maximal thickness of the subplate layer of neurons (from germinal matrix) is reached | ||
| 28–36 weeks | Blood vessel density in deep white matter is low | 3,60,89,97 | |
| 28 weeks to ? years postnatally | Neuronal and glial apoptosis | Diffuse gray matter lesions3 | |
| 36 weeks to ? years postnatally | Myelination | ||
Collateral damage to the brain related to ventilation strategy: an epigenetic hypothesis
A trend over the last decade is initial use of nasal CPAP or early extubation to nasal CPAP. The rationale is to reduce the primary injury to the preterm infant’s lungs and secondary injury to other organs, notably the brain. However, the impact of ventilation strategy on mechanisms of pathogenesis of brain injury in preterm neonates remains uncertain. To this end, insights are being made using large-animal, physiological models of neonatal chronic lung disease in which brain injury is an accompaniment. The models share the common feature of brain injury without intraventricular hemorrhage or cystic periventricular leukomalacia.
Preterm baboon model
Brain injury occurs in preterm baboons that have evolving neonatal chronic lung disease. An informative experiment compared brain injury outcomes when the preterm baboons were weaned from mechanical ventilation to nasal CPAP 52 One group was weaned at 24 hours of life (early nasal CPAP); the other starting at 5 days of life (delayed nasal CPAP). The principal results showed brain injury in both nasal CPAP groups compared to the fetal lambs that were not ventilated. In both groups, brain injury was diffuse, without hemorrhage or cystic infarction, and affected white matter and gray matter. Secondarily, the results showed that injury severity was less in the early nasal CPAP group compared to the delayed nasal CPAP group. The latter result suggests that duration of mechanical ventilation is directly related to brain injury. The molecular mechanisms of these pathological changes were not a part of the study.
Preterm lamb model
We are using chronically ventilated preterm lambs to identify molecular mechanisms that are involved in injury to multiple organs, notably the lung and brain. Our studies led us to propose an epigenetic hypothesis for the pathogenesis of neonatal chronic lung disease and its associated co-morbidities, including brain injury.
We focus on epigenetics because of its role in fetal and perinatal adaptation 58, 103. Importantly, epigenetic regulation of gene expression in the perinatal period is associated with re-adjustment in gene expression in response to changes in environment 40. For example, intrauterine growth restriction, which predisposes to preterm labor and delivery, is associated with reduced levels of insulin-like growth factor-1 (IGF-1) in the liver of rat pups 22. In addition, many of the affected epigenetic characteristics persist postnatally, in conjunction with persistently less IGF-1 mRNA and protein levels. Does the same hold true in chronically ventilated preterm lambs? Before answering that question, concepts about epigenetics will be provided.
Epigenetic regulation of gene expression uses modifications to chromatin, the unit of which is the nucleosome. Nucleosomes have 146 bp of DNA wrapped around an octomeric core of histone proteins 54. The modifications constitute an epigenetic code for regulation of gene expression by directing interactions between transcription complexes with DNA. Because the interactions are dynamic, transcriptional levels of proteins are adjusted and re-adjusted over time, including long-term (life-long).
Epigenetic regulation of gene expression can use several mechanisms, including histone modifications, DNA methylation, microRNAs, and nucleosome positioning 47, 83. Histone modifications consist of acetylation, methylation, phosphorylation, and ubiquitination. Enzymes that add histone modifications include histone acetylases and methyltransferases 57. Enzymes that take away histone modifications include histone deacetylases (HDACs) and demethylases. DNA methylation occurs on a cytosine base where cytosine precedes and is linked to a guanosine by phosphate. Therefore, the modification is referred to as methylation of CpG. Methylation involves DNA methyltransferases 47. Methyl-cytosine demethylation involves the ten-eleven translocation (Tet) gene family 38, 88. Biologically, these enzymatic processes appear to be coordinated dynamically 11. MicroRNAs, short RNA molecules that do not code for a protein, silence target genes by binding to their 3' untranslated regions 84. Nucleosome positioning regulates gene transcription by exposing or not exposing transcription start sites to transcription complexes and RNA polymerase, as will be described near the end of this review. Understanding how these processes are regulated and dysregulated will be necessary to identify their roles in health and disease.
The dynamic nature of epigenetics is complex, making generalizations difficult when comparing one cell type to another, one organ to another, one species to another, along the continuum of developmental processes, and among diseases. Difficulty is even greater in the in vivo context, where interplay among epigenetic, genetic, physiologic, and pathophysiologic processes occurs dynamically in the setting of a whole organism 1. Although daunting, testing epigenetic hypotheses in vivousing large-animal models of human disease, and in humans, is necessary to translate epigenetic concepts and principles to understand the epigenetic basis of human health and disease.
Our recent work suggests that epigenetic characteristics in the brain are affected in chronically ventilated preterm lambs with evolving neonatal chronic lung disease (unpublished data). Highlights of some of these studies will be summarized from two perspectives of epigenetic characteristics: (1) genome-wide and (2) candidate-gene-specific epigenetic characteristics. Comparative results will be highlighted for the brain and lung from the same preterm lambs.
Genome-wide epigenetic characteristics
Some clinical evidence suggests that nasal CPAP reduces the risk for premature infants to develop neonatal chronic lung disease and its co-morbidities 92. However, the biological basis for the different outcomes is not known. To address this unknown, we are pursuing studies in chronically ventilated preterm lambs. One study is assessing genome-wide epigenetic characteristics in the brain (and lung) of preterm lambs that are supported by either positive-pressure ventilation or a version of bubble nasal CPAP that delivers high-frequency ventilation at the level of the nose (nasal HFV) 80.
We measure levels of several histone modifications in homogenates of brain and lung tissue. For example lower levels of genome-wide acetylation of histone 3 lysine 14 (H3K14ac) occurred in the brain and lung of mechanically ventilated preterm lambs compared to preterm lambs supported by nasal HFV. Another histone modification that we measured is trimethylated H3K36 (H3K36me3). Lower levels of H3K36me3 are detected in the mechanically ventilated group compared to the nasal HFV group. These initial results suggest that ventilation strategy affects genome-wide histone covalent modifications.
Gene-specific epigenetic characteristics
A limitation of our aforementioned studies is that the results do not identify an epigenetically regulated gene as a molecular culprit. An alternative approach is to test the participation of a candidate gene in vivo.
Our current research efforts are focusing on IGF-1 as a prototypic, epigenetically-regulated gene 22, 40. Our rationale is a follows. First, IGF-1 is involved in brain and lung development 49. Second, IGF-1 expression is increased in the lung of preterm infants who died during acute respiratory distress or neonatal chronic lung disease 13. Whether IGF-1 expression is affected in the brain of chronically ventilated preterm infants remains to be determined. Of note, IGF-1 expression is decreased in the brain in other models of perinatal insult in which growth is disrupted and adverse long-term neurodevelopmental outcomes are detected 44, 100. Together, these characteristics make the IGF-1 gene a candidate to study in the context of brain vulnerability to injury in chronically ventilated preterm lambs.
Our initial results suggest that IGF-1 expression is affected in the brain and lung of ventilated preterm lambs. For example, the brain of mechanically ventilated preterm lambs appears to have lower IGF-1 protein levels compared to preterm lambs supported by nasal HFV. Lower levels of IGF-1 protein in the brain are consistent with the reduction that occurs in other models of perinatal insult, such as intrauterine growth restriction 22, 44. In the same preterm lambs, the lung appears to have higher levels of IGF-1 protein in the mechanically ventilated group compared to the nasal HFV group. Elevated IGF-1 in the lung of the mechanically ventilated preterm lambs is consistent with results obtained at autopsy of preterm infants who died with respiratory failure 13.
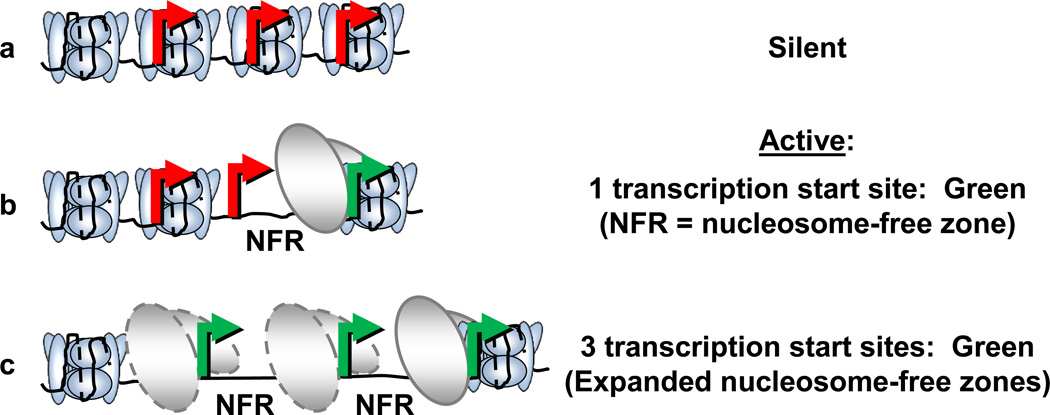
Finding less IGF-1 protein in the brain, and more in the lung, in mechanically ventilated preterm lambs prompted us to ask how the changes may occur. As a start, we are determining the pattern of histone modifications (the histone code) along the length of the IGF-1 gene locus in sheep. The rationale for assessing the histone code is because its pattern plays a prominent role in regulating gene transcription 22, 40, 44. Briefly, chromatin is condensed in the heterochromatic state. While chromatin is condensed, access of transcription complexes to transcription start sites is blocked physically by close-packing of nucleosomes (Figure 1A). For transcription to be initiated, the chromatin has to open, forming euchromatin. Opening chromatin, and therefore permitting transcription, is mediated in part by acetylation of histones. As acetylation occurs, open space is created upstream of a transcription start site or sites by unraveling a nucleosome, creating a nucleosome-free zone (Figure 1B). The open space provides access for transcription complexes to bind to the exposed transcription start site. Conversely, eventual silencing to reduce or stop transcription occurs when other histones are methylated, especially tri-methylated. A consequence of increasing tri-methylation of histones is decreasing acetylation of histones. This reciprocal shift is believed to help condense the chromatin along the body of the gene to prevent inappropriate transcription initiation from occurring downstream of the designated transcription start site.
Figure 1.
Schema transcription start sites in a promoter region. (a) Transcriptionally silent promoter. Transcription start sites are shown in red because they are covered by nucleosomes and therefore are not accessible to transcription complexes. (b) Transcriptionally active promoter. A nucleosome-free region (NFR) is shown where a nucleosome is displaced, exposing a transcription start site (green). A transcription complex (ovals) is shown immediately upstream to the exposed (green) transcription start site, where access is available for RNA polymerase. (c) Transcription is potentially enhanced by expanded nucleosome-free zones, which expose 3 transcription start sites to RNA polymerase.
With the aforementioned outline of the process of epigenetic regulation of gene expression in mind, how might we explain the results in the brain and lung of preterm lambs, depending on ventilation strategy? First, we must admit that the scenario is hypothetical. That is, we do not have direct cause-and-effect evidence. Taking into account that epigenetic regulation is organ-specific and that every cell has the same genetic information, yet each organ develops uniquely, we hypothesize that uniqueness is conferred by cell-specific or cell- and organ-specific epigenetic regulation. We specifically hypothesize that genome-wide hypoacetylation in the brain shifts the balance toward more apoptosis, and less proliferation of astrocytes and oligodendrocytes (Alvord abs). Reduced numbers of both types of glia would decrease IGF-1 locally in the brain because astrocytes and oligodendrocytes synthesize and secrete IGF-1 7, 25, 67, 101
How might we explain more IGF-1 in the lung of the same chronically ventilated preterm lambs, given that the lung also has genome-wide histone hypoacetylation? We propose that lung-specific epigenetic mechanisms may lead to more expression of pulmonary IGF-1. One mechanism is the source of IGF-1 mRNA and protein in the lung is mesenchymal cells (Heber’s refs). We showed that mesenchymal cell proliferation exceeds apoptosis in the walls of the distal airspaces of preterm lambs that are supported by mechanical ventilation compared to nasal HFV 80. Another possibility is that mechanical ventilation may lead to promiscuous transcription of IGF-1 in the lung. To explain this hypothesis, attention is drawn again to Figure 1. Figure 1C depicts several transcription start sites that are uncovered because numerous nucleosomes are absent (nucleosome-free zones). We have new preliminary data to suggest that ventilation of preterm lambs exposes multiple transcription start sites for IGF-1 in the lung. By comparison, only one site appears to be used in the lung of fetal lambs that are not allowed to breathe. We propose that exposure of more transcription start sites across a longer length of the gene locus upstream of promoter 1 may contribute to more IGF-1 transcription in the lung of chronically ventilated preterm lambs. Of course, other epigenetic mechanisms may also influence gene expression, such as regulation of elongation and/or termination of transcription. Such potential epigenetic mechanisms will need to be investigated.
A hypothesis that remains to be tested is whether early changes in the histone code provide survival advantage (adaptation) in the short-run, but also exact a cost later in life, the cost being vulnerability to subsequent insults and/or onset of adult diseases. This hypothesis will be important to test because survival advantage in the short-term may create vulnerability to disease (e.g.tumor formation), to subsequent insults later in life (e.g.recurrent respiratory tract infections), and/or to adult-onset diseases (e.g.obesity, diabetes, cardiovascular disease). To this end, our newest studies use a modified protocol whereby preterm lambs are weaned from ventilation support and fostered for the equivalent of 2 years or 6–8 years of postnatal life in humans. The intention of these new studies is to correlate epigenetic characteristics of gene regulation with long-term neurodevelopmental delays and deficits among former preterm, ventilated lambs.
Ultimately, a histone modification-specific transgene construct will be necessary to definitively show that a specific epigenetic modification causes a specific phenotype.
Summary
Structural and functional immaturity of a preterm neonate’s lung necessitates use of antenatal steroids, postnatal surfactant replacement therapy, postnatal ventilation support with oxygen-rich gas, and other measures to keep the neonate alive. Acute lung injury often ensues. If recovery from acute lung injury does not happen, endotracheal intubation and prolonged positive-pressure ventilation support with oxygen-rich gas may be necessary, leading to neonatal chronic lung disease. A frequent co-morbidity of evolving neonatal chronic lung disease is brain injury. However, the molecular basis of the brain injury remains incompletely understood. This void is addressed by use of large-animal, physiological models of brain injury in the setting of evolving neonatal chronic lung disease. An advantage of the chronically ventilated preterm baboon and lamb models is that the setting of preterm birth is uncomplicated by antenatal conditions, such as intrauterine infection or asphyxia, that may potentiate brain injury. Also, neither chronic model is associated with intraventricular hemorrhage or cystic lesions of periventricular white matter. Consequently, drawing mechanistic conclusions is more straightforward. On the other hand, the less complicated setting for the large-animal models may be viewed as a disadvantage because the models lack antenatal conditions that may potentiate injury to the lung and brain. Nonetheless, new mechanistic insights are being provided by the chronic animal models. In particular, involvement of epigenetics in the pathogenesis of lung and brain injury opens new doors for mechanistic studies that may provide opportunities for interventions. Endotracheal intubation with prolonged positive-pressure ventilation support and oxygen-rich gas appears to change epigenetic determinants of gene expression. Such ventilation support appears to scramble the histone code, at least based on results for IGF-1. This effect does not appear to occur when nasal HFV is used. A caveat, of course, will be whether altering epigenetic regulatory patterns provides short-term adaptations that improve survival during the neonatal period, but creates unintended consequences later in life. This caveat makes the pursuit a high-risk/high-potential-benefit for understanding and improving health and outcomes of chronically ventilated preterm infants.
Key Points.
Brain injury is a frequent co-morbidity in chronically ventilated preterm infants. However, the molecular basis of the brain injury remains incompletely understood.
This article focuses on the subtler (diffuse) form of brain injury that has white matter and gray matter lesions, without germinal matrix hemorrhage-intraventricular hemorrhage, posthemorrhagic hydrocephalus, or cystic periventricular leukomalacia.
This clinical review synthesizes data that suggest diffuse lesions to white matter and gray matter are collateral damage related to ventilator strategy.
Evidence is introduced from the two large-animal, physiological models of evolving neonatal chronic lung disease that suggest an epigenetic mechanism may underlie the collateral damage.
Acknowledgements
Appreciation is expressed to Dr. Robert McKnight, Dr. Ronald Bloom, Dr. Robert H. Lane, and Dr. Lisa Joss-Moore for their invaluable input.
Portions of this work were supported by NIH grants HL062875 and HL110002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Albertine has no conflicts of interest to disclose.
References
- 1.Aagaard-Tillery KM, Grove K, Bishop J, Ke X, Fu Q, McKnight R, Lane RH. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41:91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertine KH, Pysher TJ. Impaired lung growth after injury in premature lung. In: In: Polin RA, Fox WW, Abman S, editors. Fetal and Neonatal Physiology. Fourth ed. New York: Elsevier Sciences; 2011. pp. 1039–1047. [Google Scholar]
- 3.Ando M, Takashima S, Mito T. Endotoxin, cerebral blood flow, amino acids and brain damage in young rabbits. Brain Dev. 1988;10:365–370. doi: 10.1016/s0387-7604(88)80094-9. [DOI] [PubMed] [Google Scholar]
- 4.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child. 1959;97:517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 5.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, Epstein MF, Fitzhardinge PM, Hansen CB, Hansen TN, et al. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79:26–30. [PubMed] [Google Scholar]
- 6.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. doi: 10.1001/archneur.1962.04210050022004. [DOI] [PubMed] [Google Scholar]
- 7.Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death in the oligodendrocyte lineage. J Neurobiol. 1992;23:1221–1230. doi: 10.1002/neu.480230912. [DOI] [PubMed] [Google Scholar]
- 9.Battin MR, Maalouf EF, Counsell SJ, Herlihy AH, Rutherford MA, Azzopardi D, Edwards AD. Magnetic resonance imaging of the brain in very preterm infants: visualization of the germinal matrix, early myelination, and cortical folding. Pediatrics. 1998;101:957–962. doi: 10.1542/peds.101.6.957. [DOI] [PubMed] [Google Scholar]
- 10.Biagioni E, Frisone MF, Laroche S, Kapetanakis BA, Ricci D, Adeyi-Obe M, Lewis H, Kennea N, Cioni G, Cowan F, Rutherford M, Azzopardi D, Mercuri E. Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol. 2007;118:53–59. doi: 10.1016/j.clinph.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 12.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 13.Chetty A, Andersson S, Lassus P, Nielsen HC. Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr Pulmonol. 2004;37:128–136. doi: 10.1002/ppul.10415. [DOI] [PubMed] [Google Scholar]
- 14.Childs AM, Ramenghi LA, Evans DJ, Ridgeway J, Saysell M, Martinez D, Arthur R, Tanner S, Levene MI. MR features of developing periventricular white matter in preterm infants: evidence of glial cell migration. AJNR Am J Neuroradiol. 1998;19:971–976. [PMC free article] [PubMed] [Google Scholar]
- 15.Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 16.Coughtrey H, Rennie JM, Evans DH. Variability in cerebral blood flow velocity: observations over one minute in preterm babies. Early Hum Dev. 1997;47:63–70. doi: 10.1016/s0378-3782(96)01769-0. [DOI] [PubMed] [Google Scholar]
- 17.Dani C, Bertini G, Pezzati M, Cecchi A, Caviglioli C, Rubaltelli FF. Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks' gestation. Pediatrics. 2004;113:e560–563. doi: 10.1542/peds.113.6.e560. [DOI] [PubMed] [Google Scholar]
- 18.Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- 19.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neurosci. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 20.Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H. Bacterial endotoxin sensitizes the immature brain to hypoxic--ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 21.Enhorning G, Shennan A, Possmayer F, Dunn M, Chen CP, Milligan J. Prevention of neonatal respiratory distress syndrome of tracheal instillation of surfactant: a randomized clinical trial. Pediatrics. 1985;76:145–153. [PubMed] [Google Scholar]
- 22.Fu Q, Yu X, Callaway CW, Lane RH, McKnight RA. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. FASEB J. 2009;23:2438–2449. doi: 10.1096/fj.08-124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funato M, Tamai H, Noma K, Kurita T, Kajimoto Y, Yoshioka Y, Shimada S. Clinical events in association with timing of intraventricular hemorrhage in preterm infants. J Pediatr. 1992;121:614–619. doi: 10.1016/s0022-3476(05)81157-6. [DOI] [PubMed] [Google Scholar]
- 24.Gilles FH, Averill DRJ, Kerr CS. Neonatal endotoxin encephalopathy. Ann Neurol. 1977;2:49–56. doi: 10.1002/ana.410020108. [DOI] [PubMed] [Google Scholar]
- 25.Gluckman P, lempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxicischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 26.Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK. Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. NEJM. 1971;284:1333–1340. doi: 10.1056/NEJM197106172842401. [DOI] [PubMed] [Google Scholar]
- 27.Gressens P. Mechanisms and disturbances of neuronal migration. Pediatr Res. 2000;48:725–730. doi: 10.1203/00006450-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gressens P, Richelme C, Kadhim HJ, Gadisseux JF, Evrard P. The germinative zone produces the most cortical astrocytes after neuronal migration in the developing mammalian brain. Biol Neonate. 1992;61:4–24. doi: 10.1159/000243526. [DOI] [PubMed] [Google Scholar]
- 29.Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–462. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Guevara FR, Giannuzzi R, Nosralla Mde O, Vignolo P, Moriette G, Maier MA. Positive slow waves in the EEG of premature infants between 24 and 36 weeks of conceptional age. Clin Neurophysiol. 2008;119:180–189. doi: 10.1016/j.clinph.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- 32.Hambleton G, Wigglesworth JS. Origin of intraventricular haemorrhage in the preterm infant. Arch Dis Child. 1976;51:651–659. doi: 10.1136/adc.51.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haynes RL, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC. Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol. 2009;118:391–399. doi: 10.1007/s00401-009-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J. The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res. 1997;41:607–616. doi: 10.1203/00006450-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1988;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 37.Inage YW, Itoh M, Takashima S. Correlation between cerebrovascular maturity and periventricular leukomalacia. Pediatr Neurol. 2000;22:204–208. doi: 10.1016/s0887-8994(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 38.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jobe AH. Pulmonary surfactant therapy. NEJM. 1993;328:861–868. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- 40.Joss-Moore LA, Albertine KH, Lane RH. Epigenetics and the developmental origins of lung disease. Mol Genet Metab. 2011;104:61–66. doi: 10.1016/j.ymgme.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr. 2004;144:809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- 44.Ke X, Lei Q, James SJ, Kelleher SL, Melnyk S, Jernigan S, Yu X, Wang L, Callaway CW, Gill G, Chan GM, Albertine KH, McKnight RA, Lane RH. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol Genomics. 2006;25:16–28. doi: 10.1152/physiolgenomics.00093.2005. [DOI] [PubMed] [Google Scholar]
- 45.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsaki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 46.Kostovic I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- 47.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Kwong MS, Egan EA, Notter RH, Shapiro DL. Double-blind clinical trial of calf lung surfactant extract for the prevention of hyaline membrane disease in extremely premature infants. Pediatrics. 1985;76:585–592. [PubMed] [Google Scholar]
- 49.Lallemand AV, Ruocco SM, Joly PM, Gaillard DA. In vivo localization of the insulin-like growth factors I and II (IGF I and IGF II) gene expression during human lung development. Int J Dev Biol. 1995;39:529–537. [PubMed] [Google Scholar]
- 50.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–520. [PubMed] [Google Scholar]
- 51.Liu XH, Kwon D, Schielke GP, Yang GY, Silverstein FS, Barks JD. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Loeliger M, Inder T, Cain S, Ramesh RC, Camm E, Thomson MA, Coalson J, Rees SM. Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics. 2006;118:1640–1653. doi: 10.1542/peds.2006-0653. [DOI] [PubMed] [Google Scholar]
- 53.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- 54.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 55.Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 56.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. NEJM. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 57.Marmorstein R, Trievel RC. Histone modifying enzymes: structures, mechanisms, and specificities. Biochim Biophys Acta. 2009;1789:58–68. doi: 10.1016/j.bbagrm.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathers JC, McKay JA. Epigenetics - potential contribution to fetal programming. Adv Exp Med Biol. 2009;646:119–123. doi: 10.1007/978-1-4020-9173-5_13. [DOI] [PubMed] [Google Scholar]
- 59.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep. 2011;59:1–30. [PubMed] [Google Scholar]
- 60.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- 62.Merritt TA, Hallman M, Bloom BT, Berry C, Benirschke K, Sahn D, Key T, Edwards D, Jarvenpaa AL, Pohjavuori M, et al. Prophylactic treatment of very premature infants with human surfactant. NEJM. 1986;315:785–790. doi: 10.1056/NEJM198609253151301. [DOI] [PubMed] [Google Scholar]
- 63.Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- 64.Mirro R, Leffler CW, Armstead WM, Busija DW. Positive-pressure ventilation alters blood-tobrain and blood-to-CSF transport in neonatal pigs. J Appl Physiol. 1991;70:584–589. doi: 10.1152/jappl.1991.70.2.584. [DOI] [PubMed] [Google Scholar]
- 65.Miyawaki T, Matsui K, Takashima S. Developmental characteristics of vessel density in the human fetal and infant brains. Early Hum Dev. 1998;53:65–72. doi: 10.1016/s0378-3782(98)00043-7. [DOI] [PubMed] [Google Scholar]
- 66.Mollgard K, Saunders NR. The development of the human blood-brain and blood-CSF barriers. Neuropathol Appl Neurobiol. 1986;12:337–358. doi: 10.1111/j.1365-2990.1986.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 67.Mozell RL, McMorris FA. Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. J Neurosci Res. 1991;30:382–390. doi: 10.1002/jnr.490300214. [DOI] [PubMed] [Google Scholar]
- 68.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. NEJM. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 69.Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- 70.Palmer C, Menzies SL, Roberts RL, Pavlick G, Connor JR. Changes in iron histochemistry after hypoxic-ischemic brain injury in the neonatal rat. J Neurosci Res. 1999;56:60–71. doi: 10.1002/(SICI)1097-4547(19990401)56:1<60::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 71.Perlman J, Thach B. Respiratory origin of fluctuations in arterial blood pressure in premature infants with respiratory distress syndrome. Pediatrics. 1988;81:399–403. [PubMed] [Google Scholar]
- 72.Perlman JM. Neurobehavioral deficits in premature graduates of intensive care--potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339–1348. doi: 10.1542/peds.108.6.1339. [DOI] [PubMed] [Google Scholar]
- 73.Perlman JM. White matter injury in the preterm infant: an important determination of abnormal neurodevelopment outcome. Early Hum Dev. 1998;53:99–120. doi: 10.1016/s0378-3782(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 74.Perlman JM, Volpe JJ. Cerebral blood flow velocity in relation to intraventricular hemorrhage in the premature newborn infant. J Pediatr. 1982;100:956–959. doi: 10.1016/s0022-3476(82)80527-1. [DOI] [PubMed] [Google Scholar]
- 75.Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pryds O, Andersen GE, Friis-Hansen B. Cerebral blood flow reactivity in spontaneously breathing, preterm infants shortly after birth. Acta Paediatr Scand. 1990;79:391–396. doi: 10.1111/j.1651-2227.1990.tb11482.x. [DOI] [PubMed] [Google Scholar]
- 77.Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J Pediatr. 1989;115:638–645. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- 78.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 79.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 80.Reyburn B, Li M, Metcalfe DB, Kroll NJ, Alvord J, Wint A, Dahl MJ, Sun J, Dong L, Wang ZM, Callaway C, McKnight RA, Moyer-Mileur L, Yoder BA, Null DM, Lane RH, Albertine KH. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. 2008;178:407–418. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 82.Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 83.Roth DM, Balch WE. Modeling general proteostasis: Proteome balance in health and disease. Curr Opin Cell Biol. 2011;23:126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS Journal. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 85.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- 86.Smith JL, Schoenwolf GC. Neurulation: coming to closure. Trends Neurosci. 1997;20:510–517. doi: 10.1016/s0166-2236(97)01121-1. [DOI] [PubMed] [Google Scholar]
- 87.Smith ME, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 88.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tanaka F, Ozawa Y, Inage Y, Deguchi K, Itoh M, Imai Y, Kohsaka S, Takashima S. Association of osteopontin with ischemic axonal death in periventricular leukomalacia. Acta Neuropathol. 2000;100:69–74. doi: 10.1007/s004010051194. [DOI] [PubMed] [Google Scholar]
- 90.Taylor HG, Burant CJ, Holding PA, Klein N, Hack M. Sources of variability in sequelae of very low birth weight. Child Neuropsychol. 2002;8:163–178. doi: 10.1076/chin.8.3.163.13500. [DOI] [PubMed] [Google Scholar]
- 91.Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 92.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 2000;105:1194–1201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 93.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 95.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 96.Volpe JJ. Overview: normal and abnormal human brain development. Ment Retard Dev Disabil Res Rev. 2000;6:1–5. doi: 10.1002/(SICI)1098-2779(2000)6:1<1::AID-MRDD1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 97.Volpe JJ. Subplate neurons--missing link in brain injury of the premature infant? Pediatrics. 1996;97:112–113. [PubMed] [Google Scholar]
- 98.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, Wright LL, Ehrenkranz RA, Stoll BJ, Fanaroff AA. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804. doi: 10.1016/j.jpeds.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 99.Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19:103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- 100.Woods KA, Camacho-Hubner C, Barter D, Clark AJ, Savage MO. Insulin-like growth factor I gene deletion causing intrauterine growth retardation and severe short stature. Acta Paediatr Suppl. 1997;423:39–45. doi: 10.1111/j.1651-2227.1997.tb18367.x. [DOI] [PubMed] [Google Scholar]
- 101.Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshioka H, Goma H, Sawada T. [Cerebral hypoperfusion and leukomalacia] No To Hattatsu. 1996;28:128–129. [PubMed] [Google Scholar]
- 103.Zeisel SH. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am J Clin Nutr. 2009;89:1488S–1493S. doi: 10.3945/ajcn.2009.27113B. [DOI] [PMC free article] [PubMed] [Google Scholar]